Change history
5/22/2023
An Editorial Expression of Concern to this paper has been published: 10.1007/s12672-023-00668-5
Abstract
Tetraiodothyroacetic acid (tetrac) and its nanoparticle formulation (Tetrac NP) act at an integrin cell surface receptor to inhibit tumor cell proliferation and tumor-related angiogenesis. Human pancreatic cancer cell (PANC-1 and MPanc96) xenografts were established in nude mice, and the effects of tetrac versus Tetrac NP on tumor growth and tumor angiogenesis were determined. The in vitro effects of tetrac and Tetrac NP were also determined by reverse transcription polymerase chain reaction or immunoblot on gene expression or gene products relevant to cell cycle arrest, apoptosis, or angiogenesis. Tetrac and Tetrac NP reduced both PANC-1 tumor mass by 45–55 % and PANC-1 tumor hemoglobin content, a marker of angiogenesis, by 50–60 % (*P < 0.05) in treated groups vs. controls by treatment day 15. Comparable results were obtained with tetrac and Tetrac NP in suppressing tumor growth and tumor angiogenesis in MPanc96 xenografts. In vitro studies showed that tetrac and Tetrac NP caused accumulation of pro-apoptotic protein BcLx-s. Tetrac NP was more effective than tetrac in increasing cellular abundance of mRNAs of pro-apoptotic p53 and p21 and anti-angiogenesis thrombospondin 1 protein in PANC-1 and MPanc96 cancer cell lines. Tetrac NP noticeably decreased expression of EGFR and of anti-apoptosis gene XIAP; tetrac did not affect EGFR and increased XIAP mRNA in both MPanc96 and PANC-1. In conclusion, tetrac or Tetrac NP effectively inhibited human pancreatic xenograft growth and tumor angiogenesis via a plasma membrane receptor that downstream modulated cellular abundance of proteins or mRNAs relevant to apoptosis and angiogenesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-013-0137-y) contains supplementary material, which is available to authorized users.
Keywords: Epidermal Growth Factor Receptor, Thyroid Hormone, Human Pancreatic Cancer Cell, Veteran Affair Medical Center, Epidermal Growth Factor Receptor mRNA
Introduction
Human pancreatic carcinoma is an aggressive tumor with generally unsatisfactory 5-year survival responses to surgery, where feasible, or to chemotherapy or chemoradiotherapy [1–3]. There is general agreement that novel chemotherapeutic strategies are needed for this tumor [4, 5], preferably targeting multiple tumor cell survival pathways [6].
Integrin αvβ3 is a structural protein of the plasma membrane that is expressed in increased amounts by cancer cells [7] and rapidly dividing blood vessel cells [8, 9]. Thus, this heterodimer is an attractive target in cancer strategy [10–12], particularly if there is refinement of targeting to discrete domains of the protein that do not involve desirable functions of the αvβ3 that is present on osteoclasts [13] or that is expressed in small quantities on non-malignant cells and platelets [14]. We have described a cell surface receptor for thyroid hormone (l-thyroxine, T4; 3,5,3′-triiodo-l-thyronine, T3) on integrin αvβ3 [15] that mediates actions of the hormone on tumor [16–18] and blood vessel [19–21] cell proliferation. The hormone receptor domain is complex and can differentially transduce cell surface signals via extracellular signal-regulated kinase 1/2 (ERK1/2) or by phosphatidylinositol 3-kinase [22] pathways. Tetraiodothyroacetic acid (tetrac) is a naturally occurring thyroid hormone analogue that blocks binding of T4 and T3 to their receptor on αvβ3 [15] and inhibits the proliferative actions of these agonist thyroid hormones [19, 23].
In the absence of thyroid hormone, however, tetrac can block the pro-angiogenic activities of VEGF and basic fibroblast growth factor (bFGF) [24]. Thus, tetrac has discrete effects of its own at the integrin receptor that transcend inhibition of actions of agonist thyroid hormone analogues. In addition, tetrac has distinctive actions on gene transcription in tumor cells that are initiated nongenomically at the cell surface and do not require the presence of T4 or T3 [25].
Unmodified tetrac gains access to the cell interior, however, where it has low-grade thyromimetic activity [26]. This is undesirable in the setting of any clinical uses of the molecule that may be long-term and may require high doses of the agent. We have reformulated tetrac as a poly[lactic-co-glycolic acid] (PLGA) nanoparticle (NP) that we have shown does not gain access to the interior of the cell [27, 28] and whose biologic activity is thus restricted to the receptor on integrin αvβ3 for thyroid hormone and its analogues. Administered systemically, nanoparticle tetrac (Tetrac NP) has recently been shown to be effective against xenografts of human medullary thyroid carcinoma [27] and renal carcinoma [28]. In the present studies, we have determined the efficacy of Tetrac NP and tetrac against human pancreatic carcinoma xenografts in the nude mouse. Previous in vitro studies have shown that the NP formulation is up to tenfold more potent than unmodified tetrac (Table 1 of [29]), and this observation determined the dosing formula for the two compounds in the present xenograft studies.
Materials and Methods
Materials
Unmodified tetrac, Drabkin's reagent, and hemoglobin standard were obtained from Sigma Aldrich (St. Louis, MO, USA). Nanoparticulate tetrac (Tetrac NP) was synthesized at the Albany College of Pharmacy and Health Sciences by our previously published methods [27] from epoxy-activated tetrac and poly[lactic-co-glycolic acid] (PLGA) and scaled up at Azopharma (Miramar, FL, USA). Monoclonal antibodies to BcLx, X-linked inhibitor of apoptosis protein (XIAP), thrombospondin 1 (THBS1), and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit anti-mouse IgG was a product of Dako Corp. (Carpinteria, CA, USA).
Cell Preparation for Xenografts
Two human pancreatic carcinoma cell lines (PANC-1 and MPanc96) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured as recommended by the ATCC in DMEM (Invitrogen, Grand Island, NY, USA) supplemented with 10 % FBS and penicillin/streptomycin (1 %). Cells were grown to sub-confluence in a 5 % CO2/air atmosphere at 37 °C and then released from the culture vessel by treatment with 0.25 % (w/v) trypsin/EDTA. Cells were then washed with culture medium, suspended in DMEM that was free of both phenol red and FBS, and counted. These cells were then xenografted as described below.
Cell Implantation in Nude Mice
Female NCr mice (Taconic Farms, Hudson, NY, USA) were obtained at age 5–6 weeks (20 g body weight), maintained under specific pathogen-free conditions, and allowed to acclimate for 5 days prior to experimental treatments. Mice were housed four animals per cage under conditions of a 12-h light/dark cycle, 20–24 °C, and 60–70 % humidity. Food and water were provided ad libitum. Animals were supported and xenograft experiments conducted in the animal facility of the Veterans Affairs Medical Center (VAMC), Albany, NY, USA. The animal protocol was approved by the VAMC Institutional Animal Care and Use Committee (IACUC).
For cell implantations, Matrigel® (BD Biosciences, San Jose, CA, USA) was thawed at 4 °C overnight and then placed on ice. Tumor cells in exponential growth phase were harvested as described above with trypsin/EDTA and suspended in medium. Approximately 2 × 106 cells in 100 μL of medium were mixed with the same volume of Matrigel® and injected subcutaneously into the left and right flanks of each animal.
Treatment of Animals with Unmodified Tetrac or Tetrac NP
Tumors were measured daily or every other day with calipers and tumor volumes estimated by a standard formula (W × L 2/2), where W = width and L = length. The tumor volumes reached 250 mm3 by 15 days, at which time, PANC-1 animals were randomized into three groups (n = 6 animals/group): control (solvent only), unmodified tetrac (10 mg/kg body weight i.p. daily) and Tetrac NP (1 mg/kg i.p. daily) and treatment initiated. The mice were weighed daily or every other day. Mice with MPanc96 cancer cell xenografts were treated with tetrac or Tetrac NP at 1 mg/kg/day, s.c.
Once tumors were established, tetrac, Tetrac NP, or a control vehicle was administered daily for 15 days. Animals were then sacrificed humanely in a CO2 chamber, and tumor masses were excised and weighed. Tumor mass hemoglobin content was measured to estimate vascularity. For this purpose, each tumor was placed in a 0.5-mL tube with double-distilled H2O and homogenized for 5–10 min. Samples were centrifuged (4,000×g) × 10 min and supernatants collected. Fifty microliters of supernatant was mixed with 50 μL Drabkin's reagent and incubated at room temperature for 15–30 min. The mixture was then placed in a 96-well plate and absorbance measured at 540 nm with a Microplate Manager ELISA reader. Hemoglobin concentration (milligrams per milliliter) was estimated from a standard curve.
Confocal Microscopy of Fluorescently Labeled Tetrac and Tetrac NP in PANC-1 Cells
Approximately 50,000 cells in 500-μL medium were placed in each well of a 4-well Chamber Slide System (Nalge Nunc International, Naperville, IL, USA). The slides were incubated for 24 h at 37 °C in 5 % CO2/air, as previously described [27]. Cells were then treated with 20 μL of either Cy3-tagged unmodified tetrac or Cy3-tagged Tetrac NP for 2 h at 37 °C, after which the plates were washed several times with PBS, fixed in 1 % formaldehyde, and mounted (Vectashield, Vector Laboratories, Inc., Burlingame, CA, USA). Confocal images of slides were obtained with a Leica TCS SP5 confocal microscope and a ×63 objective (NA = 1.3 glycerol immersion). For excitation, a 405-nm laser was used, and emission was detected between 565 and 688 nm.
Studies of Cultured Pancreatic Cell Growth In Vitro
PANC-1 and MPanc96 cancer cells were established as recommended by ATCC in DMEM supplemented with 10 % fetal calf serum. Cells were cultured as previously described for other cell lines [23, 30], except that the medium contained 10 % FBS throughout the course of each study. Media were replenished daily, including the addition of unmodified tetrac or Tetrac NP. Aliquots of media were obtained at 3, 6, and 8 days for counts of viable cells (trypan blue exclusion) by a Countess™ Automated Cell Counter (Invitrogen).
Immunoblotting
Extracts of cytosolic proteins were obtained from control and treated cells, after which the total protein content was quantitated and proteins resolved on discontinuous PAGE. Proteins were then electroblotted to nitrocellulose membranes (Millipore, Bedford, MA, USA), as previously described [31–33]. The membranes were treated with 5 % milk in Tris-buffered saline containing 0.1 % Tween, and incubated overnight with monoclonal anti-BcLx, anti-XIAP, or anti-THBS1. Primary antibody incubation was followed by treatment with the secondary rabbit anti-mouse IgG antibody. Immunoblots of β-actin were also prepared to control for equalization of sample proteins. Results presented reflect three similar western blot experiments.
RT-PCR
Total RNA was isolated as described previously [25, 27]. First-strand cDNA templates were amplified for mRNAs by polymerase chain reaction (PCR), using a hot start (Ampliwax, Perkin Elmer, Waltham, MA, USA). Primer sequences (Invitrogen) were GAPDH [5′-AAGGTCATCCCTGAGCTGAACG-3′ (forward) and 5′-GGGTGTCGCTGTTGAAGTCAGA-3′ (reverse)], p53 [5′-TACAAGCAGTCACAGCAGATGACGGAGGTT-3′ (forward) and 5′-CTGAGACTAAGGCAGAAGAT-3′ (reverse)], p21 [5′-TACCACTCCAAACGCCGGCT-3′ (forward) and 5′-CTGAGACTAAGGCAGAAGAT-3′ (reverse)], and epidermal growth factor receptor (EGFR) [5′-CTGCCAAGGCACAAGTAACA-3′ (forward) and 5′-ATTGGGACAGCTTGGATCAC-3′ (reverse)].
Integrin αv and β3: integrin αv [5′-TGGGATTGTGGAAGGAG-3′ (forward) and 5′-AAATCCCTGTCCATCAGCAT-3′ (reverse)] and integrin β3 [5′-TGCCTCAACAATGAGGTCATCCCT-3′ (forward) and 5′-AGACACATTGACCACAGAGGCACT-3′ (reverse)]. The PCR cycle was an initial step at 95 °C for 3 min, followed by 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min, then 25 cycles; the final cycle was 72 °C for 8 min. PCR products were separated by electrophoresis on 2 % agarose gels that contained 0.2 μg ethidium bromide per milliliter. Gels were visualized under UV light and photographed with Versadoc (Bio-Rad). Photographs were scanned under direct light for quantitation and documentation. Results from PCR products of specific genes were normalized to the GAPDH signal.
Statistical Analysis
Analysis of the in vivo study results was by one-way ANOVA using StatView software (Adept Scientific, Acton, MA, USA). The mean ± SD from each experimental group was compared with its respective control, and statistical significance was defined as P < 0.05. For the in vitro studies, the unpaired t test was used for analysis.
Results
Cellular Distribution of Unmodified Tetrac and Tetrac NP in PANC-1
Studied by confocal microscopy of PANC-1 cells in vitro, Cy3-labeled unmodified tetrac was apparent in both cytoplasm and nuclei of cells; in contrast, Cy3-labeled Tetrac NP remained on the cell surface (Supplemental Figure 1), and no fluorescence clearly appeared in either cytoplasm or the nuclear compartment. Confocal microscopy was carried out at ×63 (Supplemental Figure 1, left panels) and then magnified by ×3 to ×4 (Supplemental Figure 1, right panels) to enhance cellular details.
Anti-cancer Efficacy of Tetrac and Tetrac NP on PANC-1 Xenograft Size, Weight, and Hemoglobin Content
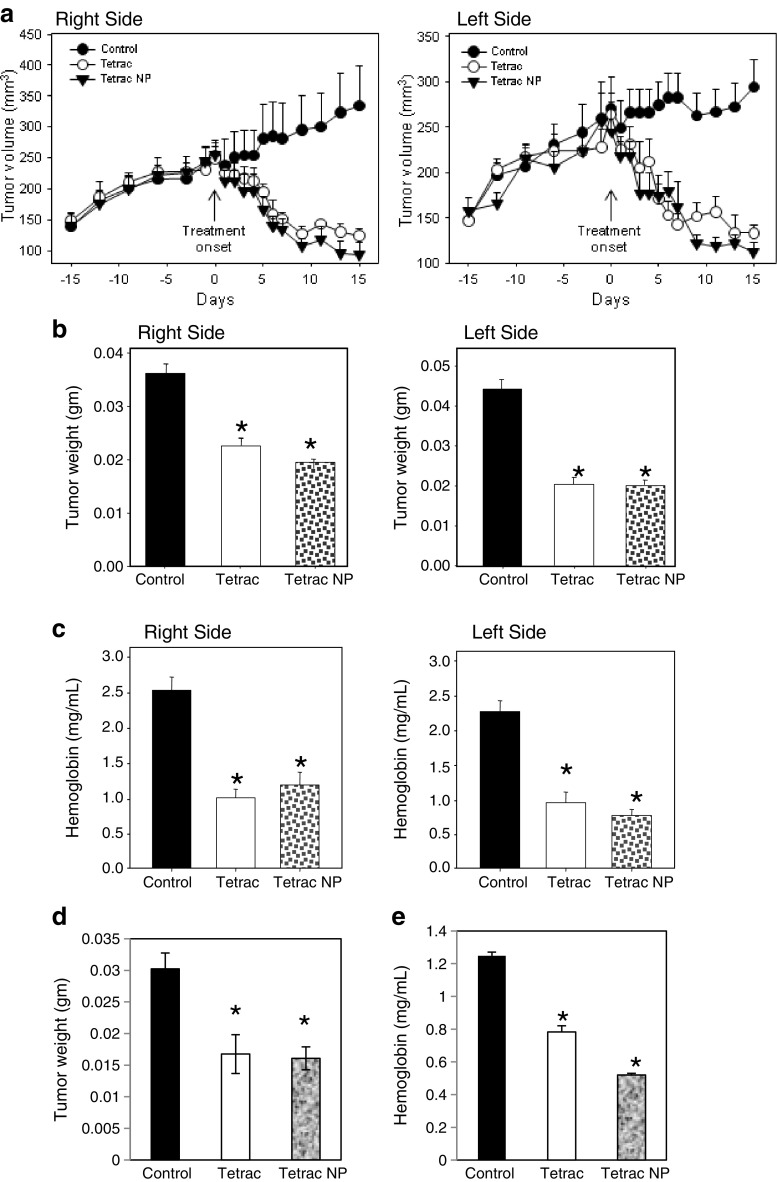
Tumor cell inoculum/xenograft volume increased by 66 % over 15 days prior to treatment. Daily i.p. administration of unmodified tetrac (10 mg/kg body weight) or Tetrac NP (1 mg/kg body weight, corrected for NP mass) resulted in a progressive reduction in xenograft tumor volume that was statistically significant by treatment days 5–6 (Fig. 1a, P < 0.05). Both agents decreased tumor volume to that of the original implant size (150 mm3) or to less than inoculum size by day 7 (P < 0.01). Measured as xenograft weight (Fig. 1b), the xenograft size reduction attributable to treatment for 15 days with either agent ranged from 43.5 % to 55.1 %, compared to the weight of tumors in control animals (*P < 0.05).
Fig. 1.
Effect of treatment with tetrac and Tetrac NP on PANC-1 tumor xenograft size, weight, and hemoglobin content. a Effect of unmodified tetrac (10 mg/kg daily, i.p.) or Tetrac NP (1 mg/kg daily, i.p.) on PANC-1 cell xenograft volumes. Volumes were measured daily or every other day. PANC-1 cells were implanted on day −15, and drug treatment was started on day 0. Significant diminution in xenograft volumes was seen in both tetrac and Tetrac NP treatment groups by days 5–6 (both flanks, P < 0.05), and by days 7–15, both treatment groups showed further size reductions significantly different from the untreated xenografts (P < 0.01). Xenograft volumes continued to increase in the control group to day +15. b Effects of tetrac or Tetrac NP on the weights of PANC-1 xenografts at the termination of the study (day +15). Treatment was as shown in (a). A decrease in xenograft weights in the animals exposed to either tetrac or Tetrac NP was observed (*P < 0.05). c Actions of unmodified tetrac or Tetrac NP on angiogenesis in PANC-1 cell xenografts, as measured by hemoglobin content of the tumors at the time of animal sacrifice. Hemoglobin content of the xenografts was reduced in the presence of both tetrac and Tetrac NP (*P < 0.05). Effect of treatment with tetrac and Tetrac NP on MPanc96 tumor xenograft weight and hemoglobin content. d Effects of tetrac or Tetrac NP on the weights of MPanc96 xenografts at the termination of the study (day +15). A decrease in xenograft weights in the animals exposed to either tetrac or Tetrac NP was observed (*P < 0.05). e Actions of unmodified tetrac or Tetrac NP on angiogenesis in MPanc96 cell xenografts, as measured by hemoglobin content of the tumors at the time of animal sacrifice. Hemoglobin content of the xenografts was reduced in the presence of both tetrac and Tetrac NP (*P < 0.05)
Hemoglobin content of xenografts as an index of tumor angiogenesis collectively decreased by 60 % in animals treated daily for 15 days with unmodified tetrac or Tetrac NP (*P < 0.05 Fig. 1c), compared with tumors in the untreated control animals.
Body Weights in PANC-1 Xenograft-bearing Animals
Control animals and animals that received tetrac or Tetrac NP gained weight comparably throughout the course of the study (data not shown).
Anti-cancer Efficacy of Tetrac and Tetrac NP on MPanc96 Xenograft Weight, Hemoglobin Content
Tetrac and Tetrac NP resulted in 40–60 % inhibition of MPanc96 tumor growth (Fig. 1d) and tumor angiogenesis (Fig. 1e), comparable to results achieved in PANC-1 xenografts. The amount of hemoglobin in PANC-1 xenografts (Fig. 1c) was substantially greater than in MPanc96 tumors (Fig. 1e; see “Discussion” section). There was no effect of tetrac or Tetrac NP treatment on animal body weight growth rate as compared to vehicle-treated animals (data not shown).
Comparison of the Actions of Tetrac and Two Tetrac NP Preparations on Proliferation of PANC-1 Cells in Cell Culture
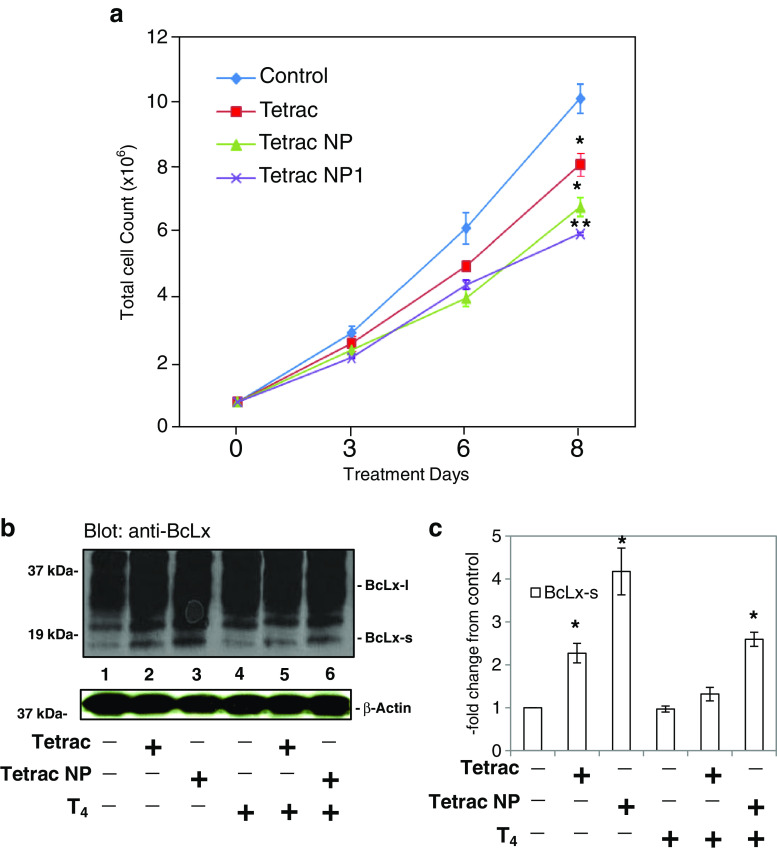
Cultured PANC-1 cell counts were reduced over 8 days by unmodified tetrac (10−6 M) and by two preparations of Tetrac NP (10−6 M; Fig. 2a). Tetrac NP is a scaled-up formulation manufactured at Azopharma, and Tetrac NP1 is a small-scale formulation of tetrac generated at Albany College of Pharmacy and Health Sciences (ACPHS). By day 8, total cell counts were reduced approximately 20 % by unmodified tetrac compared with untreated cells (P < 0.04) and by 35–40 % in the presence of each of the Tetrac NP formulations (P ≤ 0.01). Thus, the two preparations of NPs, although from different sources, were comparably effective. Tetrac NP was more effective in vitro than tetrac, consistent with what we have reported previously in other cell lines [29].
Fig. 2.
Actions of tetrac and two Tetrac NP preparations on proliferation of PANC-1 cells in cell culture, and effect of tetrac and Tetrac NP on abundance of cell proteins and mRNAs relevant to apoptosis and angiogenesis. a In vitro effects of tetrac or each of two Tetrac NP preparations on the growth of PANC-1 cells and on elaboration of the pro-apoptotic protein, BcLx-s. Tetrac NP is a scaled-up formulation manufactured by Azopharma, and Tetrac NP1 is a small-scale formulation of nanoparticulate tetrac synthesized at ACPHS. Cells were cultured for 8 days in the presence of either control solvent, 10−6 M unmodified tetrac, or an equivalent amount of tetrac as Tetrac NP or Tetrac NP1. After 3, 6, and 8 days of cell culture, representative samples from each set were counted and the results compared. By day 8, the suppressive effects of tetrac on cell growth were significant [P < 0.04 vs. control (no tetrac, Tetrac NP, or T4)], as were the effects of both NP preparations (P ≤ 0.01). Experiment was performed three times. b, c In separate studies, immunoblots of cytosolic proteins harvested from control, tetrac-treated, and Tetrac NP-treated PANC-1 cells in the presence or absence of 10−7 M l-thyroxine (T4) were prepared. BcLx isoform quantitation was performed after exposure of cells to T4 and/or tetrac or Tetrac NP for 3 days. Unmodified tetrac or Tetrac NP (10−7 M) both caused accumulation of pro-apoptotic BcLx-s (lanes 2 and 3). Abundance of anti-apoptotic BcLx-l was estimated by computer-assisted adjustment of blot background and was found to be unaffected by unmodified tetrac and Tetrac NP (results not shown). T4 (10−7 M), alone, did not significantly affect the cytosolic content of these BCL2-related proteins, but did suppress the accumulation of BcLx-s in response to Tetrac NP (lanes 5 and 6; *P < 0.05 and **P < 0.01). Experiment was performed three times
Effects of Tetrac and Tetrac NP on Abundance of Cell Proteins and mRNAs Relevant to Apoptosis and Angiogenesis
The response of pro-apoptotic protein BcLx-s to treatment of cells with unmodified tetrac or Tetrac NP was studied. PANC-1 pancreatic cancer cells were treated with T4 (10−7 M) or hormone solvent in the presence or absence of tetrac or Tetrac NP for 24 h. Studies of the harvested cytosolic proteins indicated that both unmodified tetrac and Tetrac NP in the absence of T4 caused accumulation of the pro-apoptotic BcLx-s protein in 3 days (Fig. 2b, lanes 2–3). The effect of Tetrac NP was approximately 1.9-fold greater than that of unmodified tetrac (Fig. 2c). In the presence of T4 (Fig. 2b, lanes 4–6), an inhibitory effect of the hormone on the effect of Tetrac NP was noted (80 % reduction, Fig. 2c). There was no discernible effect of T4, alone, on BcLx-s abundance in PANC-1 cells. Computer-assisted adjustment of background about BcLx-l revealed no apparent effect of T4, unmodified tetrac, or Tetrac NP on the cytosol content of this protein (results not shown).
Previous RNA microarray studies conducted in human breast cancer cells [25] or medullary thyroid carcinoma cells [26] revealed that tetrac or Tetrac NP affected expression of differentially regulated genes relevant to apoptosis, the cell cycle, and to angiogenesis. In the current studies, we measured the effects of unmodified tetrac and Tetrac NP on abundance of proteins relevant to apoptosis, cell cycle arrest, or angiogenesis. In the current studies of PANC-1 cells, Fig. 3a (upper and lower panels) reveals that cellular abundance of p53 and p21 mRNAs was significantly increased in tetrac-treated (1.5-fold, P < 0.05) and Tetrac NP-treated (twofold, P < 0.05) cells and that EGFR mRNA was not materially affected by either tetrac formulation. Concentrations of agents were 10−7 M unmodified tetrac and 10−8 M tetrac-equivalent in Tetrac NP. THBS1 is an endogenous anti-angiogenesis protein, the expression of whose gene is usually suppressed in cancer cells. The abundance of THBS1 protein was increased three- to fourfold by tetrac (P = 0.029) and Tetrac NP (P < 0.022; Fig. 3b, upper and lower panels). Figure 3b also shows that abundance of XIAP was significantly increased in cells exposed to tetrac (P = 0.006), but not in PANC-1 cells treated with Tetrac NP.
Fig. 3.
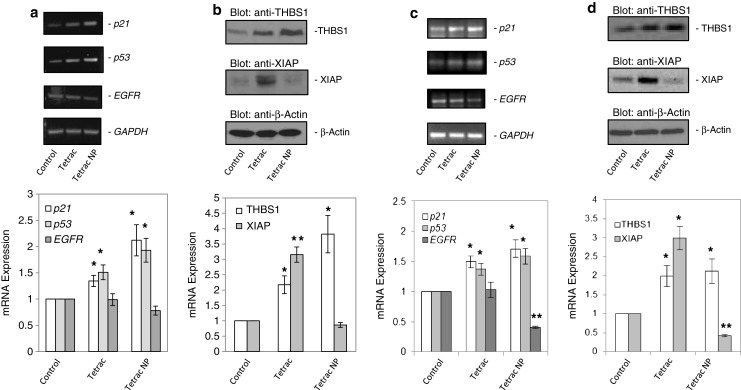
Effects of unmodified tetrac and Tetrac NP on abundance of tumor-relevant mRNAs or proteins in PANC-1 cells. a Cells were cultured for 2 days in the presence of control solvent, tetrac (10−7 M), or Tetrac NP (10−8 M). Harvest of total RNA and PT-PCR was performed as described in “Materials and Methods” section. Unmodified tetrac and Tetrac NP both increased abundance of p21 and p53 mRNAs (P < 0.05). Neither agent significantly affected expression of EGFR. GAPDH was the internal control used to correct IOD in graphed data. b Cells were cultured for 3 days in the presence of control solvent, unmodified tetrac, and Tetrac NP, as in (a). Immunoblots of total proteins harvested from the samples were prepared. Increased accumulation of THBS1 protein was obtained with tetrac (P = 0.029) and Tetrac NP (P < 0.022). Tetrac, but not Tetrac NP, increased accumulation of XIAP (P = 0.006). β-Actin was the internal control used to correct IOD in graphed data (*P < 0.05 and **P < 0.01). Experiments in (a) and (b) were performed three times. Effects of unmodified tetrac and Tetrac NP on abundance of tumor-relevant mRNAs or proteins in MPanc96 cells. c Cells were cultured for 2 days in the presence of control solvent, tetrac (10−7 M), or Tetrac NP (10−8 M). Harvest of total RNA and PT-PCR was performed as described in “Materials and Methods” section. Unmodified tetrac and Tetrac NP both increased expression of p21 and p53 mRNAs (P < 0.05). Tetrac NP (P < 0.01) but not tetrac significantly reduced the expression of EGFR. GAPDH was the internal control. d Cells were cultured for 3 days in the presence of control solvent, unmodified tetrac, and Tetrac NP, as in (a). Immunoblots of total proteins harvested from the samples were prepared. Increased accumulation of THBS1 protein was obtained with tetrac (P < 0.05) and Tetrac NP (P < 0.01). Tetrac significantly increased accumulation of XIAP (P < 0.05), while Tetrac NP decreased accumulation of XIAP (P < 0.01). β-Actin was the internal control for correction of IOD (*P < 0.05 and **P < 0.01). Experiments in (c) and (d) were carried out three times
Similar effects on MPanc96 cell lines were obtained with tetrac and Tetrac NP (Fig. 3c, d). Figure 3c (upper and lower panels) reveals that cellular abundance of p53 and p21 mRNAs was significantly increased in 10−7 M tetrac-treated and 10−8 M Tetrac NP-treated (1.5-2-fold, P < 0.05) cells, and that EGFR mRNA was significantly decreased (P < 0.01) by Tetrac NP but not by tetrac. The abundance of THBS1 protein was increased two to threefold by tetrac (P < 0.05) and Tetrac NP (P < 0.01; Fig. 3d, upper and lower panels). Abundance of XIAP was significantly increased in cells exposed to tetrac (P < 0.05), but not in cells treated with Tetrac NP (Fig. 3d).
The abundance of β3 and αv by PANC-1 and MPanc96 was comparable to the expression of these monomers in the breast cancer cell line MDA-MB 231 (Supplementary Figure 2).
Discussion
Human pancreatic carcinoma (PANC-1 and MPanc96) cell xenografts are shown in this study to respond favorably in a short-term, 15-day protocol to the parenteral administration of nanoparticulate tetrac or unmodified tetrac. We have previously shown tetrac to have anti-proliferative [27, 28, 34, 35] and anti-angiogenic [15, 19, 20, 36] activities that are initiated at a plasma membrane receptor for iodothyronine analogues on integrin αvβ3 [15, 20, 36]. The integrin is generously expressed on tumor cells and in tumor-associated, rapidly proliferating vasculature. Tumor xenograft hemoglobin content, an index of tumor vascularity in these studies, was reduced by both tetrac formulations.
The anti-angiogenic properties of tetrac and Tetrac NP are complex [21], but reflect in part the ability of these two formulations to inhibit the binding of endogenous pro-angiogenic T4 and T3 to the thyroid hormone receptor on integrin αvβ3 [15]. In addition, under thyroid hormone-free conditions, tetrac has been shown to inhibit the pro-angiogenic activities of VEGF and bFGF [24]. Epidermal growth factor (EGF) and its receptor (EGFR) may also regulate angiogenesis [37], and we have shown that thyroid hormone potentiates the actions of EGF at its receptor by several mechanisms [38, 39]. Effects of tetrac formulations on gene expression are also relevant to cancer-associated angiogenesis (see below). It is interesting to note that the vascularity of PANC-1 tumors was greater than that of MPanc96 xenografts in the current studies. The clinical variability in pancreatic cancer vascularity may contribute to the relative ineffectiveness of anti-angiogenic agents directed at a single vascular growth factor in this disease [40, 41]. In the present studies, however, a frank difference in VEGF production by PANC-1 cells vs. MPanc96 (AsPC-1) cells [42] may explain the increased vascularity of the PANC-1 xenografts.
The molecular basis of the anti-proliferative action of tetrac formulations is also complex. Principal agonist thyroid hormones, such as T4, are anti-apoptotic via an integrin receptor-mediated mechanism [16, 17], and tetrac inhibits this anti-apoptotic action. In addition, tetrac formulations may act at integrin αvβ3 in certain cancer cells to suppress expression of anti-apoptotic genes and cause transcription of pro-apoptotic genes [25, 27] in the absence or presence of agonist thyroid hormone analogues (T4 and T3). A substantial number of genes can be regulated via integrin αvβ3 [43–46], and thus, this integrin-mediated effect of tetrac is not surprising.
In the mechanistic studies of PANC-1 cells that were conducted here, Tetrac NP and unmodified tetrac induced accumulation in cytosol of pro-apoptotic BcLx-s, but did not appear to affect the amount of anti-apoptotic BcLx-l. This observation confirms the effects of tetrac on these BCL2-related proteins that were previously shown in glioma cells [17]. RNA microarray studies that we have conducted in other human cancer cell lines—breast cancer MDA-MB 231 [25] and medullary thyroid carcinoma cells [27]—show that while there is much concordance in the actions of Tetrac NP and unmodified tetrac, there are important distinctions between the agents. For example, in MDA-MB 231 cells, the expression of EGFR is downregulated by the NP formulation, but not by unmodified tetrac; further, expression of the pro-apoptotic BCL2L14 gene, although unaffected by unmodified tetrac, is upregulated by Tetrac NP [25]. Such distinctions confer a therapeutic advantage on the NP formulation of tetrac.
The microarray results just described guided measurements in the current studies of PANC-1 and MPanc96 cells of proteins relevant to cell cycle arrest, apoptosis, and angiogenesis. For example, Tetrac NP and unmodified tetrac increased the amount of the cyclin-dependent kinase inhibitor protein, p21, in these human pancreatic cancer cells. The oncogene suppressor protein and pro-apoptotic protein, p53, was increased in abundance by Tetrac NP > tetrac. The p21 gene is a target of p53 [47], and thus, the p21 effect of tetrac formulations may not represent an effect separate from that on p53 accumulation. We found that Tetrac NP, but not unmodified tetrac, promoted tumor cell accumulation of THBS1, an anti-angiogenesis factor almost invariably unexpressed in cancer cells. Effects of tetrac formulations on p53, p21, THBS1, and BcLx-s constitute a coherent complex of anti-cancer effects that are postulated to have contributed to the results of treatment with tetrac formulations in pancreatic cancer xenografts. In contrast to results we obtained previously in human breast cancer cells [25], EGFR expression was not statistically affected in PANC-1 and MPanc96 cells by tetrac, but was significantly suppressed by Tetrac NP in both cell lines. Mutations in KRAS occur in a large majority of clinical pancreatic tumors [48], but effects of Tetrac NP on KRAS were not measured in the current studies. Therapeutic modalities directed at KRAS in pancreatic cancer have so far been disappointing [49], and KRAS mutations do not satisfactorily predict survival [48].
There were disparate effects of unmodified tetrac and Tetrac NP on the anti-apoptotic protein, XIAP, in these pancreatic cancer cells that may confer an advantage on Tetrac NP in these cells. The accumulation of XIAP in tetrac-exposed cells is undesirably anti-apoptotic and was absent in Tetrac NP-treated cells. XIAP expression may be mitogen-activated protein kinase (MAPK)-regulated [50], and a number of actions of thyroid hormone initiated at αvβ3 are transduced by MAPK and result downstream in expression of specific genes [46, 47, 51, 52]. In previously studied breast cancer cells, neither Tetrac NP nor tetrac affected XIAP [25]. Thus, the actions of the two formulations of tetrac on differentially regulated genes such as EGFR and XIAP indicate existence of some cell line-specificity for the agents. A possible explanation for the difference between actions of tetrac and Tetrac NP in PANC-1 cells is the ability of unmodified tetrac to gain access to the cell interior, where it is a thyroid hormone agonist—in contrast to its function and that of Tetrac NP as thyroid hormone antagonists at the iodothyronine receptor on the cell surface integrin αvβ3.
Compared to unmodified tetrac, Tetrac NP also has an advantage in potency that we have described elsewhere [29]. In the studies of PANC-1 xenografts reported here, Tetrac NP was effective in reducing tumor volume, weight, and vascularity when administered at a dose (1 mg tetrac equivalent/kg body weight) that was 10 % of a comparably effective dose of unmodified tetrac (10 mg/kg); the latter was intended to approximate a tissue concentration of 10−7 M. Because formal dose–response studies were not a part of the studies conducted in intact animals, the xenograft experiments do not expressly prove increased potency of the formulation. However, the in vitro studies carried out here included tetrac and Tetrac NP concentrations of 10−8 to 10−6 M (Figs. 2 and 3a, b) and showed increased potency of the nanoparticulate analogue. An important attribute of Tetrac NP is its exclusion from the intracellular space, particularly from the nucleus where unmodified tetrac has been shown to be thyromimetic [26]. This may contribute an undesirable side effect profile to unmodified tetrac, particularly in the setting of in vivo studies of more extended duration. The short-term studies conducted here did not, however, reveal differences in weight gain between tetrac-treated and the control and Tetrac NP-exposed animals that would suggest hypermetabolism. Confocal microscopy of cells exposed to fluorescently labeled tetrac or Tetrac NP confirmed both the access of unmodified tetrac to the cell interior and, in contrast, the restriction of Tetrac NP to the plasma membrane of cells.
The NP used in the current studies of Tetrac NP was PLGA, whose biocompatibility and biodegradability had led to its approval by the US Food and Drug Administration for parenteral administration of drugs such as Lupron Depot® and use in grafts, sutures, and prostheses. No effects of PLGA on cell viability, proliferation, and mitochondrial activity have been detected [53, 54]. We have conducted our own studies of void PLGA on immortalized normal human cell lines and on human cancer cell lines and have found no effects on viability, cell proliferation, and morphology (S.A. Mousa, unpublished observations).
Future xenograft studies of pancreatic carcinoma that we will conduct include orthotopic tumor cell implantations. These will control for increase in tumor tissue interstitial pressure that can be seen in fibrotic lesions. We will also verify that the studies of molecular mechanism reported here in cellular studies of the action of Tetrac NP are reproduced in xenografts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 358 kb)
Acknowledgments
This work was supported by the Pharmaceutical Research Institute at ACPHS and in part by an endowment established in the laboratory of one of the authors (PJD) by Frank and Margaret Domiter Rudy. The funders had no role in study design, data collection and analysis, decision to publish, or in the preparation of the manuscript. Kelly Keating of the Pharmaceutical Research Institute at the Albany College of Pharmacy and Health Sciences edited the manuscript. The authors declare no conflicts of interest.
Abbreviations
- NP
Nanoparticle
- Tetrac
Tetraiodothyroacetic acid
- T3
3,5,3′-triiodo-l-thyronine
- T4
l-thyroxine
- THBS1
Thrombospondin 1
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
Murat Yalcin and Hung-Yun Lin contributed equally to the manuscript.
References
- 1.Philip PA, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27:5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oettle H, Neuhaus P. Adjuvant therapy in pancreatic cancer: a critical appraisal. Drugs. 2007;67:2293–2310. doi: 10.2165/00003495-200767160-00001. [DOI] [PubMed] [Google Scholar]
- 3.Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 4.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9:1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- 6.Mahalingam D, Giles F. Challenges in developing targeted therapy for pancreatic adenocarcinoma. Expert Opin Ther Targets. 2008;12:1389–1401. doi: 10.1517/14728222.12.11.1389. [DOI] [PubMed] [Google Scholar]
- 7.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 9.Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol. 2008;295:H69–76. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen X. Integrin alpha v beta 3 antagonists for anti-angiogenic cancer treatment. Recent Pat Anticancer Drug Discov. 2007;2:143–158. doi: 10.2174/157489207780832469. [DOI] [PubMed] [Google Scholar]
- 12.Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–131. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura I, le Duong T, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25:337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 14.Mousa SS, Davis FB, Davis PJ, Mousa SA. Human platelet aggregation and degranulation is induced in vitro by L-thyroxine, but not by 3,5,3'-triiodo-L-thyronine or diiodothyropropionic acid (DITPA) Clin Appl Thromb Hemost. 2010;16:288–293. doi: 10.1177/1076029609348315. [DOI] [PubMed] [Google Scholar]
- 15.Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–2871. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- 16.Lin HY, Tang HY, Shih A, Keating T, Cao G, Davis PJ, Davis FB. Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids. 2007;72:180–187. doi: 10.1016/j.steroids.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Lin HY, Tang HY, Keating T, Wu YH, Shih A, Hammond D, Sun M, Hercbergs A, Davis FB, Davis PJ. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: both actions are integrin and ERK mediated. Carcinogenesis. 2008;29:62–69. doi: 10.1093/carcin/bgm239. [DOI] [PubMed] [Google Scholar]
- 18.Davis PJ, Davis FB, Lin HY, Mousa SA, Zhou M, Luidens MK. Translational implications of nongenomic actions of thyroid hormone initiated at its integrin receptor. Am J Physiol Endocrinol Metab. 2009;297:E1238–1246. doi: 10.1152/ajpendo.00480.2009. [DOI] [PubMed] [Google Scholar]
- 19.Davis FB, Mousa SA, O’Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res. 2004;94:1500–1506. doi: 10.1161/01.RES.0000130784.90237.4a. [DOI] [PubMed] [Google Scholar]
- 20.Mousa SA, O’Connor L, Davis FB, Davis PJ. Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology. 2006;147:1602–1607. doi: 10.1210/en.2005-1390. [DOI] [PubMed] [Google Scholar]
- 21.Luidens MK, Mousa SA, Davis FB, Lin HY, Davis PJ. Thyroid hormone and angiogenesis. Vascul Pharmacol. 2010;52:142–145. doi: 10.1016/j.vph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Lin HY, Sun M, Tang HY, Lin C, Luidens MK, Mousa SA, Incerpi S, Drusano GL, Davis FB, Davis PJ. L-Thyroxine vs. 3,5,3'-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol. 2009;296:C980–991. doi: 10.1152/ajpcell.00305.2008. [DOI] [PubMed] [Google Scholar]
- 23.Tang HY, Lin HY, Zhang S, Davis FB, Davis PJ. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology. 2004;145:3265–3272. doi: 10.1210/en.2004-0308. [DOI] [PubMed] [Google Scholar]
- 24.Mousa SA, Bergh JJ, Dier E, Rebbaa A, O’Connor LJ, Yalcin M, Aljada A, Dyskin E, Davis FB, Lin HY, Davis PJ. Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis. 2008;11:183–190. doi: 10.1007/s10456-007-9088-7. [DOI] [PubMed] [Google Scholar]
- 25.Glinskii AB, Glinsky GV, Lin HY, Tang HY, Sun M, Davis FB, Luidens MK, Mousa SA, Hercbergs AH, Davis PJ. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac) Cell Cycle. 2009;8:3554–3562. doi: 10.4161/cc.8.21.9963. [DOI] [PubMed] [Google Scholar]
- 26.Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–253. doi: 10.1089/thy.2007.0248. [DOI] [PubMed] [Google Scholar]
- 27.Yalcin M, Dyskin E, Lansing L, Bharali DJ, Mousa SS, Bridoux A, Hercbergs AH, Lin HY, Davis FB, Glinsky GV, Glinskii A, Ma J, Davis PJ, Mousa SA. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 2010;95:1972–1980. doi: 10.1210/jc.2009-1926. [DOI] [PubMed] [Google Scholar]
- 28.Yalcin M, Bharali DJ, Lansing L, Dyskin E, Mousa SS, Hercbergs A, Davis FB, Davis PJ, Mousa SA. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009;29:3825–3831. [PubMed] [Google Scholar]
- 29.Lin HY, Landersdorfer CB, London D, Meng R, Lim CU, Lin C, Lin S, Tang HY, Brown D, Scoy B, Kulawy R, Queimado L, Drusano GL, Louie A, Davis FB, Mousa SA, Davis PJ. Pharmacodynamic modeling of anti-cancer activity of tetraiodothyroacetic acid in a perfused cell culture system. PLoS Comput Biol. 2011;7:e1001073. doi: 10.1371/journal.pcbi.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis PJ, Shih A, Lin HY, Martino LJ, Davis FB. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J Biol Chem. 2000;275:38032–38039. doi: 10.1074/jbc.M002560200. [DOI] [PubMed] [Google Scholar]
- 31.Lin HY, Lansing L, Merillon JM, Davis FB, Tang HY, Shih A, Vitrac X, Krisa S, Keating T, Cao HJ, Bergh J, Quackenbush S, Davis PJ. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742–1744. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Cao HJ, Davis FB, Tang HY, Davis PJ, Lin HY. Oestrogen inhibits resveratrol-induced post-translational modification of p53 and apoptosis in breast cancer cells. Br J Cancer. 2004;91:178–185. doi: 10.1038/sj.bjc.6601902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87:1223–1232. doi: 10.1210/jc.87.3.1223. [DOI] [PubMed] [Google Scholar]
- 34.Davis FB, Tang HY, Shih A, Keating T, Lansing L, Hercbergs A, Fenstermaker RA, Mousa A, Mousa SA, Davis PJ, Lin HY. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006;66:7270–7275. doi: 10.1158/0008-5472.CAN-05-4365. [DOI] [PubMed] [Google Scholar]
- 35.Rebbaa A, Chu F, Davis FB, Davis PJ, Mousa SA. Novel function of the thyroid hormone analog tetraiodothyroacetic acid: a cancer chemosensitizing and anti-cancer agent. Angiogenesis. 2008;11:269–276. doi: 10.1007/s10456-008-9110-8. [DOI] [PubMed] [Google Scholar]
- 36.Mousa SA, O’Connor LJ, Bergh JJ, Davis FB, Scanlan TS, Davis PJ. The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol. 2005;46:356–360. doi: 10.1097/01.fjc.0000175438.94906.a0. [DOI] [PubMed] [Google Scholar]
- 37.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 38.Shih A, Zhang S, Cao HJ, Tang HY, Davis FB, Davis PJ, Lin HY. Disparate effects of thyroid hormone on actions of epidermal growth factor and transforming growth factor-alpha are mediated by 3',5'-cyclic adenosine 5'-monophosphate-dependent protein kinase II. Endocrinology. 2004;145:1708–1717. doi: 10.1210/en.2003-0742. [DOI] [PubMed] [Google Scholar]
- 39.Lin HY, Shih A, Davis FB, Davis PJ. Thyroid hormone promotes the phosphorylation of STAT3 and potentiates the action of epidermal growth factor in cultured cells. Biochem J. 1999;338(Pt 2):427–432. doi: 10.1042/0264-6021:3380427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko AH, Youssoufian H, Gurtier J, Dicke K, Kayaleh O, Lenz HJ, Keaton M, Katz T, Ballai S, Rowinsky EK. A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first-line therapy for metastatic pancreatic carcinoma. Invest New Drugs. 2012;30:1597–1606. doi: 10.1007/s10637-011-9691-8. [DOI] [PubMed] [Google Scholar]
- 41.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan CQ, Zhao YP. Expression of angiogenic factors in pancreatic carcinoma cells and their significance. Zhonghua Wai Ke Za Zhi. 2009;47:787–890. [PubMed] [Google Scholar]
- 43.Lee DY, Li YS, Chang SF, Zhou J, Ho HM, Chiu JJ, Chien S. Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by alphavbeta3 and beta1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J Biol Chem. 2010;285:30–42. doi: 10.1074/jbc.M109.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, Hsu HC, Liu SH, Tang CH. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Physiol. 2009;221:98–108. doi: 10.1002/jcp.21835. [DOI] [PubMed] [Google Scholar]
- 45.Lossner D, Abou-Ajram C, Benge A, Aumercier M, Schmitt M, Reuning U. Integrin alphavbeta3 upregulates integrin-linked kinase expression in human ovarian cancer cells via enhancement of ILK gene transcription. J Cell Physiol. 2009;220:367–375. doi: 10.1002/jcp.21774. [DOI] [PubMed] [Google Scholar]
- 46.Mangale SS, Modi DN, Reddy KV. Identification of genes regulated by an interaction between alphavbeta3 integrin and vitronectin in murine decidua. Reprod Fertil Dev. 2008;20:311–319. doi: 10.1071/RD07155. [DOI] [PubMed] [Google Scholar]
- 47.Brady CA, Attardi LD. p53 at a glance. J Cell Sci. 2010;123:2527–2532. doi: 10.1242/jcs.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira-Cunha M, Hadfield KD, Siriwardena AK, Newman W. EGFR and KRAS mutational analysis and their correlation to survival in pancreatic and periampullary cancer. Pancreas. 2012;41:428–434. doi: 10.1097/MPA.0b013e3182327a03. [DOI] [PubMed] [Google Scholar]
- 49.Le A, Rajeshkumar NV, Maitra A, Dang CV. Conceptual framework for cutting the pancreatic cancer fuel supply. Clin Cancer Res. 2012;18:4285–4290. doi: 10.1158/1078-0432.CCR-12-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin H, Chen C, Li X, Chen BD. Activation of the MEK/MAPK pathway is involved in bryostatin1-induced monocytic differenciation and up-regulation of X-linked inhibitor of apoptosis protein. Exp Cell Res. 2002;272:192–198. doi: 10.1006/excr.2001.5417. [DOI] [PubMed] [Google Scholar]
- 51.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis PJ, Davis FB, Mousa SA, Luidens MK, Lin HY. Membrane receptor for thyroid hormone: physiologic and pharmacologic implications. Annu Rev Pharmacol Toxicol. 2011;51:99–115. doi: 10.1146/annurev-pharmtox-010510-100512. [DOI] [PubMed] [Google Scholar]
- 53.d'Angelo I, et al. Nanoparticles based on PLGA:Poloxmer blends for the delivery of proangiogenic growth factors. Mol Pharm. 2010;7:1724–1733. doi: 10.1021/mp1001262. [DOI] [PubMed] [Google Scholar]
- 54.Kohl Y, Kaiser C, Bost W, Stracke F, Fournelle M, Wischke C, Thielecke H, Lendlein A, Kratz K, Lemor R. Preparation and biological evaluation of multifunctional PLGA-nanoparticles designed for photoacoustic imaging. Nanomedicine. 2011;7:228–237. doi: 10.1016/j.nano.2010.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 358 kb)