Abstract
The aim of this study was to investigate the effect of dietary supplementation of sows with yeast cultures (XPC) during late gestation and lactation on the immune performance of their weaned offspring under lipopolysaccharide (LPS) stress. A total of 40 Landrace × Yorkshire sows (parity 3 to 7) with similar backfat thickness were selected and randomly divided into two treatment groups: a control group (basal diet) and a yeast culture group (basal diet + 2.0 g/kg XPC). The trial was conducted from day 90 of gestation to day 21 of lactation. At the end of the experiment, 12 piglets with similar weights were selected from each group and slaughtered 4 h after intraperitoneal injection with either saline or LPS. The results showed that the concentrations of interleukin-6 (IL-6) in the thymus and tumor necrosis factor-α in the liver increased significantly (P < 0.05) in weaned piglets after LPS injection. Maternal dietary supplementation with XPC significantly reduced the concentration of inflammatory factors in the plasma and thymus of weaned piglets (P < 0.05). LPS injection significantly upregulated the expression of some tissue inflammation-related genes, significantly downregulated the expression of intestinal tight junction-related genes, and significantly elevated the protein expression of liver phospho-nuclear factor kappa B (p-NF-κB), the phospho-inhibitory subunit of NF-κB (p-IκBα), phospho-c-Jun N-terminal kinase (p-JNK), Nuclear factor kappa-B (NF-κB), and the inhibitory subunit of NF-κB (IκBα) in weaned piglets (P < 0.05). Maternal dietary supplementation with XPC significantly downregulated the gene expression of IL-6 and interleukin-10 (IL-10) in the thymus and decreased the protein expression of c-Jun N-terminal kinase (JNK) in the liver of weaned piglets (P < 0.05). In summary, injection of LPS induced an inflammatory response in weaned piglets and destroyed the intestinal barrier. Maternal dietary supplementation of XPC improved the immune performance of weaned piglets by inhibiting inflammatory responses.
Keywords: immune performance, lipopolysaccharide, sow, weaned piglet, yeast culture
Yeast cultures are complex in composition, containing a variety of nutrients and unknown growth factors, and play an important role in maintaining intestinal health and improving immune function. This study showed that the addition of yeast cultures to the maternal feed could improve the immune status of their offspring through maternal transmission.
Introduction
Weaning is an important growth stage for piglets, and is also a key stage in livestock production. In modern intensive farming, early weaning of piglets has greatly improved production efficiency (Su et al., 2022). However, the piglet weaning process can easily cause weaning stress, due to pressures from many stressors such as separation from sows, living with other piglets, and transitioning from breast milk to solid feed (Lallès et al., 2007; Xiong et al., 2019). The structure of the gastrointestinal tract affects the body’s digestion, absorption of nutrients, and regulation of the intestinal flora making it critical to the future health and performance of young animals (Upadhaya and Kim, 2021). Furthermore, weaning stress can lead to changes in intestinal structure and function in piglets, such as destruction of the intestinal structure, decreasing digestion and absorption capacity, impairment of the intestinal barrier, loss of gut microbial diversity, and imbalance of intestinal immune homeostasis (Jayaraman and Nyachoti, 2017; Modina et al., 2019; Xiong et al., 2019), thus increasing morbidity and mortality rates (Lallès et al., 2007). The effects of weaning stress on intestinal barrier function, immune function, and nervous system function in early weaned piglets have been shown to continue into adulthood (Modina et al., 2019; Upadhaya and Kim, 2021), adversely affecting health and performance and causing serious economic losses (Modina et al., 2019). Intrauterine conditions during the critical period of fetal growth and development before birth have long-term effects on the offspring, known as “fetal imprinting” (Johnston et al., 2008). Maternal nutrition affects the development of the sow’s mammary glands, which in turn affects milk production and milk composition, and ultimately the growth and development of their piglets (Farmer, 2018). Therefore, in-depth studies of sow nutrition have been conducted to better understand its effects on fetal imprinting, and have brought long-term beneficial effects on offspring growth and development by improving maternal uterine conditions and the production and quality of milk during lactation (Jiang et al., 2020). Maternal nutritional interventions, in general, have been shown to influence gut health and immune performance in the offspring (Cao et al., 2014; Liu et al., 2016a; Chen et al., 2017) and this remains an important area of study.
Yeast culture is a biological product composed of metabolites produced during anaerobic fermentation of yeast and some live yeast cells. Yeast cultures are complex in composition, containing a variety of nutrients and unknown growth factors (Shen et al., 2009), and play an important role in maintaining intestinal health and improving immune function (Van Heugten et al., 2003; Trckova et al., 2014; Burdick et al., 2021). Lipopolysaccharide (LPS) can cause intestinal injury and inflammatory responses in animals and is often used as an experimental immune system stress agent (Yi et al., 2016). Studies have found that maternal dietary supplementation with yeast culture can improve the growth performance of their offspring during suckling (Zhao et al., 2022), and dietary supplementation with yeast extract in piglets can inhibit the inflammatory response caused by LPS and improve immune performance (Gao et al., 2008; Waititu et al., 2016). However, there is a lack of research on the effect of the addition of yeast cultures to the maternal diet on the immune performance of their weaned offspring under LPS stress. This experiment investigated the effect of adding yeast cultures to the diet of sows during late gestation and lactation on the immunoglobulin and inflammatory responses in weaned piglets under lipopolysaccharide stress.
Materials and Methods
The yeast culture (XPC) used in the experiment was provided by the Diamond V Company (Cedar Rapids, IA, USA). It contained oligosaccharides, proteins (15.21% crude protein), peptides, amino acids, yeast-derived enzymes, nucleic acids, and other nutrients. The trial was conducted at the Yile breeding pig farm (Dekang Group Co., Ltd, Yibin, China). All experimental procedures were approved by the Animal Protection and Use Committee of Sichuan Agricultural University (Ethical Approval Code: SICAU20220119).
Experimental design
A total of 40 Landrace × Yorkshire sows (parity 3 to 7) with similar backfat thickness (16.90 ± 0.42 mm) were selected and randomly divided into two treatment groups: a control group (basal diet) and a yeast culture group (basal diet + 2.0 g XPC per kilogram diet). The trial was conducted from day 90 of gestation to day 21 of lactation. On day 20 of lactation, six litters of piglets with similar average body weight were selected from each group. After fasting overnight, the piglets were individually weighed. Two piglets per litter (24 in total) close to the litter average weight were selected for further study (average weight of the control group: 5.76 ± 0.07 kg; average weight of the yeast culture group: 6.30 ± 0.14 kg). Six piglets in each group were then selected for intraperitoneal injection of 50 μg/kg body weight of Escherichia coli lipopolysaccharide (LPS, Escherichia coli 055: B5; Sigma–Aldrich, St. Louis, MO, USA) (Wang et al., 2011), and the other six piglets were injected intraperitoneally with the same dose of sterile saline. The piglets were slaughtered 4 h after injection. The trial was divided into four treatment groups: 1) sows fed the basal diet and piglets injected with saline (CON); 2) sows fed the basal diet and piglets injected with LPS (CON+LPS); 3) sows fed the basal diet + 2.0 g/kg XPC and piglets injected with saline (XPC); and 4) sows fed the basal diet + 2.0 g/kg XPC and piglets injected with LPS (XPC+LPS). There were six replicates per treatment, with one piglet per replicate. The LPS stock solution (500 µg/mL LPS) was prepared by dissolving LPS in sterile saline.
Feeding management
The basal diet of the test sows during gestation and lactation was formulated according to the nutritional requirements set out by the National Research Council (NRC, USA) (NRC, 2012). The composition and nutritional levels of the basal diet are shown in the supplementary materials (Supplementary Table S1). Gestating sows were fed 3 kg of feed per day (at 0830 and 1430 hours). The sows were transferred to a rinsed and disinfected dry farrowing room at about 110 d of gestation. Lactating sows were fed in increasing amounts, with free feeding (at 830, 1130, and 1730 hours) starting on day 7 of lactation, and a small amount of feed was always left in the trough each day. Sow milk was the only food source for lactating piglets. Sows and piglets were given ad libitum access to water throughout the trial period, and the appropriate temperature was maintained (ambient temperature 18 to 22 °C; the temperature for piglets was adjusted according to the piglets’ age). The humidity was maintained at 55% to 65%. The pens were cleaned daily, and the sows and piglets were immunized according to the pig farm immunization program. The animals were not treated with antibiotics or vaccinated at weaning, and there were no dietary changes during the experiment.
Sample collection
On the day of piglet weaning (21-d-old), 10 mL of anterior vena cava blood was collected at 0 and 4 h after saline or LPS injection, and placed into sodium heparin anticoagulation tubes (Huabo Medical Equipment Co., Ltd, Heze, China). After standing for 30 min, the plasma supernatant was centrifuged at 3,000 × g for 15 min at 4 °C and stored at −20 °C for later analysis. The piglets were anesthetized and slaughtered 4 h after the LPS challenge and the abdominal cavity was opened along the abdominal midline. The intestinal tissues were removed and the mesenteric lymph nodes and some central duodenum, jejunum, ileum, and colon tissues were also collected. In addition, thymus, liver, and spleen tissues were collected, snap frozen in liquid nitrogen, and then stored in a freezer at −80 °C for later analysis.
Plasma immune indices
The concentrations of immunoglobulin G (IgG, RX500984P), immunoglobulin A (IgA, RX500986P), immunoglobulin M (IgM, RX500977P), interleukin-1β (IL-1β, RX501070P), interleukin-6 (IL-6, RX501064P), interleukin-10 (IL-10, RX501078P), and tumor necrosis factor-α (TNF-α, RX500888P) in the plasma of the weaned piglets were determined using a double antibody sandwich enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions. All the ELISA kits were obtained from Ruixin Biotechnology Co., Ltd (Quanzhou, China).
Thymus, liver, and spleen inflammatory factors
About 0.1 g of the various tissues were ground into a powder, weighed, placed in a tube, and about 900 μL of saline was added, followed by homogenization in an ice water mixture box. The supernatant was then collected by centrifugation at 3,000 × g for 10 min at 4 °C to obtain a 10% tissue homogenate. The protein concentration of the homogenate was determined using a BCA protein concentration assay kit (Beyotime Biotechnology, Shanghai, China), according to the manufacturer’s instructions. The concentrations of the inflammatory factors IL-1β, IL-6, IL-10, and TNF-α in the thymus, liver, and spleen of the weaned piglets were determined using an ELISA kit, (Ruixin Biotechnology Co., Ltd), according to the manufacturer’s instructions, and calibrated against known protein concentrations.
Gene expression
A Quantitative Real-time PCR kit (RT-PCR, Huang et al., 2022) was used to determine the expression of all tissue inflammation-related genes: toll-like receptor-4 (TLR-4), toll-like receptor-9 (TLR-9), myeloid differentiation factor 88 (MyD88), TNF-α, IL-1β, IL-6, IL-10, nuclear factor kappa-B (NF-κB) and intestinal tissue tight junction-related genes zonula occluden-1 (ZO-1), Occludin, and Claudin-1. An RNAiso Plus kit (TaKaRa, Tokyo, Japan) was used to extract RNA from all tissues, and the concentration and quality of the RNA were then determined using an ultraviolet spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Waltham, MA, USA) and gel electrophoresis. cDNA was obtained by reverse transcription using a HiScript III RT SuperMix for qPCR (+ gDNA wiper) kit (Vazyme, R323; Vazyme Nanjing, China), according to the manufacturer’s instructions. Fluorescent quantitative PCR was performed using a ChamQ Universal SYBR qPCR Master Mix quantification kit (Vazyme, Q711; Nanjing, China) under the following reaction conditions: 1) 95 °C, 30 s for 1 cycle; 2) 95 °C, 10 s, then 60 °C, 30 s for 40 cycles; 3) 95 °C, 15 s, then 60 °C, 60 s, and finally 95 °C, 15 s for one cycle. The relative expression of the target gene was calculated using the 2−∆∆Ct method with β-actin as the internal reference gene. The target gene primer sequences are shown in the supplementary materials (Table S2) The primers were synthesized by the Tsingke Biotechnology Co., Ltd, Beijing, China.
Protein expression
Western blotting (Xu et al., 2020) was used to determine the expression of liver inflammation and the apoptosis-related proteins phospho-nuclear factor kappa B (p-NF-κB), NF-κB, the phospho-inhibitory subunit of NF-κB (p-IκBα), the inhibitory subunit of NF-κB (IκBα), phospho-c-Jun N-terminal kinase (p-JNK), c-Jun N-terminal kinase (JNK), cysteine aspastic acid-specific protease 3 (Caspase3), the phospho-p38 MAPK (p-p38), and p38 MAPK (p38) in the weaned piglets. The method was as follows: 10 mg of liver tissue was taken, and 600 μL of RIPA lysate (Beyotime Biotechnology) was added and the sample was ground and ultrasonically crushed, and then centrifuged at 8,000 × g at 4 °C for 30 min before the supernatant was collected to determine the protein concentration using the BCA method. The protein concentrations were adjusted using RIPA lysate. Loading buffer (Bio-Rad, Hercules, CA, USA) and sulfhydryl reducing agent (Solarbio Science & Technology Co., Ltd, Beijing, China) were then added proportionally, mixed well, and denatured at 98 °C for 8 min. An SDS–PAGE gel rapid preparation kit (Beyotime Biotechnology) was used to prepare a 10% separation gel and a 5% concentration gel. After uploading the samples, electrophoresis was performed, and the gel was then transferred to a PVDF membrane (Bio-Rad). After the transfer was completed, the membrane was washed with 1×TBST for 5 min, and then closed with 1% BSA for 1 h. The primary antibody was added and incubated overnight at 4 °C. The membrane was washed three times with 1×TBST and closed with 5% skimmed milk powder containing secondary antibody for 1 h. After washing the membrane three times, a color development reaction was carried out using an ultrasensitive ECL chemiluminescence kit (Beyotime Biotechnology), according to the manufacturer’s instructions. The grayscale values of the strips were analyzed using Image Lab software.
Statistical analysis
The data were analyzed by two-wayanalysis of variance using the general linear model (GLM) in the SPSS 27.0 statistical software. Shapiro-Wilk and Levene’s tests were used to test the normality and homogeneity of variance before statistical analysis. If there was no interaction between the independent variables, main effects analyses were performed; if there was an interaction between the independent variables, separate effects, and interaction control analyses were performed. The results were expressed as “mean ± standard error”, with P < 0.05 indicating a significant difference, and 0.05 ≤ P < 0.1 indicating a significant trend.
Results
Plasma immunoglobulins and inflammatory factors
LPS injection of weaned piglets tended to increase plasma concentrations of IgG (P = 0.094) and TNF-α (P = 0.097) (Tables 1, 2). Addition of XPC to the maternal diet significantly reduced plasma concentrations of IgG, IgA, IgM, IL-1β, IL-6, IL-10, and TNF-α in their weaned piglets (P < 0.05).
Table 1.
Effects of maternal dietary supplementation with XPC1 on the plasma immunoglobulins of weaned piglets after LPS challenge2
| Item3 | CON | XPC | P-value | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | XPC | LPS | XPC×LPS | |
| IgG, mg/L | |||||||
| 0 h | 3.69 ± 0.09 | 3.88 ± 0.20 | 3.33 ± 0.27 | 3.71 ± 0.36 | 0.301 | 0.273 | 0.721 |
| 4 h | 4.36 ± 0.10 | 4.64 ± 0.14 | 3.62 ± 0.28 | 4.12 ± 0.29 | 0.010 | 0.094 | 0.627 |
| IgA, μg/L | |||||||
| 0 h | 210.06 ± 7.71 | 180.30 ± 5.21 | 155.02 ± 15.92 | 166.96 ± 14.54 | 0.009 | 0.457 | 0.091 |
| 4 h | 202.17 ± 6.58 | 199.65 ± 5.49 | 183.72 ± 13.39 | 186.78 ± 2.82 | 0.079 | 0.975 | 0.744 |
| IgM, mg/L | |||||||
| 0 h | 2.24 ± 0.11 | 2.04 ± 0.09 | 1.75 ± 0.29 | 1.98 ± 0.27 | 0.198 | 0.955 | 0.326 |
| 4 h | 2.59 ± 0.10 | 2.56 ± 0.13 | 2.09 ± 0.27 | 2.24 ± 0.22 | 0.043 | 0.736 | 0.657 |
1Sows were fed yeast culture (XPC, Diamond V, Cedar Rapids, IA, USA) from day 90 of gestation to day 21 of lactation.
2CON = sows fed control diet; XPC = sows fed diet supplemented with 2.0 g/kg XPC. Piglets were weaned at 21 d of age and slaughtered 4 h after lipopolysaccharide (LPS) injection on the day of weaning.
3IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M. Data are means ± standard errors of six piglets per treatment.
Table 2.
Effects of maternal dietary supplementation with XPC1 on plasma inflammatory factors of weaned piglets after LPS challenge2
| Item3 | CON | XPC | P-value | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | XPC | LPS | XPC×LPS | |
| IL-1β, pg/mL | |||||||
| 0 h | 115.10 ± 3.37 | 124.07 ± 4.46 | 91.91 ± 9.34 | 95.43 ± 8.09 | 0.001 | 0.368 | 0.693 |
| 4 h | 113.87 ± 4.76 | 125.76 ± 2.14 | 105.36 ± 9.57 | 102.22 ± 7.51 | 0.025 | 0.516 | 0.269 |
| IL-6, pg/mL | |||||||
| 0 h | 71.31 ± 3.95 | 83.88 ± 4.88 | 66.71 ± 9.94 | 72.36 ± 11.51 | 0.339 | 0.282 | 0.679 |
| 4 h | 101.31 ± 3.76 | 98.75 ± 3.66 | 78.28 ± 10.52 | 82.97 ± 10.02 | 0.021 | 0.892 | 0.644 |
| IL-10, pg/mL | |||||||
| 0 h | 21.74 ± 0.79 | 20.91 ± 1.10 | 19.06 ± 2.72 | 19.77 ± 2.90 | 0.373 | 0.978 | 0.716 |
| 4 h | 27.45 ± 1.59 | 25.71 ± 1.56 | 19.05 ± 1.93 | 19.62 ± 2.50 | 0.001 | 0.764 | 0.555 |
| TNF-α, pg/mL | |||||||
| 0 h | 45.99 ± 1.43 | 49.78 ± 1.21 | 38.09 ± 2.90 | 43.96 ± 3.74 | 0.014 | 0.072 | 0.687 |
| 4 h | 46.34 ± 1.91 | 51.09 ± 2.07 | 38.35 ± 2.81 | 42.79 ± 3.49 | 0.006 | 0.097 | 0.953 |
1Sows were fed yeast culture (XPC, Diamond V, Cedar Rapids, IA, USA) from day 90 of gestation to day 21 of lactation.
2CON = sows fed control diet; XPC = sows fed diet supplemented with 2.0 g/kg XPC. Piglets were weaned at 21 d of age and slaughtered 4 h after lipopolysaccharide (LPS) injection on the day of weaning.
3IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α. Data are means ± standard errors of six piglets per treatment.
Tissue inflammatory factors
LPS injection significantly increased the concentrations of IL-6 in the thymus and TNF-α in the liver (P < 0.05) of weaned piglets (Table 3), tended to increase the concentrations of IL-1β (P = 0.059) and IL-10 (P = 0.051) in the liver, and significantly decreased the concentration of IL-10 in the spleen (P < 0.05) of weaned piglets. In addition, maternal dietary supplementation with XPC significantly decreased the concentrations of IL-1β, IL-6, IL-10, and TNF-α in the thymus of weaned piglets (P < 0.05), and significantly decreased the concentrations of TNF-α in the liver and IL-10 in the spleen (P < 0.05).
Table 3.
Effects of maternal dietary supplementation with XPC1 on tissue inflammatory factors of weaned piglets after LPS challenge2
| Item3 | CON | XPC | P-value | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | XPC | LPS | XPC×LPS | |
| Thymus | |||||||
| IL-1β, pg/mg | 16.59 ± 1.05 | 17.63 ± 0.87 | 13.28 ± 0.91 | 13.83 ± 0.69 | 0.001 | 0.382 | 0.788 |
| IL-6, pg/mg | 7.42 ± 0.65 | 8.90 ± 0.47 | 5.37 ± 0.37 | 6.44 ± 0.66 | 0.001 | 0.031 | 0.715 |
| IL-10, pg/mg | 1.41 ± 0.05 | 1.68 ± 0.05 | 1.23 ± 0.11 | 1.21 ± 0.09 | 0.001 | 0.155 | 0.104 |
| TNF-α, pg/mg | 4.75 ± 0.33 | 5.14 ± 0.21 | 3.24 ± 0.24 | 3.70 ± 0.31 | 0.000 | 0.135 | 0.896 |
| Liver | |||||||
| IL-1β, pg/mg | 9.92 ± 0.55 | 10.99 ± 1.04 | 9.70 ± 0.46 | 10.71 ± 0.62 | 0.637 | 0.059 | 0.945 |
| IL-6, pg/mg | 10.14 ± 0.91 | 10.75 ± 0.53 | 9.61 ± 0.82 | 10.11 ± 0.58 | 0.429 | 0.457 | 0.938 |
| IL-10, pg/mg | 1.89 ± 0.04 | 2.22 ± 0.08 | 1.88 ± 0.14 | 2.00 ± 0.12 | 0.301 | 0.051 | 0.354 |
| TNF-α, pg/mg | 3.43 ± 0.23 | 3.87 ± 0.13 | 3.01 ± 0.09 | 3.44 ± 0.17 | 0.018 | 0.015 | 0.998 |
| Spleen | |||||||
| IL-1β, pg/mg | 13.21 ± 0.68 | 11.71 ± 0.34 | 11.82 ± 0.33 | 12.24 ± 0.80 | 0.484 | 0.378 | 0.127 |
| IL-6, pg/mg | 5.44 ± 0.26 | 5.14 ± 0.23 | 5.12 ± 0.04 | 5.96 ± 0.63 | 0.506 | 0.486 | 0.147 |
| IL-10, pg/mg | 1.16 ± 0.06 | 0.98 ± 0.04 | 1.02 ± 0.01 | 0.92 ± 0.02 | 0.026 | 0.005 | 0.335 |
| TNF-α, pg/mg | 4.16 ± 0.13 | 3.59 ± 0.21 | 3.73 ± 0.31 | 3.75 ± 0.42 | 0.398 | 0.103 | 0.079 |
1Sows were fed yeast culture (XPC, Diamond V, Cedar Rapids, IA, USA) from day 90 of gestation to day 21 of lactation.
2CON = sows fed control diet; XPC = sows fed diet supplemented with 2.0 g/kg XPC. Piglets were weaned at 21 d of age and slaughtered 4 h after lipopolysaccharide (LPS) injection on the day of weaning.
3IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α. Data are means ± standard errors of six piglets per treatment.
Tissue inflammation-related gene expression
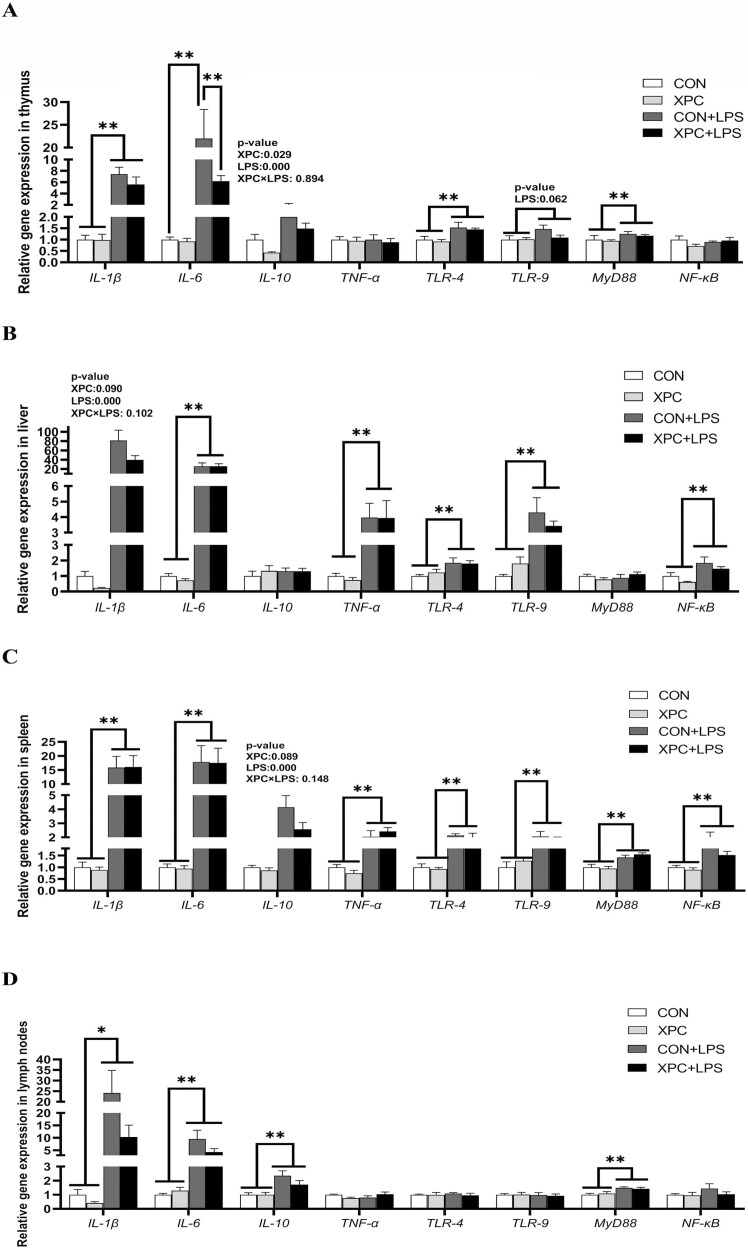
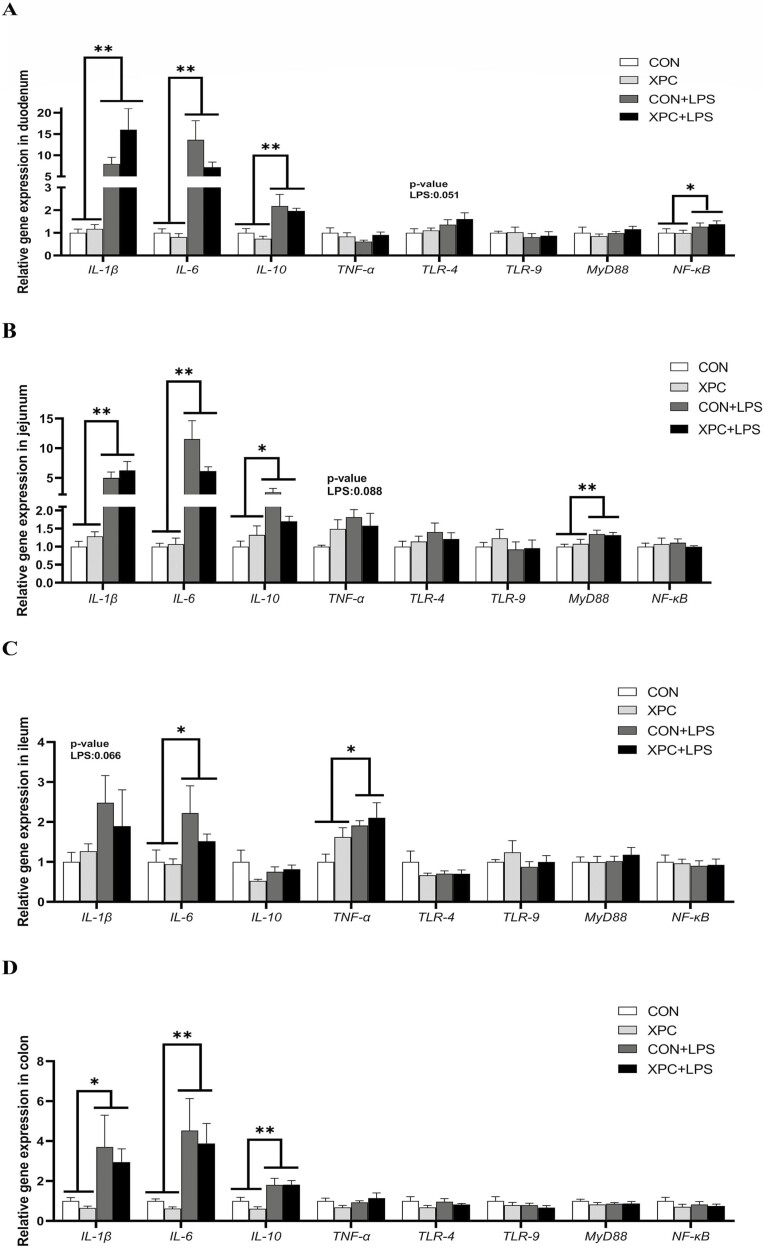
In various tissues of weaned piglets (P < 0.05), LPS injection significantly upregulated the expression of IL-1β (except in the ileum), IL-6 (except in the thymus), and IL-10 (except in the liver and ileum) (Figures 1A to D and 2A to D), and significantly upregulated the expression of TNF-α in the liver, spleen, and ileum (P < 0.05). LPS injection also significantly upregulated the expression of TLR-4 in the thymus, liver, and spleen (P < 0.05), TLR-9 in the liver and spleen (P < 0.05), MyD88 in the thymus, spleen, lymph nodes, and jejunum (P < 0.05), and NF-κB in the liver, spleen, and duodenum of weaned piglets (P < 0.05). In addition, LPS injection tended to upregulate the expression of IL-1β (P = 0.066) in the ileum, TNF-α (P = 0.088) in the jejunum, TLR-4 (P = 0.051) in the duodenum, and TLR-9 (P = 0.062) in the thymus of weaned piglets. The expression of IL-6 in the thymus of piglets in the CON group was significantly upregulated after LPS injection, and the expression of IL-6 in the XPC+LPS group was significantly lower than that in the CON+LPS group (P < 0.05). Maternal dietary supplementation with XPC significantly downregulated the expression of IL-10 in the thymus of weaned piglets (P < 0.05) and tended to downregulate the expression of IL-1β in the liver (P = 0.090) and IL-10 in the spleen (P = 0.089).
Figure 1.
Effect of XPC on the expression of tissues inflammation-related genes of weaned piglets. (A) thymus, (B) liver, (C) spleen, (D) lymph nodes. IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; TLR-4, toll-like receptor-4; TLR-9, toll-like receptor-9; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa-B. Data are means ± standard errors of six piglets per treatment. CON: sows fed basal diet, piglets injected with saline; XPC: sows fed basal diet + 2.0 g/kg XPC, piglets injected with saline; CON+LPS: sows fed basal diet, piglets injected with LPS; XPC+LPS: sows fed basal diet + 2.0 g/kg XPC, piglets injected with LPS. * P < 0.05, ** P < 0.01.
Tight junction-related gene expression in intestinal tissue
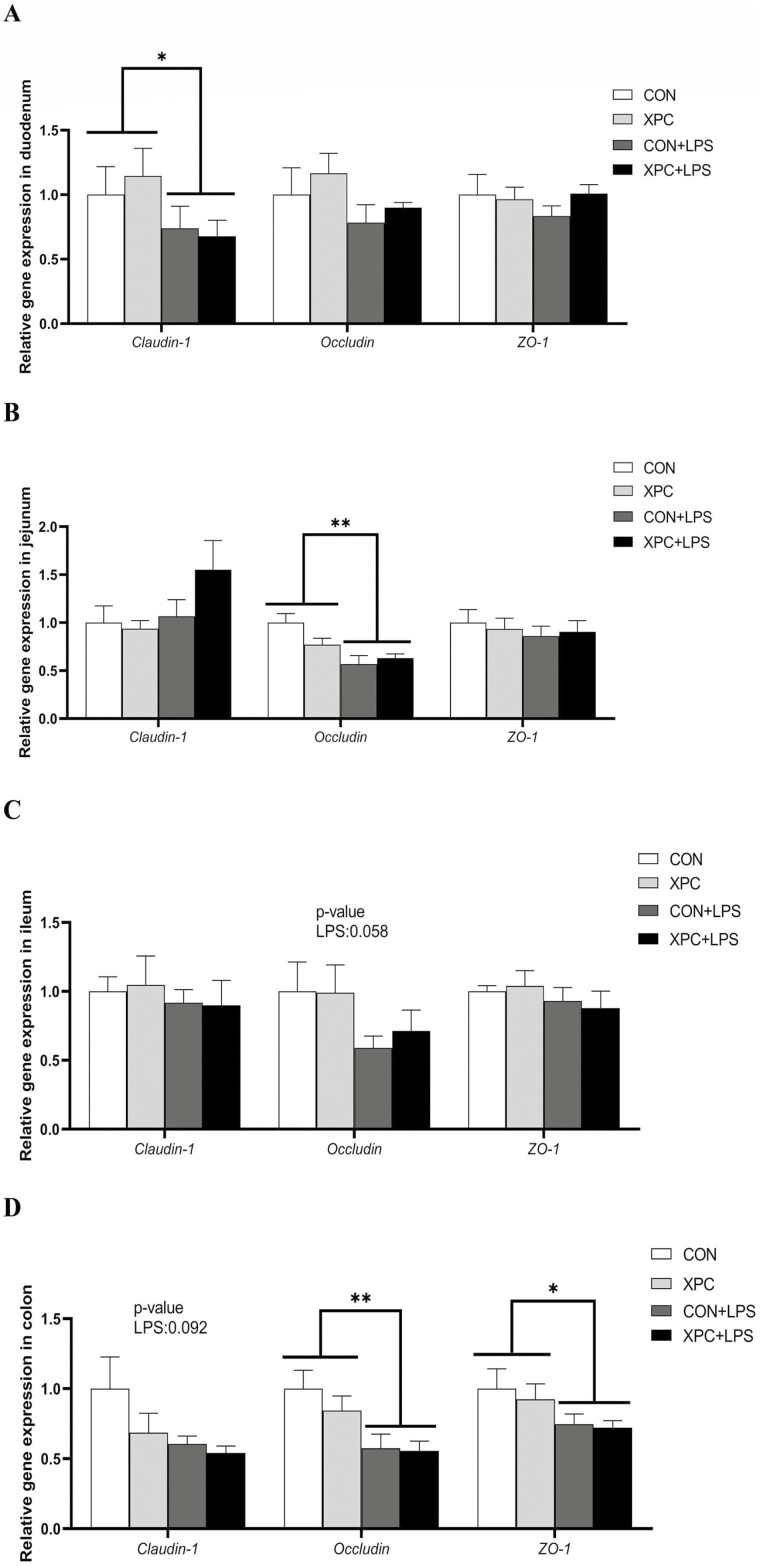
LPS injection significantly downregulated the expression of Claudin-1 in the duodenum and Occludin in the jejunum (P < 0.05), significantly downregulated the expression of Occludin and ZO-1 in the colon (P < 0.05), and tended to downregulate the expression of Occludin in the ileum (P = 0.058) and Claudin-1 in the colon (P = 0.092) (Figure 3A to D). Maternal XPC supplementation had no significant effect on intestinal tight junction-related genes in weaned piglets (P > 0.05).
Figure 3.
Effect of XPC on the expression of intestinal tight junction related genes of weaned piglets. (A) duodenum, (B) jejunum, (C) ileum, (D) colon. ZO-1, zonula occluden-1. Data are means ± standard errors of six piglets per treatment. CON: sows fed basal diet, piglets injected with saline; XPC: sows fed basal diet + 2.0 g/kg XPC, piglets injected with saline; CON+LPS: sows fed basal diet, piglets injected with LPS; XPC+LPS: sows fed basal diet + 2.0 g/kg XPC, piglets injected with LPS. * P < 0.05, ** P < 0.01.
Figure 2.
Effect of XPC on the expression of intestinal inflammation-related genes of weaned piglets. (A) duodenum, (B) jejunum, (C) ileum, (D) colon. IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; TLR-4, toll-like receptor-4; TLR-9, toll-like receptor-9; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa-B. Data are means ± standard errors of six piglets per treatment. CON: sows fed basal diet, piglets injected with saline; XPC: sows fed basal diet + 2.0 g/kg XPC, piglets injected with saline; CON+LPS: sows fed basal diet, piglets injected with LPS; XPC+LPS: sows fed basal diet + 2.0 g/kg XPC, piglets injected with LPS. * P < 0.05, ** P < 0.01.
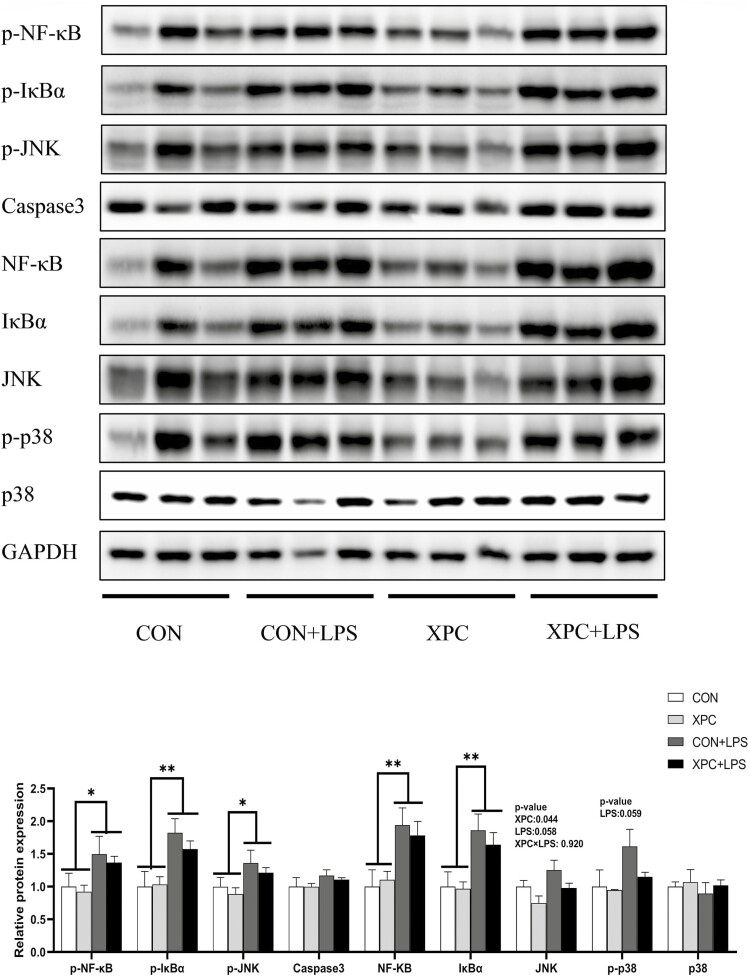
Liver immune-related protein expression
LPS injection significantly increased the protein expression of p-NF-κB, p-IκBα, p-JNK, NF-κB, and IκBα in the liver of weaned piglets (P < 0.05) (Figure 4), and there was a trend to increased protein expression of JNK (P = 0.058) and p-p38 (P = 0.059). Maternal dietary addition of XPC significantly reduced the protein expression of JNK in piglet liver (P < 0.05). The two treatments had no significant effect on the protein expression of Caspase3 and p38 (P > 0.05).
Figure 4.
Effect of XPC on liver protein expression of weaned piglets after LPS challenge. p-NF-κB, phospho-nuclear factor kappa B; p-IκBα, phospho-inhibitory subunit of NF-κB; p-JNK, phospho-c-Jun N-terminal kinase; Caspase3, cysteine aspastic acid-specific protease 3; NF-κB, nuclear factor kappa-B; IκBα, inhibitory subunit of NF-κB; JNK, c-Jun N-terminal kinase. Data are means ± standard errors of six piglets per treatment. CON: sows fed basal diet, piglets injected with saline; XPC: sows fed basal diet + 2.0 g/kg XPC, piglets injected with saline; CON+LPS: sows fed basal diet, piglets injected with LPS; XPC+LPS: sows fed basal diet + 2.0 g/kg XPC, piglets injected with LPS. * P < 0.05, ** P < 0.01.
Discussion
Yeast cultures are complex in composition and are considered beneficial biologics that play a positive role in both promoting animal growth and maintaining intestinal health (Van Heugten et al., 2003; Trckova et al., 2014). Our previous study found that maternal dietary supplementation with yeast cultures promoted offspring growth, increased the abundance of beneficial bacteria, and improved the structure of the intestinal community (Liu et al., 2023). However, the effect of maternal dietary supplementation with yeast cultures on the inflammatory response of weaned piglet offspring under lipopolysaccharide stress is so far unknown, prompting the present study.
The results of this experiment showed that maternal dietary supplementation with XPC reduced the concentrations of inflammatory factors in the plasma and thymus of their weaned piglets and reduced the concentrations of TNF-α in the liver and IL-10 in the spleen. In addition, maternal supplementation with XPC down-regulated the expression of IL-1β in the liver, IL-6 and IL-10 in the thymus, and IL-10 in the spleen of their weaned piglets. The levels of pro-inflammatory factors were lower in the XPC treatment group, leading to correspondingly lower levels of anti-inflammatory factors in their piglets. This reflected the less intense degree of piglet inflammatory response in the XPC group compared with the control group, and that XPC piglets were better able to counteract the immune stress. These results suggested that addition of XPC to the maternal diet alleviated the inflammatory response in weaned piglets and facilitated the recovery of weaned piglets after LPS challenge. In the previous phase of this project, it was found that addition of XPC to the maternal diet increased the abundance of Alloprevotella spp. in the colon of weaned piglets (Liu et al., 2023), and that Alloprevotella spp. mainly produced succinate and acetate, both of which have improved intestinal barrier and anti-inflammatory effects (Downes et al., 2013). This result further validated the statement above. It was found that feeding β-glucan to piglets suppressed the elevation of TNF-α and IL-6 in the plasma caused by LPS inoculation and further enhanced the elevation of IL-10 (Li et al., 2006). Another study found that adding yeast extract to piglet diets after challenge reduced the level of TNF-α in the serum, increased the level of IL-10 in the serum, and increased the gene expression of IL-10 in the ileum (Waititu et al., 2016). The results of our current experiment are consistent with previous studies in which yeast products inhibited the elevation of levels of pro-inflammatory factors caused by LPS. However, our results are inconsistent with previous studies in which yeast products further enhanced the levels of anti-inflammatory factors. The piglets in this trial were recently weaned at 21 d of age, and their immune system development differed from that of nursery piglets, resulting in different immune responses to inflammatory reactions between the two. The dose of LPS administered was set relatively low due to the young age of the piglets. Therefore, the different results of different studies may be related to the age of the piglets tested (21 d of age, c.f., 32 d of age and 63 d of age), the LPS dose, and differences in maternal yeast transmission.
Immunoglobulins act on antibodies and can regulate the body’s immunity. Their main function is to identify and exclude antigenic foreign bodies (Butler and Brown, 1994) and they can also interact with immune cells and other immune active substances to maintain the relative stability of an animal’s physiology (Sun et al., 2020). IgG is the major immunoglobulin in pig serum, accounting for approximately 88% of the total (Butler and Brown, 1994). IgG is produced by B lymphocytes through class switching, and IgG and IgM promote microbial phagocytosis by activating the complement system (Liu and Cao, 2016b). It has been found that IgG concentration in a piglet’s serum increased after LPS challenge, and the addition of mannan-oligosaccharide further increased IgG serum concentration (Li et al., 2021). In this study, weaned piglets were found to have increased plasma IgG concentrations after LPS injection, XPC supplantation of maternal diets decreased a piglet’s plasma IgG, IgA, and IgM concentrations. This result implied that IgG production was effective in reducing the inflammatory response. Note that the lower concentration of immunoglobulins in the XPC group may be related to the lower levels of inflammatory factors in the XPC group. The inflammatory response in the XPC group may be weaker, reducing the level of inflammatory factors in the body, alleviating the need to produce excessive immunoglobulin to suppress the inflammatory response. In addition, the XPC group may better resist the invasion of pathogens. When LPS is injected, the body can respond more quickly and mobilize the immune system for defense. The level of inflammatory factors in the tissues of piglets in the XPC group was lower. Our previous study found that addition of XPC to the maternal diet could increase the abundance of Alloprevotella spp., which have anti-inflammatory effects in the colon of piglets (Liu et al., 2023). These results supported the statement above, as both inflammatory factor levels and immunoglobulin concentrations were lower in the XPC group.
Mammalian toll-like receptors (TLR) recognize specific patterns in microbial assemblages and can activate antigen-specific immunity (Li et al., 2006). LPS is commonly used as an experimental immune stressor and has been found to induce an inflammatory response through the TLR-4-MyD88-NF-κB signaling pathway, promoting the secretion of cytokines (including the pro-inflammatory factors IL-1β, IL-6, and TNF-α and the anti-inflammatory factor IL-10) (Johnson, 1997; Webel et al., 1997; Sun et al., 2015). The increased concentrations of TNF-α in the plasma, IL-6 in the thymus, IL-1β, TNF-α, and IL-10 in the liver, and the upregulation of inflammation-related gene expression in tissues after LPS injection in weaned piglets observed in this experiment all indicated the success of LPS challenge.
Tight junction proteins play an important role in maintaining the normal intestinal barrier function, and three of them, Claudin-1, Occludin, and ZO-1, are expressed in almost all epithelial cells (Hampson, 1986; Pi et al., 2014). If the tight junction proteins are dysregulated, the intestinal barrier is disrupted, triggering intestinal damage (Slifer and Blikslager, 2020; Wen et al., 2020). The results of our experiment revealed that LPS challenge downregulated the expression of the intestinal tight junction-related genes Claudin-1, Occludin, and ZO-1 in weaned piglets, and that maternal dietary supplementation with XPC did not alter this result. Similarly, previous studies have found that LPS injection of piglets significantly downregulated the gene expression of ZO-1 and Occludin in the jejunum and ileum (Ding et al., 2021; Mou et al., 2021). This implies that LPS interferes with the expression of tight junction proteins and causes damage to the intestinal barrier of weaned piglets. In addition, maternal dietary addition of XPC did not affect the expression of tight junction proteins in weaned piglets. We hypothesize that the damage to the intestinal barrier of piglets caused by LPS is very severe and cannot be effectively mitigated by XPC.
The liver is an ideal site for detecting pathogens that enter the body through the intestine, and can remove bacteria, viruses, and some macromolecules, so that the body is in a state of low immune response, which plays a vital role in the overall health of an individual (Berg, 1995; Li et al., 2005; Paulos et al., 2007; Jiang et al., 2009; Kubes and Jenne, 2018). Therefore, our tests examined the expression of liver inflammation-related proteins to examine the immune status of the piglets. Previous studies have found that LPS challenge significantly upregulated the phosphorylation level of NF-κB in weaned piglets (Ding et al., 2021) and that the placental NF-κB, JNK, and p38 signaling pathways were activated in mice after LPS injection (Mou et al., 2021). Pathogen infection or tissue damage can stimulate pattern recognition receptors on the surface, and in the cytoplasm, of innate immune cells (Lumsden et al., 1988; Kubes and Jenne, 2018) causing the activation of mitogen-activated protein kinase (MAPK) subfamily members, and extracellular signal-regulated kinase (ERK), p38, and JNK (Webel et al., 1997). Pattern recognition receptors also activate NF-κB and interferon regulatory factor transcription factors. MAPK activation induces the expression of inflammation-related genes, thereby regulating the inflammatory response (Arthur and Ley, 2013). This experiment also found that LPS injection activated the hepatic NF-κB, JNK, and p38 signaling pathways in weaned piglets, as well as elevating the levels of inflammatory factors in the liver, indicating that LPS migrated from the intestine to the liver and triggered a hepatic inflammatory response. Maternal dietary addition of XPC reduced liver JNK protein expression in weaned piglets, suggesting that XPC may be able to alleviate the liver inflammatory response to some extent. We speculate that the liver removes the pathogens in the blood in a timely manner, so that the body maintains a state of low immune response.
Our previous study found that maternal dietary supplementation with XPC increased the weight of their weaned piglets (6.31 vs. 5.72 kg), average daily gain (0.23 vs. 0.20 kg), and average daily feed intake of sows during lactation (6.27 vs. 5.78 kg) (Liu et al., 2023). Higher feed intake of sows during lactation leads to higher milk yields, leading to higher weight of piglets at weaning (Jiang et al., 2020). We speculate that the immune system of piglets in the XPC group, with their higher body weight, was more developed and had better immunomodulatory than the control group. Therefore, the better resistance to LPS of piglets in the XPC group is strongly related to their own body weight.
Conclusion
In this study, LPS was effective in causing an inflammatory response in weaned piglets, causing intestinal and tissue damage. Maternal dietary supplementation with yeast cultures alleviated the inflammatory response in their weaned piglets by reducing plasma and tissue inflammatory factor levels and decreasing the expression of tissue inflammation-related genes and proteins, thereby improving the immune performance of piglets to some extent. Therefore, supplementation of sow diets with yeast cultures during late gestation and lactation improved the immune status of their offspring through maternal transmission.
Supplementary Material
Acknowledgments
This research was funded by Major Scientific and Technological Special Project of Sichuan Province (2021ZDZX0009), Natural Science Foundation of Sichuan Province (2022NSFSC1628), Sichuan Province “145” Breeding Tackle Project (2021YFYZ0008) and China Agriculture Research System (CARS-35). We would like to thank Diamond V, United States for kindly providing yeast culture (XPC) and Higher Education Discipline Innovation Project of China (D17015).
Glossary
Abbreviations
- Caspase3
cysteine aspartic acid-specific protease 3
- CLRs
C-type lectin receptors
- ELISA
enzyme-linked immunosorbent assay
- ERK
subfamily members – extracellular signal-regulated kinase
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IκBα
inhibitory subunit of NF-κB
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- JNK
c-Jun N-terminal kinase
- LPS
E. coli lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MyD88
myeloid differentiation factor 88
- NF-κB
nuclear factor kappa-B
- NLRs
NOD-like receptors
- p-IκBα
phospho-inhibitory subunit of NF-κB
- p-JNK
phospho-c-Jun N-terminal kinase
- p-NF-κB
phospho-nuclear factor kappa B
- RLRs
RIG-I-like receptors
- TLR
Toll-like receptors
- TLR-4
toll-like receptor-4
- TLR-9
toll-like receptor-9
- TNF-α
tumor necrosis factor-α
- XPC
yeast cultures
- ZO-1
zonula occluden-1
Contributor Information
Yalei Liu, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Xinlin Jia, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Junlei Chang, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Xunjing Pan, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Xuemei Jiang, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Lianqiang Che, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Yan Lin, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Yong Zhuo, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Bin Feng, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Zhengfeng Fang, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Jian Li, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Lun Hua, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Jianping Wang, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Mengmeng Sun, College of Science, Sichuan Agricultural University, Xin Kang Road, Yucheng District, Ya’an 625014, P.R. China.
De Wu, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Shengyu Xu, Animal Disease-Resistance Nutrition, Ministry of Education, Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, P. R. China.
Conflict of interest statement
The authors declare no conflicts of interest associated with this study.
Literature Cited
- Arthur, J. S., and Ley S. C.. . 2013. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 1.:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- Berg, R. D. 1995. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. .:149–154. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- Burdick, N. C., Broadway P. R., and Carroll J. A.. . 2021. Influence of yeast products on modulating metabolism and immunity in cattle and swine. Animals. 1.:371. doi: 10.3390/ani11020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, J. E., and Brown W. R.. . 1994. The immunoglobulins and immunoglobulin genes of swine. Vet. Immunol. Immunopathol. 4.:5–12. doi: 10.1016/0165-2427(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Cao, M., Che L., Wang J., Yang M., Su G., Fang Z., Lin Y., Xu S., and Wu D.. . 2014. Effects of maternal over- and undernutrition on intestinal morphology, enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition. 3.:1442–1447. doi: 10.1016/j.nut.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Mou D., Hu L., Zhen J., Che L., Fang Z., Xu S., Lin Y., Feng B., Li J., . et al. 2017. Effects of maternal low-energy diet during gestation on intestinal morphology, disaccharidase activity, and immune response to lipopolysaccharide challenge in pig offspring. Nutrients. .:1115. doi: 10.3390/nu9101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, D., Mou D., Zhao L., Jiang X., Che L., Fang Z., Xu S., Lin Y., Zhuo Y., Li J., . et al. 2021. Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct. 1.:11214–11228. doi: 10.1039/d1fo01653a. [DOI] [PubMed] [Google Scholar]
- Downes, J., Dewhirst F. E., Tanner A. C. R., and Wade W. G.. . 2013. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 6.:1214–1218. doi: 10.1099/ijs.0.041376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, C. 2018. Nutritional impact on mammary development in pigs: a review. J. Anim. Sci. 9.:3748–3756. doi: 10.1093/jas/sky243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J., Zhang H. J., Yu S. H., Wu S. G., Yoon I., Quigley J., Gao Y. P., and Qi G. H.. . 2008. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 8.:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Hampson, D. J. 1986. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 4.:32–40. doi: 10.1016/S0034-5288(18)30482-X. [DOI] [PubMed] [Google Scholar]
- Huang, X., He Q., Zhu H., Fang Z., Che L., Lin Y., Xu S., Zhuo Y., Hua L., Wang J., . et al. 2022. Hepatic leptin signaling improves hyperglycemia by stimulating MAPK phosphatase-3 protein degradation via STAT3. Cell. Mol. Gastroenterol. Hepatol. 1.:983–1001. doi: 10.1016/j.jcmgh.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, B., and Nyachoti C. M.. . 2017. Husbandry practices and gut health outcomes in weaned piglets: a review. Anim. Nutr. .:205–211. doi: 10.1016/j.aninu.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z. Y., Sun L. H., Lin Y. C., Ma X. Y., Zheng C. T., Zhou G. L., Chen F., and Zou S. T.. . 2009. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J. Anim. Sci. 8.:4050–4056. doi: 10.2527/jas.2008-1120. [DOI] [PubMed] [Google Scholar]
- Jiang, X., Lin S., Lin Y., Fang Z., Xu S., Feng B., Zhuo Y., Li J., Che L., Jiang X., . et al. 2020. Effects of silymarin supplementation during transition and lactation on reproductive performance, milk composition and haematological parameters in sows. J. Anim. Physiol. Anim. Nutr. 10.:1896–1903. doi: 10.1111/jpn.13425. [DOI] [PubMed] [Google Scholar]
- Johnson, R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 7.:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Johnston, L., Shurson J., and Whitney M.. . 2008. Nutritional effects on fetal imprinting in swine. Minneapolis, MN: University of Minnesota Press; p. 207–222. [Google Scholar]
- Kubes, P., and Jenne C.. . 2018. Immune responses in the liver. Annu. Rev. Immunol. 3.:247–277. doi: 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- Lallès, J. P., Bosi P., Smidt H., and Stokes C. R.. . 2007. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 6.:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- Li, J., Xing J., Li D., Wang X., Zhao L., Lv S., and Huang D.. . 2005. Effects of β-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch. Anim. Nutr. 5.:303–312. doi: 10.1080/17450390500247832. [DOI] [PubMed] [Google Scholar]
- Li, J., Li D., King J., Cheng Z., and Lai C.. . 2006. Effects of fi-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide1. J. Anim. Sci. 8.:2374–2381. doi: 10.2527/jas.2004-541. [DOI] [PubMed] [Google Scholar]
- Li, Y. S., San Andres J. V., Trenhaile-Grannemann M. D., van Sambeek D. M., Moore K. C., Winkel S. M., Fernando S. C., Burkey T. E., and Miller P. S.. . 2021. Effects of mannan oligosaccharides and Lactobacillus mucosae on growth performance, immune response, and gut health of weanling pigs challenged with Escherichia coli lipopolysaccharides. J. Anim. Sci. 9.:skab286. doi: 10.1093/jas/skab286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Che L., Yang Z., Feng B., Che L., Xu S., Lin Y., Fang Z., Li J., and Wu D.. . 2016a. A maternal high-energy diet promotes intestinal development and intrauterine growth of offspring. Nutrients. .:258. doi: 10.3390/nu8050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., and Cao X.. . 2016b. Cellular and molecular regulation of innate inflammatory responses. Cell. Mol. Immunol. 1.:711–721. doi: 10.1038/cmi.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Jia X., Chang J., Jiang X., Che L., Lin Y., Zhuo Y., Feng B., Fang Z., Li J., . et al. 2023. Effect of yeast culture supplementation in sows during late gestation and lactation on growth performance, antioxidant properties, and intestinal microorganisms of offspring weaned piglets. Front. Microbiol. 1.:1105888. doi: 10.3389/fmicb.2022.1105888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden, A. B., Henderson J. M., and Kutner M. H.. . 1988. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. .:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- Modina, S. C., Polito U., Rossi R., Corino C., and Di Giancamillo A.. . 2019. Nutritional regulation of gut barrier integrity in weaning piglets. Animals. .:1045. doi: 10.3390/ani9121045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou, D., Ding D., Yang M., Jiang X., Zhao L., Che L., Fang Z., Xu S., Lin Y., Zhuo Y., . et al. 2021. Maternal organic selenium supplementation during gestation improves the antioxidant capacity and reduces the inflammation level in the intestine of offspring through the NF-κB and ERK/Beclin-1 pathways. Food Funct. 1.:315–327. doi: 10.1039/d0fo02274h. [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington D.C: National Academies Press. [Google Scholar]
- Paulos, C. M., Wrzesinski C., Kaiser A., Hinrichs C. S., Chieppa M., Cassard L., Palmer D. C., Boni A., Muranski P., Yu Z., . et al. 2007. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 11.:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi, D., Liu Y., Shi H., Li S., Odle J., Lin X., Zhu H., Chen F., Hou Y., and Leng W.. . 2014. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2.:456–462. doi: 10.1016/j.jnutbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Shen, Y. B., Piao X. S., Kim S. W., Wang L., Liu P., Yoon I., and Zhen Y. G.. . 2009. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 8.:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- Slifer, Z. M., and Blikslager A. T.. . 2020. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int. J. Mol. Sci. 2.:972. doi: 10.3390/ijms21030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, W., Gong T., Jiang Z., Lu Z., and Wang Y.. . 2022. The role of probiotics in alleviating postweaning diarrhea in piglets from the perspective of intestinal barriers. Front. Cell. Infect. Microbiol. 1.:883107. doi: 10.3389/fcimb.2022.883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M., He C., Cong Y., and Liu Z.. . 2015. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal. Immunol. .:969–978. doi: 10.1038/mi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y., Huang T., Hammarström L., and Zhao Y.. . 2020. The immunoglobulins: new insights, implications, and applications. Annu. Rev. Anim. Biosci. .:145–169. doi: 10.1146/annurev-animal-021419-083720. [DOI] [PubMed] [Google Scholar]
- Trckova, M., Faldyna M., Alexa P., Sramkova Zajacova Z., , Gopfert E., Kumprechtova D., Auclair E., and D’Inca R.. . 2014. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 9.:767–774. doi: 10.2527/jas.2013-6793. [DOI] [PubMed] [Google Scholar]
- Upadhaya, S. D., and Kim I. H.. . 2021. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets-a review. Animals. 1.:2418. doi: 10.3390/ani11082418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heugten, E., Funderburke D., and Dorton K.. . 2003. Growth performance, nutrient digestibility, and fecal microflora in weanling pigs fed live yeast. J. Anim. Sci. 8.:1004–1012. doi: 10.2527/2003.8141004x. [DOI] [PubMed] [Google Scholar]
- Waititu, S. M., Yin F., Patterson R., Rodriguez-Lecompte J. C., and Nyachoti C. M.. . 2016. Short-term effect of supplemental yeast extract without or with feed enzymes on growth performance, immune status and gut structure of weaned pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. Biotechnol. .:64. doi: 10.1186/s40104-016-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. P., Yoo J. S., Jang H. D., Lee J. H., Cho J. H., and Kim I. H.. . 2011. Effect of dietary fermented garlic by Weissella koreensis powder on growth performance, blood characteristics, and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. 8.:2123–2131. doi: 10.2527/jas.2010-3186. [DOI] [PubMed] [Google Scholar]
- Webel, D. M., Finck B. N., Baker D. H., and Johnson R. W.. . 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 7.:1514–1520. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- Wen, C., Guo Q., Wang W., Duan Y., Zhang L., Li J., He S., Chen W., and Li F.. . 2020. Taurine alleviates intestinal injury by mediating tight junction barriers in diquat-challenged piglet models. Front. Physiol. 1.:449. doi: 10.3389/fphys.2020.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X., Tan B., Song M., Ji P., Kim K., Yin Y., and Liu Y.. . 2019. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. .:46. doi: 10.3389/fvets.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, S., Wu X., Dong Y., Li Z., Lin Y., Che L., Li J., Feng B., Fang Z., Zhuo Y., . et al. 2020. Glucose activates the primordial follicle through the AMPK/mTOR signaling pathway. Clin. Transl. Med. 1.:e122. doi: 10.1002/ctm2.122. [DOI] [Google Scholar]
- Yi, D., Hou Y., Wang L., Zhao D., Ding B., Wu T., Chen H., Liu Y., Kang P., and Wu G.. . 2016. Gene expression profiles in the intestine of lipopolysaccharide-challenged piglets. Front Biosci. 2.:487–501. doi: 10.2741/4404. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Wang Q., Zhou P., Li Z., Zhong W., Zhuo Y., Che L., Xu S., Fang Z., Jiang X., . et al. 2022. Effects of yeast culture supplementation from late gestation to weaning on performance of lactating sows and growth of nursing piglets. Animal. 1.:100526. doi: 10.1016/j.animal.2022.100526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.