ABSTRACT
Coproantigen detection by enzyme-linked immunosorbent assay (coAg ELISA) is a vital tool for detecting and treating cases of Taenia solium taeniasis. However, the assay’s procedures require costly materials and sophisticated equipment, which are typically inaccessible in rural settings where the disease is endemic. To overcome these barriers, we developed and evaluated a field-applicable coAg ELISA. The field coAg ELISA was developed and evaluated across four phases using known positive and negative stool samples collected from northern Peru. Phase I focused on field assay development, phase II on a small-scale performance evaluation, phase III on a large-scale evaluation, and phase IV on the use and reliability of a colorimetric scale card. All samples were processed using the field and standard assay procedures and compared using signal-to-noise ratios, correlation tests, performance characteristics, and agreement statistics where appropriate. The field coAg ELISA using reagents stored at −20°C and commercially available water and milk powder, and relying on spontaneous separation of the supernatant, had performance comparable to the standard assay. The field coAg ELISA was strongly correlated with the standard in both the small- and large-scale laboratory evaluation (r = 0.99 and r = 0.98, respectively). Finally, the field assay had an almost perfect agreement between independent readers (kappa = 0.975) and between each reader and the spectrophotometer. The field coAg ELISA demonstrated performance comparable to the standard, providing a low-cost alternative to the standard assay for identifying cases of intestinal taeniasis in a low-resource setting.
KEYWORDS: enzyme-linked immunosorbent assay, cysticercosis, diagnosis, Taenia solium, taeniasis, neurocysticercosis
INTRODUCTION
Taenia solium taeniasis (pork tapeworm) is a parasitic infection of the intestine that greatly affects rural populations living under conditions of poverty where free-roaming pigs are raised (1). Humans acquire taeniasis after consuming raw or undercooked pork containing cysticerci (i.e., larvae). Typically, this infection is asymptomatic or presents with minor symptoms, and therefore, it often goes undetected (2). However, if left untreated, the tapeworm will release millions of infectious eggs into the surrounding environment during its life span through the stool of the human host. When humans or pigs ingest these eggs, they go on to cause cysticercosis and neurocysticercosis (3).
Identifying and successfully treating people with T. solium tapeworm infections are critical for the control and prevention of taeniasis and cysticercosis (4–6). Several diagnostic techniques that use stool samples are available, but each approach has limitations regarding sensitivity, specificity, or accessibility for laboratories in low-resource settings (7). For example, visually inspecting the whole stool for parasitic material such as the scolex or proglottids allows for species-specific diagnosis, but obtaining these pieces of the tapeworm is uncommon and impractical for community screening. Microscopy is a commonly used technique that identifies tapeworm eggs in stool but has low sensitivity (52.5%), even in experienced laboratory settings (8). This low sensitivity is likely due to the intermittent shedding of proglottids, the small numbers of eggs shed from the proglottids within the host’s intestine, and the small volume of the stool being examined (9). Additionally, T. solium eggs are not morphologically distinguishable from those of Taenia saginata. Available molecular techniques, such as PCR, can identify and amplify T. solium DNA in stool but require parasite material to be effective. So far, PCR has not demonstrated sensitivity when evaluating T. solium egg-positive samples in clinical or community population settings (9–11). Specific antibodies to T. solium taeniasis have been identified and detected in serum using an enzyme-linked immunoelectrotransfer blot (EITB) technique, but the test cannot distinguish between current and past infections (10).
Detecting adult tapeworm T. solium antigens in stool (coproantigen [coAg]) by enzyme-linked immunosorbent assay (ELISA) is the assay of choice for intestinal taeniasis. Allan and colleagues first developed the test in 1990 (11). At the genus level, this test demonstrated high sensitivity and specificity (sensitivity, 95%; specificity, 99%) and more than doubled the case identification of people with taeniasis compared to microscopy (11). However, this test was susceptible to cross-reactions at the genus level with T. saginata and Taenia asiatica. In 2009, Guezala and colleagues developed a coAg ELISA that combined anti-T. solium whole worm extract (WWE) and excretory-secretory (ES) antibodies (12). This test provided greater specificity for T. solium taeniasis (sensitivity, 96%; specificity, 100%) but is typically unavailable outside academic research laboratories (7).
Multiple barriers preclude the use of the coAg ELISA in low-resourced settings, where most T. solium transmission occurs. These barriers include the need for sophisticated equipment, including ultralow-temperature freezers to store reagents, a centrifuge to sediment the stool samples, and a spectrophotometer to read the optical density (OD) of plates to determine results. Procurement and maintenance of this equipment require substantial resources. The test also requires high-cost materials such as ultrapurified water for buffer preparation and heat-inactivated fetal bovine serum as a blocking agent. In this work, we describe the development and evaluation of a novel, simplified, and field-applicable coAg ELISA (here called field coAg ELISA) that was modified to overcome these barriers while still maintaining performance similar to that of the standard coAg ELISA.
MATERIALS AND METHODS
Stool samples.
Stool samples used at each phase of the field assay’s development were previously collected from sites in northern Peru (range of estimated prevalence of taeniasis, 0.5 to 6.7% [13–15]) under appropriate informed consent processes approved by the Institutional Review Board of the Universidad Peruana Cayetano Heredia, with specific permission for future use. All stool samples were collected in 50-mL Falcon tubes and were diluted at a ratio of 1:5 in a solution made of phosphate-buffered saline (0.15 M PBS, pH 7) and 5% formaldehyde. Taenia coproantigens are very stable and can be detected in stool samples stored at room temperature (20°C) for several days. The samples can also be reliably stored in a 5% formalin solution for several months. This greatly simplifies the collection and storage of samples, particularly under field conditions (16).
Following their collection and preservation, samples were identified as positive or negative for taeniasis using traditional parasitological methods and standard coAg ELISA procedures (11, 17). Traditional parasitological methods included macroscopic examination of whole-stool samples for proglottids or scolexes and/or PCR-restriction enzyme analysis (targeting the T. solium ribosomal 5.8S gene plus internal transcribed spacer regions) when parasite material was available (17), as well as microscopy for the presence of Taenia eggs. For the standard coAg ELISA, the ODs of processed samples were measured using a spectrophotometer (Molecular Devices Vmax) at 650 nm (11). The OD of each sample was then used to calculate the percentage of positivity (PP) {[OD of the sample]/[OD of the positive control (P1)] × 100}. Identified samples were then stored in 5% formalin and at room temperature (20°C) in the sample repository (duration ranging from a few days to months) until their selection for assay development (Table 1). Additional details about sample selection are summarized in the supplemental material.
TABLE 1.
Summary of samples used in the development and validation of the field coAg ELISAa
| Characteristic | Phase I: test development | Phase II: small-scale validation | Phase III: large-scale validation | Phase IV: colorimetric scale evaluation |
|---|---|---|---|---|
| Objective | Identify optimal conditions for modified field coAg ELISA | Compare field and standard coAg ELISA results on a small set of known samples | Compare field and standard coAg ELISA results on a large set of known samples | Compare field coAg ELISA results using a spectrophotometer to those using a colorimetric scale |
| Stool sample source | Archived samples that were previously typified | Archived samples that were previously typified | Samples that were typified during the Cysticercosis Elimination Evaluation (15) | Archived samples that were previously typified |
| Year | 2004 | 2004 | 2009 | 2012 |
| Sample selection process | Samples were selected to generate a positive pool (P1) and a negative pool (N) | Available samples that had sufficient volume (≥30 mL of supernatant) | Systematic sampling among available samples (n = 48,648) | Available samples that had sufficient volume (≥30 mL of supernatant) |
| Positive samples | Confirmed diagnosis by microscopy, PCR, and/or gravid proglottids | coAg ELISA (PP ≥ 7.5) | coAg ELISA (positive/suspect, PP ≥ 14; weakly suspect, PP 7.5–14) | coAg ELISA (PP ≥ 7.5) |
| Negative samples | coAg ELISA (PP < 7.5) | coAg ELISA (PP < 7.5) | coAg ELISA (PP < 7.5) | coAg ELISA (PP < 7.5) |
| Selected study samples | Positive, 69; negative, 46 | Positive, 80; negative, 80 | Positive/suspect, 357b; weakly suspect, 261; negative, 737b | Positive, 308; negative, 56; T. saginata, 78 |
| Total no. of study samples | 115 | 160 | 1,355 | 442 |
Abbreviations and definitions: coAg ELISA, coproantigen enzyme-linked immunosorbent assay. Optical density (OD) values were read using a spectrophotometer (Molecular Devices Vmax) at 650 nm. Strong positive pool (P1) is the mean OD from a group of positive samples selected from participants with confirmed Taenia solium diagnosis. P1 was compared to historical P1s to ensure equivalence over time. Negative pool (N) is a group of randomly selected negative samples from regions of endemicity and regions of nonendemicity. Percentage of positivity (PP) is calculated as the OD of the sample/(OD of P1) × 100.
PCR-restriction enzyme analysis (PCR) was used for species-specific diagnosis when proglottids, scolexes, or visible parasite material was present in the stool sample (n = 51). Among the positive/suspect samples, 33 were confirmed as T. solium. One sample in the weakly suspect and 17 in the negative category were T. saginata.
Creation of sample pools for assay development in phase I.
In phase I, the field coAg ELISA was developed using positive and negative pools formed by samples stored in the repository in 2004. The positive pool was formed by selecting samples that were previously identified as positive for T. solium taeniasis using parasitological diagnosis. This was done using microscopy or PCR if proglottids or scolexes were collected in the stool sample. After selection, all samples were processed with the standard coAg ELISA protocol to construct a 16-point dilution curve. This curve was used to select the concentrations of the positive control (P1) and the intermediate control (P2). The concentration of P1 was the point of the curve with the greatest slope, while P2 was the lowest point where detection was achieved. The mean OD of the undiluted positive pool was calculated and compared to the mean OD of a historical referent. This was done to ensure that the new positive pool had the same absorbance as the previous one to maintain equivalence over time. In total, 69 stool samples were selected for the positive pool. The negative pool was formed by selecting 40 samples from areas of nonendemicity and 6 samples from areas of endemicity, all negative for Taenia spp. by microscopy or standard coAg ELISA. The remaining samples were selected and confirmed as negative using the standard coAg ELISA (PP of <7.5).
Standard coAg ELISA procedure.
Across all study phases, selected samples were processed using the coAg ELISA as described by Allan et al. (11) with modifications described by Zamora et al. (18). This study used RbATSIgG-8 (6.2 mg/mL) as the capture polyclonal antibody and GATSAWIgG-POD-400 (0.9 mg/mL) as the conjugate antibody. T. solium excretory/secretory antigens were produced in Lima, Peru, and used to produce rabbit anti-T. solium polyclonal antibodies as described by Allan and Craig (16). Polyclonal antibodies were sent to our collaborating investigators at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, USA, for purification and conjugation.
Phase I: field coAg test development.
Each modification to the standard coAg ELISA’s protocol was made systematically, meaning one condition was changed at a time while keeping all other conditions constant. In total, we developed the field coAg ELISA protocol in eight steps. For each step, we compared the OD of P1 from the modified coAg ELISA to that of the standard coAg ELISA considering the range of acceptability, which we defined as the mean OD ± 3 standard deviations (SD) (0.889 ≤ OD ≤ 1.562) per the standard coAg ELISA (11). We also considered the range of acceptability for P2, the negative pool (N), and the cutoff (mean from 6 negative controls ± 3 SD). Additionally, we calculated the signal-to-noise ratio (SNR) as a measure of dispersion between the positive and negative pools. The SNR was defined as the ratio of the mean OD of P1 to the mean OD of N.
We first evaluated three types of 96-well microtiter plates to identify the solid support that provided the highest SNR. Plates included Immulon 4HBX flat-bottom (Thermo Scientific, USA), Nunc Immuno (MG Scientific, USA), and MaxiSorp Immuno (Thermo Scientific, USA) plates.
Second, we evaluated three capture antibody binding buffers: 0.15 M PBS, carbonate-bicarbonate (pH 9.6), and sensitization buffer (pH 8.0). Each buffer was tested and incubated at 30, 60, and 120 min at agitation and ambient temperature. The binding buffer that provided the highest SNR was selected.
In the third step, the reagent storage temperature was modified. We evaluated capture and conjugate antibodies after storage at the standard temperature (−70°C) and a higher temperature more accessible in a field setting (−20°C). Each plate was sensitized with reagents with and without protease inhibitors. Half of each plate was sensitized with reagents stored at −70°C and the other half with reagents stored at −20°C. The stability of the stored antibodies was evaluated on the 4th and 7th days of the week for 6 weeks for repeatability. The ODs of P1, P2, N, and the cutoff were evaluated against the range of acceptability.
In the fourth step, we identified the initial concentrations of capture and conjugate antibodies using serial dilutions of the positive and negative pool with ortho-phenylenediamine (OPD) and 3,3′,5,5′-tetramethylbenzidine (TMB) developers (i.e., checkerboard titration). Dilutions of the capture and conjugate antibodies were selected based on the SNR and concentrations that provided optimal sensitivity for the test.
The fifth step evaluated the modification of the water type used for preparing buffers. Water types included the standard type 1 water (Milli-Q Academic system), type 3 water (Milli-RO 90 Millipore reverse osmosis system), and Aquafil/San Luis bottled water, which is readily available in local grocery and convenience stores. The plates using the three water types were run twice a week for six consecutive weeks for repeatability. The ODs of P1, P2, N, and the cutoff were evaluated to ensure values fell within the range of acceptability.
The sixth step evaluated the modification of the blocking agent. We first evaluated the SNR of Anchor skim milk powder (also commercially available) in combination with the nonionic detergent (Tween 20) at different concentrations (see Table S4 in the supplemental material). To select the optimal concentration of the blocking agent, we then compared SNR and ODs of P1, P2, N, and the cutoff for the different modified concentrations against the standard, which used inactivated fetal bovine serum (FBS).
In the seventh step, we evaluated the spontaneous separation of the supernatant without centrifugation among samples left in storage for 24 h. We compared these samples to those that used the standard process of centrifugation. Spearman’s rank correlation coefficient was used to compare the OD and PP values generated from the standard and modified protocols.
In the last development step, we identified the final concentrations of the capture and conjugate antibodies using serial dilutions with TMB. This step was done to fine-tune the concentrations identified in the fourth step. Dilutions of the capture and conjugate antibodies were selected based on the SNR and concentrations that provided optimal sensitivity for the test.
Phases II and III: field coAg ELISA performance evaluation.
The standard coAg ELISA and newly established field coAg ELISA procedures were processed in parallel for all selected samples in phases II and III of our study. We followed the procedure mentioned above for the standard coAg ELISA (11). For the field coAg ELISA, we carried the procedure out as follows: all buffers used throughout the process were prepared using commercially available bottled water (Aquafil/San Luis). Next, Immulon 4 HBX microtiter plates (Dynex) were coated with a 1:6,000 dilution of the capture antibody (RbATSIgG-8 [6.2 mg/mL]) at 100 μL/well in sensitization buffer (10 mL of sensitization buffer [pH 8.0] and 1.66 μL of the capture antibody). Plates were sealed, placed on an orbital shaker for 2 h, and then stored at 4°C overnight. Next, plates were washed 5 times with 100 μL of 0.1% Tween 20-PBS at approximately 22°C. Then, 100 μL of 0.1% Tween 20-PBS was dispensed into each well and plates were placed on an orbital shaker for 30 min and washed 5 more times. Once dried, plates were labeled to identify the wells corresponding to P1, P2, and N. Next, 50 μL of blocker made of 10% powdered milk solution (Anchor; Fonterra, New Zealand) in 0.3% Tween 20-PBS was dispensed in all wells, except for the negative controls. Then, 50 μL of stool supernatant sample was added to the wells in duplicate. Plates were then sealed, shaken, and stored for 24 h. Next, plates were placed on an orbital shaker for 1 h, removed, and then washed five times with 0.1% Tween 20-PBS. Afterward, a 1:4,000 dilution of the conjugate antibody (GATSAWIgG-POD-400 [0.9 mg/mL]) was prepared with a 5% powdered milk solution in 0.1% Tween 20-PBS and 100 μL of the dilution was dispensed into each well. Plates were incubated for 1 h on an orbital shaker. Then, plates were washed five times with 0.1% Tween 20-PBS and left to dry at room temperature. Finally, 100 μL of the substrate TMB (3,3′,5,5′-tetramethylbenzidine; Sureblue; KPL, USA) was added to each well. The plates were covered with aluminum foil and placed on an orbital shaker for 30 min.
The results from the standard and field coAg procedures were read using a spectrophotometer (SpectraMax) at 650 nm. The OD values from each test were interpreted quantitatively using the PP and compared. In phase III, the PP was further categorized into four levels to facilitate the interpretation of quantitative results: negative (PP < 7.5), weakly suspect (7.5 ≤ PP < 14), strongly suspect (14 ≤ PP < 40), and positive (PP ≥ 40).
Phase IV: colorimetric scale development and evaluation.
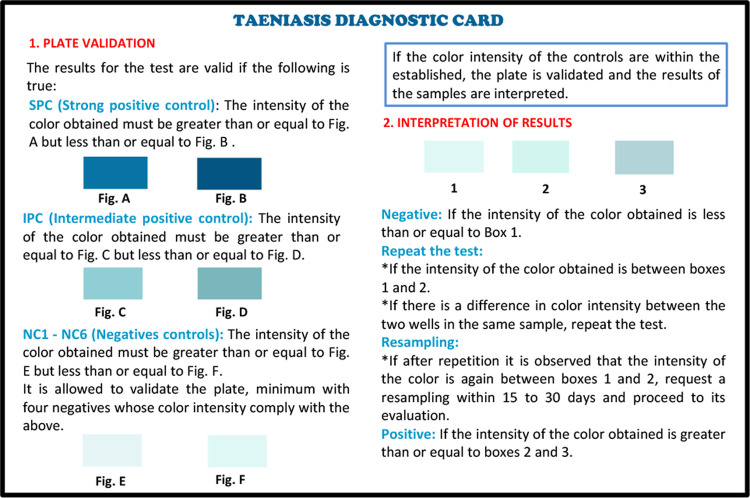
Following phase III, a colorimetric scale card was developed to allow for the qualitative interpretation of sample results according to color intensity (Fig. 1). The card presents the user with steps to assess plate validation and interpret results. For plate validation, the card displays the minimal and maximal expected color intensities for P1 and P2 (boxes A and B). Plates were considered valid if their color intensity fell within the limits of P1 or P2. For the result interpretation of valid plates, the card presents the colorimetric scale as a gradient of colors (boxes 1 to 3) that correlate with the four PP categories described above. The scale was used to interpret results as negative (well color less intense than box 1); weakly suspect—repeat the test (well-color intensity between boxes 1 and 2); strongly suspect—request and process a new sample (well-color intensity between boxes 2 and 3); and positive—proceed with anthelmintic treatment for a T. solium taeniasis infection (well color more intense than box 3).
FIG 1.
The colorimetric scale used for the interpretation of field coAg ELISA results. The original Spanish version and the back of the card are presented in the supplemental material.
In evaluating this card, samples were processed using the field coAg ELISA procedures. The results were read qualitatively by two independent readers and quantitatively by a spectrophotometer (SpectraMax) at 650 nm.
Statistical analysis.
For the small-scale evaluation (phase II), the performance characteristics (sensitivity, specificity, negative predictive value, and positive predictive value) of the coAg ELISAs were evaluated as previously described (11, 19). The cutoff point was assessed using the receiver operating characteristic (ROC) curve of the PP. Additionally, we used Pearson’s correlation to examine the agreement between PP values measured by the standard and field coAg ELISA procedures.
For the large-scale evaluation (phase III), Pearson’s correlation was also used to assess the consistency of PP values generated by each test. At this stage, we analyzed the results of the four antigen-level categories based on PP values of the standard and field coAg ELISAs. We assessed the correlation between values overall and within each category. Then, we used the weighted Cohen kappa statistic to measure the agreement of test results across the four categories. Additionally, we conducted a descriptive analysis of coAg ELISA results for samples with confirmed species diagnosis of T. solium and T. saginata.
For the colorimetric scale evaluation (phase IV), interrater reliability (IRR) was used to determine the agreement between the results obtained from two independent readers, as well as the agreement between results from each reader and the spectrophotometer. The IRR was evaluated using Cohen’s kappa coefficient (20). All statistical analysis was conducted using XLSTAT 2000.3.1.7 Trial and RStudio software (21).
Data availability.
Data used for evaluating the coAg ELISA is available in an open-access repository https://doi.org/10.7910/DVN/P1GCQS.
RESULTS
Phase I: initial test development.
The results used to inform the field coAg ELISA procedures are presented in the supplemental material. In brief, the Immulon 4 HBX microtiter plate, sensitization buffer (pH 8.0), and a 1:4,000 dilution of capture antibodies with a 1:6,000 dilution of conjugate antibodies were selected as they provided optimal conditions compared to other options. In the field modification steps, the mean ODs of the positive and negative pools were within the range of acceptability when we stored reagents at −20°C and when we used Aquafil/San Luis as the water type and Anchor skim milk powder as the blocking agent and replaced centrifugation with spontaneous separation of the supernatant. Table 2 summarizes the optimized and modified features of the field coAg ELISA compared to those used in the standard coAg ELISA.
TABLE 2.
Optimal features and modifications for the field coproantigen (coAg) ELISA compared to the standard
| Feature of the procedure | Field coAg ELISA | Standard coAg ELISA |
|---|---|---|
| Optimizations | ||
| Plate | Immulon 4 HBX microtiter plates (Dynex) | Immulon 4 HBX microtiter plates (Dynex) |
| Capture antibody fixation buffer | Sensitization buffer (pH 8.0) | Bicarbonate coating buffer (pH 9.6) |
| Concns of capture antibodies | 1:4,000 dilution | 1:4,000 dilution |
| Concns of conjugate antibodies | 1:6,000 dilution | 1:4,000 dilution |
| Field modifications | ||
| Reagent storage temp | −20°C | −70°C |
| Water type | Aquafil/San Luis bottled water | Type 1 water (Milli-Q Academic System) |
| Blocking agent | Anchor skim milk powder | Inactivated fetal bovine serum (heat-inactivated BS; Gibco) |
| Centrifuge use | Spontaneous separation of the supernatant at 24 h | Centrifuged at 3,000 × g for 30 min at 25°C |
| Spectrophotometer usea | Results were read qualitatively using a colorimetric scale | Results were read quantitatively using a spectrophotometer at 650 nm |
Evaluated in phase IV.
Phase II: small-scale performance assessment.
Following the initial development of the field coAg ELISA, 160 known positive and negative samples were processed using both standard and field coAg ELISAs. The PP values read from both procedures had a strong positive correlation (P < 0.001, Fig. 2). Further, the standard and field tests had similar performance characteristics (Table 3).
FIG 2.

Correlation between percentage of positivity (PP) using the field coAg ELISA and that using the standard coAg ELISA (n = 160).
TABLE 3.
Performance characteristics per percentage of positivity (PP) of the standard and field coproantigen (coAg) ELISAs among 160 known positive and negative stool samples collected in northern Peru, 2004a
| Characteristic | Standard |
Field |
||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Sensitivity | 96.3 | 89.4, 99.2 | 93.8 | 86.0, 97.9 |
| Specificity | 97.5 | 91.3, 100 | 96.3 | 89.4, 99.2 |
| PPV | 97.5 | 91.2, 100 | 96.2 | 89.2, 99.2 |
| NPV | 96.3 | 89.6, 99.2 | 93.9 | 86.3, 98.0 |
CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Phase III: large-scale performance assessment.
Overall, a high degree of positive correlation (Pearson’s, r = 0.98) was observed between the PP values in the 1,355 samples processed using the field and standard coAg ELISA procedures (Table 4). Further, across the four antigen level categories, there was almost perfect agreement between the two tests (weighted Kappa statistic = 0.86). Within each antigen level category, the correlation between tests remained very strong for the 104 samples in the positive category (Pearson’s, r = 0.97) and strong in other PP range categories, with the lowest correlation measured between PP values among the 261 samples in the weakly suspect category (Pearson’s, r = 0.65).
TABLE 4.
Agreement and correlation between the field and standard coAg ELISA results classified into four categories of antigen levels for 1,355 known positive and negative stool samples collected in northern Peru, 2009a
| Field coAg ELISA | Standard coAg ELISA |
Total | |||
|---|---|---|---|---|---|
| PP < 7.5 | 7.5 ≤ PP < 14 | 14 ≤ PP < 40 | PP ≥ 40 | ||
| PP < 7.5 | 720 | 81 | 0 | 0 | 801 |
| 7.5 ≤ PP < 14 | 17 | 168 | 60 | 0 | 245 |
| 14 ≤ PP < 40 | 0 | 12 | 186 | 13 | 211 |
| PP ≥40 | 0 | 0 | 7 | 91 | 98 |
| Total | 737 | 261 | 253 | 104 | 1,355 |
| Pearson correlation (r) | 0.75 | 0.65 | 0.85 | 0.97 | 0.98 |
| Weighted kappa | 0.86 | ||||
Percentage of positivity (PP): [OD of the sample]/[OD of a known positive group (P1)] × 100.
A total of 51 samples had confirmed species diagnosis: 18 (1.3%) had T. saginata and 33 (2.4%) had T. solium. Among the 18 confirmed for T. saginata, 17 had a PP value of <7.5 and one had a PP between 7.5 and 14. Among the 33 confirmed for T. solium, 31 had a PP of ≥40, one had a PP between 14 and 40, and one had a PP of <7.5 by the standard and field coAg ELISA procedures. In both the standard and field coAg assays, 94% (n = 17/18) of T. saginata samples were negative (PP value of <7.5), and 94% (n = 31/33) of T. solium samples were strongly positive (PP of ≥40).
Phase IV: colorimetric scale evaluation.
Table 5 displays the agreement among the 442 samples used to evaluate the IRR of test results using the colorimetric scale displayed on the taeniasis diagnostic card. An almost perfect agreement was observed between the two readers (kappa = 0.975) and each of the readers and the spectrophotometer (kappa = 0.981 and 0.973). Overall, the first reader misclassified 11 samples against the spectrophotometer, while the second reader misclassified 16. Most of the misclassification was in the two “suspect” categories.
TABLE 5.
Interrater agreement between independent readers using the colorimetric scale and between each reader and the spectrophotometer for 442 known positive and negative samples collected in northern Peru, 2012
| Agreement | Cohen’s kappa |
|---|---|
| Between reader 1 and reader 2 | 0.975 |
| Between spectrometer and reader 1 | 0.981 |
| Between spectrometer and reader 2 | 0.973 |
DISCUSSION
The identification of T. solium taeniasis carriers is a fundamental pillar in the control and elimination of human and porcine cysticercosis. This study developed a simple, field-applicable coAg ELISA that had performance comparable to the standard coAg ELISA in a controlled laboratory setting. These modifications were focused on using low-cost and widely available materials and limiting the use of expensive equipment. This field coAg ELISA is sensitive, practical, useful, low in cost, and capable of being used in the routine diagnosis of taeniasis in areas where the disease is endemic.
Developing the field test aimed to overcome resource barriers related to materials and equipment. Both the water type and the blocking agent were replaced in the field coAg ELISA with low-cost and widely accessible materials. San Luis bottled water is widely available in Peru at US$0.40 per 500-mL bottle, and Anchor skim milk powder is available internationally and in Peru for US$5.00 per kilogram. Commercially bottled water was used in place of ultrapurified water by reverse osmosis (Milli-Q RO and Milli Q Team). Ultrapure water is characterized by a resistivity of 18.2 MΩ/cm at 25°C. Further, it undergoes filtration to remove ionic contamination and impurities that improve the test’s performance. While the substitution of San Luis bottled water demonstrated a slight decrease in OD, the difference was negligible for the assay’s overall performance. As for the blocking agent, the proteins in FBS, when combined with nonionic detergents (e.g., Tween 20), are effective at blocking unoccupied hydrophobic sites in the microtiter plate in the standard coAg ELISA. This step ensures that nonspecific binding is minimized (22). Anchor, a skim milk powder, was used as a nonfat dry milk blocking agent in the field coAg ELISA. In our evaluation, the combination of 5% milk powder solution in 0.3% Tween 20-PBS for the sample blocker and 5% milk powder solution in 0.1% Tween 20-PBS for the conjugate blocker demonstrated performance similar to the standard procedures which used FBS. Using commercially purchased bottled water and skim milk powder instead of the standard test’s materials overcame cost and logistical barriers without compromising the performance of the coAg ELISA.
For barriers related to the availability or procurement of laboratory equipment, the procedures using an ultralow-temperature freezer, centrifuge, and spectrophotometer were modified and evaluated. The modification of the reagent storage temperature from −70°C to −20°C demonstrated no difference in the stability of the reagents until day 42. This finding was consistent with or without the addition of protease inhibitors. This modification was possible due to the chemical structure of the reagents (i.e., polyclonal antibodies), which are less susceptible to degradation when stored at concentrations of ≥1 mg/mL. However, we cannot rule out that a combination of storage duration and temperature can affect and denature antibodies, thus decreasing the absorption of the capture antibodies to the plate (22–24). Centrifuging samples in the standard coAg ELISA protocol separates the solid phase and liquid (supernatant) by applying centripetal force. Our study demonstrated that spontaneous sedimentation after 24 h produced similar results. Finally, spectrophotometers are not widely available in laboratories due to their high procurement and maintenance costs. However, they are required to read OD values and interpret test results for the standard coAg ELISA. For the field coAg ELISA, a colorimetric scale was developed with four categories (negative, suspect-retest, suspect-resample, and positive) to visually interpret the test results based on the color intensity of the wells. In our evaluation, there was an almost perfect agreement between the colorimetric scale and spectrophotometer results. Further, there was an almost perfect agreement between the two independent readers, suggesting reliable results.
Other modifications included the selection of ELISA plates and sensitization buffer. The Immulon 4 HBX flat-bottom microtiter plate was selected because it had the highest SNR. The same plate was used in methods described by Guezala et al. (12). Notably, this plate has a high surface area for protein uptake (400 to 600 ng IgG/cm2) and an affinity for hydrophilic groups capable of establishing hydrogen bonds, and it allows for their passive adsorption (23). They are designed for high binding of IgG molecules and immobilizing proteins and peptides that do not bind to passive surfaces. Its polystyrene material has excellent optical quality, facilitating stable links and mechanical hardness. Further, it forms a strong covalent bond of biomolecules (e.g., proteins, peptides, and amino acids) with free -NH2 or -SH groups (25). The selection of a neutral sensitization buffer with a pH of 8.0 was done experimentally and was unique to our study. However, the selection of a neutral sensitization buffer was consistent with buffers selected for previous coAg ELISAs based on prior knowledge that they have higher adsorption of the capture antibody (11, 12, 25).
The sensitivity of the standard coAg ELISA was 96.3%, and the specificity was 97.5%, while those of the field test were 93.8% and 96.3%, respectively, per the PP ROC curve. An area under the curve of 99.3% for the field coAg ELISA indicated an almost perfect test performance (26). From the large-scale evaluation, the two tests had a strong positive correlation within each diagnostic category and almost perfect agreement across the four categories. These results suggest considerable flexibility in choosing the positive cutoff point based on the purpose of the test. As a diagnostic test, a higher cutoff point will ensure greater specificity. As a screening test, a lower cutoff point will ensure higher sensitivity to capture most taeniasis cases in the population and avoid false negatives (27). Further, only 6% (n = 1/18) of the T. saginata confirmed samples from the large-scale evaluation had a PP value of >7.5. This finding suggested some, but minimal, cross-reactivity with other Taenia species at lower cutoff points. These findings were identical for the two tests.
Limitations.
Several limitations to the field coAg ELISA remain. First, this study evaluated the field coAg ELISA only under laboratory conditions, using known positive and negative stool samples from participants in Peru. Further data on test assessment under field conditions are required to validate these findings, including assay testing in other countries where T. solium is endemic. Second, the field coAg ELISA relies on polyclonal antibodies that vary by batch. As with the standard coAg ELISA, this factor limits the consistency of the test across batches of reagents, as described in another study (28). Third, the field test relies on materials developed for commercial consumption that have the potential to alter the field coAg ELISA's performance (e.g., lot-to-lot variability in nonfat dry milk) (25). Finally, while the field coAg ELISA overcomes barriers to performing the test in a more rural laboratory, it still requires basic laboratory infrastructure for processing. Minor delays can still occur depending on the distance between communities and the laboratory. For community screening and field studies, this will delay the identification of participants with taeniasis, the delivery of treatment, and the assessment of treatment successes. Ultimately, a rapid, point-of-care test is needed to overcome these logistical barriers and improve control strategies for taeniasis (29).
Conclusions.
The newly developed field coAg ELISA demonstrated performance comparable to the standard test in a controlled laboratory setting. Importantly, the modifications provide an inexpensive alternative that is more accessible in resource-limited settings. While further test validation is needed, the field coAg ELISA shows great potential for improving the detection of T. solium tapeworm infections in communities where the disease is endemic.
ACKNOWLEDGMENTS
This research was supported through the Fogarty International Center (training grant D43TW001140) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (grant R01A1141554).
Footnotes
[This article was published on 27 June 2023 with the Acknowledgments section missing and with a CC BY 4.0 copyright line (“Copyright © 2023 Castillo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.”). The authors elected to remove open access for the article after publication, necessitating replacement of the original copyright line, and this change was made and the Acknowledgments section was added on 5 July 2023.]
Supplemental material is available online only.
Contributor Information
Melissa T. Wardle, Email: wardlem@ohsu.edu.
Bobbi S. Pritt, Mayo Clinic Minnesota
REFERENCES
- 1.World Health Organization. 2022. Taeniasis/cysticercosis. https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis. Retrieved 7 November 2021.
- 2.Gonzales I, Rivera JT, Garcia HH, Cysticercosis Working Group in Peru . 2016. Pathogenesis of Taenia solium taeniasis and cysticercosis. Parasite Immunol 38:136–146. doi: 10.1111/pim.12307. [DOI] [PubMed] [Google Scholar]
- 3.Garcia HH, Rodriguez S, Friedland JS, for The Cysticercosis Working Group in Peru . 2014. Immunology of Taenia solium taeniasis and human cysticercosis. Parasite Immunol 36:388–396. doi: 10.1111/pim.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwape KE, Gabriël S. 2014. The parasitological, immunological, and molecular diagnosis of human taeniasis with special emphasis on Taenia solium taeniasis. Curr Trop Med Rep 1:173–180. doi: 10.1007/s40475-014-0028-5. [DOI] [Google Scholar]
- 5.Sarti E, Flisser A, Schantz PM, Gleizer M, Loya M, Plancarte A, Avila G, Allan J, Craig P, Bronfman M, Wijeyaratne P. 1997. Development and evaluation of a health education intervention against Taenia solium in a rural community in Mexico. Am J Trop Med Hyg 56:127–132. doi: 10.4269/ajtmh.1997.56.127. [DOI] [PubMed] [Google Scholar]
- 6.Escalante AH, Huamanchay CO, Davelois AK. 2001. La inmunocromatografía para el diagnóstico de la infección por Taenia solium en Mesocricetus auratus mediante la detección de coproantígenos. Rev Peru Med Exp Salud Publica 18:57–62. [Google Scholar]
- 7.Garcia HH, Gonzalez AE, Gilman RH, for the Cysticercosis Working Group in Peru . 2020. Taenia solium cysticercosis and its impact in neurological disease. Clin Microbiol Rev 33:e00085-19. doi: 10.1128/CMR.00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praet N, Verweij JJ, Mwape KE, Phiri IK, Muma JB, Zulu G, van Lieshout L, Rodriguez-Hidalgo R, Benitez-Ortiz W, Dorny P, Gabriël S. 2013. Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis. Trop Med Int Health 18:608–614. doi: 10.1111/tmi.12089. [DOI] [PubMed] [Google Scholar]
- 9.Allan JC, Velasquez-Tohom M, Torres-Alvarez R, Yurrita P, Garcia-Noval J. 1996. Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg 54:352–356. doi: 10.4269/ajtmh.1996.54.352. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins PP, Allan JC, Verastegui M, Acosta M, Eason AG, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. 1999. Development of a serologic assay to detect Taenia solium taeniasis. Am J Trop Med Hyg 60:199–204. doi: 10.4269/ajtmh.1999.60.199. [DOI] [PubMed] [Google Scholar]
- 11.Allan JC, Avila G, Noval JG, Flisser A, Craig PS. 1990. Immunodiagnosis of taeniasis by coproantigen detection. Parasitology 101:473–477. doi: 10.1017/S0031182000060686. [DOI] [PubMed] [Google Scholar]
- 12.Guezala M-C, Rodriguez S, Zamora H, Garcia HH, Gonzalez AE, Tembo A, Allan JC, Craig PS. 2009. Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am J Trop Med Hyg 81:433–437. doi: 10.4269/ajtmh.2009.81.433. [DOI] [PubMed] [Google Scholar]
- 13.O’Neal SE, Moyano LM, Ayvar V, Gonzalvez G, Diaz A, Rodriguez S, Wilkins PP, Tsang VCW, Gilman RH, Garcia HH, Gonzalez AE, The Cysticercosis Working Group in Peru . 2012. Geographic correlation between tapeworm carriers and heavily infected cysticercotic pigs. PLoS Negl Trop Dis 6:e1953. doi: 10.1371/journal.pntd.0001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, Gavidia C, Tsang VCW, Falcon N, Lescano AG, Moulton LH, Bernal T, Tovar M, Cysticercosis Working Group in Perú . 2003. Hyperendemic human and porcine Taenia solium infection in Perú. Am J Trop Med Hyg 68:268–275. doi: 10.4269/ajtmh.2003.68.268. [DOI] [PubMed] [Google Scholar]
- 15.Garcia HH, Gonzalez AE, Tsang VCW, O’Neal SE, Llanos-Zavalaga F, Gonzalvez G, Romero J, Rodriguez S, Moyano LM, Ayvar V, Diaz A, Hightower A, Craig PS, Lightowlers MW, Gauci CG, Leontsini E, Gilman RH, Cysticercosis Working Group in Peru . 2016. Elimination of Taenia solium transmission in northern Peru. N Engl J Med 374:2335–2344. doi: 10.1056/NEJMoa1515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allan JC, Craig PS. 2006. Coproantigens in taeniasis and echinococcosis. Parasitol Int 55(Suppl):S75–S80. doi: 10.1016/j.parint.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, Ruiz M, Garcia HH, Gonzalez AE. 2000. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. J Clin Microbiol 38:133–137. doi: 10.1128/JCM.38.1.133-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamora H, Rodriguez S, Garcia HH, Allan JC, Vasquez Y, Gilman RH, Gonzales AE, Noh J, Patthabi S, Tsang VCW, The Cysticercosis Working Group in Peru . 2004. Sensitivity and specificity of ELISA coproantigen detection for the diagnosis of intestinal Taenia solium taeniasis. Am J Trop Med Hyg 71(4 Suppl):42. [Google Scholar]
- 19.Fisterra. Retrieved 24 June 2021. Pruebas diagnósticas: sensibilidad y especificidad. https://www.fisterra.com/formacion/metodologia-investigacion/pruebas-diagnosticas-sensibilidad-especificidad/.
- 20.Cortés-Reyes É, Rubio-Romero JA, Gaitán-Duarte H. 2010. Métodos estadísticos de evaluación de la concordancia y la reproducibilidad de pruebas diagnósticas. Rev Colomb Obstet Ginecol 61:247–255. doi: 10.18597/rcog.271. [DOI] [Google Scholar]
- 21.R Core Team. 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 22.KPL, Inc. 2013. Technical guide for ELISA. KPL, Gaithersburg, MD. [Google Scholar]
- 23.Ochoa R, Martínez JC, Ferriol X, Estrada E, García AM, Blanco R, Sotolongo F. 2000. Guía para la estandarización de técnicas inmunoenzimáticas en ensayos de vacunas. Vaccimonitor 9:13–18. [Google Scholar]
- 24.Crowther JR. 2000. The ELISA guidebook. Humana Press, Totowa, NJ. http://link.springer.com/10.1385/1592590497. Retrieved 24 June 2021.
- 25.Thermo Fisher Scientific. 2010. ELISA technical guide and protocols. Tech Tip # 65. Thermo Fisher Scientific, Rockford, IL. [Google Scholar]
- 26.López de Ullibarri Galparsoro I, Píta Fernández S. 1998. Curvas ROC. Cad Aten Primaria 5:229–235. [Google Scholar]
- 27.Pita Fernández S, Pértegas Díaz S. 2003. Pruebas diagnosticas. Cad Aten Primaria 10:120–124. [Google Scholar]
- 28.Ramiandrasoa NS, Ravoniarimbinina P, Solofoniaina AR, Rakotomanga IPA, Andrianarisoa SH, Molia S, Labouche A-M, Fahrion AS, Donadeu M, Abela-Ridder B, Rajaonatahina D. 2020. Impact of a 3-year mass drug administration pilot project for taeniasis control in Madagascar. PLoS Negl Trop Dis 14:e0008653. doi: 10.1371/journal.pntd.0008653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donadeu M, Fahrion AS, Olliaro PL, Abela-Ridder B. 2017. Target product profiles for the diagnosis of Taenia solium taeniasis, neurocysticercosis and porcine cysticercosis. PLoS Negl Trop Dis 11:e0005875. doi: 10.1371/journal.pntd.0005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download jcm.00282-23-s0001.docx, DOCX file, 1.1 MB (1.1MB, docx)
Data Availability Statement
Data used for evaluating the coAg ELISA is available in an open-access repository https://doi.org/10.7910/DVN/P1GCQS.