Abstract
Adiponectin has been proposed to be a mediator of obesity-associated malignancies and to have direct antineoplastic effects acting via adiponectin receptors AdipoR1 and AdipoR2. We describe herein the expression of AdipoR1 and AdipoR2 in several cancers not previously studied. We used immunohistochemistry to assess expression of adiponectin receptors in archival specimens of renal cell carcinoma (n = 64), hepatocellular carcinoma (n = 123), melanoma (n = 20), cholangiocarcinoma (n = 20), transitional cell carcinoma of the bladder (n = 24), ovarian epithelial carcinoma (n = 63), cervical squamous cell carcinoma (n = 49), and adrenocortical carcinoma (n = 48). To compare expression in malignant versus nonmalignant tissues, we also studied AdipoR1 and AdipoR2 expression in pairs of renal cell carcinoma and adjacent healthy kidney tissue specimens by immunohistochemistry. We also studied mRNA expression in 45 specimens of renal cell carcinoma by real-time polymerase chain reaction. Finally, we utilized Western blotting to confirm the presence of adiponectin receptors and subsequently studied cell signaling pathways of adiponectin in the renal cancer cell line 786-O. Cancers associated with obesity were significantly more likely to express AdipoR1 than cancers not associated with obesity. Of the specimens of renal cell carcinoma, which is strongly associated with obesity, 93.8% expressed AdipoR1 compared to 44.9% of the specimens of cervical cell carcinoma, which is not associated with obesity (p < 0.001). There was no difference in the expression of adiponectin receptors or their mRNA between malignant and benign kidney tissue specimens. Overall, there were no correlations between expression of adiponectin receptors or their mRNA and tumor prognostic factors. Finally, Western blotting confirmed the presence of AdipoR1 in the renal cancer cell line 786-O, and adiponectin activates in vitro several signaling pathways in this cell line. In summary, we report for the first time expression of AdipoR1 and AdipoR2 in the above cancers and that AdipoR1 is more ubiquitously expressed in obesity-associated cancers.
Keywords: Adiponectin, Adiponectin receptor, Malignancy, Obesity
Introduction
Large prospective studies have shown a significant association between obesity and several cancers, including colorectal, postmenopausal breast, endometrial, and renal cancers [1]. Originally, this association was thought to be mediated by altered levels of insulin, insulin growth factors, and sex steroids [2]. Recently, adiponectin, a hormone that is secreted by adipose tissue in inverse relation to abdominal obesity and is upstream of all these hormonal abnormalities, has been proposed to play a role in the development and progression of these obesity-related malignancies [3]. Epidemiologic studies have reported lower serum adiponectin levels in patients with colorectal [4–6], breast [7], endometrial [8], and renal [9, 10] cancers compared to controls.
The antineoplastic effects of adiponectin include indirect effects through the above hormonal mechanisms as well as direct effects, which are mediated through adiponectin receptors AdipoR1 and AdipoR2. Our group has previously reported the presence and analyzed the expression of these adiponectin receptors in breast [7], colorectal [11], prostate [12], lung [13], and pancreatic [14] cancers. Others have also reported the presence of adiponectin receptors in renal cell carcinoma [15], gastric carcinoma [16], and chronic lymphocytic leukemia [17]. The purpose of this paper is to describe and compare AdipoR1 and AdipoR2 expression in several cancers not yet studied to date. We chose to study cancers unambiguously associated with obesity, including renal cell carcinoma, cancers likely associated with obesity, including hepatocellular carcinoma, melanoma, cholangiocarcinoma, and transitional cell carcinoma of the bladder, and cancers not associated with obesity, including ovarian epithelial carcinoma, cervical squamous cell carcinoma, and adrenocortical carcinoma.
Materials and Methods
Study Subjects
Available for analysis by immunohistochemistry were archival, formalin-fixed, and paraffin-embedded specimens of renal cell carcinoma (n = 64), hepatocellular carcinoma (n = 123), melanoma (n = 20), cholangiocarcinoma (n = 20), transitional cell carcinoma of the bladder (n = 24), ovarian epithelial carcinoma (n = 63), cervical squamous cell carcinoma (n = 49), and adrenocortical carcinoma (n = 48). Information on the presence of metastases, presence of nodal invasion, stage, and/or grade were available for the specimens studied except for those of adrenocortical carcinoma. An additional nine pairs of renal cell carcinoma and adjacent healthy kidney tissue specimens in the form of formalin-fixed tissue microarray mounted to standard salinized slides were purchased to compare expression of adiponectin receptors in malignant and benign tissues (Imgenex, San Diego, CA, USA).
For analysis by real-time quantitative polymerase chain reaction (RT-qPCR), a panel of prenormalized cDNA from 48 cancerous tissues involving the kidney was purchased as an independent confirmatory study in a different population (TissueScan Kidney Disease Tissue qPCR Array, OriGene Technologies, Rockville, MD, USA). Two of the samples were excluded as they were not of renal cell carcinoma, and one sample was used as a negative control.
Immunohistochemistry Analysis
The immunohistochemistry analysis was performed as previously described [7, 11, 12]. Five-micrometer paraffin tissue sections were deparaffinized, rehydrated, microwaved for 25 min in 10 mmol/l citrate buffer, and incubated for 30 min in methanol-containing 0.5% H2O2. After incubation in 16% normal goat serum for 1 h at room temperature, the slides were incubated with the primary antibodies at room temperature. The primary antibodies used were rabbit antihuman AdipoR1 antiserum, which was raised against amino acid residues 357–375, and rabbit antihuman AdipoR2 antiserum, which was raised against amino acid residues 374–386 (both from Phoenix Pharmaceuticals Inc., Belmont, CA, USA). The primary antibodies were diluted at 1:500 and 1:200, respectively. The secondary antibody used was a biotinylated antirabbit antibody at 1:400 dilution and was applied for 30 min at room temperature. Vectastain Elite ABC Reagent (Vector Laboratories, Burlingame, CA, USA) was added for 30 min. The horseradish peroxidase reaction was developed with diaminobenzidine. The slides were counterstained with hematoxylin. The immunostaining was evaluated semiquantitatively according to its extent and degree of intensity on a scale of “−,” “−/+,” “+,” “++,” to “+++” by two expert pathologists independently. The results were averaged. For each of the cancers studied, primary antibody was replaced with nonimmune goat serum for a slide to serve as negative control.
Real-Time Quantitative Polymerase Chain Reaction
Expression of AdipoR1 and AdipoR2 was studied in the human renal adenocarcinoma cell line 786-O (American Type Culture Collection, Manassas, VA, USA) with RT-qPCR by total RNA extraction with Trizol (Invitrogen, Carlsbad, CA, USA), cDNA synthesis using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), and qPCR with human-specific TaqMan® Gene Expression Assay (Assay ID: Hs00360422_m1 and Hs00226105_m1, Applied Biosystems, Foster City, CA) in 7500 Fast Real-Time PCR system using Standard Real-Time 7500 protocol (Applied Biosystems, Foster City, CA, USA). Expression of AdipoR1 and AdipoR2 was also studied in cDNA samples from 45 specimens of renal cell carcinoma, including five specimens of surrounding normal tissues (TissueScan Kidney Disease Tissue qPCR Array, OriGene Technologies, Rockville, MD, USA) according to the application guide. Prenormalized cDNA (2–3 ng, by β-actin) in 30-µl reaction volumes were amplified using TaqMan® Gene Expression system with the specific primers stated above. Data were analyzed using 7500 Real-Time PCR System software (Applied Biosystems, Foster City, CA, USA). Relative quantification was done using ΔΔCt method with Eukaryotic 18S rRNA (Applied Biosystems, Foster City, CA, USA) as the endogenous control and human subcutaneous fat biopsy specimen and the normal renal tissue samples as reference controls.
Western Blotting
Western blotting was performed on the renal cancer cell line 786-O (American Type Culture Collection, Manassas, VA, USA) to confirm the presence of AdipoR1 and AdipoR2. Fifty micrograms of protein were loaded. After SDS–polyacrylamide gel electrophoresis, proteins were blotted onto nitrocellulose membranes (Schleicher & Schuell, Inc., Keene, NH, USA). The membranes were blocked for 1 h in Tris-buffered saline (TBS) containing 5% nonfat dry milk and 0.1% Tween-20. Incubation with primary antibodies at a dilution of 1:1,000 was performed in TBS containing 5% nonfat dry milk overnight, which was followed by incubation with horseradish-peroxidase-labeled secondary antibodies at a dilution of 1:2,000 for 2 h. After incubation with the antibodies, membranes were washed with TBS containing 0.1% Tween-20. Enhanced chemiluminescence was used for detection. Measurement of signal intensity on nitrocellulose membranes after Western blotting was performed using Image J processing and analysis software (US National Institutes of Health, Bethesda, MD, USA).
Cell Signaling Studies
The renal cancer cell line 786-O (American Type Culture Collection, Manassas, VA, USA) was seeded in six-well tissue culture plates and grown in RPMI medium with 100 U/ml of penicillin, 100 mg/ml of streptomycin, and 1 mM of sodium pyruvate, supplemented with 10% fetal bovine serum. After 2 days of cell culture, 60% confluency was reached (referred to as day 0). The cells were then serum-starved overnight and treated with adiponectin (R&D systems, Minneapolis, MN, USA) in a time- and dose-dependent manner.
Western blotting was performed to assess for phosphorylation of signal transducer and activator of transcription 3 (STAT3), ribosomal S6 kinase (S6), extracellular signal-regulated kinases 1 and 2 (ERK1/2), and the serine/threonine kinase Akt. All cell lysates were examined by Western blotting with primary p-STAT3 (Santa Cruz Biotechnology, Inc., San Francisco, CA, USA), STAT3 (Santa Cruz Biotechnology, Inc., San Francisco, CA, USA), p-S6 (Cell Signaling Technology, Inc., Danvers, MA, USA), S6 (Cell Signaling Technology, Inc., Danvers, MA, USA), p-ERK1/2 (Santa Cruz Biotechnology, Inc., San Francisco, CA, USA), ERK1/2 (Santa Cruz Biotechnology, Inc., San Francisco, CA, USA), p-Akt (Santa Cruz Biotechnology, Inc., San Francisco, CA, USA), and Akt (Santa Cruz Biotechnology, Inc., San Francisco, CA, USA) antibodies diluted with TBS with 0.1% Tween-20 at 1:500. The secondary antibodies used were horseradish-peroxidase-conjugated antimouse, antirabbit, or antigoat antibodies and were diluted at 1:750. Measurement of signal intensity on nitrocellulose membranes was performed using Image J processing and analysis software (US National Institutes of Health, Bethesda, MD, USA). All experiments were performed in triplicate.
Statistical Analysis
For the immunohistochemistry studies, descriptive characteristics are presented as proportions and mean values ± standard deviations. Comparisons of categorical variables were conducted using Fisher’s exact test. Comparison of cases and controls on expression of adiponectin receptors were conducted using McNemar's test. Analyses were performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Statistics for the RT-qPCR data were performed using general linear models, and individual differences between groups were identified by one-way analysis of variance (ANOVA) followed by the protected least significant differences technique (SAS version 9.1; SAS Institute, Cary, NC, USA). Data are presented as means ± standard error.
For the cell signaling studies by Western blotting, all data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons with SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). Values are expressed as means ± standard deviation.
For all studies, a two-sided level of α = 0.05 was used to determine statistical significance.
Results
We first compared the percentage of specimens with positive expression of AdipoR1 and AdipoR2 in several cancers, including the kidney, liver, skin, gallbladder, bladder (transitional cell), ovarian epithelium, cervix (squamous cell), and adrenal cortex (Table 1). Kidney, skin, and bladder cancers had the greatest prevalence of AdipoR1 expression, i.e., over 80% of the specimens. Less than 50% of the specimens of cervical squamous cancer expressed AdipoR1. Renal cell carcinoma, which is strongly associated with obesity [1, 2, 18], expressed significantly more AdipoR1 compared to ovarian epithelial (p < 0.01), cervical (p < 0.001), and adrenocortical (p < 0.001) carcinomas, all of which have not been shown to be associated with obesity [1, 19–22]. There was a similar pattern in AdipoR2 expression among the studied cancers though to a lesser extent.
Table 1.
Expression of adiponectin receptors in various cancers
| Cancera | Number | Age, years (SD) | Sex (M/F/NR) | AdipoR1: positiveb, n (%) | AdipoR2: positiveb, n (%) | AdipoR1: robustly positivec, n (%) | AdipoR2: robustly positivec, n (%) |
|---|---|---|---|---|---|---|---|
| Renal | 64 | 56.2 (11.1) | 45/18/1 | 60 (93.8) | 62 (96.9) | 27 (42) | 48 (75) |
| Liver | 123 | 54.2 (11.4) | 96/27 | 90 (73.2) | 93 (75.6) | 41 (33.3) | 29 (23.6) |
| Melanoma | 20 | 52.0 (17.5) | 9/11 | 17 (85) | 14 (70) | 11 (55) | 4 (20) |
| Gallbladder | 20 | 59.4 (9.4) | 5/15 | 16 (80) | 16 (80) | 7 (35) | 4 (20) |
| Bladder | 24 | 64.0 (8.5) | 22/2 | 24 (100) | 16 (67) | 11 (46) | 4 (17) |
| Ovarian | 63 | 49.6 (12.7) | 0/63 | 47 (87.3) | 32 (54.0) | 14 (25.4) | 9 (14.3) |
| Cervix | 49 | 51.6 (10.0) | 0/49 | 22 (44.9) | 32 (65.3) | 3 (6) | 12 (24) |
| Adrenal | 48 | 52.4 (16.1) | 26/22 | 29 (60.4) | 42 (87.5) | 7 (14.6) | 15 (31.3) |
NR not recorded
aArranged by highest to lowest risk ratio of cancer per 5-kg/m2 increase in BMI as estimated in a meta-analysis by Renehan et al. [1]. Bladder cancer, adrenocortical cancer, and cervical cancer were not included in the meta-analysis and inserted accordingly by expanded literature search
bPositive indicates score of “+” and above
cRobustly positive indicates score of “++” or above
We also analyzed the relationship between intensity of AdipoR1 and AdipoR2 expression with available tumor characteristics, including presence of metastases, presence of nodal invasion, stage, and grade, in all the studied cancers except adrenocortical carcinomas as information on these cancers were not available. We only found greater AdipoR1 expression in nonmetastatic compared to metastatic renal cell carcinoma specimens (p = 0.031). There were no other significant associations.
Due to its well-acknowledged association with both obesity and circulating adiponectin levels [10], we chose to further study renal cell carcinoma. By immunohistochemistry, we directly compared expression of adiponectin receptors in nine pairs of malignant and adjacent benign tissue from the same patient (Table 2). The mean patient age was 55.9 years, and six of the nine patients were men. Six of the patients had cancers that invaded beyond the renal capsule, and one of these six patients also had invasion into the venous system. None of the patients were found to have metastases. All of the specimens, cancerous and healthy, expressed AdipoR1 and AdipoR2 with no significant difference (p = 0.13 for AdipoR1, p = 0.73 for AdipoR2).
Table 2.
Expression of adiponectin receptors in paired renal cell carcinoma and adjacent healthy tissue specimens
| Pair | Age | Sex | Tumor size (cm) | Capsule invasion | Vein invasion | Nodal invasion | Metastases | Cancerous tissue | Normal tissue | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AdipoR1 | AdipoR2 | AdipoR1 | AdipoR2 | ||||||||
| 1 | 59 | M | 7.5 | + | − | Unknown | None | + | ++ | ++ | + |
| 2 | 43 | F | 4 | + | − | None | None | ++ | ++ | ++ | +++ |
| 3 | 40 | M | 2.2 | − | − | Unknown | None | + | + | ++ | +++ |
| 4 | 52 | M | 6 | + | − | Unknown | None | + | + | ++ | +++ |
| 5 | 62 | M | 7 | + | + | Unknown | None | + | ++ | ++ | +++ |
| 6 | 72 | M | 1.5 | − | − | Unknown | None | ++ | +++ | + | ++ |
| 7 | 43 | F | 6 | − | − | None | None | + | +++ | ++ | ++ |
| 8 | 77 | M | 2.5 | + | − | Unknown | None | + | + | ++ | ++ |
| 9 | 55 | F | 3 | + | − | Unknown | None | + | + | + | + |
We then used Western blotting and real-time PCR to confirm our results. First of all, we found that AdipoR1, but not AdipoR2, was detected by Western blotting in the renal cancer cell line 786-O. We then studied the presence of mRNA in this cell line by RT-qPCR and found that the renal cancer cell line had 0.16 ± 0.02 times the expression of AdipoR1 and 0.11 ± 0.01 times the expression of AdipoR2 compared to human subcutaneous fat biopsy specimens, which are known to express adiponectin receptors [23–25] (Fig. 1).
Fig. 1.
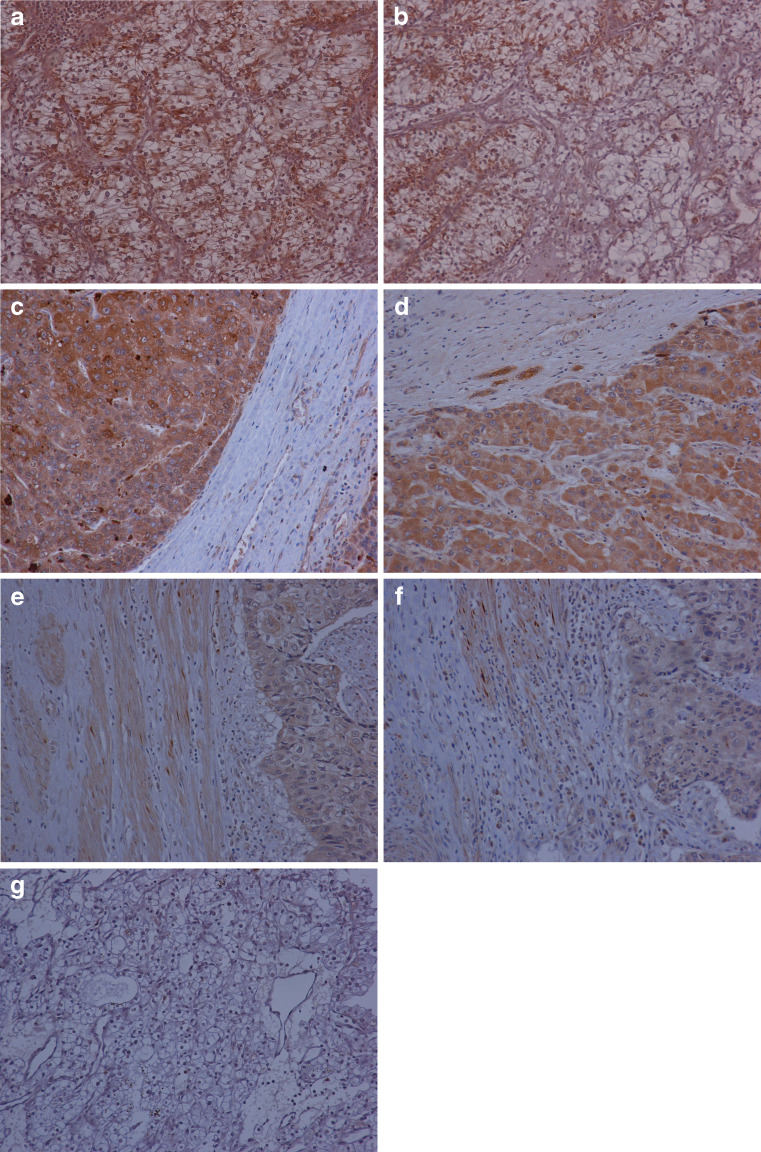
Adiponectin receptor expression is higher in obesity-related cancers than in obesity-nonrelated cancers. Immunohistochemical staining for AdipoR1 (a) and AdipoR2 (b) in renal cell carcinoma, AdipoR1 (c) and AdipoR2 (d) in hepatocellular carcinoma, and AdipoR1 (e) and AdipoR2 (f) in cervical squamous cell carcinoma. Immunohistochemical staining of negative control with no primary antibody (g). All figures are at ×250 magnification.
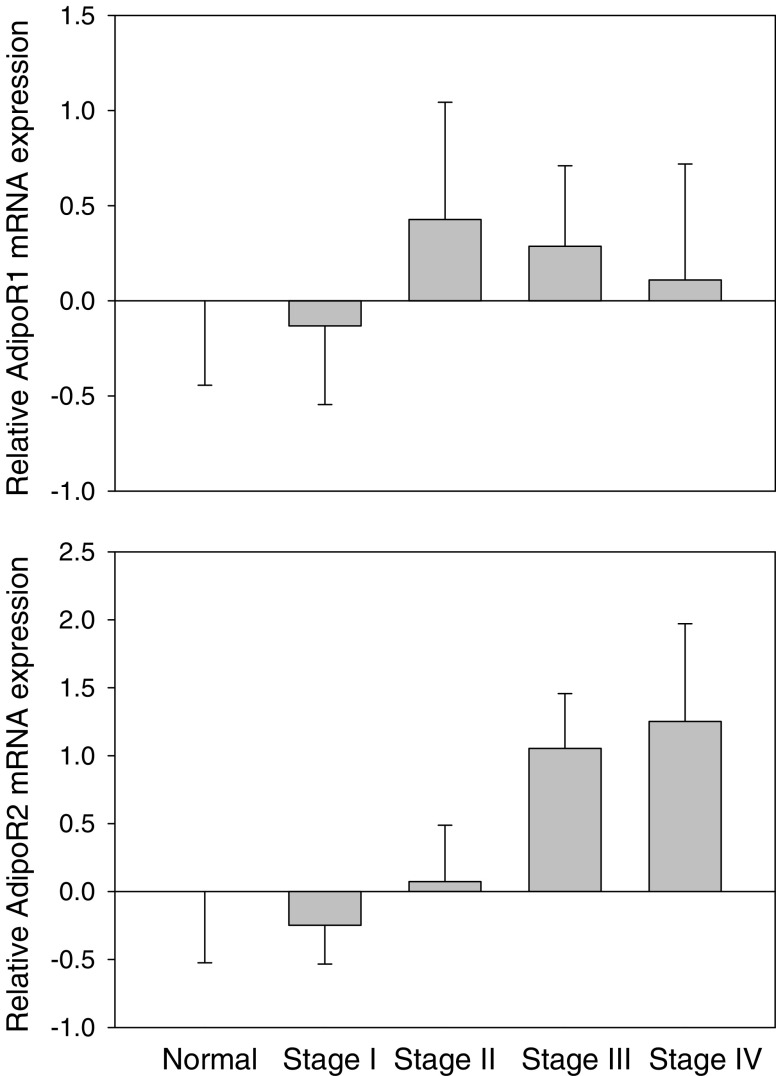
By RT-qPCR, we also analyzed the extent of mRNA expression and its relation with stage in an independent study of 45 specimens of renal cell carcinoma. The mean age was 59 with a standard deviation of 10 years, and 29 were men. Of these specimens, five were of surrounding normal tissue, 20 were of stage I, six were of stage II, eight were of stage III, and six were of stage IV. No information on other tumor characteristics was available. We found no difference in the levels of mRNA of the adiponectin receptors between benign and malignant specimens, adjusted for age and gender (p = 0.76 for AdipoR1, p = 0.32 for AdipoR2). We also found no significant correlation between mRNA expression and stage of cancer for AdipoR1 (p = 0.98; Fig. 2). We did find a nonsignificant positive trend towards increased AdipoR2 mRNA expression with more advanced stage (p = 0.21; Fig. 2).
Fig. 2.
mRNA expression (means ± standard error) of AdipoR1 and AdipoR2 in relation to stage of renal cell cancer.
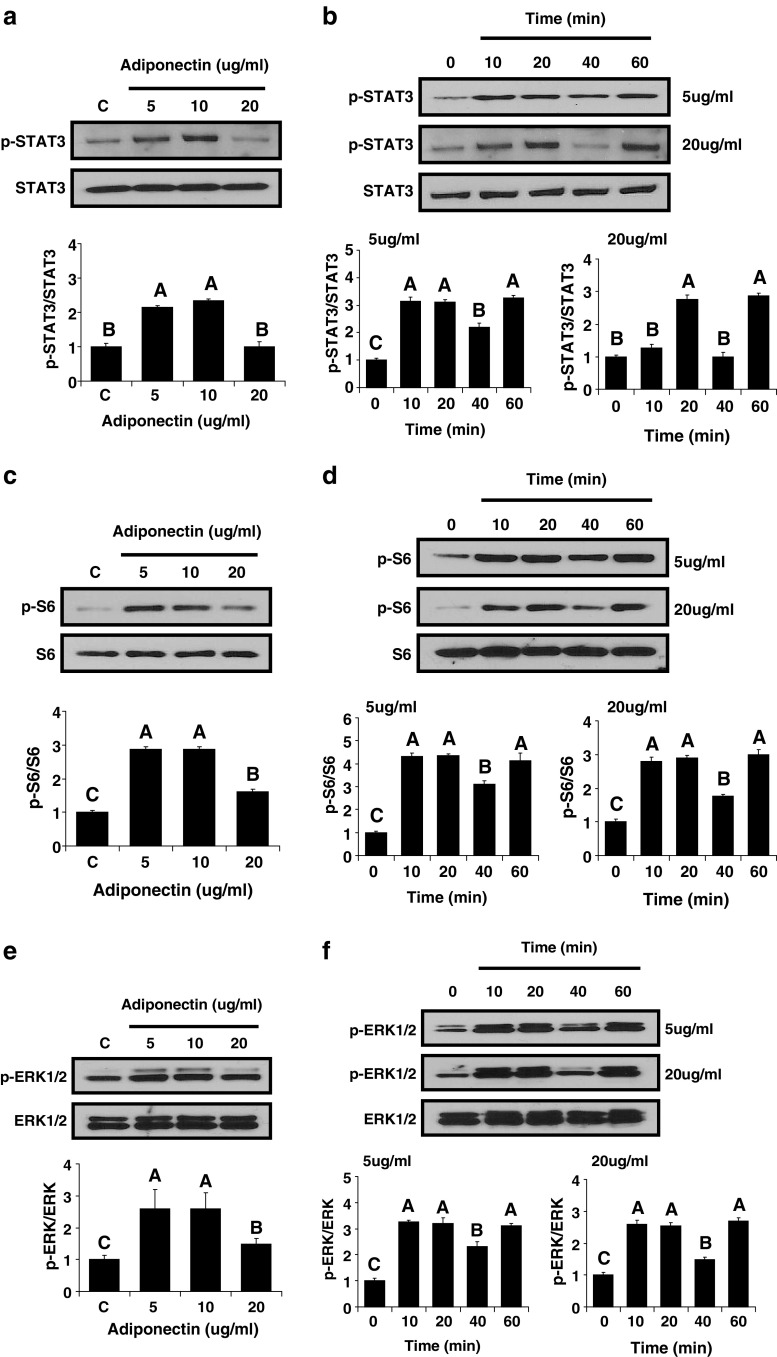
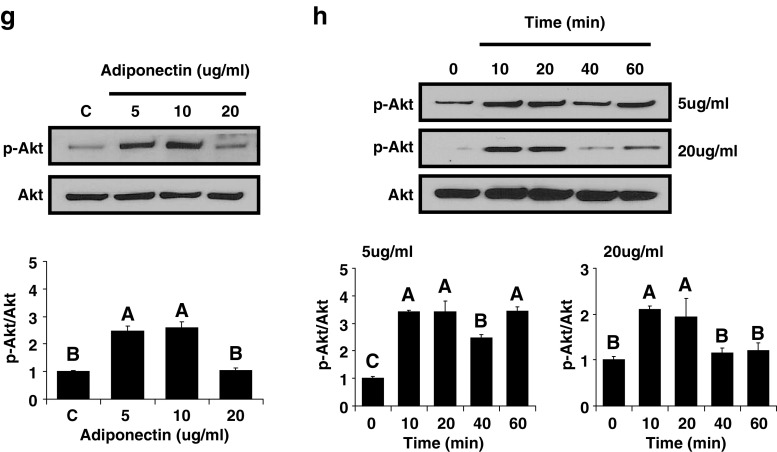
Finally, we used Western blotting to assess activation of several signaling pathways by adiponectin in the renal cancer cell line 786-O. Adiponectin phosphorylated STAT3, S6, ERK1/2, and Akt in a dose-dependent manner with the greatest activation at doses of 5 and 10 µg/ml and little or no activation at a dose of 20 µg/ml (Fig. 3). Adiponectin also activated STAT3, S6, ERK1/2, and Akt in a time-dependent manner, and the response was similar with 5 and 20 µg/ml of adiponectin (Fig. 3).
Fig. 3.
Cell signaling molecules activated by adiponectin stimulation in the human renal cancer cell line 786-O. In vitro adiponectin administration to human renal cancer cells was performed. The cells were treated with adiponectin at the indicated concentrations for 40 min (a, c, e, and g). The cells were treated with 5 and 20 µg/ml of adiponectin for the indicated time periods (b, d, f, and h). All immunoblots shown are representative of an experiment performed three times. All density values for each protein band of interest are expressed as a fold increase. All data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means ± standard deviation. Means with different letters are significantly different, p < 0.05.
Discussion
We report for the first time the presence of adiponectin receptors in hepatocellular carcinoma, melanoma, cholangiocarcinoma, transitional cell carcinoma of the bladder, ovarian epithelial carcinoma, cervical squamous cell carcinoma, and adrenocortical carcinoma. We found significantly increased expression of AdipoR1 in cancers strongly associated with obesity compared to cancers not associated with obesity; this finding was a priori expected given that circulating adiponectin levels are in general lower in obesity-associated malignancies and that lower levels of a ligand are usually associated with overexpression of the relevant receptor. For example, renal cell carcinoma, which is strongly associated with obesity [1, 2, 18] and low levels of serum adiponectin [9, 10], had significantly greater expression of AdipoR1 compared to cancers not associated with obesity. We also compared expression of AdipoR1 and AdipoR2 in malignant and adjacent healthy kidney tissues and found no difference with respect to extent of expression by immunohistochemistry and RT-qPCR; however, the sample sizes of normal specimens were small in each study.
Beyond renal cancer, other cancers studied herein have also been associated with obesity though not consistently; these cancers include hepatocellular carcinoma, melanoma, cholangiocarcinoma, and transitional cell carcinoma of the bladder [1, 2, 18, 26, 27]. Melanoma commonly expressed AdipoR1 in our specimens. However, it has been previously suggested that the increased risk for melanoma in the obese may be due to increased body surface area [28], and a case–control study did not show an association between melanoma and circulating levels of adiponectin [29].
We found that cancers that have not been shown to be related to obesity, such as ovarian epithelial carcinoma and cervical squamous cell carcinoma [19, 21, 22], had less frequent expression of AdipoR1. Adrenocortical carcinoma is a very rare cancer, resulting in few epidemiological studies; in one case–control study of 176 patients who died of adrenal cancer, there was no association with weight [20]. In our case series, adrenocortical carcinomas had lower frequency of AdipoR1 expression.
Our findings are consistent with our previous studies demonstrating adiponectin receptor expression in colorectal [11], prostate [12], and lung [13] cancers. In colorectal cancer, which is closely associated with obesity [1] and low adiponectin levels [6], we found that 95% of the specimens expressed AdipoR1 compared to 47% expression in gastrointestinal stromal tumors, which have not been previously associated with obesity [11]. On the other end of the spectrum, we have previously reported that AdipoR1 expression was found in only 64.2% of the specimens with lung cancer [13], which is not associated with body mass index except in advanced stages [1] and which has not been found to be associated with low adiponectin levels [30].
In the setting of low adiponectin levels, expression of AdipoR1 may be upregulated in a compensatory manner frequently seen in many hormonal axes. Adiponectin has been shown to downregulate AdipoR mRNA expression in breast epithelial cancer [31] and Barrett's adenocarcinoma cell lines [32]. To our knowledge, there are no studies systematically comparing serum adiponectin levels and extent of adiponectin receptor expression in malignant tissues.
We then proceeded to study whether presence or extent of AdipoR1 and AdipoR2 expression is associated with tumor prognostic factors and found no association. The only exception was less AdipoR1 expression in specimens of metastatic compared to nonmetastatic renal cell carcinoma; however, this result should be interpreted with caution as we studied multiple measures using a significant p value of <0.05. Interestingly, our RT-qPCR data, which are highly more quantitative than our immunohistochemistry data, found a nonsignificant trend towards an inverted U-shaped association between AdipoR1 mRNA expression and stage of renal cell cancer and a nonsignificant trend towards a positive correlation with AdipoR2 mRNA expression and stage. Other studies show contrasting results on the association between adiponectin receptor expression and cancer prognosis. In breast cancer, greater expression of AdipoR2 is associated with vascular invasion [33], but in gastric carcinomas, greater expression of AdipoR1 and/or AdipoR2 is positively associated with longer overall survival [16]. This is an area of active investigation; results from large-cohort studies are needed to fully elucidate a potential predictive role for either AdipoR1 or AdipoR2.
There are several proposed mechanisms for the antineoplastic properties of adiponectin. Higher levels of adiponectin have been shown to improve insulin resistance in obesity [34], thereby decreasing circulating levels of insulin and insulin-like growth factors which promote cellular proliferation [2]. In addition, adiponectin is a direct inhibitor of angiogenesis of endothelial cells [35]. Adiponectin also selectively binds to and sequesters several mitogenic growth factors [36]. Finally, adiponectin may act on tumor cells directly to regulate cell proliferation, differentiation, and apoptosis via 5′-AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) [37, 38] and c-Jun NH2-terminal kinase and STAT3 (JNK-STAT3) [35, 39].
We found that adiponectin at low concentrations activates STAT3, S6, ERK1/2, and Akt, all of which promote cancer [40–43], in the renal cancer cell line 786-O. In contrast, relatively decreased activation of these cell signaling molecules was observed with treatment of a higher concentration of adiponectin (20 µg/ml). This is consistent with clinical observations. Higher levels of adiponectin are protective of cancer [4–10]. Obese humans have been previously found to have a mean plasma adiponectin level of 3.7 ± 3.2 µg/ml, compared to nonobese humans (8.9 ± 5.4 µg/ml) [44]. In another study, the 25th, 50th, and 75th percentile values for serum adiponectin levels among healthy women were 9.97, 13.21, and 17.68 µg/ml, respectively [45]. There might also be additional signaling pathways activated by adiponectin, either known, such as the protective AMPK pathway, or yet to be identified signaling pathways, which could potentially mediate adiponectin's beneficial effects. The role that each one and/or the balance of these signaling pathways plays in the antineoplastic effects of adiponectin is an active area of investigation.
The antineoplastic effects of adiponectin on tumor promotion as well as progression have been confirmed in in vivo studies with mice models of colonic tumors [46] and gastric cancers [47], respectively.
Recent studies are also beginning to elucidate the roles of AdipoR1 and AdipoR2 in cancer. In ex vivo studies, adiponectin binds AdipoR1 to decrease esophageal adenocarcinoma cell proliferation by attenuating leptin's downstream effects [48] and to decrease breast cancer cell proliferation by inhibiting entry into S phase [49]. In a recent in vivo study, increased colonic epithelial cell proliferation and more aberrant crypt foci were observed in AdipoR1-deficient mice but not in AdipoR2-deficient mice [50]. There have not been many studies elucidating the role of AdipoR2 in cancer. In view of the above, our findings imply that treatment with either adiponectin and/or adiponectin receptor analogs may have direct effects in many cancers, especially of the bladder, kidney, and skin.
A limitation of our study is our relatively small sample sizes for most cancers except for hepatocellular and renal cell carcinomas, which are the cancers most closely associated with obesity and the metabolic syndrome. However, we were able to include a wide variety of cancers which have not been studied to date. Another limitation is the lack of follow-up data on survival; we only had prognostic markers available for analysis. Finally, studies linking obesity and cancer are largely observational, and there are less data on rarer cancers, including adrenocortical carcinoma. However, we based our results on a large, recent meta-analysis [1] and performed an expanded literature search on the cancers not included. The strengths of our study include the wide range of cancers assessed, the blinded assessment of tissue specimens by two expert pathologists, and the use of state-of-the-art techniques which have been previously published [7, 11, 12]. We also confirmed the presence of AdipoR1 in renal cancer cell line by Western blotting and mRNA of both AdipoR1 and AdipoR2 by RT-qPCR. Furthermore, we also performed an independent study to elucidate any correlation between mRNA expression and stage of renal cell carcinoma with RT-qPCR.
In summary, we report for the first time greater expression of AdipoR1 in cancers associated with obesity compared to cancers not associated with obesity, which also express adiponectin receptors but at a lower rate and intensity. The increased expression of AdipoR1 in obesity-related cancers is consistent with lower levels of the ligand adiponectin in these cancers. The pathophysiological significance of adiponectin and/or adiponectin receptors and their potential predictive value in assessment of malignancy risk, relapse, and treatment outcomes in obesity-related cancers warrant further research. Finally, further studies are necessary to elucidate the direct effects of adiponectin to prevent carcinogenesis and/or tumor progression in humans as well as the potential clinical use of adiponectin in these malignancies.
References
- 1.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 3.Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 4.Guadagni F, Roselli M, Martini F, et al. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009;29:3321–3327. [PubMed] [Google Scholar]
- 5.Kumor A, Daniel P, Pietruczuk M, et al. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis. 2009;24:275–281. doi: 10.1007/s00384-008-0605-y. [DOI] [PubMed] [Google Scholar]
- 6.Wei EK, Giovannucci E, Fuchs CS, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 7.Korner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 8.Dal Maso L, Augustin LS, Karalis A, et al. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 9.Horiguchi A, Ito K, Sumitomo M, et al. Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2008;38:106–111. doi: 10.1093/jjco/hym158. [DOI] [PubMed] [Google Scholar]
- 10.Spyridopoulos TN, Petridou ET, Skalkidou A, et al. Low adiponectin levels are associated with renal cell carcinoma: a case–control study. Int J Cancer. 2007;120:1573–1578. doi: 10.1002/ijc.22526. [DOI] [PubMed] [Google Scholar]
- 11.Williams CJ, Mitsiades N, Sozopoulos E, et al. Adiponectin receptor expression is elevated in colorectal carcinomas but not in gastrointestinal stromal tumors. Endocr Relat Cancer. 2008;15:289–299. doi: 10.1677/ERC-07-0197. [DOI] [PubMed] [Google Scholar]
- 12.Michalakis K, Williams CJ, Mitsiades N, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomark Prev. 2007;16:308–313. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 13.Petridou ET, Mitsiades N, Gialamas S, et al. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case–control studies. Oncology. 2007;73:261–269. doi: 10.1159/000127424. [DOI] [PubMed] [Google Scholar]
- 14.Dalamaga M, Migdalis I, Fargnoli JL, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case–control study. Cancer Causes Control. 2009;20:625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinthus JH, Kleinmann N, Tisdale B, et al. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol. 2008;54:866–873. doi: 10.1016/j.eururo.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Barresi V, Grosso M, Giuffre G, et al. The expression of adiponectin receptors Adipo-R1 and Adipo-R2 is associated with an intestinal histotype and longer survival in gastric carcinoma. J Clin Pathol. 2009;62:705–709. doi: 10.1136/jcp.2009.066175. [DOI] [PubMed] [Google Scholar]
- 17.Molica S, Vitelli G, Cutrona G, et al. Prognostic relevance of serum levels and cellular expression of adiponectin in B-cell chronic lymphocytic leukemia. Int J Hematol. 2008;88:374–380. doi: 10.1007/s12185-008-0165-5. [DOI] [PubMed] [Google Scholar]
- 18.Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362, 552 Swedish men. Cancer Causes Control. 2006;17:901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 19.Modesitt SC, Van Nagell JR., Jr The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60:683–692. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 20.Hsing AW, Nam JM, Co Chien HT, et al. Risk factors for adrenal cancer: an exploratory study. Int J Cancer. 1996;65:432–436. doi: 10.1002/(SICI)1097-0215(19960208)65:4<432::AID-IJC6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Lacey JV, Jr, Swanson CA, Brinton LA, et al. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer. 2003;98:814–821. doi: 10.1002/cncr.11567. [DOI] [PubMed] [Google Scholar]
- 22.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Abellan P, Gomez-Santos C, Madrid JA, et al. Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology. 2010;151:115–122. doi: 10.1210/en.2009-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nannipieri M, Bonotti A, Anselmino M, et al. Pattern of expression of adiponectin receptors in human adipose tissue depots and its relation to the metabolic state. Int J Obes (Lond) 2007;31:1843–1848. doi: 10.1038/sj.ijo.0803676. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen MS, Lihn AS, Pedersen SB, et al. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 2006;14:28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- 26.Holick CN, Giovannucci EL, Stampfer MJ, et al. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120:140–146. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]
- 27.Koebnick C, Michaud D, Moore SC, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomark Prev. 2008;17:1214–1221. doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 28.Thune I, Olsen A, Albrektsen G, et al. Cutaneous malignant melanoma: association with height, weight and body-surface area. A prospective study in Norway. Int J Cancer. 1993;55:555–561. doi: 10.1002/ijc.2910550406. [DOI] [PubMed] [Google Scholar]
- 29.Mantzoros CS, Trakatelli M, Gogas H, et al. Circulating adiponectin levels in relation to melanoma: a case–control study. Eur J Cancer. 2007;43:1430–1436. doi: 10.1016/j.ejca.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Karapanagiotou EM, Tsochatzis EA, Dilana KD, et al. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC) Lung Cancer. 2008;61:391–397. doi: 10.1016/j.lungcan.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Dos Santos E, Benaitreau D, Dieudonne MN, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- 32.Konturek PC, Burnat G, Rau T, et al. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig Dis Sci. 2008;53:597–605. doi: 10.1007/s10620-007-9922-1. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiler G, Treeck O, Wenzel G, et al. Influence of insulin resistance on adiponectin receptor expression in breast cancer. Maturitas. 2009;63:253–256. doi: 10.1016/j.maturitas.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond) 2002;103:137–142. doi: 10.1042/CS20010336. [DOI] [PubMed] [Google Scholar]
- 35.Brakenhielm E, Veitonmaki N, Cao R, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Lam KS, Xu JY, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 37.Luo Z, Saha AK, Xiang X, et al. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama M, Takahashi H, Hosono K, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol. 2009;34:339–344. [PubMed] [Google Scholar]
- 39.Miyazaki T, Bub JD, Uzuki M, et al. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 40.Bowman T, Garcia R, Turkson J, et al. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 41.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/A:1023781114568. [DOI] [PubMed] [Google Scholar]
- 43.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 44.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 45.Petridou E, Mantzoros C, Dessypris N, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case–control study in Greece. J Clin Endocrinol Metab. 2003;88:993–997. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 46.Otani K, Kitayama J, Yasuda K, et al. Adiponectin suppresses tumorigenesis in Apc(Min)(/+) mice. Cancer Lett. 2009;288:177–182. doi: 10.1016/j.canlet.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa M, Kitayama J, Yamauchi T, et al. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci. 2007;98:1120–1127. doi: 10.1111/j.1349-7006.2007.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogunwobi OO, Beales IL. Globular adiponectin, acting via adiponectin receptor-1, inhibits leptin-stimulated oesophageal adenocarcinoma cell proliferation. Mol Cell Endocrinol. 2008;285:43–50. doi: 10.1016/j.mce.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama S, Miyoshi Y, Ishihara H, et al. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res Treat. 2008;112:405–410. doi: 10.1007/s10549-007-9874-3. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa T, Endo H, Tomimoto A, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]