ABSTRACT
Macrolides are a mainstay of therapy for infections due to nontuberculous mycobacteria (NTM). Among rapidly growing mycobacteria (RGM), inducible macrolide resistance is associated with four chromosomal 23S rRNA methylase (erm) genes. Beginning in 2018, we detected high-level inducible clarithromycin resistance (MICs of ≥16μg/mL) in clinical isolates of Mycobacterium chelonae, an RGM species not previously known to contain erm genes. Using whole-genome sequencing, we identified a novel plasmid-mediated erm gene. This gene, designated erm(55)P, exhibits <65% amino acid identity to previously described RGM erm genes. Two additional chromosomal erm(55) alleles, with sequence identities of 81% to 86% to erm(55)P, were also identified and designated erm(55)C and erm(55)T. The erm(55)T is part of a transposon. The erm(55)P allele variant is located on a putative 137-kb conjugative plasmid, pMchErm55. Evaluation of 133 consecutive isolates from 2020 to 2022 revealed 5 (3.8%) with erm(55). The erm(55)P gene was also identified in public data sets of two emerging pathogenic pigmented RGM species: Mycobacterium iranicum and Mycobacterium obuense, dating back to 2008. In both species, the gene appeared to be present on plasmids homologous to pMchErm55. Plasmid-mediated macrolide resistance, not described previously for any NTM species, appears to have spread to multiple RGM species. This has important implications for antimicrobial susceptibility guidelines and treatment of RGM infections. Further spread could present serious consequences for treatment of other macrolide-susceptible RGM. Additional studies are needed to determine the transmissibility of pMchErm55 and the distribution of erm(55) among other RGM species.
KEYWORDS: Mycobacterium chelonae, clarithromycin, erm gene, macrolide resistance, mycobacterial plasmids, nanopore genomic sequencing, rapidly growing mycobacteria, whole-genomic sequencing
INTRODUCTION
Macrolide antibiotics are a cornerstone in the treatment of infections caused by nontuberculous mycobacteria (NTM) (1–6). Point mutations in the 23S rRNA gene can confer constitutive resistance to macrolides, whereas recent studies of rapidly growing mycobacteria (RGM) have identified 23S rRNA methylase (erm) genes as key mediators of inducible macrolide resistance (7–9). The chromosomally located erm(38), erm(39), erm(40), and erm(41) genes are present in Mycobacterium smegmatis, the Mycobacterium fortuitum group, M. mageritense, and M. wolinskyi, and Mycobacterium abscessus, respectively (8, 9). Phenotypic detection of inducible macrolide resistance requires extended antimicrobial susceptibility testing times (up to 14 days of incubation) (10).
The presence of erm genes has unequivocally impacted the utility of the macrolides in the treatment of infections caused by many species of RGM. A pivotal study in Korea showed that, when treated with a macrolide-containing regimen, only 25% of pulmonary patients infected with M. abscessus subsp. abscessus (with a functional erm gene) showed clinical improvement, compared with 75% of patients infected with M. abscessus subsp. massiliense (without a functional erm gene) (11). M. abscessus subsp. abscessus is the most commonly isolated RGM species in the United States, and approximately 80% of clinical isolates contain a functional erm(41) gene (9, 10).
The original studies of the erm(41) gene by Nash et al. found no evidence of inducible macrolide resistance among isolates of M. chelonae after 3 or 14 days of incubation (8, 9). Similarly, a large 2-year study by Hanson and colleagues of 45 isolates of M. chelonae also showed no inducible macrolide resistance after 14 days (12). In 2018, The Clinical and Laboratory Standards Institute (CLSI) recommended that extended incubation for detection of inducible macrolide resistance was no longer necessary for some RGM species, including M. chelonae (13).
However, beginning in 2018, the Mycobacteria/Nocardia Research Laboratory at the University of Texas Health Science Center at Tyler (UTHSCT) detected clinical isolates of M. chelonae that exhibited elevated MICs (1 to ≥16 μg/mL) to clarithromycin after 3 to 4 days of incubation and high-level resistance (≥16 μg/mL) after extended incubation for up to 14 days. Genomic approaches, including both short-read (Illumina) and long-read (Oxford Nanopore) platforms, were used to characterize these macrolide-resistant isolates of M. chelonae. Here, we describe three variants of a novel 23S rRNA methylase gene, erm(55), present both on a conjugative plasmid and on the M. chelonae chromosome.
MATERIALS AND METHODS
Collection, susceptibility testing, and selection of M. chelonae isolates.
M. chelonae reference strain CIP 104535 (also known as ATCC 35752T) was obtained from the Institute Pasteur (Paris, France) and was used as a control for all experiments. Clinical isolates of M. chelonae were obtained from culture collections at the Mycobacteria/Nocardia Research Laboratory at the UTHSCT (Laboratory A) and the Mayo Clinic in Rochester, Minnesota (Laboratory B). The clinical isolates were initially submitted for antimicrobial susceptibility testing. Species-level identification of M. chelonae was determined on all isolates by growth rate, colony morphology, pigmentation, and either targeted sequencing of the rpoB gene as previously described or matrix-assisted laser desorption ionization–time of flight mass spectrometry (14, 15). Susceptibility testing to clarithromycin was performed by broth microdilution with commercially available 96-well plates manufactured by ThermoFisher (Cleveland, OH) according to CLSI guidelines (13). Because extended incubation was not (and still is not) required according to current CLSI guidelines, screening for inducible resistance was only routinely performed in Laboratory A beginning in November 2020, upon recognition of inducible macrolide resistance in some isolates. The isolates from Laboratory B were tested for 14 days in 2021 to screen for possible resistant isolates.
Upon subsequent recognition that isolates with high-level inducible macrolide resistance at 14 days had high 3-day clarithromycin MICs of ≥1 μg/mL, isolates in Laboratory A were screened for possible macrolide-resistant isolates based on high 3-day MICs between 2018 (date of first known resistant isolate) and 2020. These isolates then underwent 14-day incubation.
Screening for the erm(41) gene and 23S rRNA (rrl) mutations.
Isolates with high clarithromycin MICs were screened for the presence of the erm(41) gene and point mutations in the 23S rRNA gene (rrl) as previously described. (7, 9) The wild-type M. chelonae CIP 104535 (ATCC 35752T) reference strain and isolates known to have the erm(41) gene or the 23S rRNA A2058 or 2059 mutations were used as controls.
Whole-genome sequencing and assembly.
M. chelonae genomes were sequenced using a combination of Illumina and Oxford Nanopore platforms. For both, DNA was extracted using the EpiCentre Masterpure Complete DNA and RNA purification kit (Mandel Scientific, ON, Canada). Illumina Nextera XT libraries were sequenced on the Illumina MiSeq (San Diego, CA) instrument using the reagent kit v2 (300 cycles). MiSeq reads were assembled and annotated using the IRIDA pipeline (16).
Nanopore libraries were prepared using the Nanopore ligation sequencing kit with the native barcoding expansion kit and sequenced on a MinION device (Oxford Nanopore, Oxford, United Kingdom). Raw Nanopore reads were base-called and demultiplexed with Guppy (5.0.16) using the superaccuracy model with default settings. Additional read trimming was carried out using Porechop (0.3.2pre) to remove remaining adapter contamination. Filtlong (0.2.1) was used for light quality filtering, discarding the lowest quality 5% of reads and any reads shorter than 1,000 bp. The filtered reads were used as the input for long-read consensus assembly using Trycycler (0.5.3) with Flye (2.9-b1768), miniasm (0.3-r179) + Minipolish (0.1.2), and raven (1.7.0) as assemblers. Medaka (1.5.0), Polpypolish (0.4.3), and POLCA from MaSuRCA (3.4.2) were used to polish the initial assembly with a combination of long-read (Nanopore) and short-read (MiSeq) data.
Phylogenetic analysis.
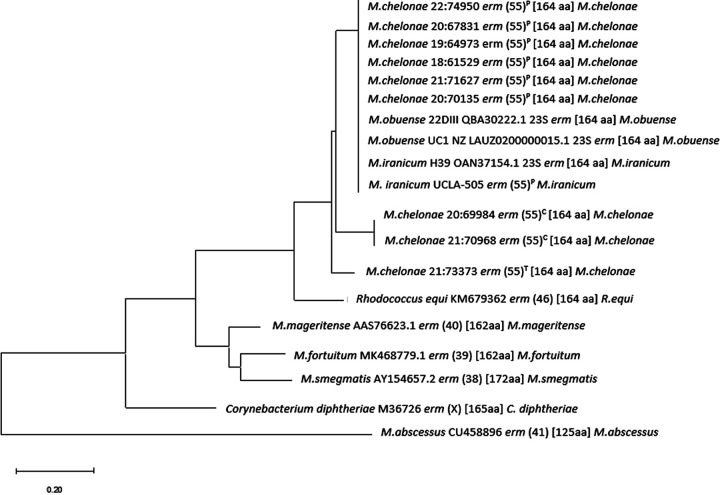
MEGA Molecular Evolutionary Genetics Analysis software (megasoftware.net) was used for construction of erm(55)P, erm(55)C, and erm(55)T dendrograms. Default parameters for the neighbor joining method were used for amino acids (Fig. 1). Sequences of erm genes from M. fortuitum subsp. fortuitum, M. smegmatis, M. abscessus, M. mageritense, M. obuense, M. iranicum, Rhodococcus equi, and Corynebacterium diphtheriae were used to illustrate erm(55) relatedness among Mycobacterium species and other relevant genera. GenBank numbers for reference sequences were included in the dendrogram.
FIG 1.
Neighbor-joining tree comparing the differences between the amino acid sequences of the erm(55)P, erm(55)C, and erm(55)T genes to those in other mycobacterial species and notable bacterial species. The scale represents percentages, i.e., 0.20 equates to 20% difference between each sequence. erm genes of organisms that has <79% amino acid similarity to one another were considered different.
Primer design and results for new erm gene PCR and gene sequencing.
Primers for amplification of erm(55)P were based on the sequence from M. chelonae strain 20:67831 designated erm55-F-1 (5′ CTT GAC TGA CCA ACC GAC GA 3′) and erm55-R-1 (5′ TGT CAT GAC CCC ACC TTT CG 3′) (Table 1). The utility of these primers for detection of erm(55)P was evaluated by PCR-based screening of M. chelonae isolates with clarithromycin MICs of 4 to >16 μg/mL, including isolates with known 23S A2058 or A2059 mutational resistance to clarithromycin. Additional primers were designed to support PCR-based screening of the chromosomal erm(55)C (primers erm55.2 Fa and erm55.2 Ra) and erm(55)T (erm55.3 Fb and erm55.3 Rb) gene variants (Tables 1 and 2) .
TABLE 1.
Primers used for PCRs
| Namea | Sequence | Direction | Target gene | Reference |
|---|---|---|---|---|
| ermF | 5′ GAC CGG GGC CTT CGT GAT 3′ | Forward | erm(41) | 10 |
| erm41-4* | 5′ CCG GCC CGT AGC GTC CAA TG 3′ | Reverse | erm(41) | 10 |
| ermR1 | 5′ GAC TTC CCC GCA CCG ATT 3′ | Reverse | erm(41) | 10 |
| erm(55)P-F-1 | 5′ CTT GAC TGA CCA ACC GAC GA 3′ | Forward | erm(55)p | Current study |
| erm(55)P -R-1 | 5′ TGT CAT GAC CCC ACC TTT CG 3′ | Reverse | erm(55)p | Current study |
| erm(55)C.2 Fa | 5′ CAA CTA CCC TGT TCG CCG TA 3′ | Forward | erm(55)C | Current study |
| erm(55)C.2 Ra | 5′ CAT CGC CAA TTC CTC GAA CG 3′ | Reverse | erm(55)c | Current study |
| erm(55)T.3 Fb | 5′ CCA TCG TAG GAA ACC TGC CA 3′ | Forward | erm(55)T | Current study |
| erm(55)T.3 Rb | 5′ CGC GAG GCA AGG ATT GAT CT 3′ | Reverse | erm(55)T | Current study |
| ermfortF | 5′ TCA CTT CTC TCG GAC CTT CC 3′ | Forward | erm(39) | Current study |
| ermfortR | 5′ CTC TAC ATC GCC TGG ACC AT 3′ | Reverse | erm(39) | Current study |
| T1 | 5′ AAG GGT GAA GCG GAG AAT 3′ | Forward | 23S rRNA | 7 |
| R1 | 5′ TGA TTG CCG TCC AGG TT 3′ | Reverse | 23S rRNA | 7 |
| pA-F | 5′ AGA GTT TGA TCC TGG CTC AG 3′ | Forward | p16S rRNA | 30 |
| pF-R | 5′ ACG AGC TGA CGA CAG CCA TG 3′ | Reverse | p16S rRNA | 30 |
| pC-F* | 5′ CTA CGG GAG GCA GTG GG 3′ | Forward | p16S rRNA | 30 |
| pD-R* | 5′ GTA TTA CCG CGG CTG 3′ | Reverse | p16S rRNA | 30 |
| 16S 830 F | 5′-GTG TGG GTT TCC TTC CTT GG 3′ | Forward | Full 16S rRNA | 30 |
| 16S pH R | R-5′-AAG GAG GTG ATC CAG CCG CA-3′ | Reverse | Full 16S rRNA | 30 |
Primer names followed by asterisks are those used for the BigDye reaction in Sanger sequencing that replaced the original PCR primers (36).
TABLE 2.
Clinical and laboratory information on 10 clarithromycin-resistant (MIC, ≥16 μg/mL) PCR erm(55)P-positive isolates (one per patient) and 6 erm(55)-negative isolates with an intermediate clarithromycin MIC (4 μg/mL) of Mycobacterium chelonaea
| Organism and patient no. | Age (yrs) | Geographic location | Strain | Culture dateb | Source | Colony morphology | Clarithromycin MIC (μg/mL) |
23S rRNA mutation | PCR result |
erm (55)P aa sequence | PCR result |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-day | Extended (days) | erm(55)P | erm(55)T | erm(55)C | |||||||||||
| M. chelonae, resistantc | |||||||||||||||
| 1 | 54 | OH | 18:60630 | 6/27/2018 | Tissue, ankle wound | Smooth 7H10, rough irregular edges on TSA | >16 | NA | − | − | 82% | + | − | ||
| 2 | 74 | WA | 18:62378 | 10/31/2018 | Nasal septal cartilage | Rough on TSA or 7H10 | 1 | (5), (8) 16, >16 | − | + | 100% | − | − | ||
| 3 | 83 | KY | 19:64973 | 2/27/2019 | Hand | Slightly smooth to smooth granular on 7H10, smooth intermediate with irregular edges on TSA | 2 | (6) >16 | − | + | 100% | − | − | ||
| 4 | 5 | MA | 20:67831 | 3/13/2020 | BAL | Smooth on TSA, rough on 7H10 | 4 | (4), (5) 8 16 | − | + | 100% | − | − | ||
| 4c | 5 | MA | 20:68529 | 3/13/2020 | BAL | Smooth on TSA or 7H10 | 0.25 | (14) 1 | − | − | NA | − | − | ||
| 5 | 69 | PA | MC6 22:74950 | 8/28/2020 | Leg | Smooth on TSA or 7H10 | 1 | >16 (×3)d | − | + | 100% | − | − | ||
| 6 | 71 | NC | 20:69984 | 12/03/2020 | Synovial fluid | Smooth on TSA, smooth irregular edges on 7H10 | >16 | NA | − | − | 86% | − | + | ||
| 7 | 75 | MA | 21:70135 | 12/23/2020 | Great toe pus | Smooth on TSA, smooth irregular edges on 7H10 | 2 | (4) >16 | − | + | 100% | − | − | ||
| 8 | 55 | IA | 21:70968 | 3/5/2021 | Leg | Smooth on TSA, smooth irregular edges on 7H10 | 16 | (4) >16 | − | − | 86% | − | + | ||
| 9 | 49 | MA | 21:71627 | 5/06/2021 | Knee biopsy | Rough on TSA or 7H10 | 2 | (6) >16 | − | + | 100% | − | − | ||
| 10 | 25 | AL | 21:73373 | 6/16/2021 | Sputum | Smooth on TSA, smooth irregular edges on 7H10 | >16 | NA | − | − | 82% | + | − | ||
| M. chelonae, intermediatee | |||||||||||||||
| 1 | 34 | NY | 21:71445 | 4/16/2021 | Soft tissue, foot | Smooth on TSA; irregular edges on 7H10 | 0.5 | 4 | NA | − | NA | − | − | ||
| 2 | 60 | MA | 21:73597 | 9/30/2021 | Sputum | Smooth irregular edges on TSA or 7H10 | 0.12 | 4 | NA | − | NA | − | − | ||
| 3f | 62 | TX | 21:74200 | 11/26/2021 | Skin | Smooth on TSA, smooth irregular edges on 7H10 | 0.25, 0.5 | 4, 4 | NA | − | NA | − | − | ||
| 4 | 76 | NY | 22:74948 MC4 | 2021 | Sputum | Smooth on TSA7 or H10 | 0.5, 0.5 | 4, 4 | NA | − | NA | − | − | ||
| 5 | 9 | PA | 22:74954MC8 | 2021 | Abdominal wound | Smooth on TSA, rough on 7H10 | 0.5, 0.25 | 8, 4 | NA | − | NA | − | − | ||
| 6 | 54 | MO | 22:74477 | 2/11/2022 | Cornea | Smooth on TSA or 7H10 | 0.5 | 4 | NA | − | NA | − | − | ||
The 10 resistant isolates (from 10 patients) were isolated in Laboratory A or B. Symbols and abbreviations: NA, not applicable; −, negative; +, positive; 7H10, Middlebrook 7H10 agar; TSA, trypticase soy agar; BAL, bronchoalveolar lavage; aa, amino acids.
Isolates are presented in chronological order of isolation.
Resistant (MIC ≥ 16 μg/mL; n = 10 patients); also includes one clarithromycin-susceptible isolate from mixed culture (number 4).
Susceptible to clarithromycin (same patient as 20:67831).
Intermediate (MIC = 4 μg/mL; n = 6 isolates from 6 patients).
Same patient had two isolates.
Patient demographics.
Information collected at the time of culture submission for patients found to be infected with M. chelonae, with intermediate susceptibility and high-level resistance, included the culture date, specimen source and geographic location, patient age, and type of infection if available (Table 2).
This study was approved by the Institutional Review Boards at the UTHSCT and the Mayo Clinic (Rochester, MN).
Data availability.
Whole-genome sequencing data sets have been deposited in GenBank as BioProject PRJNA938130. Sequences for the three erm(55) gene variants have been deposited as GenBank accession numbers OQ656455, OQ656456, and OQ656457. The sequence for plasmid pMchErm55 from M. chelonae strain 20:67831 has been deposited as accession number CP118918.1.
RESULTS
Detection of macrolide-intermediate and macrolide-resistant isolates.
A total of 133 M. chelonae isolates received in Laboratories A and B between November 2020 and March 2022 underwent extended, 14-day incubation. Five (3.8%) exhibited inducible, high-level resistance to clarithromycin (MIC of ≥16 μg/mL). In addition, five Laboratory A isolates obtained during January 2018 to October 2020 that had 3-day clarithromycin MICs of ≥1 μg/mL showed high-level inducible resistance with 14-day incubation. An additional six isolates were found to have intermediate clarithromycin MICs of 4 μg/mL at 14 days.
All M. chelonae isolates from Laboratories A and B were nonpigmented and grew upon subculture within 3 to 5 days at 30°C. Reference strain M. chelonae CIP 104535 (ATCC 35752T) had a 14-day clarithromycin MIC of ≤2 μg/mL and was classified on initial testing (3 to 5 days of incubation) as clarithromycin susceptible, according to CLSI guidelines (17).
Identification of erm(55)P in M. chelonae.
Two M. chelonae isolates with distinct colony types were recovered from one patient. Isolate 20:68529 had a smooth colony morphology and was macrolide susceptible, whereas isolate 20:67831 had a rough granular morphology and was macrolide resistant (Table 1). Both isolates were sequenced on the Illumina MiSeq and Oxford Nanopore MinION platforms. Assembly of MinION data followed by polishing with MiSeq reads generated two complete, circular chromosomal sequences that were nearly identical in size (5,143,578 bp for 20:68529 and 5,143,566 bp for 20:67831). However, the assembly for the macrolide-resistant isolate 20:67831 genome included an additional 137,526 bp circular contig that was absent from the macrolide-susceptible isolate 20:68529 data set. Annotation of this second contig, a presumptive plasmid, revealed a putative 23S rRNA methylase gene with 72% amino acid sequence identity to erm(46) from Rhodococcus and <65% amino acid identity with the four previously described RGM erm genes: erm(38), erm(39), erm(40), and erm(41) (Fig. 1). The novel erm gene from M. chelonae was designated erm(55) by Marilyn Roberts at the Nomenclature Center for MLS Genes (https://faculty.washington.edu/marilynr/ermweb1.pdf). Here, we refer to this original allele as erm(55)P, to highlight its presence on the plasmid contig, which we have named pMchErm55 (Fig. 2). The erm(55) sequence from M. chelonae strain 20:67831 was used to design oligonucleotide primers erm55-F-1 and erm55-R-1 for the erm(55)P PCR assay (Fig. 3).
FIG 2.
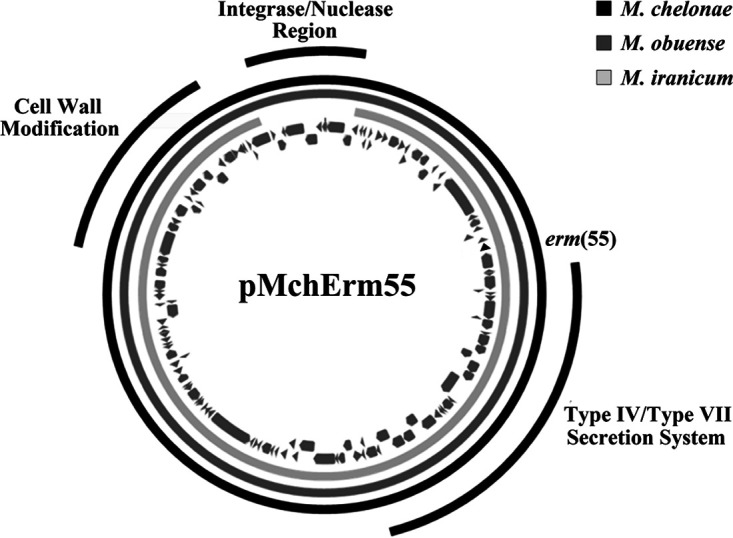
The 137,526-bp circular plasmid pMchErm55, encoding erm(55) (black triangle), is present in macrolide-resistant RGM, including M. chelonae strains 20:67832 and 18:61529 (black ring) and M. obuense strains UC1 and 22DIII (dark gray ring). In M. iranicum strains H39 and UCLA_505 (light gray ring), the ≈12-kb region encoding the integrase and nuclease region is absent.
FIG 3.
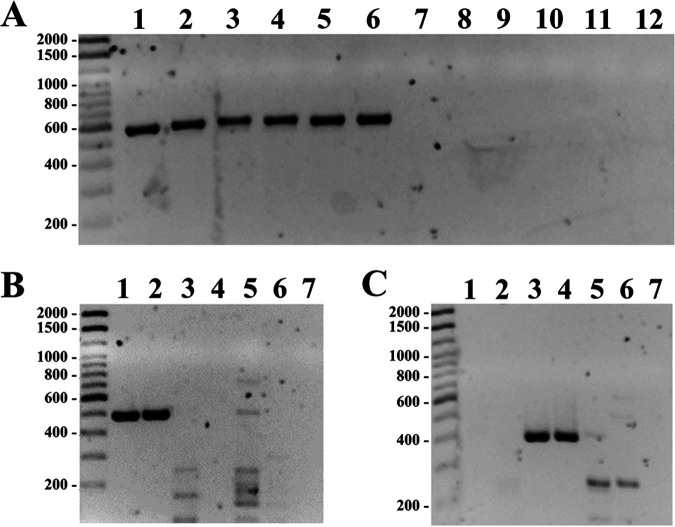
PCR assays targeting erm(55) genes. (A) PCR with the erm(55)P primer set. Isolates encoding erm(55)P (lanes 1 to 6) were positive, but isolates containing erm(55)C (lanes 7 and 8) or erm(55)T (lanes 9 and 10) were negative. Lane 11 is an isolate with no erm(55) gene. Lane 12 is the no-template (water) control. (B) PCR with the erm(55)C primer set. Isolates encoding erm(55)C (lanes 1 and 2) were positive, but isolates containing erm(55)T (lanes 3 and 4) or erm(55)P (lanes 5 and 6) were negative. Lane 7 is an isolate with no erm(55) gene. (C) PCR with the erm(55)T primer set. Isolates encoding erm(55)T (lanes 3 and 4) were positive, but isolates containing erm(55)C (lanes 1 and 2) or erm(55)P (lanes 5 and 6) were negative. Lane 7 is an isolate with no erm(55) gene.
Description of pMchErm55.
We refer to the 137-kb contig from M. chelonae 20:67831 as pMchErm55 because it encodes erm(55) as well as key features of conjugative mycobacterial plasmids, including type IV and type VII secretion system genes. A 137-kb contig with 99.9% identity to pMchErm55 is present in the genome assembly of the macrolide-resistant strain M. chelonae 18:61529. The plasmid is also present in other macrolide-resistant RGM species, as revealed by reference mapping of sequencing reads from M. iranicum strains H39 (SRA entry SRX1693165) and UCLA-505 (SRR21647431) and M. obuense strains UCI (GCF0009749252) and 22DIII(QBA30222.1) data sets (Table 3). Notably, a 12-kb region of pMchErm55 that encodes several integrase and recombinase genes is absent from both M. iranicum data sets (Fig. 2).
TABLE 3.
Clinical and laboratory information from public databases on two isolates of Mycobacterium iranicum and two isolates of Mycobacterium obuensea
| Organism and isolation yr | Strain | Patient age | Sequence accession no. | Geographic location | Source | Colony morphology | P16S CCUG 52297 | P16S M05T % | Clarithromyci MIC (μg/mL) |
erm(55)P PCR | erm(55)P sequence % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 days | 14 days | |||||||||||
| M. iranicum | ||||||||||||
| 2015 | H39 | NA | GCF_001650495.1 | CA | Environ | NA | NA | 100 | NA | NA | NA | 100 |
| 2022 | UCLA-505 | 76 | SRR21647431 | CA | Blood | NA | NA | 99.9 | 2 | 8 (7 days) | NA | 100 |
| M. obuense | ||||||||||||
| 2008 | UCI | NA | GCF_000974925.2 | CA | Sputum | NA | 100 | NA | NA | NA | NA | 100 |
| 2012 | 22DIII | NA | QBA30222.1 | Portugal | Environ (sink) | NA | 100 | NA | 16 | NA | NA | 100 |
NA, not available; Environ, environmental.
Identification of erm(55)C and erm(55)T in M. chelonae.
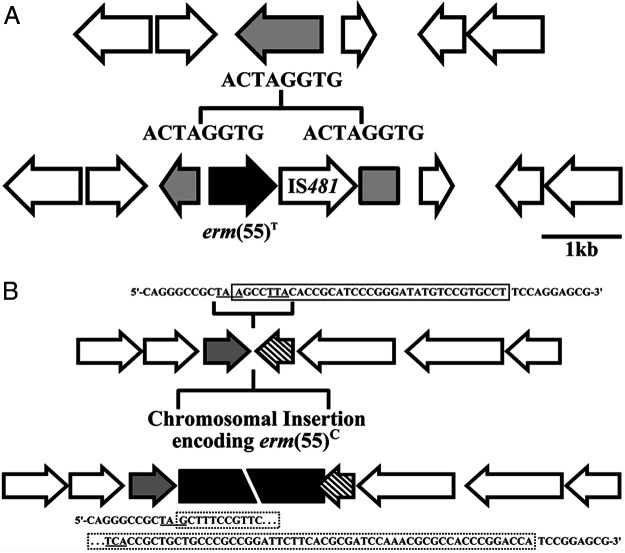
Four macrolide highly resistant isolates of M. chelonae that tested negative with the erm(55)P PCR screening were sequenced on the Illumina MiSeq platform. Contigs were assembled and annotated using the IRIDA pipeline. BLASTN analysis identified erm(55)-like sequences in all four assemblies (Fig. 1). The sequences from strains 20:69984 and 21:70968 were identical to each other but only 86% identical to erm(55)P (Table 2 and Fig. 1). Genome comparison suggests that the erm(55) gene in isolates 20:69984 and 21:70968 is part of a 37-kb chromosomal insertion (Fig. 4A). To highlight the chromosomal location of this allele, we refer to it as erm(55)C. The sequences from 18:60630 and 21:73373 were identical to each other but only 82% identical to erm(55)P. Genome annotation revealed that the erm(55) gene in 18:60630 and 21:73373 is immediately adjacent to a IS481-family transposase gene (Fig. 4B). This gene pair comprise a 2,029-bp insertion that disrupts a putative NADP oxidoreductase gene. The edges of the insertion are defined by 8-bp direct repeats (5′-ACTAGGTG-3′). These features are consistent with a transposon, such that we refer to this allele as erm(55)T.
FIG 4.
(A) Macrolide resistance in M. chelonae strain 21:73373 is associated with a ≈2-kb insertion that disrupts a NADP oxidoreductase gene (gray arrow). This putative transposon contains erm(55)T (black arrow) and an IS481 family transposase and is flanked by 8-bp direct repeats. (B) Macrolide resistance in M. chelonae strains 20:69984 and 21:70968 is associated with a chromosomal insertion (black box) that encodes erm(55)C. The insertion replaces a 35-bp sequence (solid box) that overlaps the stop codons (underlined) of two open reading frames, an uncharacterized gene (gray arrow), and a polyketide cyclase (hatched). The 5′ end of the insertion mutates the stop codon of the uncharacterized gene (dotted box); however, this does not alter the coding sequence. In contrast, the 3′ edge of insertion does change the coding sequence of the polyketide cyclase and extends the open reading frame by 8 codons.
PCR-based screening for 23S rRNA gene mutations, the erm(41) gene, and erm(55) variants.
Isolates with high-level resistance (MIC ≥ 16 μg/mL) or intermediate susceptibility (MIC = 4 μg/mL) to clarithromycin were screened by PCR. Previously described methods were used for detection of the erm(41) gene and mutations in the 23S rRNA (rrl) gene (7, 9). Novel assays were developed for detection of the erm(55)P erm(55)C, and erm(55)T gene variants (Table 1). Verification experiments confirmed that the erm(55) assays were specific, with no cross-reactivity between the three variant genes. Similarly, strains that were susceptible to clarithromycin as well as strains containing erm(41) or 23S rRNA (rrl) gene mutations were negative when tested in the erm(55) assays.
None of the 10 isolates from 10 patients with high-level clarithromycin resistance were PCR positive for erm(41) or 23S rRNA mutations, but six high-level macrolide-resistant M. chelonae isolates were positive for erm(55)P. Genome sequencing of the four M. chelonae strains that were negative for erm(55)P led to the discovery of the erm(55)T and erm(55)C gene variants (Tables 1 and 2).
The six M. chelonae isolates with intermediate susceptibility to clarithromycin tested negative for all PCR targets. The mechanism of elevated MICs in this intermediate susceptibility group is unknown at present.
Identification of erm(55)P in M. iranicum and M. obuense.
BLASTN and BLASTX analyses revealed identical matches to erm(55)P in four GenBank entries: M. iranicum strains H39 recovered in 2015 (GCF_001650495.1) and UCLA-505, published in 2023 (SRR21647431), and M. obuense strains UC1 recovered in 2008 (GCF_000974925.2) and 22DIII recovered in 2012 (QBA30222.1) (Fig. 1 and 2 and Table 3).
Colony morphology.
Macrolide-susceptible isolates of M. chelonae are typically described as smooth. Of the seven isolates of M. chelonae that were erm(55)P positive, 3/7 were rough on both trypticase soy agar (TSA) and Middlebrook 7H10 (7H10), 2/7 were smooth on TSA with granular or rough margins on 7H10, and only one was smooth on TSA and rough on 7H10. All eight of the isolates with intermediate clarithromycin MICs were smooth on TSA; 3/8 had irregular smooth borders on 7H10, 3/8 had smooth borders on both media, and 1/8 was rough on 7H10 (Table 1). Notably, over time, the rough colony morphology was less apparent.
Demographics.
The 10 M. chelonae patients with erm(55)-positive isolates were from eight states (Table 2). The isolates were from a sinus and sinus tissue (1), wounds and joints (7), sputum (1), and a bronchoalveolar lavage (1). Most (9/10) were adults and over the age of 50 years (7/10). The median age was 64 years. The dates of isolation are shown in chronological order in Table 2. Two were isolated in 2018, one in 2019, four in 2020, and three in 2021.
The six isolates with an intermediate clarithromycin MIC (4 μg/mL) were from six patients from five states (Table 2). Six were isolated in 2021 and one from 2022. Isolates were recovered from sputum (2), wounds (4), and cornea (1). One patient had two isolates from a skin wound. The median age of the patients was 60 years.
DISCUSSION
Since FDA approval of clarithromycin in 1990, macrolides have evolved as the standard of care, initially for Mycobacterium avium complex and subsequently for infections caused by many slowly growing mycobacteria (SGM) and RGM species, including M. chelonae (1–6, 18). Macrolides bind to the bacterial 23S ribosome to inhibit protein synthesis. Changes to the ribosome, due to mutation or enzyme-mediated modification (e.g., methylation), can prevent macrolide binding (7, 8). Early studies in 1996 by Wallace et al. revealed that point mutations in the A2058 and A2059 positions of the 23S rRNA gene conferred resistance to macrolides (7). This resistance is constitutive and is reliably detected by measuring MICs after 3 to 5 days of incubation (7). The subsequent discovery of inducible macrolide resistance, conferred by 23S rRNA methylase (erm) genes (especially M. fortuitum group and 80% of isolates of M. abscessus subsp. abscessus), has complicated antimicrobial susceptibility testing of RGM and compromised the usefulness of the newer macrolides, clarithromycin and azithromycin (8–10).
In contrast to erm(38), erm(39), erm(40), and erm(41) genes, which have only been found on the chromosomes of RGM, we describe here erm(55) gene variants located on an M. chelonae chromosomal insertion [erm(55)C], a putative (chromosomal) transposon [erm(55)T], and a putative 137-kb conjugative plasmid [erm(55)P]. Antibiotic resistance due to plasmid-borne R factors has been recognized since the 1950s. However, it was not until 2014 that plasmid-mediated drug resistance was first demonstrated for mycobacteria, when plasmid pMAB01, which carries multiple resistance genes and confers resistance to kanamycin, was recovered from a strain of M. abscessus subsp. massiliense (then designated M. bolletii) responsible for a nationwide outbreak of surgical infections in Brazil (19). Additional studies are required to characterize the 137-kb pMchErm55 contig for erm(55)P. In the current study, annotation of the contig revealed several features of conjugative mycobacterial plasmids, including genes associated with type IV and type VII secretion systems, which appear essential for mycobacterial conjugation (20). Moreover, the presence of pMchErm55 sequences in data sets from M. iranicum and M. obuense suggest that this is a broad-host-range plasmid that can be transferred between RGM species (21). Except for erm(55)P, pMchErm55 does not appear to contain any other antibiotic resistance genes. However, the plasmid does contain homologs to methoxymycolic acid synthase (mma4) and dimycocerosyl transferase (papA5). Cell wall modifications mediated by mma4 or papA5 may explain the rough colony morphology associated with erm(55)P (i.e., pMchErm55-containing strains).
M. iranicum is a scotochromogenic (pigmented) RGM species and a recognized human pathogen first described in 2013 (22, 23). In 2017, Lymperopoulou et al. published the draft genome of M. iranicum strain H39, an environmental isolate collected in 2015 (24). Susceptibility testing of strain H39 was not described, but sequencing reads (SRA SRX1693165) mapped to 125 kb of pMchErm55 that includes erm(55)P. The smaller plasmid size reflects a 12-kb region that includes recombinase and integrase genes which are missing (Table 3 and Fig. 2). In 2023, Ranson et al. reported a blood isolate of M. iranicum (strain UCLA-505) causing a central line infection (25). The isolate was identified by next-generation sequencing and was inducibly macrolide resistant using CLSI guidelines. Those authors were unable to detect any known erm gene. Subsequent sequencing reads (SRR21647431) mapped to pMchErm55 that included erm(55)P. Interestingly, the same 12-kb plasmid fragment was missing, as in strain H39 (Fig. 2).
Mycobacterium obuense, also a scotochromogenic RGM that is a recognized human pathogen, was first characterized by Tsukamura and Mizune in 1971 (26). The first draft genome of an M. obuense strain (UCI), recovered in 2008 from sputum, was published in 2015 (21). The sequence of M. obuense strain 22DIII was reported in 2019 in a genomic study of NTM recovered from health care-associated environments in Portugal (27, 28). Strain 22DIII was the only isolate in the study that was macrolide resistant, with a clarithromycin MIC of 16 μg/mL. Although those authors noted that the strain possessed “a classical erm gene,” additional details were not provided (27). BLAST analysis indicated that the UC1 and 22DIII strains both contained amino acid sequences identical to the translated sequence of erm(55)P. Moreover, the draft genome of strain UC1 includes >83 kb of sequence with >99% identity to pMchErm55. This suggests that pMchErm55 is a conjugative plasmid that can be transferred between multiple RGM species. Comparison of erm genes and inferred amino acid sequences indicates that erm(55) is most closely related to erm(46), initially described in Rhodococcus species (Fig. 1) (29). The erm(46) gene appears to be quite promiscuous. It belongs to a mobile element that is present on a plasmid (pRErm46).
Horizontal gene transfer (HGT) in NTM has rarely been described except under laboratory conditions (30–33). A presumptive conjugative 23-kb mercury resistance plasmid, initially found in M. marinum strain ATCC BAA-535 (32), was also described in the genomic sequence of the type strain of M. abscessus subsp. abscessus (CIP 104536T), published by Ripoll et al. in 2009 (34). The first conjugative plasmid conferring antibiotic resistance in NTM was described by Leão et al. in 2013 (35) and further characterized by Matsumoto et al. in 2014 (19). This was a strain of M. abscessus (INCQS 00594) recovered in 2008 that was responsible for a nationwide epidemic of more than 2,000 surgical infections in Brazil. The 56,267-bp circular plasmid (pMAB 01) belonged to the broad-host-range Inc P-1β subgroup and contained a complete system for conjugative DNA to transfer and two genetic load regions carrying antimicrobial resistance genes. It was successfully transferred to a modified Escherichia coli strain but not to other NTMs. (19, 35). Characterization of the M. chelonae plasmid (pMchErm55) reported here is ongoing.
There are limitations to our current study. During the early part of the study period (2018 to late 2020), Laboratories A and B were following the current CLSI recommendation to not routinely perform extended (14-day) incubations for clarithromycin susceptibility testing if molecular identification was previously performed (13). As such, all strains with inducible macrolide resistance due to erm(55) were recovered from the subset of M. chelonae isolates that were incubated for 14 days; the complete collection of clinical isolates recovered during 2018 to 2022 may also include additional isolates with erm(55). Although it is implied that all isolates with the erm(55)P allele carry a plasmid, the current PCR-based screen only detects the erm(55)P allele, not the plasmid sequence. However, of the two strains of M. chelonae, two strains of M. obuense (UCI, 22DIII) (Table 3), and two strains of M. iranicum (H39, UCLA-505) (Table 3) for which erm(55)P-flanking region analysis is available, all had plasmid sequences. Similarly, additional experiments are required to demonstrate that the 137-kb contig really is a conjugative plasmid that can be transferred within and between RGM species. Those investigations are in progress.
Our current findings have major implications for antibiotic susceptibility testing of M. chelonae and the treatment of RGM infections. The discovery of erm(55) upends the 2018 CLSI recommendations regarding testing for inducible macrolide resistance in M. chelonae (13). Extended (14-day) clarithromycin susceptibility testing should be reinstated, pending CLSI evaluation and formal recommendation, at a minimum on isolates with clarithromycin MICs of ≥0.5 μg/mL after 3-day incubation, at least until a rapid and reliable method for detection of all three variant alleles of erm(55) becomes available. The M. chelonae isolates examined in the current study were collected during 2018 to 2022. However, the identification of erm(55)P in M. obuense strain UC1, which was isolated in 2008, at least 10 years earlier (21), clearly indicates that plasmid-mediated macrolide resistance due to this erm allele is already widespread, albeit an underappreciated phenomenon among RGM.
Greater spread within M. chelonae strains and transmission to other macrolide-susceptible RGM pathogens, including M. abscessus subsp. massiliense and isolates of M. abscessus subsp. abscessus with a type II erm(41) gene (both of which are nonfunctional), will have dire consequences for antimicrobial therapy. These two subspecies of M. abscessus as well as M. chelonae are multidrug resistant, and the macrolides are the only proven effective oral antimicrobials. M. abscessus subspecies are also a major cause of chronic RGM lung disease, especially in the setting of cystic fibrosis and bronchiectasis. The potential spread of plasmids between RGM and SGM is highly likely, given the sequence identity of a 23-kb mercury resistance plasmid in M. marinum and M. abscessus subsp. abscessus (30–32, 34).
Our study is indeed a plea for newer antimicrobials which are designed to overcome these resistance mechanisms and better understanding of plasmids and their potential role in drug resistance in RGM and in NTM.
ACKNOWLEDGMENTS
We acknowledge the collaboration of Shangxin Yang and Elizabeth Ranson for providing unpublished data on their recently published macrolide-resistant strain (UCLA-505) of M. iranicum (25) and the guidance of Joseph Falkinham of Virginia Tech, Blacksburg, VA. We thank Peggy Churchman and Joanne Woodring for typing the manuscript.
This research received no external funding.
Footnotes
[This article was published on 22 June 2023 with a reference missing, an unneeded reference included, and an incorrect reference citation in the third paragraph of the Discussion section. The references were adjusted in the current version, posted on 27 June 2023.]
Contributor Information
Barbara A. Brown-Elliott, Email: Barbara.Elliott@uthct.edu.
Melissa B. Miller, The University of North Carolina at Chapel Hill School of Medicine
REFERENCES
- 1.Wallace RJ, Jr, Glassroth J, Griffith DE, Olivier KN, Cook JL, Gordin F. 1997. American Thoracic Society, diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med 156(Suppl):S1–S25. doi: 10.1164/ajrccm.156.2atsstatement. [DOI] [PubMed] [Google Scholar]
- 2.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K, Infectious Diseases Society of America . 2007. An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 3.Brown BA, Wallace RJ, Jr, Onyi GO, De Rosas V, Wallace IR. 1992. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother 36:180–184. doi: 10.1128/AAC.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace RJ, Jr, Tanner D, Brennan PJ, Brown BA. 1993. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med 119:482–486. doi: 10.7326/0003-4819-119-6-199309150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Wallace RJ, Jr, Brown BA, Onyi G. 1992. Skin, soft tissue, and bone infections due to Mycobacterium chelonae subspecies chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis 166:405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- 6.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 56:2000535. doi: 10.1183/13993003.00535-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace RJ, Jr, Meier A, Brown BA, Zhang Y, Sander P, Onyi GO, Bottger EC. 1996. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother 40:1676–1681. doi: 10.1128/AAC.40.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash KA, Andini N, Zhang Y, Brown-Elliott BA, Wallace RJ. 2006. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob Agents Chemother 50:3476–3478. doi: 10.1128/AAC.00402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash KA, Brown-Elliott BA, Wallace RJ Jr. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown-Elliott BA, Vasireddy S, Vasireddy R, Iakhiaeva E, Howard ST, Nash K, Parodi N, Strong A, Gee M, Smith T, Wallace RJ. 2016. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol 54:1172. doi: 10.1128/JCM.00192-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh WJ, Jeon K, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park Y-K, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 12.Hanson KE, Slechta ES, Muir H, Barker A. 2014. Rapid molecular detection of inducible macrolide resistance in Mycobacterium chelonae and M. abscessus strains: a replacement for 14 day susceptibility testing? J Clin Microbiol 52:1705–1707. doi: 10.1128/JCM.03464-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2018. Susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes, M-124, 3rd ed. CLSI, Wayne, PA. [PubMed] [Google Scholar]
- 14.Adékambi T, Berger P, Raoult D, Drancourt M. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., M. phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol 56:133–143. doi: 10.1099/ijs.0.63969-0. [DOI] [PubMed] [Google Scholar]
- 15.Brown-Elliott BA, Fritsche TR, Olson BJ, Vasireddy S, Vasireddy R, Iakhiaeva E, Alame D, Wallace RJ, Jr, Branda JA. 2019. Comparison of two commercial matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for identification of nontuberculous mycobacteria. Am J Clin Pathol 152:527–536. doi: 10.1093/ajcp/aqz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews TC, Bristow FR, Griffiths EJ, Petkau A, Adam J, Dooley D, Kruczkiewicz P, Curatcha J, Cabral J, Fornika D, Mabon P, Enns E, Thiessen J, Keddy A, Isaac-Renton J, Gardy JL, Tang P, Carrico JA, Chindelevitch L, Chauve C, Graham MR, McArthur AG, Taboada EN, Beiko RG, Brinkman F, Hsiao WL, Domselaar GV, The IRIDA Consortium . 2018. The integrated rapid infectious disease analysis (IRIDA) platform. bioRxiv. 10.1101/381830. [DOI]
- 17.Clinical and Laboratory Standards Institute. 2018. Performance standards for susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes. CLSI suppl. M62, 1st ed. CLSI, Wayne, PA. [PubMed] [Google Scholar]
- 18.Tebas P, Sultan F, Wallace RJ, Jr, Fraser V. 1995. Rapid development of resistance to clarithromycin following monotherapy for disseminated Mycobacterium chelonae infection in a heart transplant patient. Clin Infect Dis 20:443–444. doi: 10.1093/clinids/20.2.443. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto CK, Bispo PJ, Santin K, Nogueira CL, Leão SC. 2014. Demonstration of plasmid-mediated drug resistance in Mycobacterium abscessus. J Clin Microbiol 52:1727–1729. doi: 10.1128/JCM.00032-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortimer TD, Weber AM, Pepperell CS. 2017. Evolutionary thrift: Mycobacteria repurpose plasmid diversity during adaptation of type VII secretion systems. Genome Biol Evol doi: 10.1093/gbe/evx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greninger AL, Cunningham G, Hsu ED, Yu JM, Chiu CY, Miller S. 2015. Draft genome sequence of Mycobacterium obuense strain UC1, isolated from patient sputum. Genome Announce 3:e00612-15. doi: 10.1128/genomeA.00612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shojaei H, Daley C, Gitti Z, Hashemi A, Heidarieh P, Moore ERB, Naser AD, Russo C, van Ingen J, Tortoli E. 2013. Mycobacterium iranicum sp. nov., a rapidly-growing scotochromogenic species isolated from clinical specimens on three different continents. Int J Syst Evol Microbiol 63:1383–1389. doi: 10.1099/ijs.0.043562-0. [DOI] [PubMed] [Google Scholar]
- 23.Lapierre SG, Toro A, Drancourt M. 2017. Mycobacterium iranicum bacteremia and hemophagocytic lymphohistiocytosis: a case report. BMC Res Notes 10. doi: 10.1186/s13104-017-2684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lymperopoulou DS, Coil DA, Schichnes D, Lindow SE, Guillaume J, Eisen JA, Adams RI. 2017. Draft genome sequences of eight bacteria isolated from the indoor environment: Staphylococcus capitis strain H36, S. capitis strain H65, S. cohnii strain H62, S. hominis strain H69, Microbacterium sp. strain H83, Mycobacterium iranicum strain H39, Plantibacter sp. strain H53, and Pseudomonas oryzihabitans strain H72. Stand Genomic Sci 12:17. doi: 10.1186/s40793-017-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranson EL, Tsevat RK, von Bredow B, Kamau E, Yang S, Prabaker KK. 2023. Catheter-related bloodstream infection caused by Mycolicibacterium iranicum, California, USA. Emerg Infect Dis 29:217–219. doi: 10.3201/eid2901.220851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukamura M, Mizuno S, Tsukamura S. 1981. Numerical analysis of rapidly growing, scotochromogenic mycobacteria, including Mycobacterium obuense sp. nov., nom. rev., Mycobacterium rhodesiae sp. nov., nom. rev., Mycobacterium aichiense sp. nov., nom. rev., Mycobacterium chubuense sp. nov., nom. rev., and Mycobacterium tokaiense sp. nov., nom. rev. Int J Syst Bacteriol 31:263–275. doi: 10.1099/00207713-31-3-263. [DOI] [Google Scholar]
- 27.Pereira SG, Alarico S, Tiago I, Reis D, Nunes-Costa D, Cardoso O, Maranha A, Empadinhas N. 2019. Studies of antimicrobial resistance in rare mycobacteria from a nosocomial environment. BMC Microbiol 19. doi: 10.1186/s12866-019-1428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farias PGGF, Reis D, Alarico S, Empadinhas N, Martins JC, Almeida AF, Morais PV. 2017. Hospital microbial surface colonization revealed during monitoring of Klebsiella spp., Pseudomonas aeruginosa, and non-tuberculous mycobacteria. Antonie Van Leeuwenhoek 110:863–876. doi: 10.1007/s10482-017-0857-z. [DOI] [PubMed] [Google Scholar]
- 29.Anastasi E, Giguère S, Berghaus LJ, Hondalus MK, Willingham-Lane JM, MacArthur I, Cohen ND, Roberts MC, Vazquez-Boland JA. 2015. Novel transferable erm(46) determinant responsible for emerging macrolide resistance in Rhodococcus equi. J Antimicrob Chemother 70:3184–3190. doi: 10.1093/jac/dkv279. [DOI] [PubMed] [Google Scholar]
- 30.Rabello MCDS, Matsumoto CK, de Almeida LGP, Menendez MC, de Oliveira RS, Silva RM, Garcia MJ, Leão SC. 2012. First description of natural and experimental conjugation between mycobacteria mediated by a linear plasmid. PLoS One 7:e29884. doi: 10.1371/journal.pone.0029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schué M, Dover LG, Besra GS, Parkhill J, Brown NL. 2009. Sequence and analysis of a plasmid-encoded mercury resistance operon from Mycobacterium marinum identifies MerH, a new mercuric ion transporter. J Bacteriol 191:439–444. doi: 10.1128/JB.01063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PDR, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PLC, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray TA, Derbyshire KM. 2018. Blending genomes: distributive conjugal transfer in mycobacteria, a sexier form of HGT. Mol Microbiol 108:601–613. doi: 10.1111/mmi.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrmann J-L, Daffé M, Brosch R, Risler J-L, Gaillard J-L. 2009. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One 4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leão SC, Matsumoto CK, Carneiro A, Ramos RT, Nogueira CL, Junior JDL, Lima KV, Lopes ML, Schneider H, Azevedo VA, da Costa da Silva A. 2013. The detection and sequencing of a broad-host-range conjugative incP-1β plasmid in an epidemic strain of Mycobacterium abscessus subsp. bolletii. PLoS One 8:e60746. doi: 10.1371/annotation/5dd55ed1-2fb6-4672-9142-fb01331567e1. (Erratum 8:10.1371). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards U, Rogall T, Blöcker HME, Böttger EC. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Whole-genome sequencing data sets have been deposited in GenBank as BioProject PRJNA938130. Sequences for the three erm(55) gene variants have been deposited as GenBank accession numbers OQ656455, OQ656456, and OQ656457. The sequence for plasmid pMchErm55 from M. chelonae strain 20:67831 has been deposited as accession number CP118918.1.