Abstract
RET sequencing has become an important tool in medullary thyroid cancer (MTC) evaluation and should be performed even in the absence of family history of MTC. The most commonly studied exons in index cases are 8, 10, 11, and 13–16. To address the ATA guidelines regarding the sequencing of the entire coding region of RET, we selected 50 patients with sporadic MTC (sMTC) without mutations in the hot spot regions of RET for extended investigation of exons 1–7, 9, 12, 17, 18, and 19. Twenty-seven of 50 patients presented with one or more features suggesting familial disease. We found only a new RET variant (p.Gly550Glu) in one patient with MTC. Several polymorphisms were observed, and their frequency was histogram scaled by exons and introns. Eight patients were also included for somatic mutation search. We estimated the sequencing cost by stratifying into four investigation approaches: (1) hot spot exons in a new patient, (2) the remaining exons if the hot spots are negative in a patient with suspected familial disease, (3) a relative of a carrier for a known RET mutation, and (4) tumor sequencing. In spite of the increasing number of variants being described in MTC, it appears that there is no direct clinical benefit in extending RET germ line analysis beyond the hot spot regions in sMTC. The cost evaluation in apparent sMTC using a tiered approach may help clinicians make more suitable decisions regarding the benefits of investigating only the hot spots against the entire coding region of RET.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-012-0109-7) contains supplementary material, which is available to authorized users.
Keywords: Multiple Endocrine Neoplasia, Medullary Thyroid Cancer, Entire Code Region, Familial Disease, American Thyroid Association Guideline
Introduction
Medullary thyroid cancer (MTC) originates from parafollicular calcitonin-producing cells (C cells) and accounts for 3–7 % of all thyroid cancers. It manifests as a sporadic tumor in 75–80 % of patients and in the remainder as an autosomal dominant inherited disease [1]. The hereditary forms of the disease are caused by germ line mutations in the RET oncogene (REarranged during Transfection), resulting in familial MTC (FMTC), multiple endocrine neoplasia (MEN) 2A, and MEN 2B. In addition to a family history of MTC or MEN 2, other features suggesting familial disease include multifocal medullary tumors, C cell hyperplasia (CCH), and young age at diagnosis [2]. As for sporadic tumors, it is known that somatic RET mutations are present in as many as 64 % of cases [4, 9, 10].
Germ line RET testing should be offered to all patients presenting with MTC, primary CCH, and MEN 2 and to people with a family history of FMTC or MEN 2. Other situations that should prompt RET testing are the presence of cutaneous lichen amyloidosis in the central upper back and individuals with a diagnosis of Hirschsprung’s disease [8]. RET analysis allows the early detection of asymptomatic carriers and has become very useful for screening at risk relatives of affected patients [13], as mutation carriers have a high risk of developing MTC and, thus, can benefit from prophylactic thyroidectomy.
When investigating index cases, some centers initially test only exons 10 and 11; others additionally sequence exons 13, 14, 15, and 16, and a few also investigate exons 5 and 8 for mutations in the RET gene [8, 12]. In our laboratory, we routinely sequence exons 8, 10, 11, and 13–16. Nevertheless, when the initial genetic analysis is negative in the presence of MEN 2 or when there is a discrepancy between the genotype and the phenotype, the American Thyroid Association guidelines recommend sequencing the entire coding region of RET [8]. Given this recommendation and the clinical relevance of this issue, in this study, we aim to evaluate the benefits of sequencing the entire coding region of RET in patients with apparently sporadic MTC. In addition, we address the sequencing of selected exons using DNA from tumor tissue from sporadic MTC patients.
Patients and Methods
Patients
From our cohort followed at the Thyroid Clinic, Hospital São Paulo, Escola Paulista de Medicina, Federal University of São Paulo, we selected 50 patients with MTC and no mutations in exons 8, 10, 11, 13, 14, 15, or 16 to undergo further investigation for other mutations in RET. Of the 50 patients, 27 also presented with one or more features suggesting familial disease, such as a young age of onset of MTC (16 patients), tumor multifocality (13 patients), or the presence of CCH (10 patients). We defined young age of onset based on a cutoff that was established by a statistical analysis using an ROC curve performed by our group, which indicated that familial disease is more likely to be present when the tumor is diagnosed at the age of 34 years or under (Supplementary Fig. A). Based on this finding and including a safety margin, we considered individuals 35 years old or younger as suspected of having familial disease and selected them to undergo comprehensive RET molecular evaluation. The other 23 individuals were consecutive patients seen for sporadic MTC. All subjects signed a consent form in accordance to the policies of the university’s ethical committee.
Molecular Analysis
Genomic DNA was extracted from peripheral blood using an in-house method. Tumor DNA from eight patients who underwent thyroidectomy was extracted from paraffin-embedded tumor tissues through deparaffinization with xylol or n-octane heated baths followed by standard protocols. DNA was amplified through polymerase chain reaction with Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA, USA), and specific primers for the entire coding region of RET and for tumor hot spots were designed using the public primer design method available at http://ihg.gsf.de/ihg/ExonPrimer.html in a way to avoid SNP regions in accordance to the reference sequences from the University of California Santa Cruz database (Supplementary Table A).
PCR products were purified using Illustra GFX PCR DNA and Gel Purification Kit (GE Healthcare, Buckinghamshire, UK) and submitted to direct sequencing by the Sanger method, using Big Dye™ Terminator Cycle Sequencing Ready Reaction Kit, ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City CA, USA). Each exon was sequenced at least twice and in both directions.
The sequences were analyzed by two separate evaluators using BioEdit Sequence Alignment Editor and CLC Main Workbench 6 (http://www.clcbio.com) and compared to reference data available at the NCBI GenBank (RefSeq NG_007489) and the Ensembl Genome Browser. Somatic mutations were referred using the Catalog of Somatic Mutations in Cancer database (http://www.sanger.ac.uk/genetics/CGP/cosmic/).
Results
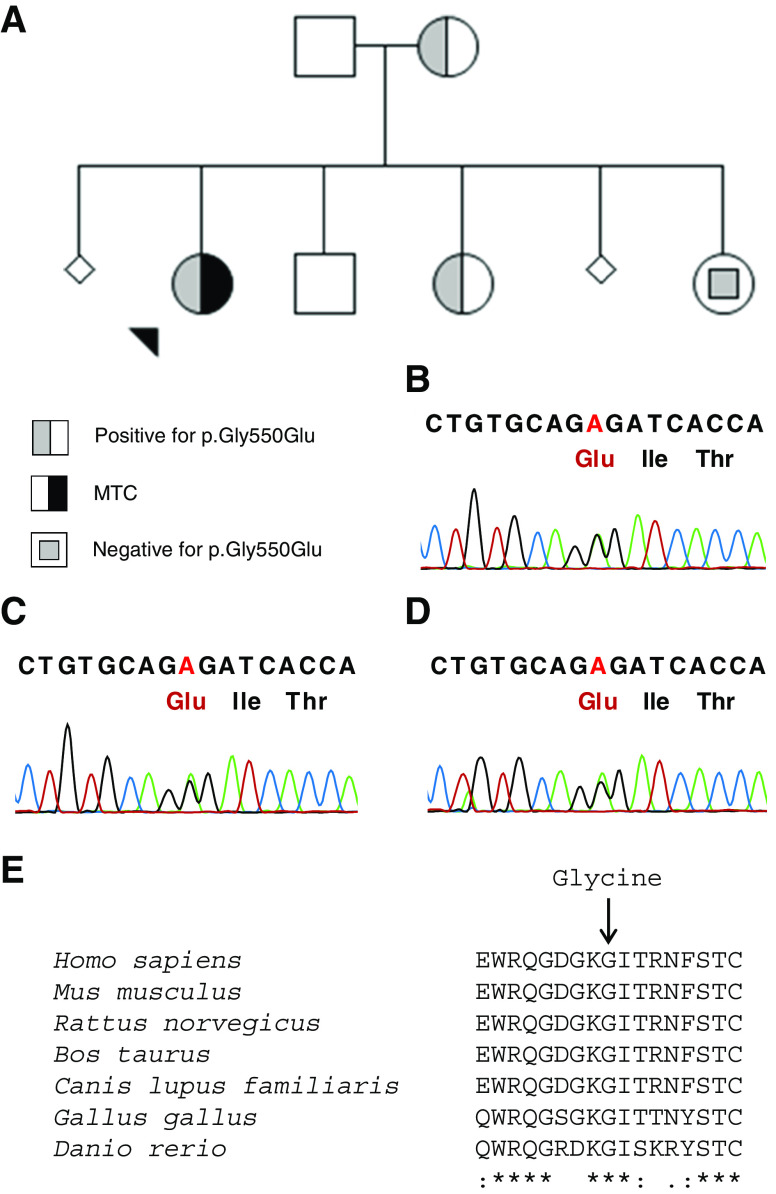
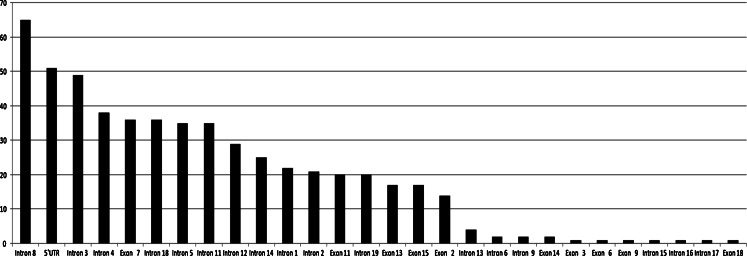
Fifty patients with MTC negative for mutations in the hot spot exons of RET were analyzed for mutations in exons 1, 2, 3, 4, 5, 6, 7, 9, 12, 17, 18, and 19. In one of these cases, a patient who had been diagnosed with a 1.3-cm MTC staged T1bN0M0 at the age of 32, a p.Gly550Glu variant was identified in the acceptor splicing region of exon 9 (Fig. 1). The patient’s disease is now in clinical and biochemical remission. Her mother and one of her sisters tested positive for this variant (Fig. 1), but their serum calcitonin was undetectable as well as the ultrasound examination revealed no thyroid nodules. All the other patients investigated were negative for RET mutations in all exons. Several polymorphisms were also found, both intronic and exonic, as depicted in Fig. 2 (details in Supplementary Table B).
Fig. 1.
Evaluation of the family presenting the p.Gly550Glu variant. a Pedigree chart; b electropherogram of germ line sequencing of the index case; c, d electropherogram of germ line sequencing of her mother and sister; e sequence alignment of human RET protein residues in which the position of the conserved glycine at residue 550 is indicated (arrow). Multiple sequence alignment was generated with Clustal Omega software available at the European Bioinformatics Institute website (http://www.ebi.ac.uk/Tools/msa/clustalo/). Asterisk indicate residues in the column are identical in all sequences in the alignment, colon indicates conserved substitutions, and period indicates semiconserved substitutions observed in the alignment
Fig. 2.
Histogram of the frequency of polymorphisms. y-axis: total number of times the polymorphisms were detected in each exon and intron, which are represented in the x-axis. Exon 1—rs10900296, rs10900297; intron 1—rs12267460; exon 2—rs1800858; intron 2—rs2435351; exon 3—rs1800859; intron 3—rs35906041, rs2472739, rs115766280; intron 4—rs2435352, rs2742243; intron 5—rs1864404, rs3026742; exon 6—new; intron 6—rs9282835; exon 7—rs1800860; intron 8—rs3026750, rs111463326; intron 9—new; exon 11—rs1799939; intron 11—rs2256550; intron 12—rs760466; exon 13—rs1800861; intron 13—rs112200125, rs111264957, rs3026767; exon 14—rs1800862; intron 14—rs111306965, rs11238441; exon 15—rs1800863; intron 15—rs3026771; intron 16—rs3026772; intron 17—new; exon 18—rs17158558; intron 18—rs2742236; intron 19—rs2075912
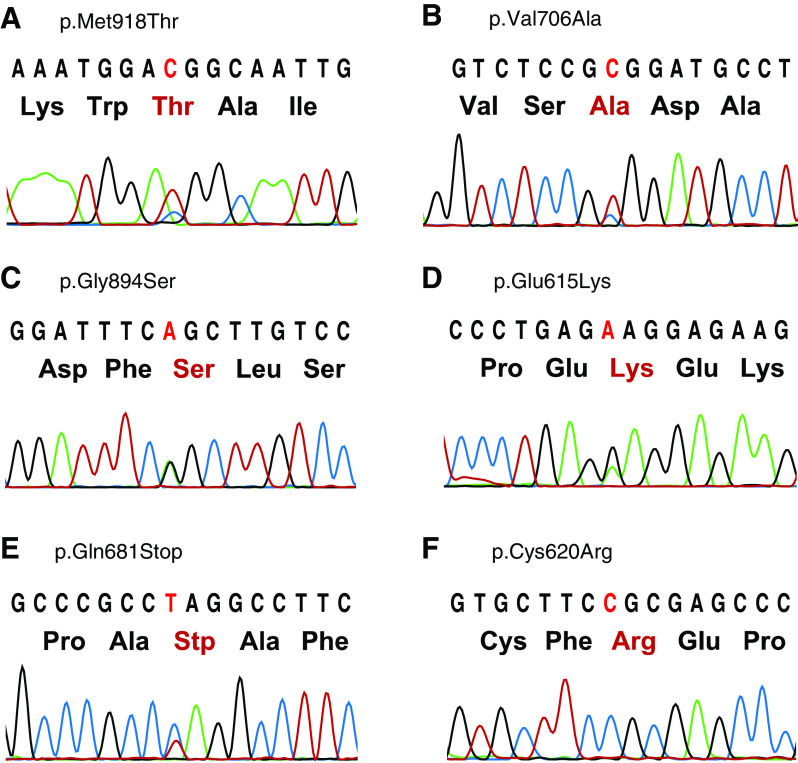
We were able to investigate tumor tissue from eight of the patients who underwent total thyroidectomy. Sequencing revealed mutations in five of these samples (Fig. 3); one of the identified mutations is the most frequent one, p.Met918Thr, whereas the other mutations have not been described previously. The presence of these mutations was confirmed by cloning the PCR products into pCR4 vector (Invitrogen) followed by sequencing. A p.Cys620Arg mutation was detected in one of the clones from the tumor sample from one of the patients (Fig. 3). The clinical characteristics of these patients are outlined in Table 1.
Fig. 3.
RET somatic sequencing analysis. a–f Electropherograms of MTC tissue sequencing of five patients
Table 1.
Clinical features of the patients who underwent tumor sequencing and the detected mutations
| Patient’s clinical features | Exon | Mutation |
|---|---|---|
| 25 years old; pT2N1bMx | 16 | p.Met918Thr |
| 27 years old; pT1N1aMx | – | – |
| 56 years old; pT4N1bMx | – | – |
| 58 years old; pT3N0Mx; 7-cm tumor | 11 | p.Val706Ala |
| 32 years old; pT2N0Mx; CCH | 15 | p.Gly894Ser |
| 59 years old; pT1mN1Mx | 10 | p.Glu615Lys, p.Cys620Arg |
| 48 years old; pT2mN1aMx | 11 | p.Gln681Stop |
| 69 years old; pT3N0Mx | – | – |
Age at diagnosis, pathological TNM staging—AJCC 2010
CCH C cell hyperplasia
Discussion
In this study, 1 out of 50 patients with sporadic MTC presented with a variant in RET that has not been described before. It is not clear whether this newly identified variant has a pathogenic role. The nucleotide change occurs in a position that has the potential to alter RNA splicing, and its analysis through the Variant Effect Predictor (www.ensembl.org) suggests that it is probably damaging. In addition, this sequence is highly conserved in several species, at both the nucleotide and amino acid levels (Fig. 1). Despite these assumptions, the clinical and biochemical investigation of the family so far suggests this is a variant unlikely to be related to MTC. This led us to presume that sequencing the entire coding region of RET in this study did not bring immediate clinical benefits, at the cost of greatly increasing the price of testing. Nevertheless, it is very important that the patient carrying the p.Gly550Glu variant and her relatives be carefully monitored for signs and symptoms of MTC or MEN.
In addition, this study demonstrated the presence of a large number of polymorphisms throughout the RET gene; to our knowledge, such a broad investigation has never been published. Whether there is a causative association between these polymorphic RET nucleotides and disease remains to be clarified [3, 5, 6, 14]. Interestingly, one of these polymorphic regions has been identified as a fragile site concerning DNA breaks [7].
Regarding the novel somatic mutations detected in our study, the patients bearing them will be closely observed during clinical follow-up and the mutations will be subject of further molecular and functional investigations. It is known that somatic RET mutations in MTC are present in as many as 64 % of cases and occur mainly in exons 16 (Met918Thr in up to 79 % of the cases) and 11 (up to 21.2 %), followed by exons 10 and 15 in similar proportions [4, 9, 10]. Based on these frequencies, tissue analysis can be performed through a tiered approach—first sequence exon 16; only if negative, proceed to exon 11; if no mutations are found, then analyze exons 10 and 15. This tiered approach would add less cost to the investigation than extended germ line sequencing would. Along with the fact that somatic mutations have been related to disease prognosis in some studies [4, 10], this information is currently used in clinical trials to evaluate any possible influence on the patients’ response to new target drug therapies, and somatic mutation analysis may be useful in the near future. Thus, although currently somatic mutation analysis has little clinical benefit to the MTC patients and their families, it offers a more complete molecular diagnosis that can be used in clinical decision making.
Considering the importance of the genetic screening for RET mutations and, on the other hand, the scarcity of trained labs in many areas and the cost for an accurate molecular diagnosis, we estimated the cost of this type of screening in an academic institution, taking into account our experience over the past 10 years in testing RET in more than 1,000 individuals. We stratified this analysis into four situations: sequencing select exons in a new patient, sequencing the remaining exons if the hot spots are negative in a patient with suspected familial disease, sequencing a relative of a carrier for a known RET mutation, and tumor sequencing (Table 2). The cost analysis for such cases may help clinicians make more suitable decisions regarding the benefits of investigating only one specific RET mutation, investigating hot spot exons, examining the entire coding region or performing tissue analysis for prognosis assessment, and predicting drug response. It is important to note that, especially in diseases caused by multiple genes or by genes with a longer coding sequence, whole exome sequencing might become a useful tool and, as its application increases, its cost will likely reduce in the forthcoming years [11].
Table 2.
Estimated costs for a stratified RET mutational analysis
| RET sequencing coverage | Technical–operational cost |
|---|---|
| Hot spots in a new patient | USD 720.00 |
| Additional exons if selected exons negative | USD 831.00 |
| Testing of a relative for a specific known mutation | USD 305.00 |
| Tumor testing (from 1 to 4 exons) | USD 305.00–513.00 |
In spite of the increasing number of variants being described in MTC and MTC tumor tissue, it appears there is no direct clinical benefit in extending RET analysis beyond the hot spot regions, reinforcing the recommendation of the American Thyroid Association guidelines. However, when assisting a young adult whose tumor histopathology reveals multifocal disease or CCH, which are more related to inheritance, complete RET testing might be able to unmask a hidden familial MTC. Therefore, the challenge for endocrinologists and biochemical and molecular diagnostic laboratories will be to determine which test(s) to offer and how to best test for these mutations in a way that is relevant to patient care and has a reasonable cost–benefit trade off.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(DOCX 185 kb)
Acknowledgments
The authors thank the team of the Laboratory of Molecular and Translational Endocrinology, especially Teresa Kasamatsu, José Gilberto Vieira, João Roberto Martins, Maria Izabel Chiamolera, and Maria Sharmila de Sousa, and the team of the Thyroid Clinic, particularly Maria da Conceição Mamone, Alberto Machado, Reinaldo Furlanetto, Luiza Matsumura, Jairo Hidal, Rosa Paula Biscolla, Maria Cecília Costa and Danielle Andreoni, as well as the Head and Neck Surgery group. We also thank Fernando Soares and Gilberto Furuzawa for the daily technical assistance. The authors’ research is supported by São Paulo State Research Foundation (FAPESP) 2009/50575-4 (to S.C.L.), 2010/08981-2 (to M.S.Y.), 2006/54922-2 (to J.M.C.), 2006/60402-1 (to R.M.B.M. and M.R.D.S.), and Fleury Group (12518). R.M.B.M. and J.M.C. are investigators of the Brazilian Research Council. R.M.B.M. is an investigator of the Fleury Group.
Disclosure
The authors have no conflict of interest to disclose.
Contributor Information
Rui M. B. Maciel, Phone: +55-11-50845231, FAX: +55-11-50845231, Email: rui.maciel@unifesp.br
Magnus R. Dias-da-Silva, Email: diasdasilvamr@gmail.com
References
- 1.Alsanea O, Clark OH. Familial thyroid cancer. Curr Opin Oncol. 2001;13(1):44–51. doi: 10.1097/00001622-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Block MA, Jackson CE, Greenawald KA, Yott JB, Tashjian AH., Jr Clinical characteristics distinguishing hereditary from sporadic medullary thyroid carcinoma. Treatment implications. Arch Surg. 1980;115(2):142–148. doi: 10.1001/archsurg.1980.01380020012004. [DOI] [PubMed] [Google Scholar]
- 3.Cebrian A, Lesueur F, Martin S, Leyland J, Ahmed S, Luccarini C, Smith PL, et al. Polymorphisms in the initiators of RET (rearranged during transfection) signaling pathway and susceptibility to sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90(11):6268–6274. doi: 10.1210/jc.2004-2449. [DOI] [PubMed] [Google Scholar]
- 4.Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93(3):682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez RM, Pecina A, Antinolo G, Navarro E, Borrego S. Analysis of RET polymorphisms and haplotypes in the context of sporadic medullary thyroid carcinoma. Thyroid. 2006;16(4):411–417. doi: 10.1089/thy.2006.16.411. [DOI] [PubMed] [Google Scholar]
- 6.Fugazzola L, Muzza M, Mian C, Cordella D, Barollo S, Alberti L, Cirello V, et al. RET genotypes in sporadic medullary thyroid cancer: studies in a large Italian series. Clin Endocrinol. 2008;69(3):418–425. doi: 10.1111/j.1365-2265.2008.03218.x. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi M, Dillon LW, Pramanik S, Nikiforov YE, Wang YH. DNA breaks at fragile sites generate oncogenic RET/PTC rearrangements in human thyroid cells. Oncogene. 2010;29(15):2272–2280. doi: 10.1038/onc.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 9.Mian C, Pennelli G, Barollo S, Cavedon E, Nacamulli D, Vianello F, Negro I, et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur J Endocrinol. 2011;164(6):971–976. doi: 10.1530/EJE-11-0079. [DOI] [PubMed] [Google Scholar]
- 10.Moura MM, Cavaco BM, Pinto AE, Domingues R, Santos JR, Cid MO, Bugalho MJ, Leite V. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer. 2009;100(11):1777–1783. doi: 10.1038/sj.bjc.6605056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi XP, Ma JM, Du ZF, Ying RB, Fei J, Jin HY, Han JS, et al. RET germline mutations identified by exome sequencing in a Chinese multiple endocrine neoplasia type 2A/familial medullary thyroid carcinoma family. PLoS One. 2011;6(5):e20353. doi: 10.1371/journal.pone.0020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romei C, Cosci B, Renzini G, Bottici V, Molinaro E, Agate L, Passannanti P, et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC) Clin Endocrinol. 2011;74(2):241–247. doi: 10.1111/j.1365-2265.2010.03900.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanso GE, Domene HM, Garcia R, Pusiol E, De M, Roque M, Ring A, et al. Very early detection of RET proto-oncogene mutation is crucial for preventive thyroidectomy in multiple endocrine neoplasia type 2 children: presence of C-cell malignant disease in asymptomatic carriers. Cancer. 2002;94(2):323–330. doi: 10.1002/cncr.10228. [DOI] [PubMed] [Google Scholar]
- 14.Weinhaeusel A, Scheuba C, Lauss M, Kriegner A, Kaserer K, Vierlinger K, Haas OA, Niederle B. The influence of gender, age, and RET polymorphisms on C-cell hyperplasia and medullary thyroid carcinoma. Thyroid. 2008;18(12):1269–1276. doi: 10.1089/thy.2008.0139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 185 kb)