Abstract
We have taxonomically and phylogenetically characterized a new aerobic bacterial strain (JF-1) that contains photosynthetic pigment-protein complexes and which was recently isolated from black smoker plume waters of the Juan de Fuca Ridge. Strain JF-1 is a gram-negative, yellow-pigmented, motile bacterium that is salt-, pH-, and thermotolerant. These properties are consistent with an oligotrophic adaptation to varied environmental conditions thought to exist around deep-sea hydrothermal vents. The analysis of 16S rDNA sequences revealed that strain JF-1 forms a separate phylogenetic branch between the genus Erythromonas and the Erythromicrobium-Porphyrobacter-Erythrobacter cluster within the α subclass of the Proteobacteria. The taxonomic name Citromicrobium bathyomarinum (gen. nov., sp. nov.) is proposed for strain JF-1.
Obligately aerobic bacteria that contain photosynthetic pigment-protein complexes are widely known as aerobic anoxygenic phototrophic bacteria (37). The first species of the aerobic anoxygenic phototrophic bacteria, Erythrobacter longus, was isolated and characterized over 15 years ago (20, 21). Since that time, additional representatives have been isolated from a variety of habitats (7, 10, 19, 26, 29–32, 36). Recently we reported on the isolation and many of the properties of the aerobic phototrophic bacterium JF-1 from samples that were obtained from the vicinity of nonbuoyant regions of plumes emitted from hydrothermal vents of the Juan de Fuca Ridge (26). Aerobic anoxygenic phototrophic bacteria are classified in two marine genera (Erythrobacter and Roseobacter) and six freshwater genera (Acidiphilium, Erythromicrobium, Erythromonas, Porphyrobacter, Roseococcus, and Sandaracinobacter), which phylogenetically belong to the α-1, α-3, and α-4 subclasses of the Proteobacteria (7, 10, 18, 20, 25, 34, 35). Although taxonomically and phylogenetically heterogeneous, these bacteria share the following distinguishing features: the presence of bacteriochlorophyll (Bchl) a incorporated into reaction center (RC) and light-harvesting (LH) complexes, a low level of the photosynthetic unit in cells, an abundance of carotenoids, a strong inhibition by light of Bchl synthesis, the inability to grow photosynthetically under anaerobic conditions, and a high mid-point potential of the RC primary electron acceptor QA (37).
The documentation of geothermal light at otherwise dark deep-sea ecosystems led to the suggestion that geothermally driven photosynthesis could exist on the seafloor (12, 23, 24). Nisbet et al. proposed that light emitted at deep-sea vents may have provided the selective force for the evolution of photosynthesis, through the initial development of phototaxis toward geothermal light followed by the evolution of rudimentary photosynthesis (16).
Strain JF-1 is the first strain containing photosynthetic pigments that has been recovered from deep-ocean (about 2,000 m depth) waters (26). The preliminary study was insufficient for taxonomic purposes. In this study we report the results of experiments that lead us to propose that the strain JF-1 is a species of a new genus within the α subclass of the Proteobacteria, which we name Citromicrobium bathyomarinum.
MATERIALS AND METHODS
Bacterial strain and cultivation.
Strain JF-1 was isolated (26) and cultivated for the purposes of this study in yeast-peptone-acetate medium. Unless otherwise noted, the bacterium was grown in Erlenmeyer flasks shaken aerobically in the dark at 30°C and pH 7.8 to 8.0 in a rich organic (RO) medium, containing (in grams per liter) the following ingredients: yeast extract, 1.0; Bacto Peptone, 1.0; sodium acetate, 1.0; KCl, 0.3; MgSO4 · 7H2O, 0.5; CaCl2 · 2H2O, 0.05; NH4Cl, 0.3; K2HPO4, 0.3; and NaCl, 20.0. This medium was supplemented with a mixture of vitamins ([per liter of medium] 20 μg of vitamin B12, 200 μg of nicotinic acid, 80 μg of biotin, and 400 μg of thiamine) and 1.0 ml per liter of a trace element solution (5). The same medium with the addition of agar (2%) was used for routine cultivation on agar plates. Liquid (taken from the late logarithmic growth phase) and agar surface cultures remained viable after storage at 4°C for at least 1 month.
Morphological and physiological tests.
The Gram test was performed by the method of Gregersen (9). The size and shape of cells were determined by phase-contrast and electron microscopy of cells from cultures grown in RO medium that contained different concentrations of NaCl (%): 0, 2, and 10. The motility of cells was determined by observation of a 24-h (log phase) culture grown in liquid RO medium.
The utilization of organic substrates for growth in the presence of NH4Cl (0.3 g per liter) or NaNO3 (0.3 g per liter) as sole sources of fixed inorganic nitrogen was investigated under low- and high-aeration conditions in the previously described liquid minimal medium (35). The organic substrates (pH 7.8 to 8.0) were added at a concentration of 1.0 g per liter. The results were recorded 5 days after inoculation.
Susceptibility to antibiotics was detected on agar plates by using discs impregnated with antibiotics in the amounts shown in Table 2. Tests for oxidase and catalase activities, reduction of nitrate, and the capacity to hydrolyze starch, gelatin, and Tweens were performed by using standard procedures (8).
TABLE 2.
Growth and physiological properties of strain JF-1 and the species phylogenetically closest to ita
| Characteristic | JF-1 | E. longus | P. neustonensis | E. ramosum | E. ursincola |
|---|---|---|---|---|---|
| Growth at pH: | |||||
| 5.0 | − | − | − | − | − |
| 5.5 | + | − | − | − | − |
| 6.0 | + | − | − | + | + |
| 7.0 | + | + | + | + | + |
| 8.0 | + | + | + | + | + |
| 9.0 | + | + | + | + | + |
| 9.5 | + | + | − | + | + |
| 10.0 | W | − | − | − | − |
| Growth at temp (°C): | |||||
| 4 | + | W | − | − | − |
| 15 | + | + | W | + | + |
| 20 | + | + | + | + | + |
| 30 | + | + | + | + | + |
| 37 | + | W | W | − | W |
| 40 | + | − | − | − | − |
| 42 | + | − | − | − | − |
| 45 | + | − | − | − | − |
| 50 | − | − | − | − | |
| Utilization of: | |||||
| Acetate | W | − | − | + | + |
| Pyruvate | − | + | + | + | + |
| Glutamate | + | + | − | + | + |
| Butyrate | + | + | − | + | + |
| Citrate | − | + | − | + | + |
| Malate | − | − | − | + | + |
| Succinate | − | − | + | + | + |
| Lactate | − | ND | − | + | + |
| Formate | − | ND | − | − | − |
| Glucose | W | + | + | + | + |
| Fructose | − | + | + | + | + |
| Methanol | − | − | − | − | − |
| Ethanol | − | ND | − | + | − |
| Yeast extract | + | + | + | + | + |
| Hydrolysis of: | |||||
| Gelatin | − | + | − | − | − |
| Tween 20 | + | ND | + | − | + |
| Tween 80 | + | + | + | − | + |
| Starch | + | − | − | − | − |
| Reduction of: | |||||
| NO3 to NO2 | − | + | ND | − | − |
| NO2 to N2 | − | − | − | − | − |
| TeO3 to Te | + | + | ND | + | + |
| Oxidase | + | + | + | + | + |
| Catalase | + | + | + | + | + |
| Susceptibility to: | |||||
| Chloramphenicol (100 μg) | + | + | + | + | + |
| Penicillin (20 μg) | − | − | + | − | − |
| Streptomycin (50 μg) | − | − | − | − | − |
| Fusidic acid (0.5 μg) | + | ND | ND | + | + |
| Polymyxin B (100 μg) | + | − | ND | + | + |
Fixation of CO2 (autotrophic growth) was investigated on agar plates of the minimal medium (35) without organic substrates by using a GasPak (marketed by BBL) system generating an atmosphere enriched in CO2-H2 (without catalyst) or aerobically in a liquid medium containing sodium bicarbonate.
The ability to grow anaerobically photosynthetically (26) or anaerobically in the presence of trimethylamine-N-oxide (TMAO) (1) and resistance to the heavy-metal oxide tellurite (33) were tested as described previously. Acid production from the fermentation of glucose was determined as described previously (7). Vitamin requirements were examined in minimal-acetate liquid medium by using four vitamin combinations, each of which lacked one of the four vitamins tested (thiamine, biotin, nicotinic acid, and B12). The final reading of culture turbidity was obtained after three successive transfers. The pH, temperature, and salinity ranges for growth were determined by measurement of the stationary-phase culture turbidity.
Analytical procedures.
Bchl and carotenoids were extracted from whole cells with acetone-methanol (7:2 [vol/vol]), and the total amount of Bchl was determined by using the method of Clayton (4). Culture turbidity was measured spectrophotometrically at the wavelength of 623 nm. Absorption spectra were recorded at room temperature with a Hitachi U-2000 spectrophotometer. The protein content of samples was determined by the Lowry assay (13).
Membrane and pigment-protein complex isolation.
Cells in the exponential growth phase were harvested by centrifugation, washed, and resuspended in 20 mM Tris-HCl buffer (pH 7.8). Cells were predominantly intact after several passages through a French pressure cell (15,000 lb/in2). However, the subsequent addition of 1.2% lauryldimethylamine-N-oxide to the cell suspension resulted in cell lysis and release of photosynthetic pigment-protein complexes from the membrane. The method of LHI-RC complex isolation by sucrose density gradient was previously described (27, 28).
Electron microscopy.
Cells from 24 h (log phase) cultures grown in liquid medium were negatively stained with 1% aqueous uranyl acetate. For thin sections, the bacteria were embedded in Epon after fixation with 1% glutaraldehyde and 1% osmium tetroxide as described by Kellenberger et al. (11).
Determination of the DNA G+C content.
DNA was isolated (3), and the G+C content of the DNA was determined by high-performance liquid chromatography (HPLC) (15).
16S rDNA sequence determination and analysis.
Extraction of genomic DNA, PCR-mediated amplification of the 16S ribosomal DNA (rDNA), and sequence analysis of the purified PCR products were done as previously reported (17). In order to determine the closest relatives of strain JF-1, the phylogenetic position was initially determined by using the ARB database (22). A fine resolution of relatedness between strain JF-1 and its closest relative within the α subclass of the class Proteobacteria was performed by using the ae2 editor (14). Phylogenetic dendrograms were constructed by using treeing algorithms contained in the PHYLIP software package, and bootstrap values were determined by using the PHYLIP package (6).
Nucleotide sequence accession numbers.
The 16S rDNA sequence determined in this study was deposited in the EMBL database (Cambridge, United Kingdom) under accession no. 16267 for C. bathyomarinum JF-1. The accession numbers for the 16S rDNAs of reference strains are as follows: for Erythromicrobium ramosum E5T, M72909; for Porphyrobacter neustonensis ACM2844T, M96747; for Erythrobacter litoralis T4T, M72962; for E. longus ATCC 33941T, M59062; for Erythromonas ursincola KR99T, Y10677; for Sphingomonas paucimobilis DSM1089T, X72722; and for Sandaracinobacter sibiricus RB16-17T, Y10678.
RESULTS AND DISCUSSION
Habitat.
The bacteria were isolated from samples obtained in August 1996 from the vicinity of nonbuoyant regions of plumes emitted from hydrothermal vents on the Juan de Fuca Ridge (Northeastern Pacific Ocean; ca. 47°57′N, 129°05′W; about 2,000 m beneath the ocean surface). Descriptions of the samples and bacterial heterotrophic population enumerated on the medium used are given in reference 26.
Culture properties.
Strain JF-1 on agar plates formed small (2 to 4 mm in diameter) citron-yellow colonies with a smooth surface. With age, the colonies became intensely yellow. After aerobic growth in liquid media aerated by shaking, cultures were slightly aggregative and intensely yellow. The culture did not grow anaerobically under light or dark conditions. In agar (0.7%) deeps, growth was observed only at the surface (the aerobic and semiaerobic zones) of agar tubes. Light was not required for growth.
Morphology and cytology.
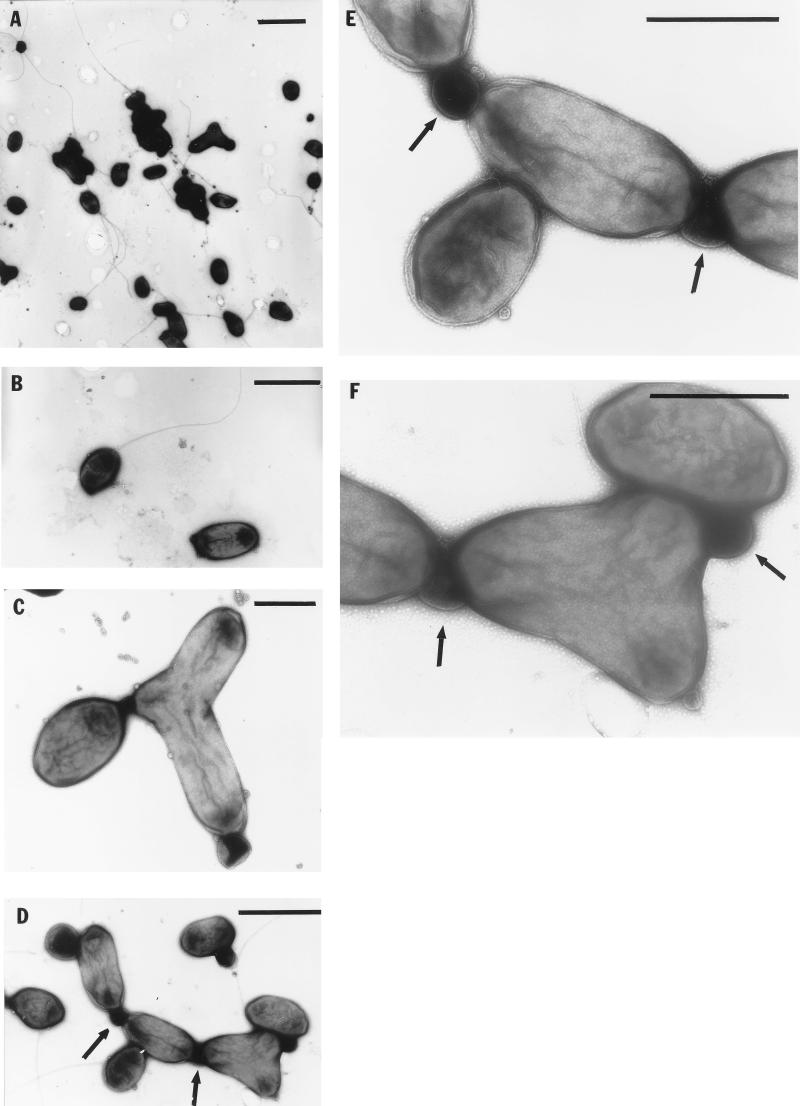
Cells of strain JF-1 grown in RO liquid medium that contained 0, 0.5, 1.0, 2.0, 3.0, or 5.0% NaCl were similar morphologically and unusually pleomorphic. The cells were gram negative. Depending on the growth phase of cultures, the morphology of cells ranged from almost coccoid (0.4 to 0.5 by 0.5 to 0.8 μm) to ovoid rods (0.4 to 0.5 μm by 1.0 to 1.2 μm) to thread-like formations of up to five cells (Fig. 1). In RO medium with a higher NaCl content (7 or 10%), bean-shaped or wavy cells as well as long, thread-like formations of up to 10 cells were found (data not shown). Coccoid cells from young cultures are motile by one polar or subpolar flagellum (Fig. 1).
FIG. 1.
Electron micrographs of negatively stained cells. (A) Pleomorphism of cells; (B) flagellation of a single cell; (C and D) cells of different morphologies connected by an unknown material (indicated by arrows); (E and F) enlarged regions of panel D, showing membranous connective material (indicated by arrows) and a bubbly substance. Bars, 2 μm (A), 1 μm (B and D), and 0.5 μm (C, E, and F).
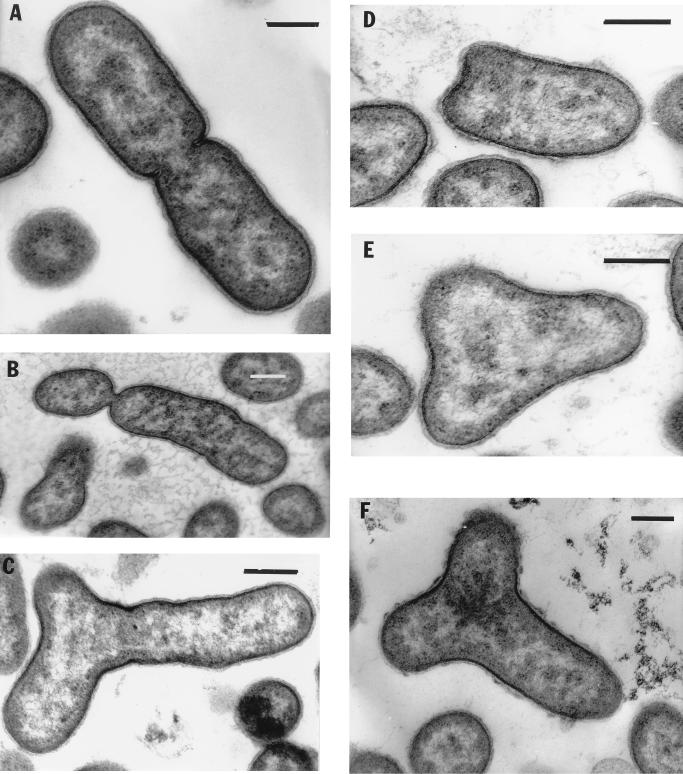
Strain JF-1 is very variable in its mode of cell division, since budding, ternary fission, binary division, and symmetric and asymmetric constrictions were observed (some examples are shown in Fig. 2). This bacterium forms Y cells, a rare type of bacterial multiplication which may result in the production of three daughter cells by one mother cell (Fig. 1 and 2). Cells often remained attached after division, apparently by means of a membranous connective material of unknown nature, and were surrounded by a thin bubbly substance (Fig. 1). Therefore, as mentioned above, cells in liquid culture were slightly aggregative, such that many individual cells remained in contact after division.
FIG. 2.
Different types of cell division revealed in electron micrographs of thin-sectioned cells. (A) Example of cell binary division with peptidoglycan layer and cytoplasmic membrane invagination. The nucleoid is seen as light zones. (B) Asymmetric constriction; (C) interesting type of cell division; (D through F) different stages of Y cell division. The nucleoids are distributed in three directions. Bars, 0.25 μm.
Electron microscopic thin sections showed that strain JF-1 has a gram-negative cell wall. The cytoplasmic membrane was visible, but no obvious intracytoplasmic membranes (ICM) were detected (Fig. 2). No inclusions indicative of storage materials were seen in cells harvested from RO medium.
Photosynthetic apparatus.
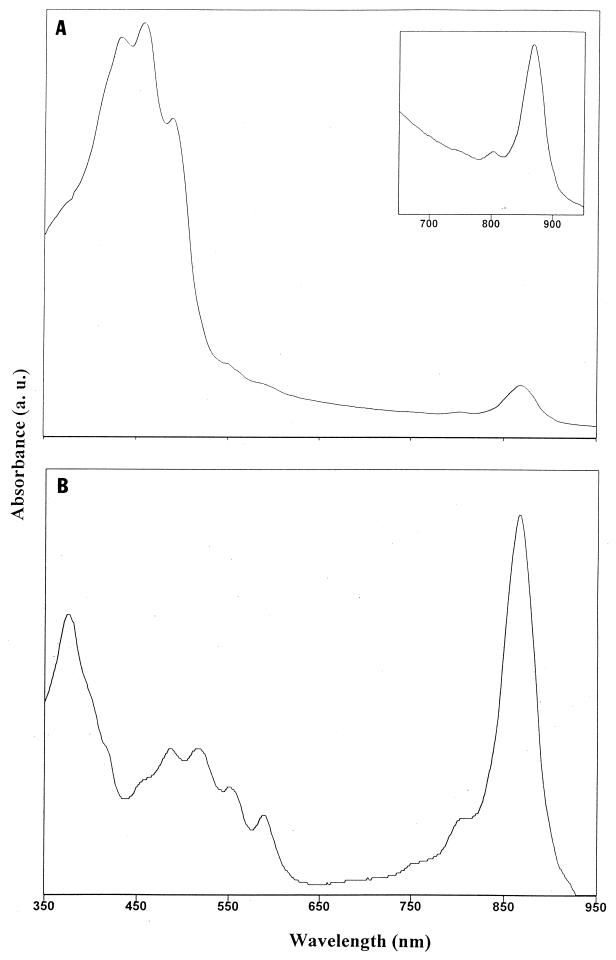
The in vivo absorption spectra of JF-1 had a major peak at 867 nm, indicating the presence of Bchl a incorporated into LH complex I (Fig. 3A and Table 1). The small peak at 800 nm indicates the presence of the photosynthetic RC. The photosynthetic apparatus organization of an LH system associated with the RC (photosynthetic unit) in strain JF-1 was indicated by isolation and purification of LHI-RC particles after lysis and treatment of cells with detergent and sucrose gradient fractionation (Fig. 3B). In addition to the LHI-RC-enriched fraction, a fraction that contained a low amount of an apparent LHII complex (absorption peaks at 799 and 849 nm) was obtained (data not shown). It seems that the amount of LHII in cells of JF-1 is so small that LHII peaks were not visible in the in vivo absorption spectra of intact cells (Fig. 3A), where the LHI Bchl a absorption peak was predominant. Additional experiments are necessary to unequivocally determine whether the 800-nm peak is due to an RC or an LHII complex.
FIG. 3.
(A) Absorption spectrum of intact cells showing carotenoid and Bchl peaks. Inset, enlarged 650- to 950-nm region of the spectrum showing the presence of the photosynthetic RC (small peak at 800 nm) and LHI (major peak at 867 nm). (B) Absorption spectrum of sucrose gradient fraction of detergent-solubilized membranes enriched in the LHI-RC complex.
TABLE 1.
Some determinative characteristics of strain JF-1 and its closest phylogenetic relatives that are aerobic anoxygenic photosynthetic bacteria
| Characteristic | Strain JF-1 | Erythrobacter | Erythromonas | Erythromicrobium | Porphyrobacter |
|---|---|---|---|---|---|
| Environment from which isolated | Deep-sea hydrothermal vent plume water | Marine surface | Freshwater | Freshwater | Freshwater |
| Cell shape and size (μm) | pleomorphic; 0.4–0.5 by 1.0–1.2 | Rods; 0.4–0.5 by 1.0–5.0 | Ovoid; 0.8–1.0 by 1.3–2.6 | Rods, branched; 0.7–1.0 by 1.6–2.5 | Pleomorphic; 0.4–0.8 by 1.1–2.0 |
| Color | Citron yellow | Orange | Orange-brown | Red-orange | Orange-red |
| Carotenoid in vivo peaks (nm) | 433, 457, 487 | 470 | 430, 458, 485 | 466, 478 | 464, 491 |
| Bchl in vivo peaks (nm) | 800, 867 | 800, 869 | 800, 867 | 798, 832, 868 | 799, 869 |
| LHI peak (nm) | 866 | 870 | 867 | 868 | NDa |
| LHII peaks (nm) | 799, 848 | Absent | Absent | 798, 832 | ND |
| DNA G+C content (mol%) | 67.5 | 60–64 | 65.4 | 64.2 | 65–66 |
ND, not determined.
The above data indicate the presence of LHI, LHII, and RC complexes in cells of JF-1, and the total number of photosynthetic units per cell is similar to the number of units determined from the spectra of other aerobic phototrophic bacteria. Cells contained 0.4 to 0.6 nmol of Bchl/mg of protein. Strain JF-1 produced Bchl when it was grown aerobically in the dark, but production of this pigment was significantly repressed on rich media such as RO or on medium containing yeast extract or Casamino Acids. The most-pronounced Bchl synthesis was detected in a minimal medium containing acetate, glutamate, or butyrate as the sole source of carbon.
The yellow color of cells and the three peaks at 433, 457, and 487 nm indicate the presence of carotenoids, apparently of the carotene type (Fig. 3A). The ratio of the absorbence at the LHI Bchl absorption peak (867 nm) to that at the main carotenoid absorption peak (457 nm) is about 1:8 (26). However, the carotenoid/Bchl ratio was much higher in intact cells than in partially purified photosynthetic complexes (Fig. 3). It seems that most of these carotenoids are not intimately bound to the components of the photosynthetic apparatus (LH and RC). As noted above, ICM invaginations were not observed in the electron microscopic thin sections. Possibly the photosynthetic apparatus of JF-1 is restricted to the cytoplasmic membrane.
Physiological and nutritional characteristics.
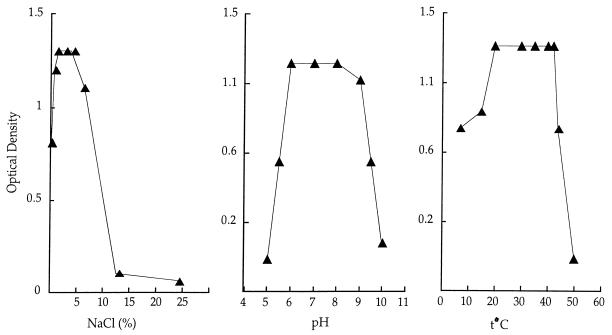
Strain JF-1 revealed a broad tolerance to culture conditions such as salinity, temperature, and pH. Thus, growth was obtained in a freshwater medium and in medium supplemented with 10% NaCl, at temperatures ranging from 4 to 45°C and at pHs of 5.5 to 10.0 (Fig. 4 and Table 2). Therefore, JF-1 is a salt-, pH- and thermotolerant strain. Such a broad tolerance of culture conditions might be the result of evolutionary adaptation to diverse environmental conditions that exist around deep-ocean hydrothermal vents.
FIG. 4.
Strain JF-1 is capable of growth over a broad range of NaCl concentrations, pH values, and temperatures in RO media.
The results of nutritional and biochemical tests performed with strain JF-1 are shown in Table 2. This isolate utilizes an unusually low number of substrates compared to other aerobic phototrophic bacteria. Glutamate, butyrate, and yeast extract are the best carbon sources for JF-1, and acetate and glucose support weak growth. This restricted metabolic capacity is consistent with the idea that JF-1 is adapted to the presumably oligotrophic environment of hydrothermal vent plume waters. The substitution of nitrate for ammonia as a nitrogen source and increased or decreased aeration did not affect the variety of organic substrates that supported growth. Gelatin was not hydrolyzed, but Tweens 20 and 80 and starch were hydrolyzed, indicating the absence of gelatinase and the presence of lipolytic and amylolytic activities (Table 2). Strain JF-1 was fermentative without gas generation, as shown by acid production in a minimal medium with glucose. Strain JF-1 did not reduce nitrate but did reduce tellurite to metallic tellurium at tellurite concentrations up to 2,000 μg/ml in acetate-glutamate-containing medium.
Strain JF-1 did not grow under anaerobic photosynthetic conditions (26) or anaerobically in the presence of TMAO. Autotrophic growth was not detected under either aerobic or anaerobic conditions in the light or the dark. No vitamins were required as growth factors, although the addition of biotin stimulated growth. Strain JF-1 was sensitive to chloramphenicol, fusidic acid, and polymyxin B and resistant to penicillin and streptomycin (Table 2).
DNA composition and phylogenetic analysis.
The DNA base composition of the new isolate was 67.5 mol% G+C (Table 1). In addition to the four main peaks of nucleotides, four small peaks were detected which could represent modified nucleotides.
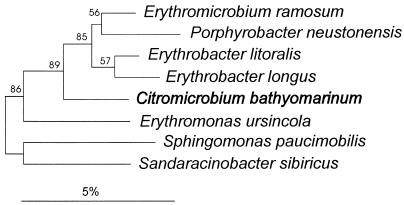
Most of the 16S rDNA sequence of strain JF-1 (about 92% by analogy with the Escherichia coli sequence [2]) was determined and compared with other sequences contained in public databases (22), including the database maintained in the Deutsche Sammlung von Mikroorganismen und Zellkulturen. The phylogenetically closest relatives were members of the genera Sphingomonas, Sandaracinobacter, Erythrobacter, Erythromonas, and Porphyrobacter. The phylogenetic position (Fig. 5) of strain JF-1 is as a branch between the genus Erythromonas and the Erythromicrobium-Porphyrobacter-Erythrobacter cluster within the α subclass.
FIG. 5.
Dendrogram showing the phylogenetic position of strain JF-1, designated C. bathyomarinum, among members of the genera Sphingomonas, Erythromonas, Erythrobacter, Sandaracinobacter, and Porphyrobacter within the α-subclass of the Proteobacteria. The sequence of A. tumefaciens was used to root the dendrogram. Bootstrap values (expressed as percentages of 500 replications) of 65% or more are indicated at the branch points. Bar, 5% sequence divergence.
Concluding remarks.
The presence of Bchl a incorporated into LH and RC complexes, the inability to grow anaerobically in the light, the abundance of carotenoids, the small number of photosynthetic units, and the absence of an extensive ICM system indicate that JF-1 is a member of the aerobic anoxygenic phototrophic bacteria. The phylogenetic analysis based on 16S rDNA sequences demonstrates that strain JF-1 has an ancestor in common with previously described aerobic anoxygenic phototrophic bacteria and other representatives of the α subclass of the Proteobacteria. At the present time it is not possible to conclude whether JF-1 developed photosynthetic properties near a hydrothermal vent or colonized this environment at a later time. However, the abundance of this bacterium (approaching 10% of all cells cultivated on the medium used) indicates that the ability to produce Bchl may be selectively advantageous (26). The answers to questions concerning the photosynthetic nature of JF-1 may improve our understanding of the possibility of the evolution of photosynthesis at deep hydrothermal vents as opposed to sun-irradiated environments. The small amount of Bchl that is present in JF-1 could be a direct result of oxygen-driven evolution preceding or closely following the light-driven evolutionary changes in a photosynthetic apparatus. Strain JF-1 may have lost most of its photosynthetic activity due to the development of an oxygen-rich environment at the ocean floor. The isolation of this aerobic phototrophic bacterium from the black smoker vicinity is indicative of the diversity of bacteria found in this extreme environment. The fact that such a bacterium was found there suggests that the ecology of that environment is even more diverse than has been imagined. We hope that our report on JF-1 will spur additional searches for frankly photosynthetic organisms in deep-ocean hydrothermal vent environments, which could provide clues about the possibility of the evolution of photosynthesis at deep-sea hydrothermal vents.
The significant ecological, morphological, physiological, and nutritional differences between strain JF-1 (Tables 1 and 2) and other aerobic photosynthetic genera as well as the results of 16S rDNA phylogenetic analysis (Fig. 5) lead us to propose a new genus for strain JF-1—Citromicrobium.
Description of Citromicrobium gen. nov.
Citromicrobium (Ci.tro.mi.cro′bi.um. Gr. n. citron, citron; Gr. adj. micros, small; Gr. n. bios, life; M. L. n. Citromicrobium, citron-colored microbe). Cells are pleomorphic, depending on the growth phase of cultures, coccoid to ovoid rods, often forming Y cells. Motile by one polar or subpolar flagellum. Gram negative, highly variable in its mode of multiplication. Cultures are an intense citron yellow because of carotenoid pigments and contain Bchl a. Photosynthetic apparatus contains RC and LH complexes. No growth occurs anaerobically in light. Incapable of autotrophic growth. Obligately aerobic but ferments glucose to acids without gas production. No dissimilatory denitrification activity detected.
DNA base composition is 67.5 mol% G+C (by HPLC). The habitat is marine. Member of the α subclass of the Proteobacteria. The type species is C. bathyomarinum.
Description of C. bathyomarinum sp. nov.
C. bathyomarinum (ba.thy.o.ma.ri′num. Gr. adj. bathys, deep; L. adj. marinum, oceanic; M. L. adj. bathyomarinum, deeply oceanic). Gram negative, citron-yellow-pigmented, pleomorphic. Cells may be almost coccoid (0.4 to 0.5 by 0.5 to 0.8 μm), ovoid rods (0.4 to 0.5 by 1.0 to 1.2 μm), or thread-like structures. Coccoid cells are motile by means of one polar or subpolar flagellum.
Cells contain Bchl a and carotenoid pigments. Bchl a bound to proteins gives in vivo absorption peaks at 800 and 867 nm. Photosynthetic apparatus is organized in RC, LHI, and LHII complexes, as evidenced by partially purified preparations in which the LHI complex yields a peak at 866 nm and the LHII complex yields peak at 799 and 849 nm.
Aerobic chemoorganotroph and presumptive facultative photoheterotroph. The best growth substrates are glutamate, butyrate, and yeast extract; weak growth on minimal media containing acetate or glucose. No growth on pyruvate, citrate, malate, succinate, lactate, formate, fructose, methanol, or ethanol.
Optimal temperature for growth is 20 to 42°C. Capable of growth over a salinity range from 0 to 10% NaCl in RO medium, with an optimum range of 1 to 5%. The pH optimum is from 6.0 to 8.0. Exhibits oxidase, catalase, lipase, and amylase activities but not gelatinase activity. No dissimilatory denitrification or anaerobic growth in the presence of TMAO. Glucose is fermented to acid products without gas generation. Demonstrates a high level of resistance to tellurite (up to 2,000 μg/ml in an acetate-glutamate minimal medium). Tellurite is reduced and transformed to metallic tellurium, causing the culture to blacken.
Resistant to penicillin and streptomycin; sensitive to chloramphenicol, fusidic acid, and polymyxin B. The DNA G+C content is 67.5 mol% (by HPLC). Habitat: the vicinity of nonbuoyant regions of plumes emitted from hydrothermal vents on the Juan de Fuca Ridge (northeastern Pacific Ocean). The type strain is JF-1.
ACKNOWLEDGMENTS
This work was supported in part by grants from NSERC (Canada) to V.V.Y. and J.T.B.
REFERENCES
- 1.Arata H, Serikawa Y, Takamiya K I. Trimethylamine-N-oxide respiration by aerobic photosynthetic bacterium, Erythrobacter sp. OCh114. J Biochem. 1988;103:1011–1015. doi: 10.1093/oxfordjournals.jbchem.a122371. [DOI] [PubMed] [Google Scholar]
- 2.Brosius J, Palmer M L, Kennedy P J, Noller H F. Complete nucleotide sequence of the 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cashion P, Holder-Franklin M A, McCully J, Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1977;81:461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- 4.Clayton R K. Spectroscopic analysis of bacteriochlorophyll in vivo and in vitro. Photochem Photobiol. 1966;5:669–677. [Google Scholar]
- 5.Drews G. Mikrobiologisches Praktikum. Berlin, Germany: Springer-Verlag; 1983. [Google Scholar]
- 6.Felsenstein J. PHYLIP (phylogenetic inference package) version 3.5.1. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 7.Fuerst J A, Hawkins J A, Holmes A, Sly L I, Moore C J, Stackebrandt E. Porphyrobacter neustonensis gen. nov., sp. nov., an aerobic bacteriochlorophyll-synthesizing budding bacterium from freshwater. Int J Syst Bacteriol. 1993;43:125–134. doi: 10.1099/00207713-43-1-125. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. [Google Scholar]
- 9.Gregersen T. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur J Appl Microbiol Biotechnol. 1978;5:123–127. [Google Scholar]
- 10.Hanada S, Kawase Y, Hiraishi A, Takaichi S, Matsuura K, Shimada K, Nagashima K V P. Porphyrobacter tepidarius sp. nov., a moderately thermophilic aerobic photosynthetic bacterium isolated from a hot spring. Int J Syst Bacteriol. 1997;47:408–413. doi: 10.1099/00207713-47-2-408. [DOI] [PubMed] [Google Scholar]
- 11.Kellenberger E, Ryter A, Sechaud J. Electron microscope study of DNA-containing plasms. J Biophys Biochem Cytol. 1958;4:671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LITE and Workshop Participants. Light in thermal environments. In: Van Dover C L, Cann J R, Cavanaugh C, Chamberlain S, Delaney J R, Janecky D, Imhoff J, Tyson A, editors. RIDGE program report. Woods Hole, Mass: Oceanographic Institution; 1994. p. 44. [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Maidak B L, Larsen N, McCaughey M J, Overbeck R, Olsen G J, Fogel K, Blandy J, Woese C R. The ribosomal database project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesbah M, Premachandran U, Whitman B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 16.Nisbet E G, Cann J R, van Dover C L. Origins of photosynthesis. Nature (London) 1995;373:479–480. [Google Scholar]
- 17.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 18.Shiba T. Roseobacter litoralis gen. nov., sp. nov. and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. Syst Appl Microbiol. 1991;14:140–145. [Google Scholar]
- 19.Shiba T, Shioi Y, Takamiya K I, Sutton D C, Wilkinson C R. Distribution and physiology of aerobic bacteria containing bacteriochlorophyll a on the East and West coasts of Australia. Appl Environ Microbiol. 1991;57:295–300. doi: 10.1128/aem.57.1.295-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiba T, Simidu U. Erythrobacter longus gen. nov., sp. nov., an aerobic bacterium which contains bacteriochlorophyll a. Int J Syst Bacteriol. 1982;32:211–217. [Google Scholar]
- 21.Shiba T, Simidu U, Taga N. Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl Environ Microbiol. 1979;38:43–45. doi: 10.1128/aem.38.1.43-45.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strunk O, Ludwig W. ARB—a software environment for sequence data. Munich, Germany: Department of Microbiology, Technical University of Munich; 1995. [Google Scholar]
- 23.Van Dover C L, Cann J R, Cavanaugh C, Chamberlain S, Delaney J R, Janecky D, Imhoff J, Tyson J A the LITE Workshop Participants. Light at deep sea hydrothermal vents. EOS Trans Am Geophys Union. 1994;75:44–45. [Google Scholar]
- 24.Van Dover C L, Reynolds G T, Chave A D, Tyson J A. Light at deep sea hydrothermal vents. Geophys Res Lett. 1996;23:2049–2052. [Google Scholar]
- 25.Wakao N, Shiba T, Hiraishi A, Ito M, Sakurai Y. Distribution of bacteriochlorophyll a in species of the genus Acidiphilium. Curr Microbiol. 1993;27:277–279. [Google Scholar]
- 26.Yurkov V, Beatty J T. Isolation of aerobic anoxygenic photosynthetic bacteria from Black Smoker plume waters of the Juan de Fuca Ridge in the Pacific Ocean. Appl Environ Microbiol. 1998;64:337–341. doi: 10.1128/aem.64.1.337-341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yurkov V, Gad’on N, Angerhofer A, Drews G. Light-harvesting complexes of aerobic bacteriochlorophyll-containing bacteria Roseococcus thiosulfatophilus, RB3 and Erythromicrobium ramosum, E5 and the transfer of excitation energy from carotenoids to bacteriochlorophyll. Z Naturforsch Teil C. 1994;49:579–586. . (In English.) [Google Scholar]
- 28.Yurkov V, Gad’on N, Drews G. The major part of polar carotenoids of the aerobic bacteria Roseococcus thiosulfatophilus, RB3 and Erythromicrobium ramosum, E5 is not bound to the bacteriochlorophyll a complexes of the photosynthetic apparatus. Arch Microbiol. 1993;160:372–376. [Google Scholar]
- 29.Yurkov V, Gorlenko V M. Erythrobacter sibiricus sp. nov., a new freshwater aerobic bacterial species containing bacteriochlorophyll a. Microbiology (New York) 1990;59:85–89. [Google Scholar]
- 30.Yurkov V, Gorlenko V M. A new genus of freshwater aerobic, bacteriochlorophyll a-containing bacteria, Roseococcus gen. nov. Microbiology (New York) 1992;60:628–632. [Google Scholar]
- 31.Yurkov V, Gorlenko V M. New species of aerobic bacteria from the genus Erythromicrobium containing bacteriochlorophyll a. Microbiology (New York) 1993;61:163–168. [Google Scholar]
- 32.Yurkov V, Gorlenko V M, Kompantseva E I. A new type of freshwater aerobic orange-coloured bacterium containing bacteriochlorophyll a, Erythromicrobium gen. nov. Microbiology (New York) 1992;61:169–172. [Google Scholar]
- 33.Yurkov V, Jappe J, Vermeglio A. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl Environ Microbiol. 1996;62:4195–4198. doi: 10.1128/aem.62.11.4195-4198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yurkov V, Stackebrandt E, Buss O, Vermeglio A, Gorlenko V M, Beatty J T. Reorganization of the genus Erythromicrobium: description of “Erythromicrobium sibiricum” as Sandaracinobacter sibiricus gen. nov., sp. nov., and of “Erythromicrobium ursincola” as Erythromonas ursincola, gen. nov., sp. nov. Int J Syst Bacteriol. 1997;47:1172–1178. doi: 10.1099/00207713-47-4-1172. [DOI] [PubMed] [Google Scholar]
- 35.Yurkov V, Stackebrandt E, Holmes A, Fuerst J A, Hugenholtz P, Golecki J, Gad’on N, Gorlenko V M, Kompantseva E I, Drews G. Phylogenetic positions of novel aerobic, bacteriochlorophyll a-containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp. nov. Int J Syst Bacteriol. 1994;44:427–434. doi: 10.1099/00207713-44-3-427. [DOI] [PubMed] [Google Scholar]
- 36.Yurkov V, van Gemerden H. Abundance and salt tolerance of obligately aerobic, phototrophic bacteria in a microbial mat. Neth J Sea Res. 1993;31:57–62. [Google Scholar]
- 37.Yurkov V V, Beatty J T. Aerobic anoxygenic phototrophic bacteria. Microbiol Mol Biol Rev. 1998;62:695–724. doi: 10.1128/mmbr.62.3.695-724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]