Abstract
Non-healing diabetic foot ulcer, a chronic inflammatory disease, is a sizable clinical and economic burden to healthcare systems around the world. Chronic inflammation plays a critical role in the nonhealing pattern due to the arrest of the cellular response during wound healing in the inflammatory phase without progressing to the proliferative and remodeling phase. Fibroblasts play a critical role in all three phases of wound healing. Activation of fibroblasts in the presence of cytokines results in the formation of myofibroblast that contributes to extracellular matrix formation. Additionally, few studies documented the presence of inflammatory, angiogenic, and angiostatic fibroblast subpopulation during wound healing. Various studies have discussed the role of transcription factors and microRNA in regulating the transdifferentiation of fibroblast to myofibroblast, however, what factors regulate the reprogramming of fibroblast to inflammatory, angiogenic, and angiostatic phenotypes have not been clearly addressed in the literature. This critical review article addresses the role of transcription factors and microRNAs in regulating fibroblast to myofibroblast transdifferentiation followed by the prediction of transcription factors and microRNAs, based on the bioinformatics analysis, in regulating transdifferentiation of fibroblasts to inflammatory, angiogenic, and angiostatic subtypes. The results of in-silico networking revealed multiple new transcription factors and microRNAs and their interaction with specific markers on other fibroblasts suggesting their role in the regulation of fibroblast reprogramming.
Keywords: Diabetes, Diabetic wound, Wound healing, Fibroblast plasticity, Fibroblast heterogeneity, Fibroblast reprogramming, Transcription factors, MicroRNA
1. Introduction
Fibroblasts are crucial for wound healing and are involved in regulating turnover of extracellular matrix (ECM), laying down new ECM, breaking fibrin clots, modulating structure and expression of collagens, supporting and interacting with other cells involved in wound healing, and producing and regulating the expression of various other factors involved in wound healing [1–3]. After an injury, the quiescent fibroblasts are activated to myofibroblasts in response to the cytokines including interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α secreted following an inflammatory immune response. These activated fibroblasts secrete various cytokines (IL-1α, IL-1β, IL-6, IL-10, TNF-α), chemokines (MCP1, MIP1- α), growth factors (CSF-1, bFGF, HGF, IGF-1, and others), matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs) to maintain an inflammatory immune response, collagen synthesis, and ECM remodeling [4–7]. Crosstalk between activated fibroblasts (myofibroblasts) and other cells including immune cells, keratinocytes, and endothelial cells, and the proliferation and migration of fibroblasts regulate wound healing and contraction in normal physiological conditions. However, this regulation is disrupted during chronic inflammation and leads to a nonhealing pattern of wound healing [2, 8–10]. This suggests that the fibroblasts are differentiated into a new phenotype to regulate wound healing. A change in fibroblast phenotype in the presence of IL-1α and TNF-α to inflammatory phenotype (CD40+) that secrete high amount of IL-6 and IL-8 [11, 12] and the presence of a significantly increased population of CD40+ fibroblasts in chronic nonhealing diabetic wounds [13] support the notion that fibroblasts heterogeneity and plasticity [3] play a critical role in wound healing. Changing the phenotype of fibroblasts from “normal” quiescent fibroblasts to activated myofibroblasts during wound healing and then again to normal fibroblasts once the wound is healed support fibroblast plasticity.
The heterogeneous population of fibroblasts in a healing wound may be due to the recruitment of a subpopulation of dermal fibroblasts located at the edge of the wound including superficial or papillary spindle-shaped fibroblasts arranged as a ridge-like structure (CD26+Lrig+ Sca1−), stellate/flattened reticular fibroblasts residing in the deep dermis (Dlk1+Sca1−) arranged parallel to the surface of the skin, and fibroblasts associated with hair follicles (Sox2+) [3, 7]. In addition to these fibroblasts, mesenchymal stem cells around the dermal papilla, fibrocytes from circulation, and phenotypic conversion of epithelial and endothelial cells through epithelial- and endothelial-to-mesenchymal transition processes can also give rise to matrix-producing myofibroblasts [7]. The expression of Twist-related protein 2 (Twist2/Dermo1), and Engrailed1 (En1) regulate the differentiation of papillary and reticular dermal fibroblasts [14]. β regulates the phenotypic conversion of proto-myofibroblasts to myofibroblasts [15]. The functional and biological role of myofibroblasts during wound healing is modulated by the differential expression of ECM components including desmin, fibronectin, collagens, and matrix composition [7, 16]. Proinflammatory cytokines play a crucial role in the phenotypic conversion of fibroblasts [11, 12], however, the regulation of the phenotypic conversion of fibroblasts at transcriptional and epigenetic levels is not precisely discussed, especially in the context of wound healing. This is important because chronic diabetic foot ulcers are infected and altered microbiota in chronic diabetic ulcers affecting wound healing [17] may change the expression levels of transcriptional and epigenetic factors [18, 19] which may ultimately affect wound healing. This review focuses on highlighting the transcriptional factors and microRNAs (miRs) involved in the phenotypic conversion of fibroblasts, mainly in the context of wound healing, discussed in the literature followed by the predicted transcription factors and miRs based on bioinformatics analysis.
Fibroblasts are crucial for wound healing and are involved in regulating turnover of extracellular matrix (ECM), laying down new ECM, breaking fibrin clots, modulating structure and expression of collagens, supporting and interacting with other cells involved in wound healing, and producing and regulating the expression of various other factors involved in wound healing [1–3]. After an injury, the quiescent fibroblasts are activated to myofibroblasts in response to the cytokines including interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α secreted following an inflammatory immune response. These activated fibroblasts secrete various cytokines (IL-1α, IL-1β, IL-6, IL-10, TNF-α), chemokines (MCP1, MIP1- α), growth factors (CSF-1, bFGF, HGF, IGF-1, and others), matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs) to maintain an inflammatory immune response, collagen synthesis, and ECM remodeling [4–7]. Crosstalk between activated fibroblasts (myofibroblasts) and other cells including immune cells, keratinocytes, and endothelial cells, and the proliferation and migration of fibroblasts regulate wound healing and contraction in normal physiological conditions. However, this regulation is disrupted during chronic inflammation and leads to a nonhealing pattern of wound healing [2, 8–10]. This suggests that the fibroblasts are differentiated into a new phenotype to regulate wound healing. A change in fibroblast phenotype in the presence of IL-1α and TNF-α to inflammatory phenotype (CD40+) that secrete high amount of IL-6 and IL-8 [11, 12] and the presence of a significantly increased population of CD40+ fibroblasts in chronic nonhealing diabetic wounds [13] support the notion that fibroblasts heterogeneity and plasticity [3] play a critical role in wound healing. Changing the phenotype of fibroblasts from “normal” quiescent fibroblasts to activated myofibroblasts during wound healing and then again to normal fibroblasts once the wound is healed support fibroblast plasticity.
The heterogeneous population of fibroblasts in a healing wound may be due to the recruitment of a subpopulation of dermal fibroblasts located at the edge of the wound including superficial or papillary spindle-shaped fibroblasts arranged as a ridge-like structure (CD26+Lrig+ Sca1-), stellate/flattened reticular fibroblasts residing in the deep dermis (Dlk1+Sca1-) arranged parallel to the surface of the skin, and fibroblasts associated with hair follicles (Sox2+) [3, 7]. In addition to these fibroblasts, mesenchymal stem cells around the dermal papilla, fibrocytes from circulation, and phenotypic conversion of epithelial and endothelial cells through epithelial- and endothelial-to-mesenchymal transition processes can also give rise to matrix-producing myofibroblasts [7]. The expression of Twist-related protein 2 (Twist2/Dermo1), platelet-derived growth factor receptor α (PDGFRα), and Engrailed1 (En1) regulate the differentiation of papillary and reticular dermal fibroblasts [14]. Transforming growth factor (TGF)-β regulates the phenotypic conversion of proto-myofibroblasts to myofibroblasts [15]. The functional and biological role of myofibroblasts during wound healing is modulated by the differential expression of ECM components including desmin, fibronectin, collagens, and matrix composition [7, 16]. Proinflammatory cytokines play a crucial role in the phenotypic conversion of fibroblasts [11, 12], however, the regulation of the phenotypic conversion of fibroblasts at transcriptional and epigenetic levels is not precisely discussed, especially in the context of wound healing. This is important because chronic diabetic foot ulcers are infected and altered microbiota in chronic diabetic ulcers affecting wound healing [17] may change the expression levels of transcriptional and epigenetic factors [18, 19] which may ultimately affect wound healing. This review focuses on highlighting the transcriptional factors and microRNAs (miRs) involved in the phenotypic conversion of fibroblasts, mainly in the context of wound healing, discussed in the literature followed by the predicted transcription factors and miRs based on bioinformatics analysis.
1.1. Dermal fibroblast: Origin and lineage tracing
As discussed above, the cellular and molecular milieu under the influence of cytokines at the injury site activate fibroblasts to differentiate into myofibroblasts, and returns to normal fibroblast after healing. This supports fibroblast plasticity which is also regulated by the wound microenvironment and mechanical stress of the matrix. For instance, in the lower wound bed, macrophages phagocytosing the Wnt inhibitor secreted frizzled-related protein 4 (SFRP4) promotes prolonged Wnt/β-catenin signaling activation in the fibrotic wound [20] and increased mechanical tension reactivates expression of En1 in reticular fibroblasts and promotes scar formation [21]. Further, the location of the angiogenesis and regenerating blood vessels determine the subpopulation of fibroblasts participating in blood vessel-associated Ng2+ pericytes formation [22]. TGF-β signaling in the wound facilitates the trans-differentiation of adipocytes of the dermal white adipose tissue to myofibroblasts [23] and these cells convert back to quiescent adipocytes under the influence of BMP signaling [24]. In addition, there are reports on the role of cell-cell interaction, soluble cytokines, ECM components, and other intrinsic and extrinsic regulatory factors in regulating fibroblast plasticity [25].
Lineage tracing suggests two types of fibroblasts originating from the precursors of dermal fibroblast (with specific markers) namely papillary and reticular fibroblast contributing to superficial and deep dermis, respectively. Based on the surface makers different subpopulation of fibroblasts playing a critical role in wound healing and fibrosis has been documented in the literature [26–28] (Figure 1). The surface markers characterizing different subpopulations of fibroblasts vary between different species such as mice and humans [25] and fibroblasts have different biologically functional characteristics (nonfibrotic, profibrotic, regeneration capacity, proliferation capacity, contractility) at different sites. Some fibroblasts with a specific marker playing a role in wound healing may not be present in other species, like Sca1+ fibroblasts are present in mice but not in humans [29]. Among the dermal fibroblasts in humans, papillary fibroblasts have a higher expression of immune- and angiogenesis-related genes while reticular fibroblasts have a higher expression of cytoskeleton organization and connective tissue formation-associated genes [30, 31]. Single-cell RNA sequencing and spatial transcriptomics have revealed different subpopulations of fibroblasts within the dermis (papillary and reticular fibroblasts) with distinguished gene expression [29, 32, 33]. These studies suggest heterogeneity of fibroblasts in skin and their differential role during wound healing. This may be due to the location/state of the tissue, regional or anatomical heterogeneity, local heterogeneity, and change in cell phenotype resulting in differential biological and molecular output [34].
Figure 1:
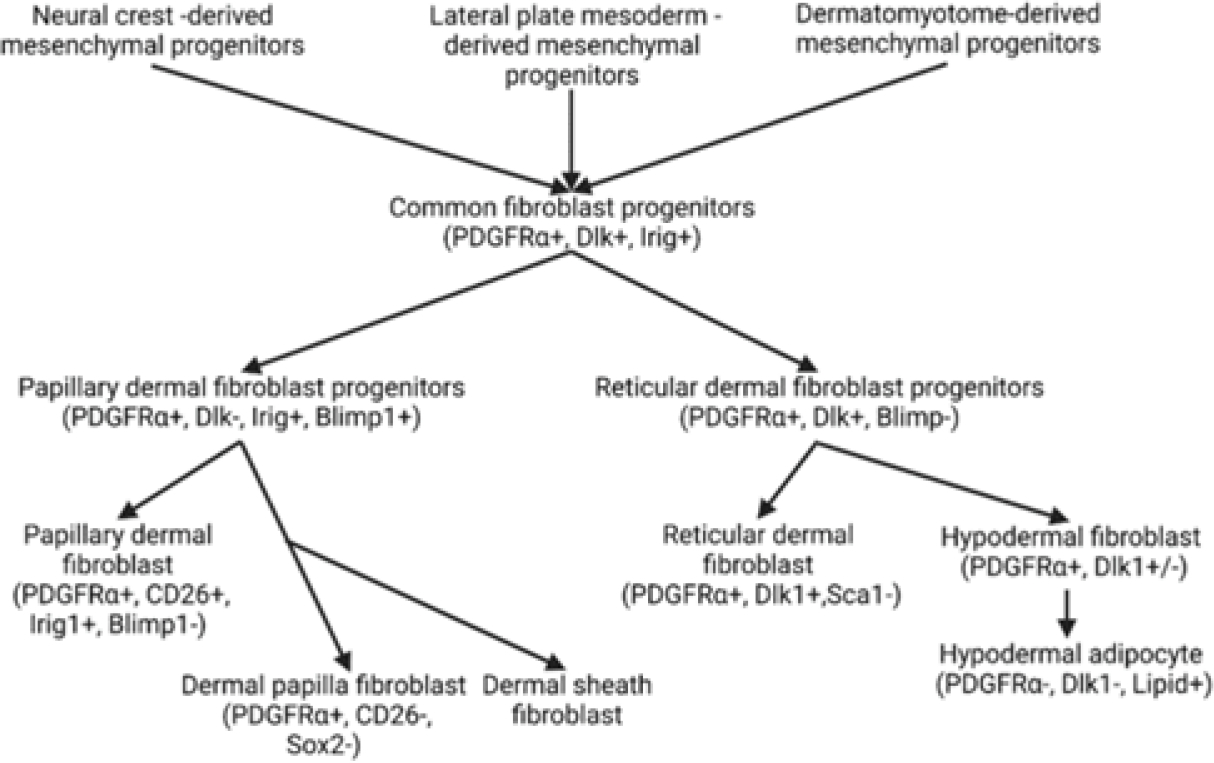
Origin and lineage of dermal fibroblast in humans and mice.
Additionally, in-vitro studies suggest that fibroblasts in the presence of IL-1 and TNF-α acquire a CD40+ phenotype secreting increased IL-8 and IL-6 and with a pro-inflammatory function. One of the explanations for CD40 positivity and inflammatory aspects of this subpopulation may be due to the interaction between the inflammatory immune cells and CD40 receptors on fibroblasts resulting in increased secretion of IL-6, IL-8, cyclooxygenase-2, and hyaluronan through activation of NF-κB signaling [35]. Furthermore, during wound healing after myocardial infarction, fibroblasts acquire an angiogenic (FSP+) and antiangiogenic (TSP+) phenotype [3, 13]. This suggests the plasticity of fibroblasts (changing to myofibroblasts or becoming adipocytes, dermal papilla cells, or cartilage-like cells) [36]; however, other than the markers of each subtype, the factors regulating the phenotypic switch are not extensively discussed in the literature.
1.2. Transcription factors regulating transdifferentiation of fibroblast
Transcription factors (TFs) also play a role in the transition from the inflammatory phase to the proliferation phase by modulating inflammation, cellular proliferation, re-epithelialization, angiogenesis, and granulation tissue formation [37]. During wound healing in neonatal and adult skin, differential expression of TFs for activator protein-1 (AP-1), c-jun and c-fos, IL-6, IL-8, and TGF-β1 regulate healing with or without scar [38]. TFs Foxn1, Foxo1, Foxo3, and Msx2 play a critical role in wound healing [39]. Studies have reported the importance of myocardin-related transcription factor (MRTF)-A for myofibroblast differentiation [40]. With the role of TFs in wound healing, it is imperative to consider that TFs play a critical role in phenotypic switching of fibroblast to the subpopulation or subtypes like inflammatory, angiogenic, and anti-angiogenic types. However, other than the transdifferentiation of fibroblast to myofibroblast, the regulatory role of TFs in reprogramming fibroblast to inflammatory, angiogenic, and anti-angiogenic subtypes has not been addressed or investigated.
Transcription factors regulate gene expression and thereby the cell characteristics. Guerrero-Juarez et al. [26], based on single cells transcriptomics analysis of murine skin, documented 12 subclusters of fibroblasts with differential gene expression profile but with a shared expression of TFs, including Cebpb, Egr1, Fosb, Fosl2, Hif1a, Klf2, Klf4, Klf6, Klf9, Nfat5, Nfatc1, Nfkb1, Nr4a1, Nr4a2, Pbx1, Prrx1, Runx1, Stat3, Tcf4, and Zeb2 with a different extent of expression in various clusters. Of these, Runx1, Tcf4, and Zeb2 play a role in myofibroblast differentiation. It was also revealed that these hierarchically distinct subclusters have distinct receptors and ligand signatures (Mcam/Pdgfrb/Fgfr1/Tgfbr2/Tgfbr3/ Ncam1/Pdgfra, and Il6/Pdgfa/Igf1/Igfbp3/Mdk/Dkk3). These findings were corroborated by differential expression of specific expression of fibroblast markers on immunostaining [26]. The analysis also revealed two distinct populations of fibroblasts upon wound healing: one population (nearly 24%) with low expression levels of TGFβ receptors Tgfbr2, Tgfbr3, and PDGF receptor Pdgfra and high levels of Pdgfrb while other population (nearly 76%) with intermediate to high expression levels of Tgfbr2, Tgfbr3, high expression levels of Pdgfra, but not of Pdgfrb. Pseudotime analysis revealed two different trajectories of fibroblasts while RNA path velocity analysis revealed three paths with the expression of Col14a1 and mesoderm-specific transcript, Mest, in the beginning with an increased density and expression for contractile genes, Acta2 and Tagln, toward the ends. These findings suggest the phenotypic change of fibroblasts to myofibroblasts and the possible underlying regulatory mechanism [26]. The study also documented that the myofibroblasts have hematopoietic features contributing to wound healing. Overall, the molecular regulators for the phenotypic conversion of fibroblasts to myofibroblasts were revealed. Another study revealed two repair trajectories with distinct molecular motifs in fibroblasts: one leading to regenerative potential and another to wound repair with fibrosis. Noizet et al. [41] reported that transcription factors, TCF4, SOX9, EGR2, and FOXS1, play a major regulatory role in the transdifferentiation of fibroblast to myofibroblast and this reprogramming can be achieved even in the absence of TGF-β if transcription factors MEOX2, SIX2, and MAF are down-regulated. A study by Phan et al. [42] revealed that fibroblasts expressing a canonical Wnt transcription factor Lef1 (neonatal papillary fibroblasts) prime the adult skin macroenvironment to enhance skin repair. However, the regulatory mechanism for the phenotypic conversion of fibroblasts to inflammatory, angiogenic, and anti-angiogenic fibroblasts remains elusive.
1.3. MicroRNA regulating wound healing and fibroblast reprogramming
Along with TFs, microRNAs (miR) also play a crucial role in wound healing by regulating inflammation (miR-146a, miR-132, miR-155, and miR-21), re-epithelialization, cell proliferation and migration, angiogenesis (miR-132, miR-21, miR-31, miR-27b, miR-483–3p, miR-203, miR-99, miR-198, miR-210, miR-146a, and miR-106b), and granulation tissue formation (miR-378a, miR-21, and miR-196a) [37]. In a diabetic wound, upregulation of miR-132 results in a decreased pro-inflammatory response, downregulation of miR-146a results in persistent inflammation, upregulation of miR-21 results in increased polarization towards M1 subtype, and upregulation of miR-155–5p impairs wound re-epithelialization. miR-223 regulates the inflammatory response by modulating cytokines expression, activation or inhibition of inflammatory pathways (NF-κB) and M2 macrophage polarization [43, 44].
Further, the role of miR-15b, miR-17, miR-18a, miR-19a, miR-19b, miR-20a, miR-21, miR-26a, miR-27b, miR-29a, miR-29b, miR-29c, miR-31, miR-34a, miR-34c, miR-92a, miR124a, miR-125b, miR-126, miR-132, miR-141, miR-146a, miR-155, miR-191, miR-198, miR-200a, miR-200b, miR-200c, miR-205b, miR-210, miR-223, miR-429, and others in regulating inflammatory, proliferative, and remodeling phases of wound healing in diabetes has been discussed in the literature [43, 45–50]. Moreover, circulating and tissue miRs may also serve as biomarkers for the early detection of diabetic foot ulcers [43]. Since miRs play a critical role in various aspects of wound healing and fibroblasts are functionally involved in all phases of wound healing, it is imperative that miRs may play a critical role in fibroblast plasticity and heterogeneity.
Fibroblasts play a critical role in wound healing. MiR-215 regulates fibroblast proliferation [51], miR-1, miR-133, miR-208, and miR-499 regulate fibroblast reprogramming to induced cardiac myocytes [52, 53], miR‑18a‑5p suppresses the proliferation of scar fibroblast and ECM deposition [54], and miR-217 [55], miR-152 and miR-181a [56], and miR-34c-5p [57] regulate fibroblast senescence in human skin. In addition to the migration of fibroblasts to the wound site, myeloid cells also convert to fibroblast-like cells and this conversion is regulated by miR-21 packaged in extracellular vesicles [58]. These findings suggest that miRs play a crucial role in fibroblast reprogramming, proliferation, migration, and its biological function of matrix secretion.
1.4. Predicted TFs and miRs associated with fibroblast gene expression
The findings of the studies discussed above suggest an obvious role of TFs and miRs in wound healing and fibroblast function. These studies have discussed various aspects of the regulatory role of TFs and miRs in three phases of wound healing and the reprogramming of fibroblast to myofibroblast in the context of wound healing. The regulatory role of inflammatory, angiogenic, and antiangiogenic (angiostatic) fibroblast subtypes has been discussed in the literature [3], however, how this reprogramming happens and what are the regulators are not clear and have not been discussed in the literature. To predict the TFs and miRs regulating the expression of various genes (markers of fibroblast) differentially expressed on quiescent fibroblasts (anti-CD34+, anti-HSP47+, anti-SFA+, S100A4+), myofibroblasts (α-SMA+, fibronectin+, cadherin+, n-caldesmon−, smoothelin−, and desmin−), fibroblasts secreting IL-8 (CD40+; inflammatory fibroblast), angiogenic fibroblasts (FSP-1+/CD31−/CD45−), and angiostatic fibroblasts (TSP-1+) [7, 11, 12, 59, 60], we performed the in-silico analysis using STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) enrichment network analysis (https://string-db.org/), the SIGnaling Network Open Resource (SIGNOR) (https://signor.uniroma2.it/) and Network analyst (https://www.networkanalyst.ca/) with the name of genes expressed on various fibroblast subtypes as input gene list. SIGNOR is both a source of signaling information and support for data analysis and is a repository of manually annotated causal relationships between human proteins, chemicals of biological relevance, stimuli, and phenotypes. While STRING is used to compute interactive networks and predict protein function.
STRING analysis (Figure 2) revealed interaction in between fibroblast subpopulation markers including cadherin (CDH), S100A4, ACTA2, smoothelin (SMTN), fibronectin (FN1), CD34, and CD40 and with cytokines (IL-6, CXCL8 (IL-8), TNF-α, IL-1A, IL-37, and IL-10), metalloproteinases (MMP2), inhibitors of metalloproteinases (TIMP1 and TIMP2), growth factors (FGF7 and HGF), along with other proteins involved in ECM remodeling.
Figure 2:
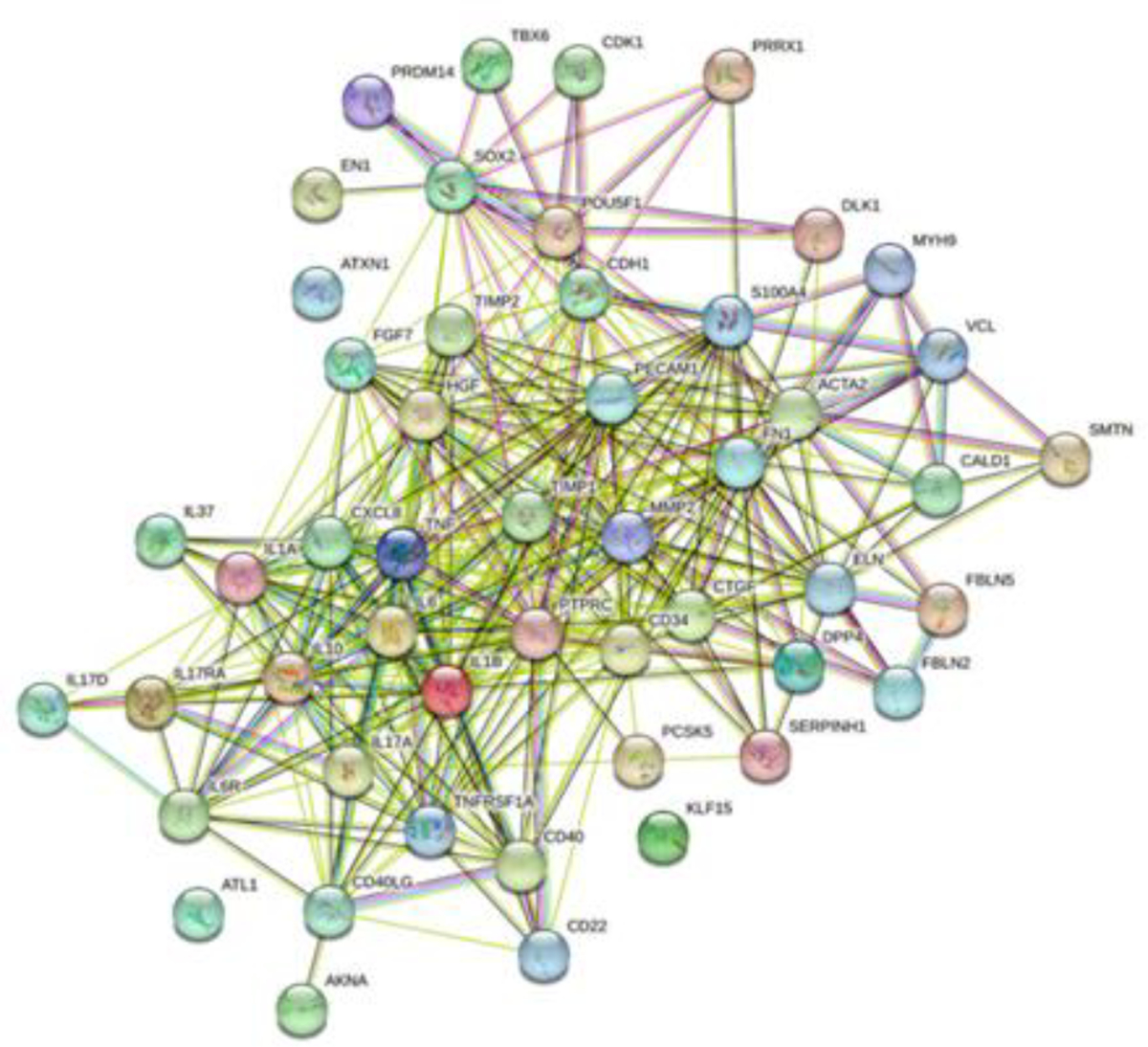
STRING network showing interaction between fibroblast markers, cytokines, inflammatory mediators, and ECM remodeling mediators.
The interaction between various factors involved in wound healing, ECM remodeling, and the genes expressed on fibroblasts suggest that the wound microenvironment may play a crucial role in fibroblast reprogramming. To further understand the regulation of various fibroblast markers and whether they could regulate reprogramming, we performed the network analysis (Networkanalyst.ca) and the results (Figure 3) revealed various TFs interacting with fibroblast specific genes including CDH1, FN1, CD34, CD40, S100A4, SMTN, desmin (DES), ACTA2, and CALD1. It should be noted that CD40 and S100A4 (FSP1) are markers specifically expressed on inflammatory and angiogenic fibroblast subpopulation [11–13, 59].
Figure 3:
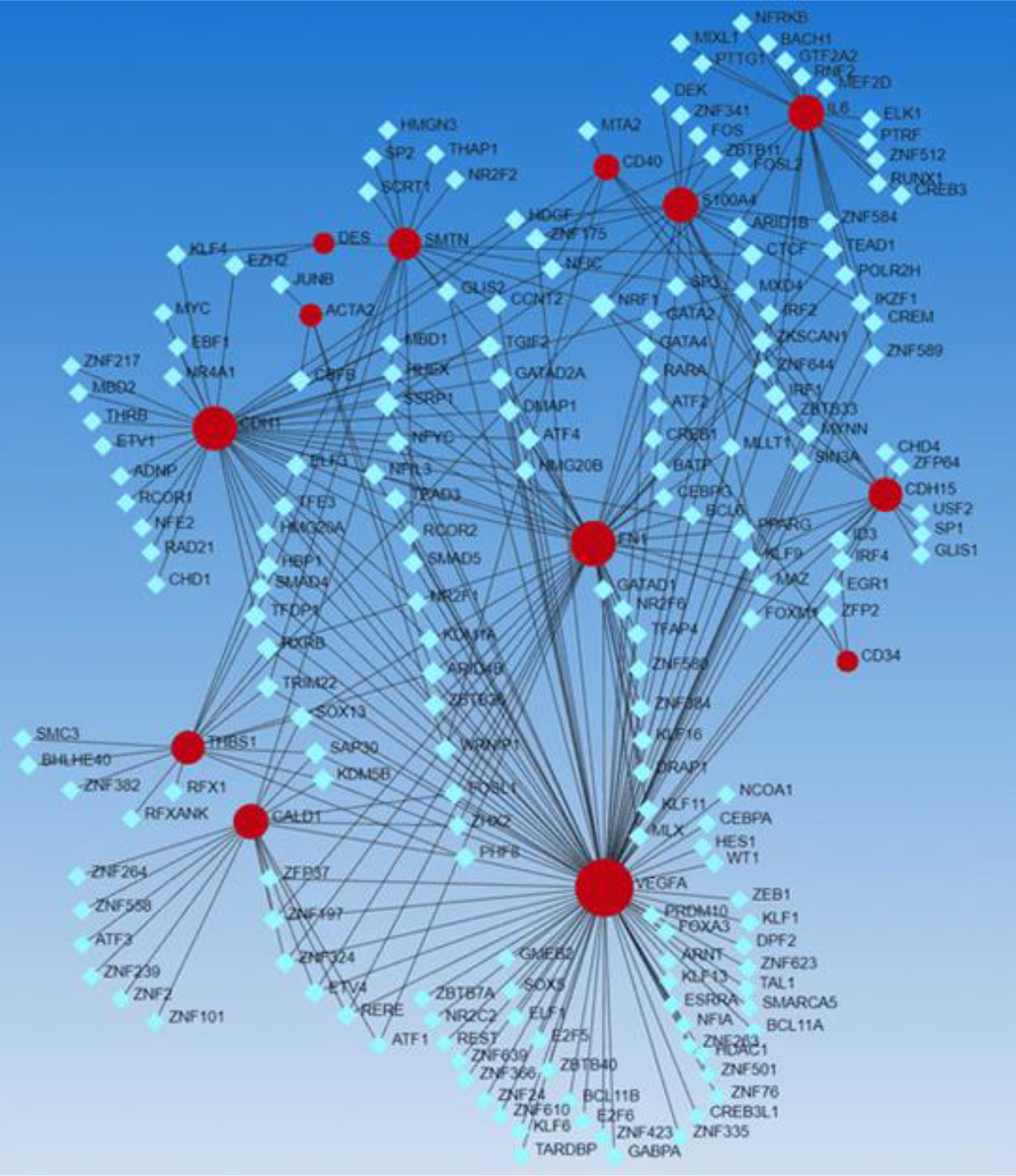
Network analysis showing interactive network between transcription factors (light blue squares) and gene expressed (red dots) on various fibroblast subpopulations including quiescent fibroblasts (anti-CD34+, anti-HSP47+, anti-SFA+, S100A4+), myofibroblasts (α-SMA+, fibronectin+, cadherin+, n-caldesmon-, smoothelin-, and desmin-), fibroblasts secreting IL-8 (CD40+), angiogenic fibroblasts (FSP-1+/CD31-/CD45-), and angiostatic fibroblasts (TSP-1+).
Further, we noted the presence of antiangiogenic fibroblast marker thrombospondin 1 (THBS1) [60] and angiogenic mediator vascular endothelial growth factor (VEGF) within the interacting network in association with various TFs (Figure 3). This suggests that TFs may regulate the expression of various genes expressed in the subpopulation of fibroblast including inflammatory, angiogenic, and angiostatic subtypes, and thus may regulate reprogramming. To further validate our findings on the interaction of genes expressed on various fibroblast subpopulations and transcription factors and other factors playing a role in wound healing and ECM remodeling, we did SIGNOR networking (using Networkanalyst.ca). The findings (Figure 4) revealed the interaction between genes (blue circles) expressed on various fibroblast subpopulations (FN1 and CDH1) with many of the transcription factors revealed in Figure 2. These findings support the assumption that transcription factors may play a critical role in the reprogramming of fibroblasts but need in-depth in-vitro and in-vivo studies. The hypothesis that TFs may regulate the reprogramming of fibroblasts to other phenotypes including inflammatory, angiogenic, and angiostatic phenotypes is supported by the findings discussed above that transcription factors regulate the reprogramming of fibroblasts to myofibroblasts and fibroblast phenotype [26, 37–42].
Figure 4:
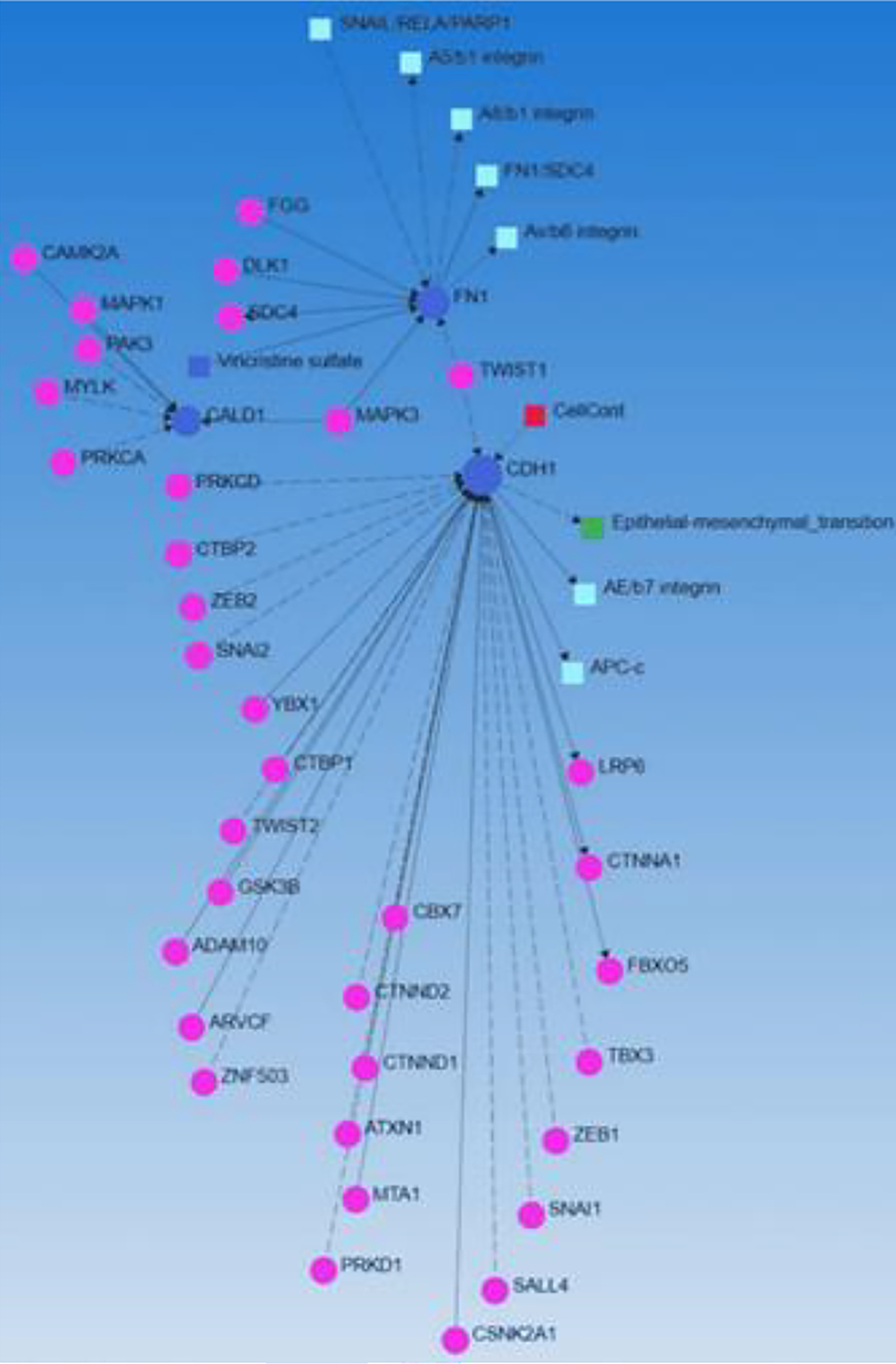
SIGNOR networking representing interactive network between transcription factors (pink circles) and gene expressed (blue dots) on various fibroblast subpopulations.
This review aimed to elucidate the transcription factors regulating the reprogramming of fibroblast to inflammatory (CD40+), angiogenic (FSP1+), and angiostatic (THBS1+) phenotypes [7, 11, 12, 59], so we focused on these genes and their correlation with transcription factors (Figure 5).
Figure 5:
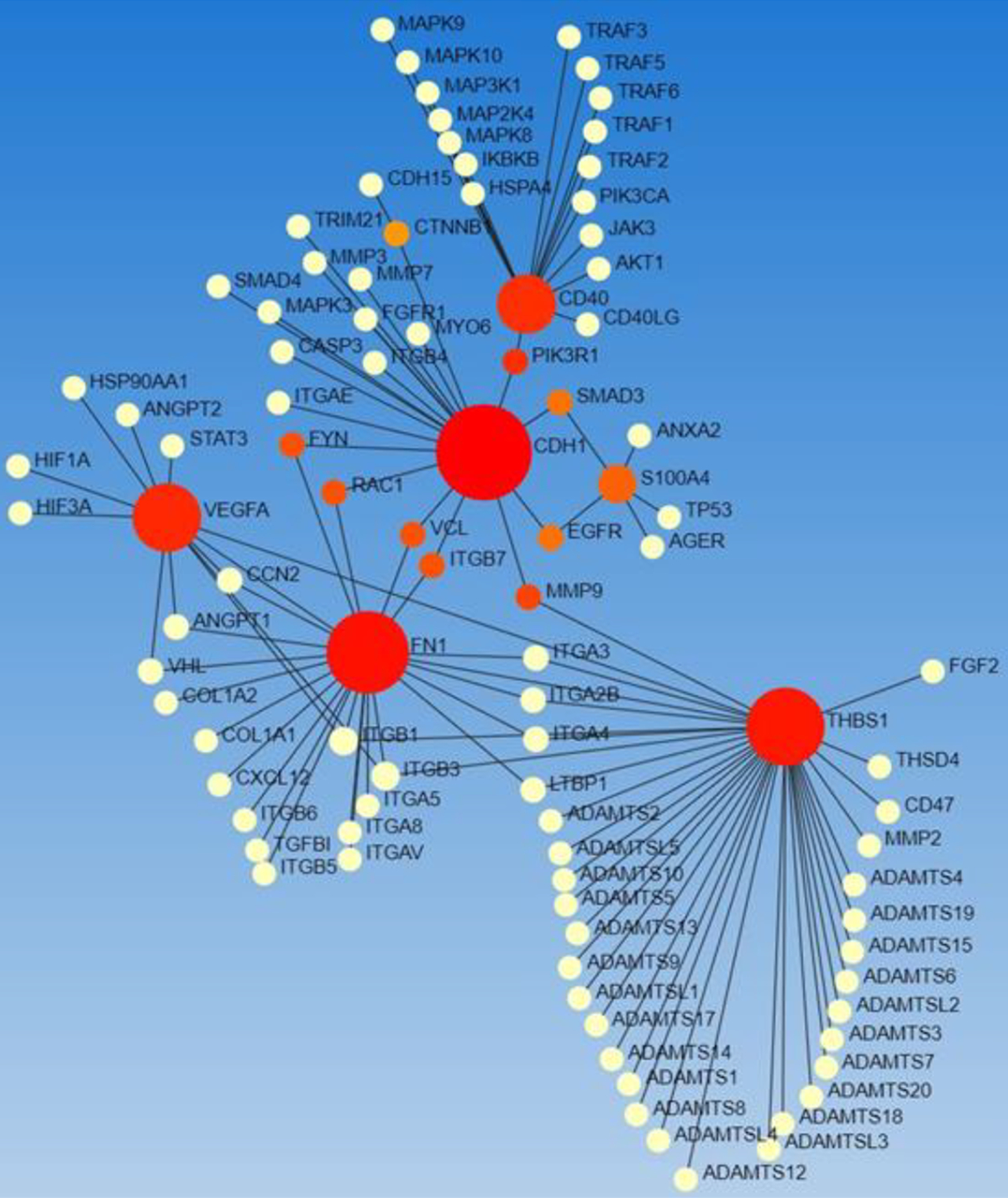
STRING network analysis for CD40, THBS1, and FSP1 (S100A4).Genes (red circles) and transcription factors (yellow circles).
The networking showed an interaction between these genes and TFs and interestingly also with mediators regulating angiogenesis (VEGF, ANGPT1, HIF1A), an important regulator of wound healing in chronic diabetic foot ulcers [61]. Another interesting finding was the interaction between mediators of inflammation, metalloproteinases, cytoplasmic kinases, and ADAMS (Figure 5) suggesting the possible role of various regulators during angiogenesis and wound healing [3, 9, 13, 62].
We previously reported the presence of an increased CD40+ subpopulation of fibroblasts in chronic nonhealing diabetic foot ulcers in humans [13] and findings from a study [35] suggest that activation of NF-κB induces increased expression of CD40 positivity in fibroblast. We found the correlation between IL-6, NF-κB, CD40, and inflammation (Figure 6) supporting the hypothesis that chronic inflammation due to IL-6 and other cytokines may induce or regulate fibroblast reprogramming towards inflammatory phenotype [11, 12]. Further, it was also revealed that IL-6 may activate ACTA2 (alpha-smooth muscle actin) (Figure 6) through serum response factor (SRF). This suggests the correlation between inflammation and proliferation of fibroblasts.
Figure 6:
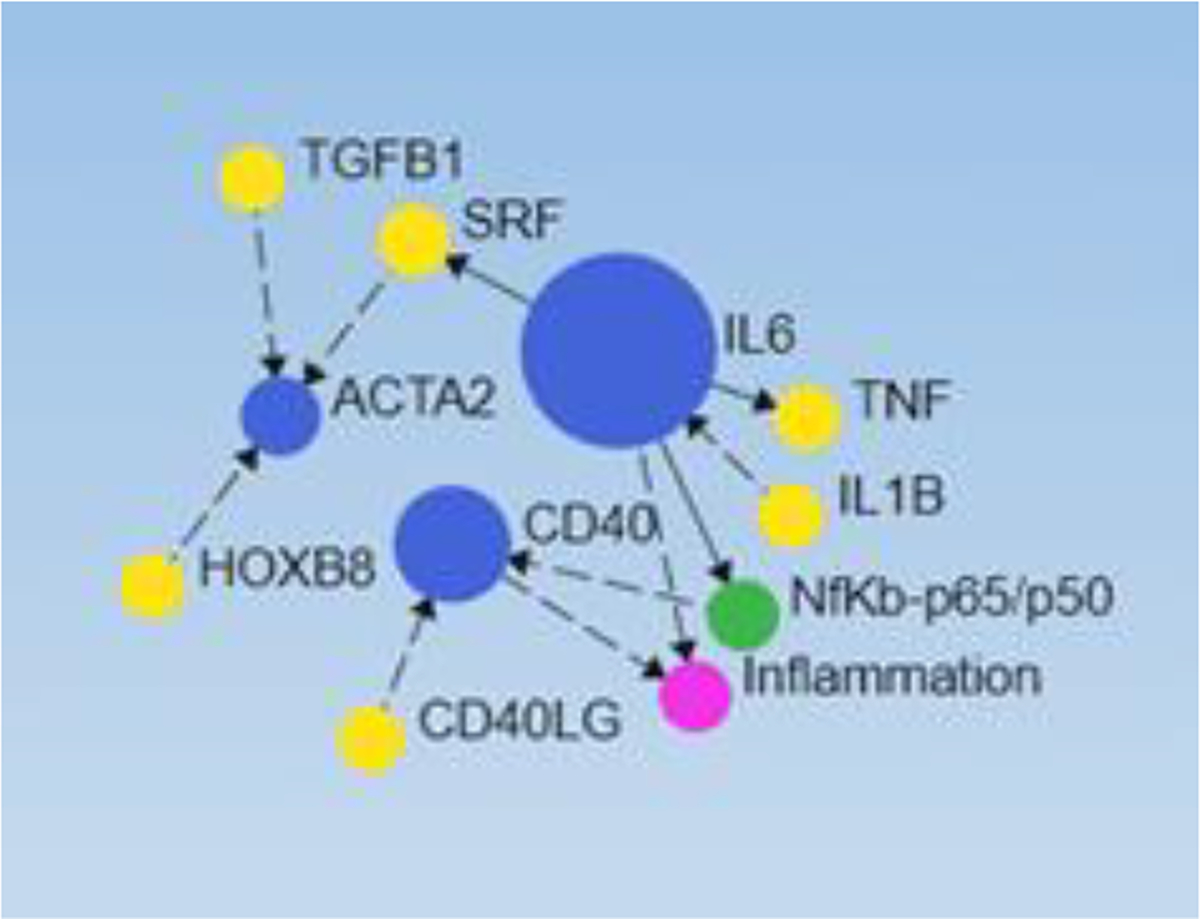
Network analysis depicting the correlation between interleukin (IL)-6, nuclear factor kappa beta (NF-κB), CD40, and inflammation. Blue circles (genes), yellow circles (transcription factors), red (inflammation), and NF-κB (green).
Next, since miRs play a crucial role in various aspects of wound healing, we delineated the probable role of miRs in regulating fibroblast reprogramming to inflammatory, angiogenic, and angiostatic phenotypes. We focused on CD40, THBS1, VEFG, and other matrix proteins and their interaction with miRs (using Networkanalyst.ca) (Figure 7). The results revealed an interaction between these genes and multiple miRs including miRs described in the section above “MicroRNA regulating wound healing and fibroblast reprogramming”. These findings suggest that in addition to the discussed miRs, other miRs are also involved in fibroblast reprogramming towards myofibroblast, inflammatory, angiogenic, and angiostatic phenotypes. It was also revealed that many miRs regulate gene expression in common (one miR regulating the expression of more than one gene).
Figure 7:
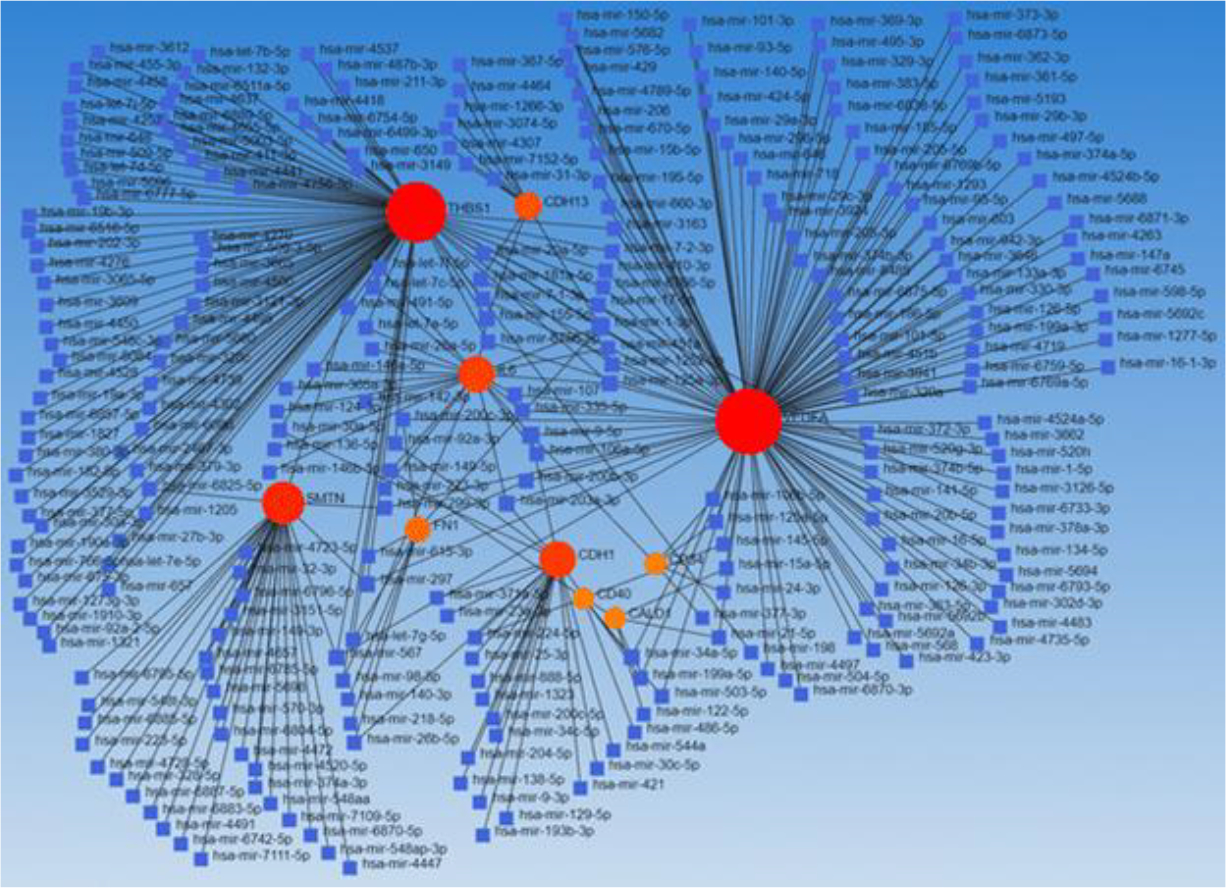
Network depicting the correlation between miRs (blue square) and gene expressed (red/orange dots) on various fibroblast subpopulations
The findings of in-silico network analysis suggest that TFs and miRs may regulate the reprogramming of fibroblast to inflammatory, angiogenic, and angiostatic phenotypes. Although these findings are based only on in-silico bioinformatics analysis, validation of these findings needs in-depth in-vitro and in-vivo studies. Despite these limitations, the data add various TFs and miRs which may play a role in fibroblast reprogramming and thus in wound healing.
2. Conclusion
Inflammation and angiogenesis play an important role in wound healing and are dysregulated in diabetes resulting in chronic nonhealing diabetic foot ulcers. The micro-environment of diabetic foot ulcer and the presence of gram-positive and gram-negative bacteria and fungal infection alter microbiota. Altered microbiota is associated with altered gene expression and epigenetic modification. Thus, identification of the transcription factors and miRs regulating the phenotypic change of fibroblasts to angiogenic, antiangiogenic, and inflammatory subtypes is important to understand the genetic and epigenetic factors regulating wound healing. Network analysis in this article with the genes expressed on different fibroblast phenotypes is important in paving the way to design future research and therapeutics to enhance wound healing.
Funding
This work was supported by research grants R01 HL144125 and R01 HL147662 to D.K. Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this critical review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Bainbridge P Wound healing and the role of fibroblasts. J Wound Care 22 (2013): 407–8. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 20 (2011): 108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai V, Moellmer R, Agrawal DK. Role of fibroblast plasticity and heterogeneity in modulating angiogenesis and healing in the diabetic foot ulcer. Mol Biol Rep 50 (2023): 1913–29. [DOI] [PubMed] [Google Scholar]
- 4.Thankam FG, La V, Agrawal DK. Single-cell genomics illustrates heterogeneous phenotypes of myocardial fibroblasts under ischemic insults. Biochem Cell Biol 101 (2023): 12–51 [DOI] [PubMed] [Google Scholar]
- 5.Thankam FG, Sedighim S, Kuan R, et al. Ischemia challenged epicardial adipose tissue stem cells-derived extracellular vesicles alter the gene expression of cardiac fibroblasts to cardiomyocyte like phenotype. Transl Res 254 (2022): 54–67. [DOI] [PubMed] [Google Scholar]
- 6.Thankam FG, Larsen NK, Varghese A, et al. Biomarkers and heterogeneous fibroblast phenotype associated with incisional hernia. Mol Cell Biochem 476 (2021): 3353–3363. [DOI] [PubMed] [Google Scholar]
- 7.Darby IA, Laverdet B, Bonte F, et al. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7 (2014): 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cialdai F, Risaliti C, Monici M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front Bioeng Biotechnol 10 (2022): 958381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shofler D, Rai V, Mansager S, et al. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Expert Rev Clin Immunol 17 (2021): 681–90. [DOI] [PubMed] [Google Scholar]
- 10.Addis R, Cruciani S, Santaniello S, et al. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int J Med Sci 17 (2020): 1030–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen CG, Anderson AO, Oppenheim JJ, et al. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology 68 (1989): 31–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Dongari-Bagtzoglou AI, Ebersole JL. Increased presence of interleukin-6 (IL-6) and IL-8 secreting fibroblast subpopulations in adult periodontitis. J Periodontol 69 (1998): 899–910. [DOI] [PubMed] [Google Scholar]
- 13.Littig JPB, Moellmer R, Estes AM, et al. Increased Population of CD40+ Fibroblasts Is Associated with Impaired Wound Healing and Chronic Inflammation in Diabetic Foot Ulcers. J Clin Med 11 (2022): 6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai V, Sharma P, Agrawal S, et al. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem 424 (2017): 123–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes RN, Manuel F, Nascimento DS. The bright side of fibroblasts: molecular signature and regenerative cues in major organs. NPJ Regen Med 6 (2021): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira SG, Moura J, Carvalho E, et al. Microbiota of Chronic Diabetic Wounds: Ecology, Impact, and Potential for Innovative Treatment Strategies. Front Microbiol 8 (2017): 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards AL, Muehlbauer AL, Alazizi A, et al. Gut Microbiota Has a Widespread and Modifiable Effect on Host Gene Regulation. mSystems 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin Y, Wade PA. Crosstalk between the microbiome and epigenome: messages from bugs. J Biochem 163 (2018): 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gay D, Ghinatti G, Guerrero-Juarez CF, et al. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci Adv 6 (2020): 3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascharak S, desJardins-Park HE, Davitt MF, et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goss G, Rognoni E, Salameti V, et al. Distinct Fibroblast Lineages Give Rise to NG2+ Pericyte Populations in Mouse Skin Development and Repair. Front Cell Dev Biol 9 (2021): 675080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Shao M, Hepler C, et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 129 (2019): 5327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plikus MV, Guerrero-Juarez CF, Ito M, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355 (2017): 748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganier C, Rognoni E, Goss G, et al. Fibroblast Heterogeneity in Healthy and Wounded Skin. Cold Spring Harb Perspect Biol 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero-Juarez CF, Dedhia PH, Jin S, et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun 10 (2019): 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504 (2013): 277–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinkevich Y, Walmsley GG, Hu MS, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348 (2015): 2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.desJardins-Park HE, Chinta MS, Foster DS, et al. Fibroblast Heterogeneity in and Its Implications for Plastic and Reconstructive Surgery: A Basic Science Review. Plast Reconstr Surg Glob Open 8 (2020): 2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nauroy P, Barruche V, Marchand L, et al. Human Dermal Fibroblast Subpopulations Display Distinct Gene Signatures Related to Cell Behaviors and Matrisome. J Invest Dermatol 137 (2017): 1787–9. [DOI] [PubMed] [Google Scholar]
- 31.Janson DG, Saintigny G, van Adrichem A, et al. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol 132 (2012): 2565–72. [DOI] [PubMed] [Google Scholar]
- 32.Philippeos C, Telerman SB, Oules B, et al. Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J Invest Dermatol 138 (2018): 811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabib T, Morse C, Wang T, et al. SFRP2/DPP4 and FMO1/LSP1 Define Major Fibroblast Populations in Human Skin. J Invest Dermatol 138 (2018): 802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw TJ, Rognoni E. Dissecting Fibroblast Heterogeneity in Health and Fibrotic Disease. Curr Rheumatol Rep 22 (2022): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RS, Smith TJ, Blieden TM, et al. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol 151 (1997): 317–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Barallobre-Barreiro J, Woods E, Bell RE, et al. Cartilage-like composition of keloid scar extracellular matrix suggests fibroblast mis-differentiation in disease. Matrix Biol Plus 4(2019): 100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 73 (2016): 3861–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moretti L, Stalfort J, Barker TH, et al. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J Biol Chem 298 (2022): 101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bukowska J, Kopcewicz M, Walendzik K, et al. Foxn1 in Skin Development, Homeostasis and Wound Healing. Int J Mol Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velasquez LS, Sutherland LB, Liu Z, et al. Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc Natl Acad Sci USA 110 (2013): 16850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noizet M, Lagoutte E, Gratigny M, et al. Master regulators in primary skin fibroblast fate reprogramming in a human ex vivo model of chronic wounds. Wound Repair Regen 24 (2016): 247–62. [DOI] [PubMed] [Google Scholar]
- 42.Phan QM, Fine GM, Salz L, et al. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petkovic M, Sorensen AE, Leal EC, et al. Mechanistic Actions of microRNAs in Diabetic Wound Healing. Cells 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalgaard LT, Leal EC, Svendsen R, , et al. Effects of the diabetes-induced microrna-155 on wound healing and fibroblast growth factor 7 expression. Diabetes 67 (2018). [Google Scholar]
- 45.Li D, Li XI, Wang A, et al. MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. J Invest Dermatol 135 (2015): 1676–85. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Li X, Li D, et al. MicroRNA-34 Family Enhances Wound Inflammation by Targeting LGR4. J Invest Dermatol 140 (2020): 465–76. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Li D, Wikstrom JD, et al. MicroRNA-132 promotes fibroblast migration via regulating RAS p21 protein activator 1 in skin wound healing. Sci Rep 7 (2017): 7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Li D, Wang A, et al. MicroRNA-132 with Therapeutic Potential in Chronic Wounds. J Invest Dermatol 137 (2017): 2630–8. [DOI] [PubMed] [Google Scholar]
- 49.Ozdemir D, Feinberg MW. MicroRNAs in diabetic wound healing: Pathophysiology and therapeutic opportunities. Trends Cardiovasc Med 29 (2019): 131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Xu X, Xiao L, et al. The Role of microRNA in the Inflammatory Response of Wound Healing. Front Immunol 13 (2022): 852419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan W, Chen S, Tong L. MicroRNA-215 Regulates Fibroblast Function: Insights from a Human Fibrotic Disease. Cell Cycle 14 (2015): 1973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paoletti C, Divieto C, Tarricone G, et al. MicroRNA-Mediated Direct Reprogramming of Human Adult Fibroblasts Toward Cardiac Phenotype. Front Bioeng Biotechnol 8 (2020): 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res 110 (2012): 1465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li T, Wu Y, Liu D, et al. MicroRNA-18a-5p represses scar fibroblast proliferation and extracellular matrix deposition through regulating Smad2 expression. Exp Ther Med 22 (2021): 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang B, Du R, Xiao X, et al. Microrna-217 modulates human skin fibroblast senescence by directly targeting DNA methyltransferase 1. Oncotarget 8 (2017): 33475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mancini M, Saintigny G, Mahe C, et al. MicroRNA-152 and −181a participate in human dermal fibroblasts senescence acting on cell adhesion and remodeling of the extra-cellular matrix. Aging (Albany NY) 4 (2012): 843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou BR, Guo XF, Zhang JA, et al. Elevated miR-34c-5p mediates dermal fibroblast senescence by ultraviolet irradiation. Int J Biol Sci 9 (2013): 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinha M, Sen CK, Singh K, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun 9 (2018): 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saraswati S, Marrow SMW, Watch LA, et al. Identification of a pro-angiogenic functional role for FSP1-positive fibroblast subtype in wound healing. Nat Commun 10 (2019): 3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venugopal H, Hanna A, Humeres C, et al. Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction. Cells 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rai V, Moellmer R, Agrawal DK. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mauch C, Zamek J, Abety AN, et al. Accelerated wound repair in ADAM-9 knockout animals. J Invest Dermatol 130 (2010): 2120–30. [DOI] [PubMed] [Google Scholar]


