Abstract
The expression of growth hormone-releasing hormone (GHRH) splice variant 1 (SV1) receptor in neoplastic lesions of the oral cavity was assessed. The sensitivity of HaCaT keratinocytes to GHRH analogs was also evaluated. Thirty-three benign precancerous oral lesions and 27 squamous cell carcinomas of the oral cavity were evaluated by immunohistochemistry for SV1 expression. SV1 expression in HaCaT keratinocytes was assessed by western blot. HaCaT proliferation was evaluated by cell counting. Anti-SV1 immunoreactivity was detected in only 9 % (three of 33) precancerous lesions (one hyperplasia and two dysplasias), while 44 % (12 of 27) carcinomas were positive for SV1 (p < 0.002). GHRH(1–29)NH2 and GHRH agonist JI-38 stimulated HaCaT proliferation in vitro, and this effect was blocked by GHRH antagonists. These results indicate that SV1 expression may be associated with the transition of precancerous lesions to carcinomas of the oral epithelium. GHRH antagonists may be useful for the management of the disease.
Keywords: Oral Cancer, Oral Squamous Cell Carcinoma, HaCaT Cell, Oral Squamous Cell Carcinoma, Precancerous Lesion
Introduction
Oral cancer is among the ten most common cancers in the world, with broad differences in geographic distribution [1, 2]. The major risk factors for oral neoplasms are chronic exposure of oral mucosa to consumption of tobacco and alcohol [3, 4]. The most common type of oral cancer is squamous cell carcinoma (SCC) with dysplasias being considered as the histological precursor lesions for this malignancy [5]. Several molecular alterations have been associated with development of oral cancer including aberrant expression of p53, epidermal growth factor receptor, matrix metalloproteinases, Ras oncoproteins, and others [6–15]. In certain cases, these molecular changes may reflect etiology and ethnic origin [16]. A link between the development of oral cancer and human papilloma virus infection has also been proposed [17, 18]. Besides these autonomous cellular lesions, recent evidence has also revealed an important role for stroma activation in development of oral cancer [19–21]. Despite the progress made in the identification of the molecular alterations that are linked to oral SCC (OSCC) and their potential susceptibility to the development of targeted therapies [22, 23], the identification of additional alterations that are causally linked to the disease is imperative.
Growth hormone-releasing hormone (GHRH) is a neuroendocrine peptide hormone which regulates the production and release of GH in the pituitary and in peripheral tissues plays various roles that range from stimulation of cardiac and pancreatic cell to wound healing and tissue repair [24–27]. In addition, overexpression of GHRH by various neoplastic tissues has also been reported. Importantly, the inhibition of autocrine GHRH activity by specific GHRH antagonists which has been found to inhibit cancer cell growth in vitro and in vivo underlines the potential use of these peptide analogs as a novel class of anticancer drugs [28–30]. The pituitary type of GHRH receptors and its splice variants (SVs) in the peripheral tissues and in the malignant tumors are likely to mediate the effects of GHRH. Among them, SV1 possesses the greatest similarity to pituitary GHRH receptor and has been shown to be expressed in several primary human tumors and many human cancer cell lines [31–36]. SV1 corresponds to a truncated form of GHRH receptor lacking a segment of the extracellular portion of the receptor [30]. However, it remains functional as it binds GHRH analogs and elicits mitogenic signals upon GHRH stimulation [37]. In addition, SV1 also possesses activity that is independent of GHRH ligand stimulation as its expression is sufficient to stimulate cell proliferation in the absence of GHRH [38, 39].
In the present study, we evaluated the expression of anti-SV1 immunoreactivity in a bank of primary oral lesions including squamous cell carcinomas and dysplasias. We also evaluated the effects of GHRH agonists and antagonists in vitro in HaCaT keratinocytes. Our results suggest that oral cancers express receptors for GHRH more commonly than do dysplasias and suggest that therapy with GHRH antagonists should be considered for the therapy of oral SCCs.
Materials and Methods
Specimens and Immunohistochemistry
A bank of 60 oral tissue specimens consisting of 27 squamous cell carcinomas and 33 precancerous lesions (five hyperplasias and 28 dysplasias) was collected from the archives of the Laboratory of Histology and Embryology, University of Athens Medical School and was analyzed by immunohistochemistry for SV1 expression by using the antibody JH2317/5 [32]. The local Institutional Review Board for use of human subjects at Athens University approved the protocol for collection and use of these specimens and authorized the study. Human pituitary tissue of normal adult individuals was obtained from the tissue archives of the Department of Forensic Medicine and Toxicology. Immunostaining was performed by using the Superpicture Polymer (Dab) Kit (Novocastra), following the manufacturer’s instructions. Before evaluations, a weak counterstaining with hematoxylin was performed. Specimens were evaluated for positive immunohistochemical staining and classified according to the percentage of positive cells, into the following categories: 1–10 %, ±; 11–30 %, +; 31–70 %, ++; and 71–100 %, +++.
Peptides and Cell Culture
hGHRH(1–29) NH2 was obtained from Sigma. Agonist JI-38 [40] and GHRH antagonists JMR-132 and MIA-602 were synthesized as reported [28, 41, 42]. The peptides were dissolved in phosphate buffered saline at the indicated concentrations. Controls contained solvent alone. HaCaT cells were maintained in Dulbecco’s modified Eagle medium containing 10 % fetal bovine serum (FBS) and antibiotics/antimycotics. Tissue culture reagents were obtained from Invitrogen.
Cell Proliferation and Migration Assays
The rate of cell proliferation was evaluated by cell counting under inverted microscope. Dead cells were identified by the Trypan blue exclusion staining and were not included in the analysis; 104 cells were seeded per well and were treated for 5 days with the peptides at the indicated concentrations. Cell migration was evaluated by the “scratch” assay as described, exposing cells to the peptide analogs for 24 h [43]. All in vitro experiments were performed in triplicates and yielded similar results.
Western Blot Analysis for SV1
HaCaT cells were seeded at a level of 30–40 % of confluence and cultured for 3 days in order to achieve a low-density cell culture. For the high-density cell culture, cells were seeded at about 90 % confluence and cultured subsequently for 3 days prior to protein isolation. Total proteins were extracted from cultured HaCaT cells as previously described [43]. Briefly, the cells were lysed at 4°C for 20 min using a lysis buffer (radioimmunoprecipitation assay, Invitrogen, and proteinase inhibitor). Whole cell lysates were subsequently centrifuged at 13,000 rpm for 10 min at 4°C, and the supernatants were collected. Protein content in the supernatants was determined by the Bradford assay. One hundred-microgram aliquots of the proteins extracted from cells were electrophoresed through a 10 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions. The proteins were electrophoretically transferred from gels onto nitrocellulose membranes. The blots were exposed overnight to primary antibodies, followed by 1 h of incubation with secondary antibodies. The antigen–antibody complexes were detected with the enhanced chemiluminescence detection system (Thermo Scientific). The experiments were repeated with at least three different cultured specimens with similar results, and the reported results are representative. Antibody for actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and SV1 antibody is the same as was used in immunohistochemistry [32].
Statistical Analysis
Immunohistochemical data were analyzed by the chi-squared test while the statistical analysis of tissue culture experiments was by the two-tailed Student’s t test.
Results
SV1 Expression in Oral Cancers and Dysplasias
We have evaluated by immunohistochemistry the expression of the GHRH receptor SV1 in a bank of 60 neoplastic lesions of the oral cavity that included 33 precancerous lesions (five hyperplasias and 28 dysplasias) and 27 OSCCs. SV1 immunopositivity was detected in 15 specimens and was cytoplasmic in all cases (Fig. 1). In all cases, immunopositivity was localized in the neoplastic epithelial cells and was undetectable in the stromal cells of the tumors. Those positive (positivity ≥ ++) for SV1 comprised 12 of 27 (44 %) OSCCs as compared to only three of 33 (9 %) precancerous lesions (p < 0.002) (Tables 1 and 2). Thus, expression of SV1 is significantly increased in oral neoplastic lesions as they progress into malignancy. Among the three precancerous lesions in which SV1 immunoreactivity was detected, two were dysplasias and one was hyperplasia. This implies that prior to malignant degeneration, expression of SV1 is not increased in dysplasias as compared to the hyperplasias despite that the former histopathological entity is considered to be the precursor of OSCC. No correlation between SV1 expression and the differentiation level or anatomic site of OSCCs was observed. As a control for the immunohistochemical analysis, human pituitary tissue was used which showed positive staining for SV1 receptors (Fig. 2a). Omission of the primary antibody abolished the immunoreactivity against SV1 (Fig. 2b).
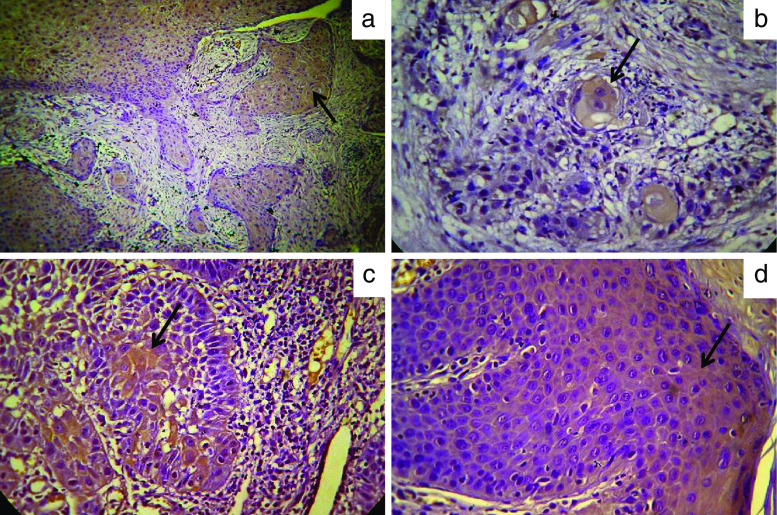
Fig. 1.
Representative microphotographs of SV1 immunopositivity (brown staining indicated by arrow) in primary oral neoplastic lesions. a Well-differentiated SCC that is characterized as +++ for SV1 expression (×20 magnification). b Well-differentiated SCC that is characterized as + for SV1 expression (×40 magnification). c Well-differentiated SCC that is characterized as ++ for SV1 expression (×40 magnification). d Moderate epithelial dysplasia that is characterized as ++ for SV1 expression (×40 magnification)
Table 1.
Clinicopathological data and anti-SV1 immunoreactivity in pre-neoplastic oral lesions
| Specimen no. | Gender | Age | Anatomical location | Histopathology | SV1 |
|---|---|---|---|---|---|
| 1 | M | 68 | Floor of the mouth | Mild epithelial dysplasia | + |
| 2 | M | 71 | Gingiva | Mild epithelial dysplasia | − |
| 3 | F | 68 | Buccal mucosa | Mild epithelial dysplasia | ± |
| 4 | M | 72 | Gingiva | Mild epithelial dysplasia | ± |
| 5 | F | 53 | Lower lip | Epithelial hyperplasia | ± |
| 6 | F | 57 | Buccal mucosa | Mild epithelial dysplasia | − |
| 7 | F | 78 | Tongue | Epithelial hyperplasia | ++ |
| 8 | F | 76 | Alveolar ridge | Mild epithelial dysplasia | ++ |
| 9 | M | 53 | Lower lip | Severe epithelial dysplasia | ++ |
| 10 | F | 52 | Gingiva | Moderate epithelial dysplasia | ± |
| 11 | F | 67 | Tongue, gingiva | Moderate epithelial dysplasia | − |
| 12 | M | 35 | Tongue | Mild epithelial dysplasia | − |
| 13 | F | 42 | Palate | Epithelial hyperplasia | + |
| 14 | M | 58 | Tongue | Mild epithelial dysplasia | − |
| 15 | F | 70 | Tongue | Mild epithelial dysplasia | − |
| 16 | M | 50 | Floor of the mouth | Mild epithelial dysplasia | − |
| 17 | F | 53 | Gingiva | Mild epithelial dysplasia | − |
| 18 | M | 62 | Tongue | Mild epithelial dysplasia | − |
| 19 | M | 38 | Tongue | Mild epithelial dysplasia | + |
| 20 | F | 42 | Tongue | Mild epithelial dysplasia | − |
| 21 | F | 49 | Tongue | Mild epithelial dysplasia | − |
| 22 | F | 53 | Gingiva | Mild epithelial dysplasia | − |
| 23 | F | 63 | Buccal mucosa | Mild epithelial dysplasia | ± |
| 24 | F | 69 | tongue | Mild epithelial dysplasia | − |
| 25 | M | 58 | Buccal mucosa | Mild epithelial dysplasia | ± |
| 26 | F | 38 | Palate | Mild epithelial dysplasia | ± |
| 27 | F | 53 | Tongue | Mild epithelial dysplasia | − |
| 28 | F | 52 | Buccal mucosa | Moderate epithelial dysplasia | + |
| 29 | F | 60 | Tongue | Mild epithelial dysplasia | − |
| 30 | F | 58 | Buccal mucosa | Mild epithelial dysplasia | − |
| 31 | M | 34 | Tongue | Epithelial hyperplasia | − |
| 32 | M | 58 | Tongue | Moderate epithelial dysplasia | |
| 33 | M | 29 | Gingiva | Epithelial hyperplasia | + |
Table 2.
Clinicopathological data and anti-SV1 immunoreactivity in OSCC
| Specimen no. | Gender | Age | Anatomical location | Histopathology | SV1 |
|---|---|---|---|---|---|
| 1 | M | 73 | Buccal mucosa | Superficially invasive | ++ |
| 2 | M | 55 | Tongue | Moderate differentiated | ++ |
| 3 | M | 96 | Alveolar ridge | Well differentiated | ++ |
| 4 | M | 63 | Tongue | Well differentiated | ++ |
| 5 | M | 82 | Tongue | Well differentiated | − |
| 6 | F | 35 | Tongue | Well differentiated | − |
| 7 | M | 48 | Soft palate | Moderately differentiated | ++ |
| 8 | M | 77 | Tongue | Well differentiated | − |
| 9 | M | 60 | Tongue | Superficially invasive | − |
| 10 | F | NA | Tongue | Well differentiated | ++ |
| 11 | F | 40 | Lower lip | Well differentiated | +++ |
| 12 | M | 32 | Tongue | Moderately differentiated | − |
| 13 | F | 70 | Tongue | Well differentiated | ++ |
| 14 | F | 59 | Tongue | Well differentiated | − |
| 15 | M | 73 | Soft palate | Well differentiated | ++ |
| 16 | F | 54 | Tongue | Well differentiated | − |
| 17 | M | 78 | Tongue | Well differentiated | ++ |
| 18 | F | NA | Alveolar ridge | Well differentiated | − |
| 19 | M | NA | Palate | Well differentiated | − |
| 20 | F | 68 | Palate | Moderately differentiated | − |
| 21 | M | 69 | Tongue | Well differentiated | ++ |
| 22 | F | 53 | Tongue | Well differentiated | − |
| 23 | M | 72 | Tongue | Well differentiated | − |
| 24 | M | 48 | Lip | Moderately differentiated | − |
| 25 | F | 55 | Lip | Moderately differentiated | − |
| 26 | M | 49 | Lip | Moderately differentiated | − |
| 27 | F | 52 | Alveolar ridge | Well differentiated | +++ |
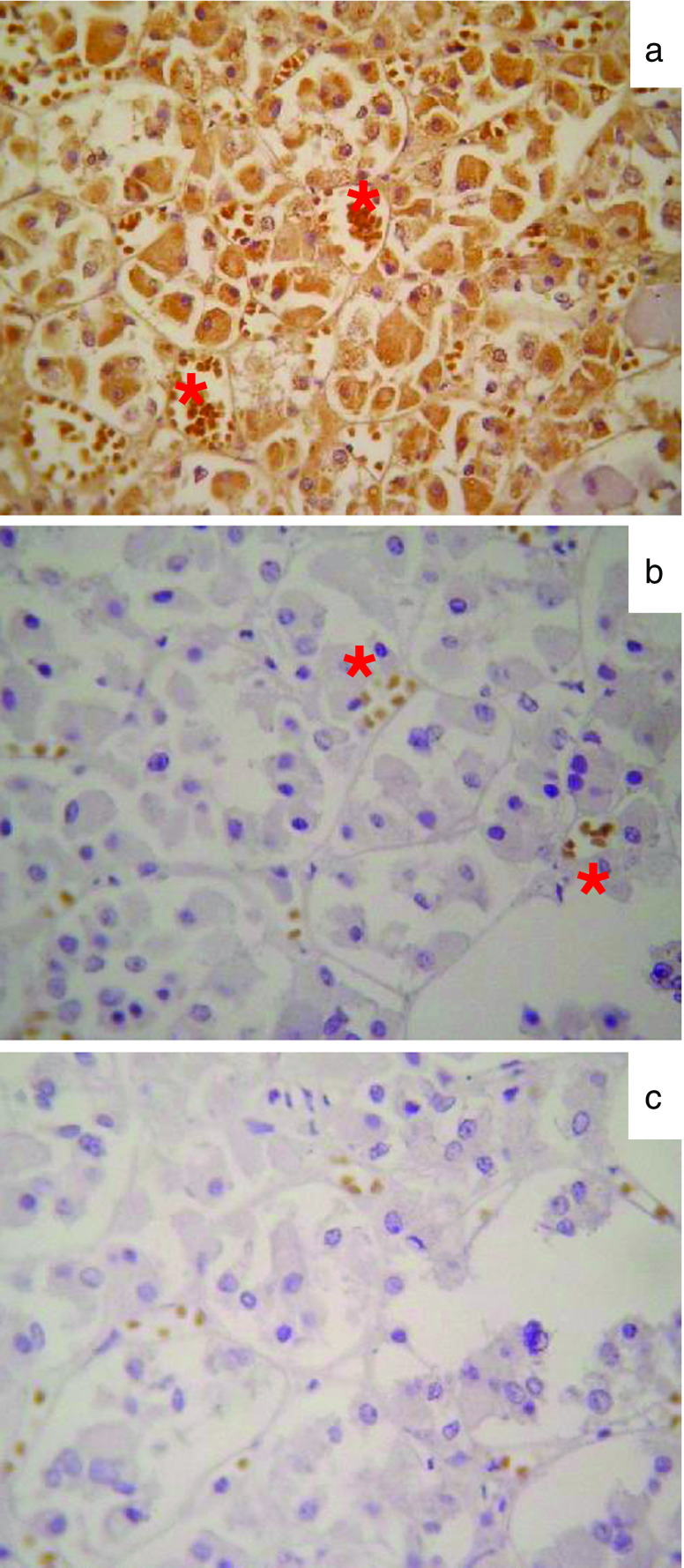
Fig. 2.
Representative microphotographs of SV1 immunopositivity (brown staining) in normal human pituitary in the presence (a) or absence of the primary anti-SV1 (b) or secondary (c) antibody. Red asterisks in both panels indicate non-specific immunopositivity that developed against red blood cells
Effect of GHRH Analogs on the Proliferation and Migration of HaCaT Keratinocytes
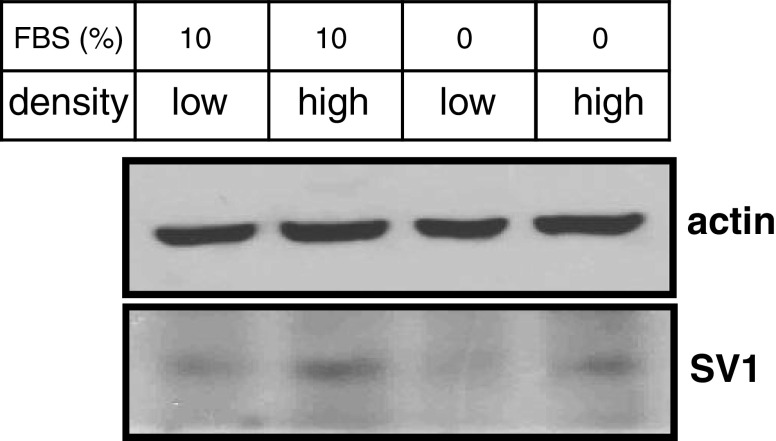
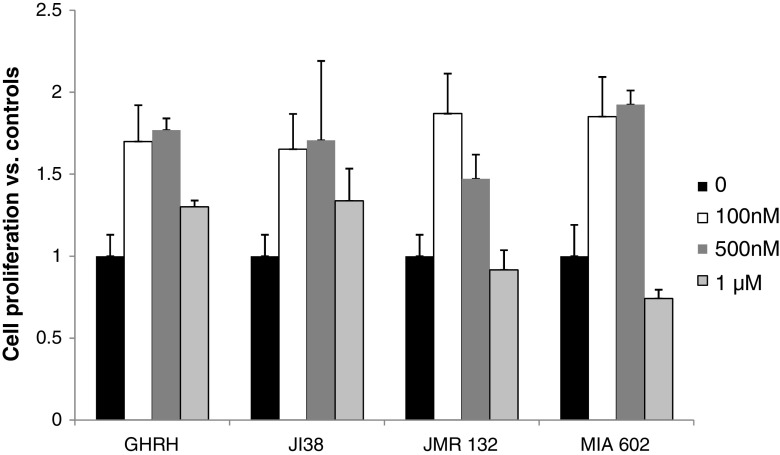
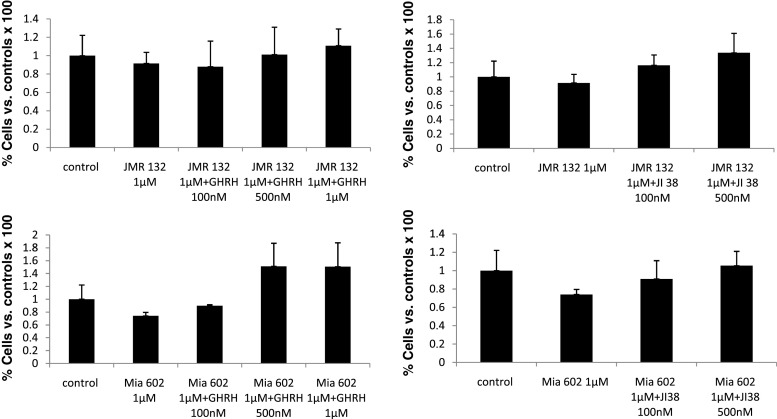
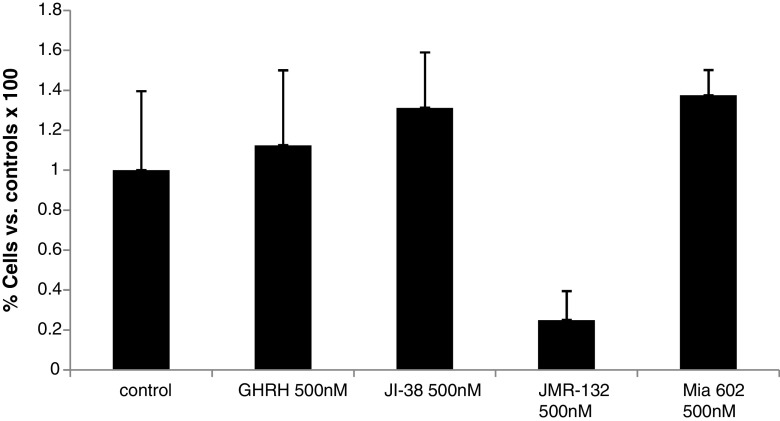
In view of the increased anti-SV1 immunoreactivity in OSCCs, we then explored the effects of GHRH agonists and antagonists on the rate of proliferation of HaCaT keratinocytes in vitro. HaCaT cells are transformed keratinocytes of human origin that are not tumorigenic in vivo [44]. Therefore, we considered them as a reliable in vitro model for studies related to SCCs proliferation. Initially, we tested whether HaCaT cells express SV1. As shown in Fig. 3, western blot analysis indicated that, indeed, HaCaT cells are positive for SV1. We also noted that SV1 expression was dependent on cell density: When cells were cultured at high density, SV1 expression was elevated as compared to that of cells growing at low-density conditions. In order to clarify whether this effect was due to contact inhibition at high density, we also explored the levels of SV1 expression in cells cultured at high or low density in the absence of serum. As shown in Fig. 3, upregulation of SV1 was detectable even in serum starved cells implying that the stage of cell cycle is not the determining factor in SV1 expression. Some inhibition though of SV1 in serum arrested, as compared to proliferating cells, cultured at low density was noted (Fig. 3). These observations are indicative of a dynamic role of cell contact in the regulation of SV1 expression. Subsequently, we tested whether HaCaT cells respond to GHRH analogs. We found that both GHRH and agonistic analog JI-38 as well as GHRH antagonists JMR-132 and MIA-602 were mitogenic at concentrations of 100 and 500 nM; however, at 1 μΜ only GHRH and agonist JI-38 stimulated cell growth while JMR-132 and the more potent MIA-602 inhibited it (Fig. 4). We then attempted to further explore the specificity of the GHRH antagonists used in this study. To that end, we tested whether increasing concentrations of GHRH and agonist JI-38 are capable of overcoming the inhibitory activities of JMR-132 and MIA-602 elicited at 1 μΜ. Indeed, both GHRH and agonist JI-38 caused a dose-dependent increase of cell proliferation in HaCaT cells in the presence of JMR-132 and MIA-602 at 1 μΜ (Fig. 5). Furthermore, while the agonists alone were highly mitogenic at 100 and 500 nM (Fig. 4), their mitogenic activity was diminished in the presence of the antagonists (Fig. 5).
Fig. 3.
Western blot analysis for SV1 expression in HaCaT keratinocytes cultured at low or high density and in the absence or presence of serum (FBS). Actin levels are shown as a loading control
Fig. 4.
Proliferation of HaCaT keratinocytes in the presence of GHRH, agonist JI38, and antagonists JMR132 or MIA602 supplemented in the culture medium at 100 nM, 500 nM, or 1 μΜ
Fig. 5.
Proliferation of HaCaT keratinocytes in the presence of antagonists JMR132 (upper panel) or MIA602 (lower panel) in combination with increasing concentrations of GHRH (left panel) or agonist JI38 (right panel)
The elevated SV1 expression under dense culture conditions prompted us to address whether GHRH analogs affect other properties of HaCaT cells besides cell proliferation, such as the rate of cell migration. Therefore, we performed an in vitro wound healing assay that is indicative of the ability of cells to migrate and is associated with the metastatic ability of the cancer cells. We chose to expose cells at 500 nM since at this concentration, all analogs exhibited mitogenic activity (Fig. 4), and thus, potential anti-migratory activity could be identified. As shown in Fig. 6, for GHRH, JI-38, and MIA-602, some inhibition in the rate of cell migration was detected which paralleled inversely the mitogenic activity of these analogs at equimolar concentration. However, JMR-132 at 500 nM potently accelerated cell migration despite that at this concentration, it was less potent in terms of mitogenic activity as compared to the other three peptides tested. These results, besides their importance in the regulation of cell migration, indicate that regulation of cell proliferation and migration by GHRH analogs are not tightly related.
Fig. 6.
Cell migration (scratch) assay performed with HaCaT keratinocytes in the presence of GHRH, agonist JI38, and antagonists JMR132 or MIA602 supplemented in the culture medium at 500 nM
Discussion
Receptors for GHRH, particularly SV1, are expressed in several human cancers. Furthermore, antagonists of GHRH are potent inhibitors of cancer cell growth in vitro and in vivo and have emerged as a promising therapeutic strategy for the treatment of several of these malignancies. In view of the absence of experimental results regarding the expression of GHRH receptors in oral neoplastic lesions, in the present study we evaluated the presence of SV1 immunoreactivity in OSCCs and dysplastic lesions by immunohistochemistry and in HaCaT keratinocytes by western blot analysis. Our results indicated that expression of SV1 in OSCCs is about five times more common than in the pre-malignant lesions (44 % in OSCCs vs. 9 % in precancerous lesions) suggesting that SV1 activity may be associated with malignant transition of oral squamous epithelium. This finding is in agreement with our previous findings on other cancers, such as melanomas, in which SV1 expression was significantly more common in the malignant than in the dysplastic lesions [36].
In view of the increased immunopositivity for SV1 in OSCCs, we subsequently explored the antineoplastic activity of GHRH antagonists in vitro, by using HaCaT human keratinocytes as a model. While HaCaT cells are immortal keratinocytes of skin origin, the fact that the oral epithelium also consists of keratinocytes justifies the use of these cells in order to assess the responsiveness to analogs of GHRH. We evaluated the effects of GHRH agonists and antagonists on two major phenotypic properties of these cancer cells such as the proliferation rate and the rate of cell migration. Our results confirmed that, indeed, GHRH antagonists inhibited the proliferation of HaCaT cells while GHRH and agonists stimulated it, at 1 μΜ. We also noted that at lower concentrations, both agonists and antagonists were mitogenic. This stimulatory activity of GHRH antagonists at 100 and 500 nM has been noted by some of us (HK and AVS) using other antagonists in different cell lines. This might be associated with the sensitization of GHRH receptors such as SV1 by exposure at lower concentrations of GHRH analogs. This hypothesis that is consistent with the previously reported ligand-independent activity of SV1 [38] has to be confirmed by additional studies. The fact that GHRH and agonist JI-38 at increasing concentrations overcame the antiproliferative activity of GHRH antagonists in a dose-dependent manner argues in favor of the specificity of our findings. Despite that GHRH analogs clearly affected cell proliferation, cell migration was not affected considerably by GHRH, JI-38, and MIA-602. On the contrary, all these three analogs slightly inhibited the closure in vitro of the wound, despite that at the same concentration all three were mitogenic. GHRH antagonist JMR-132 though, which at 500 nM was less potently mitogenic as compared to the other three analogs, caused strong stimulation in cell migration. These discrepancies between the activities of the GHRH analogs used in the present study as regards on the effects on cell proliferation compared to migration indicate that these properties do not necessarily parallel each other. In addition, they may indicate that specific antagonists of GHRH may target additional receptors besides SV1 in the HaCaT keratinocytes and likely in other cells. These receptors upon their activation or downregulation differentially regulate that specific effect measured that is due to SV1 suppression. Finally, it may be due to the fact that cell migration and cell proliferation are occasionally opposing cellular activities, and thus, stimulation of cell proliferation may limit the capacity of these cells to migrate.
Collectively, our results demonstrate, for the first time, that OSCCs but not pre-malignant lesions such as dysplasias and hyperplasias frequently express the SV1 receptor for GHRH which suggests that this receptor splice variant might be associated with the progression of the disease. This finding, in addition with the responsiveness of transformed keratinocytes to GHRH analogs, indicates that antagonists for GHRH should be considered for the treatment of OSCCs.
Acknowledgments
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010;46(6):407–410. doi: 10.1016/j.oraloncology.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Lambert R, Sauvaget C, de Camargo CM, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 3.Znaor A, Brennan P, Gajalakshmi V, Mathew A, Shanta V, Varghese C, Boffetta P. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105(5):681–686. doi: 10.1002/ijc.11114. [DOI] [PubMed] [Google Scholar]
- 4.Muwonge R, Ramadas K, Sankila R, Thara S, Thomas G, Vinoda J, Sankaranarayanan R. Role of tobacco smoking, chewing and alcohol drinking in the risk of oral cancer in Trivandrum, India: a nested case–control design using incident cancer cases. Oral Oncol. 2008;44(5):446–454. doi: 10.1016/j.oraloncology.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Izumo T. Oral premalignant lesions: from the pathological viewpoint. Int J Clin Oncol. 2011;16(1):15–26. doi: 10.1007/s10147-010-0169-z. [DOI] [PubMed] [Google Scholar]
- 6.Ogmundsdóttir HM, Björnsson J, Holbrook WP. Role of TP53 in the progression of pre-malignant and malignant oral mucosal lesions. A follow-up study of 144 patients. J Oral Pathol Med. 2009;38(7):565–571. doi: 10.1111/j.1600-0714.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarkis SA, Abdullah BH, Abdul Majeed BA, Talabani NG. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in oral squamous cell carcinoma in relation to proliferation, apoptosis, angiogenesis and lymphangiogenesis. Head Neck Oncol. 2010;2:13. doi: 10.1186/1758-3284-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taoudi Benchekroun M, Saintigny P, Thomas SM, El-Naggar AK, Papadimitrakopoulou V, Ren H, Lang W, Fan YH, Huang J, Feng L, Lee JJ, Kim ES, Hong WK, Johnson FM, Grandis JR, Mao L. Epidermal growth factor receptor expression and gene copy number in the risk of oral cancer. Cancer Prev Res (Phila) 2010;3(7):800–809. doi: 10.1158/1940-6207.CAPR-09-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardes VF, Gleber-Netto FO, Sousa SF, Silva TA, Aguiar MC. Clinical significance of EGFR, Her-2 and EGF in oral squamous cell carcinoma: a case control study. J Exp Clin Cancer Res. 2010;29:40. doi: 10.1186/1756-9966-29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteiro LS, Diniz-Freitas M, Garcia-Caballero T, Forteza J, Fraga M. EGFR and Ki-67 expression in oral squamous cell carcinoma using tissue microarray technology. J Oral Pathol Med. 2010;39(7):571–578. doi: 10.1111/j.1600-0714.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 11.Tilakaratne WM, Kobayashi T, Ida-Yonemochi H, Swelam W, Yamazaki M, Mikami T, Alvarado CG, Shahidul AM, Maruyama S, Cheng J, Saku T. Matrix metalloproteinase 7 and perlecan in oral epithelial dysplasia and carcinoma in situ: an aid for histopathologic recognition of their cell proliferation centers. J Oral Pathol Med. 2009;38(4):348–355. doi: 10.1111/j.1600-0714.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomas GT, Lewis MP, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncol. 1999;35(3):227–233. doi: 10.1016/S1368-8375(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 13.Kuo MY, Chang HH, Hahn LJ, Wang JT, Chiang CP. Elevated ras p21 expression in oral premalignant lesions and squamous cell carcinoma in Taiwan. J Oral Pathol Med. 1995;24(6):255–260. doi: 10.1111/j.1600-0714.1995.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiaris H, Spandidos DA, Jones AS, Vaughan ED, Field JK. Mutations, expression and genomic instability of the H-ras proto-oncogene in squamous cell carcinomas of the head and neck. Br J Cancer. 1995;72(1):123–128. doi: 10.1038/bjc.1995.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiaris H, Spandidos D, Jones A, Field J. Loss of heterozygosity and microsatellite instability of the H-ras gene in cancer of the head and neck. Int J Oncol. 1994;5(3):579–582. doi: 10.3892/ijo.5.3.579. [DOI] [PubMed] [Google Scholar]
- 16.Paterson IC, Eveson JW, Prime SS. Molecular changes in oral cancer may reflect aetiology and ethnic origin. J Cancer B Oral Oncol. 1996;32B(3):150–153. doi: 10.1016/0964-1955(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 17.Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, Challacombe S, Ficarra G, Flaitz C, Zhou HM, Maeda H, Miller C, Jontell M. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 18.Angiero F, Gatta LB, Seramondi R, Berenzi A, Benetti A, Magistro S, Ordesi P, Grigolato P, Dessy E. Frequency and role of HPV in the progression of epithelial dysplasia to oral cancer. Anticancer Res. 2010;30(9):3435–3440. [PubMed] [Google Scholar]
- 19.Chatzistamou I, Dioufa N, Trimis G, Sklavounou A, Kittas C, Kiaris H, Papavassiliou AG. p21/waf1 and smooth-muscle actin α expression in stromal fibroblasts of oral cancers. Anal Cell Pathol (Amst) 2010;33(1):19–26. doi: 10.3233/ACP-CLO-2010-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, Upile T, Kalavrezos N, Violette SM, Weinreb PH, Chester KA, Chana JS, Marshall JF, Hart IR, Hackshaw AK, Piper K, Thomas GJ. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223(4):470–481. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- 21.Meng W, Xia Q, Wu L, Chen S, He X, Zhang L, Gao Q, Zhou H. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi: 10.1186/1471-2407-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraishi Y, Wada T, Nakatani K, Tojyo I, Matsumoto T, Kiga N, Negoro K, Fujita S. EGFR inhibitor enhances cisplatin sensitivity of oral squamous cell carcinoma cell lines. Pathol Oncol Res. 2008;14(1):39–43. doi: 10.1007/s12253-008-9020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holsinger FC, Doan DD, Jasser SA, Swan EA, Greenberg JS, Schiff BA, Bekele BN, Younes MN, Bucana CD, Fidler IJ, Myers JN. Epidermal growth factor receptor blockade potentiates apoptosis mediated by paclitaxel and leads to prolonged survival in a murine model of oral cancer. Clin Cancer Res. 2003;9(8):3183–3189. [PubMed] [Google Scholar]
- 24.Ludwig B, Ziegler CG, Schally AV, Richter C, Steffen A, Jabs N, Funk RH, Brendel MD, Block NL, Ehrhart-Bornstein M, Bornstein SR. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci U S A. 2010;107(28):12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanashiro-Takeuchi RM, Tziomalos K, Takeuchi LM, Treuer AV, Lamirault G, Dulce R, Hurtado M, Song Y, Block NL, Rick F, Klukovits A, Hu Q, Varga JL, Schally AV, Hare JM. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107(6):2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granata R, Trovato L, Gallo MP, Destefanis S, Settanni F, Scarlatti F, Brero A, Ramella R, Volante M, Isgaard J, Levi R, Papotti M, Alloatti G, Ghigo E. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia–reperfusion injury in rat heart. Cardiovasc Res. 2009;83(2):303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 27.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: not only a neurohormone. Trends Endocrinol Metab. 2011;22:311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4(1):33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 29.Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 30.Barabutis N, Schally AV. Growth hormone-releasing hormone: extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 31.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci U S A. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, Szepeshazi K, Köster F, Kovitz K, Groot K, Zarandi M, Kanashiro CA. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci U S A. 2005;102(48):17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theophanous E, Petraki C, Scorilas A, Komborozos V, Veloudis G, Varga JL, Zarandi M, Schally AV, Koutsilieris M. The immunohistochemical expression of growth hormone-releasing hormone receptor splice variant 1 is a favorable prognostic marker in colorectal cancer. Mol Med. 2009;15(7–8):242–247. doi: 10.2119/molmed.2008.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Köster F, Engel JB, Schally AV, Hönig A, Schröer A, Seitz S, Hohla F, Ortmann O, Diedrich K, Buchholz S. Triple-negative breast cancers express receptors for growth hormone-releasing hormone (GHRH) and respond to GHRH antagonists with growth inhibition. Breast Cancer Res Treat. 2009;116(2):273–279. doi: 10.1007/s10549-008-0120-4. [DOI] [PubMed] [Google Scholar]
- 35.Schulz S, Röcken C, Schulz S. Immunocytochemical localisation of plasma membrane GHRH receptors in human tumours using a novel anti-peptide antibody. Eur J Cancer. 2006;42(14):2390–2396. doi: 10.1016/j.ejca.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Chatzistamou I, Volakaki AA, Schally AV, Kiaris H, Kittas C. Expression of growth hormone-releasing hormone receptor splice variant 1 in primary human melanomas. Regul Pept. 2008;147(1–3):33–36. doi: 10.1016/j.regpep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Kiaris H, Schally AV, Busto R, Halmos G, Artavanis-Tsakonas S, Varga JL. Expression of a splice variant of the receptor for GHRH in 3T3 fibroblasts activates cell proliferation responses to GHRH analogs. Proc Natl Acad Sci U S A. 2002;99(1):196–200. doi: 10.1073/pnas.012590999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiaris H, Chatzistamou I, Schally AV, Halmos G, Varga JL, Koutselini H, Kalofoutis A. Ligand-dependent and -independent effects of splice variant 1 of growth hormone-releasing hormone receptor. Proc Natl Acad Sci U S A. 2003;100(16):9512–9517. doi: 10.1073/pnas.1533185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barabutis N, Tsellou E, Schally AV, Kouloheri S, Kalofoutis A, Kiaris H. Stimulation of proliferation of MCF-7 breast cancer cells by a transfected splice variant of growth hormone-releasing hormone receptor. Proc Natl Acad Sci U S A. 2007;104:5575–5579. doi: 10.1073/pnas.0700407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izdebski J, Pinski J, Horvath JE, Halmos G, Groot K, Schally AV. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci U S A. 1995;92(11):4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letsch M, Schally AV, Busto R, Bajo AM, Varga JL. Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and -independent prostate cancers. Proc Natl Acad Sci U S A. 2003;100:1250–1255. doi: 10.1073/pnas.0337496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchholz S, Schally AV, Engel JB, Hohla F, Heinrich E, Koester F, Varga JL, Halmos G. Potentiation of mammary cancer inhibition by combination of antagonists of growth hormone-releasing hormone with docetaxel. Proc Natl Acad Sci U S A. 2007;104(6):1943–1946. doi: 10.1073/pnas.0610860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dioufa N, Schally AV, Chatzistamou I, Moustou E, Block NL, Owens GK, Papavassiliou AG, Kiaris H. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc Natl Acad Sci U S A. 2010;107(43):18611–18615. doi: 10.1073/pnas.1013942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]