Abstract
Aryl hydrocarbon receptor (AhR) has been reported to exert various anticancer effects upon breast carcinoma cells in vitro but its details have remained largely unknown. Therefore, we first examined the AhR status in 90 invasive ductal carcinoma patients using immunohistochemistry. We then performed in vitro studies including wound healing assay, invasion assay, and matrix metalloproteinase (MMP) protein array in order to further elucidate the roles of AhR signaling in breast carcinoma. The status of AhR immunoreactivity was inversely correlated with histological grade (P = 0.0135) and Ki-67 labeling index (LI; P = 0.0087) of the patients. In addition, results of both uni- and multivariate analyses revealed that AhR in carcinoma cells turned out an independent prognostic factor with a protective relative risk (P = 0.0179). An administration of 10 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a ligand of AhR, significantly decreased Ki-67 LI in an AhR-dependent fashion in MCF-7, T47D, ZR75-1, and MDA-MB-231. Wound healing and invasion assays performed in T47D and ZR75-1 further demonstrated that 10 nM TCDD inhibited estrogen-induced migration and invasion of cells. MMP proteins associated with AhR in breast carcinoma cells were also firstly identified. These results demonstrated that AhR in breast carcinoma cells is considered a newly defined histological prognostic parameter of the breast cancer patients and effects of AhR activation on proliferation and MMPs expression may be related to the relatively good clinical outcome of AhR-positive breast cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-013-0160-z) contains supplementary material, which is available to authorized users.
Keywords: Invasive Ductal Carcinoma, Wound Healing Assay, Aryl Hydrocarbon Receptor, Breast Carcinoma Cell Line, Aryl Hydrocarbon Receptor Ligand
Introduction
Aryl hydrocarbon receptor (AhR) is a ligand-dependent basic helix–loop–helix–Per/Arnt/Sim (PAS)-containing transcription factor, and its expression has been detected in a wide range of species and tissues [8, 10, 25]. AhR binds to both exogenous and endogenous ligands including halogenated aromatic hydrocarbons (HAHs) such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most potent exogenous ligand of this receptor, polycyclic aromatic hydrocarbons (PAHs), PAH-like chemicals such as beta-naphthoflavone, as well as endogenous ligands such as indirubin, prostaglandin E2, and others [2, 7, 38]. In addition, many dietary compounds were also reported to possess AhR agonist activities [28]. The activation of AhR is generally followed by the formation of a heterodimer with one of the AhR nuclear translocator proteins (ARNT1 or 2) and the subsequent induction of cytochrome P450 (CYP) 1A1 and CYP1B1 [3, 19].
AhR activation is also known to regulate the expression of numerous genes and result in its specie- and tissue-specific toxic and/or biological effects. The results of previously published studies all demonstrated that AhR activation promoted the carcinogenesis, growth, or invasion of carcinoma cells in urogenital tracts, lung, stomach, and others [6, 16, 32, 33]. In addition, an upregulation of matrix metalloproteinase (MMP), an inhibitor of cell to cell contact [9] or cell cycle regulation [24] have been all proposed to account for these AhR-related processes above.
Expression of AhR protein in breast cancer patients and its possible clinical significance have, however, remained largely unknown. Epidemiological studies following the Seveso incidence, one of the most well-known industrial incidents, demonstrated increased risks of developing some neoplasm following exposure to TCDD in the victims of this incidence [4, 33]. In particular, the incidence of breast cancer significantly increased even after 15 years of the accident, in the zones where high levels of TCDD exposure were documented. However, it is also true that an activation of AhR has been also known to exert inhibitory effects on breast carcinoma cell growth in vitro [27, 36]. Several in vitro studies have therefore been focused on intrinsic AhR signaling as an anticancer therapeutics [44]. It then becomes important to study the status of AhR in breast cancer patients but this has not been studied at all in human breast cancer.
AhR and its ligands are also well known to disturb estrogen receptor (ER)-dependent signals in vitro [18, 30, 43]. For instance, TCDD was reported to exert anti-estrogen effects through competitive inhibition of ERα binding to estrogen response element (ERE) and stimulation of ERα degradation [18, 43]. However, it is also true that AhR ligands were reported to induce an activation of ERE in the presence of ERα without ER ligands [30]. MMP have been well-known as one of the key enzymes involved in carcinoma invasion and to be induced by both AhR and ER pathways for their transcriptional target genes. However, the effects of AhR ligands on MMPs expression through ER or AhR signals in breast carcinoma cells have not been studied. Therefore, it is not known whether AhR ligands could elicit any effects upon MMPs expression through ER pathway or not.
Therefore, in this study, we first examined the status of AhR immunoreactivity and its clinical significance in breast invasive ductal carcinoma patients. We then studied the effects of TCDD, which is one of the most potent and also most commonly used AhR ligand in previous studies [1, 14, 44], upon cell proliferation and invasion in breast carcinoma cell lines. We subsequently examined whether estrogen-induced MMPs expression is suppressed by AhR ligands treatment or not in breast carcinoma cells in order to further evaluate the cross-talks between AhR- and ER-mediated intracellular signaling systems.
Materials and Methods
Patients and Tissue Preparation
Surgical pathology specimens of invasive ductal carcinoma of the breast were retrieved from Japanese female patients from 1995 to 1999 at the Department of Surgery, Tohoku University Hospital (Sendai, Japan). The patients did not receive chemotherapy or irradiation prior to surgery. The number of patients examined in this study was 90 [55.5 years (range, 31–81; standard deviation (SD), 11.2)]. These specimens had been all fixed in 10 % formalin and embedded in paraffin wax. Relevant clinical data were retrieved from the review of the patients' charts. The histological grade of each case was evaluated according to the report of Elston and Ellis [11]. The Ethics Committee of the Tohoku University School of Medicine approved the research protocol (2010-573).
Immunohistochemistry
Rabbit polyclonal antibody against AhR was purchased from Enzo Life Sciences, Inc. (Farmingdale, USA). This antibody was reported to specially recognize human AhR by both immunoblotting and immunohistochemistry [21]. Immunohistochemical absorption test using AhR-recombinant protein (Abnova, Taipei, Taiwan) was employed as negative control of immunostaining (Fig. 1e) as well as using Rabbit Immunoglobulin Fraction (Solid-Phase Absorbed; Dako, Carpinteria, CA, USA) instead of primary antibody (Fig. 1f). No specific immunoreactivity was detected in these negative control specimens. Normal liver tissue was used as a positive control of AhR immunostaining. A Histofine Kit (Nichirei, Tokyo, Japan) based on the streptavidin–biotin amplification method was used in this study. Antigen retrieval was performed by microwave treatment for AhR in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate (pH 6.0)). The dilution of primary AhR antibody was 1:500. The antigen–antibody complex was visualized using 3,3′-diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris–HCl buffer (pH 7.6), and 0.006 % H2O2) and counterstained with hematoxylin.
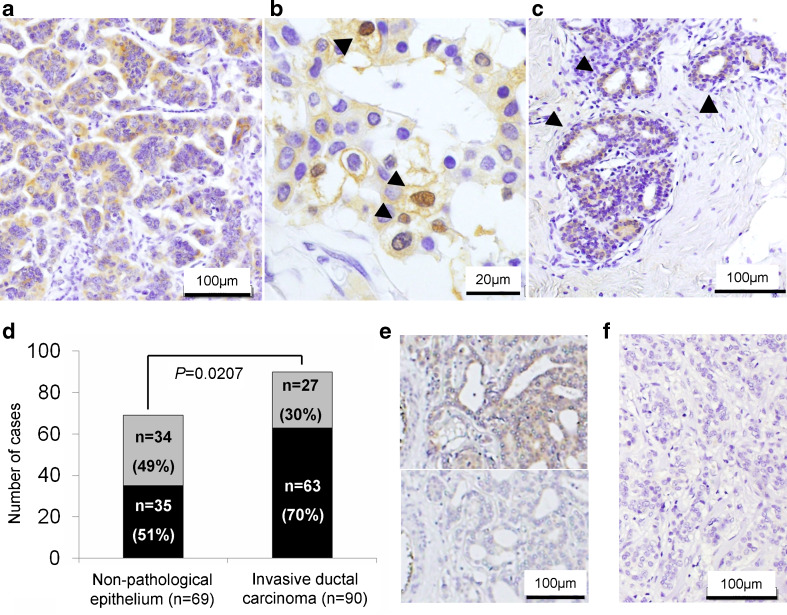
Fig. 1.
a–c Immunohistochemistry of AhR in human invasive ductal carcinoma (a, b) and nonpathological ductal epithelial cells (arrowhead; c). AhR immunoreactivity was mainly detected in the cytoplasm (a) and/or nuclei (arrowhead; b). d Black AhR positive, Gray AhR negative. The rate of AhR immunopositivity in carcinoma was significantly higher than that in nonpathological ductal epithelium (P = 0.0207). The statistical analysis was performed using χ 2 test. e The representative images of immunohistochemical absorption test using specimen of breast carcinoma and AhR antibody. Upper panel no AhR recombinant protein. Lower panel 10 μg/ml AhR recombinant protein. f The negative staining control using rabbit immunoglobulin fraction
In this study, we tentatively defined the cells demonstrating more marked immunointensity than background immunointensity as positive cells. The percentage of positive cells was also calculated by the ratio of immunopositive area to the whole invasive ductal carcinoma or nonpathological ductal epithelium relatively in each slide. The percentage of immunopositive cells was tentatively classified into the following two groups: negative, <10 %; positive, ≥10 %.
Other antibodies used in this study for characterizing clinicopathological parameters of the cases were as follows: monoclonal antibodies for ERα (ER1D5), progesterone receptor (PR; MAB429), and Ki-67 (MIB1) were purchased from Immunotech (Marseille, France), Chemicon (Temecula, CA, USA) and Dako, respectively. We used a HercepTest for Immunoenzymatic Staining (Dako) for evaluation of HER2 status. The representative illustrations of low and high values of ERα and HER2 were presented in Electronic Supplementary (ESM) Fig. 1.
Real-time RT-PCR
Total RNA was carefully extracted from cultured cells, using the TRI reagent (COSMO BIO, Molecular Research Center, Inc). A reverse transcription kit (QuantiTect Reverse Transcription Kit; QIAGEN) was used in the synthesis of cDNA.
The LightCycler System (Roche Diagnositics GmbH) was used to semiquantify the mRNA expression levels using real-time RT-PCR. The PCR mixture (20 μl) contained 0.5 μM of each primers, 1 μl cDNA, 3 mM of MgCl2, and 2 μl LightCycler (Fast Start DNA Master SYBR Green I; Roche Diagnostics) for ribosomal protein L 13a (RPL13A) and CYP1A1. Setting for the PCR thermal profile were as follows: initial denaturation at 95 °C for 10 min followed by 40 amplification cycles of 95 °C for 10 s, annealing at 68 °C (RPL13A and CYP1A1), and elongation at 72 °C for 10 s. The following primers were used: RPL13A, forward: 5′-CCTGGAGGAGAAGAGGAAAG-3′ and reverse: 5′-TTGAGGACCTCTGTGTATTT-3′; CYP1A1, forward: 5′-GCCTATGTGGTCTAAGATTCA-3′ and reverse: 5′-CCTGTTTTACCTGTTGTCTC-3′.
Breast Carcinoma Cell Line and Cell Culture
Human breast carcinoma cell lines MCF-7, T47D, ZR75-1, and MDA-MB-231 were all obtained from Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). These cells were maintained in RPMI 1640 (Sigma-Aldrich Co., St. Louis, MO, USA) with 10 % fetal bovine serum (FBS; Nichirei Co., Ltd., Tokyo, Japan) and 1 % penicillin/streptomycin in a 5 % CO2 humidified incubator at 37 °C.
Immunocytochemistry of Breast Carcinoma Cell Lines
MCF-7, T47D, ZR75-1, and MDA-MB-231 (5 × 105 cells/ml) were treated with DMSO for control or 10 nM TCDD in phenol red- and FBS-free RPMI 1640 medium for 24 h. The concentration was selected based on the results of previous reports [14, 44] and our preliminary data, in which CYP1A1 was significantly induced by both 1 and 10 nM TCDD in MCF-7 (ESM Fig. 2). These cells were washed twice by phosphate-buffered saline (PBS), and were subsequently fixed in 10 % formalin for 5 min. After washing three times with PBS, a Histofine Kit (Nichirei, Tokyo, Japan) and the monoclonal antibody for Ki-67 (Marseille, France) diluted by 1/100 were used without antigen retrieval. More than 500 cells were counted in three areas of the slide, and the Ki-67 labeling index (LI) was calculated as the percentage of positively stained nuclei.
Wound Healing Assay
Both T47D and ZR75-1 were seeded onto 24-well dishes (5 × 105 cells/ml) in charcoal-stripped RPMI 1640 medium with 10 % FBS. After several hours in culture, scratch wound was created using a p200 micropipette tip into confluent cells. Ten micromolar CH-223191 (Calbiochem., Germany), a potent and specific AhR antagonist [17], was added. Later, after 2 h, 10 nM TCDD, 10 nM estrogen (E2), 10 μM CH-223191, and/or DMSO were added in phenol red- and FBS-free RPMI 1640 medium for 24 h. Images were subsequently captured in four different fields per well using phase-contrast microscopy at 0 and 24 h after wounding.
Invasion Assay
Invasion assays were performed using BioCoat Matrigel Invasion Chamber (Becton Dickinson, Bedford, MA, USA), which consisted of a 24-well companion plate with cell culture inserts containing 8 mm pore size filters coated with the basement membrane Matrigel. After incubation in charcoal-stripped RPMI 1640 medium containing 10 % charcoal-stripped FBS for several hours, both T47D and ZR75-1 were treated with DMSO for control, 10 nM TCDD and/or 10 nM E2 in phenol red- and FBS-free RPMI 1640 medium. After 24 h, the cells were trypsinized and resuspended in phenol red- and FBS-free RPMI 1640 medium. The cells were then plated in the upper chambers (5 × 105 cells/ml). Phenol red-free charcoal-stripped RPMI 1640 medium with 10 % FBS was used as a chemoattractant in the lower chamber. After 24 h incubation in a 5 % CO2 humidified incubator at 37 °C, the cells on the upper surface of the membrane were mechanically removed with cotton swabs. The invading cells on the under surface were fixed in 100 % methanol and stained with Toluidine Blue. The membranes were subsequently mounted on glass slides and the cells from 3–5 random microscopic fields at hot spot areas (×400 magnification) were counted.
Cell Proliferation Assay
T47D and ZR75-1 were seeded in 96-well plate (5 × 103 cells/well) in phenol red- and FBS-free RPMI 1640 medium. After several hours, DMSO for control or 10 nM E2 were administered in phenol red- and FBS-free RPMI 1640 medium (n = 6). After 24 h, the cell proliferation was evaluated using the WST-8 method (Cell Counting Kit-8; Dojindo Inc., Kumamoto, Japan).
MMP Array
In order to assess the expression of MMPs in T47D and ZR75-1 treated with DMSO as control, 10 nM TCDD and/or 10 nM E2 in charcoal-stripped RPMI 1640 medium containing 10 % charcoal-stripped FBS for 72 h, we used the Human Matrix Metalloproteinase Antibody Array (RayBiotech, Norcross, GA, USA), which can detect seven MMPs and three tissue inhibitors of metalloproteinases. The membranes were spotted in duplicate with MMP-specific antibodies. Membranes were also analyzed according to the manufacturer's instructions. The signal intensities were analyzed using a LAS-3000mini imaging analyzer and Multi Gauge software version 3.0 (Fujifilm, Tokyo, Japan).
Statistical Analysis
Statistical analysis was performed using the StatView 5.0 J software (SAS Institute Inc., Cary, NC, USA). An association between AhR immunoreactivity and clinicopathological factors was evaluated using a Student's t test and cross-table using the χ 2 test. Overall and disease-free survival curves were generated according to the Kaplan–Meier method and the statistical significance was calculated using the logrank test. Both uni- and multivariate analyses were performed using a Cox's proportional hazard model. Ki-67 LI of cultured cells, wound healing assay, invasion assay, proliferation tests, signal intensities of MMP array, and mRNA levels of CYP1A1 were evaluated using ANOVA and Bonferroni test.
Results
AhR Immunohistochemistry
AhR immunoreactivity was detected in the cytoplasm of carcinoma cells in 63/90 cases (70 %) (Fig. 1a, b). Sixty-nine of 90 cases contained nonpathological breast tissues in the slides, in which 35 cases demonstrated AhR immunoreactivity in nonpathological ductal epithelium (51 %; Fig. 1c). The AhR-positive rate in invasive ductal carcinoma was significantly higher than that in nonpathological ductal epithelium (P = 0.0207; Fig. 1d).
Correlation between AhR and Clinicopathological Parameters or Clinical Outcome of the Patients
Table 1 summarized the correlation of AhR immunoreactivity in carcinoma cells with the clinicopathological parameters of the cases examined. A statistically significant negative association was detected between the status of AhR immunoreactivity and age (P = 0.0048), clinical stage (P = 0.0395), tumor size (P = 0.0372), histological grade (P = 0.0135), and Ki-67 LI (P = 0.0087). The significantly positive correlation was detected between the status of AhR immunoreactivity and ERα LI (P = 0.0243) or PR LI (P = 0.0128).
Table 1.
Association between AhR immunoreactivity and clinicopathological parameters in breast cancer patients (n = 90)
| AhR immunoreactivity | |||
|---|---|---|---|
| Positive (n = 63) | Negative (n = 27) | P value | |
| Age (years) | 53.3 ± 9.7 | 60.5 ± 12.8 | 0.0048* |
| Menoposal status | |||
| Premenopausal | 27 | 6 | 0.1046 |
| Postmenopausal | 36 | 21 | |
| Stage | |||
| I | 25 | 5 | 0.0395* |
| II | 26 | 13 | |
| III | 8 | 6 | |
| IV | 4 | 3 | |
| Tumor size | |||
| <2.0 cm | 33 | 7 | 0.0372* |
| ≧2.0 cm | 30 | 20 | |
| Lymph node status | |||
| Positive | 27 | 17 | 0.1289 |
| Negative | 36 | 10 | |
| Histological grade | |||
| I | 14 | 3 | 0.0135* |
| II | 34 | 10 | |
| III | 15 | 14 | |
| ER alpha LI (%) | 56.2 ± 34.0 | 37.2 ± 40.6 | 0.0243* |
| PR LI (%) | 30.5 ± 30.1 | 13.6 ± 25.9 | 0.0128* |
| HER2 status | |||
| Positive | 9 | 7 | 0.3064 |
| Negative | 54 | 20 | |
| Ki-67 LI (%) | 15.1 ± 12.3 | 23.0 ± 13.9 | 0.0087* |
Data are presented as mean ± 95 % confidence interval (95 % CI) or the number of cases
*P < 0.05, significant
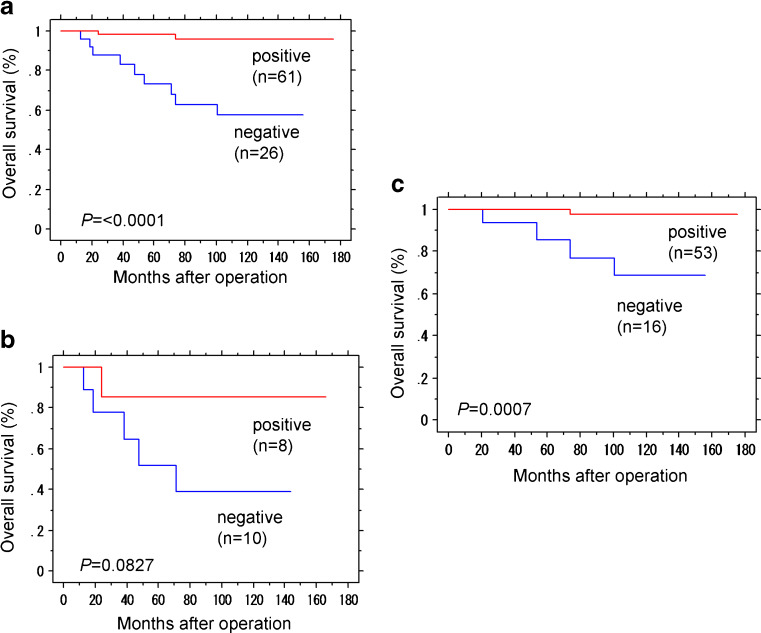
Overall survival curve was illustrated in Fig. 2a–c. A significant correlation was detected between the status of AhR immunoreactivity and clinical outcome of all the patients examined (P < 0.0001 in the logrank test) or the group of the patients with ER-positive carcinoma cells (ER LI >1 %; P = 0.0007). The group of the ER-negative patients also demonstrated the similar tendency but the correlation did not reach statistical significance (P = 0.0827). The results of univariate analysis (in the logrank or Cox test) demonstrated that AhR immunoreactivity (P = 0.0008), tumor size (P = 0.0357), histological grade (P = 0.0015), ERα LI (P = 0.0068), HER2 status (P = 0.0100), and Ki-67 LI (P = 0.0007) all turned out significant prognostic factors for overall survival in the patients examined. However, subsequent multivariate analysis revealed that only AhR immunoreactivity was an independent prognostic factor with a significant protective relative risk (P = 0.0179, relative risk (95%CI) = 0.110 (0.018–0.684); Table 2).
Fig. 2.
Overall survival of 87 out of 90 cases with invasive ductal carcinoma according to AhR status of carcinoma cells (Kaplan–Meier method). a AhR immunoreactivity was significantly associated with better prognosis (P < 0.0001, logrank test). b, c Overall survival according to AhR with the ERα status separated. b In 18 ERα-negative cases, AhR immunoreactivity tend to be associated with better prognosis but the correlation did not reach the statistical significance (P = 0.0827). c In 69 ERα-positive cases, AhR immunoreactivity was significantly associated with better prognosis (P = 0.0007)
Table 2.
Uni- and multivariate analyses of overall survival in breast cancer patients examined (n = 87)
| Univariate | Multivariate | ||
|---|---|---|---|
| P value | P value | Relative risk (95 % CI) | |
| AhR (positive/negative) | 0.0008* | 0.0179* | 0.110 (0.018–0.684) |
| Tumor size | 0.0357* | 0.6761 | |
| Lymph node status | 0.1390 | 0.7086 | |
| Histological grade (III/ I, II) | 0.0015* | 0.2658 | |
| ER alpha | 0.0068* | 0.9677 | |
| HER2 | 0.0100* | 0.1128 | |
| Ki-67 | 0.0007* | 0.4480 | |
*P < 0.05, significant in the univariate analyses and were examined in the multivariate analyses
AhR Suppressed Cell Proliferating in ER-Positive and ER-Negative Breast Carcinoma In Vitro
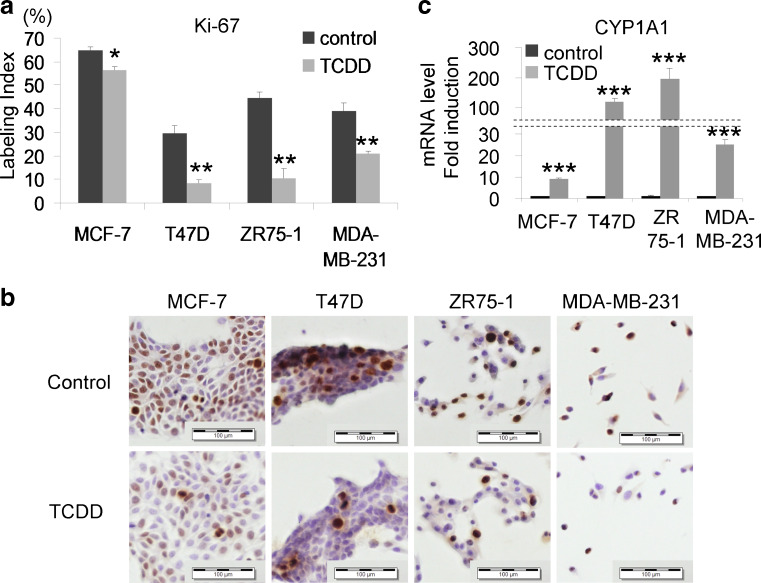
A 10 nM TCDD administration for 24 h in phenol red- and FBS-free medium decreased Ki-67 LI of both ER-positive and ER-negative breast carcinoma cell lines (MCF7 (P = 0.0135), T47D (P = 0.0026), ZR75-1 (P = 0.0024), and MDA-MB-231 (P = 0.0082)) in a significant manner (Fig. 3a, b). CYP1A1 induction by TCDD also indicated AhR activation by TCDD (Fig. 3c).
Fig. 3.
MCF-7, T47D, ZR75-1, and MDA-MB-231 were all treated with 10 nM TCDD for 24 h in phenol red- and FBS-free medium. a, b Ki-67 LI of both ER-positive and ER-negative breast cancer cell lines decreased by TCDD administration. Bars mean ± SE (n = 3). *P < 0.05, **P < 0.01 compared with control. c CYP1A1 was induced following TCDD administration, consistent with AhR activation by TCDD. Bars mean ± SE (n = 3). ***P < 0.001 compared with control. Scale bars 100 μm
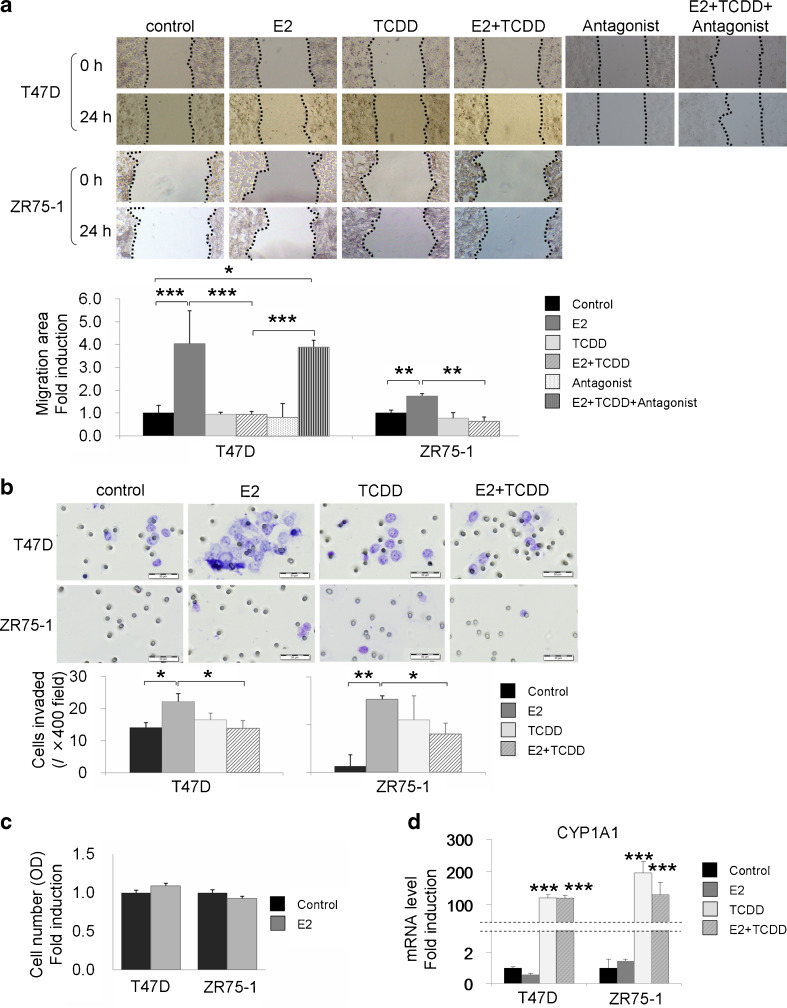
AhR Inhibited E2-Induced Cell Migration and Invasion
We investigated the effects of AhR on migration ability of breast carcinoma cells using wound healing assay (Fig. 4a). The 10 nM TCDD alone in phenol red- and FBS-free medium for 24 h demonstrated no significant changes. An exposure to 10 nM E2 in phenol red- and FBS-free medium for 24 h significantly induced the migration of T47D and ZR75-1 (P = 0.0003, P = 0.0083, respectively). In addition, 10 nM TCDD significantly inhibited E2-dependent induction of migration of T47D and ZR75-1 (P = 0.0003 and P = 0.0015, respectively). Inhibitory effects of TCDD upon E2-dependent cell migration were significantly reduced by specific AhR antagonist in T47D (P = 0.0009).
Fig. 4.
a Wound healing assay in T47D and ZR75-1. T47D were exposed to 10 nM TCDD, 10 nM estrogen (E2) and/or 10 μM AhR antagonist (CH-223191), and ZR75-1 were exposed to 10 nM E2 and/or 10 nM TCDD in phenol red- and FBS-free medium for 24 h. Exposure to 10 nM E2 in serum-free medium for 24 h significantly induced migration of T47D and ZR75-1. A 10 nM TCDD administration significantly inhibited E2-dependent induction of cell migration in both cell lines. The inhibitory effects of TCDD on E2-dependent cell migration were significantly reduced by specific AhR antagonist in T47D cells. Bars mean ± SE (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001. b We examined the effects of AhR on cell invasion using Matrigel-coated transwell chambers. T47D and ZR75-1 were treated with 10 nM E2 and/or 10 nM TCDD in phenol red- and FBS-free medium. After 24 h, the cells were plated in the upper chambers (5 × 105 cells/ml). Phenol red-free charcoal-stripped RPMI 1640 medium with 10 % FBS was used as a chemoattractant in the lower chamber. After 24 h, the cells invaded were counted. With 10 nM E2 administration, T47D and ZR75-1 invaded with forming larger clusters, and the number of invaded cells counted at three to five random fields in hotspot areas was significantly higher than that in control. A 10 nM TCDD administration significantly suppressed E2-dependent induction of cell invasion of T47D (P = 0.0431) and ZR75-1 (P = 0.0452). Bars mean ± SE (n = 3–5). *P < 0.05, **P < 0.01. c Cell proliferation assay using WST-8 method. Both T47D and ZR75-1 demonstrated no significant changes of the number of the cells between control and 10 nM E2 administration in serum-free medium for 24 h. Bars mean ± SE (n = 6). d We confirmed the activation of AhR by examining CYP1A1 induction. Bars mean ± SE (n = 3). ***P < 0.001 compared with control
Cell migration is an important process promoting tumor invasion. Therefore, we further examined the effects of AhR upon cell invasion using Matrigel-coated transwell chambers (Fig. 4b). With 10 nM E2 in phenol red- and FBS-free medium for 24 h, both T47D and ZR75-1 invaded with making larger clusters than control, and the number of invaded cells counted at some fields at hot spot areas were significantly larger than the control (P = 0.0358 and P = 0.0024, respectively). The 10 nM TCDD also significantly suppressed E2-dependent induction of cell invasion in T47D (P = 0.0431) and ZR75-1 (P = 0.0452).
We performed the following cell proliferation assay in order to investigate the correlation between the status of cell proliferation and the results of wound healing assay and invasion assay, using WST-8 method (Fig. 4c). Both T47D and ZR75-1 demonstrated no significant changes of the cell number (optical density) between control and 10 nM E2 administration in serum-free medium for 24 h, which indicated that the effects of cell proliferation upon the migration and invasion assays were negligible in this condition. We confirmed AhR activation by examining CYP1A1 induction (Fig. 4d).
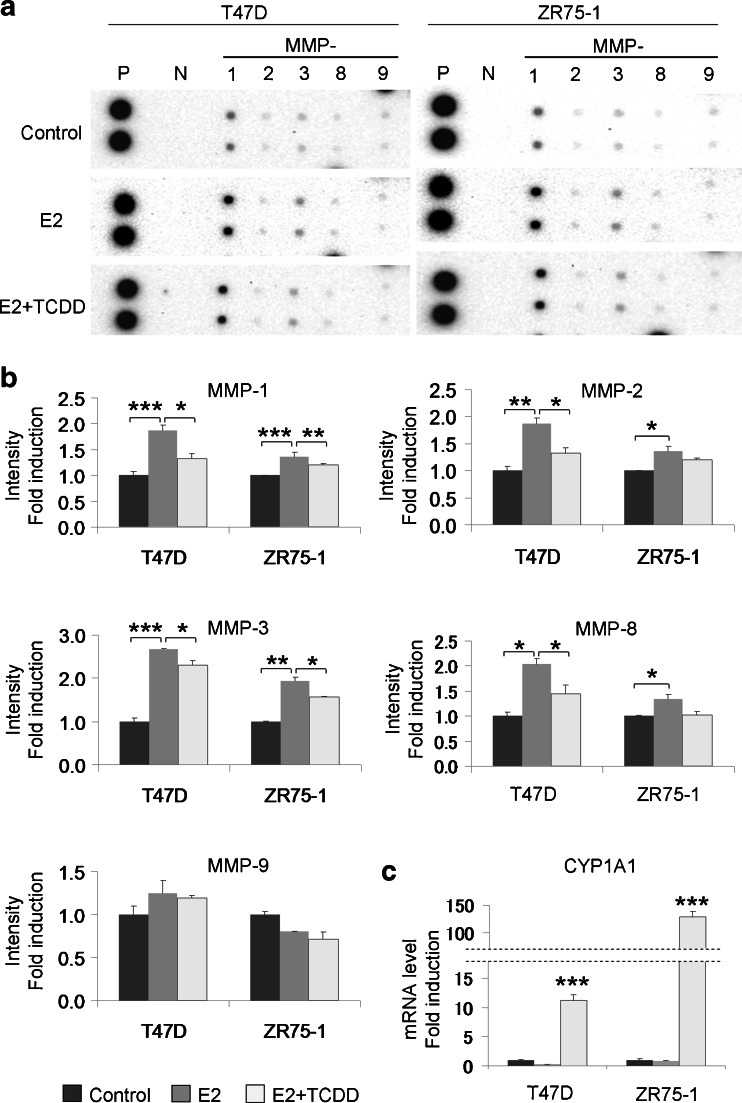
MMP Protein Array—AhR Inhibited E2-Induced MMPs Expression
MMPs are well-known and important factors involved in the process of cell migration and invasion. We therefore examined the effects of E2 and AhR on MMPs protein expression, using the human matrix metalloproteinase antibody array. We evaluated the protein expression levels by measuring corrected intensity of each spots.
Results were summarized in Fig. 5a, b. Exposure to 10 nM E2 in charcoal-stripped medium containing FBS for 72 h significantly increased MMP-1, MMP-2, MMP-3, and MMP-8 in T47D (P < 0.0001, P = 0.0073, P = 0.0004, and P = 0.0110, respectively), and in ZR75-1 (P = 0.0003, P = 0.0254, P = 0.0011, and P = 0.0463, respectively). In addition, 10 nM of TCDD significantly suppressed these E2-dependent induction of MMP-1, MMP-2, MMP-3, and MMP-8 in T47D (P = 0.0252, P = 0.0265, P = 0.0313, and P = 0.0480, respectively), and MMP-1 and MMP-3 in ZR75-1 (P = 0.0085 and P = 0.0161, respectively). The same tendency was also detected in MMP-8 of ZR75-1 but the degrees of suppression did not reach the statistical significance (P = 0.0524). In ZR75-1, 10 nM TCDD did not suppress E2-induced MMP-2 expression. We also confirmed the activation of AhR by examining CYP1A1 induction (Fig. 5c).
Fig. 5.
a We examined MMPs protein expression and the effects of E2 and AhR on MMPs, using the human matrix metalloproteinase antibody array. b We examined the protein expression levels by measuring corrected relative immunointensity of each spots. Bars mean ± SE (n = 2). *P < 0.05, **P < 0.01, and ***P < 0.001. c We confirmed the activation of AhR by examining CYP1A1 induction. Bars mean ± SE (n = 3). ***P < 0.001 compared with control
Migration and invasion under TCDD alone condition demonstrated no significant changes. Therefore we presented only three conditions above. MMP array using TCDD alone demonstrated no significant change, although performed in different condition (10 nM TCDD in charcoal stripped RPMI 1640 medium with 10 % FBS for 48 h; ESM Fig. 3).
Discussion
Results of our present study did demonstrate that AhR status in carcinoma cells is considered a newly identified prognostic marker of breast cancer patients. Results of our in vitro studies also demonstrated that AhR significantly suppressed cell proliferation in both ER-positive and ER-negative cells as well as migration and invasion of ER-positive cells possibly due to ER-AhR cross-talk action, which may be related to an induction of MMPs expression.
Results of our immunohistochemical study also did demonstrate that the number of the AhR positive cells was significantly higher in carcinoma cells than in nonpathological epithelium. This finding also indicated that an upregulation of AhR may be caused by the factors increasing AhR expression, which were present in cancer microenvironment such as interleukin-4, interleukin-13, tumor growth factor beta, and Wnt/beta-catenin [15, 39, 42]. In our study, the status of AhR immunoreactivity was also inversely associated with histological grade and Ki-67 LI, and positively with ERα and PR LIs of the patients. In invasive ductal carcinoma, well-differentiated carcinoma tends to be associated with high ER and PR LIs, and low Ki-67 LI or luminal A type [5, 20]. AhR signaling was also reported to promote breast carcinoma cell differentiation [14]. These results all indicated that AhR expression in breast carcinoma cells could represent the novel marker of cell differentiation of invasive ductal carcinoma. AhR immunoreactivity in breast carcinoma cells was significantly associated with age of the patients. AhR can therefore be considered the target of specific therapy in pre- or perimenopausal breast cancer patients but it awaits further investigations for clarification.
Results of univariate analysis in our present study did demonstrate that the groups of patients with positive AhR status was significantly associated with a good clinical outcome and subsequent multivariate analysis also did reveal that the status of AhR immunoreactivity in individual patients turned out an independent prognostic factor of the patients. AhR status was significantly associated with a good clinical outcome in ERα positive cases, and similar tendency was also detected in ERα negative cases. Therefore, the effects of AhR upon clinical course of breast cancer patients are reasonably postulated to be due to the results of ER-AhR cross-talks but further investigations are required for clarification.
AhR activation was also reported to induce an inhibition of breast carcinoma cell proliferation, especially in ER-negative cell lines [41, 44] or putative breast cancer stem-like cells [35]. It is well known that the E2-induced cell proliferation of breast carcinoma cells is inhibited by AhR activation primarily through its effects on cell cycle progression [1, 13, 37]. However, some studies also did demonstrate that AhR-mediated actions stimulated the growth of the breast carcinoma cell lines in E2-free medium [22, 34]. In our present immunocytochemical studies using both ER-positive and-negative cell lines and TCDD in E2-free medium, TCDD did decrease Ki-67 LI of all four breast carcinoma cell lines examined. We further confirmed the inhibitory effects of TCDD on breast carcinoma cell proliferation in both ER-positive and ER-negative cell lines.
Results of our present studies also demonstrated that AhR signaling suppressed E2-induced migration, invasion, and some MMP subtypes expression in ER-positive cells. AhR-induced MMPs expression and enhanced invasiveness in some malignant tumor cells, including urothelial carcinoma [16], gastric carcinoma [32], melanoma [40], and others. However, the studies investigating AhR actions on MMPs expression, migration, and invasiveness of breast cancer have not been reported to the best of our knowledge. TCDD-activated AhR complex was reported to inhibit ER-dependent gene expression in vitro [30]. It is also suggested that MMPs play important roles in the process of breast carcinoma cell invasion [12, 14, 29, 31]. In addition, treatment of E2 increased MMP subtypes such as MMP-2 and MMP-9 in human neuroblastoma cells [26]. However, details on the relation between E2, AhR, and other MMPs have remained largely unknown. We therefore examined cell migration and invasiveness of ER-positive breast cancer cell lines (T47D and ZR75-1), which were also associated with higher invasive potentials than MCF-7 [12], using 10 nM E2, 10 nM TCDD, and/or 10 μM AhR antagonist (CH-223191) in serum-free medium. E2 induced cell migration and invasiveness. TCDD significantly suppressed these inductions above. In addition, the inhibitory effects of TCDD on E2-dependent cell migration were significantly reduced by specific AhR antagonist in T47D. These findings all indicated that TCDD may suppress or decrease the migration of carcinoma cells induced by E2 treatment through its binding to AhR. We then studied all major MMPs protein expression and identified MMPs protein associated with AhR and E2 effects. Exposure to E2 significantly increased expression of MMP-1, MMP-2, MMP-3, and MMP-8 in both cell lines. TCDD also significantly inhibited E2-dependent induction of MMP-1 and MMP-3 in both cell lines, and MMP-2 and MMP-8 in T47D. In ZR75-1, MMP-8 was increased by E2 treatment and TCDD suppressed this increase of MMP-8. These findings above all indicated that TCDD suppressed migration and invasiveness of carcinoma cells possibly through MMPs protein expression following ER-AhR cross-talk in ER-positive breast carcinoma cells.
TCDD was reported to cause AhR-mediated DNA damages, which subsequently resulted in increased risk of developing several types of human malignancies [23]. However, the mechanisms of TCDD-induced carcinogenesis or mutagenesis have not been fully elucidated. AhR is also known to possess ER-AhR cross-talk actions resulting in an inhibition of activities of E2-dependent tumors. These findings suggest that TCDD inhibits breast carcinoma progression in vitro because ER-AhR cross-talk effect overwhelmed toxic effects of AhR.
In summary, AhR status in carcinoma cells is considered a newly identified prognostic marker of breast cancer patients. Results of our in vitro studies also demonstrated that AhR suppressed cell proliferation in both ER-positive and ER-negative carcinoma cells, and AhR also suppressed migration and invasion of ER-positive cells possibly due to ER-AhR cross-talk actions through the regulation of MMPs expression. These effects through AhR could reflect relatively favorable clinical outcome of the AhR-positive breast cancer patients, especially among ER-positive cases.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Immunohistochemistry of ERα and HER2 in human invasive ductal carcinoma. (A) A case with abundant immunoreactivity of ERα. (B) A case with negative ERα immunoreactivity. (C) A case positive for HER2 immunoreactivity. (D) A case negative for HER2 immunoreactivity. Scale bar, 100 μm (JPEG 236 kb)
The mRNA induction of CYP1A1 in MCF-7 by TCDD in charcoal stripped RPMI 1640 medium with 10% FBS for 48 h depends on TCDD concentration (1 pM, 10 pM, 1 nM and 10 nM). Bars, mean ± SE (n = 3). **P < 0.01, ***P < 0.001 compared with control (JPEG 42 kb)
MMP array in different conditions. T47D were treated with 10 nM TCDD in charcoal stripped RPMI 1640 medium with 10% FBS for 48 h. No significant changes were detected (JPEG 32 kb)
Acknowledgments
We gratefully acknowledge Erina Iwabuchi and Kazue Ise (Department of Pathology, Tohoku University School of Medicine) for their excellent technical support.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- 1.Abdelrahim M, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol Pharmacol. 2003;63(6):1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 2.Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA, 3rd, Kato T, Saeki K, Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001;276(34):31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- 3.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–250. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertazzi PA, Zocchetti C, Guercilena S, Consonni D, Tironi A, Landi MT, Pesatori AC. Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident”. Epidemiology. 1997;8(6):646–652. [PubMed] [Google Scholar]
- 5.Caldarella A, Crocetti E, Bianchi S, Vezzosi V, Urso C, Biancalani M, Zappa M. Female breast cancer status according to ER, PR and HER2 expression: a population based analysis. Pathol Oncol Res. 2011;17(3):753–758. doi: 10.1007/s12253-011-9381-z. [DOI] [PubMed] [Google Scholar]
- 6.Chang JT, Chang H, Chen PH, Lin SL, Lin P. Requirement of aryl hydrocarbon receptor overexpression for CYP1B1 up-regulation and cell growth in human lung adenocarcinomas. Clin Cancer Res. 2007;13(1):38–45. doi: 10.1158/1078-0432.CCR-06-1166. [DOI] [PubMed] [Google Scholar]
- 7.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 8.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell–cell contact and tumor growth. Carcinogenesis. 2010;31(8):1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993;44(5):911–917. [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 12.Figueira RC, Gomes LR, Neto JS, Silva FC, Silva ID, Sogayar MC. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer. 2009;9:20. doi: 10.1186/1471-2407-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gierthy JF, Bennett JA, Bradley LM, Cutler DS. Correlation of in vitro and in vivo growth suppression of MCF-7 human breast cancer by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res. 1993;53(13):3149–3153. [PubMed] [Google Scholar]
- 14.Hall JM, Barhoover MA, Kazmin D, McDonnell DP, Greenlee WF, Thomas RS. Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol Endocrinol. 2010;24(2):359–369. doi: 10.1210/me.2009-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper PA, Riddick DS, Okey AB. Regulating the regulator: factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol. 2006;72(3):267–279. doi: 10.1016/j.bcp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Ishida M, Mikami S, Kikuchi E, Kosaka T, Miyajima A, Nakagawa K, Mukai M, Okada Y, Oya M. Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis. 2010;31(2):287–295. doi: 10.1093/carcin/bgp222. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69(6):1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 18.Klinge CM, Bowers JL, Kulakosky PC, Kamboj KK, Swanson HI. The aryl hydrocarbon receptor (AHR)/AHR nuclear translocator (ARNT) heterodimer interacts with naturally occurring estrogen response elements. Mol Cell Endocrinol. 1999;157(1–2):105–119. doi: 10.1016/S0303-7207(99)00165-3. [DOI] [PubMed] [Google Scholar]
- 19.Larsen MC, Angus WG, Brake PB, Eltom SE, Sukow KA, Jefcoate CR. Characterization of CYP1B1 and CYP1A1 expression in human mammary epithelial cells: role of the aryl hydrocarbon receptor in polycyclic aromatic hydrocarbon metabolism. Cancer Res. 1998;58(11):2366–2374. [PubMed] [Google Scholar]
- 20.Laurinavicius A, Laurinaviciene A, Ostapenko V, Dasevicius D, Jarmalaite S, Lazutka J. Immunohistochemistry profiles of breast ductal carcinoma: factor analysis of digital image analysis data. Diagn Pathol. 2012;7(1):27. doi: 10.1186/1746-1596-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin P, Chang H, Tsai WT, Wu MH, Liao YS, Chen JT, Su JM. Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol Pathol. 2003;31(1):22–30. doi: 10.1080/01926230309746. [DOI] [PubMed] [Google Scholar]
- 22.Lin PH, Lin CH, Huang CC, Chuang MC, Lin P. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly(ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol Lett. 2007;172(3):146–158. doi: 10.1016/j.toxlet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B. 2005;175(4):221–230. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- 24.Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem. 2005;96(6):1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- 25.Mason ME, Okey AB. Cytosolic and nuclear binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to the Ah receptor in extra-hepatic tissues of rats and mice. Eur J Biochem. 1982;123(1):209–215. doi: 10.1111/j.1432-1033.1982.tb06518.x. [DOI] [PubMed] [Google Scholar]
- 26.Merlo S, Sortino MA. Estrogen activates matrix metalloproteinases-2 and -9 to increase beta amyloid degradation. Mol Cell Neurosci. 2012;49(4):423–429. doi: 10.1016/j.mcn.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Morrow D, Qin C, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J Steroid Biochem Mol Biol. 2004;88(1):27–36. doi: 10.1016/j.jsbmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson UW, Garvin S, Dabrosin C. MMP-2 and MMP-9 activity is regulated by estradiol and tamoxifen in cultured human breast cancer cells. Breast Cancer Res Treat. 2007;102(3):253–261. doi: 10.1007/s10549-006-9335-4. [DOI] [PubMed] [Google Scholar]
- 30.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 31.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10(22):7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 32.Peng TL, Chen J, Mao W, Song X, Chen MH. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;10:27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: twenty years of follow-up. Environ Health. 2009;8:39. doi: 10.1186/1476-069X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pliskova M, Vondracek J, Vojtesek B, Kozubik A, Machala M. Deregulation of cell proliferation by polycyclic aromatic hydrocarbons in human breast carcinoma MCF-7 cells reflects both genotoxic and nongenotoxic events. Toxicol Sci. 2005;83(2):246–256. doi: 10.1093/toxsci/kfi040. [DOI] [PubMed] [Google Scholar]
- 35.Prud'homme GJ, Glinka Y, Toulina A, Ace O, Subramaniam V, Jothy S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One. 2010;5(11):e13831. doi: 10.1371/journal.pone.0013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safe S, McDougal A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers (review) Int J Oncol. 2002;20(6):1123–1128. [PubMed] [Google Scholar]
- 37.Safe S, Wormke M, Samudio I. Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J Mammary Gland Biol Neoplasia. 2000;5(3):295–306. doi: 10.1023/A:1009550912337. [DOI] [PubMed] [Google Scholar]
- 38.Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, Denison MS. Activation of the Ah receptor signaling pathway by prostaglandins. J Biochem Mol Toxicol. 2001;15(4):187–196. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka G, Kanaji S, Hirano A, Arima K, Shinagawa A, Goda C, Yasunaga S, et al. Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int Immunol. 2005;17(6):797–805. doi: 10.1093/intimm/dxh260. [DOI] [PubMed] [Google Scholar]
- 40.Villano CM, Murphy KA, Akintobi A, White LA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol Appl Pharmacol. 2006;210(3):212–224. doi: 10.1016/j.taap.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Wang WL, Porter W, Burghardt R, Safe SH. Mechanism of inhibition of MDA-MB-468 breast cancer cell growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Carcinogenesis. 1997;18(5):925–933. doi: 10.1093/carcin/18.5.925. [DOI] [PubMed] [Google Scholar]
- 42.Wolff S, Harper PA, Wong JM, Mostert V, Wang Y, Abel J. Cell-specific regulation of human aryl hydrocarbon receptor expression by transforming growth factor-beta(1) Mol Pharmacol. 2001;59(4):716–724. doi: 10.1124/mol.59.4.716. [DOI] [PubMed] [Google Scholar]
- 43.Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol Cell Biol. 2003;23(6):1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Lei P, Liu X, Li X, Walker K, Kotha L, Rowlands C, Safe S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr Relat Cancer. 2009;16(3):835–844. doi: 10.1677/ERC-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry of ERα and HER2 in human invasive ductal carcinoma. (A) A case with abundant immunoreactivity of ERα. (B) A case with negative ERα immunoreactivity. (C) A case positive for HER2 immunoreactivity. (D) A case negative for HER2 immunoreactivity. Scale bar, 100 μm (JPEG 236 kb)
The mRNA induction of CYP1A1 in MCF-7 by TCDD in charcoal stripped RPMI 1640 medium with 10% FBS for 48 h depends on TCDD concentration (1 pM, 10 pM, 1 nM and 10 nM). Bars, mean ± SE (n = 3). **P < 0.01, ***P < 0.001 compared with control (JPEG 42 kb)
MMP array in different conditions. T47D were treated with 10 nM TCDD in charcoal stripped RPMI 1640 medium with 10% FBS for 48 h. No significant changes were detected (JPEG 32 kb)