Abstract
The present study aimed to investigate the effects of lipopolysaccharide (LPS) stimulation on oxidative damage, apoptosis, and glutamine (Gln) transporter Alanine-Serine-Cysteine transporter 2 (ASCT2) expression in porcine small intestinal epithelial cells (IPEC-J2), and preliminarily elucidated the relationship between ASCT2 expression level and oxidative damage and apoptosis of IPEC-J2 cells. IPEC-J2 cells were treated without (control group, CON, N = 6) or with 1 μg/mL LPS (LPS group, LPS, N = 6). Cell viability, lactate dehydrogenase (LDH) content, malonaldehyde (MDA), anti-oxidant enzymes (superoxide dismutase [SOD], catalase [CAT], glutathione peroxidase [GSH-Px], and total anti-oxidant capacity [T-AOC]), apoptosis of IPEC-J2 cells, the expression of Caspase3, the expression of ASCT2 mRNA and ASCT2 protein was detected. The results showed that LPS stimulation of IPEC-J2 cells significantly reduced the cell viability, and anti-oxidant enzymes activity (SOD, CAT, and GSH-Px), and significantly increased LDH and MDA release. Flow cytometry results showed that LPS stimulation significantly increased the late apoptosis rate and the total apoptosis rate of IPEC-J2 cells. The immunofluorescence results showed that the fluorescence intensity of LPS stimulated IPEC-J2 cells was significantly enhanced. LPS stimulation significantly decreased the mRNA and protein expression of ASCT2 in IPEC-J2 cells. The correlation analysis showed that ASCT2 expression was negatively correlated with apoptosis, and positively correlated with the anti-oxidant capacity of IPEC-J2 cells. According to the results of this study, it can be preliminarily concluded that LPS promotes the apoptosis and oxidative injury of IPEC-J2 cells by down-regulating the expression of ASCT2.
Keywords: apoptosis, ASCT2, cell injury, lipopolysaccharide, porcine small intestinal epithelial cells
Due to long-term exposure to a complex environment composed of various food components, antigens, symbiotic micro-organisms and pathogens, intestinal epithelial cells are often subjected to various stresses, which may lead to intestinal oxidative damage and promote cell apoptosis. Therefore, maintaining the dynamic balance of intestinal redox state and apoptosis is of great significance for intestinal health. The results of this study can provide a new idea for the nutritional regulation of intestinal repair, that is, by upregulating intestinal ASCT2 expression to alleviate oxidative damage and reduce cell apoptosis, and thus maintain intestinal homeostasis.
Introduction
Intestinal epithelial barrier is an important intestinal barrier formed primarily from a continuous monolayer of proliferating and differentiating intestinal epithelial cells (IECs), which selectively allows the absorption of nutrients, while limits the entry of pathogenic substances (Catalioto et al., 2011; Schoultz and Keita, 2020). The integrity of intestinal epithelial barrier structure and function depends on the dynamic balance between proliferation and apoptosis of IECs (Cai et al., 2016; Tang et al., 2018). Excessive apoptosis can disturb the intestinal mucosa homeostasis, and eventually result in intestinal barrier dysfunction (Tang et al., 2018). Thus, the dynamic balance between proliferation and apoptosis of IECs is contributed to intestinal mucosa homeostasis maintenance. However, due to the long-term exposure to a complex environment consisting of various food components, antigens, symbiotic micro-organisms, and pathogens, intestinal epithelial cells are frequently subjected to various stresses, which may cause intestinal oxidative damage and promote apoptosis of IECs (Tang et al., 2018).
Lipopolysaccharide (LPS), a major integral component of the Gram-negative bacteria outer membrane, can induce oxidative injury and apoptosis of IECs (Zhang et al., 2019), and ultimately result in intestinal dysfunction, which makes it easier for intestinal pathogenic bacteria and endotoxins to break through the intestinal mucosal barrier and enter other tissues, organs, and blood circulation system (Tang et al., 2018; Zhang et al., 2019; Stephens et al., 2020). Hence, LPS is commonly used to construct animal or cell injury models for experimental studies.
Glutamine (Gln) is a conditionally essential amino acid that serves important roles in maintaining intestinal integrity and healthy gastrointestinal function (Scalise et al., 2018; Tang et al., 2022a). It is commonly accepted that Gln is the main energy source for animal eukaryotic cells including IECs, and contributes to the maintenance of intestinal structure and function and the repair of mammalian intestinal barrier damage caused by various stresses (Achamrah et al., 2017; Li et al., 2021). The transportation of Gln across intestinal epithelium is mainly mediated by Alanine-Serine-Cysteine transporter 2 (ASCT2), a kind of Na+-dependent neutral amino acid transporter (Nakaya et al., 2014; Tang et al., 2022a). Studies have shown that ASCT2 expression is decreased in injured human intestinal epithelial cells caused by ischemic (Huang et al., 2007), injured porcine small intestinal epithelial cells (IPEC-J2) induced by LPS (Tang et al., 2022a), and injured jejunum of piglets caused by intrauterine growth retardation (Tang and Xiong, 2022a), suggesting that ASCT2 is involved in intestinal injury. What’s more, several different studies have demonstrated that LPS stimulation resulted in increased oxidative injury and apoptosis of IPEC-J2 cells (Tang et al., 2018; Dong et al., 2019; Zhang et al., 2019). It can be speculated that the expression level of ASCT2 is related to oxidative injury and apoptosis of IPEC-J2 cells, but this relationship has not been confirmed by studies. In this study, we hypothesized that cells under stress promoted oxidative injury and apoptosis by down-regulating the expression of ASCT2. To test the above hypothesis, we used LPS to construct a cell injury model, and studied the effect of LPS stimulation on oxidative damage, apoptosis and Gln transporter ASCT2 expression in IPEC-J2 cells, and preliminarily elucidated the relationship between ASCT2 expression level and oxidative damage and apoptosis of IPEC-J2 cells. The results of this study can provide a new idea for the nutritional regulation of intestinal repair, that is, by upregulating intestinal ASCT2 expression to alleviate oxidative damage and reduce cell apoptosis, and thus maintain intestinal homeostasis.
Materials and Methods
Cell culture and experimental design
The IPEC-J2 cells, a stably passaged cell lines isolated from the jejunal epithelium of newborn piglets, were purchased from Abiowell (Changsha, China), and cultured as described previously (Tang et al., 2018; Tang and Xiong, 2022b). Briefly, cells were seeded in DMEM/F12 medium (Sigma-Aldrich, Saint Louis, MO, USA) which contains 10% fetal bovine serum (FBS; GIBCO, Carlsbad, CA, USA) and 1% antibiotics (Abiowell), and cultured in a humidified incubator at 37 °C with 5% CO2 and 95% air. After 80% to 90% of fusion, the cells were digested with 0.25% trypsin-EDTA (Abiowell, Changsha, USA), then counted the cell numbers under a microscope, and the collected cells were used for cell passage or for subsequent assays. Digested IPEC-J2 cells were seeded in 96-well plates (1 × 104/well) or 6-well plates (1 × 105/well; NEST, Wuxi, China), and cultured in DMEM/F12 medium with 10% FBS and 1% antibiotics for 24 h. Then cells were treated without (control group, CON) or with 1 μg/mL LPS (LPS group, LPS) for 24 h according to our previous studies (Tang et al., 2018; Tang and Xiong, 2022b). LPS from Escherichia coli serotype 055: B5 was purchased from Sigma-Aldrich.
Cell viability assay
The cell viability was assessed by the CCK-8 assay (kit no. NU679; Dojindo, Japan) in accordance with the protocol of the manufacturer. Briefly, IPEC-J2 cells were treated with or without LPS treatment for 24 h, then added 10 mL CCK-8 reagent, and further cultured for 3 h. Optical density (OD) was read at a wavelength of 450 nm by microplate reader (Huisong, Shenzhen, China). In this study, OD value was used to represent cell viability. The experiments were performed six times.
Cytotoxicity assay
Cytotoxicity was evaluated by measuring lactate dehydrogenase (LDH) and malonaldehyde (MDA) released in the media (Tang et al., 2018). Briefly, IPEC-J2 cells were treated with or without LPS treatment for 24 h, the culture medium was collected, then the LDH content was measured using an LDH Assay Kit (kit no. A020-2; Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and the MDA content was measured using an MDA Assay Kit (kit no. A003-1-2; Nanjing Jiancheng Bioengineering Institute) in accordance with the protocol of the manufacturer. The experiments were performed six times.
Flow cytometry
Flow cytometry was used to detect the apoptosis of IPEC-J2 cells challenged by LPS according to a previous study (Tang et al., 2018; Tang and Xiong, 2022a). After a 24-h incubation with or without LPS stimulation, IPEC-J2 cells were digested with trypsin without EDTA. Cells were washed twice with phosphate buffered solution (PBS; Abiowell), centrifuged at 2000 rpm for 5 min each time, and about 3.2 × 105 cells were collected for subsequent assays. Collected cells were incubated with 5 μl of Annexin V-APC and 5ul of Propidium Iodide (kit no. KGA1030; KeyGEN BioTECH, Nanjing, China) for 10 min at room temperature in the dark, and apoptotic cells were detected by flow cytometer (BD Biosciences, San Diego, CA, USA). Data were analyzed using the software CELLQuest. The experiments were performed six times.
Immunofluorescence staining analysis
The distribution of Caspase3 in IPEC-J2 cells were determined with immunofluorescence staining (Luo et al., 2019). In brief, digested IPEC-J2 cells were seeded in 6-well plates (1 × 105/well) with coverslips, and cultured in DMEM/F12 medium with 10% FBS and 1% antibiotics for 24 h, then cells were treated with or without 1 μg/mL LPS for 24 h, the coverslips were removed and gently washed with PBS for 2~3 times. Then the cells on the coverslips were fixed with 4% paraformaldehyde for 30 min and gently washed with PBS for 2~3 times, followed by the use of 0.3% Triton-X100 (Beyotime, Shanghai, China) to permeabilize the cells for 30 min at 37 °C and blocked with 5% bovine serum albumin (Sigma-Aldrich) for 60 min and gently washed with PBS thrice. Thereafter, the cells were incubated with Caspase3 primary antibody (1:50 dilution; Proteintech, Rosemont, IL, USA) at 4 °C overnight and gently washed with PBS thrice, then continue to incubate with secondary antibody (goat antirabbit IgG (H + L); 1:5000 dilution; Abiowell) at 37 °C for 90 min and gently washed with PBS thrice, and stained with DAPI solution (Abiowell) at 37 °C for 10 min and washed with PBS thrice. Finally, the slices were sealed with 90% buffered glycerin (Abiowell), and fluorescence images were captured using confocal microscopy (MoticBA210T; Xiamen, China). The experiments were performed six times.
Determination of T-AOC, CAT, GSH-Px, and SOD levels
After a 24 h of incubation with or without LPS stimulation, the culture medium was collected, then the total anti-oxidant capacity (T-AOC), catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) levels were measured using T-AOC (kit no. A015-1), CAT (kit no. A007-1-1), GSH-Px (kit no. A005), SOD (kit no. A001-3) assay kit, respectively (Tang et al., 2018). All kits were purchased from Nanjing Jiancheng Bioengineering Institute in accordance with the protocol of the manufacturer. The experiments were performed six times.
Real-time PCR
After a 24 h of incubation with or without LPS stimulation, cells were collected, and the total cell RNA was extracted using TRIzol Reagent (Thermo, San Jose, CA, USA) in accordance with the manufacturer’s protocol. The real-time PCR procedure was performed according to our previous studies (Tang et al., 2018; Tang and Xiong, 2022a). Primers used in this study were synthesized by Tsingke Biotechnology Co., Ltd. (Beijing, China), and the sequences were listed as follows: ASCT2 forward 5ʹ- GATCCCCATTGGCACCGAGA -3ʹ, reverse 5ʹ- CATGACACCAGCACCATCGTT -3ʹ; β-actin forward 5ʹ-CATCCTGCGTCTGGACCTGG-3ʹ, reverse 5’-TAATGTCACGCACGATTTCC-3ʹ. The data were analyzed by the 2- (ΔΔCt) method (Tang et al., 2018). The experiments were performed six times.
Western blot analysis
After a 24 h of incubation with or without LPS stimulation, the cells were gently washed with PBS for twice, then lysed in RIPA Lysis Buffer (Abiowell, Changsha, China) in the presence of protease inhibitors (Gentihold, Beijing, China) to obtain the total protein samples. Cellular protein concentration was determined with a bicinchoninic acid (BCA) protein assay kit (Beyotime). Western blot procedure was performed as described previously (Tang et al., 2018). Briefly, equal amount of protein samples was loaded on the SDS-PAGE gels and subsequently the proteins were transferred to PVDF membrane. Thereafter, membranes were blocked with PBST buffer containing 5% for 90 min at room temperature, and then incubated overnight at 4 °C with different primary antibodies: anti-ASCT2 (1:1000 dilution; Cell Signaling Technology, Beverly, MA, USA), and anti-β-actin (1:5000 dilution; Proteintech), and then continue to incubate with secondary antibody (goat antirabbit IgG (H + L); 1:5000 dilution; Abiowell, Changsha, USA) at 37 °C for 90 min. Finally, enhanced chemiluminescence kits (ECL-Plus, Thermo, Waltham, MA, USA) was used to identify immunoreactive bands, and then the densitometry analysis of the immunoreactive bands was performed using the chemiluminescence imaging system (ChemiScope6100; Clinx, Shanghai, China). The experiments were performed six times.
Statistical analysis
Data were presented as the mean ± SEM. Data were subjected to the unpaired t-test using SPSS 21.0 programs (SPSS, Inc., Chicago, IL, USA). The results of all data analyses were input into GraphPad Prism 9.0 (GraphPad Software, Inc., San Diego, CA) software for graphical display (Tang and Xiong, 2022a). The correlations analysis was performed by the genescloud tools, a free online platform for data analysis (https://www.genescloud.cn). P < 0.05 was considered to be statistically significant.
Results
Effects of LPS stimulation on cell viability of IPEC-J2 cells
The effects of LPS on cell viability of IPEC-J2 cells were evaluated by CCK-8 assay. As shown in Figure 1, LPS stimulation significantly reduced the cell viability of IPEC-J2 cells (P < 0.0001), indicating that LPS had strong toxicity to cells.
Figure 1.
Effects of LPS stimulation on cell viability of IPEC-J2 cells. Values are expressed as means ± SEM, N = 6. CON: control group, IPEC-J2 cells without LPS stimulation; LPS: IPEC-J2 cells challenged by LPS. ****P < 0.0001.
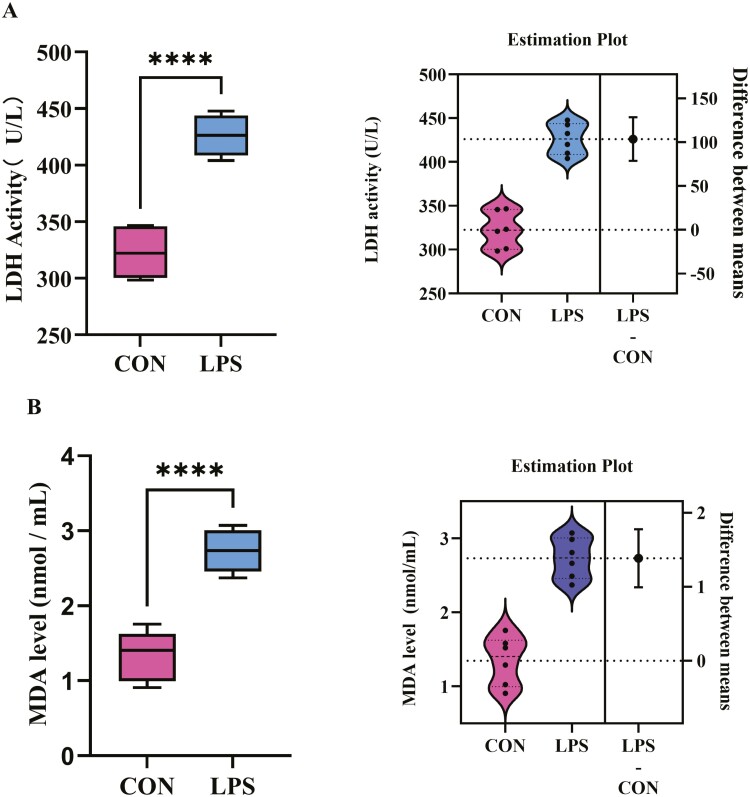
Effects of LPS stimulation on LDH and MDA release of IPEC-J2 cells
To further explore the damage effect of LPS stimulation on IPEC-J2 cells, the LDH and MDA content released from cells into the medium, the indicators of cell injury were examined. Results showed that, LPS stimulation significantly (P < 0.0001) increased LDH content (Figure 2A) and MDA content (Figure 2B) in the medium, which means that LPS stimulation caused cell injury.
Figure 2.
Effects of LPS stimulation on (A) LDH content and (B) MDA content of IPEC-J2 cells. Values are expressed as means ± SEM, N = 6. CON: control group, IPEC-J2 cells without LPS stimulation; LPS: IPEC-J2 cells challenged by LPS. ****P < 0.0001.
Effect of LPS stimulation on the apoptosis of IPEC-J2 cells
The apoptosis of IPEC-J2 cells stimulated by LPS was presented in Figure 3. The results of flow cytometry showed that LPS stimulation significantly increased (P < 0.0001) the late apoptosis rate (2.24% ± 0.12% vs. 16.27% ± 0.31%; Figure 3C) and total apoptosis rate (3.81% ± 0.24% vs. 17.06% ± 0.76%; Figure 3D) of IPEC-J2 cells, but significantly decreased (P < 0.0001) the early apoptosis rate (1.57% ± 0.24% vs. 0.78% ± 0.01%; Figure 3B) of IPEC-J2 cells.
Figure 3.
Effects of LPS stimulation on apoptosis of IPEC-J2 cells. (A) Representative charts of flow cytometry analyses of apoptosis; (B) early apoptosis rate; (C) late apoptosis rate; (D) total apoptosis rate; (E) Representative charts of immunofluorescence; (F) Fluorescence intensity. Values are expressed as means ± SEM, N = 6. CON: control group, IPEC-J2 cells without LPS stimulation; LPS: IPEC-J2 cells challenged by LPS. ****P < 0.0001.
The distribution of Caspase 3 protein was detected by immunofluorescence. As shown in Figure 3E and F, the fluorescence intensity of LPS group was significantly higher than that of control group (P < 0.0001). It means that LPS induced a significant decrease of Caspase3 expression in IPEC-J2 cells. These results indicated that the cells were basically in late apoptosis after 24 h of LPS treatment.
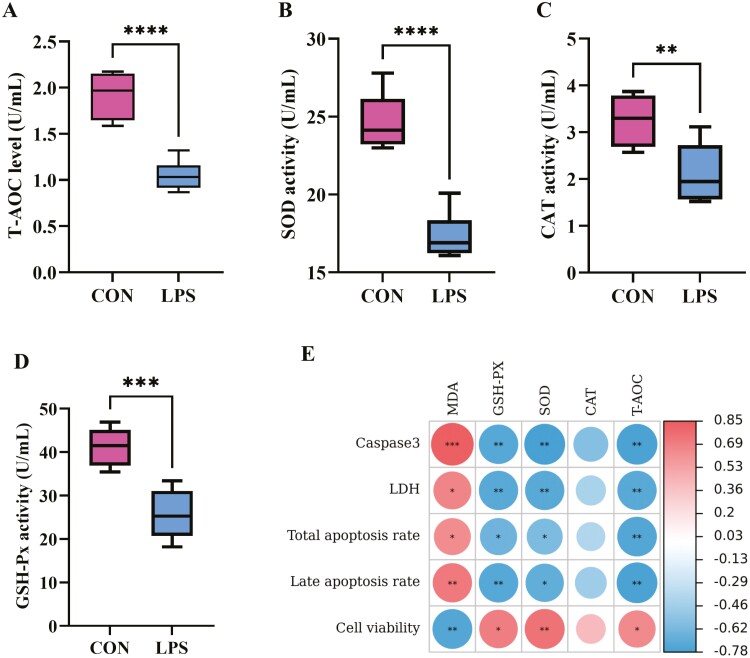
Effect of LPS stimulation on anti-oxidant capacity of IPEC-J2 cells
To investigate the effect of LPS on the anti-oxidant ability of IPEC-J2 cells, the T-AOC, CAT, GSH-Px, and SOD levels in cell culture medium were measured (Figure 4). Results showed that, LPS stimulation significantly reduced the T-AOC levels (P < 0.0001; Figure 4A), SOD activity (P < 0.0001; Figure 4B), CAT activity (P < 0.01; Figure 4C), and GSH-Px activity (P < 0.001; Figure 4D). Pearson’s correlation analysis showed that the redox status was closely related to apoptosis and cell viability of IPEC-J2 cell. MDA level was positively correlated with Caspase3 expression (P < 0.001), LDH content (P < 0.05), total apoptosis rate (P < 0.05), and late apoptosis rate (P < 0.01), and was negatively correlated with cell viability (P < 0.01); GSH-Px activity (P < 0.01, P < 0.01, P < 0.05, P < 0.01), SOD activity (P < 0.01, P < 0.01, P < 0.05, P < 0.05) and T-AOC level (P < 0.01, P < 0.01, P < 0.01, P < 0.01) was negatively correlated with Caspase3 expression, LDH content, total apoptosis rate and late apoptosis rate, and was positively correlated with the cell viability (P < 0.05, P < 0.01, P < 0.05; (Figure 4E). Thus, the results of this study indicated that LPS promoted cell apoptosis by inducing oxidative damage.
Figure 4.
Effects of LPS stimulation on anti-oxidation capacity of IPEC-J2 cells. (A) T-AOC level, (B) SOD activity, (C) CAT activity, (D) GSH-Px activity; (E) the correlations between anti-oxidant capacity and apoptosis, and cell viability of IPEC-J2 cells were analyzed using Pearson’s correlation analysis method performed by the genescloud tools (https://www.genescloud.cn). Values are expressed as means ± SEM, N = 6. CON: control group, IPEC-J2 cells without LPS stimulation; LPS: IPEC-J2 cells challenged by LPS. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Effect of LPS stimulation on the expression of ASCT2 in IPEC-J2 cells
The expression of ASCT2 mRNA was detected by real-time PCR, and the expression of ASCT2 protein was detected by western blot. As shown in Figure 5, LPS stimulation significantly decreased (P < 0.0001) the expression of ASCT2 mRNA (Figure 5A) and ASCT2 protein (Figure 5B). We analyze the correlation between the ASCT2 expression and apoptosis, and the correlations analysis between ASCT2 expression and anti-oxidant capacity of IPEC-J2 cells. Pearson’s correlation analysis showed that ASCT2 mRNA expression (P < 0.001, P < 0.05, P < 0.01, P < 0.01) and ASCT2 protein expression (P < 0.001, p < 0.01, P < 0.05, P < 0.05) was negatively correlated with Caspase3 expression, LDH content, total apoptosis rate and late apoptosis rate, and was positively correlated with the cell viability (P < 0.001, P < 0.001; Figure 5C). ASCT2 mRNA expression (P < 0.05, P < 0.01, P < 0.05) was positively correlated with GSH-Px activity, SOD activity and T-AOC level, and ASCT2 protein expression (P < 0.01, P < 0.001, P < 0.05, P < 0.01) was positively correlated with GSH-Px activity, SOD activity, CAT activity, and T-AOC level; ASCT2 mRNA expression and ASCT2 protein expression was negatively correlated with MDA level (P < 0.01, P < 0.001; Figure 5D).
Figure 5.
Effect of LPS stimulation on the expression of ASCT2 in IPEC-J2 cells. (A) Relative expression of ASCT2 mRNA; (B) Relative expression of ASCT2 protein; (C) The correlations analysis between ASCT2 expression and apoptosis and cell viability of IPEC-J2 cells; (D) the correlations between ASCT2 expression and anti-oxidant capacity of IPEC-J2 cells were analyzed using Pearson’s correlation analysis method performed by the genescloud tools (https://www.genescloud.cn). Values are expressed as means ± SEM, N = 6. CON: control group, IPEC-J2 cells without LPS stimulation; LPS: IPEC-J2 cells challenged by LPS. ****P < 0.0001.
Discussion
The IECs play a critical role in the selective absorption of nutrients and resistance to toxins, allergens and pathogens, and its dysfunction directly affects intestinal barrier function (Tang et al., 2022b). Therefore, maintaining the normal renewal of IECs is very important for intestinal health. As we know, the renewal of IECs mainly involves in the dynamic and strictly regulated proliferation and apoptosis (Cai et al., 2016; Xiong et al., 2016; Tang and Xiong, 2022a). LPS has strong cytotoxicity and can cause cell injury by inducing the release of pro-inflammatory cytokines (Liu et al., 2019; Luo et al., 2019; Zhang et al., 2021) and promoting oxidative stress (Tang et al., 2018; Bao et al., 2022). The most obvious manifestation of LPS-induced cell damage is the low cell viability. Zhang et al. (2019) showed that the cell viability decreased significantly when ovine intestinal epithelial cells (IOECs) were stimulated with LPS; Bao et al. (2022) and Wu et al. (2020) indicated that LPS stimulation significantly decreased the cell viability of IPEC-J2 cells; Li et al. (2018) demonstrated that LPS inhibited cell viability of rat intestinal epithelial cells (IEC-6) in a dose-dependent and a time-dependent manner; similarly, our previous study (Tang et al., 2018) as well as the present study also showed that LPS significantly decreased the cell viability of IPEC-J2 cells. All these studies indicated that LPS has strong cytotoxicity, with serious negative effect on cell viability of IECs.
To further explore the cytotoxicity effect of LPS on IPEC-J2 cells, the release of LDH from cells into the medium, the markers of cell damage, were detected. LDH is a stable glycolytic enzyme, which can be released from the cell rapidly when cells or organs suffered from stress (Todd et al., 2016; Tang et al., 2018; Zhang et al., 2019). Previous studies have shown that LPS stimulation of IOECs cells (Zhang et al., 2019), IPEC-J2 cells (Tang et al., 2018; Wu et al., 2020), and intestinal epithelial cell line HT-29 (Lu et al., 2022) can lead to a large release of LDH from cells. Similar to previous studies, the current study demonstrated that the LDH content was raised after LPS stimulation, indicating that LPS caused severe cell damage.
Apoptosis, also known as programmed cell death, is a physiological process of cell suicide, which plays an important role in the growth and development of the body (Zaman et al., 2014; Sangaran et al., 2021). Uncontrolled apoptosis can lead to disturbance of intestinal mucosal epithelial cell homeostasis and damage intestinal barrier function (Souza et al., 2005; Xiao et al., 2018). When cells are stimulated by antigenic substances, such as LPS, it will lead to intestinal inflammation (Dong et al., 2019; Liu et al., 2019; Zhang et al., 2021) and oxidative damage (Tang et al., 2018; Zhang et al., 2019; Bao et al., 2022), thus promoting cell apoptosis. Previous studies have shown that LPS stimulation leads to apoptosis (Tang et al., 2018; Dong et al., 2019; Zhang et al., 2019). In this study, the results of flow cytometry showed that LPS stimulation caused an increased apoptosis rate, which is similar to previous studies (Li et al., 2018; Tang et al., 2018; Guo et al., 2021). The apoptosis is usually activated by extrinsic (mediated by caspase-8) and intrinsic (mediated by caspase-9) pathways, and either pathway ultimately involves in Caspase3 activation, which subsequently activates the remaining caspase cascade and finally results in apoptotic cell death (Ramachandran et al., 2000; Ghobrial et al., 2005; Li et al., 2018; Tang et al., 2018). In the present study, we detected the expression of Caspase3, a key gene of apoptosis by immunofluorescence staining technique, and the results showed that the expression of Caspase3 was significantly decreased in IPEC-J2 cells stimulated by LPS. Consistent with our present results, Li et al. (2018) showed that the Caspase3 activity was increased in IEC-6 cells treated with LPS; Zhang et al. (2019) reported that the expression of Caspase3 mRNA and protein was significantly increased when IOECs cells were stimulated by LPS; and Tang et al. (2018), Liu et al. (2019), Xiao et al. (2018), and Zhang et al. (2021) showed that LPS stimulation of IPEC-J2 cells significantly increased the expression of Caspase3 mRNA and protein, which indicated that LPS induced apoptosis.
Oxidative stress is characterized by an imbalance between anti-oxidants and pro-oxidants, which has been widely implicated in intestinal epithelium apoptosis (Zhu et al., 2013; Tang et al., 2018; Tang and Xiong, 2022c). The increased MDA level and decreased SOD, CAT, and GSH-Px levels are generally considered as markers of intestinal oxidative injury (Qiu et al., 2019; Tang and Xiong, 2022a). Previous studies have indicated that LPS impaired anti-oxidant defense system by increasing MDA production, and decreasing anti-oxidant enzymes activity, such as, SOD, CAT, and GSH-Px (Tang et al., 2018; Qiu et al., 2019; Zhang et al., 2019; Sun et al., 2020). Similarly, in the present study, IPEC-J2 cells challenged by LPS produced a large amount of MDA, while significantly inhibited the expression of SOD, CAT, and GSH-Px, which demonstrated that LPS caused a serious oxidative injury. Oxidative damage is closely related to apoptosis (Tang et al., 2018; Tang and Xiong, 2022a). Pearson’s correlation analysis showed that the redox status was closely related to apoptosis and cell viability of IPEC-J2 cells. Thus, the results of this study indicated that LPS promoted cell apoptosis by inducing oxidative damage.
Gln is the main energy source of eukaryotic cells, which has important roles in intestinal homeostasis (Tang and Xiong, 2021; Tang et al., 2022a). The transportation of Gln across the intestinal epithelium is mainly mediated by ASCT2 (Nakaya et al., 2014; Tang et al., 2022a), which can be speculated that ASCT expression has a certain effect on intestinal homeostasis. Our previous studies have shown that LPS stimulation can lead to decreased glutamine absorption (Tang and Xiong, 2021), and down-regulated ASCT2 mRNA and protein expression in IPEC-J2 cells (Tang et al., 2022a); on the other hand, LPS stimulation led to increased apoptosis of IPEC-J2 cells (Tang et al., 2018; Zhang et al., 2019). Is there a correlation between the ASCT2 expression and apoptosis in IPEC-J2 cells? It has not been reported yet. This study preliminarily investigated the correlation between ASCT2 expression and apoptosis. The present study showed that LPS stimulation significantly decreased ASCT2 expression, and significantly increased the cell injury and apoptosis of IPEC-J2 cells. We analyze the correlation between the ASCT2 expression and apoptosis, and the results show that ASCT2 expression was negatively correlated with Caspase3 expression, total apoptosis rate, late apoptosis rate, LDH content, and MDA content; and was positively correlated with the cell viability, GSH-Px activity, SOD activity, CAT activity, and T-AOC level, suggesting that ASCT2 is associated with cell oxidative damage and apoptosis of IPEC-J2 cells. It can be preliminarily concluded that LPS promotes the apoptosis and oxidative injury of IPEC-J2 cells by down-regulating the expression of ASCT2, but the specific mechanism needs further study.
Conclusions
In summary, LPS stimulation of IPEC-J2 cells significantly reduced the cell viability and anti-oxidant enzymes activity (SOD, CAT, and GSH-Px), and significantly increased LDH and MDA release; significantly increased apoptosis rate and Caspase 3 expression; and significantly decreased ASCT2 expression. The correlation analysis showed that ASCT2 expression was negatively correlated with apoptosis, and positively correlated with anti-oxidant capacity of IPEC-J2 cells. According to the results of this study, it can be preliminarily concluded that LPS promotes the apoptosis and oxidative injury of IPEC-J2 cells by down-regulating the expression of ASCT2.
Acknowledgments
This research was supported by grants from the China Overseas Expertise Introduction Program for Discipline Innovation (D17016); Guizhou Provincial Science and Technology Foundation (Qiankehe Jichu-ZK [2023] Yiban 267); Guizhou Normal University Academic New Seedling Fund project (Qianshi Xinmiao [2021] B16); and the Natural Science Research Project of Education Department of Guizhou Province (Qianjiaohe KY Zi [2021] 294).
Glossary
Abbreviations
- ASCT2
Alanine-Serine-Cysteine transporter 2
- BCA
bicinchoninic acid
- CAT
catalase
- FBS
fetal bovine serum
- Gln
glutamine
- GSH-Px
glutathione peroxidase
- IECs
intestinal epithelial cells
- IPEC-J2
porcine small intestinal epithelial cells
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MDA
malonaldehyde
- OD
optical density
- SOD
superoxide dismutase
- T-AOC
total anti-oxidant capacity
Contributor Information
Xiaopeng Tang, State Engineering Technology Institute for Karst Desertfication Control, School of Karst Science, Guizhou Normal University, Yunyan District, Guiyang 550001, China.
Kangning Xiong, State Engineering Technology Institute for Karst Desertfication Control, School of Karst Science, Guizhou Normal University, Yunyan District, Guiyang 550001, China.
Jia Liu, Livestock and Poultry Genetic Resources Management Station of Guizhou Province, Yunyan District, Guiyang 550001, China.
Meijun Li, College of Animal Science and Technology, Hunan Biological and Electromechanical Polytechnic, Furong District, Changsha 410127, China.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Achamrah, N., Déchelotte P., and Coëffier M.. . 2017. Glutamine and the regulation of intestinal permeability: from bench to bedside. Curr. Opin. Clin. Nutr. Metab. Care. 2.:86–91. doi: 10.1097/MCO.0000000000000339 [DOI] [PubMed] [Google Scholar]
- Bao, M., Liang M., Sun X., Mohyuddin S. G., Chen S., Wen J., Yong Y., Ma X., Yu Z., Ju X., . et al. 2022. Baicalin alleviates LPS-induced oxidative stress via NF-κB and Nrf2-HO1 signaling pathways in IPEC-J2 cells. Front. Vet. Sci. .:808233. doi: 10.3389/fvets.2021.808233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, X., Zhu L., Chen X., Sheng Y., Guo Q., Bao J., and Xu J.. . 2016. X/XO or H2O2 induced IPEC-J2 cell as a new in vitro model for studying apoptosis in post-weaning piglets. Cytotechnology. 6.:713–724. doi: 10.1007/s10616-014-9823-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalioto, R. M., Maggi C. A., and Giuliani S.. . 2011. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 1.:398–426. doi: 10.2174/092986711794839179 [DOI] [PubMed] [Google Scholar]
- Dong, N., Xu X., Xue C., Wang C., Li X., Bi C., and Shan A.. . 2019. Ethyl pyruvate inhibits LPS induced IPEC-J2 inflammation and apoptosis through p38 and ERK1/2 pathways. Cell Cycle. 1.:2614–2628. doi: 10.1080/15384101.2019.1653106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial, I. M., Witzig T. E., and Adjei A. A.. . 2005. Targeting apoptosis pathways in cancer therapy. CA. Cancer J. Clin. 5.:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- Guo, H., Gao J., Qian Y., Wang H., Liu J., Peng Q., Zhou Y., and Wang K.. . 2021. miR-125b-5p inhibits cell proliferation by targeting ASCT2 and regulating the PI3K/AKT/mTOR pathway in an LPS-induced intestinal mucosa cell injury model. Exp. Ther. Med. 2.:838. doi: 10.3892/etm.2021.10270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Q., Li N., Zhu W., Li Q., and Li J.. . 2007. Glutamine transporter ASCT2 was down-regulated in ischemic injured human intestinal epithelial cells and reversed by epidermal growth factor. J. Parenter. Enteral. Nutr. 3.:86–93. doi: 10.1177/014860710703100286 [DOI] [PubMed] [Google Scholar]
- Li, M., Oshima T., Ito C., Yamada M., Tomita T., Fukui H., and Miwa H.. . 2021. Glutamine blocks interleukin-13-induced intestinal epithelial barrier dysfunction. Digestion. 10.:170–179. doi: 10.1159/000502953 [DOI] [PubMed] [Google Scholar]
- Li, L., Wan G., Han B., and Zhang Z.. . 2018. Echinacoside alleviated LPS-induced cell apoptosis and inflammation in rat intestine epithelial cells by inhibiting the mTOR/STAT3 pathway. Biomed. Pharmacother. 10.:622–628. doi: 10.1016/j.biopha.2018.05.072 [DOI] [PubMed] [Google Scholar]
- Liu, H., Kai L., Du H., Wang X., and Wang Y.. . 2019. LPS inhibits fatty acid absorption in enterocytes through TNF-α secreted by macrophages. Cells. .:1626. doi: 10.3390/cells8121626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D., Yu M., Chen L., Ye J., Huang L., Zhu G., and Lan B.. . 2022. EB1089 promotes the expression of vitamin D receptor in the intestinal epithelial cell line HT-29 and reduces lipopolysaccharide-induced inflammatory response. Ann. Transl. Med. 1.:476. doi: 10.21037/atm-22-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, L., Liu Y., Cai X., Wang Y., Xue J., Zhang J., and Yang F.. . 2019. Bletilla striata polysaccharides ameliorates lipopolysaccharide-induced injury in intestinal epithelial cells. Saudi J. Gastroenterol. 2.:302–308. doi: 10.4103/sjg.SJG_520_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya, M., Xiao Y., Zhou X., Chang J. H., Chang M., Cheng X., Blonska M., Lin X., and Sun S. C.. . 2014. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 4.:692–705. doi: 10.1016/j.immuni.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y., Yang X., Wang L., Gao K., and Jiang Z.. . 2019. L-arginine inhibited inflammatory response and oxidative stress induced by lipopolysaccharide via arginase-1 signaling in IPEC-J2 cells. Int. J. Mol. Sci. 2.:1800. doi: 10.3390/ijms20071800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran, A., Madesh M., and Balasubramanian K. A.. . 2000. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J. Gastroenterol. Hepatol. 1.:109–120. doi: 10.1046/j.1440-1746.2000.02059.x [DOI] [PubMed] [Google Scholar]
- Sangaran, P. G., Ibrahim Z. A., Chik Z., Mohamed Z., and Ahmadiani A.. . 2021. LPS preconditioning attenuates apoptosis mechanism by inhibiting NF-κB and Caspase-3 activity: TLR4 pre-activation in the signaling pathway of LPS-induced neuroprotection. Mol. Neurobiol. 5.:2407–2422. doi: 10.1007/s12035-020-02227-3 [DOI] [PubMed] [Google Scholar]
- Scalise, M., Pochini L., Console L., Losso M. A., and Indiveri C.. . 2018. The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front. Cell Dev. Biol. .:96. doi: 10.3389/fcell.2018.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoultz, I., and Keita V.. . 2020. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. .:1909. doi: 10.3390/cells9081909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, H. S., Tortori C. J., Castelo-Branco M. T., Carvalho A. T., Margallo V. S., Delgado C. F., Dines I., and Elia C. C.. . 2005. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways. Int. J. Colorectal Dis. 2.:277–286. doi: 10.1007/s00384-004-0639-8 [DOI] [PubMed] [Google Scholar]
- Stephens, M., and von der Weid P. Y.. . 2020. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes. 1.:421–432. doi: 10.1080/19490976.2019.1629235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L., Xu G., Dong Y., Li M., Yang L., and Lu W.. . 2020. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules. 2.:1053. doi: 10.3390/molecules25051053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Liu B., Wang X., Yu Q., and Fang R.. . 2018. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int. J. Mol. Sci. 1.:848. doi: 10.3390/ijms19030848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., and Xiong K.. . 2021. Effects of epidermal growth factor on glutamine and glucose absorption by IPEC-J2 cells challenged by lipopolysaccharide using the Ussing chamber system. Pakistan J. Zool. 5.:417–422. doi: 10.17582/journal.pjz/20200117080156 [DOI] [Google Scholar]
- Tang, X., and Xiong K.. . 2022a. Intrauterine growth retardation affects intestinal health of suckling piglets via altering intestinal antioxidant capacity, glucose uptake, tight junction, and immune responses. Oxid. Med. Cell. Longev. 202.:2644205. doi: 10.1155/2022/2644205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., and Xiong K.. . 2022b. Epidermal growth factor activates EGFR/AMPK signalling to up-regulate the expression of SGLT1 and GLUT2 to promote intestinal glucose absorption in lipopolysaccharide challenged IPEC-J2 cells and piglets. Ital. J. Anim. Sci. 2.:943–954. doi: 10.1080/1828051x.2022.2073832 [DOI] [Google Scholar]
- Tang, X., and Xiong K.. . 2022c. Dietary epidermal growth factor supplementation alleviates intestinal injury in piglets with intrauterine growth retardation via reducing oxidative stress and enhancing intestinal glucose transport and barrier function. Animals. 1.:2245. doi: 10.3390/ani12172245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Xiong K., and Fang R.. . 2022a. Effects of epidermal growth factor on ASCT2 expression in IPEC-J2 cells challenged by lipopolysaccharide. Pakistan J. Zool. 5.:1969–1972. doi: 10.17582/journal.pjz/20210325000338 [DOI] [Google Scholar]
- Tang, X., Xiong K., Fang R., and Li M.. . 2022b. Weaning stress and intestinal health of piglets: a review. Front. Immunol. 1.:1042778. doi: 10.3389/fimmu.2022.1042778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, K., Ghiso J., and Rostagno A.. . 2016. Oxidative stress and mitochondria-mediated cell death mechanisms triggered by the familial Danish dementia ADan amyloid. Neurobiol. Dis. 8.:130–143. doi: 10.1016/j.nbd.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Yang C. L., Sha Y. K., Wu Y., Liu Z. Y., Yuan Z. H., and Sun Z. L.. . 2020. Koumine alleviates lipopolysaccharide-induced intestinal barrier dysfunction in IPEC-J2 cells by regulating Nrf2/NF-κB pathway. Am. J. Chin. Med. 4.:127–142. doi: 10.1142/S0192415X2050007X [DOI] [PubMed] [Google Scholar]
- Xiao, Z., Liu L., Tao W., Pei X., Wang G., and Wang M.. . 2018. Clostridium Tyrobutyricum protect intestinal barrier function from LPS-induced apoptosis via P38/JNK signaling pathway in IPEC-J2 cells. Cell. Physiol. Biochem. 4.:1779–1792. doi: 10.1159/000489364 [DOI] [PubMed] [Google Scholar]
- Xiong, X., Yang H., Hu X., Wang X., Li B., Long L., Li T., Wang J., Hou Y., Wu G., . et al. 2016. Differential proteome analysis along jejunal crypt-villus axis in piglets. Front. Biosci. 2.:343–363. doi: 10.2741/4392 [DOI] [PubMed] [Google Scholar]
- Zaman, S., Wang R., and Gandhi V.. . 2014. Targeting the apoptosis pathway in hematologic malignancies. Leuk. Lymphoma. 5.:1980–1992. doi: 10.3109/10428194.2013.855307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Peng A., Yu Y., Guo S., Wang M., and Wang H.. . 2019. L-arginine protects ovine intestinal epithelial cells from lipopolysaccharide-induced apoptosis through alleviating oxidative stress. J. Agric. Food Chem. 67.:1683–1690. doi: 10.1021/acs.jafc.8b06739 [DOI] [PubMed] [Google Scholar]
- Zhang, J., Wan J., Chen D., Yu B., and He J.. . 2021. Low-molecular-weight chitosan attenuates lipopolysaccharide-induced inflammation in IPEC-J2 cells by inhibiting the nuclear factor-κb signalling pathway. Molecules. 2.:569. doi: 10.3390/molecules26030569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L., Cai X., Guo Q., Chen X., Zhu S., and Xu J.. . 2013. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br. J. Nutr. 11.:1938–1947. doi: 10.1017/S0007114513001608 [DOI] [PubMed] [Google Scholar]