Abstract
The insulin analog glargine has a higher binding affinity than regular insulin for the insulin-like growth factor 1 receptor in vitro, raising questions about increased mitogenicity in vivo. Observational studies in humans have recently reported a potential differential association between insulin glargine and malignancies, but available evidence remains inconclusive. We directly compared glargine vs. neutral protamine Hagedorn (NPH) insulin’s effects on cell proliferation in colonic mucosa and on formation of aberrant crypt foci in diabetic mice, i.e., early stages of colorectal cancer development. Mice (BKS.Cg-+Lepr db/+Lepr db/OlaHsd) were treated with insulin glargine (G), NPH insulin (NPH), or saline (NaCl). We assessed epithelial proliferation after long-term insulin treatment (18 weeks) by 5-bromo-2′-deoxyuridine and Ki67 staining and analyzed the formation of aberrant crypt foci (ACF) in mice treated with insulin glargine or NPH insulin or 10 weeks after initiation with 1,2-dimethylhydrazine. Insulin glargine treatment did not result in significantly different epithelial colonic proliferation compared to NPH insulin (G, 137 ± 22; NPH, 136 ± 15; NaCl, 100 ± 20 (relative proliferation index)), but both insulin-treated groups of mice had a higher proliferation index compared to the NaCl control group (p < 0.001). Similarly, we observed no difference in ACF formation between glargine- and NPH-insulin-treated mice (G, 132 ± 12; NPH, 138 ± 9; NaCl, 100 ± 7 (relative number of ACF)), but ACF formation was significantly higher in insulin-treated mice than in NaCl-treated control mice (p = 0.001). Chronic insulin treatment results in higher colonic epithelial proliferation and ACF formation, but the use of insulin glargine vs. NPH insulin is not associated with increased risk.
Keywords: Type 2 diabetes mellitus, Glargine, NPH insulin, Proliferation, Aberrant crypt foci
Introduction
Insulin resistance has been associated with an increased risk of several cancers, including colorectal cancer [1–3]. Elevated plasma levels of insulin promote the proliferation of epithelial colon cells [4, 5]. In high concentrations, insulin binds and activates not only its cognate insulin receptor but also the insulin-like growth factor receptor 1 (IGF-R1) [6]. It may also increase circulating levels of free IGF-1 by altering the levels of IGF-binding proteins (IGFBP) [7]. IGF-1 is a more potent growth factor than insulin, promoting proliferation and inhibiting apoptosis [8], and plays an important role in facilitating malignant cell survival and metastasis in colorectal cancer [9].
Observational studies in humans have demonstrated a ~30% higher risk for colorectal cancer in patients with type 2 diabetes mellitus [1]. Chronic insulin therapy further increases the risk of colorectal cancer by twofold to threefold compared to the general population [10]. Moreover, use of insulin and/or insulin secretagogues are associated with increased cancer-specific mortality [11]. Treatment of rodents with insulin increases proliferation in the healthy colonic epithelium and, after chemical initiation, may increase the number of aberrant crypt foci (ACF) [12, 13], putative precursor lesions of colorectal cancer [14].
More recently, insulin analogs have also been implicated in carcinogenesis. Insulin glargine (A21Gly,B31Arg,B32Arg human insulin) is a widely used insulin analog in which a 24-h action profile is achieved by altering the amino acid sequence of the alpha (α) and beta (β) chains of the C terminus [15]. Given the increased affinity of insulin glargine to the IGF-1 receptor [16], concerns have been raised about its safety with regard to the development of malignancies. In a safety study, no increased spontaneous development of tumors in rodents treated with nonmodified human insulin or insulin glargine was detected. Final conclusions could not be drawn, however, since the study had been performed in nondiabetic mice and rats and was therefore unable to use relevant doses of insulin or take any effects of insulin resistance into account [17]. Moreover, a significant proportion of animals died (25% and >50%, respectively).
In humans, four recently published observational studies raised further controversy [18–21]. The largest study was interpreted by the authors to demonstrate a dose-dependent increase in cancer risk associated with the use of insulin glargine [20]. This conclusion was challenged by the other three original papers, which could not replicate these data (or could only replicate them for the subset of patients with breast cancer). Only a prospective head-to-head comparison could conclusively and unambiguously elucidate whether these concerns are substantiated. Since it is not feasible to do such a study in humans, we used an established diabetic mouse model to gain insight into any potential differential effects of insulin glargine on early stages of colorectal carcinogenesis.
To date, no animal studies have been performed comparing insulin glargine treatment with a nonmodified long-acting insulin. We used the db/db (BKS.Cg-+Lepr db/+Lepr db/OlaHsd) mouse model which is characterized by hyperphagia, leading to obesity, hyperglycemia, and hyperinsulinemia [22], mimicking the conditions in patients with type 2 diabetes mellitus. We studied the effects of treatment with insulin glargine vs. neutral protamine Hagedorn (NPH) insulin in two different models of early colorectal carcinogenesis. Increased proliferation in healthy colonic mucosa, while not mitogenic per se, increases the risk of mutations, thus potentially leading to an increased risk for colon cancer. Proliferation has been shown to be acutely increased by both insulin treatment [12] and long-term [23], but not short-term, IGF-1 treatment [24]. After a mutation occurs, ACF represent the earliest step in colorectal carcinogenesis. ACF can be found in humans and in rodents, and ACF formation is therefore widely used as an established experimental model [14].
The aim of our study was to determine whether, in comparison to NPH insulin, insulin glargine increases cell proliferation in healthy colonic mucosa and whether it alters ACF formation after induction with a carcinogen in diabetic mice in vivo.
Materials and Methods
Animals and Diets
Four-week-old female db/db (BKS.Cg-+Lepr db/+Lepr db/OlaHsd) mice were purchased from Harlan Winkelmann (Borchen, Germany) and housed under controlled conditions of a 12-h light/dark cycle. Food (standard chow V1126, ssniff, Soest, Germany) and water were provided ad libitum, and body weight and food intake were measured daily. All experiments were carried out in accordance with the German Animal Protection Law (55.2-1-54-2531-75-07).
Insulin Treatment
Mice were randomized to treatment groups after stratification for body weight and blood glucose levels. Insulin glargine (Lantus®, Sanofi-Aventis, Germany) and NPH insulin (Insuman basal®, Sanofi-Aventis, Germany) were administered once or twice daily, respectively. Insulin doses were adjusted to control blood glucose levels below 150 mg/dl (Precision Xceed, Abbott Diabetes Care, Wiesbaden, Germany) as hyperglycemia increased with age. For the proliferation experiment, doses from 20 to 150 IU kg−1 day−1 were administered s.c. Because of concerns of hypoglycemia, the maximum dose of 150 IU kg−1 day−1 (from day 47 on) was not further increased. All mice were killed after 18 weeks of treatment (day 126).
In the ACF formation study, doses from 5 to 20 IU kg−1 day−1 s.c. were sufficient to maintain blood glucose control. Blood glucose levels were monitored once a week, and insulin doses were adjusted accordingly. The control groups of both experiments received volume-matched isotonic sodium chloride solution (0.9% NaCl) injections s.c. once daily. Since all experimental conditions were otherwise the same, the application of the procarcinogen 1,2-dimethylhydrazine (DMH) presumably led to lower insulin requirements. DMH is metabolized into the active metabolite, azoxymethane, in the liver, and may have influenced glucose levels.
Analysis of Colonic Epithelial Proliferation
Mice were killed after 18 weeks of treatment with insulin glargine (G), NPH insulin (NPH), or saline (NaCl) as control (G: n = 6; NPH: n = 6; NaCl: n = 4). One hour to 2 h before sacrificing the animals, 5-bromo-2′-deoxyuridine (BrdU; 30 mg/kg; Roche, Germany) was injected. The colons were removed, rinsed (Tris buffer pH 7.4), cut open along the longitudinal median axis, placed on microscopic slides (mucosal side up), and fixed flat between the microscopic slide and filter paper in 4% neutral-buffered formalin overnight. Then, colons were rolled with the mucosa facing inwards and paraffin-embedded. Proliferating cells were detected using 5-bromo-2′-deoxyuridine labeling and detection kit II (Roche Applied Science, Germany), and Ki67 staining was performed as described before [25]. For quantitative assessment, the labeling index of BrdU- and Ki76-positive nuclei was calculated as the number of BrdU-labeled cells divided by the total number of cells in the crypt and normalized to the saline-treated control group (100%). All intact, longitudinally sectioned U-shaped crypts that extended from the lamina muscularis to the lumen were evaluated. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using the Apoptag Peroxidase in situ kit (Millipore, MA, USA) using tonsil as positive control. All analyses were performed in a blinded manner.
Analysis of ACF Formation
To induce formation of ACF, mice (G: n = 15; NPH: n = 18; NaCl: n = 10) received weekly intraperitoneal injections of DMH (40 mg/kg body weight; in 0.9% NaCl, 1 mM EDTA) immediately after receiving insulin or saline injections (9–11 a.m.) for 10 weeks starting at the age of 4 weeks as previously described [26]. Colons were removed and prepared as described above. Following fixation, fixed colons were washed with 0.01 M phosphate-buffered saline (PBS; pH 7.5) and stained with 2 g/l methylene blue in 0.01 M PBS for approximately 10 min. The number and crypt multiplicity of ACF were analyzed by light microscopy at 25-fold magnification (Stemi SV 6, Zeiss, Germany) by two blinded observers. Colons were then rolled, and formation of dysplastic ACF in hematoxylin and eosin (H&E)-stained slides was independently verified by a blinded pathologist.
Measurement of Serum Parameters
Blood samples were collected at the beginning and the end of experiments by retro-orbital puncture (proliferation study: days 5 and 126; ACF formation study: days 3 and 77). Serum samples were stored at −80°C until further analysis. Concentrations of IGF-1 were determined using a standardized radioimmunoassay as previously described [27]; IGFBP-2 and IGFBP-3 were determined by enzyme-linked immunosorbent assay (ELISA; mediagnost, Reutlingen, Germany). The molar ratio of IGF-1 to IGFBP-3 was calculated by 3.7 × IGF-1 (ng/ml)/IGFBP-3 (ng/ml) [28]. Levels of glycosylated hemoglobin (HbA1c) were determined by high-performance liquid chromatography (HPLC kit for the analysis of Hemoglobin Variants in whole blood, Chromsystems, Germany). Insulin and adiponectin levels in mouse sera were determined by enzyme immunoassay (mouse Insulin Ultrasensitive and mouse Adiponectin EIA; Alpco, Salem, NH, USA). For the quantitative determination of nonesterified fatty acids (NEFA), an enzymatic colorimetric method assay was used (HR Series NEFA-HR (2), Wako Pure Chemical Industries, Osaka, Japan).
Assessment of Perigonadal Fat and Liver Histology
The weight of perigonadal white adipose tissue (perigonadal fat) and livers was measured upon autopsy. Liver morphology was evaluated by H&E staining according to the method described by Brunt [29].
Statistical Analysis
All data are presented as the mean ± SEM of the mean and were analyzed with SPSS (version 11.5, SAS Institute, Cary, NC, USA). Two-tailed t test was performed to assess differences between animals treated with insulin glargine and NPH insulin and between insulin groups and saline-treated control animals. To confirm the above data adjusting for multiple comparisons, we used analysis of variance (ANOVA) and Scheffe post hoc testing. Differences were considered statistically significant at p < 0.05.
Results
Long-Term Treatment of db/db Mice with Insulin Glargine or NPH Insulin
Body Weight, Food Intake, Blood Glucose Control
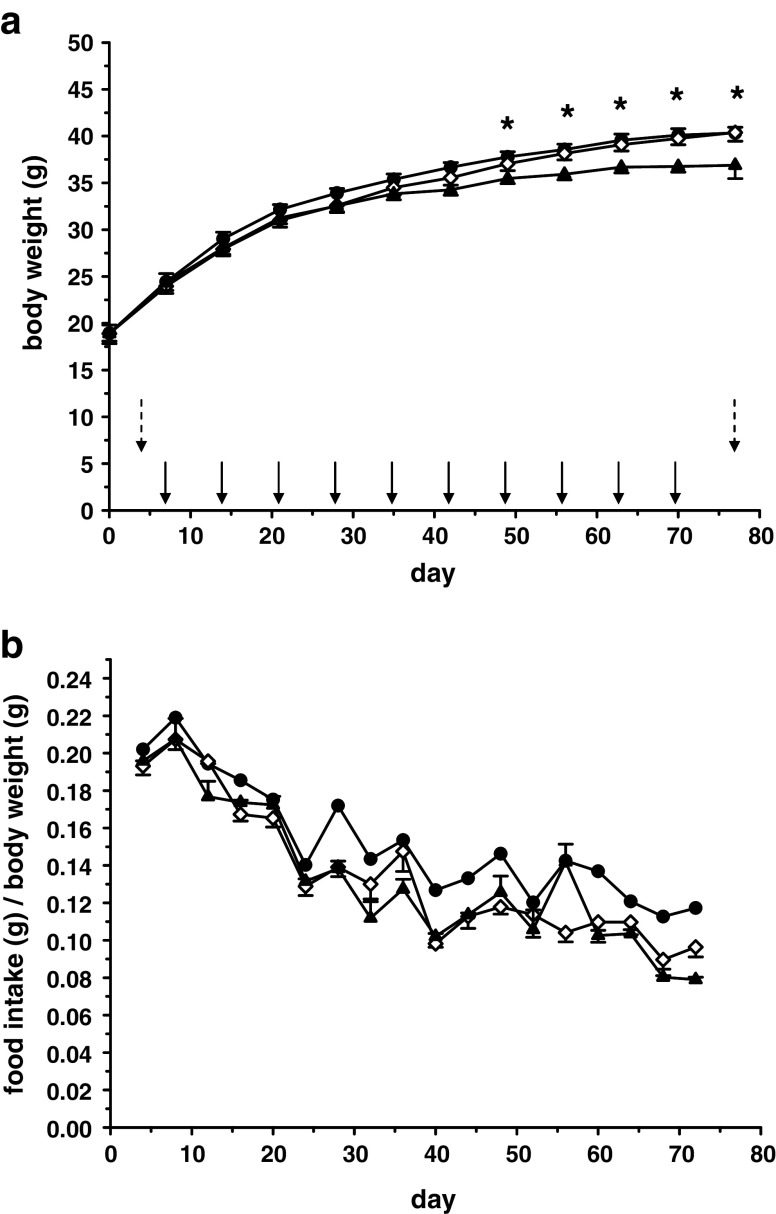
Over the course of the experiment (126 days), all animals gained weight, but animals treated with insulin (insulin glargine or NPH insulin, 150 IU/kg body weight) gained significantly more weight than saline-treated mice (Fig. 1a, Table 1). Neither body weights nor food intake differed between insulin-treated groups (G vs. NPH, Fig. 1b). Animals treated with saline gained significantly less weight despite the fact that, adjusted for body weight, saline-treated animals had a higher food intake than animals treated with either insulin. At the beginning of the experiment, all animals had HbA1c levels within the normal range. Blood glucose levels were consistently higher in mice treated with NaCl, but not different in mice treated with either NPH insulin or insulin glargine, which maintained good blood glucose control (data not shown). This resulted in twice as high HbA1c levels at 126 days in the saline-treated control group (G vs. NPH p = ns; insulin vs. NaCl p < 0.001; Table 2). Insulin levels decreased in saline-treated animals, and levels were higher in animals treated with insulin. Interestingly, insulin levels in animals treated with insulin glargine tended to be higher than those in the group treated with NPH insulin, although differences did not reach statistical significance (Table 2). IGF-1 and IGFBP-3 levels at autopsy were slightly higher in the insulin glargine group than in the NPH insulin group (p = 0.021), but the molar IGF-1/IGFBP-3 ratio remained unaltered. IGFBP-3, but not IGF-1 levels at autopsy were significantly lower in the insulin-treated groups, leading to a significantly higher molar IGF-1/IGFBP-3 ratio than in control mice. In addition, IGFBP-2 levels were significantly higher in mice treated with NPH insulin than in mice treated with insulin glargine and significantly higher in control mice than in insulin-treated mice.
Fig. 1.
Development of body weight (a) and food intake relative to body weight (b) of db/db mice under treatment with insulin glargine (n = 6; solid circles), NPH insulin (n = 6; open diamond), or saline as a control (n = 4; solid triangles) over 126 days. Data are mean ± SE. Food intake differed significantly (*p < 0.05). a Dates of the blood draw for serum analyses (days 5 and 126) are indicated by vertical arrows. Insulin glargine, NPH insulin, or saline were injected daily
Table 1.
Body, liver, and perigonadal fat weights of db/db mice treated with increasing insulin doses (glargine or NPH) or saline (control) at autopsy
| Glargine (n = 6) | NPH (n = 6) | Saline (n = 4) | ||
|---|---|---|---|---|
| Final body weight | (g) | 73.38 ± 1.80 | 71.10 ± 0.95 | 48.78 ± 5.52a |
| Perigonadal fat | Absolute (g) | 4.64 ± 0.16 | 4.19 ± 0.22 | 4.02 ± 0.33 |
| % of body weight | 6.35 ± 0.32 | 5.89 ± 0.26 | 8.36 ± 0.39a | |
| Liver | Absolute (g) | 4.74 ± 0.40 | 3.89 ± 0.21 | 3.21 ± 0.47a |
| % of body weight | 6.48 ± 0.56 | 5.48 ± 0.30 | 6.51 ± 0.41 | |
No significant differences between groups treated with NPH insulin or insulin glargine
aSignificant difference (p < 0.05) between saline-treated control group and insulin-treated animals
Table 2.
HbA1c and serum levels of insulin, IGF-1, IGFBP-3, IGFBP-2, and adiponectin and NEFA in db/db mice treated with increasing insulin doses (glargine or NPH) or saline (control)
| Parameter | Glargine (n = 6) | NPH (n = 6) | Saline (n = 4) | |||
|---|---|---|---|---|---|---|
| Day 5 | Day 126 | Day 5 | Day 126 | Day 5 | Day 126 | |
| HbA1c (%) | 2.66 ± 0.14 | 4.37 ± 0.29 | 2.75 ± 0.21 | 3.83 ± 0.19 | 2.80 ± 0.26 | 7.95 ± 0.68b |
| Insulin (ng/ml) | 24.7 ± 7.65 | 1181.2 ± 261.67 | 17.5 ± 7.01 | 453.4 ± 202.79 | 25.5 ± 5.28 | 3.1 ± 1.28b |
| IGF-1 (ng/ml) | 245.83 ± 28.20 | 211.00 ± 18.93a | 191.00 ± 14.33 | 147.17 ± 13.73a | 213.50 ± 29.45 | 188.75 ± 40.86 |
| IGFBP-3 (ng/ml) | 434.76 ± 64.33 | 265.61 ± 21.00a | 334.96 ± 26.52 | 185.66 ± 22.38a | 358.44 ± 49.60 | 331.77 ± 66.96b |
| Molar ratio IGF-1/IGFBP-3 | 2.20 ± 0.32 | 2.97 ± 0.23 | 2.13 ± 0.13 | 3.02 ± 0.22 | 2.21 ± 0.06 | 2.06 ± 0.18b |
| IGFBP-2 (ng/ml) | ND | 34.77 ± 3.94a | ND | 45.74 ± 1.76a | ND | 64.33 ± 15.37b |
| Adiponectin (μg/ml) | 55.02 ± 8.46 | 40.25 ± 5.61 | 50.87 ± 3.70 | 42.09 ± 5.07 | 30.41 ± 7.38b | 47.24 ± 17.44 |
| NEFA (mmol/l) | ND | 2.70 ± 0.18 | ND | 2.59 ± 0.22 | ND | 2.87 ± 0.28 |
aSignificant differences (p < 0.05) between groups treated with NPH insulin or insulin glargine
bSignificant difference (p < 0.05) between saline-treated control group and insulin-treated animals
Liver Histology, Perigonadal Fat, Adiponectin, and NEFA
In all treatment groups, mice showed a high degree of steatohepatitis involving over 66% of hepatocytes with higher absolute liver weight in insulin-treated animals. Upon autopsy, no difference in perigonadal fat was found between insulin-glargine- and NPH-insulin-treated groups. Relative to body weight, insulin groups had a significantly lower percentage of perigonadal fat (p < 0.001) compared to the saline-treated control group (Table 1). Adiponectin levels in the insulin treatment groups decreased over time. In saline-treated animals, adiponectin levels were significantly lower at day 5 but increased over time, resulting in adiponectin levels without differences between insulin-treated and control groups at autopsy. NEFA levels at autopsy did not differ between groups (Table 2).
No Differences in Proliferation or Apoptosis in Colonic Epithelium After Long-Term Treatment with Either Insulin Glargine or NPH Insulin
Colonic epithelial proliferation assessed using Ki67 staining (Fig. 2a) was not different between insulin-glargine- (G) and NPH-insulin-treated (NPH) groups but was significantly higher than in saline-treated controls (G 136.56 ± 3.75; NPH 135.93 ± 2.63; NaCl 100 ± 4.16; G vs. NPH: p = 0.89; insulin vs. NaCl: p < 0.001; Fig. 2c). Results were confirmed by BrdU staining (Fig. 2b; G: 114.8 ± 2.87; NPH: 116.8 ± 3.27; NaCl: 100 ± 4.84; G vs. NPH: p = 0.64; insulin vs. NaCl: p < 0.05).
Fig. 2.
Colonic epithelial proliferation after long-term treatment (18 weeks) with insulin glargine or NPH insulin. Immunohistochemical staining of Ki67 (a) and BrdU (b) to evaluate proliferation indices in intact colonic crypts, extending from the lamina muscularis to the lumen. c The Ki67 proliferation index is shown for insulin glargine (n = 6; 165 crypts) and NPH insulin (n = 6; 199 crypts) treatment groups relative to the NaCl control group (n = 4; 88 crypts). No differences in colonic epithelial proliferation were found between insulin-glargine- and NPH-treated groups (p = ns). In animals treated with insulin glargine or NPH insulin, colonic epithelial proliferation was increased by approximately 35% compared to a saline-treated control group (*p < 0.01). Data are mean ± SE
Although our primary aim was to compare animals treated with insulin glargine vs. NPH insulin, we not only analyzed the data by t tests but we also confirmed the above data, adjusting for multiple comparisons using ANOVA. We found no difference in Ki67 staining (ANOVA <0.001) between G and NPH (p = 0.99), but we detected highly significant differences between either G or NPH and NaCl, respectively (both p < 0.001). Likewise, the ANOVA (p = 0.004) revealed no differences in BrdU staining between G and NPH (p = 0.909) but significant differences between G and NaCl (p = 0.017) and NPH and NaCl (p = 0.008). TUNEL staining revealed hardly any apoptotic cells without differences between treatment groups.
Treatment of db/db Mice with Either Insulin Glargine or NPH Insulin in a Model of ACF Formation
Body Weight, Food Intake, Blood Glucose Control
The experimental procedures are outlined within Fig. 3a. Animals treated with NPH insulin or insulin glargine (20 IU/kg body weight) did not differ significantly in terms of weight gain, body weight, or food intake, but insulin-treated animals had a slightly higher final body weight compared to saline-treated controls (Fig. 3a, b, Table 3). All animals in this experiment had lower blood glucose levels than animals in the first experiment, resulting in only mildly increased HbA1c level at the end of the experiment (Table 4). Insulin levels in saline-treated animals remained low over time, whereas higher levels were detected in animals treated with insulin. No statistically significant differences in insulin, IGF-1, IGFBP-3, or IGFBP-2 levels were found between animals treated with NPH insulin and insulin glargine or between animals treated with insulin and saline (Table 4).
Fig. 3.
Development of body weight (a) and food intake relative to body weight (b) of db/db mice under treatment with insulin glargine (n = 15; solid circles), NPH insulin (n = 18; open diamond), or saline as a control (n = 10; solid triangles) during the ACF formation study. Body weight development differed between animals treated with insulin (insulin glargine or NPH insulin) and saline-treated controls at the end of the experiment (*p < 0.05), but no differences in food intake between groups (G, NPH, NaCl) were observed. Data are mean ± SE. a The experimental outline is illustrated by vertical arrows: Upper row of dotted arrows indicates blood draws for serum analyses (days 3 and 77), the lower row of solid arrows represents weekly i.p. DMH administrations. Insulin glargine, NPH insulin, or saline were injected daily
Table 3.
Body, liver, and perigonadal fat weights of db/db mice treated with DMH and increasing insulin doses (glargine or NPH) or saline (control) at autopsy (day 77)
| Glargine (n = 15) | NPH (n = 18) | Saline (n = 10) | ||
|---|---|---|---|---|
| Final body weight | (g) | 39.84 ± 0.62 | 40.93 ± 0.78 | 37.95 ± 0.83a |
| Perigonadal fat | Absolute (g) | 3.01 ± 0.09 | 3.15 ± 0.08 | 2.95 ± 0.07 |
| % of body weight | 7.56 ± 0.16 | 7.71 ± 0.16 | 7.81 ± 0.19 | |
| Liver | Absolute (g) | 1.30 ± 0.05 | 1.31 ± 0.05 | 1.16 ± 0.04a |
| % of body weight | 3.29 ± 0.09 | 3.19 ± 0.10 | 3.09 ± 0.15 | |
No significant differences between groups treated with NPH insulin or insulin glargine
aSignificant difference (p < 0.05) between saline-treated control group and insulin-treated animals
Table 4.
Percentage of HbA1c and serum levels of insulin, IGF-1, IGFBP-3, IGFBP-2, adiponectin, and NEFA found in db/db mice treated with DMH and increasing insulin doses (glargine or NPH) or saline (control)
| Parameter | Glargine (n = 15) | NPH (n = 18) | Saline (n = 10) | |||
|---|---|---|---|---|---|---|
| Day 3 | Day 77 | Day 3 | Day 77 | Day 3 | Day 77 | |
| HbA1c (%) | 2.08 ± 0.11 | 2.86 ± 0.16 | 2.14 ± 0.08 | 2.69 ± 0.13 | 2.48 ± 0.17a | 3.34 ± 0.26a |
| Insulin (ng/ml) | 6.87 ± 3.17 | 403.28 ± 121.20 | 6.39 ± 2.97 | 575.46 ± 138.35 | 4.39 ± 1.43 | 4.38 ± 1,76a |
| IGF-1 (ng/ml) | 122.73 ± 5.84 | 57.89 ± 3.83 | 119.78 ± 7.38 | 60.33 ± 4.51 | 128.8 ± 7.62 | 64.50 ± 8.48 |
| IGFBP-3 (ng/ml) | 227.53 ± 15.09 | 142.54 ± 8.70 | 229.35 ± 20.04 | 156.15 ± 4.24 | 227.41 ± 12.08 | 174.67 ± 3.16 |
| Molar ratio IGF-1/IGFBP-3 | 2.12 ± 0.18 | 1.58 ± 0.13 | 2.05 ± 0.09 | 1.59 ± 0.17 | 2.06 ± 0.09 | 1.46 ± 0.21 |
| IGFBP-2 (ng/ml) | ND | 51.66 ± 3.61 | ND | 53.77 ± 4.10 | ND | 52.14 ± 4.30 |
| Adiponectin (μg/ml) | 74.41 ± 2.32 | 75.63 ± 6.46 | 72.53 ± 2.24 | 89.38 ± 7.97 | 81.25 ± 2.28a | 122.99 ± 21.06a |
| NEFA (mmol/l) | ND | 0.97 ± 0.08 | ND | 0.82 ± 0.06 | ND | 0.86 ± 0.06 |
No significant differences between groups treated with NPH insulin or insulin glargine
aSignificant difference (p < 0.05) between saline-treated control group and insulin-treated animals
Liver Histology, Perigonadal Fat, Adiponectin, and NEFA
Livers of all mice showed signs of both chronic and comparable (moderate) grades of mixed (acute–chronic) liver inflammation, with slightly higher absolute liver weight in insulin-treated groups. At the end of the experiment, no absolute or relative (adjusting for body weight) differences in perigonadal fat were found between insulin-glargine- and NPH-insulin-treated groups (Table 3) or between insulin- and saline-treated animals. Adiponectin levels between insulin glargine and NPH insulin groups were not different and remained largely unchanged over the course of the experiment but increased to significantly higher levels in the group of saline-treated mice. NEFA levels did not differ between groups (Table 4).
No Differences in ACF Formation After Treatment with Either Insulin Glargine or NPH Insulin
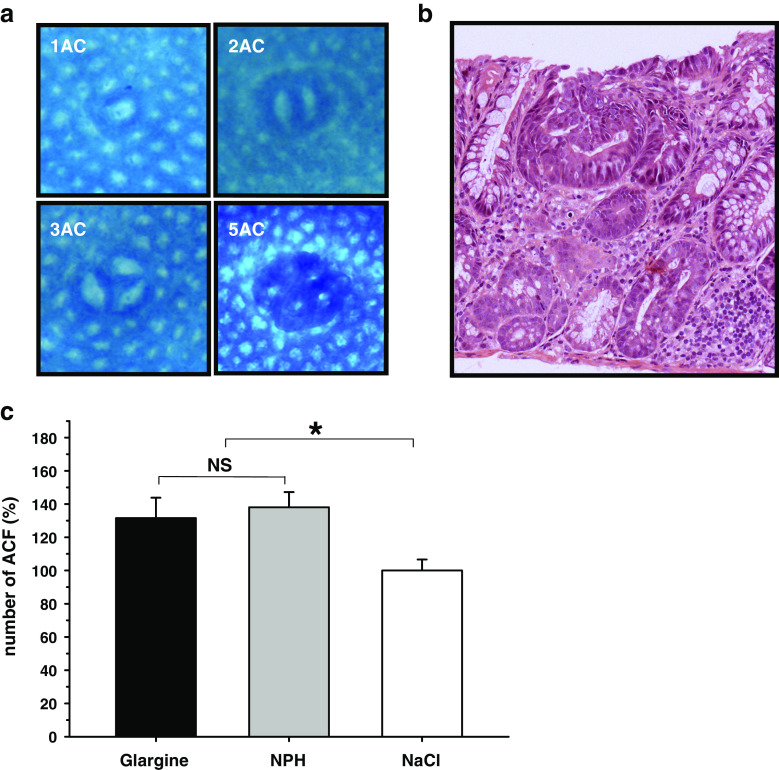
Methylene-blue-stained aberrant crypt foci and dysplastic aberrant crypts (Fig. 4a, b) were found in all animals following treatment with DMH. There was no difference in the number or multiplicity of ACF between insulin glargine- and NPH insulin-treated mice, but the number of ACF was significantly higher in insulin-treated than in control animals (G, 132 ± 12; NPH, 138 ± 9; NaCl, 100 ± 7; G vs. NPH, p = 0.67; insulin vs. NaCl, p < 0.05; Fig. 4c). Absolute numbers of ACFs and relative distribution of AC multiplicity in treatment groups are shown in Fig. 5a, b, respectively. These results were confirmed by histological quantification of ACF, which showed no difference in the occurrence of dysplastic aberrant crypt foci between insulin-glargine- and NPH-insulin-treated mice but significantly more dysplastic ACF in insulin-treated groups compared to the control group (G, 144 ± 15; NPH, 164 ± 16; NaCl, 100 ± 20; G vs. NPH, p = 0.40; insulin vs. NaCl, p < 0.05).
Fig. 4.
ACF development in diabetic mice (db/db) treated with insulin glargine or NPH insulin. a Methylene-blue-stained colonic mucosa showing ACF with different multiplicity. b Dysplastic aberrant crypt foci identified by H&E staining in the colon epithelium of DMH-treated db/db mice. c Percentage of ACF formation in insulin-glargine- or NPH-insulin-treated db/db mice relative to NaCl control group. No differences in the number of ACF were found between insulin-glargine- and NPH-insulin-treated groups of mice (p = ns). The number of ACF was increased by 32–38% in insulin-treated groups (for insulin glargine and NPH insulin, respectively) compared to the saline-treated control group (*p < 0.05). Data are mean ± SE
Fig. 5.
Absolute numbers of ACF and AC multiplicity. a Absolute numbers of ACF with one to five or more aberrant crypts per focus in groups treated with insulin glargine (black bars), NPH insulin (gray bars), and saline (white bars). Saline-treated groups have a lower number of absolute ACF per mouse, but there is no difference in absolute numbers of ACF in mice treated with insulin glargine or NPH insulin. b Relative distribution of crypt multiplicity per treatment group (G, NPH, and NaCl): Black 1 AC, light gray 2 AC, medium gray 3 AC, white 4 AC, dark gray 5 or more AC. The same distribution of crypt multiplicity was found in mice treated with insulin glargine or NPH insulin, but mice treated with NaCl had less advanced ACF with more single aberrant crypts
When evaluating the number of ACF using ANOVA (p = 0.052), the above was essentially confirmed. G vs. NPH groups again did not differ (p = 0.89), and there was a trend for more ACF with NPH vs. NaCl (p = 0.059). We also confirmed the histological analysis of dysplastic crypt foci using ANOVA (p = 0.049). Again, there were more ACF in the NPH insulin treatment group compared to the NaCl control group (p = 0.05) whereas differences between insulin-glargine- and NaCl-treated groups did not reach statistical significance (p = 0.241).
Discussion
Utilizing the diabetic db/db mouse model, we showed that long-term treatment with insulin led to an increase in colonic epithelial proliferation without differences between the two long-acting insulins, i.e., insulin glargine and nonmodified, long-acting human insulin (NPH insulin). Likewise, we found no evidence for increased formation or multiplicity of ACF, putative precursors of colorectal cancer, in animals treated with insulin glargine when compared with NPH insulin. Similar to the increase in colonic epithelial proliferation, insulin treatment (with either insulin glargine or NPH insulin) led to increased formation of ACF when compared to the saline-treated control group.
This is the first study to demonstrate in a head-to-head comparison that insulin glargine does not increase risk when compared to a nonmodified long-acting human insulin in models of early stages of colorectal carcinogenesis in vivo, a malignancy that has been linked with insulin resistance [1, 2] and in which the insulin/IGF-1 pathway plays a critical role [3, 30]. No difference between insulin glargine and NPH insulin was demonstrated using two independent models and two different readout methods which yielded comparable results in each experiment. In addition, we found no evidence for lymphadenopathy or spontaneous formation of solid tumors in any treatment group after 18 weeks of insulin treatment or placebo, but we did not perform a bone marrow examination.
Our finding that both insulin treatments increased proliferation and ACF formation in comparison to saline treatment alone is in concordance with previous findings in nondiabetic rats, showing that acute exogenous insulin administration increases proliferation [12] and ACF multiplicity [31]. It has been argued that optical evaluation of ACF represents a summary of both hyperplastic and dysplastic ACF. With hyperplastic foci being prone to regression, we also performed a histologic evaluation of dysplastic lesions only. Although even dysplastic lesions may sometimes regress, they reflect a more accurate measure of tumor risk [14]. Evaluation of dysplastic lesions confirmed optical findings. Similar to a previous study [32], we observed an increase in ACF formation in groups treated with insulin vs. saline-treated controls, without differences between the two insulin treatment groups.
In vitro studies using an osteosarcoma cell line with high IGF-1 receptor expression have previously suggested that insulin glargine may increase mitogenic potency [16]. Slightly increased proliferation with a trend towards decreased apoptosis was reported in a colon carcinoma cell line [33], and similar findings were reported in breast cancer cell lines [34]. We did not detect any differences between insulin-glargine- and NPH-insulin-treated mice, and the differences between those in vitro and our in vivo observations may be due to several factors.
One potential limitation of our studies is that mice were treated with human insulin (insulin glargine or NPH insulin). The insulin/IGF-1 system represents a highly conserved system with 94.2% homology between the mouse and the human insulin receptor. Consequently, both insulin formulations controlled blood glucose comparably well as documented by HbA1c levels. The differences in IGF-1 receptor binding affinity that have been reported for insulin glargine vs. NPH insulin can be expected to be similar in the mouse as in humans. The human IGF-1 receptor shares an overall homology with the mouse IGF-1 receptor of 95.7%, and similar to humans, both the mouse IGF-1 receptor and the insulin receptor share 84% homology in the substrate-binding domain (http://ca.expasy.org/tools/blast/). Therefore, the mouse model is highly suited for interventional studies addressing this question.
In our study, we deliberately chose long time periods of insulin treatment in order to account for potential changes of IGF1-R and IR expression level with chronic treatment. However, potential changes could not be quantified by immunohistochemistry because of IGF1-R and IR cross-reactivity. Thus, it cannot be determined whether insulin glargine and NPH insulin have the same pro-proliferative and ACF formation stimulating activity per se or whether stronger (IGF-1 mediated) stimulation by insulin glargine may have led to a compensatory downregulation of IGF-1 receptors. Similarly, because of the manipulation of the tissue following autopsy, we could not perform intracellular signaling studies in the target cells of the mucosa in our study.
It can be argued that the insulin measurements were limited by the fact that we were measuring a mixture of endogenous and exogenous (human) insulin (in NPH insulin- or insulin glargine- treated mice), so that the individual contribution of exogenous and endogenous insulin and c-peptide, respectively, cannot be determined. In our study, however, exogenous insulin accounted for the vast majority of insulin levels: in both experiments and both insulin-treated groups, insulin levels were ≥100-fold higher than the endogenous insulin (and by extension, c-peptide) levels measured in the control group, so effects must mainly have been mediated through exogenously administered insulin.
The insulin ELISA used in this study has an approximately threefold higher affinity for NPH insulin than for regular mouse insulin, whereas its sensitivity to glargine is only slightly altered (~109%; data not shown). Following s.c. injection, insulin glargine is metabolized into the metabolites M1 (A21-Gly-insulin) and M2 (A21-Gly-des-30B-Thr-insulin) [35]. Both are bioactive products but lack the diarginine residues at B31 and B32 and hence are less mitogenic. All three forms (glargine, M1, and M2) can be found in the circulation, and their relative affinity for the ELISA used herein remains poorly studied. While it can be assumed that a substantial amount of glargine will reach the colon in its unaltered form, substantial interindividual variation in metabolite (M1 and M2) formation has been described in humans [36]. This may explain why we found (nonsignificantly) higher levels of insulin glargine in the proliferation study with high interindividual variation. We conclude that treatment with insulin glargine in doses resulting in similar blood glucose control reflected by comparable blood glucose levels (data not shown) and HbA1c as with NPH insulin treatment does not lead to increased colonic epithelial proliferation.
We did not observe a feedback mechanism regulating IGF-1 in mice treated with insulin glargine, which is in line with a recent study in humans, in which insulin glargine treatment did not lead to reduced IGF-1 levels [37]. In the proliferation study, insulin glargine- and NPH insulin-treated mice had a significantly higher molar ratio of IGF-1/IGFBP-3, a surrogate marker of free IGF-1. The same pattern was observed in the ACF formation study, in which lower doses of insulin were given, but differences did not reach statistical significance. Thus, increased levels of free IGF-1 may have contributed to the increased proliferation seen in both insulin-treated groups and, potentially, to the increased ACF formation. Moreover, we cannot exclude that higher levels of IGFBP-2, a molecule that has been implicated in reducing proliferation in vitro and ACF formation in vivo [38, 39], may have contributed to the lower proliferation in saline-treated control mice.
Proliferation and ACF have not been described to be angiogenesis-dependent. Thus, angiogenesis was not a primary outcome of the study. However, angiogenesis plays an important role in later stages, and insulin and IGF-1 have been shown to upregulate vascular endothelial growth factor (VEGF) in endometrial adenocarcinomas cells [40, 41]. Using a semiquantitative mouse angiogenesis array to identify VEGF and other angiogenic proteins, we found that, in both studies, expression levels of VEGF were below the detection level (data not shown). No differences were detected in the ACF formation study, and immunohistochemical staining for CD31 revealed no differences between groups in accordance with the above. In the proliferation study, the proangiogenic factor PDGF-AB/BB was upregulated by approximately fourfold, and the antiangiogenic factor Timp-1 was downregulated by >20-fold in both insulin-treated groups of mice. Although proliferation in the nontransformed epithelium is angiogenesis- independent, we cannot exclude that these factors, possibly through angiogenesis-independent effects, may have contributed to the higher proliferation in the insulin-treated groups.
A recently published observational study in humans suggested an increased risk for cancer with insulin glargine treatment [20], but these findings have subsequently been questioned by several other studies [18, 19, 21]. Moreover, the observed increase in cancer risk was associated with and possibly due to higher insulin doses [42]. Our study, by providing direct evidence from two experimental animal models, bridges a gap between in vitro observations and observational studies in humans. Even though our data cannot be directly extrapolated to humans, they do not support any evidence for an increased risk associated with the use of insulin glargine in early models of colorectal cancer in vivo. Instead, high physiologic doses of insulin in the presence of insulin resistance were associated with increased proliferation and ACF formation, irrespective of the type of insulin (insulin glargine or NPH insulin) used. This study contributes towards increasing scientists’ and physicians’ awareness regarding the potential mitogenic properties of insulin and consequently, increased cancer risk. Although insulin treatment is indispensable in the treatment of type 2 diabetes mellitus, the risk increase demonstrated in early models of colorectal carcinogenesis should prompt more studies in mice and humans. Further studies are needed to confirm and extend our findings in long-term models of adenoma formation and in other cancer models.
Acknowledgements
We thank P. Renner and T. Mittmann for expert animal care, A. Schäfer, A. Sendelhofert, and A. Heier for H&E and immunohistochemical stainings, and E. Egeler who was supported by the Hans-Fischer-Gesellschaft Munich, Germany, for analyzing HbA1c levels.
Declarations of interest
There are no conflicts of interest to report.
Funding
This study was supported by Studiengang Molekulare und Systembiologische Medizin to J.M.N. and F.T.K. and a project grant of the Stiftungsfonds to J.M.N. (both Medical Faculty, LMU Munich, Germany) as well as a project grant of the German Diabetes Society (DDG) to J.M.N and by AICR grant MG 08A001 as well as a discretionary grant from BIDMC to C.S.M. David Horst was supported by a fellowship from Deutsche Forschungsgemeinschaft (DFG).
References
- 1.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 2.Berster JM, Goke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch Physiol Biochem. 2008;114:84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- 3.Manousos O, Souglakos J, Bosetti C, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999;83:15–17. doi: 10.1002/(SICI)1097-0215(19990924)83:1<15::AID-IJC4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Keown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- 5.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Barrett EJ, Wang H, et al. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- 7.Strasser-Vogel B, Blum WF, Past R, et al. Insulin-like growth factor (IGF)-I and -II and IGF-binding proteins-1, -2, and -3 in children and adolescents with diabetes mellitus: correlation with metabolic control and height attainment. J Clin Endocrinol Metab. 1995;80:1207–1213. doi: 10.1210/jc.80.4.1207. [DOI] [PubMed] [Google Scholar]
- 8.Michell NP, Dent S, Langman MJ, et al. Insulin-like growth factor binding proteins as mediators of IGF-I effects on colon cancer cell proliferation. Growth Factors. 1997;14:269–277. doi: 10.3109/08977199709021525. [DOI] [PubMed] [Google Scholar]
- 9.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 10.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 12.Tran TT, Naigamwalla D, Oprescu AI, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147:1830–1837. doi: 10.1210/en.2005-1012. [DOI] [PubMed] [Google Scholar]
- 13.Hirose Y, Hata K, Kuno T, et al. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis. 2004;25:821–825. doi: 10.1093/carcin/bgh059. [DOI] [PubMed] [Google Scholar]
- 14.Roncucci L, Pedroni M, Vaccina F, et al. Aberrant crypt foci in colorectal carcinogenesis. Cell and crypt dynamics. Cell Prolif. 2000;33:1–18. doi: 10.1046/j.1365-2184.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerich JE. Insulin glargine: long-acting basal insulin analog for improved metabolic control. Curr Med Res Opin. 2004;20:31–37. doi: 10.1185/030079903125002711. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzhals P, Schaffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- 17.Stammberger I, Bube A, Durchfeld-Meyer B, et al. Evaluation of the carcinogenic potential of insulin glargine (LANTUS) in rats and mice. Int J Toxicol. 2002;21:171–179. doi: 10.1080/10915810290096306. [DOI] [PubMed] [Google Scholar]
- 18.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 20.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;53:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonasson JM, Ljung R, Talback M, et al. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 22.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 23.Steeb CB, Trahair JF, Tomas FM, et al. Prolonged administration of IGF peptides enhances growth of gastrointestinal tissues in normal rats. Am J Physiol. 1994;266:G1090–G1098. doi: 10.1152/ajpgi.1994.266.6.G1090. [DOI] [PubMed] [Google Scholar]
- 24.Steeb CB, Trahair JF, Read LC. Administration of insulin-like growth factor-I (IGF-I) peptides for three days stimulates proliferation of the small intestinal epithelium in rats. Gut. 1995;37:630–638. doi: 10.1136/gut.37.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deindl E, Zaruba MM, Brunner S, et al. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:956–958. doi: 10.1096/fj.05-4763fje. [DOI] [PubMed] [Google Scholar]
- 26.Diehl D, Oesterle D, Elmlinger MW, et al. IGF-II transgenic mice display increased aberrant colon crypt multiplicity and tumor volume after 1,2-dimethylhydrazine treatment. J Carcinog. 2006;5:24. doi: 10.1186/1477-3163-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf E, Kramer R, Blum WF, et al. Consequences of postnatally elevated insulin-like growth factor-II in transgenic mice: endocrine changes and effects on body and organ growth. Endocrinology. 1994;135:1877–1886. doi: 10.1210/en.135.5.1877. [DOI] [PubMed] [Google Scholar]
- 28.Ju JH, Nolan B, Cheh M, et al. Voluntary exercise inhibits intestinal tumorigenesis in Apc(Min/+) mice and azoxymethane/dextran sulfate sodium-treated mice. Bmc Cancer. 2008;8:316. doi: 10.1186/1471-2407-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Yakar S, Zhao L, et al. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 31.Koohestani N, Tran TT, Lee W, et al. Insulin resistance and promotion of aberrant crypt foci in the colons of rats on a high-fat diet. Nutr Cancer. 1997;29:69–76. doi: 10.1080/01635589709514604. [DOI] [PubMed] [Google Scholar]
- 32.Tran TT, Gupta N, Goh T, et al. Direct measure of insulin sensitivity with the hyperinsulinemic–euglycemic clamp and surrogate measures of insulin sensitivity with the oral glucose tolerance test: correlations with aberrant crypt foci promotion in rats. Cancer Epidemiol Biomarkers Prev. 2003;12:47–56. [PubMed] [Google Scholar]
- 33.Weinstein D, Simon M, Yehezkel E, et al. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev. 2009;25:41–49. doi: 10.1002/dmrr.912. [DOI] [PubMed] [Google Scholar]
- 34.Shukla A, Grisouard J, Ehemann V, et al. Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer. 2009;16:429–441. doi: 10.1677/ERC-08-0240. [DOI] [PubMed] [Google Scholar]
- 35.Kuerzel GU, Shukla U, Scholtz HE, et al. Biotransformation of insulin glargine after subcutaneous injection in healthy subjects. Curr Med Res Opin. 2003;19:34–40. doi: 10.1185/030079902125001416. [DOI] [PubMed] [Google Scholar]
- 36.Agin A, Jeandidier N, Gasser F, et al. Glargine blood biotransformation: in vitro appraisal with human insulin immunoassay. Diabetes Metab. 2007;33:205–212. doi: 10.1016/j.diabet.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Slawik M, Schories M, Busse Grawitz A, et al. Treatment with insulin glargine does not suppress serum IGF-1. Diabet Med. 2006;23:814–817. doi: 10.1111/j.1464-5491.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- 38.Diehl D, Hessel E, Oesterle D, et al. IGFBP-2 overexpression reduces the appearance of dysplastic aberrant crypt foci and inhibits growth of adenomas in chemically induced colorectal carcinogenesis. Int J Cancer. 2009;124:2220–2225. doi: 10.1002/ijc.24193. [DOI] [PubMed] [Google Scholar]
- 39.Hoflich A, Lahm H, Blum W, et al. Insulin-like growth factor-binding protein-2 inhibits proliferation of human embryonic kidney fibroblasts and of ICF-responsive colon carcinoma cell lines. Febs Letters. 1998;434:329–334. doi: 10.1016/S0014-5793(98)01011-4. [DOI] [PubMed] [Google Scholar]
- 40.Bermont L, Lamielle F, Fauconnet S, et al. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-1 in endometrial adenocarcinoma cells. Int J Cancer. 2000;85:117–123. doi: 10.1002/(SICI)1097-0215(20000101)85:1<117::AID-IJC21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Bermont L, Lamielle F, Lorchel F, et al. Insulin up-regulates vascular endothelial growth factor and stabilizes its messengers in endometrial adenocarcinoma cells. J Clin Endocrinol Metab. 2001;86:363–368. doi: 10.1210/jc.86.1.363. [DOI] [PubMed] [Google Scholar]
- 42.Nagel JM, Mansmann U, Wegscheider K, et al. Insulin resistance and increased risk for malignant neoplasms: confounding of the data on insulin glargine. Diabetologia. 2010;53:206–208. doi: 10.1007/s00125-009-1535-0. [DOI] [PubMed] [Google Scholar]