Abstract
Clinical studies have shown that progestins increase breast cancer risk in hormone replacement therapy, while we and others have previously reported that progestins stimulate invasive properties in progesterone receptor (PR)-rich human breast cancer cell lines. Based on others’ reports that omega-3 fatty acids inhibit metastatic properties of breast cancer, we have reviewed the literature for possible connections between omega-3 fatty-acid-driven pathways and progestin-stimulated pathways in an attempt to suggest theoretical mechanisms for possible omega-3 fatty acid inhibition of progestin stimulation of breast cancer invasion. We also present some data suggesting that fatty acids regulate progestin stimulation of invasive properties in PR-rich T47D human breast cancer cells, and that an appropriate concentration of the omega-3 fatty acid eicosapentaenoic acid inhibits progestin stimulation of invasive properties. It is hoped that focus on the inter-relationship between pathways by which omega-3 fatty acids inhibit and progestins stimulate breast cancer invasive properties will lead to further in vitro, in vivo, and clinical studies testing the hypothesis that omega-3 fatty acids can inhibit progestin stimulation of invasive properties in breast cancer, and ameliorate harmful effects of progestins which occur in combined progestin–estrogen hormone replacement therapy.
Keywords: Breast Cancer, Focal Adhesion Kinase, Human Breast Cancer Cell, Human Breast Cancer Cell Line, Invasive Property
Introduction
Breast cancer is the most common cancer (other than non-melanoma skin cancer), the second most common cause of cancer death among women in the USA, and a major cause of cancer death among women worldwide [1, 2]. We and others have shown that progestins can stimulate cell proliferation [3–11], inhibit cell death [7, 12–14], and enhance invasive properties [15–19] in human breast cancer cells in vitro. In vivo also, various researchers have reported similar breast cancer stimulatory effects of progestins [20–29]. Still, others have reported inhibitory effects of progestins toward breast cancer in vitro and in vivo [30–39] and in women [40, 41] and the reasons for these discrepancies are unclear. However, progestins, including micronized progesterone [42, 43] (although micronized progesterone may be safer than other progestins [44, 45]) have been repeatedly shown to increase the risk of breast cancer in postmenopausal women when included with estrogen in hormone replacement therapy (HRT), an increase significantly greater than that incurred in HRT with estrogen alone [46–53].
Omega-3 fatty acids have been widely reported to have inhibitory effects on breast cancer. Connolly and Rose [54] reported that 0.25 μg/ml eicosapentaenoic acid (EPA; about 1 μM) inhibited in vitro invasive properties of MDA-MB-435 human breast cancer cells, whereas the same concentration of the omega-6 fatty acid linoleic acid stimulated invasive properties of these cells. Rose et al. also reported that a high-fat diet rich in omega-3 fatty acids can suppress MDA-MB-435 growth and metastases in nude mice [55]. However, it is now controversial whether MDA-MB-435 cells are a breast cancer or a melanoma line [56, 57]. In other studies, Horia and Watkins found that the omega-3 fatty acid docosahexaenoic acid (DHA) decreased invasive properties in the ER-negative, PR-negative human breast cancer cell line MDA-MB-231 [58] and Senzaki et al. reported that the omega-3 fatty acid EPA reduced metastasis of the human breast cancer cell line KPL-1 in nude mice [59]. Further, Mandal et al. found that fish oil rich in omega-3 fatty acids prevented MDA-MB-231 human breast cancer cell metastasis to bone in nude mice [60], and Kim et al. reported that high intake of omega-3 fatty acids from fatty fish decreased the risk of breast cancer in both pre- and post-menopausal Korean women [61].
Canola oil, a plant-derived oil high in the omega-3 fatty acid alpha linolenic acid, which in humans can be converted to the omega-3 fatty acids EPA and DHA has been shown by Hardman to slow growth of implanted MDA-MB-231 human breast tumors in nude mice [62]. In addition, Ion and Hardman found that dietary walnut, a nut high in omega-3 fatty acids, also suppressed growth of the same tumors, while increasing the EPA and DHA contents of the animals’ livers [63]. Further, maternal consumption of canola oil suppressed mammary gland tumorigenesis in offspring of C3(1) Tag mice, as shown by Ion et al. [64]. Still another report showed that a rat maternal diet high in omega-3 fatty acids from fish oil decreased carcinogen-induced mammary cancer in offspring [65], and a case–control study by Maillard et al. suggests omega-3 fatty acids reduce breast cancer risk and that the balance between omega-6 and omega-3 fatty acids is a key factor [65]. Ratios of omega-6 to omega-3 lower than that found in humans on the western diet have been repeatedly reported to promote health, protecting against various diseases, including breast cancer [66 and references therein].
Still, a comprehensive review of the literature on the effects of omega-3 fatty acids on cancer risk found no significant association between omega-3 fatty acids and cancer risk, and stated that dietary supplementation with omega-3 fatty acids is unlikely to prevent cancer [67]. However, a more recent review by MacLennan and Ma suggests that a diet rich in omega-3 fatty acids, especially when started early in life, has promise for cancer prevention [68]. Inconsistencies in experimental protocol and data from preclinical studies on the effects of omega-3 fatty acids on breast cancer have been pointed out by Signori et al., who have suggested improved strategies for future experiments [69].
While the present review focuses on possible inhibition by omega-3 fatty acids of progestin stimulation of breast cancer invasive properties, there are many other documented beneficial effects associated with their increased levels. These include improvements in cardiovascular health [70–72], diabetes [73], rheumatoid arthritis [74], asthma [75], bone mineral density [76, 77], depression [78–80], dry eye syndrome [81], and age-related macular degeneration [82]. Simopolous has recently thoroughly reviewed the many beneficial effects associated with increased levels of omega-3 fatty acids, lowering the ratio of omega-6 to omega-3 fatty acids in the diet [66].
The average concentrations of EPA and arachidonic acid (AA) in serum of “healthy” (without chronic illnesses) humans were reported by Bailey and Southon to be 76 and 751 μM, respectively. The total concentration of all measured omega-3 fatty acids (alpha-linolenic acid (ALA), EPA, and DHA) was 341 μM and of all measured omega-6 fatty acids (linoleic acid (LA) and AA) was 5,105 μM, a ratio of omega-6/omega-3 of 15:1 [83] (our calculation from their data). In patients with multiple illnesses, including hypertension and diabetes, the average concentrations were reported to be around 24 μM (EPA) and 650 μM (AA) by Harper et al. [84]. In these patients, the total concentration of all measured omega-3 fatty acids was 123 μM, and of all measured omega-6 fatty acids was 2,137 μM, a ratio of omega-6/omega-3 of about 17:1 (our calculation from their data). Patients with early stage chronic lymphocytic leukemia had 65.9 μM EPA and 666 μM AA (Hardman E et al., unpublished data). The total concentration of all measured omega-3’s was 206 μM and of omega-6’s was 3,101 μM, giving an omega-6/omega-3 ratio of 15:1. Thus all three studies show similar very high ratios of omega-6 to omega-3 fatty acids, around 16:1, and the average omega-6 to omega-3 ratio in the diet of the western world is around 16:1, as compared to a ratio of about 1:1 in the diet humans evolved on [66]. Lower ratios of omega-6 to omega-3 have been repeatedly reported to promote health, protecting against various diseases, including breast cancer [66 and references therein]. Omega-3 fatty acids seem to counteract harmful effects of omega-6 fatty acids by competitive inhibition of the enzymes responsible for conversion of omega-6 fatty acids to harmful metabolites [85]. Thus, an important component in the inclusion of omega-3 fatty acids in the diet is lowering the ratio of omega-6 fatty acids to omega-3 fatty acids [66].
In view of our own and others’ findings that progestins stimulate invasive properties of the progestin-responsive human breast cancer cell line T47D, and the above reports suggesting that omega-3 fatty acids are helpful against breast cancer invasive properties, we decided to review what is known about the mechanisms for progestin stimulation of invasive properties in breast cancer and omega-3 fatty acid inhibition of breast cancer invasion, and speculate on how common components of these pathways might enable the use of omega-3 fatty acids to inhibit progestin stimulation of invasion in human breast cancer. We also present some data consistent with this hypothesis, showing that, in the PR-rich human breast cancer cell line T47D, while 200 and 75 μM EPA surprisingly stimulate invasive properties on their own, 40 μM EPA, while having no significant effect alone, significantly inhibits progestin stimulation of invasion.
Exploiting the Interrelationships Between Omega-3 Fatty-Acid-Related Pathways and Progestin-Related Pathways
In order to predict possible fruitful ways of exploiting the interrelationships between omega-3 fatty acid-related pathways and progestin-related pathways to promote breast health and prevent and treat breast cancer, one can consider what is known about these interrelationships. One strategy to accomplish this is to review the literature on how omega-3 fatty acids inhibit and how progestins stimulate breast cancer invasion with an eye toward connections between these two.
How Do Omega-3 Fatty Acids Inhibit Breast Cancer Invasion?
Studies indicate that omega-3 fatty acids act largely by binding to and activating peroxisome proliferator-activated receptor γ (PPARγ) which hetero-dimerizes with the retinoid X receptor (RXR) and binds to peroxisome proliferator response elements (PPREs) to regulate expression of its target genes [86]. Comba et al. [87] have reviewed how omega-3 and omega-6 fatty acids act in tumor cells as ligands for peroxisome proliferator-activated receptors (PPARs), in signal transduction through protein kinase C, and through mechanisms involving transcription factors including nuclear factor kappa B. The action of omega-3 fatty acids through PPARγ in cancer has been reviewed by Edwards and O’Flaherty [88], and Sun et al. [89] have shown that in MCF-7 human breast cancer cells the omega-3 fatty acid DHA up-regulates the pro-apoptotic, cell surface glycoprotein syndecan-1 through a PPRE in the syndecan-1 gene promoter. We postulate that the above-described activation of PPARγ by omega-3 fatty acids may be a key event in the mechanism by which they inhibit progestin stimulation of invasion, as detailed below.
Dimri et al. [90] have found that omega-3 fatty acids inhibit invasion of the human breast cancer cell lines MCF-7, T47D, and MDA-MB-231 in part by down-regulation of the epigenetically active, histone-methylating polycomb group protein enhancer of zeste homolog 2 (EZH2), whose over-expression is correlated with metastasis in breast cancer. Experiments with the proteasome inhibitor MG132 suggested that this lowering of EZH2 level occurs through a post-translational mechanism leading to degradation of the EZH2 protein, accompanied by decreased histone 3 lysine 27 trimethylation (H3K27me3) activity of EZH2, and up-regulation of the EZH2 targets E-cadherin and insulin-like growth factor binding protein 3. The down-regulation of EZH2 thus is part of the mechanism by which omega-3 fatty acids inhibit breast cancer invasion, but, to our knowledge, there is no evidence as yet that progestins up-regulate EZH2.
Omega-3 fatty acids also have in common with progestins effects on signal transduction. Lu et al. [91] have reported that treatment of MCF-7 cells with the omega-3 fatty acid DHA inhibits p42/44 MAPK signaling, decreasing its phosphorylation, while Schley et al. [92] found that omega-3 fatty acids decreased the level of epidermal growth factor receptor (EGFR) in lipid rafts and increased EGFR activation and that of p38 MAPKinase by phosphorylation. The omega-3 fatty acid ALA down-regulates Her2 (EGFR2) by suppressing its transcription, as demonstrated by Menendez et al. [93]. Rogers et al. [94] confirmed that DHA disrupts EGFR’s association with lipid rafts in MDA-MB-231 cells, finding decreased levels of p42/44 MAPK phosphorylation/activation, and Sauer et al. [95] have found that EPA decreases cell proliferation in MCF-7 human breast cancer xenografts in nude rats, decreasing intra-tumoral levels of cAMP and p42/44 MAPK phosphorylation. Using combined data from the human breast cancer cell lines MCF-7 and SK-BR-3 in vitro and Fat-1 mice in vivo, Sun and co-workers [96] have shown that the omega-3 fatty acid DHA up-regulates syndecan-1, which in turn results in dephosphorylation/deactivation of MEK (MAPKinase kinase), MAPKinase, and Bad, inducing apoptosis of the breast cancer cells. As described below, progestins also are well known to act through the above signal transduction mechanisms, generally affecting signal transduction components in a fashion opposite to the action of omega-3 fatty acids.
While omega-3 fatty acids have in general been found to inhibit breast cancer invasive properties, omega-6 fatty acids have been found to stimulate invasion. Navarro-Tito et al. [97] have shown that the omega-6 fatty acid arachidonic acid promotes migration of MDA-MB-231 cells in part through phosphorylation of the protein tyrosine kinase c-Src and focal adhesion kinase (FAK). With this in mind, it seems plausible that omega-3 fatty acids, acting contrary to omega-6 fatty acids, may also act in part through down-regulation of FAK and c-Src activity. Progestins, on the other hand, have been shown to act oppositely, activating FAK and c-Src, as described below.
Isbilen et al. [98] have shown that a very low concentration (0.5 μM) of DHA suppressed MDA-MB-231 human breast cancer cell migration in part through down-regulation of voltage-gated Na(+) channel (neonatal Nav1.5) mRNA and protein, while Gillet and co-workers [99] have proposed that omega-3 fatty acids act through some common mechanisms, including voltage-gated sodium channels, to prevent post-myocardial infarction arrhythmias and inhibit invasiveness, in cardiac cells and breast cancer, respectively. To our knowledge, it is so far unknown whether progestins act in an opposite fashion, to up-regulate voltage-gated sodium channels.
Using cDNA microarrays and quantitative polymerase chain reaction with several human breast cancer cell lines, Hammamieh et al. have demonstrated differences in the effects of omega-6 and omega-3 fatty acids on gene expression [100]. Altenburg and colleagues [101] have shown that combined DHA and curcumin up-regulated expression of genes involved in cell cycle arrest, apoptosis, inhibition of metastasis, and cell adhesion, and down-regulated expression of genes with roles in cancer development and progression, metastasis, and cell cycle progression in the human breast cancer cell line SK-BR-3. Progestins have been shown by cDNA microarray to regulate the expression of many genes in human breast cancer cells, including genes involved in the metastasis-related [102] phenomenon adhesion [103].
While progestins have been shown to stimulate breast cancer cell invasive properties in part through a mechanism involving lipid rafts, the data of Altenburg and Siddiqui [104] suggest that omega-3 fatty acids inhibit migration of MDA-MB-231 human breast cancer cells through disruption of lipid rafts and down-regulation of the expression and function of the cell surface chemokine receptor CXCR4, while Young et al. [105] investigated the effects of omega-3 fatty acids on sex hormone concentrations in postmenopausal women, finding lowered levels of estrogens and androgens, but did not report progestin levels.
In addition to omega-3 fatty acid interaction with progesterone-related pathways and PPARγ, there is also evidence for cross-talk between the estrogen receptor and PPARγ pathways in breast cancer cells [106, 107]. Further, Manni and colleagues have shown that omega-3 fatty acids can act together with the anti-estrogen tamoxifen to exert greater anti-tumor action on N-methyl-N-nitrosourea-induced rat mammary carcinogenesis than either compound alone [108], and Lu et al. [91] have reported that the omega-3 fatty acid DHA inhibits estrogen action in breast cancer cells by inducing degradation of the estrogen receptor. The above reports suggest the possibility that omega-3 fatty acids may inhibit not only the harmful effects of progestins but also those of estrogens in breast cancer. Still, it must be stated that it is unknown whether omega-3 fatty acids may also inhibit beneficial effects of both hormones.
How Progestins Stimulate Invasive Properties in Breast Cancer
The following describes pathways various researchers have shown are involved in progestin stimulation of invasive properties, most of which have been shown, as described above, to be affected in an opposite manner by omega-3 fatty acids. Kato et al. have shown that progesterone increased invasive properties of the human breast cancer cell line ZR-75-1 in part by up-regulating levels of tissue factor (TF) [15], while more recently, workers in the same group [109] have demonstrated the involvement of lipid rafts in progestin stimulation of TF and invasive properties. Many groups have shown that progestins act in part through cytoplasmic signal transduction pathways [110 and references therein]. Progestin stimulation of metastatic properties of breast cancer cells depends on progesterone receptor action through cytoplasmic signal transduction pathways involving c-Src, as demonstrated by Carnevale et al. [11]. Fu and co-workers [17, 18] as well have shown that progestins stimulate breast cancer invasive properties through cytoplasmic signal transduction pathways, involving the G protein Gα13, the tyrosine kinase c-Src, phosphoinositidyl-3 kinase, and RhoA, resulting in the activation of the actin regulatory protein moesin, and remodeling of the actin cytoskeleton. These workers have demonstrated that progesterone leads to rapid extra-nuclear phosphorylation/activation of focal adhesion kinase in T47D human breast cancer cells, leading to formation of focal adhesion complexes important for cell movement and invasion [111]. We have found that progestin stimulation of invasive properties in the human breast cancer cell line T47D involves up-regulation of the enzyme manganese superoxide dismutase which occurs through the MAPKinase signal transduction pathway [19].
It is well known that progestins stimulate activity of the EGF pathway in progestin responsive human breast cancer cell lines [112, 113], and that EGF stimulates invasive properties of breast cancer cells [114]. In view of the above-referenced inhibition of EGFR activity by omega-3 fatty acids [94], it is possible that these counteracting effects on the EGF pathway may help explain how omega-3 fatty acids inhibit progestin stimulation of invasive properties.
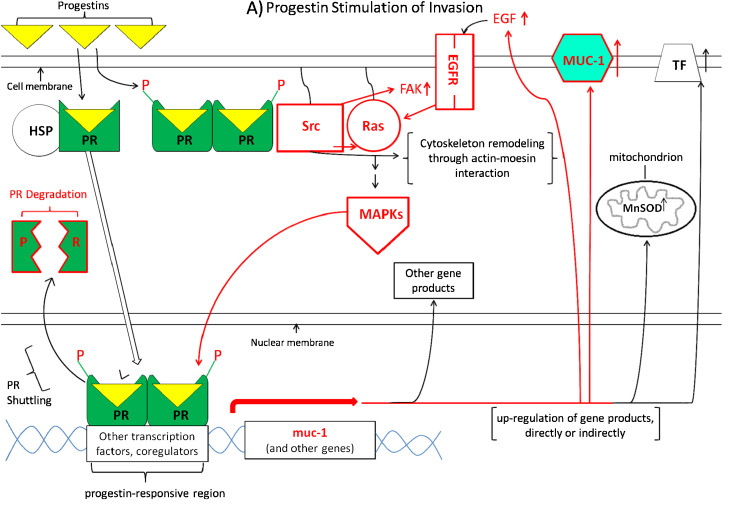
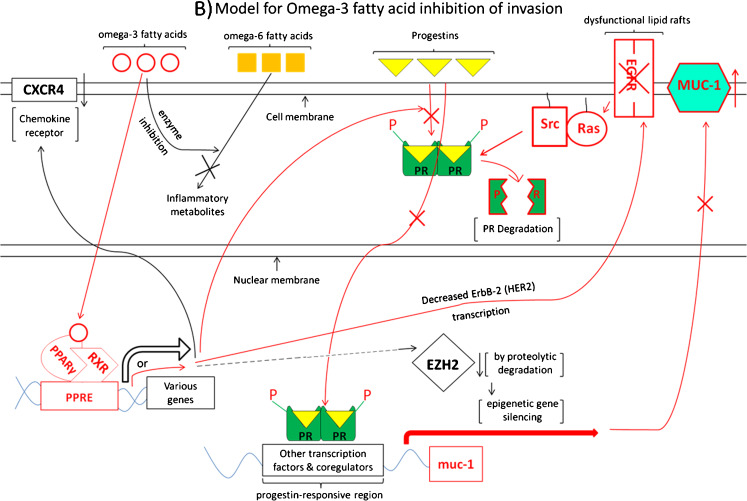
It is particularly intriguing that Carson and his co-workers have shown that progesterone up-regulates levels of the transmembrane glycoprotein mucin1 (MUC-1) in human uterine epithelial cells and breast cancer cells, including the breast cancer cell line T47D [115, 116]. High levels of MUC-1 have been shown to stimulate cancer cell invasive properties [117, 118], including invasion of human breast cancer cell lines, and to be found at higher levels in metastatic human breast tumors than in non-metastatic tumors [119]. Interestingly, progesterone stimulation of MUC-1 has been shown to be inhibited by rosiglitazone activation of PPARγ in T47D human breast cancer cells and human uterine epithelial cells. This inhibition by PPARγ activation occurs through inhibition of progesterone receptor phosphorylation and through stimulation of progesterone receptor degradation [116]. Since omega-3 fatty acids act in large part through activation of PPARγ, it is reasonable to speculate, as depicted in Fig. 2, that the mechanism by which omega -3 fatty acids can inhibit progestin stimulation of invasive properties may also involve omega-3 fatty acid activation of PPARγ, which inhibits progestin stimulation of the metastasis-enhancing protein MUC-1, decreasing metastasis.
Fig. 2.
Working model for the hypothesis that omega-3 fatty acids can inhibit progestin stimulation of breast cancer invasion. The model is separated into two parts for clarity. a Depicts progestin stimulation of invasion, while b diagrams omega-3 fatty acid pathways which may act to inhibit progestin stimulation of invasive properties. Necessarily, the model is incomplete, yet testable. Parts of the model are colored red to emphasize pathways for which there is the most evidence suggesting interaction of omega-3 fatty acid pathways to inhibit progestin-driven pathways, in particular progestin stimulation of MUC1. Small upward-pointing arrows to the right of gene products indicate up-regulation of these products, whereas downward-pointing arrows indicate down-regulation. Abbreviations: HSP heat shock proteins, PR progesterone receptor, P phosphate group, Src the tyrosine–protein kinase c-Src, Ras the G-protein Ras, EGFR epidermal growth factor receptor 1, EGF epidermal growth factor, MUC-1 mucin-1, TF tissue factor, MAPKs mitogen-activated protein kinases, MnSOD manganese superoxide dismutase, CXCR4 a transmembrane G-protein-coupled chemokine receptor for CXCL-12/stromal cell-derived factor 1, PPARγ peroxisome proliferator-activated receptor gamma, RXR retinoid X receptor, PPRE peroxisome proliferator response element, EZH2 enhancer of zeste homolog 2 (a histone-methylating polycomb group protein), ErbB-2/Her2 human epidermal growth factor receptor 2
As stated above, it has been convincingly shown that progestins can stimulate invasive properties in breast cancer, and that omega-3 fatty acids can inhibit these invasive properties. While it is presently unknown whether omega-3 fatty acids used as described above may also inhibit beneficial effects of progestins, we present, in this review, some tantalizing evidence that omega-3 fatty acids, at proper concentrations, may be effective against progestin stimulation of metastatic properties, and have attempted to analyze the literature to present some possible parts of the mechanism by which this may occur. A model showing how omega-3 fatty acids might inhibit progestin stimulation of invasive properties in breast cancer is shown in Fig. 2. This model is explained in detail in the legend for Fig. 2 and toward the end of this review. These ideas are testable, and suggest there may be an expanded role for omega-3 fatty acids in promotion of breast health and prevention and treatment of progestin-responsive breast cancer. Further, these ideas may provide a rationale for testing the use of omega-3 fatty acids to inhibit the harmful effects of progestins in combined (estrogen and progestin) hormone replacement therapy. Further research, in vitro, in vivo, and clinical trials, will be necessary to test these ideas.
Effects of EPA on Progestin Stimulation of Invasion in T47D Cells
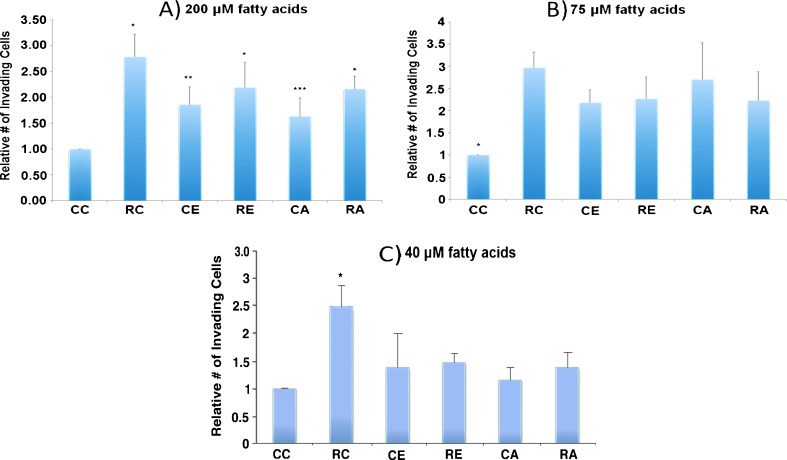
In order to test our hypothesis that the omega-3 fatty acid EPA would inhibit progestin stimulation of invasion in PR-rich T47D human breast cancer cells, we used EPA at several concentrations: 200, 75, 40, and 1 μM, since concentrations of omega-3 fatty acid in this range have previously been shown to have effects on human breast cancer cells in culture [52, 54, 57, 58, 120]. The highest concentration we used, 200 μM, can be reached in the serum of humans with fish oil supplementation (Hardman, WE, unpublished results). We also tested the omega-6 fatty acid AA at these concentrations as a control, because we expected AA to stimulate invasive properties on its own, as reported by others in the human breast cancer cell line MDA-MB-231 [58]. As shown in Fig. 1a and b, under conditions in which progestins stimulate invasion, 200 and 75 μM EPA do not inhibit progestin stimulation of invasion, but, much to our surprise, stimulate invasion on their own, while AA, as expected, also does not inhibit progestin stimulation of invasion. We detected no effect on viability of the cells under these conditions. The results of two separate experiments testing a much lower concentration suggest that neither 1 μM EPA nor AA affect invasion, either alone or in the presence of progestin (data not shown). However, upon testing the intermediate concentration 40 μM, we found, as shown in Fig. 1c, that 40 μM EPA significantly inhibits progestin stimulation of invasion, while having no significant effect without progestin.
Fig. 1.
a Effect of 200 μM fatty acid on T47D invasion. T47D cells, obtained from the American Type Culture Collection, and found to exhibit the exquisite progestin-responsiveness characteristic of these cells, were grown in plastic flasks in 5 % CO2 in air in minimal essential medium, powdered (autoclavable) plus non-essential amino acids, 2 mM l-glutamine, 10 % fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), and 6 ng/ml insulin (Sigma-Aldrich) until about 90 % confluence. The medium was then changed to fresh and made 200 μM in EPA, AA, or 0.1 % in ethanol (vehicle). Cells were incubated for 72 h, and the medium was made 10 μM in ara-C (arabinofuranosyl cytidine) for the last hour, to stop DNA replication. EPA, AA, and ara-C were purchased from Sigma-Aldrich. The cells of each flask were then harvested and single cell suspensions at 106 cells/ml (300 μl) in the same medium as above (containing ara-C), except without serum and phenol red and with or without 10 nM R5020 (dissolved in ethanol) were placed in the upper insert of a modified Boyden chamber (Cell Biolabs, catalog # CBA-110) to measure invasion through a layer of extracellular matrix and a membrane with 8-μm pores, to a lower chamber containing 500 μl of twice charcoal-stripped serum-containing medium without phenol red. Final ethanol concentration was 0.2 % in all samples. As stated above, the cells were grown to around 90 % confluency in complete growth medium (containing 10 % fetal bovine serum and phenol red), and then incubated with the tested fatty acids in fresh complete growth medium for 72 h. They were then made into single cell suspensions and incubated in the presence of the fatty acids with or without progestin for a further 48 h in the same medium as above, except serum-free and without phenol red, while invading through a layer of extracellular matrix and a porous membrane into 10 % charcoal-stripped serum-containing medium without phenol red. After 48 h, invading cells were stained and counted, according to the manufacturer’s instructions. Results are the average plus SEM of six independent experiments, and were analyzed by ANOVA followed by the Fisher’s LSD multiple comparison procedure. CC no fatty acid or R5020, RC R5020 alone, CE EPA alone, RE R5020 plus EPA, CA arachidonic acid alone, RA R5020 plus arachidonic acid. All samples are different from control (CC) at p < 0.05 except for CA. All other samples are statistically the same except RC, CE, and CA (p < 0.05). *Different from control. **Different from RC and control. ***Different from RC. b Effect of 75 μM fatty acid on T47D invasion. Protocol was the same as in (a), except that fatty acid concentration was 75 μM. Results are the average plus SEM of nine independent experiments for CC and RC, and three experiments for the remaining samples. Results were analyzed by ANOVA followed by the Fisher’s LSD multiple comparison procedure. Abbreviations are the same as in (a). All samples are different from control at p < 0.05. *Different from all others. No other differences are significant. c Effect of 40 μM fatty acid on T47D invasion. Protocol was the same as in (a), except that fatty acid concentration was 40 μM. Results are the average plus SEM of nine experiments for CC and RC, and three experiments for the remaining samples. Abbreviations are the same as in (a). Results were analyzed by ANOVA followed by Fisher’s LSD multiple comparison procedure. *Different from all others at p < 0.05
It is unclear why the higher concentrations of EPA (200 and 75 μM) stimulated invasion on their own. One possibility is that at these high concentrations EPA, in the artificial absence of omega-6 fatty acids, actually might serve as a substrate, in addition to its normal role as an inhibitor, of the enzymes which convert omega-6 fatty acids to harmful metabolites. Seventy-five micromolar is about 40 % above the average EPA concentration (55 μM, average of the above three studies [83, 84, and Hardman WE, unpublished observations]) and 200 μM, a concentration achievable in human serum with fish oil supplementation (Hardman WE, unpublished results) is about fourfold above this average level of EPA for humans without fish oil supplementation. However, 75 and 200 μM AA are much lower than the average human physiological concentration of AA, which is around 689 μM (average of the above three studies). Interestingly, the omega-6 fatty acid AA also, at 40 μM, has no significant effect on invasive properties, but inhibits progestin stimulation of invasion. However, 40 μM is only about 6 % of the average AA concentration (689 μM) in human serum. The content of omega-6 fatty acids in the western world diet is high and the omega-3 content comparatively low, resulting in a high ratio of omega-6/omega-3 of around 16:1, whereas humans evolved at a ratio of around 1:1 [66]. This high ratio has been shown to be associated with inflammation in the pathogenesis of several diseases, including cancer, cardiovascular disease, and autoimmune diseases [66]. Since the omega-6 fatty acid content of the western diet is already very high, and has been shown to be associated with disease pathogenesis, it would be unreasonable to suggest addition of omega-6 fatty acids to the western diet. As stated earlier, omega-3 fatty acids seem to counteract harmful effects of omega-6 fatty acids by competitive inhibition of the enzymes responsible for conversion of omega-6 fatty acids to harmful metabolites [84].
Our data showing that 40 μM EPA had no significant effect on invasive properties alone, but inhibited progestin stimulation of breast cancer cell invasion, are consistent with the notion that omega-3 fatty acids, in the right concentration range, might be helpful in preventing metastasis of progestin-responsive breast cancer. While these relatively simple experiments suggest the possible value of omega-3 fatty acids in inhibition of progestin stimulation of breast cancer invasion, others have shown that it is lowering the ratio of omega-6 to omega-3 fatty acids that is effective against several diseases [66 and references therein], and future experiments with varying ratios of omega-6 to omega-3 will be required to address this. Still, these results make it tempting to speculate that omega-3 fatty acids such as EPA, at the right concentrations, may have a role in the treatment of progestin-responsive breast cancer and/or in combating the progestin-induced increased risk of breast cancer from combined (estrogen plus progestin) hormone replacement therapy. Further studies, in vitro, in vivo, and in humans, involving various concentrations and ratios of combined omega-6 and omega-3 fatty acids, including that of the currently prevalent western diet, 16:1, and that on which humans evolved, 1:1, will be necessary to determine whether omega-3 fatty acids can inhibit progestin exacerbation of breast cancer in humans.
Clinical studies could determine whether greater intake of omega-3 fatty acids would inhibit the well-documented [46–53] increased risk of breast cancer in women on combined (progestin plus estrogen) hormone replacement therapy. It seems possible this could allow women to get the benefits of hormone replacement therapy while decreasing its harmful side effects. Omega-3 fatty acids are widely available without a prescription from a variety of sources including fish oil, canola oil, and oil from algae, facilitating such a study. There is already a wealth of data from the above studies on incidence of breast cancer in women who have been on HRT with estrogen alone, estrogen plus progestin, or placebo [46–53]. These women from past studies could serve as the controls without omega-3 fatty acid supplementation. Their incidences of breast cancer and metastasis could be compared with that in new women on HRT supplemented with omega-3 fatty acids with no hormone, estrogen alone, or estrogen plus progestin. This type of study would test the hypothesis that omega-3 fatty acids would inhibit the increased risk of breast cancer from combined HRT with progestin plus estrogen over that with estrogen alone. It would simultaneously test the effect of omega-3 fatty acids alone on breast cancer incidence and the effect of omega-3 fatty acids on breast cancer in women on HRT with estrogen alone. Clinical studies could also be done to determine whether omega-3 fatty acids added to conventional breast cancer therapy such as tamoxifen would be helpful. This idea has recently been suggested by others, including Manni and his co-workers [69, 108] and Bidinotto et al. [121]. Once again, the controls could be the many women from previous studies done with tamoxifen and other drugs without addition of omega-3 fatty acids.
A Working Model for the Hypothesis That Omega-3 Fatty Acids Can Inhibit Progestin Stimulation of Breast Cancer Invasive Properties
Figure 2 is a working model for how omega-3 fatty acids may inhibit progestin stimulation of breast cancer invasive properties. For clarity, the model is separated into two parts. Figure 2a depicts progestin stimulation of invasion, while Fig. 2b diagrams omega-3 fatty acid pathways which may act to inhibit progestin stimulation of invasive properties. Necessarily, the model is incomplete, yet testable. Small upward-pointing arrows to the right of gene products indicate up-regulation of these products, whereas downward-pointing arrows indicate down-regulation, and the abbreviations used are explained in the figure legend. Parts of the model are colored red to emphasize pathways for which there is the most evidence (our inference from their data) suggesting possible interaction of omega-3 fatty acid pathways to inhibit progestin-driven pathways, in particular progestin stimulation of MUC-1.
Focusing on the parts of the model shown in red in Fig. 2a, an important part of the mechanism of progestin stimulation of invasion appears to be up-regulation of the cell membrane glycoprotein MUC-1, involving a progestin-responsive region in the upstream control region of the muc-1 gene [115, 116]. High levels of MUC-1 have been shown to stimulate cancer cell invasive properties [117, 118], including invasion of human breast cancer cell lines, and MUC-1 has been found at higher levels in metastatic human breast tumors than in non-metastatic tumors [119]. PR phosphorylation and PR degradation are shown in red because Carson and his co-workers have shown that control of these processes is involved in progestin stimulation of MUC-1 [115, 116], which is also shown in red.
Also shown in red is the pathway for progestin stimulation of the EGF pathway. It is well known that progestins stimulate activity of the EGF pathway in progestin responsive human breast cancer cell lines [112, 113], and that EGF stimulates invasive properties of breast cancer cells [114]. Many groups have shown that progestins act in part through cytoplasmic signal transduction pathways involving Ras and MAPKinase [111 and references therein]. Carnevale et al. [11] have demonstrated that progestin stimulation of metastatic properties of breast cancer cells depends on progesterone receptor action through cytoplasmic signal transduction pathways involving c-Src, and Fu et al. have shown the involvement of progestin stimulation of focal adhesion kinase in breast cancer cell invasive properties [111]. Once again, these pathways are shown in red to emphasize the fact that their influence in opposite directions by progestins and omega-3 fatty acids constitutes circumstantial evidence that they may be involved in omega-3 fatty acid inhibition of progestin stimulation of breast cancer invasive properties.
Other gene products up-regulated by progestins which have been shown to be involved in enhancement of breast cancer invasive properties are tissue factor [15, 109] manganese superoxide dismutase [19], and cytoskeleton remodeling through actin–moesin interaction [17, 18]. These pathways are shown in black because, to our knowledge, there are no reports as yet that omega-3 fatty acids act to inhibit these processes.
Shown in Fig. 2b are pathways by which omega -3 fatty acids have been shown to inhibit breast cancer invasive properties; again certain pathways are shown in red to emphasize the circumstantial evidence (our inference from the data) that they may be involved in inhibition by omega-3 fatty acids of progestin stimulation of breast cancer invasion. After entering the cell, omega-3 fatty acids bind to and activate PPARγ, which in complex with RXR regulates various genes. Also shown in red is the pathway by which progestins enter the cell, bind to the progesterone receptor, and stimulate expression of the muc-1 gene, leading to increased levels of MUC-1 [115, 116]. Carson and his colleagues have shown that rosiglitazone binds to and activates PPARγ, resulting in inhibition of progestin stimulation of MUC-1 and invasive properties in T47D human breast cancer cells through decreased PR phosphorylation and increased PR degradation [115, 116], also shown in red. Since omega-3 fatty acids too act through activation of PPARγ, we speculate that part of the mechanism by which omega-3 fatty acids inhibit progestin stimulation of breast cancer invasion is through this same pathway.
Shown in red as well is the pathway involving omega-3 fatty acid inhibition of ErbB-2/HER2 gene transcription [93] and omega-3 inhibition of the activity of EGF and its receptor, involving disruption of lipid raft function [92, 94]. Signal transduction pathways involving Ras and Src are included, since EGF and progestins are known to act through these proteins.
Pathways shown in black are those in which it has been shown that omega-3 fatty acids act, but for which, to our knowledge, there is no evidence yet that progestins act in an opposite fashion. Omega-3 fatty acids have been shown to act as competitive inhibitors of enzymes which convert omega-6 fatty acids to cancer-associated inflammatory metabolites [84], and, as shown by Altenburg and Siddiqui [104], to inhibit migration of MDA-MB-231 human breast cancer cells through disruption of lipid rafts and down-regulation of the expression and function of the cell surface chemokine receptor CXCR4. Dimri et al. [90] have found that omega-3 fatty acids inhibit invasion of the human breast cancer cell lines MCF-7, T47D, and MDA-MB-231 in part by down-regulation of the epigenetically active, histone-methylating polycomb group protein EZH2 (enhancer of zeste homolog 2), whose over-expression is correlated with metastasis in breast cancer. Experiments with the proteasome inhibitor MG132 suggested that this lowering of EZH2 level occurs through a post-translational mechanism leading to degradation of the EZH2 protein, accompanied by decreased H3K27me3 activity of EZH2.
Summary
Much research suggests that progestin action is like a powerful current moving through the cell, which can cause both good and harmful outcomes, including the stimulation of cell proliferation, inhibition of cell death, and enhancement of invasive properties in breast cancer cells. Many other studies show that omega-3 fatty acids can inhibit breast cancer, including its invasive properties. The fatty acid content of cells is largely dependent on diet, and the western diet includes a high ratio of omega-6 to omega-3 fatty acids, which high ratio has been shown to be associated with inflammation and the pathogenesis of diseases including cardiovascular disease, autoimmune disease, and cancer. Bearing in mind the idea that progestins can stimulate breast cancer cell invasive properties and that omega-3 fatty acids can inhibit invasion, we have begun to test the hypothesis that omega-3 fatty acids can inhibit progestin stimulation of breast cancer invasion, and initial in vitro experiments suggest they can. Review of the literature with an eye for how this might occur suggests that omega-3 fatty acids, acting through activation of PPARγ, may interfere with progestin stimulation of invasion by several signal transduction and genomic pathways. These pathways may involve decreased phosphorylation and enhanced degradation of progesterone receptor, resulting in inhibition of progestin stimulation of the invasion stimulatory protein MUC-1 and other invasion-related gene products and processes. We propose that these ideas merit further testing, in vitro, in vivo, and in the clinic, and hold promise for promotion of breast health, prevention and treatment of breast cancer, and safer hormone replacement therapy by simply including the right amount of omega-3 fatty acids in the diet.
Acknowledgments
The authors would like to thank Drs. W. Elaine Hardman and Gabriela Ion for helpful discussions.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.American Cancer Society . Cancer facts and figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 2.World Cancer Report (2008) International Agency for Research on Cancer
- 3.Hissom JR, Moore MR. Progestin effects on growth in the human breast cancer cell line T47D-possible therapeutic implications. Biochem Biophys Res Commun. 1987;145:706–711. doi: 10.1016/0006-291x(87)91022-9. [DOI] [PubMed] [Google Scholar]
- 4.Moore MR, Hagley RD, Hissom JR. Progestin effects on lactate dehydrogenase and growth in the human breast cancer cell line T47D. In: Hankins HD, Puett D, editors. Hormones, cell biology and cancer, potentials. New York: Alan R. Liss, Inc; 1988. pp. 161–179. [PubMed] [Google Scholar]
- 5.Hissom JR, Bowden RT, Moore MR. Effects of progestins, estrogens and antihormones on growth and lactate dehydrogenase in the human breast cancer cell line T47D. Endocrinology. 1989;125:418–423. doi: 10.1210/endo-125-1-418. [DOI] [PubMed] [Google Scholar]
- 6.Bowden RT, Hissom JR, Moore MR. Growth stimulation of T47D human breast cancer cells by the antiprogestin RU486. Endocrinology. 1989;124:2642–2644. doi: 10.1210/endo-124-5-2642. [DOI] [PubMed] [Google Scholar]
- 7.Moore MR, Hathaway LD, Bircher JA. Progestin stimulation of thymidine kinase in the human breast cancer cell line T47D. Biochim Biophys Acta. 1991;1096:170–174. doi: 10.1016/0925-4439(91)90056-f. [DOI] [PubMed] [Google Scholar]
- 8.Moore MR, Zhou J-L, Blankenship KA, Strobl JS, Edwards DP, Gentry RN. A sequence in the 5′-flanking region confers progestin responsiveness on the human c-myc gene. J Steroid Biochem Mol Biol. 1997;62:243–252. doi: 10.1016/s0960-0760(97)00036-8. [DOI] [PubMed] [Google Scholar]
- 9.Moore MR, Conover JL, Franks KM. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem Biophys Res Commun. 2000;277:650–654. doi: 10.1006/bbrc.2000.3728. [DOI] [PubMed] [Google Scholar]
- 10.Manni A, Badger B, Wright C, Ahmed SR, Dehmers LM. Effects of progestins on growth of experimental breast cancer in culture: interaction with estradiol and prolactin and involvement of the polyamine pathway. Cancer Res. 1987;47:3066–3071. [PubMed] [Google Scholar]
- 11.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, de Kier B, Joffe E, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endo. 2007;21:1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 12.Moore MR, Spence JB, Kiningham KK, Dillon JL. Progestin inhibition of cell death in human breast cancer cell lines. J Steroid Biochem Mol Biol. 2006;98:218–227. doi: 10.1016/j.jsbmb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Ory K, Lebeau J, Levalois C, Bishay K, Fouchet P, Allemand I, Therwath A, Chevillard S. Apoptosis inhibition mediated by medroxyprogesterone acetate treatment of breast cancer cell lines. Breast Canc Res Treat. 2001;68:187–198. doi: 10.1023/a:1012288510743. [DOI] [PubMed] [Google Scholar]
- 14.Vares G, Ory K, Lectard B, Levalois C, Altmeyer-Morel S, Chevillard S, Lebeau J. Progesterone prevents radiation-induced apoptosis in breast cancer cells. Oncogene. 2004;23:4603–4613. doi: 10.1038/sj.onc.1207601. [DOI] [PubMed] [Google Scholar]
- 15.Kato S, Pinto M, Carvajal A, Espinoza N, Monso C, Sadarangani A, Villalon M, Brosens JJ, White JO, Richer JK, Horwitz KB, Owen GI. Progesterone increases tissue factor gene expression, procoagulant activity, and invasion in the breast cancer cell line ZR-75-1. J Clin Endocrinol Metab. 2005;90:1181–1188. doi: 10.1210/jc.2004-0857. [DOI] [PubMed] [Google Scholar]
- 16.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier JE, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endo. 2007;21:1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 17.Fu XD, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T. Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS One. 2008;3(7):e2790. doi: 10.1371/journal.pone.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu XD, Giretti MS, Goglia L, Flamini M, Sanchez AM, Baldacci C, Garibaldi S, Sitruk Ware R, Genazzani AR, Simoncini T. Comparative actions of progesterone, medroxyprogesterone acetate, drospirenone and nestorone on breast cancer cell migration and invasion. BMC Cancer. 2008;8:166. doi: 10.1186/1471-2407-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holley AK, Kiningham KK, Spitz DR, Edwards DP, Jenkins JT, Moore MR. Progestin stimulation of manganese superoxide dismutase and invasive properties in T47D human breast cancer cells. J Steroid Biochem Mol Biol. 2009;117:23–30. doi: 10.1016/j.jsbmb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huggins C. Two principles in endocrine therapy of cancers: hormone deprival and hormone interference. Cancer Res. 1965;25:1163–1167. [PubMed] [Google Scholar]
- 21.Huggins C, Moon RC, Morii S. Extinction of experimental mammary cancer. I. Estradiol-17β and progesterone. Proc Natl Acad Sci U S A. 1962;48:379–386. doi: 10.1073/pnas.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huggins C, Yang NC. Induction and extinction of mammary cancer. A striking effect of hydrocarbons permits analysis of mechanisms of causes and cure of breast cancer. Science. 1962;137:257–262. doi: 10.1126/science.137.3526.257. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzawa A (1982) Hormonal regulation of mammary tumors. In: Leung BS (ed) Eden, St. Albans, 183–215
- 24.Robinson SP, Jordan VC. Reversal of the antitumor effects of tamoxifen by progesterone in the 7,12 dimethylbenzanthracene-induced rat mammary carcinoma model. Cancer Res. 1987;47:5386–5390. [PubMed] [Google Scholar]
- 25.Kiss R, Paridaens RJ, Henson JC, Danguy AJ. Effect of progesterone on cell proliferation in the MXT mouse hormone-sensitive mammary neoplasm. J Natl Cancer Inst. 1986;77:173–178. [PubMed] [Google Scholar]
- 26.Lanari C, Molinolo AA, Dosene PC. Induction of mammary adenocarcinomas by medroxyprogesterone acetate in Balb/c female mice. Cancer Lett. 1986;33:215–223. doi: 10.1016/0304-3835(86)90027-3. [DOI] [PubMed] [Google Scholar]
- 27.Lanari C, Kordon E, Molinolo A, Dosene PC, Charreau EH. Mammary adenocarcinomas induced by medroxyprogesterone acetate: hormone dependence and EGF receptors of Balb/c in vivo sublines. Int J Cancer. 1989;43:845–850. doi: 10.1002/ijc.2910430518. [DOI] [PubMed] [Google Scholar]
- 28.Lamb C, Simian M, Molinolo A, Pazos P, Lanari C. Regulation of cell growth of a progestin-dependent murine mammary carcinoma in vitro: progesterone receptor involvement in serum or growth factor-induced cell proliferation. J Steroid Biochem Mol Biol. 1999;70:133–142. doi: 10.1016/s0960-0760(99)00108-9. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res. 2007;67:9929–9936. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz KB, Friedenberg GR. Growth inhibition and increase of estrogen receptors in anti-estrogen resistant T47Dco human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985;45:167–173. [PubMed] [Google Scholar]
- 31.Murphy LC, Dotzlaw H. Endogenous growth factor expression in T47D human breast cancer cells, associated with reduced sensitivity to anti-proliferative effects of progestins and anti-progestins. Cancer Res. 1989;49:599–604. [PubMed] [Google Scholar]
- 32.Dunning WF, Curtis MR, Segaloff A. Strain differences in response to diethylstilbestrol and the induction of mammary gland and bladder cancer in the rat. Cancer Res. 1947;7:51–521. [Google Scholar]
- 33.Jabara AG, Toyne PH, Harcourt AG. Effects of time and duration of progesterone administration on mammary tumors induced by 7,12-dimethylbenz(a)anthracene in Sprague–Dawley rats. Br J Canc. 1973;27:63–71. doi: 10.1038/bjc.1973.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kledzik GS, Bradley CJ, Meites J. Reduction of carcinogen-induced mammary cancer incidence in rats by early treatment with hormones or drugs. Cancer Res. 1974;34:2953–2956. [PubMed] [Google Scholar]
- 35.Welsch CW, Clemens JA, Meites J. Effects of multiple pituitary homografts or progesterone on 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. J Natl Cancer Inst. 1968;41:465–471. [PubMed] [Google Scholar]
- 36.Li S, Lepage M, Merand Y, Belanger A, Labrie F. Growth inhibition of 7,12 dimethylbenz(a)anthracene-induced rat mammary tumors by controlled release low-dose medroxyprogesterone acetate. Breast Canc Res Treat. 1993;24:127–137. doi: 10.1007/BF01961245. [DOI] [PubMed] [Google Scholar]
- 37.Formby B, Wiley TS. Bcl-2, survivin and variant CD44 v7–v10 are down-regulated and p53 is up-regulated in breast cancer cells by progesterone: inhibition of cell growth and induction of apoptosis. Mol Cell Biochem. 1999;202:53–61. doi: 10.1023/a:1007081021483. [DOI] [PubMed] [Google Scholar]
- 38.Gompel A, Somai S, Chaouat M, Kazem A, Kloosterboer HJ, Beusman I, Forgez P, Mimoun M, Rostene W. Hormonal regulation of apoptosis in breast cells and tissues. Steroids. 2000;65:593–598. doi: 10.1016/s0039-128x(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 39.Sumida T, Itahana Y, Hamakawa H, Desprez PY. Reduction of human metastatic breast cancer cell aggressiveness on introduction of either form A or B of the progesterone receptor and then treatment with progestins. Cancer Res. 2004;64:7886–7892. doi: 10.1158/0008-5472.CAN-04-1155. [DOI] [PubMed] [Google Scholar]
- 40.Blumenschein GR. The role of progestins in the treatment of breast cancer. Semin Oncol. 1983;10:7–10. [PubMed] [Google Scholar]
- 41.Parnes HL, Abrams JS, Tchekmedyian NS, Tait N, Ainsner JA. Phase I/II study of high-dose megestrol acetate in the treatment of metastatic breast cancer. Breast Canc Res Treat. 1991;18:171–177. doi: 10.1007/BF01990033. [DOI] [PubMed] [Google Scholar]
- 42.Flesch-Janys D, Slanger T, Mutsschelknauss E, et al. Risk of different histological types of postmenopausal breast cancer by type and regiman of menopausal hormone therapy. Int J Cancer. 2008;123:933–941. doi: 10.1002/ijc.23655. [DOI] [PubMed] [Google Scholar]
- 43.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH, Endocrine Society Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1–S66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(Suppl 1):3–10. doi: 10.3109/13697137.2012.669332. [DOI] [PubMed] [Google Scholar]
- 45.Gompel A. Micronized progesterone and its impact on the endometrium and breast vs. progestogens. Climacteric. 2012;15(Suppl 1):18–15. doi: 10.3109/13697137.2012.669584. [DOI] [PubMed] [Google Scholar]
- 46.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen–progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 47.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen–progestin replacement. Canc Causes Contr. 1999;10:253–260. doi: 10.1023/a:1008909128110. [DOI] [PubMed] [Google Scholar]
- 48.Ross RK, Paganini-Hill PCW, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Canc Inst. 2000;92:328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 49.Li CI, Weiss NS, Stanford JL, Daling JR. Hormone replacement therapy in relation to risk of lobular and ductal breast carcinoma in middle-aged women. Cancer. 2000;88:2570–2577. doi: 10.1002/1097-0142(20000601)88:11<2570::aid-cncr20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 50.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559–4565. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 51.Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 52.Million Women Study Collaborators Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 53.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, Manson JE, Stefanick ML, Ockene J, Sarto GE, Johnson KC, Wactawski-Wende J, Ravdin PM, Schenken R, Hendrix SL, Rajkovic A, Rohan TE, Yasmeen S, Prentice RL, WHI Investigators Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connolly JM, Rose DP. Effects of fatty acids on invasion through reconstituted basement membrane (‘Matrigel’) by a human breast cancer cell line. Cancer Lett. 1993;75:137–142. doi: 10.1016/0304-3835(93)90198-i. [DOI] [PubMed] [Google Scholar]
- 55.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–1747. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 56.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 Melanoma cells—a loss for breast cancer, but a boon for melanoma research. Breast Canc Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 57.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 58.Horia E, Watkins BA. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis. 2007;28:809–815. doi: 10.1093/carcin/bgl183. [DOI] [PubMed] [Google Scholar]
- 59.Senzaki H, Iwamoto S, Ogura E, Kiyozuka Y, Kurebayashi J, Takada H, Hioki K, Tsubura A. Dietary effects of fatty acids on growth and metastasis of KLP-1 human breast cancer cells in vivo and in vitro. Anticancer Res. 1998;18:1621–1627. [PubMed] [Google Scholar]
- 60.Mandal CC, Ghosh-Choudhury T, Yoneda T, Ghosh-Choudhury G, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–607. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Lim S-Y, Shin A, Sung M-K, Ro J, Kang H-S, Lee KS, Kim S-W, Lee E-S. Fatty fish and omega-3 fatty acid intakes decrease the breast cancer risk: a case–control study. BMC Cancer. 2009;9:216–225. doi: 10.1186/1471-2407-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardman WE. Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr Canc. 2007;57:177–183. doi: 10.1080/01635580701277445. [DOI] [PubMed] [Google Scholar]
- 63.Hardman WE, Ion G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Canc. 2008;60:666–674. doi: 10.1080/01635580802065302. [DOI] [PubMed] [Google Scholar]
- 64.Ion G, Akinsete JA, Hardman WE. Maternal consumption of canola oil suppressed mammary gland tumorigenesis in C3(1) Tag mice offspring. BMC Cancer. 2010;10:81–92. doi: 10.1186/1471-2407-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hilakivi-Clarke L, Cho E, Cabanes A, de Assis S, Olivo S, Helferich W, Lippman ME, Clarke R. Dietary modulation of pregnancy estrogen levels and breast cancer risk among female rat offspring. Clin Cancer Res. 2002;8:3601–3610. [PubMed] [Google Scholar]
- 66.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 67.Maclean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim Y-W, Traina SB, Hilton L, Garland R, Morton SC. Effects of omega-3 fatty acids on cancer risk, a systematic review. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 68.Maclennan M, Ma DWL. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Canc Res. 2010;12:211–220. doi: 10.1186/bcr2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Signori C, El-Bayoumy K, Russo J, Thompson HJ, Richie JP, Hartman TJ, Manni A. Chemoprevention of breast cancer by fish oil in preclinical models: trials and tribulations. Cancer Res. 2011;71:6091–6096. doi: 10.1158/0008-5472.CAN-11-0977. [DOI] [PubMed] [Google Scholar]
- 70.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin J-L, Monjaud I, Guidollet J, Toubul P, Delaye J. Mediterranean alpha-linoleic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 71.GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 72.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimoda K, Shirata K, for the Japan EPA lipid intervention study (JELIS) investigation Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 73.Raheja BS, Sadikot SM, Phatak RB, Rao MB. Significance of the n-6/n-3 ratio for insulin action in diabetes. Ann New York Acad Sci. 1993;683:258–271. doi: 10.1111/j.1749-6632.1993.tb35715.x. [DOI] [PubMed] [Google Scholar]
- 74.James MJ, Cleland LG. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum. 1997;27:85–97. doi: 10.1016/s0049-0172(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 75.Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. 1997;65:1011–1017. doi: 10.1093/ajcn/65.4.1011. [DOI] [PubMed] [Google Scholar]
- 76.Weiss LA, Barret-Connor E, von Muhlen D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr. 2005;81:934–938. doi: 10.1093/ajcn/81.4.934. [DOI] [PubMed] [Google Scholar]
- 77.Hogstrom M, Nordstrom P, Nordstrom A. n-3 fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 study. Am J Clin Nutr. 2007;85:803–807. doi: 10.1093/ajcn/85.3.803. [DOI] [PubMed] [Google Scholar]
- 78.Locke CA, Stoll AL. Omega-3 fatty acids in major depression. World Rev Nutr Diet. 2001;89:173–185. doi: 10.1159/000059784. [DOI] [PubMed] [Google Scholar]
- 79.Stoll AL, Severus WE, Freeman MP, Reuter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega-3 fatty acid sin bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 80.Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration. The US Twin Study of age-related macular degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 83.Bailey AL, Southon S. Determination of total long chain fatty acids in human plasma and lipoproteins, before and during copper-stimulated oxidation, by high performance liquid chromatography. Anal Chem. 1998;70:415–419. doi: 10.1021/ac970585o. [DOI] [PubMed] [Google Scholar]
- 84.Harper CR, Edwards MJ, DeFilipis AP, Jacobson TA. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr. 2006;136:83–87. doi: 10.1093/jn/136.1.83. [DOI] [PubMed] [Google Scholar]
- 85.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemotherapeutic agents. Pharmacol Ther. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 86.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 87.Comba A, Lin YH, Eynard AR, Valentich MA, Fernandez-Zapico ME, Pasqualini ME. Basic aspects of tumor cell fatty acid-regulated signaling and transcription factors. Cancer Metastasis Rev. 2011;30:325–342. doi: 10.1007/s10555-011-9308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards IJ, O’Flaherty JT (2008) Omega-3 fatty acids and PPARgamma in cancer. PPAR Res. doi:10.1155/2008/358052 [DOI] [PMC free article] [PubMed]
- 89.Sun H, Berquin IM, Owens RT, O’Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor γ-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis. 2010;31:489–495. doi: 10.1093/carcin/bgp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu IF, Hasio AC, Hu MC, Yang FM, Hm S. Docosahexaenoic acid induces proteasome-dependent degradation of estrogen receptor alpha and inhibits the downstream signaling target in MCF-7 breast cancer cells. J Nutr Biochem. 2010;21:512–517. doi: 10.1016/j.jnutbio.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of MDA-MB-231human breast cancer cells. J Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 93.Menendez JA, Vazquez-Martin A, Ropero S, Colomer R, Lupu R. Her2 (erbB-2)-targeted effects of the omega-3 polyunsaturated fatty acid, alpha-linoleic acid (ALA; 18:3n-3), in breast cancer cells: the “fat features” of the “Mediterranean diet” as an “anti-Her2 cocktail”. Clin Transl Oncol. 2006;8:812–820. doi: 10.1007/s12094-006-0137-2. [DOI] [PubMed] [Google Scholar]
- 94.Rogers KR, Kikawa KD, Mouradian M, Hernandez K, McKinnon KM, Ahwah SM, Pardini RS. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31:1523–1530. doi: 10.1093/carcin/bgq111. [DOI] [PubMed] [Google Scholar]
- 95.Sauer LA, Dauchy RT, Blask DE, Krause JA, Davidson LK, Dauchy EM. Eicosapentaenoic acid suppresses cell proliferation in MCF-7 human breast cancer xenografts in nude rats via a pertussis toxin-sensitive signal transduction pathway. J Nutr. 2005;135:2124–2129. doi: 10.1093/jn/135.9.2124. [DOI] [PubMed] [Google Scholar]
- 96.Sun H, Hu Y, Gu Z, Owens RT, Chen YQ, Edwards IJ. Omega-3 fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecan-1 inhibition of the MEK-Erk pathway. Carcinogenesis. 2011;32:1518–1524. doi: 10.1093/carcin/bgr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Navarro-Tito N, Robledo T, Salazar EP. Arachidonic acid promotes FAK activation and migration in MDA-MB-231 breast cancer cells. Exp Cell Res. 2008;314:3340–3355. doi: 10.1016/j.yexcr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 98.Isbilen B, Fraser SP, Djamgoz MB. Docosahexaenoic acid (omega-3) blocks voltage-gated sodium channel activity and migration of MDA-MB-231 human breast cancer cells. Int J Biochem Cell Biol. 2006;38:2173–2182. doi: 10.1016/j.biocel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 99.Gillet L, Roger S, Bougnox P, Le Guennec JY, Besson P. Beneficial effects of omega-3 long chain fatty acids in breast cancer and cardiovascular diseases: voltage-gated sodium channels as a common feature. Biochimie. 2011;93:4–6. doi: 10.1016/j.biochi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Hammamieh R, Chakraborty N, Miller S-A, Waddy E, Barmada M, Das R, Peel SA, Day AA, Jett M. Differential effects of omega-3 and omega-6 fatty acids on gene expression in breast cancer cells. Breast Canc Res Treat. 2007;101:7–16. doi: 10.1007/s10549-006-9269-x. [DOI] [PubMed] [Google Scholar]
- 101.Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z, Jo Davisson V, Siddiqui RA. A synergistic anti-proliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149–164. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cavallaro U, Christofori G. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Acta. 2001;1552:39–45. doi: 10.1016/s0304-419x(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 103.Graham JD, Yager ML, Hill HD, Byth K, O’Neill GM, Clarke CL. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endo. 2005;19:2713–2735. doi: 10.1210/me.2005-0126. [DOI] [PubMed] [Google Scholar]
- 104.Altenburg JD, Siddiqui RA. Omega-3 polyunsaturated fatty acids down modulate CXCR4 expression and function in MDA-MB-231 breast cancer cells. Mol Canc Res. 2009;7:1013–1020. doi: 10.1158/1541-7786.MCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 105.Young LR, Kurzer MS, Thomas W, Redmon JB, Raatz SK. Effect of dietary fat and omega-3 fatty acids on urinary eicosanoids and sex hormone concentrations in postmenopausal women: a randomized controlled feeding trial. Nutr Canc. 2011;63:930–939. doi: 10.1080/01635581.2011.589957. [DOI] [PubMed] [Google Scholar]
- 106.Bonofiglio D, Gabriele S, Aquila S, et al. Estrogen receptor α binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor γ signaling in breast cancer cells. Clin Cancer Res. 2005;11:6139–6147. doi: 10.1158/1078-0432.CCR-04-2453. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Kilgore MW. Signal cross-talk between estrogen receptor α and β and the peroxisome proliferator-activated receptor γ1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol. 2002;194:123–133. doi: 10.1016/s0303-7207(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 108.Manni A, Xu H, Washington S, Aliaga C, Cooper T, Richie JR, Jr, Bruggeman R, Prokopczyk B, Calcagnotto A, Trushin N, Mauger D, Venderame MF, El-Bayoumy K. The impact of fish oil on the chemopreventive efficacy of tamoxifen against development of N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Canc Prev Res. 2010;3:322–330. doi: 10.1158/1940-6207.CAPR-09-0173. [DOI] [PubMed] [Google Scholar]
- 109.Henriquez S, Calderon C, Quezada M, Oliva B, Bravo ML, Aranda E, Kato S, Cuello MA, Gutierrez J, Quest AF, Owen GI. Progesterone utilizes distinct membrane pools of tissue factor to increase coagulation and invasion and these effects are inhibited by TFPI. J Cell Physiol. 2011;226:3278–3285. doi: 10.1002/jcp.22689. [DOI] [PubMed] [Google Scholar]
- 110.Lange CA. Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol. 2008;188:203–212. doi: 10.1016/j.jsbmb.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fu XD, Goglia L, Sanchez AM, Flamini M, Giretti MS, Tosi V, Genazanni AR, Simoncini T. Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase. Endocr Relat Canc. 2010;17:431–443. doi: 10.1677/ERC-09-0258. [DOI] [PubMed] [Google Scholar]
- 112.Carvajal A, Espinoza N, Kato S, Pinto M, Sadarangani A, Monso C, Aranda E, Villalon M, Richer JK, Horwitz KB, Brosens JJ, Owen GI. Progesterone pre-treatment potentiates EGF pathway signaling in the breast cancer cell line ZR-75. Breast Canc Res Treat. 2005;94:171–183. doi: 10.1007/s10549-005-7726-6. [DOI] [PubMed] [Google Scholar]
- 113.Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:31308–31316. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 114.Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol. 2006;20:2278–2291. doi: 10.1210/me.2005-0343. [DOI] [PubMed] [Google Scholar]
- 116.Wang P, Dharmaraj N, Brayman MJ, Carson DD. Peroxisome proliferator-activated receptor γ activation inhibits progesterone-stimulated human MUC1 expression. Mol Endocrinol. 2010;24:1368–1379. doi: 10.1210/me.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ligtenberg MJ, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:2318–2324. [PubMed] [Google Scholar]
- 118.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sachdeva M, Mo Y-Y. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting Mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5(4):e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bidinotto LT, Lopez de Cicco R, Russo J. Omega-3 fatty acids: a potential booster for tamoxifen therapy? Expert Rev Anticancer Ther. 2011;11:1151–1153. doi: 10.1586/era.11.106. [DOI] [PubMed] [Google Scholar]