Abstract
This study aims to evaluate the incidence of non-thyroid illness syndrome (NTIS) among patients diagnosed as lung cancer and its association with the stage of the disease, Eastern Cooperative Oncology Group (ECOG) performance score, nutritional parameters, and survival. We enrolled 120 patients that 71 of them with newly diagnosed and staged non-small cell lung cancer and 49 of them small-cell lung cancer. The cases were examined for thyroid function tests, ECOG performance score, and nutritional evaluation before treatment. Also, cases were evaluated for their overall survival rates. NTIS was identified in 30 (42 %) of the 71 non-small cell lung cancer patients and 22 (44 %) of the 49 small-cell lung cancer patients. NTIS was more frequent among advanced stage of cases. Serum albumin level, cholesterol level, lymphocyte level, and body mass index were detected to be significantly low and ECOG performance score was significantly high in cases with NTIS when compared to cases without NTIS. NTIS was found to be negatively correlated with body mass index, ECOG performance score, and serum albumin level, and it was positively correlated with disease stage. NTIS was detected significantly as a poor prognostic factor for lung cancer. NTIS was frequently seen in cases with non-small cell lung cancer and small-cell lung cancer. NTIS can be used as a predictor of poor prognosis for lung cancer patients.
Keywords: Lung Cancer, Lung Cancer Patient, NSCLC Patient, Lymphocyte Count, Serum Albumin Level
Introduction
Non-thyroidal illness syndrome (NTIS) is a variable situation of abnormal thyroid hormone concentration that can rise in the serum following any acute or chronic illness that is not caused by an intrinsic abnormality in thyroid function [1]. Euthyroid sick syndrome (ESS) and non-thyroidal illness syndrome (NTIS) are terms used alternatively in the literature [2]. The laboratory parameters of NTIS include low serum levels of triiodothyronine (T3) and high levels of reverse T3, with normal or low levels of thyroxine (T4), and normal or low levels of thyroid-stimulating hormone (TSH) [3, 4].
The pathogenesis of the syndrome is still not well known; an imbalance between the activity of type I and type II deiodinase, a decreased hypothalamus and pituitary sensibility to thyroid hormones, and a reduced T4 protein binding and cellular uptake have been proposed [5]. It has been much debated whether NTIS represents a physiologic adaptive response to systemic illness, by which it lowers tissue energy requirements or conversely a maladaptive state, which induces a damaging hypothyroid state at tissue level [5]. The NTIS has been described in liver disease, renal failure, after stress or surgery, in elderly sick, malnutrition, malignancy, and after ingestion of drugs like amiodarone, corticosteroids, and propranolol [6]. The magnitude of changes in serum T3 and T4 do not depend on the type of illness but on its severity [7].
Prognosis of patients with lung cancer is related to the stage of the disease, gender, age, histological type, proliferate fraction, performance score, and nutritional and psychological state of the patients. A few studies showing that NTIS is observed in cancer patients and that prognosis is poorer for these patients have been published elsewhere [8–11].
In this study, we aimed to determine the incidence of NTIS, to identify its relation with the Eastern Cooperative Oncology Group (ECOG) performance score, nutritional parameters such as body mass index (BMI), lymphocyte count, serum albumin and cholesterol levels, the stage of the disease, and to clarify whether it can be used as a predictor of survival in non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) patients.
Materials and Methods
The study was conducted in one of the pulmonary divisions of a teaching hospital specialized in pulmonary diseases and thoracic surgery, located in the third biggest city of Turkey. Patients diagnosed histologically and sitologically as lung cancer between January 2007 and February 2009 were enrolled. Exclusion criteria were the following: intrinsic thyroid or pituitary-hypothalamic disease, use of special drugs known to affect serum thyroid hormone concentration such as glucocorticoids, amiodorone, β blockers, sucralfate, phenytoin, salicilates and rifampin, and any disease (such as secondary malignancy, diabetes mellitus, nephrotic syndrome, AIDS, chronic hepatic, renal disease, or other systemic infectious diseases) associated with thyroid function anomalies. Before treatment, all subjects were evaluated with ECOG performance scale [12]. The subjects underwent thyroid function tests, nutritional evaluation, and staging of the disease. Patients who missed their appointment were communicated via telephone. General survival time was described as the time passed from the time of diagnosis until death.
Diagnosis of NTIS was established when free triiodothyronine (f T3) and/or free thyroxin (f T4) levels were below the lower limit and TSH levels were within the normal or low limits mentioned. Patients with NTIS were classified into two groups as follows: (1) low serum level of f T3, normal serum level of f T4, and normal level of TSH as Type 1, (2) low serum level of f T3, low serum level of f T4, and normal and low serum level of TSH as type 2, (3) low serum level of f T3, high serum level of f T4, and normal serum level of TSH as type 3 [13]. The study was approved by the Ethics Committee of our hospital.
Thyroid Laboratory Tests
The normal range of serum hormone concentrations for our laboratory are as follows: 2.3–4.2 pg/mL for f T3, 0.89–1.76 ng/dL for f T4, and 0.35–5.50 mIU/mL for TSH. Serum thyroid hormone concentrations were analyzed by chemo-luminescence, immune-assay method in an ADVIA Centaur hormone analyzer.
Nutritional-Biochemical Evaluation
Anthropometric and biochemical measurements were done to assess the nutritional and biochemical state of the cases. For anthropometric measurement, BMI [weight (kg)/height (m2)] was calculated. Subjects were categorized according to World Health Organization classification (BMI <18 kg/m2 thin, 18–25 kg/m2 of acceptable weight, 25–30 kg/m2 of excessive weight, and >30 kg/m2 obese) [14]. For biochemical measurements albumin level (N 3.5–5.2 g/dL), cholesterol level (0–200 mg/dL), and lymphocyte count (N 1.0–5.0 × 103/mL) were quantified. Serum albumin and cholesterol levels were measured by a photometric method (Olympus Auto analyzer AU 2700) and lymphocyte counts were performed with ABX Pentra DX 120 device in the biochemistry laboratory of our hospital.
Tumor Staging
Cases with NSCLC were staged by using the TNM system proposed by the “American Joint Committee on Cancer” [14]. Cases with SCLC were staged by using the IASLC classification [15]. Positron emission tomography and CT or magnetic resonance imaging scan of the brain were used for staging in most of the patients. Transbronchial fine needle aspiration biopsy or mediastinoscopy was done for staging tumors of patients without any distant metastasis.
Statistical Evaluation
Statistical analyses were performed with SPSS 15.0 (statistical package for social sciences) software. Categorical data are expressed as numbers with frequencies and continuous data are expressed as medians with interquartile range (IQR). Comparisons between groups were done by Fisher’s exact test or Mann-Whitney U test where appropriate. For survival analysis, Kaplan-Meier method was used and distinction between the groups was compared by log rank test. Cox proportional hazard model as a multivariate analysis method was used in order to evaluate the factors affecting survival. A p value of <0.05 was considered as statistically significant.
Results
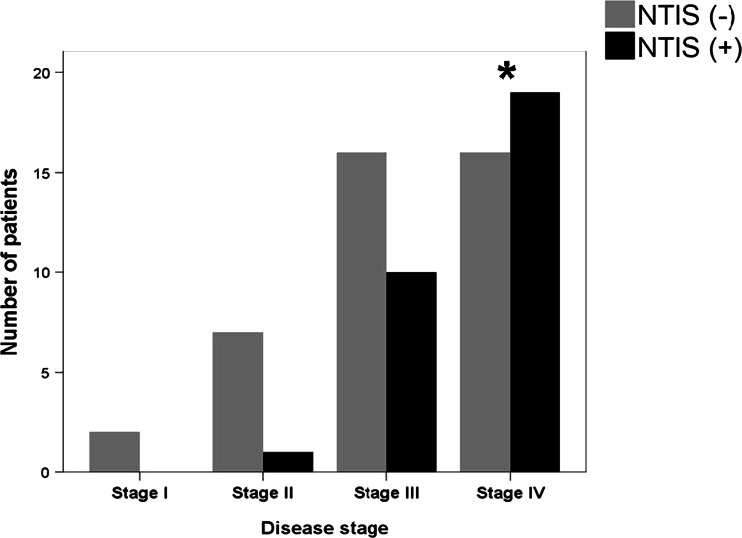
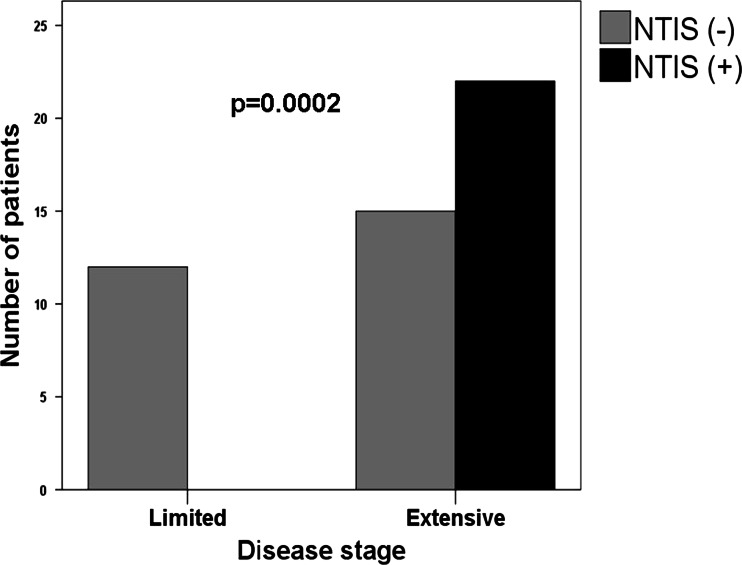
One hundred and twenty consecutive patients were enrolled during the study period. Out of 120 patients, 71 [62 (87 %) male, 9 (12 %) female] of them were diagnosed as NSCLC and 49 [45 (91 %) male, 4 (8.2 %) female] of them were diagnosed as SCLC. The stages of NSCLC were stage 1 in 2, stage 2 in 8, stage 3 in 26, and stage 4 in 35 patients. The stages of SCLC were limited disease in 12 and extensive disease in 37 patients. NTIS has been identified in 52 (43 %) of all patients, 30 (42 %) of them with case with NSCLC and 22 (45 %) of them case with SCLC. As the stage of the disease increased, NTIS was more frequent. NTIS incidence was significantly higher in stage 4 (54 %) compared to stage 2 (38 %) in cases with NSCLC (p = 0.032, Fig. 1) and in extensive disease (59 %) compared to limited disease (0 %) of SCLC (p = 0.0002, Fig. 2). Among the cases, 20 (66 %) with NSCLC and 14 (63 %) with SCLC were NTIS type 1; 10 (34) with NSCLC and 8 (37) with SCLC were NTIS type 2.
Fig. 1.
Distribution of non-thyroid illness syndrome among non-small cell lung cancer patients according to the stage of the disease. (NTIS non-thyroid illness syndrome, *p = 0.032 when compared with stage II)
Fig. 2.
Distribution of non-thyroid illness syndrome among small-cell lung cancer patients according to the stage of the disease. (NTIS non-thyroid illness syndrome)
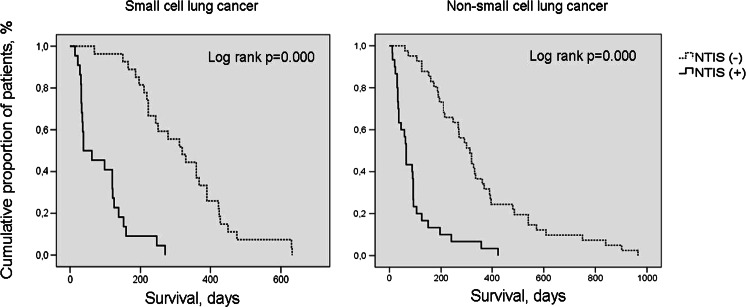
Considering the gender and age of the patients, there was no statistically significant difference between the subjects with or without NTIS in patients with lung cancer. BMI, serum albumin levels, cholesterol level, and lymphocyte counts were detected significantly lower and ECOG performance scores were detected significantly higher in cases with NTIS when compared to cases without NTIS in both NSCLC and SCLC patients. Median survival times were 65 vs. 314 days in cases with and without NTIS in NSCLC patients and 50 vs. 320 days in cases with and without NTIS in SCLC patients, respectively (Tables 1 and 2, Figs. 3 and 4). And these deaths of the patients are related to lung cancer like local relapse or metastases not dying for other causes. Cases with NTIS type 1 and type 2 showed no difference in general survival rate in patients with NSCLC and SCLC.
Table 1.
Comparison of NSCLC patients according to the presence of NTIS
| Patient characteristics | NTIS (−) | NTIS (+) | p |
|---|---|---|---|
| Age (years) | 62 (54–71) | 65 (58–72) | 0.34 |
| BMI (kg/m2) | 24 (22–27) | 18 (15–21) | 0.000 |
| Albumin (g/dl) | 4 (3.6–4.2) | 3.1 (2.1–3.4) | 0.000 |
| Cholesterol (mg/dl) | 185 (155–205) | 161 (111–176) | 0.003 |
| ECOG score | 1 (1–1) | 3 (1–4) | 0.000 |
| Hb (g/dl) | 12.3 (11.2–13.5) | 12.2 (9–13) | 0.12 |
| Lymphocyte (cell/mm3) | 1,860 (1,440–2,140) | 1,320 (1,127–1,730) | 0.006 |
| Survival (days) | 314 (194–396) | 65 (32–93) | 0.000 |
NSCLC non-small cell lung cancer, NTIS non-thyroid illness syndrome, BMI body mass index, ECOG eastern cooperative oncology group, Hb hemoglobin
Table 2.
Comparison of SCLC patients according to the presence of NTIS
| Patient characteristics | NTIS (−) | NTIS (+) | p |
|---|---|---|---|
| Age (years) | 57 (49–65) | 61 (53–67) | 0.17 |
| BMI (kg/m2) | 27 (25–29) | 15 (22–25) | 0.000 |
| Albumin (g/dl) | 4.1 (3.8–4.5) | 2.7 (2.2–3.4) | 0.000 |
| Cholesterol (mg/dl) | 213 (196–241) | 183 (166–234) | 0.013 |
| ECOG score | 1 (1–2) | 3 (2–4) | 0.000 |
| Hb (g/dl) | 13.2 (11.9–13.4) | 11.8 (10.2–13.9) | 0.19 |
| Lymphocyte (cell/mm3) | 1,456 (1,332–1,546) | 1,320 (1,127–1,730) | 0.048 |
| Survival (days) | 320 (218–423) | 50 (32–125) | 0.000 |
SCLC small-cell lung cancer, NTIS non-thyroid illness syndrome, BMI body mass index, ECOG eastern cooperative oncology group, Hb hemoglobin
Fig. 3.
Survival of small-cell and non-small cell lung cancer patients according to the presence of NTIS by Kaplan-Meier method. (NTIS non-thyroid illness syndrome)
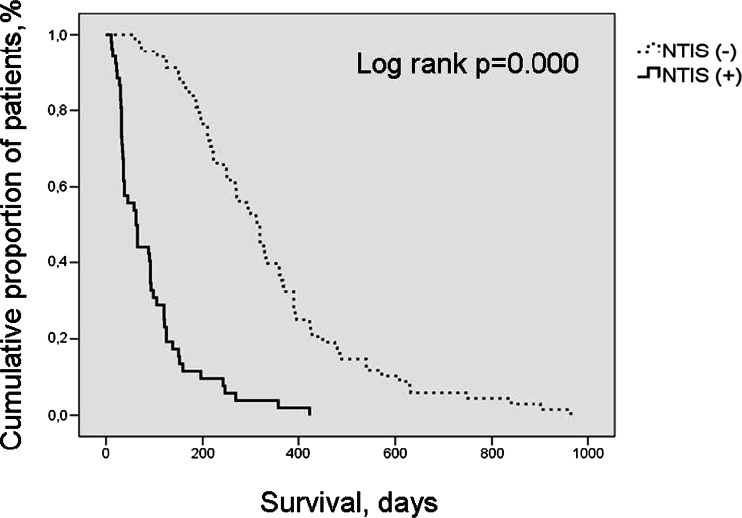
Fig. 4.
Overall survival of cancer patients according to the presence of NTIS by Kaplan-Meier method. (NTIS non-thyroid illness syndrome)
Factors found to be significant in the univariate analysis were introduced into a Cox proportional hazard model to detect the effect of NTIS on survival. After adjusting for these factors, we found that the presence of NTIS was still an independent risk factor for mortality (HR 4, 95 % CI 2.3–6.9, p = 0.000) in all cancer patients.
Discussion
The main finding of this study was that NTIS was frequently seen in cases with lung cancer, both in NSCLC and SCLC patients. As the stage of the disease increased, NTIS was more frequent. Also, NTIS can be used as a predictor of poor prognosis for lung cancer patients, especially a significant prognostic factor for patients with NSCLC.
Several studies described the presence of NTIS in pulmonary diseases and malignancies [8, 10, 16–20]. Chow et al. reported a 63 % incidence of NTIS in 40 pulmonary TB patients [16]. Kırkıl et al. reported a 74.3 % incidence of NTIS in 39 COPD patients [17]. Tellini et al. investigated thyroid hormone levels in 220 cases with malignancy in different organs and found out NTIS in 58 % of the patients [8]. Wehmann et al. found NTIS incidence as 54 % in cases with hematological malignancies [10]. Seyhan et al. found NTIS incidences as 35 % and patients with NTIS were elderly than patients without NTIS in NSCLC patients [18]. Our finding of incidence of NTIS was less than Tellini et al.’s and Wehmann et al.’s results. However, these studies did not include lung cancer patients. Our result for NSCLC is higher than what was found by Seyhan et al. This may be due to the high number of patients with advanced stage in our study. Although they reported high prevalence of NTIS in elderly subjects, we did not detect any relationship between age and NTIS.
We found that, as the stage of disease increased, NTIS was more frequent in patients with lung cancer. A positive association between the magnitude of thyroid hormone reduction and severity of disease is generally reported. Moreover, NTIS have been recently reported as the predictor of mortality [10, 19–25]. Wawrzynska et al. found out in their survey that NTIS is frequent in patients with severe respiratory failure requiring intensive care [19]. Karadağ et al. examined patients with COPD in attacks and stable periods. They found that thyroid hormones were significantly low in attack periods [20]. Caregaro et al. found that the frequency of NTIS in cirrhotic patients was about 30 % and that it was correlated with child classification defining severity of cirrhosis [21]. Moreover, they pointed out that survival rates were lower in NTIS cases. Opasich et al. examined patients with chronic heart failure and reported that NTIS cases have a higher mortality rate during follow-up period [22]. In a study including subjects in intensive care unit, mortality was significantly higher in cases with low serum level of f T3 compared with normal hormone test [23]. Schulte et al. examined patients with hematological malignancy who underwent bone marrow transplantation and reported that cases with NTIS have a higher mortality rate compared to those without NTIS [24]. Seyhan et al. pointed out that NTIS was more frequent in advanced stages of disease in their study population [18]. Our study showed consistent results with the previous ones, suggesting that NTIS is associated with disease severity and may be a predictor of high mortality.
Additionally, we found that the survival time is significantly low in cases with NTIS in both NSCLC and SCLC patients. Several studies demonstrating the presence of NTIS in malignant diseases and its association with poor prognosis have been published [8, 9, 18, 25]. Tognini et al. demonstrated that NTIS is an independent predictor of short-term survival in acutely ill hospitalized older patients [26]. Seyhan et al. reported that NTIS used to be a prognostic factor for NSCLC patients [18]. Our results confirm these findings. In the consensus reports on prognostic factors of lung cancer, stage of the disease and performance status were indicated as the main prognostic factors determining survival; it was also emphasized that cell type, tumor proliferative fraction, gender, weight loss, serum albumin level, and nutritional and psychological states were significant prognostic factors that can affect survival [27]. In this study, Seyhan et al. put forward that low survival time in cases with NTIS was associated with poor performance state and low albumin levels [18]. We also demonstrated that there was an association between presence of NTIS and initial performance score and low albumin level that confirms previous studies.
The most prominent alteration are low f T3 alone or with f T4 and normal or inappropriately low TSH. Low serum f T3 is the most common type according to the literature as justified by our data. Twenty (66 %) of cases with NSCLC patients and 14 (63 %) of cases with SCLC had only decreased f T3. Wang et al. reported that low serum f T3 level was only an independent predictor of ICU mortality among the complete thyroid indicators [28]. However, it was reported that TSH levels were normal or just below normal in 80 % of NTIS patients but decreased in 10 % and that prognosis was poorer in the later [29, 30]. In this study, TSH levels were within normal limits in all of the patients.
Nutritional insufficiencies, fasting, and calorie limitation have been reported to play a role in NTIS development. Because of the loss of appetite and increased catabolism in lung cancer, feeding disorders and nutritional insufficiencies develop. Schulte et al., in their study on NTIS incidence among bone marrow transplant patients, found that NTIS cases had lower BMI and serum albumin levels [24]. Tellini et al. described the correlation between NTIS and albumin level and degree of weight loss in oncology patients [8]. Seyhan et al. showed significant correlations between NTIS and nutritional parameters including BMI and serum albumin level [18]. In our study similar to the previous ones, we found BMI, serum albumin levels, and cholesterol level were significantly lower in cases with NTIS when compared to cases without NTIS in both NSCLC and SCLC patients.
There has been much interest recently in the roles of cytokines in the pathogenesis of NTIS. However, their significance remains unclear. Several inflammatory cytokines, such as IL1b, IL6, and TNF-α, can suppress, via direct or indirect pathways, the thyroid function at different levels. These cytokines appears to be more frequently involved in the reduction of the deiodinase 1 activity [31]. Feelders et al. detected that oncology patients with NTIS had higher TNF-α and IL-6 levels compared to those without NTIS [11]. We could not measure any cytokine level but lymphocyte counts were detected significantly lower in cases with NTIS when compared to cases without NTIS in both NSCLC and SCLC patients in our study.
Additionally, it has been hypothesized that the immune system cell can affect the systemic thyroid hormone activity. Various immune system cells can produce biologically active TSH and have also been found to express T3 [32]. We count the number of lymphocytes that are known to be associated with immune status. We found statistically significant difference in lymphocyte counts between cases with NTIS and cases without NTIS in both SCLC and NSCLC patients. Lymphocyte counts were detected significantly lower in cases with NTIS. Seyhan et al. found no correlation between NTIS and lymphocyte count. This difference might be due to the high number of patients with advance stage in our study.
This study has several limitations. First one is the sample size which is small. Second, nutritional insufficiencies, arise in especially advanced stage of disease, affects the evaluation of association between NTIS and lung cancer. It is known that nutritional insufficiencies and calorie limitation have a role in NTIS development. And also, we included our cases referred to our specific clinic with a high interest in lung cancer. This should be taken into consideration about our prevalence of NTIS.
In conclusion, we determined that NTIS may be frequently seen in cases with lung cancer. Presence of NTIS is associated with severity of illness and low survival time in both cases with NSCLC and SCLC. Furthermore, NTIS can be used as a prognostic factor for lung cancer patients. Further studies with greater sample sizes are needed to confirm these results.
Acknowledgments
Conflict of Interest
All authors have no conflicts of interest.
References
- 1.Warner MH, Beckett GJ. Mechanism behind the non thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 2.Chopra IJ. Non-thyroidal illness syndrome or euthyroid sick syndrome? Endocr Pract. 1996;2:45–52. doi: 10.4158/EP.2.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Farwell AP. Nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2013;20(5):478–484. doi: 10.1097/01.med.0000433069.09294.e8. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol. 2000;143:1–13. doi: 10.1530/eje.0.1430001. [DOI] [PubMed] [Google Scholar]
- 5.De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic–pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin. 2006;22:57–86. doi: 10.1016/j.ccc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Zargar AH, Ganie MA, Masoodi SR, Laway BA, Bashir MI, Wani AI, Salahuddin M. Prevalence and pattern of sick euthyroid syndrome in acute and chronic non-thyroidal illness. Its relationship with severity and outcome of the disorder. JAPI. 2004;52:27–31. [PubMed] [Google Scholar]
- 7.Kaptein EM (1986) Thyroid hormone metabolism in illness. In: Hennemann G (ed) Thyroid hormone metabolism Marcel Dekker. New York 297–334
- 8.Tellini U, Pellizzari L, Pravadelli B. Euthyroid sick syndrome in elderly subjects with cancer. Minerva Med. 1999;90:111–121. [PubMed] [Google Scholar]
- 9.Kami M, Tanaka Y, Chiba S, Matsumura T, Machida U, Kanda Y, et al. Thyroid function after bone marrow transplantation: possible association between immune-mediated thyrotoxicosis and hypothyroidism. Transplantation. 2001;71(3):406–411. doi: 10.1097/00007890-200102150-00012. [DOI] [PubMed] [Google Scholar]
- 10.Wehmann RE, Gregerman RI, Burns WH, Saral R, Santos GW. Suppression of thyrotropin in the low-thyroxin state of severe nonthyroidal illness. N Engl J Med. 1985;9:546–552. doi: 10.1056/NEJM198502283120904. [DOI] [PubMed] [Google Scholar]
- 11.Feelders RA, Swaak AJ, Romijn JA, Eggermont AM, Tielens ET, Vreugdenhil G, et al. Characteristics of recovery from the euthyroid sick syndrome induced by tumor necrosis factor alpha in cancer patients. Metabolism. 1999;48:324–329. doi: 10.1016/S0026-0495(99)90080-X. [DOI] [PubMed] [Google Scholar]
- 12.Okenn MM, Crecc RH, Torney DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Dillmann WH. The thyroid. In: Cecil RL, Bennett JC, editors. Textbook of medicine. Philadelphia: Saunders; 1996. pp. 1391–1396. [Google Scholar]
- 14.American Joint Committee on Cancer (AJCC) Lung. In: Fleming ID, Cooper JS, Hensen DE, editors. Manual for staging cancer. Philadelphia: JB Lippincott; 1997. [Google Scholar]
- 15.Stahel RA, Ginsberg R, Havemann K, et al. Staging and prognostic factors in small cell lung carcinoma of the lung. Consensus report. Lung Cancer. 1989;5:119–126. doi: 10.1016/0169-5002(89)90156-6. [DOI] [Google Scholar]
- 16.Chow CC, Mak TW, Chan CH, Cockram CS. Euthyroid sick syndrome in pulmonary tuberculosis before and after treatment. Ann Clin Biochem. 1995;32:385–391. doi: 10.1177/000456329503200406. [DOI] [PubMed] [Google Scholar]
- 17.Kırkıl G, Deveci F, Turgut T, Turkoglu S, Muz MH (2006) Euthyroid sick syndrome in cases with COPD attack and evaluation of the response of thyroid function tests to attack therapy. F.Ü. Saglık Bil Dergisi 20(2):143–147 (in Turkish)
- 18.Seyhan EC, Çetinkaya E, Altın S, Gunluoğlu Z, Demir A, Gunluoğlu G, Epozturk K. Nutritional and prognostic significance of sick euthyroid syndrome in non-small cell lung cancer patients. Intern Med. 2008;47:211–216. doi: 10.2169/internalmedicine.47.0703. [DOI] [PubMed] [Google Scholar]
- 19.Wawrzynska L, Sokowicz A, Filipecki S. Euthyroid sick syndrome with respiratory failure. Pneumonol Alergol Pol. 1996;64:193–196. [PubMed] [Google Scholar]
- 20.Karadağ F, Özcan H, Karul AB, Yılmaz M, Çildağ O. Correlates of nonthyroidal illness syndrome in chronic obstructive pulmonary disease. Respir Med. 2007;101:1439–1446. doi: 10.1016/j.rmed.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Caregaro L, Alberino F, Amodio P, Merkel C, Angeli P, Plebani M, Gatta A. Nutritional and prognostic significance of serum hypothyroxinemia in hospitalized patients with liver cirrhosis. J Hepatol. 1998;1(28):115–121. doi: 10.1016/S0168-8278(98)80210-9. [DOI] [PubMed] [Google Scholar]
- 22.Opasich C, Pacini F, Ambrosino N, Riccardi PG, Febo O, Ferrari R, et al. Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure. Eur Heart J. 1996;17:160–166. doi: 10.1093/eurheartj/17.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- 23.Bello G, Pennisi MA, Montini L, Silva S, Maviglia R, Cavallaro F, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest. 2009;135:1448–1454. doi: 10.1378/chest.08-1816. [DOI] [PubMed] [Google Scholar]
- 24.Schulte C, Reinhardt W, Beelen D, Mann K, Schaefer U. Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 1998;22:1171–1178. doi: 10.1038/sj.bmt.1701502. [DOI] [PubMed] [Google Scholar]
- 25.Kumar KV, Kapoor U, Kalia R, Chandra NS, Singh P, Nangia R. Low triiodothyronine predicts mortality in critically ill patients. Indian J Endocrinol Metab. 2013;17(2):285–288. doi: 10.4103/2230-8210.109715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tognini S, Marchini F, Dardano A, Polini A, Ferdeghini M, Castiglioni M, Monzani F. Non-thyroidal illness syndrome and short-term survival in hospitalised older population. Age Ageing. 2010;39:46–50. doi: 10.1093/ageing/afp197. [DOI] [PubMed] [Google Scholar]
- 27.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 28.Wang F, Pan W, Wang H, Wang S, Pan S, Ge J. Relationship between thyroid function and ICU mortality: a prospective observation study. Crit Care. 2012;16:R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaptein EM. Clinical application of free thyroxine determinations. Clin Lab Med. 1993;13:653–672. [PubMed] [Google Scholar]
- 30.Chopra IJ. Clinical review 86: euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab. 1997;82:329–334. doi: 10.1210/jcem.82.2.3745. [DOI] [PubMed] [Google Scholar]
- 31.Papanicolaou DA. Euthyroid sick syndrome and the role of cytokines. Rev Endocr Metab Disord. 2000;1:43–48. doi: 10.1023/A:1010060303031. [DOI] [PubMed] [Google Scholar]
- 32.Csaba G, Pállinger E. Thyrotropic hormone (TSH) regulation of triiodothyronine (T3) concentration in immune cells. Inflamm Res. 2009;58:51–154. doi: 10.1007/s00011-009-2004-4. [DOI] [PubMed] [Google Scholar]