Abstract
High-mobility group box 1 (HMGB1) is a dynamic nuclear protein participating in transcription, chromatin remodelling, and DNA recombination and repair processes. Accumulating evidence indicates that its function now extends beyond the nucleus, notably its extracellular role in inflammation. HMGB1 is implicated as a late mediator of sepsis and is also believed to promote atherosclerosis and other inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus. Interestingly, deregulation of HMGB1 is shown to be associated with the hallmarks of cancer development. Moreover, several clinical studies have shown that HMGB1 is a promising biomarker for a variety of cancer types. In this review, we provide novel insights into the role and mechanisms of HMGB1, in particular, to hormone-related cancers and its potential to serve as a therapeutic target.
Keywords: Ethyl Pyruvate, Clear Cell Renal Cell Carcinoma, Glycyrrhetinic Acid, Cervical Squamous Cell Carcinoma, HMGB1 Level
Introduction
High-mobility group box 1 (HMGB1) was first discovered as a non-chromosomal DNA-binding protein in calf thymus [1], a specialized endocrine organ vital for T cell homeostasis. HMGB1 is one of the most important and highly conserved chromatin proteins that organizes DNA and regulates transcription [2]. It interacts with the nucleosome, transcription factors, and histones in a dynamic and reversible manner and thus supports the transcription of many genes [3]. HMGB1 employs several mechanisms to promote transcription of different genes. The first mechanism by which they manipulate the transcription process is by inducing changes in nucleosome structure [4]. HMGB1 increases access to DNA for chromatin modelling complexes and transcription factors by loosening the wrapped DNA; by doing so, HMGB1 proteins may be able to bring about the interaction between histones and DNA causing an overall change in nucleosome structure. The second mechanism of HMGB1-promoted transcription of genes is through interaction with transcription-binding proteins and other transcription factors. The third mechanism uses HMGB1 as an adapter to establish protein–protein interaction with transcription factors, including all class I steroid receptors [5], HOX [6], p53 and p73 [7], and several NF-κB subunits [8]. This association helps HMGB1 bend the DNA and recruits partner or targets proteins to manipulate the gene expression. For example, HMGB1 has been shown to functionally interact with steroid hormone receptors to increase the DNA binding and transcription activation of these steroid receptors [5]. Similarly, HMGB1 was also shown to facilitate HOX-mediated transcription activation by establishing a protein–protein interaction between the HMG box domains and homeodomains which increases accessibility of the HOX proteins to the targeted sites at the DNA [6]. Previously, HMGB1 was also demonstrated to promote the NF-κB-dependent expression of VCAM-1 by enhancing the DNA-binding activity of NF-κB subunits [8]. On the other hand, Stros et al. [7] demonstrated that HMGB1can either decrease or increase the transcriptional activity of p53 and p73 in a cell-specific manner. Thus, modulation of transcriptional activity of these transcription factors by HMGB1 may have functional relevance in the disease context. Of note, transcription factors such as p53 and Kruppel-like factor (KLF) are shown to regulate the secretion of HMGB1 in various cell types [9, 10]. Interestingly, HMGB1 has been shown to rescue multiple phenotypes in a c-Myc independent fashion [11] suggesting that HMGB1 might be functioning as an oncogene.
HMGB1 undergoes several extensive post translational modifications such as acetylation, phosphorylation, methylation, ADP–ribosylation, and glycosylation to a certain extent [12, 13]. Acetylation of HMGB1 has several functional implications; when certain lysine residues of HMGB1 are acetylated, it is relocated from the nucleus to the cytoplasm and is eventually secreted. HMGB1 has been detected in extra nuclear fraction showing tissue-specific localization [14]. HMGB1 is found at high levels in the nuclei and cytoplasm of lymphoid tissue, while it is found at low levels in brain and liver cells [15]. Acetylated HMGB1 also facilitates histone assembly during replication. Phosphorylation of HMGB1 is reported to affect its binding affinity for DNA, its stability and conformation, and its regulatory effect on transcription factors [14]. The clinical significance of post translational modifications of HMGB1 is not clearly known at this time.
Extracellular HMGB1 and Immune Responses
HMGB1 protein released by necrotic cells acts as a signal that stimulate innate immune system and recruits mononuclear cells to the sites of tissue damage, where the immune cells clear the damaged cells and protect the tissue from further infection [16]. In addition, HMGB1 has also been found to be secreted by cells of an innate immune system as a response to pathogens and pro-inflammatory stimuli [16]. Outside the cell, HMGB1 binds to receptors such as receptor for advanced glycation end products (RAGE) and Toll-like family of receptors. RAGE is a transmembrane receptor of the immunoglobulin superfamily that binds to ligands such as advanced glycation end products [17], HMGB1 [18], S100A4 [19], and S100P [19]. Association of RAGE with its ligand is thought to activate the pro-inflammatory genes [20]. Specifically, the interaction between HMGB1 and RAGE promotes chemotaxis and production of cytokines through activation of transcription factor NF-κB [20]. This interaction also promotes maturation and migration of immune cells to the target sites [20].
HMGB1 is also known to bind to multiple Toll-like receptors (TLRs) [21]. TLRs are a type of pattern recognition receptor which plays a crucial role in pathogen defense and innate immune system. Particularly, TLR2, TLR4, and TLR9 [22, 23] are implicated as HMGB1 receptors. Binding of HMGB1 to TLR2 and TLR4 has been shown to activate MyD88 signalling pathway, thus resulting in the release of pro-inflammatory cytokines [23]. Activation of TLR9 is also shown to be stimulated by HMGB1-DNA complex that results in maturation of immune cells and secretion of cytokines [22]. In addition, HMGB1 stimulation increases adhesion capacity of monocytes and release of inflammatory mediators [24]. When neutrophils are stimulated with HMGB1, reactive oxygen species are produced by the cells via TLR4 dependent activation of NAD(P)H oxidase with upregulation of NF-κB which in turn increases the production of cytokines [24]. Furthermore, HMGB1 also stimulates T cells which results in increased proliferation, survival, and Th1 polarization [24]. Thus, HMGB1 seems to play a vital role in orchestrating different immune responses.
HMGB1 and Cancer Association
HMGB1 is expressed in higher levels in most tumor types compared to the normal tissue [18]. Localization of HMGB1 at the leading edge has been suggested to enhance invasion in various cells [25]. HMGB1 promotes cell proliferation and outgrowth of cells via RAGE signalling pathway [26]. This implies that HMGB1 may have a prominent function in cellular growth. HMGB1 plays an important role as an extracellular signalling molecule apart from its function as a chromatin protein. HMGB1 as a signalling molecule is involved in inflammation, cell differentiation, cell migration, and tumor metastasis. HMGB1 is released from necrotic cells passively and actively from inflammatory cells. Extracellular HMGB1 binds to various receptors such as RAGE, Toll-like receptors, and CD24 [27]. HMGB1 is associated with many diseases such as sepsis [28], ischemia-reperfusion [29], arthritis [30], meningitis [31], neurodegeneration [32], and cancer [33, 34].
Elevated expression of HMGB1 occurs in certain types of primary tumors, including melanoma, colon, prostate, pancreatic, and breast cancer; in the majority of cases, HMGB1 is associated with invasion and metastasis [18]. Regarding tumor cells, HMGB1 seems to have a dual effect: it promotes neoangiogenesis as well as regulates immunity against the cancer. Tumor cells that are dying release HMGB1 which then interacts with TLR4, a receptor whose signalling is critical for innate and adaptive immune responses, stimulate mature dendritic cell tumor antigen processing which results in anti-cancer immune response [35]. However, a different study showed that HMGB1 released by necrotic keratinocytes promotes skin carcinogenesis by triggering TLR4 dependent inflammatory response [36]. HMGB1 interaction with RAGE also results in the inflammatory pathway activation and seems to be associated with tumor growth, invasion, and metastasis [37–39].
According to Hanahan and Weinberg, tumors can be characterized by six properties [40]: unlimited replicative potential, angiogenesis, evasion of apoptosis, self sufficiency in growth signals, insensitivity to inhibitors of growth, and tissue invasion and metastasis. The seventh property is suggested to be inflammation [40]. Alterations in HMGB1 seem to affect all of these properties, suggesting that HMGB1 is involved in carcinogenesis.
High levels of HMGB1 have been associated with several cancer types including gastrointestinal stromal tumors [41], colorectal [42], prostate [43, 44], and cervical cancer [45]. Some epithelial malignancies are shown to have increased HMGB1 messenger RNA (mRNA) expression [43, 46–48]. Necrotic cells that have undergone chemotherapy release HMGB1 which promotes growth and metastasis and thus cancer survival [49]. Specifically, CT26 cells treated with doxorubicin, a necrosis inducer, show increased release of HMGB1 compared to CT26 cells treated with trichostatin A (an apoptosis inducer) [49]. Further, in a mouse model treated with doxorubicin showed enhanced liver and lung metastasis which was abrogated by anti-HMGB1 antibody treatment [49]. Also, HMGB1 seems to be involved in restricting immune activation by decreasing the number of U937 cells via RAGE; suggesting that HMGB1 may be involved in promoting survival of cancer through RAGE activation after chemotherapy [49]. HMGB1 mediates autophagy which results in resistance to drugs such as cisplatin, doxorubicin, and methotrexate in osteosarcoma [50], a type of cancer that occurs in childhood and adolescence. These drugs upregulate HMGB1 via an unknown mechanism. Upregulated HMGB1 competes with BCL-2 and binds with BECN1; this enhances the formation of BECN1/PI3KC3 complex, stimulating autophagy [50]. Knockdown of HMGB1 or inhibition of autophagy increases response to the drug treatment in osteosarcoma cells both in vitro and in vivo [50]. Another study has suggested that a reduced form of HMGB1 binds to RAGE, promoting beclin1-mediated autophagy conferring resistance to chemotherapy drugs and ionizing radiation in pancreatic and colon cancer cell lines [51]. HMGB1 is also shown to regulate tumor angiogenesis. High expression of HMGB1 was detected in esophageal squamous cell carcinoma (ESCC) and was found to regulate expression of VEGF-C, promoting lymphangiogenesis and metastasis of lymph nodes, resulting in the poor prognosis (surgical outcome) of the disease [52]. As HMGB1 is involved in multiple processes of cancer development and chemoresistance, it may represent an attractive oncotarget.
HMGB1 and the Endocrine System
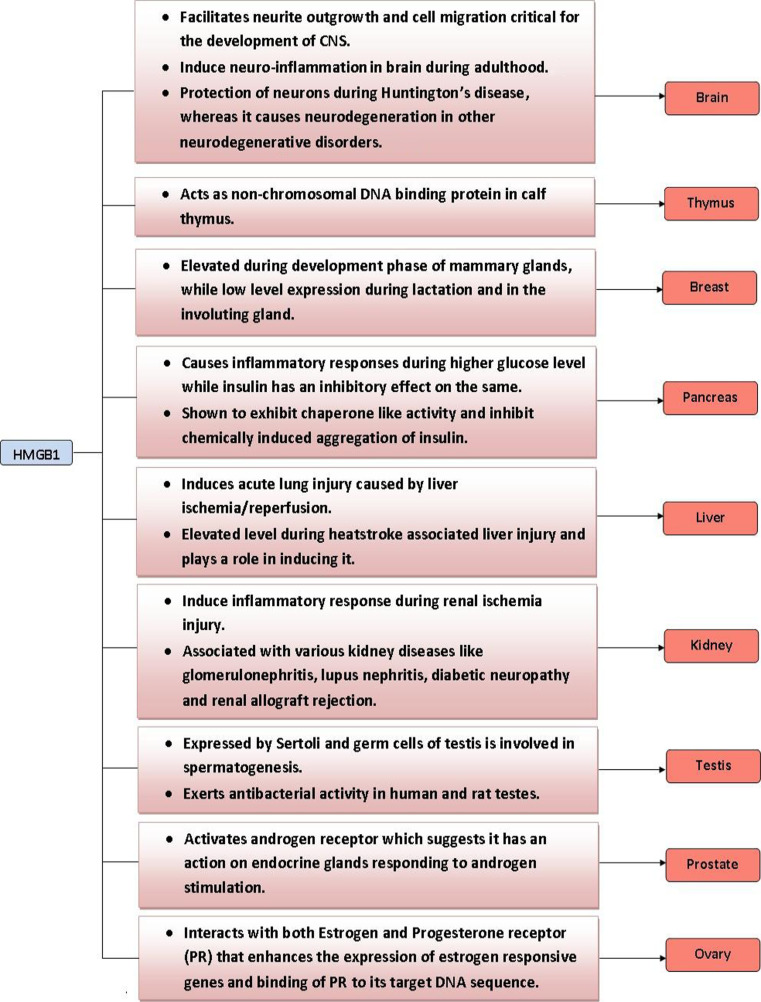
Endocrine organs that are known to express HMGB1 include the thymus [53], testes [54], pancreas [55], ovary [56], prostate [57], breasts [58], and kidneys [59]. One study found an age-dependent variation in the level of HMGB1 protein in various brain regions [60]. Specifically, HMGB1 was found to be reduced in neurons, while increased in astrocytes during aging. Quantification of mRNA levels of HMGB1 in different stages of mammary gland development using pregnant and non-pregnant mouse models done by Northern blot revealed that HMGB1 levels are elevated during the development phase and decreased during lactation and in the involuting gland (which undergoes apoptosis) [58]. This observation implies that HMGB1 may be involved in regulating development and involution of the mammary gland by modulating cellular apoptotic processes [58]. Expression analysis in the testicular system showed that both Sertoli cells and germ cells express HMGB1 [61, 62]. During rooster spermatogenesis, the ratio of HMGB1 to nucleosomal histone seemed to increase markedly at the end of spermatogenesis [62]. Surprisingly, HMGB1 purified from human and rat testes has been shown to exert anti-bacterial activity towards Bacillus megaterium in an inhibition zone assay [61]. This study suggests that HMGB1 may act as a paracrine host defense factor in the testes [61]. It is possible HMGB1 may have important cytoprotective functions in other endocrine organs as well. HMGB1 has been shown to play a key role in the interaction between the neuroendocrine and the innate and acquired immune pathways that regulate neutrophil mobilization from bone marrow following hemorrhagic shock [63]. Furthermore, HMGB1 is also released by pituicytes when exposed to inflammatory cytokines such as TNFα and interleukin1 [64]. Secreted HMGB1 is suggested to participate in the regulation of neuroendocrine and immune responses to infection or injury. These studies indicate that HMGB1 may have a vital role in both normal and pathophysiological functions of endocrine organs (Fig. 1).
Fig. 1.
Role of HMGB1 in endocrine organs
HMGB1 Interaction with Endocrine-Related Genes
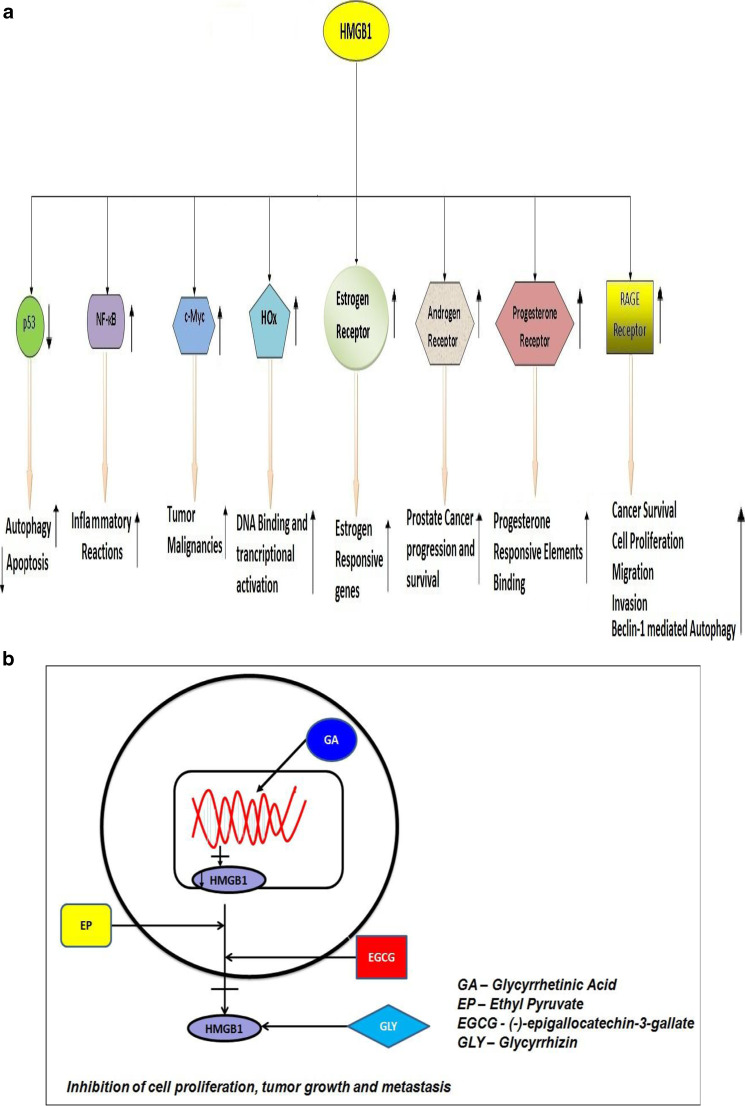
HMGB proteins, particularly 1 and 2, facilitate stronger binding of estrogen receptor to estrogen responsive element [65] which enhances the expression of estrogen responsive genes. HMGB1 also enhances the binding of progesterone receptor to its target DNA sequence [66, 67]. Sex steroid hormones such as estradiol and progesterone regulate the expression of HMGB1 in the endometrium [68]; while estradiol increases expression of HMGB1, progesterone decreases the expression of HMGB1. Interestingly, co-transfection studies showed that HMGB1 can also activate androgen receptor [69] suggesting that it may have an important role in endocrine organs that respond to androgen stimulation. HMGB1 potentiates the effect of EGF on A431 vulval cells by specifically activating extracellular-signal-regulated kinase 1/2 and remodelling the actin cytoskeleton which enhances cell motility [70].
Extracellular HMGB1 is known to interact with TLR4 and RAGE receptors [71]. Both TLR4 and RAGE are known to be expressed in endocrine cells [72, 73]. Therefore, interaction of HMGB1 with TLR4 or RAGE on endocrine cells may have clinical implications. HMGB1 that is passively released from damaged islet cells binds to TLR4 and selectively damages beta cells rather than alpha cells in early stages of diabetes [74]. HMGB1 and RAGE are involved in development of chronic diabetic complications [75]. They cause progressive pancreatic beta cell loss in type 2 diabetes by intracellular oxidative stress. RAGE ligands such as S100B and HMGB1 induce cell apoptosis of INS-1 cells [76] which is accompanied by oxidative stress. Therefore, deregulation of HMGB1 and its associated proteins may result in adverse outcomes in endocrine-related diseases such as diabetes and cancer.
HMGB1 and Hormone-Related Cancers
HMGB1 in Breast, Ovarian, and Prostate Cancers
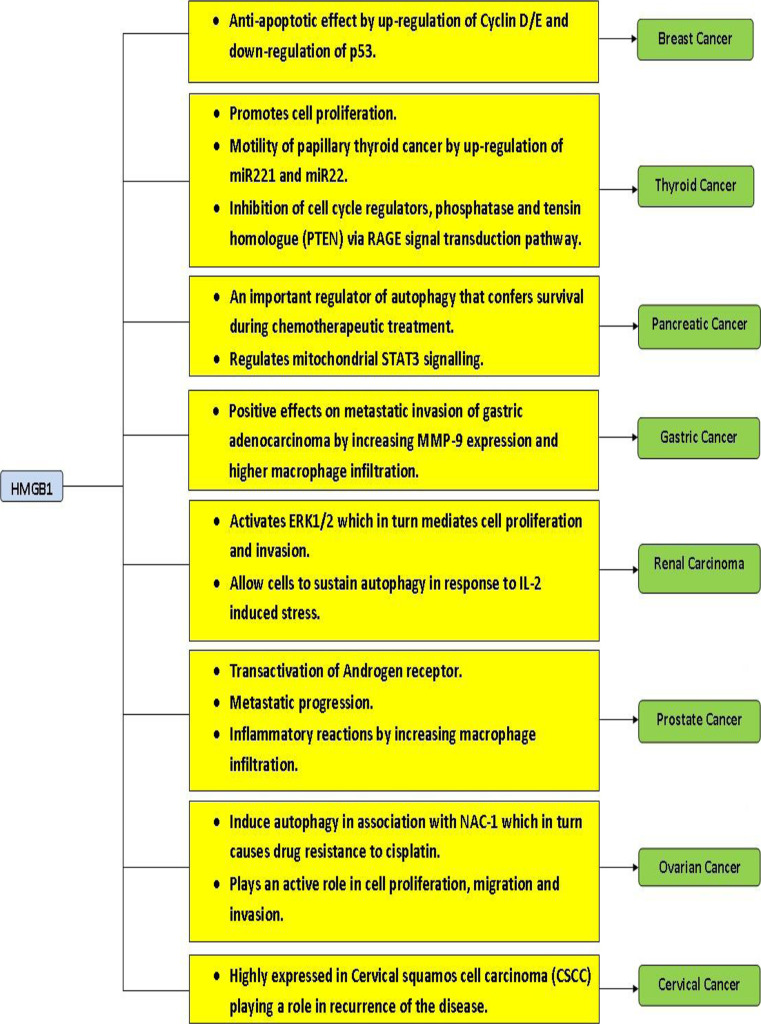
Breast cancer is one of the aggressive hormone-related cancers causing high mortality rates in women. Resistance to cell death is the underlying cause for treatment failure in breast cancer. The factors that confer chemoresistance in breast cancer are not clearly known; studies conducted by Brezniceanu et al. [58] showed that HMGB1 is an anti-apoptotic protein that confers resistance to cell death following treatment with various apoptosis-inducing agents such as ultraviolet radiation, CD95-, TRAIL-, Caspase-8-, and Bax. Further, in silico approach analysis showed that if HMGB1 is upregulated, then CyclinD/E levels are predicted to be increased and p53 levels to be downregulated in cancer cells. However, other studies advocate that extracellular HMBG1 is required for achieving optimal outcome of anti-cancer chemotherapy and radiotherapy. Therefore, regulating the localization of HMGB1 may be beneficial in breast cancer treatment outcome.
HMGB1 expression is detected at higher levels in primary breast carcinoma when compared to the normal tissue [15]. This observation is supported by another study which confirmed that there is indeed high HMGB1 expression in human breast carcinoma and tumor transplantation in a mouse model [77]. In vitro, HCC1143 breast ductal carcinoma cells treated with epirubicin showed significant release of HMGB1 [77]. In vivo, dynamic changes in plasma levels of HMGB1 were observed. Responders (pathological complete response or partial remission) showed increased HMGB1 release while the non-responders (stable or progressive disease) did not. It was suggested that early changes in the levels of HMGB1 can be useful in predicting the fate of the neoadjuvant chemotherapy [77]. Interestingly, Northern blot analysis of 1.4 and 2.4 kb transcripts of HMGB1 from 13 breast cancer samples showed a strong intertumoral variation by a factor of 8.5 and 14.5, respectively. The response to the endocrine therapy may be varied in estrogen receptor positive breast cancer due to this reason [34]. Human HMGB1 gene was cloned and the regulation of transcription was analyzed in breast cancer MCF-7 cells [78]. It was found that transcription of the HMGB1 gene starts at one major site 57 nucleotides upstream from the first exon–intron junction; HMGB1 gene expression is controlled by a very strong TATA-less promoter with a silencer present immediately upstream to it. The study postulated that the basal activity may be due to the activity of the strong promoter, which is regulated by the silencer, while increased expression of HMGB1 in growing breast cancer induced by estrogen may be due to enhancers in the intron 1 region. Overall, expression of HMGB1 seems to play an important role in breast cancer progression (Fig. 2) and in predicting treatment outcome.
Fig. 2.
Association of HMGB1 expression and hormone-related cancers
Diagnosis and treatment of ovarian cancer is a major challenge, and as such, the resulting survival rates of patients are extremely low. Chen et al. [79] recently showed that HMGB1 is a novel biomarker for human ovarian cancer. Their studies also showed that knockdown of HMGB1 resulted in reduced cell proliferation and cell migration and invasion suggesting that HMGB1 may serve as a therapeutic target for ovarian cancer [80]. Interestingly, this study suggests that HMGB1 can serve as a serum marker for ovarian cancer. In ovarian cancer cells, activation of autophagy is caused by treatment with cisplatin [81]. This autophagy regulation was shown to be enforced by nucleus accumbens-1 (NAC-1) in cooperation with HMGB1 as function of NAC-1 and is correlated with expression, translocation, and release of HMGB1. NAC-1 is a transcriptional core processor protein necessary for sustaining the cell proliferation and migration in cancer [81]. Hence, HMGB1 seems to mediate drug resistance to cisplatin in ovarian cancer cell lines through NAC-1 causing autophagy and drug resistance. Therefore, HMGB1 may prove to be a valuable tool for the diagnosis and treatment of ovarian cancer.
In case of prostate cancer, androgen, and its receptor, androgen receptor (AR) plays a crucial role in the malignancy. As HMGB1 is known to transactivate AR [69], HMGB1 may play a vital role in the progression of prostate cancer (Fig. 3a). In a pilot clinical study [57], prostatectomy specimens from 40 patients with pT3 prostate cancer (18 non-metastatic and 22 metastatic) were examined for the expression of HMGB1 and RAGE. They were previously treated with luteinizing hormone-releasing hormone agonist. HMGB1 was found to be expressed in tumor cells of 6 metastatic and 0 non-metastatic specimens, as well as in prostatic stromal cells of 14 metastatic and 2 non-metastatic cases. RAGE expression was also detected in both metastatic (16) and non-metastatic (6) cases. In another evaluation, co-expression of HMGB1 and its receptor, RAGE, were positively associated with the progression of prostate cancer [43]. Further, untreated tissue of prostate cancer showed higher levels of both HMGB1 and RAGE compared to the normal prostate tissue [43]. Using TRAMP animal model, one study [82] showed tumor progression can be disrupted by targeting HMGB1. The effect of downregulation of HMGB1 in LNCaP cells was studied by transfecting plasmid-encoding short hairpin RNAs targeting HMGB1 [83]. Through RT-PCR and immunoblotting, the downregulation of expression of HMGB1 was confirmed, and the decrease in HMGB1 expression correlated with cell growth inhibition in the transfected LNCaP cells [83]. We also showed that glycyrrhetinic acid treatment reduced the expression of HMGB1 in advanced prostate cancer cells [84], resulting in the inhibition of invasion of these cells. Silencing of HMGB1 gene by antisense targeting in PC-3 cells has also shown to eliminate the invasion and metastatic properties of PC-3 cells [57]. This is achieved by disrupting the HMGB1–RAGE signalling pathway in prostate cancer cells. Targeting HMGB1 by siRNA suppresses the genes in cancer cells with high osseous metastatic potential and effectively inhibits osseous metastasis [85]. Taken together, HMGB1 represents an important diagnostic and therapeutic target for prostate cancer.
Fig. 3.
a Cellular outcome of HMGB1 interactions with transcription factors and hormonal receptors. b Hypothetical mode of action of HMGB1 inhibitors
Cervical Cancer
HMGB1 expression was also shown to be associated with cervical carcinoma [45]. A comparison of expression level of HMGB1 was performed in 64 samples of recurrent cervical squamous cell carcinomas (CSCC), 72 cases with non-recurrent carcinoma, and 28 normal tissues. Overexpression of HMGB1 was observed in recurrent CSCC tissue sections compared to non-recurrent CSCC sections in immunostaining analysis. Furthermore, high serum HMGB1 levels were inversely correlated with disease-free survival (P = 0.009, Pearson chi square test) and overall survival (P = 0.018). In another study, expression of HMGB1 and RAGE mRNA was found to be elevated in metastatic CSCC compared to non-metastatic cases [48]. These data suggests that HMGB1 may be useful as a biomarker to diagnose the recurrence of the disease and to predict the prognosis in CSCC patients [45, 48]. Despite its association with disease progression, the molecular mechanisms of how HMGB1 promotes cervical cancer is not known; given that estrogen receptor and RAGE are both expressed in cervical cancer and both are known to be activated by HMGB1, it can be postulated that HMGB1 may promote cervical cancer through these receptors.
Gastric and Pancreatic Cancers
Expression of HMGB1 in gastric cancer was first reported by Xiang et al. using a differential screening technique in human gastric carcinoma cell line complementary DNA library [86]. It was found that HMGB1 was encoded by FM1 cDNA clone of 1,194 nucleotides in length. Furthermore, Northern blot analysis revealed that HMGB1 was expressed in all the analyzed gastric cancer samples (n = 34) at higher levels compared to benign mucosa, with two transcripts of approximately 1.4 and 2.4 kb, respectively. A subsequent study by Kuniyasu et al. [87] reported that HMGB1 is overexpressed in 85 % of gastric cancers regardless of histological type and disease progression. These findings suggest that HMGB1 may be involved in all stages of gastric carcinoma development. On the other hand, its cognate receptor RAGE was found to be associated with invasion and metastases of gastric carcinoma patients. HMGB1 is also implicated in Helicobacter pylori-mediated gastric cancer, as the VacA toxin of H. pylori is shown to release HMGB1 from AZ-521 human gastric epithelial cells [88] which may lead to the pathogenesis of gastric cancer. Akaike et al. [89] showed that there was a positive correlation of HMGB1 expression and macrophage infiltration inside the tumor microenvironment of gastric cancer cells. In another study, HMGB1 overexpression was suggested to be a good prognostic marker for resectable gastric adenocarcinoma in combination with adjuvant chemotherapy [90]. In this work, gastric adenocarcinoma of patients (n = 78) was surgically removed, and immunohistochemical analysis was performed to analyze the expression of HMGB1 in comparison to non-cancer cases (n = 78). The immunohistochemistry analysis revealed that there was a high reactivity of HMGB1 (score ≥ 9) in 32 cancerous tissue samples out of total 78 cases analyzed (i.e., 42 % of cancerous tissue samples showing high HMGB1reactvity by immunohistochemistry method), while only 7 cases out of total 78 non-cancerous tissue samples showed HMGB1 reactivity (i.e., 9 % of non-cancerous samples showing HMGB1 reactivity). Strikingly, HMGB1 overexpression was found to be positively associated with cancer-free survival of resectable gastric adenocarcinomas. Overexpression of HMGB1 and its receptor, RAGE, was also confirmed in another pilot study employing 45 human samples of gastric adenocarcinoma samples of different grades [91]. It was found that 73.3 and 68.9 % of the gastric adenocarcinoma tissues were positive for HMGB1 and RAGE expression, respectively, and it seemed to correlate with an increased level of malignancy. In order to validate HMGB1 as a biomarker for early diagnosis of gastric cancer, Chung et al. [92] analyzed 227 patients divided into 5 different disease groups according to sequence of gastric carcinogenesis, and HMGB1 level in their serum was tested by ELISA. Data obtained in this study suggested that levels of HMGB1 were significantly associated with depth of invasion, lymph node metastasis, tumor size, and poor recovery.
HMGB1 as a potential therapeutic target for gastric cancer was demonstrated by Song et al. [93] using a gene-targeting approach. Silencing of HMGB1 in the MGC-803 metastatic cell line by administration of lentiviral vector encoding HMGB1-specific RNA interference (RNAi) resulted in reduced cell proliferation. Alterations in the cell cycle were observed in which there was an increase in the number of cells in G0/G1 phase, while a decrease in the number of cells in the S-phase and G2/M-phase. In addition, cells became more sensitive to apoptosis induced by oxaliplatin and showed decreased metastatic ability and MMP-9 expression [93]. The therapeutic effect of ethyl pyruvate, a potent inhibitor of HMGB1, was assessed in gastric adenocarcinomas in a subcutaneous xenograft tumor model [91]. Interestingly, ethyl pyruvate administration decreased the expression levels of HMGB1, RAGE, Akt, p-Akt, Ki-67, and MMP-9, while it increased the expression of tumor suppressor, p53. Ethyl pyruvate was also found to be associated with inhibition of cell proliferation and invasion by inducing cell cycle arrest, apoptosis, and slowing growth rate [91].
A decade ago, HMGB1 was identified as a metastasis-associated gene in rat mammary and pancreatic adenocarcinomas using subtractive technology [94]. Pancreatic cancer can be characterized by delay in diagnosis, rapid metastasis, early mutation of Kras oncogene, and chemotherapy resistance. Recently, HMGB1 was shown to be a promising serum biomarker for predicting prognosis in pancreatic ductal adenocarcinoma [95]. Experimental studies support HMGB1 as an important regulator of autophagy in pancreatic cancer cells to survive chemotherapeutic treatment [51]. A transgenic murine model was created by back crossing RAGE-null mice to a spontaneous mouse model of pancreatic cancer, (Pdx1-Cre:KrasG12D/+(KC)) to understand the correspondence between RAGE, autophagy, and pancreatic cancer [96]. RAGE and its ligand HMGB1 is known to be associated with several types of cancers. Delayed pancreatic neoplasia development, decreased levels of autophagy, and inhibited mitochondrial STAT3 activity and subsequent ATP production was achieved by targeted ablation of RAGE in the KC mice model [96]. The study suggests that RAGE might have a critical role to play in the early stages of pancreatic carcinogenesis, stimulation of autophagy, and regulation of mitochondrial STAT3 signalling. Another study suggests that HMGB1 protein binds to RAGE promoting pancreatic and colon cancers [97]. Further, it highlights that redox status of HMGB1 protein triggers either autophagy or apoptosis suggesting that it plays an important role in cross regulating the apoptosis and autophagy processes.
Selective knockdown of autophagy regulators such as beclin 1 and ATG-5 by RNAi result in decreased HMGB1 release and enhanced apoptosis in Panc02 cells [97]. This suggests that regulation of HMGB1 may be enforced by autophagic stimuli. Similarly, Bax and p53 are regulators of apoptosis in cells that have undergone stress. It was found that cells that lack these regulators do not show apoptotic response to chemotherapeutic drug in colorectal cancer cell line HCT-116. Inhibition of HMGB1 in Panc2.03 cells and HCT-116 cell line that have undergone chemotherapy by HMGB1inhibitors, such as ethyl pyruvate and glycyrrhizin, showed enhanced apoptosis and inhibited autophagy [97]. Thus, targeting HMGB1 may represent an attractive treatment strategy for both pancreatic and gastric cancer types.
Renal Cell Carcinoma
The contribution of RAGE and its ligand HMGB1 in causing the clear cell renal cell carcinoma (CCRCC) was explored by Lin et al. [98]. Authors of this study found that HMGB1 and RAGE correspond to the tumor growth and clinical stage of CCRCC patients. We also analyzed the HMGB1and RAGE gene expression in CCRCC using data archive available at The Cancer Genome Atlas (TCGA) data portal [99] for any correlation with the above studies. However, HMGB1 and RAGE gene expression between cancer and normal samples did not show any significant difference with the available data in TCGA suggesting that further studies are required to validate the expression of HMGB1 and RAGE in CCRCC. However, Lin and colleagues [98] further showed that HMGB1 expression in CCRCC causes activation of ERK1/2, a protein kinase that mediates cell proliferation and apoptosis; this activation can be blocked by U0126 (MEK1/2 inhibitor) and knockdown of RAGE, which partially reverses the activation. The cell proliferation, migration, and invasion induced by HMGB1 are reversed by the knockdown of RAGE. Interestingly, another study showed that HMGB1 is upregulated as an immunoevasion gene in dendritic cell based-treatment of renal cell carcinoma [100]. For renal cell carcinoma treatment, IL-2 is used as an immunotherapy [101]. However, it was shown that HMGB1 is found in high levels in serum of mice treated with IL-2 [102]. Specifically, HMGB1 is released and translocated from nucleus to cytoplasm as a response to IL-2-induced stress which enables the cells to sustain autophagy. In order to limit autophagy, chloroquinone was co-administered along with IL-2 which resulted in lower levels of HMGB1, cytokines such as IFN-γ and IL6, weight gain, and enhanced production of T cell and NK cells promoting long term tumor control in murine hepatic metastases model. Chloroquinone causes autophagic flux by increasing autophagic vacuoles and inhibiting oxidative phosphorylation, thus limiting ATP production and promoting apoptosis [103]. Therefore, targeting HMGB1 may improve the IL-2 treatment efficacy in renal cell carcinoma.
Thyroid Cancer
The role of HMGB1 in thyroid cancer is now beginning to evolve; a recent study performed by Mardente et al. [104] showed that HMGB1 is associated with increased papillary thyroid malignancy scores. Their study also revealed that the HMGB1 gene in papillary thyroid carcinomas not only cause indirect inhibition of cell cycle regulators such as phosphatase and tensin homologue (PTEN) via RAGE signal transduction pathway, but may also be involved in increasing the levels of miR221 and miR222 which results in cell growth and motility of papillary thyroid cancer cells. The miR221 and miR222 promote cell proliferation by inhibiting p27kip1, a cell cycle regulator found in human papillary carcinomas. Increased expression of these microRNAs by HMGB1 was found in primary cultures of excised papillary lesions and in BC PAP cell line. This upregulated expression of HMGB1 corresponds to the increase in growth and motility leading to papillary thyroid malignancy. Inflammatory mediators such as NO and HMGB1 that are released into the microenvironment of thyroiditis are known to attract macrophages. This macrophage activation could in turn be involved in angiogenesis, remodelling of matrix, and suppression of the anti-cancer immune response; this may contribute to the cellular transformation process increasing the risk of cancer in patients affected by autoimmune lymphocytic thyroiditis [105].
HMGB1 as a Therapeutic Target
HMGB1 is considered to be a potential therapeutic target for several cancer types [33, 44]. Many strategies have been employed to target HMGB1 to contain cancer in pre-clinical studies. Gene targeting has emerged as one of the most promising approaches to target HMGB1. Antisense and RNA interference techniques have been shown to target HMGB1 in prostate cancer [57, 83, 85]. Using antibodies against HMGB1 has also shown to be a potential treatment approach. Antibody-based approaches to target HMGB1 have led to a significant improvement in malignant mesothelioma [106], colon cancer [107], and prostate cancer [82]. Another promising approach is the use of naturally occurring products such as glycyrrhizin, glycyrrhetinic acid, (−)-epigallocatechin-3-gallate (EGCG), and ethyl pyruvate (Fig. 3b), all of which have shown to work against HMGB1 in several cell/disease models [84, 108–112]. We recently showed that glycyrrhetinic acid, a derivative of glycyrrhizin found in liquorice root, reduced prostate cancer cell proliferation by decreasing gene expression of HMGB1 [84]. In another study, when glycyrrhizin (a direct inhibitor of HMGB1 protein [113, 114]) was administered with necrosis-inducing agent such as CAMEL peptide showed a significant tumor growth inhibition accompanied with diminished tumor levels of HMGB1 and reduced inflammatory condition [108]. Since ethyl pyruvate (EP) is a known inhibitor of HMGB1 release [115, 116], a study explored the anti-cancer effects of EP in a mouse liver tumor model injected intraportally with MC39 colorectal cancer cells [109]. In this model, mice treated with EP prior to infusion of tumor cells and with EP treatment continued for 9 days after the infusion of cancer cells showed more than 70 % tumor reduction compared to untreated mice. Interestingly, mice treated with EP for 9 days showed decreased levels of HMGB1 and reduced inflammation in the liver correlating with the tumor inhibition [109]. Green tea polyphenol especially (−)-epigallocatechin-3-gallate (EGCG) has been shown to inhibit the release of HMGB1 following endotoxemia and rescued the mice from lethal sepsis [112]. Although EGCG is known to have anti-cancer effects in vivo [117, 118], whether EGCG can target HMGB1 release from tumor cells in animal models and the associated anti-cancer mechanisms need to be elucidated in detail. A novel, but yet to be tested, approach involves targeting HMGB1 through A-Box domain derived from HMGB1 protein. Specifically, A-Box domain has been shown to downregulate the inflammatory activity of HMGB1 [119]. This A-box domain could disrupt HMGB1–RAGE signalling, and thus be a potential treatment for cancer. HMGB1 as a vaccine is also suggested as a feasible immunotherapeutic strategy and is supported by a study in B16-OVA melanoma model, where HMGB1 peptides engrafted in liposomes showed immunity against HMGB1[120]. These strategies to target HMGB1 could lead to finding a possible cure of endocrine cancers harboring high levels of HMGB1 expression.
Concluding Remarks
HMGB1 is well known as an inflammation-associated protein deregulated in several diseases such as arthritis, sepsis, and atherosclerosis. Increasing evidence now suggests that HMGB1 also has a major role in cancer development. In this review, we have summarized and provided novel insights into the role of HMGB1 in several hormone-related cancers. As HMGB1 is shown to be targeted by variety of agents, combining it with hormonal therapy may offer new avenues for therapeutic development against these cancers.
Acknowledgments
Some of the author’s published studies cited in this review were partially supported by American Cancer Society, IL Division, and Excellence in Academic Medicine (EAM) award. We apologize to the authors for whose valuable published information on HMGB1 is not cited in this article.
Conflicts of Interest Statement
Authors have no competing interests to declare.
Footnotes
Madhuwanti Srinivasan and Souresh Banerjee contributed equally.
References
- 1.Walker JM, Goodwin GH, Johns EW, Wietzes P, Gaastra W. A comparison of the amino-terminal sequences of two calf-thymus chromatin non-histone proteins. Int J Pept Protein Res. 1977;9:220–223. doi: 10.1111/j.1399-3011.1977.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 2.Muller S, Bianchi ME, Knapp S. Thermodynamics of HMGB1 interaction with duplex DNA. Biochemistry. 2001;40:10254–10261. doi: 10.1021/bi0100900. [DOI] [PubMed] [Google Scholar]
- 3.Strichman-Almashanu LZ, Bustin M, Landsman D. Retroposed copies of the HMG genes: a window to genome dynamics. Genome Res. 2003;13:800–812. doi: 10.1101/gr.893803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agresti A, Scaffidi P, Riva A, Caiolfa VR, Bianchi ME. GR and HMGB1 interact only within chromatin and influence each other’s residence time. Mol Cell. 2005;18:109–121. doi: 10.1016/j.molcel.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol. 1998;18:4471–4487. doi: 10.1128/mcb.18.8.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zappavigna V, Falciola L, Helmer-Citterich M, Mavilio F, Bianchi ME. HMG1 interacts with HOX proteins and enhances their DNA binding and transcriptional activation. EMBO J. 1996;15:4981–4991. [PMC free article] [PubMed] [Google Scholar]
- 7.Stros M, Ozaki T, Bacikova A, Kageyama H, Nakagawara A. HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem. 2002;277:7157–7164. doi: 10.1074/jbc.M110233200. [DOI] [PubMed] [Google Scholar]
- 8.Agresti A, Lupo R, Bianchi ME, Muller S. HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun. 2003;302:421–426. doi: 10.1016/s0006-291x(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 9.J. Liu, Y. Liu, H. Zhang, G. Chen, K. Wang, X. Xiao. KLF4 promotes the expression, translocation, and release of HMGB1 in RAW264.7 macrophages in response to LPS, Shock 30 (2008) 260–266. [DOI] [PubMed]
- 10.Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothermund K, Rogulski K, Fernandes E, Whiting A, Sedivy J, Pu L, Prochownik EV. C-Myc-independent restoration of multiple phenotypes by two C-Myc target genes with overlapping functions. Cancer Res. 2005;65:2097–2107. doi: 10.1158/0008-5472.CAN-04-2928. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe G, Talcott KE, Bhattacharya SK, Crabb JW, Sears JE. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res. 2006;312:3526–3538. doi: 10.1016/j.yexcr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Erlandsson Harris H, Andersson U. Mini-review: the nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 15.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 16.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 18.Todorova J, Pasheva E. High mobility group B1 protein interacts with its receptor RAGE in tumor cells but not in normal tissues. Oncol Lett. 2012;3:214–218. doi: 10.3892/ol.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L. Padilla, S. Dakhel, J.L. Hernandez, S100 to receptor for advanced glycation end-products binding assay: Looking for inhibitors, Biochem Biophys Res Commun (2014). [DOI] [PubMed]
- 20.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Haring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 24.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander EO, Baumann M, Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268:19726–19738. [PubMed] [Google Scholar]
- 26.Guo ZS, Liu Z, Bartlett DL, Tang D, Lotze MT. Life after death: targeting high mobility group box 1 in emergent cancer therapies. Am J Cancer Res. 2013;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Liang X, Lotze MT. HMGB1: the central cytokine for all lymphoid cells. Front Immunol. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson U, Tracey KJ. HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheum Dis Clin North Am. 2004;30:627–637. doi: 10.1016/j.rdc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, Wang H, Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med. 2008;36:291–295. doi: 10.1097/01.CCM.0000295316.86942.CE. [DOI] [PubMed] [Google Scholar]
- 32.Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012;45:499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- 33.Lotze MT, DeMarco RA. Dealing with death: HMGB1 as a novel target for cancer therapy. Curr Opin Investig Drugs. 2003;4:1405–1409. [PubMed] [Google Scholar]
- 34.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010;29:2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 38.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 39.Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33:567–577. doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 41.Choi YR, Kim H, Kang HJ, Kim NG, Kim JJ, Park KS, Paik YK, Kim HO. Overexpression of high mobility group box 1 in gastrointestinal stromal tumors with KIT mutation. Cancer Res. 2003;63:2188–2193. [PubMed] [Google Scholar]
- 42.Livesey KM, Kang R, Zeh HJ, 3rd, Lotze MT, Tang D. Direct molecular interactions between HMGB1 and TP53 in colorectal cancer. Autophagy. 2012;8:846–848. doi: 10.4161/auto.19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 44.Gnanasekar M, Kalyanasundaram R, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A. HMGB1: a promising therapeutic target for prostate cancer. Prostate Cancer. 2013;2013:157103. doi: 10.1155/2013/157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng X, Du X, Zhang X, Li D, Lu C, Li Q, Ma Z, Song Q, Wang C. Clinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: comparison with serum SCCA, CYFRA21-1, and CEA levels. Croat Med J. 2009;50:455–464. doi: 10.3325/cmj.2009.50.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F, Zhang Y, Peng Z, Gao H, Xu L, Chen M. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012;10:135. doi: 10.1186/1479-5876-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu PL, Tsai JR, Hwang JJ, Chou SH, Cheng YJ, Lin FY, Chen YL, Hung CY, Chen WC, Chen YH, Chong IW. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am J Respir Cell Mol Biol. 2010;43:530–538. doi: 10.1165/rcmb.2009-0269OC. [DOI] [PubMed] [Google Scholar]
- 48.Hao Q., Du X.Q., Fu X., Tian J. Expression and clinical significance of HMGB1 and RAGE in cervical squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2008;30:292–295. [PubMed] [Google Scholar]
- 49.Luo Y, Chihara Y, Fujimoto K, Sasahira T, Kuwada M, Fujiwara R, Fujii K, Ohmori H, Kuniyasu H. High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. Eur J Cancer. 2013;49:741–751. doi: 10.1016/j.ejca.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012;72:230–238. doi: 10.1158/0008-5472.CAN-11-2001. [DOI] [PubMed] [Google Scholar]
- 51.Tang D, Loze MT, Zeh HJ, Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 2010;6:1181–1183. doi: 10.4161/auto.6.8.13367. [DOI] [PubMed] [Google Scholar]
- 52.Chuangui C, Peng T, Zhentao Y. The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res. 2012;18:1021–1027. doi: 10.1007/s12253-012-9539-3. [DOI] [PubMed] [Google Scholar]
- 53.Abdul-Razzak KK, Garg L, Wen L, Reeck GR. Fetal and newborn calf thymus as a source of chromatin proteins: purification of HMG-1 and HMG-2. Prep Biochem. 1987;17:51–61. doi: 10.1080/00327488708062476. [DOI] [PubMed] [Google Scholar]
- 54.Kimura K, Katoh N, Sakurada K, Kubo S. Phosphorylation of high mobility group 1 protein by phospholipid-sensitive Ca2+-dependent protein kinase from pig testis. Biochem J. 1985;227:271–276. doi: 10.1042/bj2270271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nano R, Racanicchi L, Melzi R, Mercalli A, Maffi P, Sordi V, Ling Z, Scavini M, Korsgren O, Celona B, Secchi A, Piemonti L. Human pancreatic islet preparations release HMGB1: (Ir)relevance for graft engraftment, cell transplant. 2012. [DOI] [PubMed] [Google Scholar]
- 56.Lee KL, Pentecost BT, D’Anna JA, Tobey RA, Gurley LR, Dixon GH. Characterization of cDNA sequences corresponding to three distinct HMG-1 mRNA species in line CHO Chinese hamster cells and cell cycle expression of the HMG-1 gene. Nucleic Acids Res. 1987;15:5051–5068. doi: 10.1093/nar/15.13.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuniyasu H, Chihara Y, Kondo H, Ohmori H, Ukai R. Amphoterin induction in prostatic stromal cells by androgen deprivation is associated with metastatic prostate cancer. Oncol Rep. 2003;10:1863–1868. [PubMed] [Google Scholar]
- 58.Brezniceanu ML, Volp K, Bosser S, Solbach C, Lichter P, Joos S, Zornig M. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003;17:1295–1297. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 59.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enokido Y, Yoshitake A, Ito H, Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun. 2008;376:128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]
- 61.Zetterstrom CK, Strand ML, Soder O. The high mobility group box chromosomal protein 1 is expressed in the human and rat testis where it may function as an antibacterial factor. Hum Reprod. 2006;21:2801–2809. doi: 10.1093/humrep/del256. [DOI] [PubMed] [Google Scholar]
- 62.Chiva M, Mezquita C. Quantitative changes of high mobility group non-histone chromosomal proteins HMG1 and HMG2 during rooster spermatogenesis. FEBS Lett. 1983;162:324–328. doi: 10.1016/0014-5793(83)80781-9. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Yuan Y, Li Y, Zhang J, Xiao G, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol. 2009;182:572–580. doi: 10.4049/jimmunol.182.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–392. [PubMed] [Google Scholar]
- 65.Das D, Peterson RC, Scovell WM. High mobility group B proteins facilitate strong estrogen receptor binding to classical and half-site estrogen response elements and relax binding selectivity. Mol Endocrinol. 2004;18:2616–2632. doi: 10.1210/me.2004-0125. [DOI] [PubMed] [Google Scholar]
- 66.Onate SA, Prendergast P, Wagner JP, Nissen M, Reeves R, Pettijohn DE, Edwards DP. The DNA-bending protein HMG-1 enhances progesterone receptor binding to its target DNA sequences. Mol Cell Biol. 1994;14:3376–3391. doi: 10.1128/mcb.14.5.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prendergast P, Onate SA, Christensen K, Edwards DP. Nuclear accessory factors enhance the binding of progesterone receptor to specific target DNA. J Steroid Biochem Mol Biol. 1994;48:1–13. doi: 10.1016/0960-0760(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 68.Zicari A, Centonze C, Realacci M, Buchetti B, Pietropolli A, Ticconi C. Estradiol 17-beta and progesterone modulate inducible nitric oxide synthase and high mobility group box 1 expression in human endometrium. Reprod Sci. 2008;15:559–566. doi: 10.1177/1933719107312560. [DOI] [PubMed] [Google Scholar]
- 69.Verrijdt G, Haelens A, Schoenmakers E, Rombauts W, Claessens F. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochem J. 2002;361:97–103. doi: 10.1042/0264-6021:3610097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sparatore B, Patrone M, Passalacqua M, Pedrazzi M, Ledda S, Pontremoli S, Melloni E. Activation of A431 human carcinoma cell motility by extracellular high-mobility group box 1 protein and epidermal growth factor stimuli. Biochem J. 2005;389:215–221. doi: 10.1042/BJ20050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008;10:209. doi: 10.1186/ar2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SM, Choi SE, Lee JH, Lee JJ, Jung IR, Lee SJ, Lee KW, Kang Y. Involvement of the TLR4 (Toll-like receptor4) signaling pathway in palmitate-induced INS-1 beta cell death. Mol Cell Biochem. 2011;354:207–217. doi: 10.1007/s11010-011-0820-7. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Y, Shu T, Lin Y, Wang H, Yang J, Shi Y, Han X. Inhibition of the receptor for advanced glycation endproducts (RAGE) protects pancreatic beta-cells. Biochem Biophys Res Commun. 2011;404:159–165. doi: 10.1016/j.bbrc.2010.11.085. [DOI] [PubMed] [Google Scholar]
- 74.Li M, Song L, Gao X, Chang W, Qin X. Toll-like receptor 4 on islet beta cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes. Exp Mol Med. 2012;44:260–267. doi: 10.3858/emm.2012.44.4.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee BW, Chae HY, Kwon SJ, Park SY, Ihm J, Ihm SH. RAGE ligands induce apoptotic cell death of pancreatic beta-cells via oxidative stress. Int J Mol Med. 2010;26:813–818. [PubMed] [Google Scholar]
- 77.Arnold T, Michlmayr A, Baumann S, Burghuber C, Pluschnig U, Bartsch R, Steger G, Gnant M, Bergmann M, Bachleitner-Hofmann T, Oehler R. Plasma HMGB-1 after the initial dose of epirubicin/docetaxel in cancer. Eur J Clin Invest. 2013;43:286–291. doi: 10.1111/eci.12043. [DOI] [PubMed] [Google Scholar]
- 78.Lum HK, Lee KL. The human HMGB1 promoter is modulated by a silencer and an enhancer-containing intron. Biochim Biophys Acta. 2001;1520:79–84. doi: 10.1016/s0167-4781(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Xi B, Zhao Y, Yu Y, Zhang J, Wang C. High-mobility group protein B1 (HMGB1) is a novel biomarker for human ovarian cancer. Gynecol Oncol. 2012;126:109–117. doi: 10.1016/j.ygyno.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 80.Chen J, Liu X, Zhang J, Zhao Y. Targeting HMGB1 inhibits ovarian cancer growth and metastasis by lentivirus-mediated RNA interference. J Cell Physiol. 2012;227:3629–3638. doi: 10.1002/jcp.24069. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He Y, Zha J, Wang Y, Liu W, Yang X, Yu P. Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013;73:629–639. doi: 10.1158/0008-5472.CAN-12-2704. [DOI] [PubMed] [Google Scholar]
- 83.Gnanasekar M, Thirugnanam S, Ramaswamy K. Short hairpin RNA (shRNA) constructs targeting high mobility group box-1 (HMGB1) expression leads to inhibition of prostate cancer cell survival and apoptosis. Int J Oncol. 2009;34:425–431. [PubMed] [Google Scholar]
- 84.Shetty AV, Thirugnanam S, Dakshinamoorthy G, Samykutty A, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A, Gnanasekar M. 18alpha-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol. 2011;39:635–640. doi: 10.3892/ijo.2011.1061. [DOI] [PubMed] [Google Scholar]
- 85.D.X. Song, A.M. Chen, F.J. Guo, H. Liao, B.Z. Xie, B. Zhu, C. Chen. Differential proteomic analysis and function study of human prostate carcinoma cells with different osseous metastatic tendency. Zhonghua Yi Xue Za Zhi 88 (2008) 1197–1201. [PubMed]
- 86.Xiang YY, Wang DY, Tanaka M, Suzuki M, Kiyokawa E, Igarashi H, Naito Y, Shen Q, Sugimura H. Expression of high-mobility group-1 mRNA in human gastrointestinal adenocarcinoma and corresponding non-cancerous mucosa. Int J Cancer. 1997;74:1–6. doi: 10.1002/(sici)1097-0215(19970220)74:1<1::aid-ijc1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 87.Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, Ito R, Yokozaki H, Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–170. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- 88.Radin JN, Gonzalez-Rivera C, Ivie SE, McClain MS, Cover TL. Helicobacter pylori VacA induces programmed necrosis in gastric epithelial cells. Infect Immun. 2011;79:2535–2543. doi: 10.1128/IAI.01370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akaike H, Kono K, Sugai H, Takahashi A, Mimura K, Kawaguchi Y, Fujii H. Expression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancer. Anticancer Res. 2007;27:449–457. [PubMed] [Google Scholar]
- 90.Bao G, Qiao Q, Zhao H, He X. Prognostic value of HMGB1 overexpression in resectable gastric adenocarcinomas. World J Surg Oncol. 2010;8:52. doi: 10.1186/1477-7819-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J, Zhu JS, Zhou Z, Chen WX, Chen NW. Inhibitory effects of ethyl pyruvate administration on human gastric cancer growth via regulation of the HMGB1-RAGE and Akt pathways in vitro and in vivo. Oncol Rep. 2012;27:1511–1519. doi: 10.3892/or.2012.1623. [DOI] [PubMed] [Google Scholar]
- 92.Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.B. Song, W.G. Song, Z.J. Li, Z.F. Xu, X.W. Wang, C.X. Wang, J. Liu, Effect of HMGB1 silencing on cell proliferation, invasion and apoptosis of MGC-803 gastric cancer cells, Cell Biochem Funct (2011). [DOI] [PubMed]
- 94.Nestl A, Von Stein OD, Zatloukal K, Thies WG, Herrlich P, Hofmann M, Sleeman JP. Gene expression patterns associated with the metastatic phenotype in rodent and human tumors. Cancer Res. 2001;61:1569–1577. [PubMed] [Google Scholar]
- 95.Chung HW, Lim JB, Jang S, Lee KJ, Park KH, Song SY. Serum high mobility group box-1 is a powerful diagnostic and prognostic biomarker for pancreatic ductal adenocarcinoma. Cancer Sci. 2012;103:1714–1721. doi: 10.1111/j.1349-7006.2012.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, Krasinskas A, Lotze MT, Zeh HJ., 3rd The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012;109:7031–7036. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin L, Zhong K, Sun Z, Wu G, Ding G. Receptor for advanced glycation end products (RAGE) partially mediates HMGB1-ERKs activation in clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2012;138:11–22. doi: 10.1007/s00432-011-1067-0. [DOI] [PubMed] [Google Scholar]
- 99.Comprehensive molecular characterization of clear cell renal cell carcinoma, Nature 499 (2013) 43–49. [DOI] [PMC free article] [PubMed]
- 100.Birkhauser FD, Koya RC, Neufeld C, Rampersaud EN, Lu X, Micewicz ED, Chodon T, Atefi M, Kroeger N, Chandramouli GV, Li G, Said JW, McBride WH, Kabbinavar FF, Ribas A, Pantuck AJ, Belldegrun AS, Riss J. Dendritic cell-based immunotherapy in prevention and treatment of renal cell carcinoma: efficacy, safety, and activity of Ad-GM·CAIX in immunocompetent mouse models. J Immunother. 2013;36:102–111. doi: 10.1097/CJI.0b013e31827bec97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fishman M, Seigne J. Immunotherapy of metastatic renal cell cancer. Cancer Control. 2002;9:293–304. doi: 10.1177/107327480200900404. [DOI] [PubMed] [Google Scholar]
- 102.Liang X, De Vera ME, Buchser WJ, Romo de Vivar A, Chavez P, Loughran D, et al. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res. 2012;72:2791–2801. doi: 10.1158/0008-5472.CAN-12-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lotze MT, Buchser WJ, Liang X. Blocking the interleukin 2 (IL2)-induced systemic autophagic syndrome promotes profound antitumor effects and limits toxicity. Autophagy. 2012;8:1264–1266. doi: 10.4161/auto.20752. [DOI] [PubMed] [Google Scholar]
- 104.Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, Zicari A, Antonaci A. HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep. 2012;28:2285–2289. doi: 10.3892/or.2012.2058. [DOI] [PubMed] [Google Scholar]
- 105.Mardente S, Zicari A, Consorti F, Mari E, Di Vito M, Leopizzi M, Della Rocca C, Antonaci A. Cross-talk between NO and HMGB1 in lymphocytic thyroiditis and papillary thyroid cancer. Oncol Rep. 2010;24:1455–1461. doi: 10.3892/or_00001005. [DOI] [PubMed] [Google Scholar]
- 106.Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, Pass HI, Gaudino G, Carbone M, Yang H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72:3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ohmori H, Luo Y, Fujii K, Sasahira T, Shimomoto T, Denda A, Kuniyasu H. Dietary linoleic acid and glucose enhances azoxymethane-induced colon cancer and metastases via the expression of high-mobility group box 1. Pathobiology. 2010;77:210–217. doi: 10.1159/000296305. [DOI] [PubMed] [Google Scholar]
- 108.Smolarczyk R, Cichon T, Matuszczak S, Mitrus I, Lesiak M, Kobusinska M, Kamysz W, Jarosz M, Sieron A, Szala S. The role of glycyrrhizin, an inhibitor of HMGB1 protein, in anticancer therapy. Arch Immunol Ther Exp (Warsz) 2012;60:391–399. doi: 10.1007/s00005-012-0183-0. [DOI] [PubMed] [Google Scholar]
- 109.Liang X, Chavez AR, Schapiro NE, Loughran P, Thorne SH, Amoscato AA, Zeh HJ, Beer-Stolz D, Lotze MT, de Vera ME. Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol. 2009;86:599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 110.Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M, Matsuoka Y, Isohama Y, Izumi Y, Kume T, Inoue A, Akaike A. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61:975–980. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 111.Saiwichai T, Sangalangkarn V, Kawahara K, Oyama Y, Chaichalotornkul S, Narkpinit S, Harnyuttanakorn P, Singhasivanon P, Maruyama I, Tancharoen S. Green tea extract supplement inhibition of HMGB1 release in rats exposed to cigarette smoke. Southeast Asian J Trop Med Public Health. 2010;41:250–258. [PubMed] [Google Scholar]
- 112.Li W, Ashok M, Li J, Yang H, Sama AE, Wang H. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS One. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Girard JP. A direct inhibitor of HMGB1 cytokine. Chem Biol. 2007;14:345–347. doi: 10.1016/j.chembiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dave SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv Clin Chem. 2011;53:155–177. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faham A, Bennett D, Altin JG. Liposomal Ag engrafted with peptides of sequence derived from HMGB1 induce potent Ag-specific and anti-tumour immunity. Vaccine. 2009;27:5846–5854. doi: 10.1016/j.vaccine.2009.07.053. [DOI] [PubMed] [Google Scholar]