Abstract
Granulosa cell tumors of the ovary (GCT) represent ~5% of malignant ovarian tumors. The adult form is defined by a mutation in the FOXL2 gene. GCT exhibit many of the features of normal proliferating granulosa cells. We have profiled the expression of the 48 human nuclear receptors (NR) by quantitative RT-PCR in a panel of GCT and in two GCT-derived cell lines, COV434 and KGN. The highest level of expression is seen for COUP-TF2 with abundant expression of PPARγ, SF-1, and TR-α. Estrogen receptor (ER)-β is the most abundant of the steroid receptors with relatively high expression also of AR, ER-α, and PR. The concordance of expression for each NR across the tumors is remarkably high with same discordance between the cell lines and the tumors, particularly the COV434 line. No significant differences were observed with respect to tumor stage for NR expression. These findings provide a full profile of NR expression in GCT which will enable full characterization of their roles and potential as therapeutic targets.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-011-0069-3) contains supplementary material, which is available to authorized users.
Keywords: Steroid receptors, Ovarian, Tumor, FOXL2
Introduction
Granulosa cell tumors of the ovary (GCT) are a specific subset of malignant ovarian tumors which, in contrast to the more common epithelial tumors, arise from the stromal cells of the ovary. GCT are the predominant form of ovarian stromal tumor, representing ~5% of all malignant ovarian tumors [1, 2]. Although GCT are generally thought to have a better prognosis than epithelial ovarian tumors, ~80% of patients with advanced or recurrent tumors die from their disease [3].
GCT exhibit many morphological, biochemical, and hormonal features of normal proliferating pre-ovulatory granulosa cells (GC), including both estrogen and inhibin biosynthesis [1, 4, 5]. The latter hormone has proven to be a useful tumor marker in post-menopausal women and in women post-oophorectomy [5, 6]. The peak incidence for GCT is in the early 50’s but they may arise at any age; a much less common juvenile form of the disease occurs representing ~5% of GCT. Identification of the molecular pathogenesis of these tumors has proven elusive despite extensive investigation [6, 7]. In the case of juvenile GCT and in contrast to adult GCT, a recent study found that 30% contain the gsp oncogene, an activating mutation of Gαs [8]. In the case of adult GCT, the landscape has recently changed with the finding that 97% of a large series of GCT contain a missense mutation (C134W) in the FOXL2 gene [9]. Interestingly, this mutation is not found in juvenile GCT or in other stromal tumors, except where they contain GCT-like elements. This finding has been confirmed in two subsequent independent studies [10, 11]. Curiously, in juvenile GCT, advanced stage is associated with loss of FOXL2 expression [12]. It can be argued that the presence of this mutation defines adult GCT. The significance of the mutation remains to be determined. Given that the mutation is present in all adult GCT, irrespective of stage, it may be aetiologic but it does not explain the differing patterns of pathogenesis.
The NR superfamily members are usually defined as ligand-dependent transcription factors, although in some cases their ligand remains to be identified [13]. The canonical receptor consists of a central cysteine-rich DNA binding domain of 66 or 68 amino acids with a C-terminal, ligand-binding domain (LBD). The LBD consists of 12 or 11 α-helices in three anti-parallel layers; this tertiary structure is also highly conserved. N-terminal to the DBD is the N-terminal domain whose length and sequence is not conserved between receptors [14]. NR play a critical role in endocrine signaling and in hormone-dependent malignancies such as cancers of the breast, prostate and uterus.
Granulosa cells both synthesize steroid hormones and respond to steroid hormones. Whilst a potential role for some NR such as the steroidogenic factor-1 (SF-1) has been well characterized in the biology of GC, the patterns of expression of the other NR have not been systematically examined. Similarly, there is limited information on the role of NR in GCT; we have characterized the estrogen receptor (ER)-β in GCT [15] and others have examined ER-α, the progesterone receptor (PR), and SF-1 [16–18]. The role of other NR known to be expressed in GC has not been examined nor has there been a systematic expression profiling of the entire NR superfamily. Although such data might in principle be obtained from analysis of published microarray data sets, the relatively low abundance of transcription factors means that NR are often not captured in microarray analysis. In addition, with the exception of the sequencing data from four tumors in the FOXL2 study [9], microarray analyses of GCT have not been reported [2].
Therefore, in order to systematically evaluate the expression of NR in GCT, we used the commercial, ABI TaqMan Low-Density Nuclear Receptor Gene Signature Array (TLDA) to screen a well-defined cohort of GCT together with the two GCT-derived cell lines, COV434 and KGN. The relative expression across the various adult GCT has been analyzed against a commercial universal reference sample which is a tumor sample (derived from ten human cell lines). Subsequent to the analysis, we were also able to confirm the FOXL2 mutation status of the GCT examined. This dataset confirms and extends previous studies of individual nuclear receptors in granulosa cells. In several cases, these NR, represent potential therapeutic targets for the treatment of GCT.
Materials and Methods
Isolation of RNA from Tissue and Cell Line Species
RNA was isolated from GCT (n = 14) collected sequentially at our institution [10] and from the two GCT-derived cell lines COV434 [19] and KGN [20] which have been described previously [21]. RNA was extracted using the guanidine thiocyanate/caesium chloride method as previously described [22]. The RNA quality was checked by electrophoresis using a Bio-Rad Experion™ automated electrophoresis system (Bio-Rad Laboratories, Hercules, CA). The details of the individual tumors and their FOXL2 mutational status are shown in Table 1. The collection and use of this tissue is approved by the Research and Ethics Committee of Monash Medical Centre, and all women gave written informed consent for collection of the tissue. The human tumor RNA reference sample is an FDA-approved reference control from Stratagene (catalog no. 740000).
Table 1.
Clinical information of the patients studied
| Sample | FOXL2 status (*) | Patient age | Tumor stage | Menopausal status |
|---|---|---|---|---|
| 1 | Het | 31 | I | Pre |
| 2 | Het | 48 | Recurrent, metastatic | Pre |
| 3 | Het | 66 | Recurrent, metastatic | Post |
| 4 | Het | 58 | Recurrent, metastatic | Post |
| 5 | Hemi | 50 | Recurrent | Pre |
| 6 | Het | 53 | I | Pre |
| 7 | Het | 54 | I | Pre |
| 8 | Het | 45 | Recurrent | Pre |
| 9 | WT | 71 | Recurrent | Post |
| 10 | Het | 56 | Recurrent | Post |
| 11 | WT | 80 | High grade (1/2) | Post |
| 12 | Het | 50 | Ic | Post |
| 13 | Het | 54 | Recurrent | Post |
| 14 | Het | 79 | 1 | Post |
| COV434 | WT | 27 | Metastatic | Pre |
| KGN | Het | 73 | III, recurrent | Post |
Het heterozygous for the FOXL2 mutation, WT wild-type FOXL2 sequence only, Hemi FOXL2 mutation only (a distinction between homozygous and hemizygous is not possible), see Jamieson et al. [10]
TaqMan Low-Density Array
Commercial micro-fluidic cards, the TLDA (Applied Biosystems, catalog no. 4379961), that contain an exclusive set of TaqMan gene expression assays for the 48 NR and 16 internal controls (eukaryotic 18S rRNA (18S), beta-actin (ACTB), beta-2-microglubulin (B2M), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glucuronidase, beta (GUSB), hydroxymethylbilane synthase (HMBS), hypoxanthine phosphoribosyltransferase 1 (HPRT1; Lesch-Nyhan syndrome), importin 8 (IPO8), phosphoglycerate kinase 1 (PGK1), polymerase (RNA) II (DNA directed) polypeptide A, 220 kDa (POLR2A), peptidylprolyl isomerase A (cyclophilin A) (PPIA), ribosomal protein, large, P0 (RPLP0; same as 36B4 in mouse), TATA box binding protein (TBP), transferrin receptor (p90, CD71) (TFRC), ubiquitin C (UBC), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ)) were used to profile gene expression. These controls span the relative abundance/C t range of the genes on the card, and three (18S rRNA, GAPDH, and RPLPO) are validated real-time PCR controls from Nuclear Receptor Signaling Atlas (NURSA)-supported NR studies [23, 24] The geNorm software imbedded within the ABI/Intergromics StatMiner V4.1 software package was used to compute least expression variation and select the most appropriate, stable, and robust combination of internal control genes with which to normalize the expression data (against the mean of the most stable controls).
For each sample, 1.5 μg of total RNA was reverse transcribed using random hexamers with SuperScript III reverse transcriptase (Invitrogen) in a total volume of 45 μl. A total of 100-μl reaction mixture containing 50-μl cDNA template (333 ng) in RNase-free water and an equal volume of TaqMan® universal master mix (Applied Biosystems, Foster City, CA) was added to each TLDA fill reservoir. Each GCT sample was run once while the cell lines were run as three biological replicates. Four reservoirs per sample were filled. The TLDA includes all NR and endogenous controls in triplicate. After sealing the plate, it was run on an ABI 7900HT Real-Time instrument (Applied Biosystems).
Statistical Analysis
The TLDAs were analyzed for Fig. 1 using the relative quantification method of ΔC t. The geNorm software imbedded within the ABI/Intergromics StatMiner V4.1 software package was used to compute least expression variation and select the appropriate and most stable combination of internal control genes with which to normalize the expression data. The expression data were normalized to the median of GAPDH, HMBS, RPLPO, and TBP. A calibrator/reference sample was not used and hence no significance between samples was calculated. The data are expressed as percentage of the geNorm-selected controls.
Fig. 1.
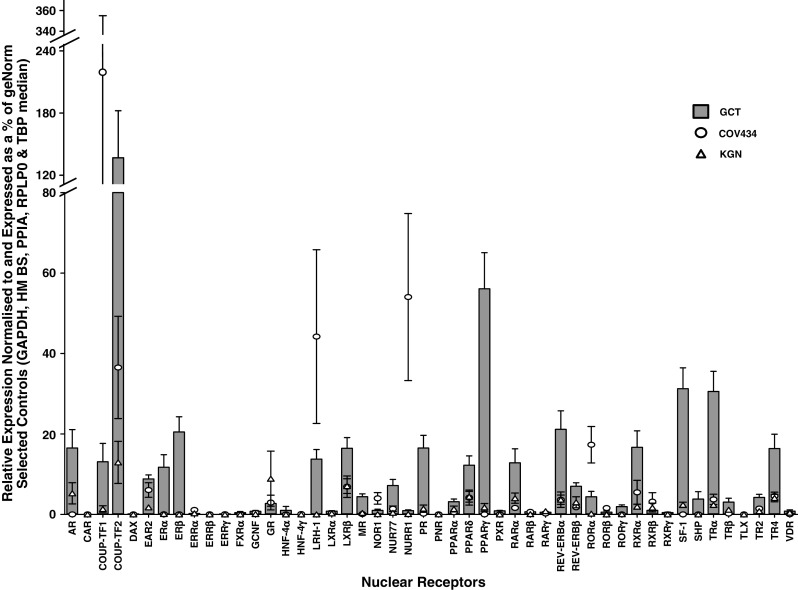
Nuclear receptor gene expression analysis in Granulosa cell tumors of the ovary (GCT) and GCT-derived cell lines, COV434 and KGN. Values were averaged over 14 GCT samples and over three replicate experiments for the COV434 and KGN cell lines. Samples with a CT value of ≥35 were given a value of 0. Data are presented as the relative expression normalized to and expressed as a percentage of the median of the geNorm-selected controls of GAPDH, HMBS, RPLPO, and TBP. The bar graphs represent mean ± SEM for the GCT; the values for the COV434 and KGN cells are the mean of three independent determinations. Actual values are shown in the Electronic Supplementary Material (Online Resource 1)
The TLDA were analyzed as described in Raichur et al. [25] for Online Resources 1, 3, and 4 (Electronic Supplementary Material). Briefly, significant changes in expression relative to reference samples or an FDA-approved universal human tumor RNA sample (Stratagene) were analyzed using the ABI/Integromics “StatMiner” software package. Differentially expressed genes were identified by the comparative C t method and significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [26]. Furthermore, we performed conservative data filtering (Benjamin–Hochberg) to control for false-discovery rate (FDR), and further refined the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target and the calibrator/reference sample/tissue) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples was <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected, and no detection (i.e., expression in either tissue), when the C t value of the gene(s) in 50% or more of the target samples, calibrator, and/or both samples was >35 cycles (i.e., the arbitrarily selected threshold limit). Hence, the reported quantitative “fold change of a gene that is not expressed in some of the biological conditions may not be reliable” [27], however, it does reflect a qualitative difference. The data generated by the ABI SDS software from the ABI7900 instrument do not normally contain missing C t values, and C t values are assigned beyond the arbitrarily set threshold (C t 35) up to a maximum C t 40. In situations where the sample is undetected and the C t values are beyond the maximum C t 40, the StatMiner software imputes a value, set to the maximum C t.
Analysis of Relative Expression
Calculation of relative expression by StatMiner is not supported, so a workflow was developed to automate the processing of raw TLDA results files and generate N·X relative expression values, where N is the number of genes in the TLDA experiment, and X is the number of samples. For small experiments, these calculations can be done by hand, but in large studies such as this, where N·X = 960, automation is required. We have designed a Pipeline Pilot workflow to read the results files from the TLDA analysis, normalize against a set of endogenous control genes (E; in this case, the set is selected by geNorm embedded within StatMiner, see above) and generate the relative expression value for each detected gene N in each submitted sample X. These data are presented as percentages in Table 1 in the Electronic Supplementary Material and Fig. 1. The workflow also assigns samples to groups according to the sample identifiers used in the results files, and visualizes descriptive statistics for the groups (Online Resources 1 and 2 (Electronic Supplementary Material)). Relative expression (R) of each gene N, in sample X was calculated as follows:
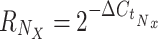 |
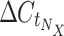 is calculated by:
is calculated by:
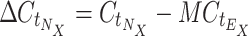 |
where 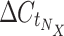 is the C
t value of gene N in sample X taken from the raw TLDA results file, and
is the C
t value of gene N in sample X taken from the raw TLDA results file, and 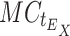 is the median C
t value from the set of selected endogenous controls. E is determined by StatMiner for the whole experiment, but C
t values of genes in E used to determine the median are specific to each sample.
is the median C
t value from the set of selected endogenous controls. E is determined by StatMiner for the whole experiment, but C
t values of genes in E used to determine the median are specific to each sample.
FOXL2 Mutation Detection
The identification by Shah et al. [9] of the FOXL2 C134W mutation in 97% of adult GCT led us to retrospectively assess the mutational status of our GCT panel [10]. The presence of the mutation (Table 1) was determined by RT-PCT with direct sequencing of the amplicon with the primers described by Shah et al. [9].
Results
The details of the GCT tissues examined and their FOXL2 mutational status are shown in Table 1. The tumors were selected on the basis that they were adult GCT however two were FOXL2 mutation negative. The significance of this is uncertain but it may be that they are a late-onset juvenile form of GCT [9]. Sample 11 is curious in that it exhibited very abundant FOXL2 expression levels [10]. The KGN cell line is heterozygous for the C134W mutation [10, 28, 29] whereas we found the COV434 line to be wild-type for FOXL2 and to have low to no expression [10], consistent with it being derived from an advanced juvenile GCT [12].
The relative expression of each NR normalized against the median of geNorm software selected controls (and expressed as a mean percentage) is shown in Fig. 1. It is in effect the inverse of the C t value. The software selects the most appropriate (and most stable) combination of internal control genes with which to normalize the expression data. The manufacturer states that the amplification efficiency of TaqMan gene expression assays is ~100 ± 10%, which suggests that the relative expression of each NR is an accurate reflection of the mRNA levels (normalized against control mRNA) in the tissue. The numerical values as well as the trivial gene names of each NR are given in Online Resource 1 in the Electronc Supplementary Material.
The highest level of expression in the tumors was observed for chicken ovalbumin upstream promoter-transcription factor 2 (COUP-TF2). Peroxisome proliferator-activated receptor (PPAR)-γ, SF-1 and thyroid hormone receptor (TR)-α also exhibited abundant expression. ER-β, perhaps not unexpectedly, was the most abundantly expressed steroid receptor although there is also expression of the androgen receptor (AR), ER-α, and the PR. Although the results represent the mean for the entire tumor panel, the concordance between tumors is extraordinarily high as is indicated by the small standard errors (Fig. 1). Although the negative FOXL2 mutation status of tumors 9 and 11 suggests that they may not be true adult GCT, their removal from the analysis does not materially alter the result.
The two cell lines generally parallel the results for the tumor panel although for several genes, there is discordance either between the lines and the GCT or between one line and the other. The relative expression for each NR between the cell lines and the GCT has been analyzed using the StatMiner software (Online Resource 2 in the Electronic Supplementary Material) to identify statistically significant differences. For the KGN cells, the results parallel that of the tumor panel being either equivalent or lower. In particular, the values for the most abundantly expressed genes in the GCT panel, COUP-TF2, PPAR-γ, TR-α, and SF-1 are significantly lower as are the two ER. The COV434 cells are significantly less concordant, with the expression of many genes being significantly lower than in the GCT (Online Resource 2b in the Electronic Supplementary Material). Retinoic acid receptor-related orphan receptor (ROR)-α, COUP-TF1 and nuclear receptor-related protein 1 (NURR1) expression was significantly elevated against the GCT panel.
Online Resource 3 (Electronic Supplementary Material) presents the box plots of the relative expression of the 48 NR in the GCT, the GCT derived cell lines and the FDA-approved universal human tumor RNA sample (Stratagene) normalized against the median of geNorm software selected controls, in this case 18S, HBMS, IPO8, and UBC. Furthermore, the expression data were also normalized against NURSA-utilized controls [30], for example, 18S rRNA (that was also selected as a stable control) and 36B4/RPLP0 which has a similar relative abundance to the genes on the card.
Analyses have also been performed using StatMiner software to compare the tumors and the cell lines with an accepted reference sample. The results in which the GCT are compared for each NR against the tumor reference are shown (Online Resource 4 in the Electronic Supplementary Material). This analysis allows the data to be compared against other similar data sets which have been standardized using this RNA sample.
The pattern of NR expression was compared between the stage 1 GCT and those that were more advanced or recurrent (Online Resource 5 in the Electronic Supplementary Material). For this analysis, only the FOXL2 mutant positive tumors (Table 1) were examined. As might be predicted from the standard errors obtained for the group data (Fig. 1), no significant differences for a given NR were identified between these two groups. The exception is FXR-α, however its absolute levels of expression (Fig. 1) are extraordinarily low. The pattern of NR expression therefore does not appear to predict stage or behavior.
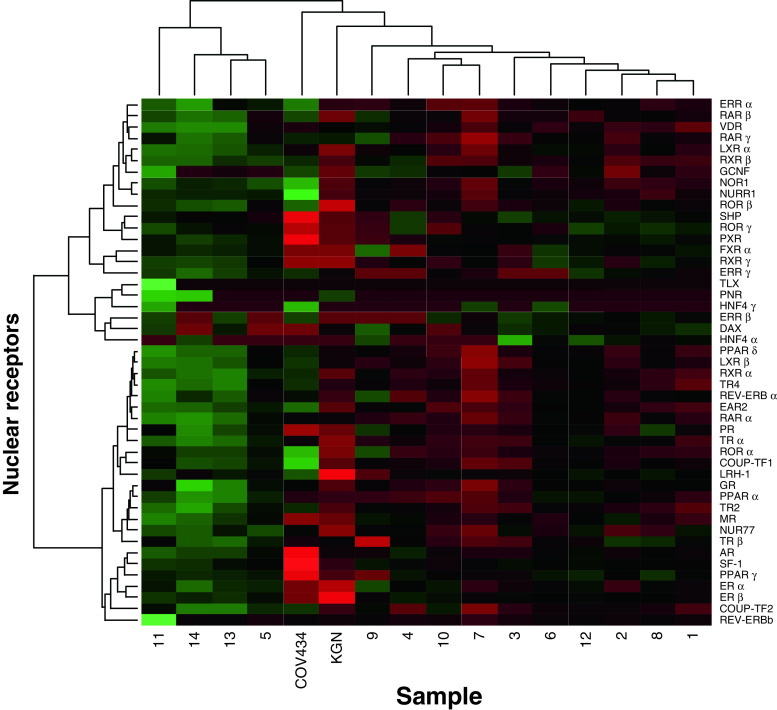
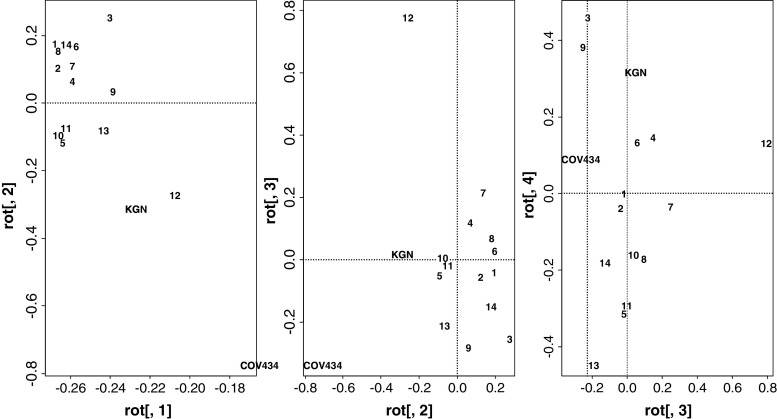
Hierarchical cluster analysis (Fig. 2) for each of the GCT and the two cell lines against the expression levels suggest some separation of the tumors into two principal groups, however this does not correspond to either FOXL2 mutation status, stage or clinical outcome. The rather anomalous tumor, sample 11, which has no FOXL2 mutation, high FOXL2 expression and a juvenile GCT-like morphology yet arose in an elderly woman [10], differs most from the group data. Principal component analysis (Fig. 3) similarly did not identify specific clusters. Surprisingly, the COV434 and KGN cell lines did not consistently separate from the tumors nor did tumor 11 consistently separate from others. The principal component analysis did not, as with the analysis of the individual NR (Online Resource 5 in the Electronic Supplementary Material), separate the tumors by stage.
Fig. 2.
Hierarchical cluster analysis. The data are presented as a heatmap and have been clustered according to sample type and gene expression
Fig. 3.
Principal component analysis for the granulosa cell tumors of the ovary (GCT) and the GCT-derived cell lines, COV434 and KGN. The first principal component accounts for as much of the variability in the data as possible, and each succeeding component accounts for as much of the remaining variability as possible. The first three components of 16 are presented
Discussion
Nuclear receptors play a fundamental role in many aspects of cellular metabolism, proliferation and homeostasis [13, 14]. In hormone-dependent cancers, they sometimes have a central pathogenic role, making them important therapeutic targets. GCT have not been well characterized with respect to NR. Indeed studies in normal GC are, with some exceptions, very limited. In this study, we have systematically determined the expression levels of the 48 NR in 14 GCT and in two GCT-derived cell lines. The data are remarkable on two counts, firstly for the wide variety in expression levels between the 48 NR and secondly, for the extraordinary concordance obtained between tumors for NR expression. In previous studies examining the expression of specific genes in GCT, we have observed remarkable homogeneity between GCT [4, 31] and that is again seen for the NR. Abundant expression is seen in decreasing order of COUP-TF2, PPAR-γ, SF-1, TR-α, and ER-β. A significant number of NR show very low/no expression.
Chicken Ovalbumin Upstream Promoter-Trancription Factor
COUP-TF2 is the most abundantly expressed NR in the GCT and indeed in both cell lines. COUP-TF2 is a ubiquitously expressed orphan member of the NR superfamily. Together with COUP-TF1 and v-erbA-related protein 2 (EAR2) it is a type III nuclear receptor. The role of COUP-TF2 has been characterized in GC where it is reported to negatively regulate SF-1-dependent transcription of steroidogenic enzymes [32]. In normal human GC, COUP-TF2 immunoreactivity is predominantly located to the thecal cells with high levels maintained across the menstrual cycle. In vitro studies suggest that COUP-TF2 is also a repressor of the FSH receptor promoter [33]. Superficially therefore, it might be concluded that COUP-TF2 antagonizes the induction of the differentiated phenotype in GC, a phenotype which ultimately leads to luteinization and terminal differentiation. The ubiquitous nature of its expression and its lack of ligands make it an interesting but challenging therapeutic target. It is tempting to speculate that the expression of COUP-TF2 may be driven by FOXL2, given the fundamental role of both factors in GC development. Expression of COUP-TF2 in human breast cancer has been associated with negative prognostic markers including VEGF-C expression [34]. Other studies in breast cancer have demonstrated interactions between COUP-TF2 and estrogen receptor signaling at estrogen response elements [35, 36]. Expression of COUP-TF2 is induced in breast cancer cell lines by the MAP kinase pathway; conversely, Lee et al. [37] reported that COUP-TF2 expression was down regulated in ovarian epithelial tumors. Our finding that COUP-TF2 was also abundantly expressed in both cell lines provides an experimental system in which the role of COUP-TF2 might be defined.
Peroxisome Proliferator Activating Receptor
In the GCT, expression of all three PPAR genes (α, δ, and γ) is observed with PPAR-γ expression being particularly abundant. Expression of PPAR-γ has been reported in a number of tumor types. PPAR-γ is the second most abundant NR in the GCT, it is modestly expressed in the KGN cells and arguably absent from the COV434 cells. Ligand-induced PPAR-γ activation has been explored as a therapeutic strategy in a number of tumors including endocrine malignancies such as thyroid cancer [38, 39]. There are only limited studies of PPAR-γ in GC [40, 41]. PPAR-γ acts as a heterodimer with its partner retinoid X receptor (RXR)-α and indeed there is significant expression of RXR-α in the GCT and also in both cell lines. Characterization of the role of PPAR-γ in GC has been aided by the availability of agonist ligands. These studies have primarily used isolated rat GC but in several studies the KGN cell line has also been examined, usually as a model of normal GC rather than as a model of GCT. Several studies have demonstrated a role for PPAR-γ in inhibiting proliferation and inducing apoptosis in cultured rat granulosa cells [40, 42]. It may also have a role in gonadotropin-induced steroidogenesis. PPAR-γ is expressed in GC in the rat where it promotes steroidogenesis [43]; its levels are decreased by LH [44], and PPAR-γ mRNA levels have a reciprocal relationship with those of P450SCC [45]. Seto-Young et al. [46] found that in isolated human GC obtained at the time of in vitro fertilization, PPAR-γ agonists markedly upregulated steroidogenic acute regulatory protein. This finding was also reported in murine GC [47]. The response of cultured human GC and KGN cells to PPAR-γ agonists and/or an RXR agonist has been examined [48]. Fan et al. [49] reported that PPAR-γ/RXR activation down-regulates aromatase gene expression through suppression of NF-κB-dependent aromatase activity. The findings of both increased [340] and decreased [49] estrogen biosynthesis in response to PPAR-γ activation are dichotomous. Whether they reflect species difference or the difference between measuring estrogen levels and aromatase promoter activity is not clear. Gasic et al. [50, 51] reported that PPAR-γ agonist treatment inhibits progesterone synthesis in porcine GC. Kim et al. [52] found that PPAR-γ was regulated by PR in the ovulatory follicle and that granulosa cell-specific deletion of PPAR-γ resulted in a failure of pre-ovulatory follicle receptors. They concluded that PPAR-γ was a key mediator of the action of PR in GC.
By analogy with other tissues, data in GC although limited, suggests primarily an anti-proliferative role for PPAR-γ which makes the high levels observed in the tumors unexpected. It is possible that there is some degree of resistance to the actions of PPAR-γ in the tumors. It also suggests that treatment with combined PPAR-γ/RXR ligands might be of therapeutic benefit.
PPAR-δ levels are 4-fold lower than those of PPAR-γ and 4-fold higher than those for PPAR-α. In the rat ovary, both PPAR-δ and PPAR-α are expressed primarily in the theca cells and in the stroma [43]. PPAR-δ is principally expressed in muscle where it has a role in regulating energy utilization. Whether it fills a similar role in the ovary remains to be established
Retinoid X Receptor
The retinoid X receptors form heterodimers with a number of NR including PPAR as discussed above. RXR-α is abundantly expressed whereas both RXR-β and RXR-γ expression is very low to absent. As noted, the possibility that activation of PPAR-γ ± RXR might have a therapeutic utility makes this finding of some interest. There is abundant and modest expression of RXR-α in the COV434 and KGN cells, respectively. Unfortunately, the COV434 cells lack PPAR-γ and the levels are only moderate in the KGN cells so it may be difficult to adequately address the therapeutic potential of combined PPAR-γ and RXR-α ligand treatment using these cell lines.
Thyroid Hormone Receptor
Thyroid hormone receptors are ubiquitously expressed. Falzacoppa et al. [53] examined the effect of T 3 on COV434 cells. They found both TR-α and TR-β in the COV434 and reported increased proliferation and decreased apoptosis in response to T 3 treatment. We found little TR-β in the tumors whereas TR-α was very abundant and also present in both the COV434 and KGN albeit at lower levels. In contrast to the study of Falzacoppa et al. [53], we found very little TR-β in either cell line. Several studies have reported that both TR-α and TR-β are expressed in normal GC [54, 55] although their functions are not clear. TR-α null mice are reported to have normal reproduction [56] so the specific role of ovarian TR-α expression is obscure, as is its role in GCT. In breast cancer there is evidence that TR-β may be a tumor suppressor gene [57] and it is interesting to reflect that the v-erb A oncogene is a dominant negative form of the thyroid receptor found in the avian erythroblastosis virus. The potential therefore of TR-α as a therapeutic target is not clear.
Steroidogenic Factor-1/Liver Receptor Homolog-1
The NR5A proteins, SF-1 and liver receptor homolog-1 (LRH-1), have important roles in the ovarian function. SF-1 null mice fail to develop ovaries [58], and granulosa cell-specific SF-1 null mice are infertile with hypoplastic ovaries, reduced follicle number and fail to develop corpora lutea [59, 60]. Humans with inactivating mutations in SF-1 exhibit a spectrum of phenotypes ranging from normal to primary ovarian insufficiency and disorders of sex development [61, 62]. Deletion of LRH-1 in mice is embryonic lethal [63], and granulosa cell-specific loss of LRH-1 results in annovulation [64]. LRH-1 expression is much higher than that of SF-1 in the corpus luteum, and mRNA for SF-1, but not LRH-1 is selectively expressed in theca cells [65]. Granulosa cells express both LRH-1 and SF-1 [66, 67]. SF-1 and LRH-1 bind to the same enhancer elements in a number of genes, with overlapping but distinct effects on FSH-stimulated estrogen and progesterone synthesis [68]. SF-1 appears to selectively regulate estrogen synthesis in that LRH-1 does not appear to be essential for estrogen synthesis, whereas LRH-1 is involved in progesterone production [69], consistent with the reduced intrafollicular progesterone levels seen in granulosa cell-specific LRH-1 null mice [64]. This regulation of progesterone synthesis has recently been reported to involve the NR co-activator PGC1-α [70]. Both LRH-1 and SF-1 have a role in the transcriptional activation of the inhibin α-subunit gene [71]; LRH-1 synergizes with the transcription factor GATA4 in the regulation of the inhibin α-promoter [72]. GATA4 expression is upregulated in GCT and is thought to have a role in their pathogenesis [73]. The significance of SF-1 and LRH-1 in GCT, however, is unknown. Interestingly, both the COV434 cells and the GCT have abundant LRH-1 expression whereas the KGN cells do not; for SF-1 the pattern is the converse with the COV434 cells having much lower levels than both the GCT and the KGN cells which have equivalent levels. In both cases, the question arises as to whether the expression of SF-1 and LRH-1 simply correlates with the cell type of origin of the tumors, or whether they have a pathogenic role. Since GCT express aromatase and synthesize estrogen, the presence of the NR5A family members is not unexpected. SF-1 immunohistochemistry has been advocate as a marker of sex-cord stromal tumors [17, 18]. Both SF-1 and LRH-1 positively regulate cell proliferation in other tumors (e.g., adrenal and colon) [74–78] and it is possible therefore that either or both receptors may influence proliferation of GCT.
Estrogen Receptor
Of the classical steroid receptors, the receptors for the gonadal steroids predominate. GC are the predominant site of expression of ER-β in the human female [79] and we have previously demonstrated expression of ER-β in GCT [15]. We also found that, in both cell lines, ER-β signaling was inhibited by constitutive activation of NF-κB signaling which provides at least circumstantial evidence that ER-β may be a tumor suppressor gene [18]. ER-α expression is ~50% that of ER-β. The significance of ER-α in GC and/or GCT is uncertain. In transgenic mice, deletion of either estrogen receptor induces an ovarian phenotype, however in the case of ER-α, this is an indirect effect via pituitary gonadotropins, whereas for ER-β, it is a direct effect via the GC [80]. It is curious that both tamoxifen therapy and aromatase treatment have been reported to have a therapeutic benefit in recurrent GCT [6].
Androgen Receptor
After ER-β, AR and PR are the most abundant of the steroid receptors in the GCT. The AR has also been reported to be abundantly expressed in the GC of pre-antral and antral follicles of the primate ovary [81]. The role of androgens and the AR in the ovary has recently been reviewed by Waters et al. [82]. Female mice null for the AR have subfertility secondary to increased GC apoptosis in the pre-ovulatory follilcles [83]. Sen and Hammes [84] have recently demonstrated that GC-specific AR gene deletion largely phenocopies the global knockout mice indicating that the reproductive phenotype reflects the role of the AR in GC. The AR appears to play a fundamental role in GC growth and in preventing follicular atresia [84]. Activating and inactivating mutations of the AR are features of prostate and breast cancer respectively; mutations in the AR have not been systematically sought in GCT.
Progesterone Receptor
GC expression of the progesterone receptor has long been recognized as a feature of the GC response to LH. Farinola et al. [16] reported that PR immunohistochemistry was positive in 98% of a series of GCT, which is consistent with our results for PR mRNA. Activation of the PR can be seen as both anti- and pro-proliferative in uterus and breast respectively. In addition, the pattern of response will be determined by the PR isoform expressed [84]. At this stage we have not established whether the PR in GCT is PRA, PRB, or both. Studies in murine systems [85] suggest that progesterone plays an essential role in ovulation, primarily via PRA. In addition, Friberg et al. [86] have reported that activation of the PR in rat pre-ovulatory GC decreases apoptosis through induction of genes involved in the control of apoptosis. This latter property may be relevant to GCT; however, a relative lack of ligand makes this of uncertain pathogenetic significance.
Testicular Receptor 4
Testicular receptor 4 (TR4) is an orphan nuclear receptor which has a role in spermatogenesis. It is however also expressed in GC. TR4 has been reported to repress ER-mediated transcription [87]. Chen et al. [88] have recently reported that female mice null for TR4 have poor follicular development and increased GC apoptosis. These authors suggest that it may also be involved in LH receptor expression. The levels of expression are similar in both the GCT and both cell lines, it is interesting to speculate that it may have an anti-apoptotic role in GCT.
Liver X Receptor
The liver X receptors (LXR)-α and LXR-β have a role in cholesterol homeostasis particularly in the liver [13]. Expression of the oxysteroid receptor LXR-β is relatively ubiquitous and has been reported in the rodent ovary [89]. The relatively greater abundance of LXR-β than LXR-α is consistent with results for the normal mouse ovary. Mice null for either LXR-α or LXR-β have enlarged ovaries, haemorrhagic corpus luteum and increased estradiol levels, suggesting that LXR normally play a role in ovarian steroidogenesis presumably through modulation of ovarian cholesterol metabolism [89, 90]. Drovineaud et al. [91] examined LXR function in human luteinized granulosa cells in vitro, LXR-β was the more abundant isoform and its levels were increased during luteinization. Treatment with LXR agonist resulted in decreased progesterone synthesis, secondary to increased cholesterol efflux through induction of LXR target genes. The expression of LXR-β in GCT would therefore seem consistent with their ability to actively synthesize estrogen.
Rev-erbA
Rev-erbAα and β are widely expressed orphan receptors. They have a role as “clock genes” [92] and may link circadian rhythms to metabolic pathways. The significance of the expression of Rev-erbAα expression in GCT raises interesting questions regarding its role in both GCT and normal GC. Rev-erbAα null female mice are fertile and have only a very mild reproductive phenotype [93]. Both Rev-erbA genes are expressed in the cell lines albeit at lower levels than in the GCT.
Retinoic Acid Receptor
Although expression of these receptors is generally low or absent, there is modest expression of retinoic acid receptor (RAR)-α in the GCT and in the two cell lines. The RAR also generally act via heterodimerization with RXR and in other systems are anti-proliferative and/or promote differentiation.
RAR-Related Orphan Receptor Alpha/Nuclear Receptor-Related 1 Protein
Several genes are relatively abundantly expressed in the COV434 cell line but not in the KGN cell line. This difference, we suspect, reflects the fact that they derive from different sub-types of GCT. The COV434 line appears, on the basis of the FOXL2 mutation studies to be derived from a juvenile GCT. In addition to prominent expression of several of the NR already discussed, including COUP-TF1 and LRH-1, the COV434 cells have very high expression of ROR-α and NURR1. ROR-α has not been associated with either GC or malignancy and their expression status in juvenile GCT has not been documented. ROR-α has been associated with the regulation of steroidogenesis [94] including the regulation of aromatase expression [95]. ROR-α is expressed in lung cancer, melanoma and some epithelial ovarian cancer-derived cell lines [30]. In breast cancer cell lines, it has been reported to inhibit proliferation [96]. NURR1 is a member of the NR4A subfamily which has been shown to play a role in the regulation of steroidogenesis. NURR1 has been reported to inhibit aromatase gene expression in KGN cells [97]. NURR1 has been reported to interact with p53 to block the induction of the pro-apoptotic gene BAX [98].
Of the genes that are not expressed in GCT, there are none that have been either characterized in GC or might have been predicted to be expressed. It would of course be desirable to be making this comparison with normal human GC but this turns out to be extremely difficult. For various ethical reasons, proliferating GC of the pre-antral follicle, which, on the basis of previous studies [4] would be appropriate comparators, are extremely difficult to obtain. Although GC can, in principal, be obtained at the time of egg collection in an IVF cycle, these GC have been exposed to a hyper-stimulation regimen that renders them at least partially luteinized.
Holbeck et al. [30] have performed a similar profiling of NR expression for the NC160 cancer cell panel. This panel does not include the COV434 or KGN cells. They found COUP-TF2 to be abundantly expressed in a range of cell lines as is COUP-TF1, albeit with a more restricted pattern. PPAR-γ also has a widespread expression which is particularly prominent in renal, colon and lung cancer-derived lines. LRH-1 expression is highly variable with most lines having little or no expression. TR-α expression is prominent across all the cell lines whereas, conversely ER-β and SF-1 are virtually absent from all of the cell lines examined. The GCT-derived cell lines and indeed the GCT show a distinct pattern of NR expression when compared with the NC160 panel, which serves to emphasize the unique nature of this disease.
A subset of the NR differ between the GCT and the cell lines and indeed between the two cells lines. Both the hierarchical clustering (Fig. 2) and the principal component analysis ( Fig. 3) however do not clearly separate the lines from the tumors reinforcing the relative homogeneity of this tumor type and also arguing for the validity of the COV434 and KGN cells for the study of GCT.
The identification of NR with abundant expression in GCT will stimulate further examination of their roles in GCT, in normal GC and their potential as therapeutic targets. In several cases (e.g., PPAR, LRH-1, and ER) there is existing data in other tumors but this potential has been little, if at all, explored in GCT.
The results of these analyses represent the first systematic review of NR gene expression in human GCT. The findings also have implications for non-malignant granulosa cells, providing an important resource for future studies as well as identifying specific targets. The analysis of the two cell lines, in the context of the tumors, similarly provides important background information for these two widely used lines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Relative gene expression levels. The nuclear receptor common name and alternative is shown. The number of samples whose C t value was below 35 is shown. Those samples whose C t value was greater than or equal to 35 or whose C t value was unable to be determined by the ABI software package SDS2.3 were given a value of 0. (PDF 24 kb)
StatMiner analysis of a KGN versus GCT (n = 14) and b COV434 versus GCT (n = 14). Data are presented as relative quantification (log10) utilizing GCT as the reference/calibrator normalized to the geNorm-selected controls HMBS, PPIA, RPLPO, and UBC. The TLDAs were analyzed using the relative quantification method of ΔC t, and comparing the normalized C t values (∆C t) of the replicates between the control/calibrator and the target sample. The geNorm software imbedded within the ABI/Integromics StatMiner V4.1 software package was used to compute least expression variation and select the appropriate and most stable combination of internal control genes with which to normalize the expression data. The expression data were normalized to the median of HMBS, PPIA, RPLPO, and UBC. The TLDAs were analyzed as described in Raichur et al. [23]. Briefly, significant changes in expression in the KGN and COV434 cell lines relative to GCT tumors were analyzed using the ABI/Integromics “StatMiner” software package. Differentially expressed genes were identified by the comparative C t method and significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [24]. Furthermore, we performed conservative data filtering (Benjamin–Hochberg) to control for FDR and further refined the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target and the calibrator/reference sample (GCT)) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples is <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected and no detection (i.e., expression in either tissue), when the C t value of the gene(s) in 50% or more of the target, calibrator and/or both tissues/samples are >35 cycles (i.e., the arbitrarily selected threshold limit). Hence, the reported quantitative “fold change of a gene that is not expressed in some of the biological conditions may not be reliable” [25], however, it does reflect a qualitative difference. The data generated by the ABI SDS software from the ABI7900 instrument do not normally contain missing C t values, and C t values are assigned beyond the arbitrarily set threshold (C t 35) up to a maximum C t 40. In situations where the sample is undetected and the C t values are beyond the maximum C t 40, the StatMiner software imputes a value, set to the maximum C t threshold. (PDF 77 kb)
Box Plot analysis. The bottom of each box is the 25th percentile, the top is the 75th percentile and the line in the middle represents the median. The whiskers that extend up and down are the highest and lowest values that are not outliers. Outliers are represented by circles above or below the whiskers. (PDF 379 kb)
StatMiner analysis of GCT (n = 14) versus Tumor Reference. The geNorm software imbedded within the ABI/Integromics StatMiner V4.1 software package was used to compute least expression variation and the expression data were normalized to the median of HMBS, PPIA, and RPLP0. Significant changes in expression in the GCT relative to the calibrator/reference human tumors were analyzed using the ABI/Integromics “StatMiner” software package. Significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [24]. Furthermore, we performed conservative data filtering (Benjamin–Hochberg) to control for FDR and further refined the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target and the calibrator/reference sample (human tumors)) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples is <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected and no detection (i.e., expression in either cell/tissue), when the C t value of the gene(s) in 50% or more of the target, calibrator and/or both tissues/samples are >35 cycles (i.e., the arbitrarily selected threshold limit). Hatched bars indicate that the target was not detected (see “Materials and Methods”) in the GCT. **p = <0.001; *p = <0.01; ƚp = <0.05 significant differences are indicated. (PDF 30 kb)
StatMiner analysis of stage 1 GCT (n = 5) versus Recurrent GCT (n = 7). The geNorm software imbedded within the ABI/Integromics StatMiner V4.1 software package was used to compute least expression variation and the expression data were normalized to the median of ACTB, GUSB, and POLR2A. Significant changes in expression in the GCT relative to the calibrator/reference human tumors were analyzed using the ABI/Integromics “StatMiner” software package. Significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [24], and conservative data filtering (Benjamin–Hochberg) was applied to control for FDR, correct/adjust p values and further refine the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target (stage 1 GCT) and the calibrator/reference sample (Recurrent GCT)) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples is <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected and no detection (i.e., expression in either cell/tissue), when the C t value of the gene(s) in 50% or more of the target, calibrator and/or both tissues/samples are >35 cycles (i.e., the arbitrarily selected threshold limit). (PDF 29 kb)
Acknowledgments
The authors thank Dr. Nishi Yanase for the KGN cell line and Sue Panckridge and Claudette Thiedeman for help with the preparation of the manuscript. The authors also wish to thank the Nuclear Receptors in Breast Cancer Research Consortium for their help and support with these studies. This work was supported by the National Health and Medical Research Council through Fellowships to PJF, CDC, and GEM and a scholarship to SJ; by the Cancer Council of Victoria, the NAB Ovarian Cancer Research Foundation and by the Granulosa Cell of the Ovary Foundation. SJ is also supported by the Monash University through a Faculty of Medicine Postgraduate Excellence Award. Prince Henry’s Institute is supported by the Victorian Governments Operational Infrastructure Program.
Conflicts of Interest
The authors declare that they have no conflict of interest
Footnotes
Maria Alexiadis and Natalie Eriksson equally contributed to this work.
References
- 1.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Colombo N, Parma G, Zanagnolo V, Insinga A. Management of ovarian stromal cell tumors. J Clin Oncol. 2007;25:2944–2951. doi: 10.1200/JCO.2007.11.1005. [DOI] [PubMed] [Google Scholar]
- 3.Young RH, Scully RE. Endocrine tumours of the ovary. Curr Top Path. 1992;85:114–164. doi: 10.1007/978-3-642-75941-3_5. [DOI] [PubMed] [Google Scholar]
- 4.Chu S, Rushdi S, Zumpe ET, Mamers P, Healy DL, Jobling T, Burger HG, Fuller PJ. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol Hum Reprod. 2002;8:426–433. doi: 10.1093/molehr/8.5.426. [DOI] [PubMed] [Google Scholar]
- 5.Healy DL, Burger HG, Mamers P, Jobling T, Bangah M, Quinn M, Grant P, Day AJ, Rome R, Campbell JJ. Elevated serum inhibin concentrations in postmenopausal women with ovarian tumors. New Eng J Med. 1993;329:1539–1542. doi: 10.1056/NEJM199311183292104. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson S, Fuller PJ. Management of granulose cell tumour of the ovary. Curr Opin Oncol. 2008;20:560–564. doi: 10.1097/CCO.0b013e328306316f. [DOI] [PubMed] [Google Scholar]
- 7.Fuller PJ, Chu S. Signalling pathways in the molecular pathogenesis of ovarian granulosa cell tumours. Trends Endocrinol Metab. 2004;15:122–128. doi: 10.1016/j.tem.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Kalfa N, Ecochard A, Patte C, Duvillard P, Audran F, Pienkowski C, Thibaud E, Brauner R, Lecointre C, Plantaz D, Guedj AM, Paris F, Baldet P, Lumbroso S, Sultan C. Activating mutations of the stimulatory g protein in juvenile ovarian granulosa cell tumors: a new prognostic factor? J Clin Endocrinol Metab. 2006;91:1842–1847. doi: 10.1210/jc.2005-2710. [DOI] [PubMed] [Google Scholar]
- 9.Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provender D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao YJ, Zeng T, Glover JNM, Vanderhyden B, Zhao CQ, Parkinson CA, Jimenez-Linan M, Bowtell DDL, Mes-Masson AM, Brenton JD, Aparico SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG. Mutation of FOXL2 in granulosa-cell tumors of the ovary. New Engl J Med. 2009;360:2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ, Anttonen M. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary: independent confirmation from two centers. Mod Pathol. 2010;23:1477–1485. doi: 10.1038/modpathol.2010.145. [DOI] [PubMed] [Google Scholar]
- 11.Kim MS, Hur SY, Yoo NJ, Lee SH. Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of ovary and other human cancers. J Pathol. 2010;221:147–152. doi: 10.1002/path.2688. [DOI] [PubMed] [Google Scholar]
- 12.Kalfa N, Philibert P, Patte C, Ecochard A, Duvillard P, Ecochard A, Duvillard P, Baldet P, Jaubert F, Fellous M, Sultan C. Extinction of FOXL2 expression in aggressive ovarian granulosa cell tumors in children. Fertil Steril. 2007;87:896–901. doi: 10.1016/j.fertnstert.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chmbon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 15.Chu S, Mamers P, Burger HG, Fuller PJ. Estrogen receptor isoform gene expression in ovarian stromal and epithelial tumors. J Clin Endocrinol Metab. 2000;85:1200–1205. doi: 10.1210/jc.85.3.1200. [DOI] [PubMed] [Google Scholar]
- 16.Farinola MA, Gown AM, Judson K, Ronnett BM, Barry TS, Movahedi-Lankarani S, Vang R. Estrogen receptor alpha and progesterone receptor expression in ovarian adult granulosa cell tumors and Sertoli–Leydig cell tumors. Int J Gynecol Pathol. 2007;26:375–382. doi: 10.1097/pgp.0b013e31805c0d99. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, Vang R. Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. Am J Surg Pathol. 2009;33:354–366. doi: 10.1097/PAS.0b013e318188373d. [DOI] [PubMed] [Google Scholar]
- 18.Anttonen M, Unkila-Kallio L, Leminen A, Butzow R, Heikinheimo M. High GATA-4 expression associates with aggressive behavior, whereas low anti-Mullerian hormone expression associates with growth potential of ovarian granulosa cell tumors. J Clin Endocrinol Metab. 2005;90:6529–6535. doi: 10.1210/jc.2005-0921. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg-Bakker CAM, Hagemeijer A, Franken-Postma EM, Smit VTHBM, Kuppen PJK, Claasen HHVR, Cornelisse CJ, Schrier PI. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: growth features and cytogenetics. Int J Cancer. 1993;53:613–620. doi: 10.1002/ijc.2910530415. [DOI] [PubMed] [Google Scholar]
- 20.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle stimulating hormone receptor. Endocrinol. 2001;142:437–445. doi: 10.1210/en.142.1.437. [DOI] [PubMed] [Google Scholar]
- 21.Chu S, Nishi Y, Yanase T, Nawata H, Fuller PJ. Transrepression of estrogen receptor b signalling by nuclear factor k-b in ovarian granulosa cells. Mol Endocrinol. 2004;18:1919–1928. doi: 10.1210/me.2004-0021. [DOI] [PubMed] [Google Scholar]
- 22.Fuller PJ, Chu S, Jobling T, Mamers P, Healy DL, Burger HG. Inhibin subunit gene expression in ovarian cancer. Gynecol Oncol. 1999;73:273–279. doi: 10.1006/gyno.1999.5356. [DOI] [PubMed] [Google Scholar]
- 23.Hussein-Fikret S, Fuller PJ. Expression of the nuclear receptor coregulators in ovarian stromal and epithelial tumours. Mol Cell Endocrinol. 2005;229:149–160. doi: 10.1016/j.mce.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Raichur S, Fitzsimmons RL, Myers SA, Pearen MA, Lau P, Eriksson N, Wang SM, Muscat GE. Identification and validation of the pathways and functions regulated by the orphan nuclear receptor, ROR alpha1, in skeletal muscle. Nucleic Acids Res. 2010;38:4296–4312. doi: 10.1093/nar/gkq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover T, Mitchell K. An introduction to Biostatistics. 2. Long Grove: Waveland Press; 2008. [Google Scholar]
- 27.Goni R, Garcia P, Foissac S (2009) The qPCR data statistical analysis. In: Integromics White Paper: Integromics SL. pp 1–9
- 28.Schrader KA, Gorbatcheva B, Senz J, Heravi-Moussavi A, Melnyk N, Salamanca C, Maines-Bandiera S, Cooke SL, Leung P, Brenton JD, Gilks CB, Monahan J, Huntsman DG. The specificity of the FOXL2 c.402C>G somatic mutation: a survey of solid tumors. PLoS ONE. 2009;4(11):e7988. doi: 10.1371/journal.pone.0007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benayoun BA, Caburet S, Dipietromaria A, Georges A, D'Haene B, Pandaranayaka PJ, L'Hôte D, Todeschini AL, Krishnaswamy S, Fellous M, De Baere E, Veitia RA. Functional exploration of the adult ovarian granulosa cell tumor-associated somatic FOXL2 mutation p.Cys134Trp (c.402C>G) PLoS ONE. 2010;5(1):e8789. doi: 10.1371/journal.pone.0008789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holbeck S, Chang J, Best AM, Bookout AL, Mangelsdorf DJ, Martinez ED. Expression profiling of nuclear receptors in the NC160 cancer cell panel reveals receptor-drug and receptor-gene interactions. Mol Endo. 2010;24:1287–1296. doi: 10.1210/me.2010-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller PJ, Zumpe ET, Chu S, Mamers P, Burger HG. Inhibin-activin receptor subunit gene expression in ovarian tumors. J Clin Endocrinol Metab. 2002;87:1395–1401. doi: 10.1210/jc.87.3.1395. [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Suzuki T, Hidaka K, Sato H, Ito K, Ito S, Sasano H. Immunolocalization of nuclear transcription factors, DAX-1 and COUP-TF II, in the normal human ovary: correlation with adrenal 4 binding protein/steroidogenic factor-1 immunolocalization during the menstrual cycle. J Clin Endocrinol Metab. 2003;88:3415–3420. doi: 10.1210/jc.2002-021723. [DOI] [PubMed] [Google Scholar]
- 33.Xing W, Danilovich N, Sairam MR. Orphan receptor chicken ovalbumin upstream promoter transcription factors inhibit steroid factor-1, upstream stimulatory factor, and activator protein-1 activation of ovine follicle-stimulating hormone receptor expression via composite cis-elements. Biol Reprod. 2002;66:1656–1666. doi: 10.1095/biolreprod66.6.1656. [DOI] [PubMed] [Google Scholar]
- 34.Nagasaki S, Suzuki T, Miki Y, J-i A, Shibata H, Ishida T, Ohuchi N, Sasano H. Chicken ovalbumin upstream promoter transcription factor II in human breast carcinoma: possible regulator of lymphangiogenesis via vascular endothelial growth factor-C expression. Cancer Sci. 2009;100:639–645. doi: 10.1111/j.1349-7006.2008.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klinge CM, Silver BF, Driscoll MD, Sathya G, Bambara RA, Hilf R. Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites, and inhibits estrogen-induced gene expression. J Bio Chem. 1997;272:31465–31474. doi: 10.1074/jbc.272.50.31465. [DOI] [PubMed] [Google Scholar]
- 36.Riggs KA, Wickramasinghe NS, Cochrum RK, Watts MB, Klinge CM. Decreased chicken ovalbumin upstream promoter transcription factor II expression in tamoxifen-resistant breast cancer cells. Cancer Res. 2006;66:10188–10198. doi: 10.1158/0008-5472.CAN-05-3937. [DOI] [PubMed] [Google Scholar]
- 37.Lee BC, Cha K, Avraham S, Avraham HK. Microarray analysis of differentially expressed genes associated with human ovarian cancer. Int J Oncol. 2004;24:847–851. [PubMed] [Google Scholar]
- 38.Tepmongkol S, Keelawat S, Honsawek S, Ruangvejvorachai P. Rosiglitazone effect on radioiodine uptake in thyroid carcinoma patients with high thyroglobulin but negative total body scan: a correlation with the expression of peroxisome proliferator-activated receptor-gamma. Thyroid. 2008;18:697–704. doi: 10.1089/thy.2008.0056. [DOI] [PubMed] [Google Scholar]
- 39.Klopper JP, Hays WR, Sharma V, Baumbusch MA, Hershman JM, Haugen BR. Retinoid X receptor-gamma and peroxisome proliferator-activated receptor-gamma expression predicts thyroid carcinoma cell response to retinoid and thiazolidinedione treatment. Mol Cancer Ther. 2004;3:1011–1020. [PubMed] [Google Scholar]
- 40.Zhang H, Li Q, Lin H, Yang Q, Wang H, Zhu C. Role of PPARgamma and its gonadotrophic regulation in rat ovarian granulosa cells in vitro. Neuro Endocrinol Lett. 2007;28:289–294. [PubMed] [Google Scholar]
- 41.WuQiang F, Yanase T, Morinaga H, Mu Y-M, Nomura M, Okabe T, Goto K, Harada N, Nawata H. Activation of peroxisome proliferator-activated receptor-g and retinoid X receptor inhibits aromatase transcription via nuclear factor-kB. Endocrinology. 2004;146:85–92. doi: 10.1210/en.2004-1046. [DOI] [PubMed] [Google Scholar]
- 42.Lovekamp-Swan T, Chaffin CL. The peroxisome proliferator-activated receptor gamma ligand troglitazone induces apoptosis and p53 in rat granulosa cells. Moll Cell Endo. 2005;233:15–24. doi: 10.1016/j.mce.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Komar CM, Braissant O, Wahli W, Curry TE., Jr Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology. 2001;142:4831–4838. doi: 10.1210/en.142.11.4831. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee J, Komar CM. Effects of luteinizing hormone on peroxisome proliferator-activated receptor gamma in the rat ovary before and after the gonadotropin surge. Reprod. 2006;131:93–101. doi: 10.1530/rep.1.00730. [DOI] [PubMed] [Google Scholar]
- 45.Komar CM, Curry TE., Jr Inverse relationship between the expression of messenger ribonucleic acid for peroxisome proliferator-activated receptor gamma and P450 side chain cleavage in the rat ovary. Biol Reprod. 2003;69:549–555. doi: 10.1095/biolreprod.102.012831. [DOI] [PubMed] [Google Scholar]
- 46.Seto-Young D, Avtanski D, Strizhevsky M, Parikh G, Patel P, Kaplun J, Holcomb K, Rosenwaks Z, Poretsky L. Interactions among peroxisome proliferator activated receptor-gamma, insulin signaling pathways, and steroidogenic acute regulatory protein in human ovarian cells. J Clin Endocrinol Metab. 2007;92:2232–2239. doi: 10.1210/jc.2006-1935. [DOI] [PubMed] [Google Scholar]
- 47.Kowalewski MP, Dyson MT, Manna PR, Stocco DM. Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod Fertil Dev. 2009;21:909–922. doi: 10.1071/RD09027. [DOI] [PubMed] [Google Scholar]
- 48.Mu YM, Yanase T, Nishi Y, Waseda N, Oda T, Tanaka A, Takayanagi R, Nawata H. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun. 2000;271:710–713. doi: 10.1006/bbrc.2000.2701. [DOI] [PubMed] [Google Scholar]
- 49.Fan W, Yanase T, Morinaga H, Mu YM, Nomura M, Okabe T, Goto K, Harada N, Nawata H. Activation of peroxisome proliferator-activated receptor-g and retinoid X receptor inhibits aromatase transcription via nuclear factor-kB. Endocrinology. 2005;146:85–92. doi: 10.1210/en.2004-1046. [DOI] [PubMed] [Google Scholar]
- 50.Gasic S, Bodenburg Y, Nagamani M, Green A, Urban RJ. Troglitazone inhibits progesterone production in porcine granulosa cells. Endocrinology. 1998;139:4962–4966. doi: 10.1210/en.139.12.4962. [DOI] [PubMed] [Google Scholar]
- 51.Gasic S, Nagamani M, Green A, Urban RJ. Troglitazone is a competitive inhibitor of 3beta-hydroxysteroid dehydrogenase enzyme in the ovary. Am J Obstet Gynceol. 2001;184:575–579. doi: 10.1067/mob.2001.111242. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Sato M, LiQ LJP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–1782. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falzacappa CV, Mangialardo C, Patriarca V, Bucci B, Amendola D, Raffa S, Torrisi MR, Silvestrini G, Ballanti P, Moriggi G, Stigliano A, Brunetti E, Toscano V, Misiti S. Thyroid hormones induce cell proliferation and survival in ovarian granulosa cells COV434. J Cell Physiol. 2009;221:242–253. doi: 10.1002/jcp.21849. [DOI] [PubMed] [Google Scholar]
- 54.Zhang SS, Carrillo AJ, Darling DS. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol Hum Reprod. 1997;3:555–562. doi: 10.1093/molehr/3.7.555. [DOI] [PubMed] [Google Scholar]
- 55.Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, Hovatta O, Skjöldebrand-Sparre L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online. 2009;18:337–347. doi: 10.1016/S1472-6483(10)60091-0. [DOI] [PubMed] [Google Scholar]
- 56.Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennstrom B, Aranda A. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69:501–509. doi: 10.1158/0008-5472.CAN-08-2198. [DOI] [PubMed] [Google Scholar]
- 58.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 59.Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, de Rooij DG, Themmen AP, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- 60.Pelusi C, Ikeda Y, Zubair M, Parker KL. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008;79:1074–1083. doi: 10.1095/biolreprod.108.069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biason-Lauber A, Schoenle EJ. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet. 2000;67:1563–1568. doi: 10.1086/316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lourenco D, Brauner R, Lin L, De PA, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, iel-Guerra AT, Achermann JC, McElreavey K, Bashamboo A. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360:1200–1210. doi: 10.1056/NEJMoa0806228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pare JF, Malenfant D, Courtemanche C, Jacob-Wagner M, Roy S, Allard D, Belanger L. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis, and regulated by a DR4 element. J Biol Chem. 2004;279:21206–21216. doi: 10.1074/jbc.M401523200. [DOI] [PubMed] [Google Scholar]
- 64.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endo. 2003;207:39–45. doi: 10.1016/S0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- 66.Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144:3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- 67.Takayama K, Sasano H, Fukaya T, Morohashi K, Suzuki T, Tamura M, Costa MJ, Yajima A. Immunohistochemical localization of Ad4-binding protein with correlation to steroidogenic enzyme expression in cycling human ovaries and sex cord stromal tumors. J Clin Endocrinol Metab. 1995;80:2815–2821. doi: 10.1210/jc.80.9.2815. [DOI] [PubMed] [Google Scholar]
- 68.Saxena D, Escamilla-Hernandez R, Little-Ihrig L, Zeleznik AJ. Liver receptor homolog-1 and steroidogenic factor-1 have similar actions on rat granulosa cell steroidogenesis. Endocrinology. 2007;148:726–734. doi: 10.1210/en.2006-0108. [DOI] [PubMed] [Google Scholar]
- 69.Saxena D, Safi R, Little-Ihrig L, Zeleznik AJ. Liver receptor homolog-1 stimulates the progesterone biosynthetic pathway during follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 2004;145:3821–3829. doi: 10.1210/en.2004-0423. [DOI] [PubMed] [Google Scholar]
- 70.Yazawa T, Inaoka Y, Okada R, Mizutani T, Yamazaki Y, Usami Y, Kuribayashi M, Orisaka M, Umezawa A, Miyanoto K. PPAR-g coactivator-1a regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endo. 2010;24:485–496. doi: 10.1210/me.2009-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weck J, Mayo KE. Switching of NR5A proteins associated with the inhibin alpha-subunit gene promoter after activation of the gene in granulosa cells. Mol Endo. 2006;20:1090–1103. doi: 10.1210/me.2005-0199. [DOI] [PubMed] [Google Scholar]
- 72.Robert NM, Miyamoto Y, Taniguchi H, Viger RS. LRH-1/NR5A2 cooperates with GATA factors to regulate inhibin a-subunit promoter activity. Mol Cell Endo. 2006;257:65–74. doi: 10.1016/j.mce.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Kyronlahti A, Kauppinen M, Lind E, Unkila-Kallio L, Butzow R, Klefstrom J, Wilson DB, Anttonen M, Heikinheimo M. GATA4 protects granulosa cell tumors from TRAIL-induced apoptosis. Endocr Relat Cancer. 2010;17:709–717. doi: 10.1677/ERC-10-0041. [DOI] [PubMed] [Google Scholar]
- 74.Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 75.Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonist. J Clin Endocrinol Metab. 2009;94:2178–2183. doi: 10.1210/jc.2008-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15:499–509. doi: 10.1016/j.molcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Schoonjans K, Dubuquoy L, Mebis J, Fayard E, Wendling O, Haby C, Geboes K, Auwerx J. Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc Natl Acad Sci USA. 2005;102:2058–2062. doi: 10.1073/pnas.0409756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Modica S, Gofflot F, Murzilli S, D'Orazio A, Salvatore L, Pellegrini F, Nicolucci A, Tognoni G, Copetti M, Valanzano R, Veschi S, Mariani-Costantini R, Palasciano G, Schoonjans K, Auwerx J, Moschetta A. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 2010;138:636–648. doi: 10.1053/j.gastro.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 79.Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/S0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 80.Drummond AE, Fuller PJ. The importance of ERbeta signaling in the ovary. J Endocrinol. 2010;205:15–23. doi: 10.1677/JOE-09-0379. [DOI] [PubMed] [Google Scholar]
- 81.Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jc.83.7.2479. [DOI] [PubMed] [Google Scholar]
- 82.Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78:380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- 83.Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu O, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA. 2004;101:11209–11214. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sriraman V, Sinha M, Richards JS. Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures. Biol Reprod. 2010;82:402–412. doi: 10.1095/biolreprod.109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friberg PA, Larsson DG, Billig H. Dominant role of nuclear progesterone receptor in the control of rat periovulatory granulosa cell apoptosis. Biol Reprod. 2009;80:1160–1167. doi: 10.1095/biolreprod.108.073932. [DOI] [PubMed] [Google Scholar]
- 87.Shyr CR, Hu YC, Kim E, Chang C. Modulation of estrogen receptor-mediated transactivation by orphan receptor TR4 in MCF-7 cells. J Biol Chem. 2002;277:14622–14628. doi: 10.1074/jbc.M110051200. [DOI] [PubMed] [Google Scholar]
- 88.Chen LM, Wang RS, Lee YF, Liu NC, Chang YJ, Wu CC, Xie S, Hung YC, Chang C. Subfertility with defective folliculogenesis in female mice lacking testicular orphan nuclear receptor 4. Mol Endo. 2008;22:858–867. doi: 10.1210/me.2007-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steffensen KR, Robertson K, Gustafasson JA, Andersen CY. Reduced fertility and inability of oocytes to resume meiosis in mice deficient of the Lxr genes. Mol Cell Endo. 2006;256:9–16. doi: 10.1016/j.mce.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 90.Mouzat K, Volat F, Baron S, Alves G, Pommier AJ, Volle DH, Marceau G, Dehaze A, DŠchelotte P, Duggavathi R, Caira F, Lobaccaro JM. Absence of nuclear receptors for oxysteroid Liver X Receptor (LXR) induces ovarian hyperstimulation syndrome in mice. Endocrinology. 2009;150:3369–3375. doi: 10.1210/en.2008-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drouineaud V, Sagot P, Garrido C, Logette E, Deckert V, Gambert P, Jimenez C, Staels B, Lagrost L, Masson D. Inhibition of progesterone production in human luteinized granulosa cells treated with LXR agonists. Mol Hum Reprod. 2007;13:373–379. doi: 10.1093/molehr/gam019. [DOI] [PubMed] [Google Scholar]
- 92.Teboul M, Guillaumond F, GrŠchez-Cassiau A, Delaunay F. The nuclear hormone receptor family round the clock. Mol Endo. 2008;22:2573–2582. doi: 10.1210/me.2007-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chomez P, Neveu I, Mansén A, Kiesler E, Larsson L, Vennstrom B, Arenas E. Increased cell death and delayed development in the cerebellum of mice lacking the rev-erbA(alpha) orphan receptor. Development. 2000;127:1489–1498. doi: 10.1242/dev.127.7.1489. [DOI] [PubMed] [Google Scholar]
- 94.Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM. Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics. 2007;31:281–294. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 95.Odawara H, Iwasaki T, Horiguchi J, Rokutanda N, Hirooka K, Miyazaki W, Koibuchi Y, Shimokawa N, Iino Y, Takeyoshi I, Koibuchi N. Activation of aromatase expression by retinoic acid receptor-related orphan receptor (ROR) alpha in breast cancer cells: identification of a novel ROR response element. J Biol Chem. 2009;284:17711–17719. doi: 10.1074/jbc.M109.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu Y, McAvoy S, Kuhn R, Smith DI. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene. 2006;25:2901–2908. doi: 10.1038/sj.onc.1209314. [DOI] [PubMed] [Google Scholar]
- 97.Wu Y, Ghosh S, Nishi Y, Yanase T, Nawata H, Hu Y. The orphan nuclear receptors NURR1 and NGFI-B modulate aromatase gene expression in ovarian granulosa cells: a possible mechanism for repression of aromatase expression upon luteinizing hormone surge. Endocrinology. 2005;146:237–246. doi: 10.1210/en.2004-0889. [DOI] [PubMed] [Google Scholar]
- 98.Zhang T, Wang P, Ren H, Fan J, Wang G. NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res. 2009;7:1408–1415. doi: 10.1158/1541-7786.MCR-08-0533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative gene expression levels. The nuclear receptor common name and alternative is shown. The number of samples whose C t value was below 35 is shown. Those samples whose C t value was greater than or equal to 35 or whose C t value was unable to be determined by the ABI software package SDS2.3 were given a value of 0. (PDF 24 kb)
StatMiner analysis of a KGN versus GCT (n = 14) and b COV434 versus GCT (n = 14). Data are presented as relative quantification (log10) utilizing GCT as the reference/calibrator normalized to the geNorm-selected controls HMBS, PPIA, RPLPO, and UBC. The TLDAs were analyzed using the relative quantification method of ΔC t, and comparing the normalized C t values (∆C t) of the replicates between the control/calibrator and the target sample. The geNorm software imbedded within the ABI/Integromics StatMiner V4.1 software package was used to compute least expression variation and select the appropriate and most stable combination of internal control genes with which to normalize the expression data. The expression data were normalized to the median of HMBS, PPIA, RPLPO, and UBC. The TLDAs were analyzed as described in Raichur et al. [23]. Briefly, significant changes in expression in the KGN and COV434 cell lines relative to GCT tumors were analyzed using the ABI/Integromics “StatMiner” software package. Differentially expressed genes were identified by the comparative C t method and significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [24]. Furthermore, we performed conservative data filtering (Benjamin–Hochberg) to control for FDR and further refined the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target and the calibrator/reference sample (GCT)) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples is <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected and no detection (i.e., expression in either tissue), when the C t value of the gene(s) in 50% or more of the target, calibrator and/or both tissues/samples are >35 cycles (i.e., the arbitrarily selected threshold limit). Hence, the reported quantitative “fold change of a gene that is not expressed in some of the biological conditions may not be reliable” [25], however, it does reflect a qualitative difference. The data generated by the ABI SDS software from the ABI7900 instrument do not normally contain missing C t values, and C t values are assigned beyond the arbitrarily set threshold (C t 35) up to a maximum C t 40. In situations where the sample is undetected and the C t values are beyond the maximum C t 40, the StatMiner software imputes a value, set to the maximum C t threshold. (PDF 77 kb)
Box Plot analysis. The bottom of each box is the 25th percentile, the top is the 75th percentile and the line in the middle represents the median. The whiskers that extend up and down are the highest and lowest values that are not outliers. Outliers are represented by circles above or below the whiskers. (PDF 379 kb)
StatMiner analysis of GCT (n = 14) versus Tumor Reference. The geNorm software imbedded within the ABI/Integromics StatMiner V4.1 software package was used to compute least expression variation and the expression data were normalized to the median of HMBS, PPIA, and RPLP0. Significant changes in expression in the GCT relative to the calibrator/reference human tumors were analyzed using the ABI/Integromics “StatMiner” software package. Significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [24]. Furthermore, we performed conservative data filtering (Benjamin–Hochberg) to control for FDR and further refined the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target and the calibrator/reference sample (human tumors)) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples is <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected and no detection (i.e., expression in either cell/tissue), when the C t value of the gene(s) in 50% or more of the target, calibrator and/or both tissues/samples are >35 cycles (i.e., the arbitrarily selected threshold limit). Hatched bars indicate that the target was not detected (see “Materials and Methods”) in the GCT. **p = <0.001; *p = <0.01; ƚp = <0.05 significant differences are indicated. (PDF 30 kb)
StatMiner analysis of stage 1 GCT (n = 5) versus Recurrent GCT (n = 7). The geNorm software imbedded within the ABI/Integromics StatMiner V4.1 software package was used to compute least expression variation and the expression data were normalized to the median of ACTB, GUSB, and POLR2A. Significant changes in expression in the GCT relative to the calibrator/reference human tumors were analyzed using the ABI/Integromics “StatMiner” software package. Significance was assigned by the application of the non-parametric Wilcoxon’s (Mann–Whitney U) test [24], and conservative data filtering (Benjamin–Hochberg) was applied to control for FDR, correct/adjust p values and further refine the subset of differentially expressed genes. Relative quantification, i.e., the calculated fold differences (between the target (stage 1 GCT) and the calibrator/reference sample (Recurrent GCT)) are displayed as valid, when the C t values of the gene in the target and calibrator/reference samples is <35 cycles. Moreover, fold differences are flagged as target not detected, calibrator not detected and no detection (i.e., expression in either cell/tissue), when the C t value of the gene(s) in 50% or more of the target, calibrator and/or both tissues/samples are >35 cycles (i.e., the arbitrarily selected threshold limit). (PDF 29 kb)