Abstract
Recent epidemiological studies suggest that treatment with insulin may promote cancer growth. The present systematic review and meta-analysis of published observational studies was conducted to assess the risk of cancer during treatment with insulin. A search of online database through January 2011 was performed and examined the reference lists of pertinent articles, limited to observational studies in humans. Pooled relative risks (RRs) and 95 % confidence intervals (CIs) were calculated with a random-effects model. Fifteen studies (five case–control and ten cohort studies) were included, with 562,043 participants and 14,085 cases of cancer. Insulin treatment was associated with an increased risk of overall cancer [summary RR (95 % CI) = 1.39 (1.14, 1.70)]. Summary RR (95 % CI) for case–control studies was 1.83 (0.99, 3.38), whereas RR for cohort studies was 1.28 (1.03, 1.59). These results were consistent between studies conducted in the USA and in Europe. For studies that included combined type 1 and 2 diabetes, the summary estimate was stronger than studies including only type 2 diabetes mellitus. The association between insulin treatment and cancer was stronger for pancreatic cancer [summary RR (95 % CI) = 4.78 (3.12, 7.32)] than for colorectal cancer [1.50 (1.08, 2.08)]. Insulin treatment was not associated with breast, prostate, and hepatocelluar cancer, and their effect estimates were not statistically significant. Our findings support an association between insulin use and increased risk of overall, pancreatic, and colorectal cancer.
Keywords: Pancreatic Cancer, Insulin Therapy, Insulin Treatment, Insulin Regimen, North American Population
Introduction
Diabetes mellitus (DM) and cancer are major causes of morbidity and premature mortality worldwide. A substantial proportion of patients with diabetes will ultimately be prescribed insulin, and the reports that insulin therapy may be associated with cancer are of significant concern [1, 2]. Although several observational studies have investigated the association between insulin-treated DM and risk of cancer, the role of insulin as a risk factor for cancer remains unknown. There are conflicting reports about risk of cancer in insulin-treated patients [1, 3–17]; in some studies, risk of cancer was reduced [10]; in other studies, it was increased [1, 3–6, 8, 9, 11, 14, 16] or unchanged [7, 12, 13, 15] or an association was observed only in men or women [18–20]. The interpretation of these findings, however, has been hampered by the low frequency of occurrence of both conditions in the same individual, which results in the lack of statistical power to adequately analyze this association in many studies. Whether insulin treatment increases risk of cancer is an important question because almost all patients with DM will eventually require insulin treatment [21].
We report here on a systematic review and meta-analysis of case–control and cohort studies to summarize the epidemiologic evidence on the risk of cancer associated with insulin-treated DM and to identify possible sources of heterogeneity between studies. We also aimed to evaluate whether the association varied by study design, type of DM, and site of cancer.
Methods
The systematic review was undertaken following MOOSE guidelines for reviews of observational studies [22].
Search Strategy
A search of the online databases (PUBMED, ISI, and EMBASE) through January 2011 was performed using key words “cancer,” “carcinoma” or “malignancy” combined with “diabetes mellitus,” “diabetes,” “glucose,” or “insulin therapy”, limited to observational studies in humans. We also reviewed reference lists of the identified publications for additional pertinent studies. No language restrictions were imposed. Titles and available abstracts were scanned for relevance, identifying papers requiring further consideration.
Eligibility Criteria
To be included in the meta-analysis, a published study had to meet the following criteria: (1) original article, (2) cohort or case–control study, (3) adult human population, and (4) insulin or one of insulin products as the main independent variable. The 15 epidemiological studies [1, 3, 6, 7, 9–11, 13–17, 23–25] included in this meta-analysis were five case–control and ten cohort studies on the association between insulin treatment and the incidence and mortality of all cancers, breast, colorectal, hepatocellular, or pancreatic cancer. Studies that did not provide data that allowed calculation of standard errors for effect estimates and if the estimates were not adjusted for age and gender were excluded. When there were multiple publications from the same population or cohort, only data from the most recent report were included. We excluded one study [4] because of overlapping publications and two studies [8, 26] that reported only crude data that were not adjusted for age and gender and one study that reported insulin therapy and colorectal adenoma [5].
Data Extraction
The following characteristics of included studied were recorded: publication reference (the first author’s last name, year of publication, and country of population studied), study design, number of exposed and unexposed subjects, control source (in case–control studies), follow-up period (for cohort studies), age, gender, type of DM (type 2 or combined type 1 and type 2), type of cancer investigated (total, breast, pancreas, hepatocellular, and colorectal), risk estimates with their corresponding confidence intervals, and variables controlled for by matching or in the multivariable model. For each study, we extracted the risk estimates that reflected the greatest degree of control for potential confounders. From each study, the information on study design, participant characteristics, site of cancers, adjustment for potential confounders, and estimates of associations was independently extracted by two investigators (MJ and MD), and in case of divergent evaluation, discrepancies were resolved by discussion.
Statistical Analysis
Three measures of association were used for the meta-analysis: odds ratio (case–control studies), incidence rate ratio (cohort studies), and standardized incidence or mortality ratio (cohort studies with an external comparison group). For simplicity, we refer to relative risk (RR) for all three types of measures of association. Because the frequency at which cancers occur is relatively low, the odds ratio in case–control studies and rate ratios in cohort studies yield similar estimates of RR [27].
We used the logarithm of the RR with its standard error for the meta-analysis. Summary RR estimates with their corresponding 95 % CIs were derived by the method of DerSimonian and Laird [28] using both fixed and random effects models. Eventually, the summary RR estimate from random effects models were used, which incorporates between-study variability, because the test for heterogeneity were statistically significant in all analysis. Statistical heterogeneity between studies was evaluated with Cochran’s Q test and quantified by I 2 statistic [29]. To assess sources of heterogeneity, we conducted a meta-regression and subgroup analyses. Publication bias was assessed by visual inspection of funnel plot [30]. In these funnel plots, the RRs were displayed against the inverse of the square of the standard error (a measure of the precision of the studies). Formal statistical assessment of funnel plot asymmetry was done with Egger’s regression asymmetry test and adjusted rank correlation test [31]. In addition, Begg’s adjusted rank correlation test and the trim-and-fill method were used [31, 32]. Sensitivity analysis was used to explore the extent to which inferences might depend on a particular study or group of studies. Statistical analyses were carried out with Stata, version 10.0 (Stata Corp, College Station, TX, USA). P < 0.05 were considered statistically significant. All statistical tests were two-sided.
Results
Study Characteristics
Fifteen independent studies met the predefined inclusion criteria. Of these 15 studies, five were case–control studies [3, 7, 9, 23, 24] (Table 1), seven were cohort studies that used incidence rate ratios or hazard ratio as the measure of RR [1, 6, 10, 13–15, 25], and three were cohort study that used standardized mortality ratio as the measure of RR [11, 16, 17] (Table 2). Five studies were conducted in the USA or Canada [3, 9, 11, 15, 24], nine in Europe [1, 6, 7, 13, 14, 16, 17, 23, 25], and one in Asia [10]. Eight studies included type 2 DM and seven studies included combined type 1 and 2 DM. All cancers combined was an outcome in eight [1, 10, 11, 13, 14, 16, 17, 25], breast cancer in three [13, 14, 25], colorectal cancer in five [6, 13–15, 24], hepatocellular cancer [3, 23] and pancreatic cancer [9, 14] in two, and prostate cancer in three [13, 14, 25]. In the meta-analysis of insulin treatment and cancer, we included five case–control studies [3, 7, 9, 23, 24] and the ten cohort studies [1, 6, 10, 11, 13–16, 25]. These 15 studies comprised 562,043 participants and 14,085 cases of cancer.
Table 1.
Main characteristics of case–control studies of insulin treatment and cancer risk
| Source | Country | Gender | Age (year) | Number of controls (selection methods) | Type of DM | Cancer site: number of cases | OR (95 % CI) | Controlled variables | Insulin regimen |
|---|---|---|---|---|---|---|---|---|---|
| Hassan et al. [3] | USA | M/F | All ages | 1,104 (clinic-based controls matched by catchment) | Self-reported types 1 and 2 combined | Hepatocellular carcinoma: 420, | 1.90 (0.80, 4.60) | Age, gender, race, educational level, cigarette smoking, alcohol drinking, hepatitis C virus, hepatitis B virus, family history of cancer | Insulin |
| Li et al. [9] | USA | M/F | All ages | 863 (healthy controls matched by age, gender and race) | Self-reported types 1 and 2 combined | Pancreatic adenocarcinoma: 973 | 4.99 (2.59, 9.61) | Age, gender, race, smoking, alcohol, BMI, family history of cancer, duration of diabetes, concomitant hypoglycemic medications | Insulin |
| Monami et al. [7] | Italy | M/F | All ages | 195 (clinic-based controls matched by age, gender, duration of diabetes, BMI, HbA1c, comorbidity, smoking, alcohol) | Type 1 and 2 (medical records) | All cancers: 195 | 1.01 (0.64, 1.59) | Age, gender, duration of diabetes, BMI, HbA1c, comorbidity, smoking, alcohol, concomitant hypoglycemic medications | Insulin |
| Donadon et al. [23] | Italy | M/F | All ages | 490 (clinic-based controls matched by age, gender, BMI, history of diabetes, time of admission) | Type 2 (ADA criteria) | Hepatocellular carcinoma: 465 | 1.24 (0.46, 3.36) | Age, gender, BMI, alcohol use, hepatitis C virus, hepatitis B virus, triglyceride, cholesterol, concomitant hypoglycemic medications, duration of diabetes | Insulin vs. sulphonylureas |
| Vinikoor et al. [24] | United States | M/F | 40–80 | 988 (matched by age, sex and race) | Type 1 and 2 combined | Colorectal: 1,007 | White:1.74 (0.92, 3.31) | Age, gender, NSAID use, calcium intake, family history of colorectal cancer, and education | Insulin |
| African: 0.94 (0.33, 2.71) |
Table 2.
Main characteristics of cohort studies of insulin use and cancer risk
| Source | Country | Average follow-up period (year) | Gender | age at enrollment (year) | Study population | Cancer site: number of cases | RR (95 % CI) | Controlled variables | |
|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [6] | UK | 5.6 | M/F | 74.9 | UK nested case–control study: 24,918 patients with medical recorded type 2 DM (mean age, 61) | Colorectal: 125 | 2.1 (1.2, 3.4) | Age, gender, smoking | |
| Exposed group: 3,160 | |||||||||
| Comparison group: 21,758 men and women without insulin use | |||||||||
| Yang et al. [10] | Hong Kong | 3.0 | M/F | 35–49 | The Hong Kong Diabetes registry: 4,623patients with type 2 DM | All cancers: 152 | 0.17 (0.09, 0.32) | Age, gender, smoking, alcohol, duration of diabetes, systolic BP, LDL, HDL, triglycerides, use of anti-hypertensive drugs | |
| Exposed group: 971 using insulin | Digestive system: 119 | 0.19 (0.08, 0.46) | |||||||
| Comparison group: 1,935 not using insulin | Other cancers: 87 | 0.20 (0.10, 0.41) | |||||||
| Bowker et al. [11] | Canada | 5.4 | M/F | 63.4 | Population-based retrospective cohort study: 10,309 new users of metformin or sulfonylurea | Cancer- related mortality: 84 | 1.90 (1.50, 2.40) | Age, gender, metformin use, sulfonylurea use, chronic disease score | |
| Exposed group: 1,443 | |||||||||
| Comparison group: 8,866 men and women not insulin user | |||||||||
| Hemkens et al. [1] | Germany | 1.63 | M/F | ≥18 | The German statutory health insurance fund: 127,031 insulin-treated diabetic patients | All cancers: 5,009 | 1.19 (1.09, 1.29) | Age, gender, BMI | |
| Comparison group | |||||||||
| Human insulin: 95,804 | |||||||||
| Aspart: 4,103 | |||||||||
| Lispro: 3,269 | |||||||||
| Glargine:23,855 | |||||||||
| Colhoun, et al. [13] | Scotland | 4.0 | M/F | ≥65 | The Scottish Diabetes Research network Epidemiology Group: 49,197 patients with insulin-treated DM | All cancers: 715 | 1.73 (0.98, 3.06) | Age, gender, type of diabetes, calendar time prior cancer, metformin, diabetes duration, HbA1c, BP, BMI, smoking | |
| Comparison group: insulin glargine users alone: 447 | Breast: 92 | 3.65 (1.05, 12.68) | |||||||
| Insulin glargine other insulin users: 3,512 | Prostate: 48 | 1.16 (0.16, 8.50) | |||||||
| Other insulin users: 32,295 | Colorectal 109 | 1.43 (0.45, 4.57) | |||||||
| Lung: 149 | 1.43 (0.53, 3.88) | ||||||||
| Jonasson et al. [25] | Sweden | 2.0 | M/F | 35–84 | The population-based follow-up study in Sweden: 114,841 | All cancers: 2,348 | 1.06 (0.90, 1.25) | Age, gender, BMI, smoking | |
| Comparison group: insulin glargine users alone: 5,970 | All cancers and insitu tumors: 2,509 | 1.04 (0.88, 1.22) | Age at onset of DM and CVD, calendar period | ||||||
| Insulin glargine and other insulin users: 20,316 | Breast: 208 | 1.97 (1.30, 3.00) | |||||||
| Other insulins users: 88,555 | Gastrointestinal: 454 | 0.91 (0.61, 1.38) | |||||||
| Prostate: 464 | 1.26 (0.88, 1.80) | ||||||||
| Currie et al. [14] | UK | – | M/F | >40 | Retrospective cohort study of people treated in UK general practice: 62,809 | All cancers: 2,106 | 1.42 (1.27, 1.60) | Age, gender, smoking, prior cancer, antidiabetic treatment | |
| Insulin:10,067 | Breast: 305 | 1.07 (0.79, 1.44) | |||||||
| Comparison group | Colorectal: 292 | 1.69 (1.23, 2.33) | |||||||
| Metformin monotherapy:31,421 | Pancreas: 89 | 4.63 (2.64, 8.10) | |||||||
| Sulfonylurea monthearp:7,439 | Prostate: 301 | 1.10 (0.79, 1.52) | |||||||
| Metformin plus sulfonyurea:13,882 | |||||||||
| Campbell et al. [15] | USA | 15 | M/F | 50–74 | Cancer Prevention Study II Nutrition Cohort: 73,312 men and 81,663 women | Colorectal cancer | 1.02 (0.79, 1.30) | Age, BMI, Education, physical activity, drug use, alcohol use, NSAID use, family history of colorectal cancer | |
| Men: 218 | 1.36 (1.05, 1.78) | ||||||||
| Women: 103 | 0.95 (0.64, 1.41) | ||||||||
| Monami et al. [17] | Italy | 4.3 | M/F | 63.4 | City Registry Office: 3,002 patients with type 2 DM | Cancer-related mortality: 87 | 2.11 (1.01, 4.50) | Age, gender, BMI, smoking, HbA1c, | 1.01, 4.50 |
| Exposed group: 764 | |||||||||
| Comparison group: 2,553 | |||||||||
| Baur et al. [16] | Germany | 4–5 | M/F | 70.4 | Diabetes Cardiovascular Risk and Evaluation: Targets and Essential Data for Commitment of Treatment (DETECT) study from German primary care practices: 6,789 | Cancer-related mortality: 78 | 3.87 (1.53, 9.81) | Age, gender, HbA1c, smoking, and BMI | |
| Insulin users:329 | |||||||||
| Comparison group: noninsulin users, 6,460 | |||||||||
* RR relative risk; CI confidence interval; DM diabetes mellitus
All Cancer
Individual study results and the overall summary results for the 15 case–control and cohort studies of insulin treatment and cancer risk are shown in Fig. 1. Eight of these 15 studies found a statistically significant positive association between insulin treatment and cancer. One of the cohort studies [10] reported a significant inverse association between insulin and cancer [RR (95 % CI) 0.17 (0.09, 0.32)]. Insulin treatment was associated with an increased risk of overall cancer [summary RR (95 % CI) = 1.39 (1.14, 1.70)]. Heterogeneity among studies was found (I 2 = 85.3 %; P heterogeneity <0.001). In a sensitivity analysis, we found a statistically significant positive association between DM and cancer (range of summary RRs, 1.29–1.50). The studies by Yang et al. [10], Hemkens et al. [11], Jonasson et al. [25], Campbell et al. [15], and Li et al. [9] contributed most to heterogeneity. In an analysis excluding these studies, the association between diabetes and cancer was somewhat stronger [summary RR (95 % CI) = 1.65 (1.38, 1.98)], and the test for heterogeneity was not statistically significant (I 2 = 39.1 %; P heterogeneity = 0.10).
Fig. 1.
Association between insulin use and cancer risk. RR relative risk, CI confidence interval. Square study-specific RR estimate (size of the square reflects the study specific statistical weight, i.e., the inverse of variance); horizontal line 95 % CI, diamond summary RR estimate and its corresponding 95 % CI. All statistical tests were two-sided. Statistical heterogeneity between studies was assessed with I 2 test
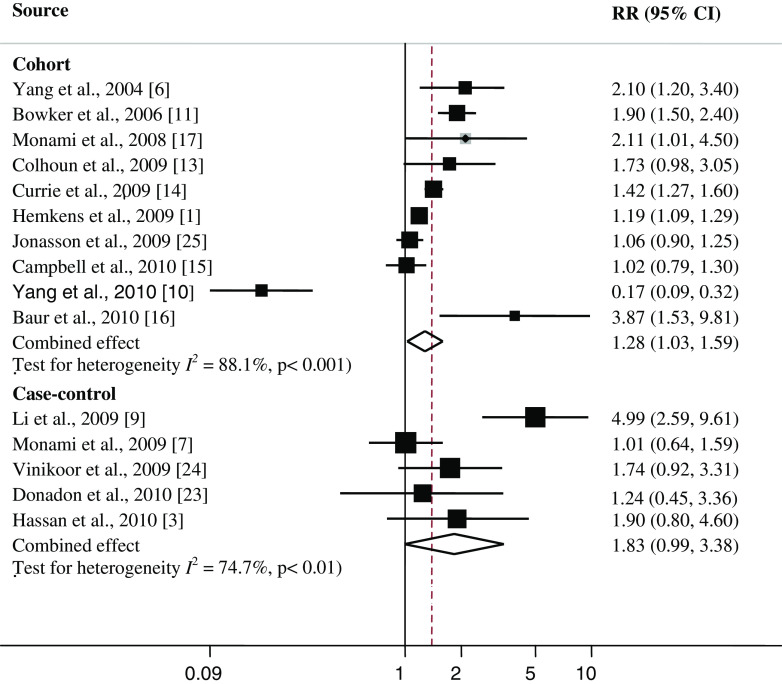
Individual study results and the overall summary results for the five case–control and ten cohort studies of insulin treatment and cancer are shown in Fig. 2. Six of ten cohort studies found a statistically significant positive association between insulin treatment and cancer (range of individual RRs, 1.19–3.87); the summary RR (95 % CI) for all ten cohort studies combined was 1.28 (1.03, 1.59). Heterogeneity among studies was significant (I 2 = 88.1; P heterogeneity <0.001). In a sensitivity analysis excluding one study at a time, we consistently found a statistically significant positive association between insulin use and cancer (range of summary RRs, 1.68–1.97). The cohort study by Yang et al. [10], Hemkens et al. [11], Jonasson et al. [25], and Campbell et al. [15] contributed most to heterogeneity. In an analysis excluding these studies, the association between insulin and cancer became stronger [summary RR (95 % CI) = 1.80 (1.43, 2.26)], and the test for heterogeneity was not statistically significant (I 2 = 54.2 %; P heterogeneity = 0.053).
Fig. 2.
Association between insulin use and cancer risk in cohort and case–control studies. RR relative risk, CI confidence interval. Square study-specific RR estimate (size of the square reflects the study specific statistical weight, i.e., the inverse of variance); horizontal line 95 % CI; diamond summary RR estimate and its corresponding 95 % CI. All statistical tests were two-sided. Statistical heterogeneity between studies was assessed with I 2 test
One of five case–control studies found a statistically significant positive association between insulin treatment and cancer incidence (RR 4.99); the summary RR (95 % CI) for all five case–control studies combined was 1.83 (0.99, 3.38). Heterogeneity among studies was significant (I 2 = 74.7; P heterogeneity < 0.01). The case–control study by Li et al. [9] contributed most to heterogeneity. In an analysis excluding this study, the association between insulin and cancer became weaker [summary RR (95 % CI) = 1.29 (0.94, 1.79)], and the test for heterogeneity was not statistically significant (I 2 = 0.0 %; P heterogeneity = 0.44).
Subgroup meta-analyses by study design, geographical area, type of DM, and duration of follow-up also conducted (Table 3). All included studies considered both gender, and only one study provided gender-specific RR [15]. With regard to the geographical area, insulin use seemed to be a better predictor of the risk of cancer in North American populations (summary RR = 1.90) than in European populations (RR = 1.34). The RR for one study conducted in Asian population was negative (RR = 0.17). The summary estimate from the case–control studies was higher than in cohort studies (1.83 vs. 1.28). For type of DM, the summary estimate was stronger [summary RR (95 % CI) = 1.44 (1.12, 1.85)] for combined type 1 and 2 DM than for only type 2 DM [summary RR (95 % CI) = 1.30 (0.89, 1.90)], there was heterogeneity among type of DM (P heterogeneity < 0.01). None of the included studies provided RR for type 1 diabetes alone due to the small number of patients with type 1 diabetes. Finally, the summary estimate was stronger [summary RR (95 % CI) = 1.82 (1.20, 2.76)] for the five cohorts with ≥4 years of follow-up than for the five cohorts with follow-up duration <4 years [1.02 (0.77, 1.35)]; there was heterogeneity among studies with <4 years and ≥4 years of follow-up (P heterogeneity < 0.006).
Table 3.
Summary relative risk (RR) estimates [95 % confidence intervals (CIs)] for case–control and cohort studies of the association between insulin treatment and cancer incidence and mortality by study design, geographical area, and duration of follow-up
| Subgroup | Number of studies | Summary RR (95 % CI) | Between studies | Between subgroups | ||
|---|---|---|---|---|---|---|
| I 2 | P heterogeneity | Q | P heterogeneity | |||
| Study design | ||||||
| Case–control | 5 | 1.83 (0.99, 3.38) | 74.7 % | 0.003 | 3.80 | 0.051a |
| Cohort studies | 10 | 1.28 (1.03, 1.59) | 88.1 % | 0.001 | ||
| Geographical area | ||||||
| USA | 5 | 1.90 (1.17, 3.09) | 84.9 % | 0.001 | 45.20 | 0.001b |
| Europe | 9 | 1.34 (1.14, 1.57) | 65.7 % | 0.003 | ||
| Asia | 1 | 0.17 (0.09, 0.32) | – | – | ||
| Type of DM | ||||||
| Type 2 | 8 | 1.30 (0.89, 1.90) | 89.0 % | 0.001 | 6.64 | 0.010c |
| Type 1 and 2 | 7 | 1.44 (1.12, 1.85) | 75.9 % | 0.001 | ||
| Follow-up duration | ||||||
| ≤4 years | 5 | 1.02 (0.77, 1.35) | 91.7 % | 0.001 | 7.47 | 0.006d |
| >4 years | 5 | 1.82 (1.20, 2.76) | 79.5 % | 0.001 | ||
All statistical tests were two-sided
aTest for heterogeneity between case–control and cohort studies
bTest for heterogeneity between USA and combined Europe and Asia
cTest for heterogeneity between combined type 1 and 2 and type 2 diabetes
dTest for heterogeneity between follow-up duration ≤4 and >4 years
The most important known confounders for the positive association between insulin use and cancer risk are age, gender, and body mass index (BMI). When we restricted the meta-analysis to studies that controlled for these variables [7, 9, 13, 16, 17, 23, 25], the association between insulin and cancer remained [summary RR (95 % CI) = 1.81 (1.15, 2.86)].
Other Cancer Sites
Of one case–control and four cohort studies of insulin use and colorectal cancers [6, 13–15, 24], two [6, 14] reported a statistically significant positive association, and three [13, 15, 24] reported no association (Tables 1 and 2). When all five studies were analyzed, a statistically significant association between insulin use and colorectal cancer was found [summary RR (95 % CI) = 1.50 (1.08, 2.08)]. There was statistically significant heterogeneity among studies (I 2 = 61.0 %; P heterogeneity < 0.05). There was no significant association between insulin use and breast cancer [summary RR (95 % CI) = 1.65 (0.92, 2.98)], prostate cancer [1.17 (0.92, 1.49)], or hepatocellular carcinoma [1.58 (0.82, 3.06)]. The association between insulin use and pancreatic cancer [4.78 (3.12, 7.32)] was statistically significant but was based on only two studies [9, 14].
Publication Bias
There was no evidence of publication bias for the association between insulin use and cancer risk (data not shown; P = 0.46, for Begg’s adjusted rank correlation test and P = 0.38 for Egger’s regression asymmetry test). No missing study was identified by the trim-and-fill method.
Discussion
Findings from this meta-analysis indicate that insulin use was associated with significantly higher risk of overall cancer. Based on the pooled estimate of risk from cohort studies, insulin-treated DM patients had 28 % greater probability of cancer compared with nonusers. The association with pancreatic cancer was stronger than colorectal. The observation that insulin-treated DMs were more likely to develop pancreatic cancer is based on a few epidemiologic studies [9, 14]. Because pancreatic cancer is a rapidly fatal but a relatively uncommon cancer, epidemiologic research on this disease is challenging. The geographic origin of the study population appeared to be an important source of heterogeneity, with the impact of insulin use being positively significant in European and North American studies but negatively associated in Asian populations. The results were stronger for case–control studies. The association was observed in combine type 1 and 2 DM but not in type 2 DM.
Our analysis must be interpreted in the context of the limitations of the available data. Seven of the studies (47 %) did not distinguish between type 1 and 2 DM [1, 3, 7, 9, 13, 24, 25]. Because type 1 DM (which accounts for 5–10 % of all diagnosed cases of DM [33]) may be related more strongly to cancer [34], the magnitude of the association between insulin use and cancer risk may have been slightly overestimated if some diagnoses of type 2 DM were truly type 1 DM. As in any meta-analysis, the possibility of publication bias is of concern. However, the results obtained from funnel plot analysis and formal statistical tests did not provide evidence for such bias. Three studies [11, 16, 17] examined only cancer mortality and not cancer incidence; differences in patient characteristics could intervene as confounding factors in the relationship between cancer and insulin treatment. Furthermore, some studies do not indicate whether their results come from a protective effect of metformin or deleterious effects of sulfonylurea. All of the studies reported are observational, making it difficult to conclude in terms of causality. It should be supposed that the described association of insulin with cancer could have been determined by some unexplored prescription bias. Diabetic patients receiving a prescription of insulin are likely to have a more “severe” form of type 2 diabetes, a greater duration of diabetes, or comorbidities contraindicating treatment with oral agents. Actually, the issue of an effect of hypoglycemic treatments on the risk of cancer has been raised by observational studies, which can be biased by uncontrolled confounders. Thus, in observational studies, insulin-treated groups may differ at baseline, and adjustments (or matching in case–control studies) for known confounders can only reduce, not eliminate, biases, and unknown confounders cannot be adjusted for. Insulin-treated patients are likely to be sicker and have more complications from their diabetes. Hence, their cancer may have progressed more and/or they may be less able to tolerate cancer-related complications or anticancer treatment. Nevertheless, it seems that a negative effect of insulin on cancer risk is highly consistent with the pathophysiological mechanisms to explain the increase risk of cancer in diabetic patients. No confirmatory studies using a long-term randomized, controlled design can be performed to validate or to refute the hypothesis of a causal association between insulin and cancer risk, for obvious ethical and practical reasons. All trials of cancer incidence or mortality are comparing one insulin regimen versus another type of diabetes therapy or insulin regimen, and the results are inconsistent. Most of the reported evidence comes from cohort or case–control observational studies. The case–control studies do not incorporate the time sequence criteria for causality. Most studies do not provide data on insulin dose or type; thus, we were not able to test the hypothesis that higher doses of insulin are associated with higher cancer rates or whether the slope of such relationship varies by insulin regimen. A major limitation for any of the cohort studies is surveillance bias: Individuals with diabetes are more likely to be seen by medical personnel on a more frequent basis compared with otherwise healthy individuals and therefore the chance of their cancer being detected sooner is greater. This could result in an overestimation of the effect. Another limitation of cohort studies was short follow-up for evaluating cancer effects.
Insulin use appeared to be a better predictor of cancer risk in North American populations than in European populations. We do not have an explanation for this association, but it may reflect increased metabolic syndrome in North American populations, discrepancies in patient characteristics, or due to higher prevalence of insulin use in type 2 diabetes in North American populations.
In contrasts with other studies included, Yang et al. [10], by analyzing data in two unmatched subcohorts with different follow-up patterns from the Hong Kong Diabetes Registry, claims an inverse relation between insulin therapy and cancer. This study presents serious methodological problems regarding patient matching as well as follow-up. From the original cohort of 4,623 diabetic patients, including 169 patients who developed cancer among insulin nonusers, a smaller new insulin user cohort, with 1:2 matched control subjects, was selected on the basis of the likelihood of initiating insulin therapy. When these new-user subcohorts were considered, a cancer-enriched population was selected from insulin nonusers but not from insulin users. After this selection, the two “matched” groups were significantly different for nearly all the variables considered, including the clinical, biochemical, BMI, and treatment parameters. This may indicate that the propensity score procedures had not allowed adequate case/control matching. The difference in clinical, biochemical, BMI, and treatment as well as follow-up may explain the very controversial findings by Yang et al. [10].
Several mechanisms for the effect of insulin on cancer risk are proposed. It is believe that insulin resistance and consequence hyperinsulinemia, which are typical features in the majority of patients with diabetes [35], plays a major role in the association between diabetes and cancer. In diabetic patients, insulin plasma levels are chronically increased by therapies based on both exogenous insulin and insulin secretogogues. Numerous lines of evidence from animal and preclinical models indicate that insulin and insulin receptor play an important role in both cancer initiation and progression [36, 37]. Protracted exposure to hyperinsulinemia increases the level of insulin-like growth factor-1, which plays a pivotal role in carcinogenesis [36, 37]. In addition, the predictive value of hyperinsulinemia on the total cancer mortality [38] and fatal liver tumor incidence [39] has been demonstrated in nondiabetic subjects.
Cancers and DM is an important medical, social, and economic concern to the society, and the prevalence of DM is increasing in developed and in many developing countries. The worldwide DM epidemic will continue to escalate as a result of the increasing proportion of older people and growing obesity epidemic, and thus, it will further contribute to the public health burden of cancer. The observational studies provide the advantages of the possibility of collecting large samples with a long duration of follow-up; however, in observational studies, multiple adjustments for confounders can never fully eliminate the prescription bias. The number of events included in the present meta-analysis does not allow a reliable analysis on specific types of cancer. Taking into account that the pathogenesis of different forms of cancer is very diverse, the insulin could have divergent effects on different malignancies. To clarify the risk profile for individual forms of cancer in insulin-treated patients, a very large database is needed.
In conclusion, there may be a link between insulin use and cancer and that the association probably is not just due to confounding factors, despite the fact that these studies are observational. Regarding the current DM epidemic, these results reinforced the claim in favor of greater public awareness about healthy lifestyle to prevent these two major increasing public health problems.
Acknowledgments
This work was partially supported by funds from Isfahan University of Medical Sciences. This research was performed as a part of the academic activity of the university.
Conflicts of Interest
The authors declared no conflict of interest.
References
- 1.Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 3.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donadon V, Balbi M, Casarin P, Vario A, Alberti A. Association between hepatocellular carcinoma and type 2 diabetes mellitus in Italy: potential role of insulin. World J Gastroenterol. 2008;14:5695–5700. doi: 10.3748/wjg.14.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung YW, Han DS, Park KH, Eun CS, Yoo KS, Park CK. Insulin therapy and colorectal adenoma risk among patients with type 2 diabetes mellitus: a case–control study in Korea. Dis Colon Rectum. 2008;51:593–597. doi: 10.1007/s10350-007-9184-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case–control study. Acta Diabetol. 2009;46:279–284. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 8.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Ko GT, So WY, Ma RC, Yu LW, Kong AP, et al. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes. 2010;59:1254–1260. doi: 10.2337/db09-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 12.Kath R, Schiel R, Müller UA, Höffken K. Malignancies in patients with insulin-treated diabetes mellitus. J Cancer Res Clin Oncol. 2000;126:412–417. doi: 10.1007/s004320050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colhoun HM, SDRN Epidemiology Group Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 15.Campbell PT, Deka A, Jacobs EJ, Newton CC, Hildebrand JS, McCullough ML, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology. 2010;139:1138–1146. doi: 10.1053/j.gastro.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 16.Baur DM, Klotsche J, Hamnvik OP, Sievers C, Pieper L, Wittchen HU, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism. 2011;60:1363–1371. doi: 10.1016/j.metabol.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Monami M, Lamanna C, Pala L, Bardini G, Cresci B, Francesconi P, et al. Treatment with insulin secretagogues and cancer-related mortality in type 2 diabetic patients a retrospective cohort study. Exp Clin Endocrinol Diabetes. 2008;116:184–189. doi: 10.1055/s-2007-992157. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92:2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Will JC, Galuska DA, Vinicor F, Calle EE. Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol. 1998;147:816–825. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 20.Lindblad P, Chow WH, Chan J, Bergström A, Wolk A, Gridley G, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999;42:107–112. doi: 10.1007/s001250051122. [DOI] [PubMed] [Google Scholar]
- 21.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025–3032. doi: 10.3748/wjg.v16.i24.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinikoor LC, Long MD, Keku TO, Martin CF, Galanko JA, Sandler RS. The association between diabetes, insulin use, and colorectal cancer among Whites and African Americans. Cancer Epidemiol Biomarkers Prev. 2009;18:1239–1242. doi: 10.1158/1055-9965.EPI-08-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steinbeck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 26.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ; 2001. [Google Scholar]
- 32.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 34.Zendehdel K, Nyrén O, Östenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;23:1797–1800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 35.Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A. Cancer and diabetes–a follow-up study of two population-based cohorts of diabetic patients. J Intern Med. 1997;241:471–475. doi: 10.1111/j.1365-2796.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 36.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 37.Novosyadlyy R, Leroith D (2012) Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/gls065 [DOI] [PubMed]
- 38.Balkau B, Kahn HS, Courbon D, Eschwege E, Ducimetiere P. Hyperinsulinemia predicts fatal liver cancer but is inversely associated with fatal cancer at some other sites: the Paris Prospective Study. Diabetes Care. 2001;24:843–849. doi: 10.2337/diacare.24.5.843. [DOI] [PubMed] [Google Scholar]
- 39.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2001;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]