Abstract
Mitotic checkpoint is a fundamental mechanism involved in fidelity mitotic chromosome segregation, and its alteration results in progression of human malignancies. In this study, we examined expression profiles of seven mitotic checkpoint genes in 20 breast carcinomas using microarray analysis. Results demonstrated that BUB1 expression level was closely correlated with the proliferation activity evaluated by Ki-67 labeling index (LI) of individual cases. Therefore, we further immunolocalized BUB1 in 104 breast carcinoma tissues in order to evaluate its clinicopathological significance. BUB1 immunoreactivity was detected in the nucleus and/or cytoplasm of carcinoma cells, and nuclear and cytoplasmic BUB1 status were positive in 40% and 58% of the cases examined, respectively. In particular, nuclear BUB1 status was significantly associated with stage, pathological tumor factors, lymph node metastasis, distant metastasis, histological grade, and Ki-67 LI, but cytoplasmic BUB1 status was not significantly associated with any of the parameters examined. Subsequent multivariate analysis revealed that nuclear BUB1 status turned out an independent prognostic factor for both disease-free and breast cancer-specific survival of the patients examined. These results all indicated that BUB1 played important roles in the proliferation and/or progression of the breast carcinoma, and nuclear BUB1 immunohistochemical status is also considered a potent prognostic factor in human breast cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-012-0130-x) contains supplementary material, which is available to authorized users.
Keywords: Breast Carcinoma, Estrogen Receptor Status, Spindle Assembly Checkpoint, Mitotic Checkpoint, Breast Carcinoma Patient
Introduction
Breast cancer is one of the most common malignancies in women. Invasive breast cancer is generally regarded as a disease that metastasizes in an early phase [1], and clinical outcome of the patients is markedly influenced not only by metastasis but also by proliferative activity of the carcinoma cells [2, 3]. A multitude of prognostic factors identified in breast cancer patients have been demonstrated to be directly or indirectly correlated with carcinoma cell proliferation.
Cell proliferation is closely associated with altered regulation of the cell cycle [4]. Progression of the cell cycle is regulated by three major checkpoint mechanisms, i.e., G1/S, G2/M, and mitotic checkpoints, which subsequently ensure that each step takes place only once and in the right sequence [5]. Among these factors, the mitotic checkpoint, also known as spindle assembly checkpoint, is to ensure accurate chromosome segregation by inducing mitotic arrest when errors occur in the spindle structure or in the alignment of the chromosomes on the spindle formation [6]. Defective mitotic checkpoint genes have been reported to be implicated as one of the mechanisms of chromosomal instability [5], but significance of alternations of mitotic checkpoint themselves have remained largely unknown in human cancer tissues compared with other checkpoints. Genomic studies in mammals implicated at least seven genes including BUB1, BUB1B (BUBR1 or MAD3), BUB3, MAD1, MAD2, CDC20, and TTK (MPS1) [5, 7, 8] in the mitotic checkpoint. Therefore, in this study, we first evaluated expression profiles of mitotic checkpoint genes in the breast carcinoma based on microarray data and did demonstrate that BUB1 expression level was closely correlated with the proliferative activity of carcinoma cells.
BUB1 is also well-known as a key component of mitotic checkpoint. BUB1 mutations were originally reported in a subset of aneuploid colorectal carcinoma cell lines [9], suggesting that low expression of BUB1 could contribute to defective mitotic checkpoint control in human malignancies. However, subsequent studies in various human cancer tissues demonstrated that the mutations of BUB1 were extremely rare or not detected at all [10–13]. However, Yuan et al. [14] reported that both mRNA and protein levels for mitotic checkpoint genes including BUB1 were significantly higher in the breast carcinoma cell lines than normal mammary epithelial cells. In addition, Shigeishi et al. [15] reported a positive significant correlation between BUB1 expression levels and proliferative activity in the salivary gland tumors. These findings all indicated that BUB1 plays important roles in the proliferation and/or progression of the breast carcinoma. However, BUB1 immunolocalization has been reported only in the gastric cancer among human malignancies [16] to the best of our knowledge, and clinical significance of BUB1 has remained unknown in the breast carcinoma. Therefore, in this study, we immunolocalized BUB1 in human breast cancer tissues in order to clarify its clinicopathological significance.
Materials and Methods
Patients and Tissues
Two sets of tissue specimens were evaluated in this study. As a first set, 20 specimens of invasive breast carcinoma were obtained from women (age, 40–74 years) who underwent surgical treatment from 2000 to 2003 in the Department of Surgery, Tohoku University Hospital, Sendai, Japan. These cases were all estrogen receptor (ER)-positive breast carcinoma patients, and the percentage of ER-positive carcinoma cells (i.e., ER labeling index (LI)) was 4–95% in these cases [17]. These specimens were kept both at−80°C for microarray analysis and fixed in 10% formalin and embedded in paraffin wax for immunohistochemistry for Ki-67.
As a second set, 104 specimens of invasive breast carcinoma were obtained from Japanese female patients who underwent surgical treatment from 1988 to 1999 in the Department of Surgery, Tohoku University Hospital, Sendai, Japan. The mean age of these patients was 55 (range, 22–81 years), and these patients did not receive chemotherapy, irradiation, or hormonal therapy prior to the surgery. Review of the charts revealed that 79 patients received adjuvant chemotherapy and 69 patients received tamoxifen therapy following the surgery. The clinical outcome was evaluated by disease-free and breast cancer-specific survival of the stages I–III patients in this study, and the mean follow-up time was 95 (range, 0–175 months). All the specimens were fixed in 10% formalin and embedded in paraffin wax.
Research protocols for this study were approved by the Ethics Committee at Tohoku University School of Medicine.
Laser Capture Microdissection/Microarray Analysis
Gene expression profiles of laser capture microdissection samples in 20 invasive breast carcinoma cases were examined using microarray analysis. A part of gene expression profile data was assembled in our previous study [18, 19]. Briefly, frozen-specimens of the breast carcinoma were sectioned at a thickness of 8 μm; approximately 5,000 breast carcinoma cells were laser-transferred, and total RNA was extracted. Sample preparation and processing were performed as described in the Affymetrix GeneChip Expression Analysis Manual (Affymetrix), with the exception that the labeled cRNA samples were hybridized to the complete human U133 GeneChip set (Affymetrix), including 22,215 and 22,577 genes. We focused on expression of seven representative mitotic checkpoint genes in this study.
Immunohistochemistry
Rabbit polyclonal antibodies for human BUB1 (LS-C118685) and γ-tubulin (GTX115850) were purchased from LifeSpan BioSciences (Seattle, WA, USA) and GeneTex (Irvine, CA, USA), respectively. Monoclonal antibodies for ER (ER1D5), progesterone receptor (PR; MAB429), and Ki-67 (MIB1) were purchased from Immunotech (Marseille, France), Chemicon (Temecula, C, USA), and DAKO (Carpinteria, CA, USA), respectively. Rabbit polyclonal antibodies for HER2 (A0485) were obtained from DAKO.
A Histofine Kit (Nichirei Bioscience, Tokyo, Japan), which employs the streptavidin-biotin amplification method was used in this study. Antigen retrieval was performed by heating the slides in an autoclave at 120°C for 5 min in antigen retrieval solution (pH 9.0; Nichirei Bioscience) for BUB1 immunostaining or citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate (pH 6.0)) for other antibodies. Dilutions of primary antibodies used in this study were as follows: BUB1, 1/200; ER, 1/50; PR, 1/30; HER2, 1/200; Ki-67, 1/50; and γ-tubulin, 1/500. The antigen–antibody complex was visualized with 3,3’-diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris–HCl buffer (pH 7.6), and 0.006% H2O2) and counterstained with hematoxylin. Human gastric carcinoma tissue was used as a positive control for BUB1 antibody [16]. As negative controls of BUB1 immunostaining, we used normal rabbit IgG instead of the primary antibody or no secondary antibody in this study.
Scoring of Immunoreactivity and Statistical Analysis
Immunoreactivity of BUB1 was detected in the nucleus and/or cytoplasm of the breast carcinoma cells. Therefore, we separately evaluated BUB1 immunoreactivity in the nucleus and cytoplasm, and the cases that had more than 10% of positive carcinoma cells were considered positive for nuclear and cytoplasmic BUB1status, respectively. Immunoreactivity for ER, PR, and Ki-67 was detected in the nucleus, and the immunoreactivity was evaluated in more than 1,000 carcinoma cells for each case, and their LI was subsequently determined. Cases with ER LI of more than 1% were considered ER-positive breast carcinoma in this study [17]. HER2 immunoreactivity was evaluated according to the grading system proposed in HercepTest (DAKO), and strongly circumscribed membrane-immunoreactivity of HER2 present in more than 10% carcinoma cells (score 3+) were considered positive. γ-Tubulin immunoreactivity was classified into three groups according to a previous report [20]. Briefly, percent of the positive cells in each case was scored 0 (less than 5%), 1 (5–25%), 2, (26–50%), 3 (51–75%), or 4 (more than 75%), as well as its immunointensity (0, negative; 1, weak; 2, moderate; and 3, intense). These scores were multiplied (range, 0–12) and then classified into the following three groups: low (the multiplied score 0–4), moderate (score 5–8), and high (score 9–12).
An association between signal intensity of the mitotic checkpoint genes and Ki-67 LI was evaluated using correlation coefficient (r) and regression equation. An association between BUB1 immunohistochemical status and clinicopathological factors was evaluated by the Student’s t test or a cross-table using the chi-square test. Disease-free and breast cancer-specific survival curves were generated according to the Kaplan–Meier method, and statistical significance was calculated using the log-rank test. Uni- and multivariate analyses were evaluated by a proportional hazard model (COX). P values of less than 0.05 were considered significant in this study. The statistical analyses were performed using the StatView 5.0J software (SAS Institute, Cary, NC, USA).
Results
Association Between Expression of Mitotic Checkpoint Genes and Proliferative Activity in the Breast Carcinoma Cases
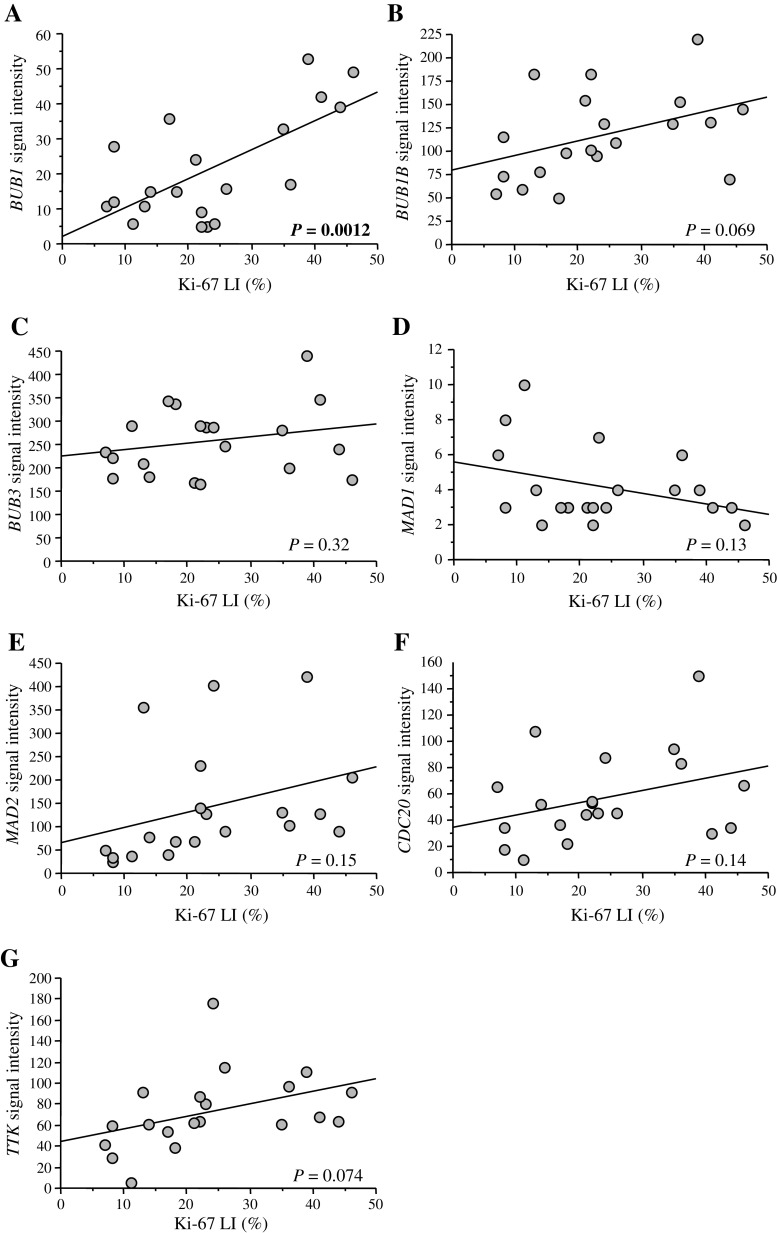
Ki-67 antibody recognizes cells in all phases of the cell cycle except G0 (resting) phase [21], and Ki-67 LI is well-known to be closely correlated with the S phase fraction and mitotic index in the breast carcinoma [2]. When we examined an association between expression level of seven representative mitotic checkpoint genes evaluated by microarray and Ki-67 LI (Fig. 1), BUB1 was positively associated with Ki-67 LI (P = 0.0012, r = 0.67) (Fig. 1a). Similar tendency was also detected in BUB1B (P = 0.069) (Fig. 1b), MAD2 (P = 0.15) (Fig. 1e), CDC20 (P = 0.14) (Fig. 1f), and TTK (P = 0.074) (Fig. 1g) and reverse tendency in MAD1 (P = 0.13) (Fig. 1d), but these did not reach statistical significance. The status of BUB3 (Fig. 1c) was not associated with Ki-67 LI in this study. The microarray data of these genes were provided in Supplementary Table S1.
Fig. 1.
Association between expression of mitotic checkpoint genes (i.e., BUB1 (a), BUB1B (b), BUB3 (c), MAD1 (d), MAD2 (e), CDC20 (f), and TTK (g)) and Ki-67 LI in the breast carcinoma. Signal intensity of each gene was obtained from microarray, and Ki-67 LI was evaluated by immunohistochemistry. Statistical analysis was evaluated using correlation coefficient (r) and regression equation. P values less than 0.05 were considered significant and described as boldface
Associations of expression levels among these mitotic checkpoint genes were summarized in Table 1. Statistically significant positive associations were detected between BUB1B and MAD2 (P = 0.0002), CDC2 (P = 0.0009), or TTK (P = 0.0095), between MAD2 and CDC20 (P < 0.0001) or TTK (P = 0.0001), and between CDC20 and TTK (P = 0.0059). BUB1 expression was not significantly associated with other genes examined.
Table 1.
Association among expression levels of seven mitotic checkpoint genes in 20 breast carcinomas
| BUB1B | BUB3 | MAD1 | MAD2 | CDC20 | TTK | |
|---|---|---|---|---|---|---|
| BUB1 | 0.37 | 0.17 | 0.25 | 0.61 | 0.25 | 0.96 |
| BUB1B | 0.88 | 0.19 | 0.0002 | 0.0009 | 0.0095 | |
| (r = 0.74) | (r = 0.68) | (r = 0.57) | ||||
| BUB3 | 0.99 | 0.25 | 0.36 | 0.74 | ||
| MAD1 | 0.26 | 0.53 | 0.082 | |||
| MAD2 | <0.0001 | 0.0001 | ||||
| (r = 0.80) | (r = 0.76) | |||||
| CDC20 | 0.0059 | |||||
| (r = 0.59) |
Data are presented as P values. P values less than 0.05 were considered significant and described as boldface
BUB1 Immunolocalization in Human Breast Carcinoma Cases
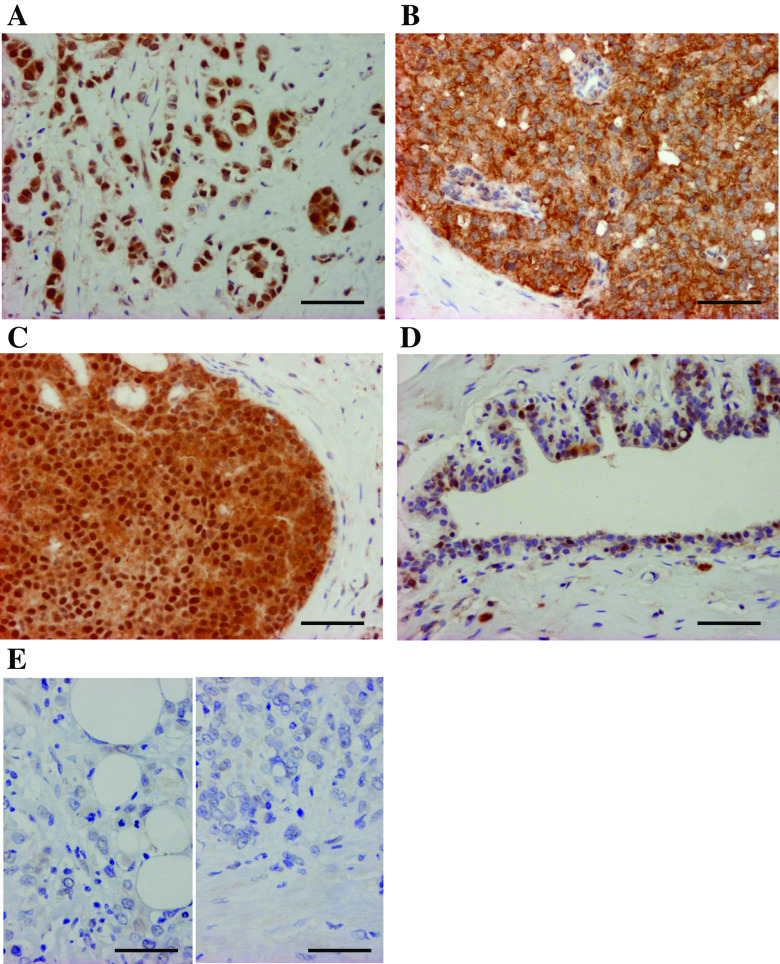
Immunoreactivity of BUB1 was detected in the nuclei and/or cytoplasm of breast carcinoma cells (Fig. 2a–c). BUB1 immunoreactivity was also focally detected in the nuclei of epithelial cells in morphologically normal glands (Fig. 2d), while negative in the stroma. No significant BUB1 immunoreactivity was detected in the negative control sections in this study (Fig. 2e).
Fig. 2.
Immunohistochemistry for BUB1 in the breast carcinoma. BUB1 immunoreactivity was detected in the nucleus (a), cytoplasm (b), or both nucleus and cytoplasm (c) of the carcinoma cells. BUB1 immunoreactivity was focally detected in the nucleus of morphologically normal mammary epithelium (d). e Negative control sections of BUB1 immunohistochemistry (left panel: normal rabbit IgG used instead of the primary antibody and right panel: no secondary antibody). Bar = 100 mm, respectively
Associations between nuclear BUB1 immunohistochemical status and various clinicopathological parameters in breast carcinomas were summarized in Table 2. The number of BUB1-positive breast carcinomas was 42 out of 104 (40%) cases. Nuclear BUB1 status was significantly associated with stage (P = 0.0070), pathological tumor factor (pT) (P = 0.023), lymph node metastasis (P = 0.016), distant metastasis (P = 0.041), histological grade (P = 0.009), Ki-67 LI (P = 0.003), and cytoplasmic BUB1 status (P = 0.0017), while no significant association was detected in patients’ age, menopausal status, ER status, PR LI, and HER2 status.
Table 2.
Association between nuclear BUB1 immunohistochemical status and clinicopathological parameters in 104 breast carcinomas
| Nuclear BUB1 status | P value | ||
|---|---|---|---|
| + (n = 42) | − (n = 62) | ||
| Patient agea (years) | 54.2 ± 1.6 | 56.0 ± 1.5 | 0.44 |
| Menopausal status | |||
| Premenopausal | 17 (16%) | 21 (20%) | 0.49 |
| Postmenopausal | 25 (24%) | 41 (39%) | |
| Stage | |||
| I | 6 (6%) | 23 (22%) | 0.0070 |
| II | 19 (18%) | 28 (27%) | |
| III | 7 (7%) | 8 (7%) | |
| IV | 10 (10%) | 3 (3%) | |
| Pathological tumor factor (pT) | |||
| pT1 | 11(11%) | 30 (29%) | 0.023 |
| pT2-4 | 31 (30%) | 32 (31%) | |
| Lymph node metastasis | |||
| Positive | 25 (24%) | 22 (21%) | 0.016 |
| Negative | 17 (16%) | 40 (38%) | |
| Distant metastasis | |||
| Positive | 10 (10%) | 3 (3%) | 0.041 |
| Negative | 32 (31%) | 59 (57%) | |
| Histological grade | |||
| 1 (well) | 1 (1%) | 19 (18%) | 0.009 |
| 2 (moderate) | 21 (20%) | 27 (26%) | |
| 3 (poor) | 20 (19%) | 16 (15%) | |
| ER status | |||
| Positive | 32 (31%) | 50 (48%) | 0.58 |
| Negative | 10 (10%) | 12 (12%) | |
| PR LIa (%) | 28.0 ± 3.7 | 21.5 ± 4.6 | 0.27 |
| HER2 status | |||
| Positive | 14 (14%) | 12 (12%) | 0.13 |
| Negative | 28 (27%) | 50 (48%) | |
| Ki-67 LIa (%) | 26.8 ± 2.7 | 14.6 ± 1.9 | 0.0003 |
| Cytoplasmic BUB1 status | |||
| Positive | 32 (31%) | 28 (27%) | 0.0017 |
| Negative | 10 (10%) | 34 (33%) | |
| γ-Tubulin immunoreactivity | |||
| Low | 16 (15%) | 18 (17%) | 0.46 |
| Moderate | 15 (14%) | 21 (20%) | |
| High | 11 (11%) | 23 (22%) | |
P values less than 0.05 were considered significant and described as boldface
aData are presented as mean ± SEM. All other values represent the number of cases and percentage
Previous studies demonstrated that γ-tubulin immunoreactivity was closely associated with aberrations of centrosomes and/or chromosomes in the breast carcinoma [20, 22]. However, no significant association was detected between γ-tubulin immunoreactivity and nuclear BUB1 status (P = 0.46) in this study (Table 2).
The positive association between nuclear BUB1 status and stage or distant metastasis was significant regardless of ER status of these cases, while significant association between nuclear BUB1 status and pT, lymph node metastasis, histological grade, Ki-67 LI, or cytoplasmic BUB1 status was detected only in ER-positive group (Table 3).
Table 3.
Association between nuclear BUB1 status and clinicopathological parameters according to ER status in 104 breast carcinomas
| Variable | Nuclear BUB1 status (positive/negative) | |
|---|---|---|
| ER-positive group (n = 82) | ER-negative group (n = 22) | |
| Patient age | 0.49 | 0.84 |
| Menopausal status | 0.75 | 0.43 |
| Stage | 0.0081 | 0.025 |
| pT | 0.034 | 0.19 |
| Lymph node metastasis | 0.018 | 0.57 |
| Distant metastasis | 0.032 | 0.041 |
| Histological grade | 0.0006 | 0.53 |
| HER2 status | 0.083 | 0.94 |
| Ki-67 LI | 0.0005 | 0.28 |
| Cytoplasmic BUB1 status | 0.0015 | 0.39 |
| γ-Tubulin immunoreactivity | 0.55 | 0.42 |
Data are presented as P values. P values less than 0.05 were considered significant, and described as boldface
Cytoplasmic BUB1 immunoreactivity was detected in 60 out of 104 (58%) breast carcinoma cases. Cytoplasmic BUB1 status was marginally associated with Ki-67 LI in the breast carcinoma (P = 0.052), but no significant association was detected between cytoplasmic BUB1 status and clinicopathological parameters examined in this study (Table 4).
Table 4.
Association between cytoplasmic BUB1 immunohistochemical status and clinicopathological parameters in 104 breast carcinomas
| Cytoplasmic BUB1status | P value | ||
|---|---|---|---|
| + (n = 60) | − (n = 44) | ||
| Patient agea (years) | 55.9 ± 1.4 | 54.5 ± 1.8 | 0.53 |
| Menopausal status | |||
| Premenopausal | 18 (17%) | 20 (19%) | 0.11 |
| Postmenopausal | 42 (40%) | 24 (23%) | |
| Stage | |||
| I | 12 (12%) | 17 (16%) | 0.11 |
| II | 30 (29%) | 17 (16%) | |
| III | 8 (8%) | 7 (7%) | |
| IV | 10 (10%) | 3 (3%) | |
| Pathological tumor factor (pT) | |||
| pT1 | 39 (38%) | 24 (23%) | 0.28 |
| pT2-4 | 21 (20%) | 20 (19%) | |
| Lymph node metastasis | |||
| Positive | 28 (27%) | 19 (18%) | 0.72 |
| Negative | 32 (16%) | 25 (24%) | |
| Distant metastasis | |||
| Positive | 10 (10%) | 3 (3%) | 0.13 |
| Negative | 50 (48%) | 41 (39%) | |
| Histological grade | |||
| 1 (well) | 8 (8%) | 12 (12%) | 0.19 |
| 2 (moderate) | 29 (28%) | 19 (18%) | |
| 3 (poor) | 23 (22%) | 13 (13%) | |
| ER status | |||
| Positive | 49 (47%) | 33 (32%) | 0.41 |
| Negative | 11 (11%) | 11 (12%) | |
| PR LIa (%) | 26.8 ± 4.1 | 23.1 ± 3.8 | 0.53 |
| HER2 status | |||
| Positive | 16 (15%) | 10 (10%) | 0.74 |
| Negative | 44 (42%) | 34 (33%) | |
| Ki-67 LIa (%) | 22.4 ± 2.2 | 15.8 ± 2.5 | 0.052 |
| γ-Tubulin immunoreactivity | |||
| Low | 16 (15%) | 18 (17%) | 0.14 |
| Moderate | 20 (19%) | 16 (15%) | |
| High | 24 (23%) | 10 (10%) | |
P values less than 0.05 were considered significant
aData are presented as mean ± SEM. All other values represent the number of cases and percentage
Association Between BUB1 Status and Clinical Outcome of the Patients
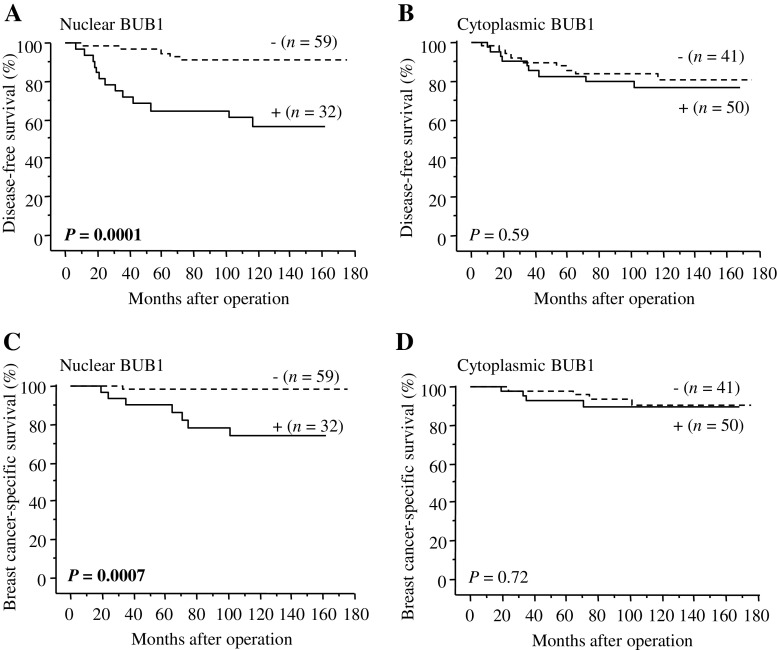
In order to examine an association between BUB1 status and prognosis of the patients precisely, we excluded stage IV cases and used stages I to III breast carcinoma patients (n = 91) in the following analyses. Nuclear BUB1 status was significantly associated with an increased incidence of recurrence (P = 0.0001) as demonstrated in Fig. 3a, whereas cytoplasmic BUB1 status was not (P = 0.59) (Fig. 3b). The multivariate analysis revealed that lymph node metastasis (P = 0.0022) and nuclear BUB1 status (P = 0.0056) were independent prognostic factors for disease-free survival with relative risks over 1.0 (Table 5).
Fig. 3.
Disease-free (a, b) and breast cancer-specific survival (c, d) of stages I-III breast carcinoma patients according to BUB1 status studied by Kaplan–Meier method (n = 91). Statistical analysis was evaluated by the log-rank test. P values less than 0.05 were considered significant and described as boldface
Table 5.
Uni- and multivariate analyses of disease-free survival in stages I-III breast cancer patients examined
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| P value | P value | Relative risk (95% CI) | |
| Lymph node metastasis (positive/negative) | 0.0005 | 0.0022 | 7.1 (2.0–25.1) |
| Nuclear BUB1 status (positive/negative) | 0.0007 | 0.0056 | 4.5 (1.6–13.0) |
| Pathological tumor factor (pT) (pT2-4/pT1) | 0.045 | 0.39 | |
| Adjuvant chemotherapy (yes/no) | 0.15 | ||
| Ki-67 LIa (78%–0%) | 0.23 | ||
| HER2 status (positive/negative) | 0.29 | ||
| Cytoplasmic BUB1 status (positive/negative) | 0.59 | ||
| Histological grade (3/1,2) | 0.74 | ||
Data considered significant (P < 0.05) in the univariate analyses were described as boldface and were examined in the multivariate analyses
aData were evaluated as continuous variables. All other data were evaluated as dichotomized variables
Breast cancer-specific survival curves of BUB1 status were summarized in Fig. 3c and d. A significantly positive correlation (P = 0.0007) was detected between nuclear BUB1 status and adverse clinical outcome of the patients examined, but cytoplasmic BUB1 status was not associated (P = 0.72). In the univariate analysis (Table 6), nuclear BUB1 status (P = 0.011), histological grade (P = 0.018), Ki-67 LI (P = 0.026), and lymph node metastasis (P = 0.043) were all detected as significant prognostic variables for breast cancer-specific survival in this study. However, a following multivariate analysis revealed that only nuclear BUB1 status was independent prognostic factor with a relative risk over 1.0 (P = 0.043), whereas histological grade (P = 0.21), Ki-67 LI (P = 0.75), and lymph node metastasis (P = 0.087) were all not significant.
Table 6.
Uni- and multivariate analyses of breast cancer-specific survival in stages I-III breast cancer patients examined
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| P value | P value | Relative risk (95% CI) | |
| Nuclear BUB1 status (positive/negative) | 0.011 | 0.043 | 9.4 (1.1–83.2) |
| Histological grade (3/1,2) | 0.018 | 0.21 | |
| Ki-67 LIa (78%–0%) | 0.026 | 0.75 | |
| Lymph node metastasis (positive/negative) | 0.043 | 0.087 | |
| Pathological tumor factor (pT) (pT2-4/pT1) | 0.091 | ||
| HER2 status (positive/negative) | 0.23 | ||
| Cytoplasmic BUB1 status (positive/negative) | 0.72 | ||
aData were evaluated as continuous variables. All other data were evaluated as dichotomized variables
Data considered significant (P < 0.05) in the univariate analyses were described as boldface, and were examined in the multivariate analyses
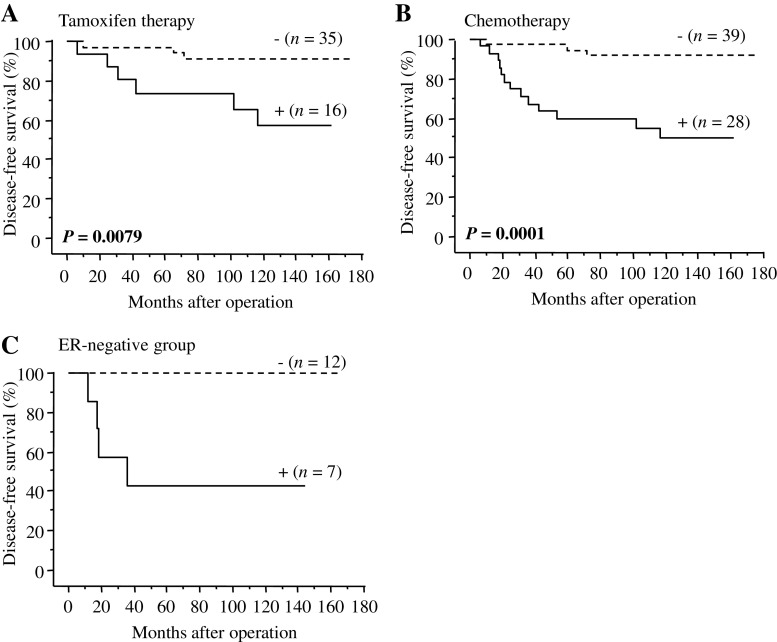
In our present study, 51 patients received tamoxifen therapy following surgery as an adjuvant treatment in ER-positive stages I-III breast carcinoma cases, and nuclear BUB1 status was significantly associated with an increased risk of recurrence in these patients (P = 0.0079) (Fig. 4a). Similar tendency was detected between nuclear BUB1 status and breast cancer-specific survival of the patients, although P value did not reach statistical significance (P = 0.14). Significant association between nuclear BUB1 status and clinical outcome of the patients was also detected in 67 patients who received adjuvant chemotherapy (P = 0.0001 for disease-free survival (Fig. 4b) and P = 0.0028 for breast cancer-specific survival). Nuclear BUB1 status was significantly associated with an increased risk of recurrence (Fig. 4c) and worse prognosis in the ER-negative stages I-III cases (n = 19), although P values were not available because no patient had recurrent disease or died in the group of these nuclear BUB1-negative cases.
Fig. 4.
Association between nuclear BUB1 status and disease-free survival in a subset of stages I-III breast carcinoma cases (Kaplan–Meier method). a ER-positive breast carcinoma cases received tamoxifen therapy (n = 51), b patients who received adjuvant chemotherapy (n = 67), and c ER-negative breast carcinoma cases (n = 19). Statistical analysis was evaluated by the log-rank test. P values less than 0.05 were considered significant and described as boldface. c P values were not available because no patient had recurrent disease in the group of nuclear BUB1-negative cases
Discussion
Results of our present study demonstrated that BUB1 expression level was significantly associated with Ki-67 LI in the breast carcinoma cells, and similar tendency was also detected in BUB1B, MAD2, CDC20, and TTK. Yuan et al. [14] previously reported that mRNA levels of mitotic checkpoint genes, such as BUB1, BUB1B, BUB3, MAD1, MAD2, CDC20, and TTK, were almost uniformly increased in breast carcinoma cell lines compared with MCF10A and mammary epithelial cells. Overexpression of BUB1, BUB1B, BUB3 [23, 24], and MAD2 [25] was also reported in the gastric carcinoma cells. In particular, Grabsch et al. [24] did report a positive association between BUB1, BUB1B, or BUB3 and Ki-67 mRNA levels in the gastric carcinoma. Association between BUB1 mRNA level and Ki-67 LI was also reported in the salivary gland tumors [15]. Results of these studies above are all consistent with those of our present study. However, MAD1 expression tended to be inversely associated with Ki-67 LI in our present study. Han et al. [26] reported that MAD1 expression was significantly reduced in poorly differentiated breast carcinomas, which may partly explain our present finding. These results also indicated that amounts of mitotic checkpoint proteins were increased in their expression in breast carcinoma cells according to their proliferative activity, and in particular, BUB1 was most pronouncedly increased among these proteins.
This is a first study to demonstrate immunolocalization of BUB1 in human breast cancer patients. BUB1 immunoreactivity was detected in both the nuclei and/or cytoplasm of the carcinoma cells. BUB1 protein is involved in the spindle assembly checkpoints, and therefore, its intracellular localization is postulated to be the nucleus. Grabsch et al. [16] demonstrated nuclear BUB1 immunolocalization in the gastric carcinoma cells, which is consistent with our present findings. However, cytoplasmic immunolocalization was also reported in some mitotic checkpoint proteins in carcinoma cells. For instances, cytoplasmic BUB1B immunoreactivity was detected in the breast [14] and colon [27] carcinomas, and cytoplasmic MAD2 immunolocalization was shown in the colon [28] and gastric [29] carcinomas. In addition, Burum-Auensen et al. [30] reported that subcellular localization of BUB1B shifted from the cytoplasm to nucleus during the malignant transformation. Results of our present study did demonstrate that BUB1 expression was correlated with Ki-67 LI in the microarray analysis, and nuclear BUB1 immunoreactivity was also associated with Ki-67 LI and cytoplasmic BUB1 status. Therefore, BUB1 immunoreactivity is required to be evaluated in the nucleus in the breast carcinoma tissues.
In our present study, nuclear BUB1 immunoreactivity was positively associated with stage, pT, lymph node metastasis, distant metastasis, histological grade, and Ki-67 LI in the 104 breast cancer patients. Shigeishi et al. [15] reported that BUB1 protein level evaluated by immunoblot analysis was significantly associated with stage (P = 0.02) and marginally associated with pT (P = 0.11) or lymph node metastasis (P = 0.14) in ten salivary gland carcinomas, which is consistent with results of our present study. Results of our present study also revealed that nuclear BUB1 status was not significantly associated with γ-tubulin immunoreactivity, which is reported to reflect centrosome aberrations [22] or chromosomal changes [20] in the breast cancer. Grabsch et al. [16] previously reported that BUB1 immunoreactivity was not associated with DNA ploidy or microsatellite instability in the gastric carcinoma, which is consistent with the findings in our present study. Decreased expression level of mitotic checkpoint proteins may result in defective spindle checkpoint controls, but further investigations are required to determine whether BUB1 expression level reflects spindle checkpoint function or not in human malignancies. Overexpression of BUB1 lead to chromosome instability of the cells [31], and BUB1 was also reported to negatively regulate p53-mediated early cell death [8, 32]. Therefore, BUB1 may have various biological functions in addition to mitotic checkpoint and play important roles in the cell proliferation and/or progression of the breast carcinoma.
Results of our present study also indicated that an association between nuclear BUB1 status and aggressive phenotype of breast carcinoma was more pronounced in ER-positive cases (Table 3). BUB1 gene has a functional estrogen-responsive element at 4,500 bp from the most upstream mRNA 5’-end of the gene [33], and BUB1 mRNA expression was upregulated by estradiol in MCF-7 breast carcinoma cells [34]. Ebata et al. [35] recently reported that expression profiles of estrogen-induced genes in ER-positive breast carcinomas were different between noninvasive and invasive cases, and BUB1 mRNA level was much higher in invasive carcinoma. Therefore, BUB1 may also play important roles especially in the estrogen-mediated progression of the breast carcinoma.
In our study, nuclear BUB1 immunoreactivity was significantly associated with recurrence and aggressive clinical course in the breast cancer patients, and similar tendency was also detected in ER-positive cases that received tamoxifen therapy or chemotherapy. In addition, results of multivariate analyses clearly demonstrated that nuclear BUB1 immunoreactivity was an independent prognostic factor for both recurrence and breast cancer-specific survival. Dai et al. [36] reported the occurrence of metastasis is strongly predicted by a homogeneous gene expression pattern almost entirely consisting of cell cycle genes within a subset of breast carcinoma patients characterized by relatively abundant ER expression for their age, and BUB1 was included in these genes. In addition, Suzuki et al. [37] very recently identified BUB1 as a gene associated with recurrence of ER-positive breast carcinomas patients who received tamoxifen as a result of microarray analysis. The nuclear BUB1 status was not necessarily associated with ER status in the breast carcinoma in our study, which also indicated that nuclear BUB1 immunoreactivity at the time of surgery may reflect the increased basal level of BUB1 rather than the level induced by estrogens in the breast carcinoma, and residual carcinoma cells following surgical treatment in BUB1-positive breast carcinomas could still have the potential to rapidly grow and/or metastasize, despite of the tamoxifen or chemotherapy. The expression of other mitotic checkpoint protein MAD2 was reported to be associated with resistance to neoadjuvant chemotherapy in the uterine cervical cancer [38], and an orally bioavailable TTK inhibitor (NMS-P715) selectively reduced carcinoma cell proliferation [39]. Results of our present study may serve as a starting point for clarification of biological functions and possible therapeutic potential of BUB1 in breast carcinoma, but it awaits further investigations for clarification.
In summary, we examined expression profiles of mitotic checkpoint genes using microarray analysis. Results demonstrated that BUB1 expression was closely associated with Ki-67 LI in the breast carcinoma cells. A subsequent immunohistochemical analysis did demonstrate that nuclear BUB1 immunoreactivity was detected in 40% of breast carcinoma cases and was significantly associated with stage, pT, lymph node metastasis, distant metastasis, histological grade, Ki-67 LI, and cytoplasmic BUB1 status of breast cancer cases. In addition, multivariate analysis further revealed that the nuclear BUB status was an independent prognostic factor of the patients. These findings all suggest that BUB1 plays important roles in the proliferation and/or progression of breast carcinoma, and nuclear BUB1 immunoreactivity is a potent prognostic factor in the breast cancer patients regardless of ER status.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(DOC 25 kb)
Acknowledgments
We appreciate skillful technical assistance of Mr. Katsuhiko Ono (Department of Anatomic Pathology, Tohoku University Graduate School of Medicine).
Declaration of Interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol. 2004;57:675–681. doi: 10.1136/jcp.2003.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Azambuja E, Cardoso F, de Castro G, Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 5.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musacchio A. Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond B Biol Sci. 2011;366:3595–3604. doi: 10.1098/rstb.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondal G, Sengupta S, Panda CK, Gollin SM, Saunders WS, Roychoudhury S. Overexpression of Cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis. 2007;28:81–92. doi: 10.1093/carcin/bgl100. [DOI] [PubMed] [Google Scholar]
- 8.Williams GL, Roberts TM, Gjoerup OV. Bub1: escapades in a cellular world. Cell Cycle. 2007;6:1699–1704. doi: 10.4161/cc.6.14.4493. [DOI] [PubMed] [Google Scholar]
- 9.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 10.Haruki N, Saito H, Harano T, Nomoto S, Takahashi T, Osada H, Fujii Y, Takahashi T. Molecular analysis of the mitotic checkpoint genes BUB1, BUBR1 and BUB3 in human lung cancers. Cancer Lett. 2001;162:201–205. doi: 10.1016/S0304-3835(00)00675-3. [DOI] [PubMed] [Google Scholar]
- 11.Olesen SH, Thykjaer T, Ørntoft TF. Mitotic checkpoint genes hBUB1, hBUB1B, hBUB3 and TTK in human bladder cancer, screening for mutations and loss of heterozygosity. Carcinogenesis. 2001;22:813–815. doi: 10.1093/carcin/22.5.813. [DOI] [PubMed] [Google Scholar]
- 12.Shigeishi H, Yokozaki H, Kuniyasu H, Nakagawa H, Ishikawa T, Tahara E, Yasui W. No mutations of the Bub1 gene in human gastric carcinomas. Oncol Rep. 2001;8:791–794. doi: 10.3892/or.8.4.791. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang B, Knauf JA, Ain K, Nacev B, Fagin JA. Mechanisms of aneuploidy in thyroid cancer cell lines and tissues: evidence for mitotic checkpoint dysfunction without mutations in BUB1 and BUBR1 . Clin Endocrinol (Oxf) 2002;56:341–350. doi: 10.1046/j.1365-2265.2002.01475.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuan B, Xu Y, Woo JH, Wang Y, Bae YK, Yoon DS, Wersto RP, Tully E, Wilsbach K, Gabrielson E. Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin Cancer Res. 2006;12:405–410. doi: 10.1158/1078-0432.CCR-05-0903. [DOI] [PubMed] [Google Scholar]
- 15.Shigeishi H, Yoneda S, Taki M, Nobumori T, Ohta K, Higashikawa K, Yasui W, Kamata N. Correlation of human Bub1 expression with tumor-proliferating activity in salivary gland tumors. Oncol Rep. 2006;15:933–938. [PubMed] [Google Scholar]
- 16.Grabsch HI, Askham JM, Morrison EE, Pomjanski N, Lickvers K, Parsons WJ, Boecking A, Gabbert HE, Mueller W. Expression of BUB1 protein in gastric cancer correlates with the histological subtype, but not with DNA ploidy or microsatellite instability. J Pathol. 2004;202:208–214. doi: 10.1002/path.1499. [DOI] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al. (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. 134:e48-72 [DOI] [PubMed]
- 18.Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535–542. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 19.Nagasaki S, Suzuki T, Miki Y, Akahira J, Kitada K, Ishida T, Handa H, Ohuchi N, Sasano H. 17β-Hydroxysteroid dehydrogenase type 12 in human breast carcinoma: a prognostic factor via potential regulation of fatty acid synthesis. Cancer Res. 2009;69:1392–1399. doi: 10.1158/0008-5472.CAN-08-0821. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Niu Y, Wang X, Wei L, Zhang R, Lv S, Yu Q, Yang X. Chromosome aberrations associated with centrosome defects: a study of comparative genomic hybridization in breast cancer. Hum Pathol. 2011;42:1693–1701. doi: 10.1016/j.humpath.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Niu Y, Yu Y, Liu Y, Zhang F. Increased gamma-tubulin expression and P16INK4A promoter methylation occur together in preinvasive lesions and carcinomas of the breast. Ann Oncol. 2009;20:441–448. doi: 10.1093/annonc/mdn651. [DOI] [PubMed] [Google Scholar]
- 23.Shigeishi H, Oue N, Kuniyasu H, Wakikawa A, Yokozaki H, Ishikawa T, Yasui W. Expression of Bub1 gene correlates with tumor proliferating activity in human gastric carcinomas. Pathobiology. 2001;69:24–29. doi: 10.1159/000048754. [DOI] [PubMed] [Google Scholar]
- 24.Grabsch H, Takeno S, Parsons WJ, Pomjanski N, Boecking A, Gabbert HE, Mueller W. Overexpression of the mitotic checkpoint genes BUB1, BUBR1, and BUB3 in gastric cancer—association with tumour cell proliferation. J Pathol. 2003;200:16–22. doi: 10.1002/path.1324. [DOI] [PubMed] [Google Scholar]
- 25.Wu CW, Chi CW, Huang TS. Elevated level of spindle checkprotein MAD2 correlates with cellular mitotic arrest, but not with aneuploidy and clinicopathological characteristics in gastric cancer. World J Gastroenterol. 2004;10:3240–3244. doi: 10.3748/wjg.v10.i22.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Park K, Kim HY, Lee MS, Kim HJ, Kim YD, Yuh YJ, Kim SR, Suh HS. Clinical implication of altered expression of Mad1 protein in human breast carcinoma. Cancer. 2000;88:1623–1632. doi: 10.1002/(SICI)1097-0142(20000401)88:7<1623::AID-CNCR17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Shin HJ, Baek KH, Jeon AH, Park MT, Lee SJ, Kang CM, Lee HS, Yoo SH, Chung DH, Sung YC, et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell. 2003;4:483–497. doi: 10.1016/S1535-6108(03)00302-7. [DOI] [PubMed] [Google Scholar]
- 28.Li GQ, Zhang HF. Mad2 and p27 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol. 2004;10:3218–3220. doi: 10.3748/wjg.v10.i21.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka K, Nishioka J, Kato K, Nakamura A, Mouri T, Miki C, Kusunoki M, Nobori T. Mitotic checkpoint protein hsMAD2 as a marker predicting liver metastasis of human gastric cancers. Jpn J Cancer Res. 2001;92:952–958. doi: 10.1111/j.1349-7006.2001.tb01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burum-Auensen E, Deangelis PM, Schjølberg AR, Røislien J, Andersen SN, Clausen OP. Spindle proteins Aurora A and BUB1B, but not Mad2, are aberrantly expressed in dysplastic mucosa of patients with longstanding ulcerative colitis. J Clin Pathol. 2007;60:1403–1408. doi: 10.1136/jcp.2006.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren CD, Brady DM, Johnston RC, Hanna JS, Hardwick KG, Spencer FA. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol Biol Cell. 2002;13:3029–3041. doi: 10.1091/mbc.E02-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao F, Ponte JF, Levy M, Papageorgis P, Cook NM, Ozturk S, Lambert AW, Thiagalingam A, Abdolmaleky HM, Sullivan BA, et al. hBub1 negatively regulates p53 mediated early cell death upon mitotic checkpoint activation. Cancer Biol Ther. 2009;8:548–556. [PubMed] [Google Scholar]
- 33.Bourdeau V, Deschênes J, Métivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genomewide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 34.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinol. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 35.Ebata A, Suzuki T, Takagi K, Miki Y, Onodera Y, Nakamura Y, Fujishima F, Ishida K, Watanabe M, Tamaki K, et al. Oestrogen-induced genes in ductal carcinoma in situ: their comparison with invasive ductal carcinoma. Endocr Relat Cancer. 2012;19:485–496. doi: 10.1530/ERC-11-0345. [DOI] [PubMed] [Google Scholar]
- 36.Dai H, van’t Veer L, Lamb J, He YD, Mao M, Fine BM, Bernards R, van de Vijver M, Deutsch P, Sachs A, et al. A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Res. 2005;65:4059–4066. doi: 10.1158/0008-5472.CAN-04-3953. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Takagi K, Miki Y, Onodera Y, Akahira J, Ebata A, Ishida T, Watanabe M, Sasano H, Suzuki T. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2012;103:136–143. doi: 10.1111/j.1349-7006.2011.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morishita M, Sumi T, Nakano Y, Teramae M, Fukuda T, Nobeyama H, Yoshida H, Matsumoto Y, Yasui T, Ishiko O. Expression of mitotic-arrest deficiency 2 predicts the efficacy of neoadjuvant chemotherapy for locally advanced uterine cervical cancer. Exp Ther Med. 2012;3:341–346. doi: 10.3892/etm.2011.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colombo R, Caldarelli M, Mennecozzi M, Giorgini ML, Sola F, Cappella P, Perrera C, Depaolini SR, Rusconi L, Cucchi U, et al. Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. 2010;70:10255–10264. doi: 10.1158/0008-5472.CAN-10-2101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 25 kb)