Abstract
ComP is a sensor histidine kinase of Bacillus subtilis required for the signal transduction pathway that initiates the development of competence for genetic transformation. It is believed that ComP senses the presence of ComX, a modified extracellular peptide pheromone, and donates a phosphate to ComA, thereby activating this transcription factor for binding to the srfA promoter. In the present study, fusions to the Escherichia coli proteins PhoA and LacZ and analysis of its susceptibility to the protease kallikrein were used to probe the membrane topology of ComP. These data suggest that ComP contains six or eight membrane-spanning segments and two large extracytoplasmic loops in its N-terminal membrane-associated domain. Deletions were introduced involving the large extracellular loops to explore the role of the N-terminal domain of ComP in signal transduction. The absence of the second loop conferred a phenotype in which ComP was active in the absence of ComX. The implications of these data are discussed.
Competence development in Bacillus subtilis is controlled by a complex signal transduction pathway that culminates with the activation of genes encoding the machinery for DNA binding and uptake (for reviews, see references 11, 12, and 16).
The histidine-kinase ComP (64) and its cognate response regulator ComA (63), members of the family of two-component regulatory proteins (45), are required for competence. ComP is a membrane-bound protein with a C-terminal domain highly conserved among histidine kinases, whereas ComA is a cytoplasmic protein. It is likely, by analogy with other two-component regulatory systems, that ComP autophosphorylates a conserved histidine and subsequently donates its phosphoryl group to the cognate response regulator ComA (64). For competence development, ComA-PO4 must bind to the promoter region of srfA (43, 44, 50) thereby activating its transcription. Embedded in the srfA operon is comS, a small open reading frame required to activate ComK, the competence transcription factor (7, 17, 59, 60).
Two B. subtilis extracellular peptide factors accumulate in the medium as cells grow to high density and act via converging signal transduction pathways to activate the transcription of srfA (35, 55). ComX, a modified 9- to 10-amino-acid peptide (35) acts via ComP, presumably to increase the phosphorylation of ComA. The response to the other competence pheromone CSF (competence and sporulation stimulating factor) does not require ComP but also depends on ComA (55). CSF, imported by an oligopeptide permease (48, 56), inhibits a phosphoprotein phosphatase which otherwise dephosphorylates ComA (29, 56). Both pheromone pathways therefore regulate the level of ComA-PO4 and consequently determine the rate of srfA transcription.
ComP and ComA play a more general role than merely to regulate competence. In addition to srfA, several other loci are dependent on these proteins for their growth-stage-dependent induction. For instance, degQ (41) and several phosphatases which act to dephosphorylate response regulator proteins (including ComA) (42, 47) are regulated by this two-component system. ComP and ComA are therefore important players in the adaptation of B. subtilis to conditions of high population density.
The amino acid sequence of the large hydrophobic N-terminal domain of ComP does not resemble that of any known protein, whereas the C-terminal moiety of ComP shares similarity with the transmitter domain of other sensor kinase proteins (64). Moreover, this domain has been predicted to contain multiple membrane-spanning segments (64), a topological feature different from most membrane-localized sensors which possess only two transmembrane segments (45, 57).
Since ComX is an extracellular pheromone, it is likely that the membrane domain of ComP plays an important role in ComX detection. In this study we have examined the membrane topology of ComP and tested the functional relevance of portions of ComP predicted to be extracellular, thereby laying the basis for future mechanistic studies. We demonstrate that removal of one of the extracellular loops of the ComP membrane domain renders its activity independent of ComX.
MATERIALS AND METHODS
Bacterial strains and growth media.
The plasmids and B. subtilis strains used are described in Table 1. Escherichia coli strains are derivatives of DH5α and JM109 carrying plasmids pUCCMPHOA and pJF751, respectively (see below). E. coli was grown in Luria-Bertani medium (52) with ampicillin (Sigma) (100 μg/ml). B. subtilis strains were derivatives of strain 168 and were isogenic with IS75 (hisA1 leu-8 metB5). B. subtilis strains harboring fusions were obtained by transformation of IS75 with derivatives of pUCCMPHOA or pJF751 (carrying comP-phoA or comP-lacZ, respectively) by using selection for chloramphenicol (5 μg/ml; Sigma). The resulting transformants were derived from single crossover events at the comP locus and carry the comP fusions in single copy on the chromosome downstream of the endogenous regulatory sequences. Transformation of B. subtilis was carried out as described previously (1). For the assay of enzymatic activities, B. subtilis was grown at 37°C in competence medium (1) containing chloramphenicol (5 μg/ml). The comX comP::spc deletion strain was constructed by a double-crossover event and replaced all of comP and the last four codons of comX by a spectinomycin resistance cassette.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype and/or characteristics | Source or reference |
|---|---|---|
| B. subtilis strains | ||
| IS75 | his leu met | Laboratory strain |
| BD1853 | comPΔBclI orf2::kan | Laboratory strain |
| BD1890 | srfA-lacZ | 23 |
| BD2356 | comXP::spc | This work |
| BD2358 | comXP::spc carrying plasmid Pspac comP | This work |
| BD2362 | comXP::spc carrying plasmid Pspac | This work |
| BD2744 | amyE::ΔLabc comP comXP::spc | This work |
| BD2745 | amyE::ΔLbc comP comXP::spc | This work |
| BD2746 | amyE::ΔLc comP comXP::spc | This work |
| BD2747 | amyE::Xba comP comXP::spc | This work |
| BD2757 | amyE::ΔLabc comP comP ΔBclI | This work |
| BD2758 | amyE::ΔLbc comP comP ΔBclI | This work |
| BD2759 | amyE::ΔLc comP comP ΔBclI | This work |
| BD2760 | amyE::XbaI comP comP ΔBclI | This work |
| Plasmidsa | ||
| Pspac | B. subtilis vector | |
| Pspac comP | Pspac plasmid carrying comP | This work |
| pUCCMPHOA | Integrative vector for construction of in-frame fusions to the phoA gene of E. coli | 21 |
| pAH (H) | comP (D106)b cloned in pUCCMPHOA | This work |
| pAF (F) | comP (R112) cloned in pUCCMPHOA | This work |
| pAI (I) | comP (R143) cloned in pUCCMPHOA | This work |
| pAL (L) | comP (E223) cloned in pUCCMPHOA | This work |
| pAM (M) | comP (K272) cloned in pUCCMPHOA | This work |
| pAN (N) | comP (Y299) cloned in pUCCMPHOA | This work |
| pAZ (Z) | comP (P314) cloned in pUCCMPHOA | This work |
| pAO (O) | comP (Y338) cloned in pUCCMPHOA | This work |
| pAP (P) | comP (I362) cloned in pUCCMPHOA | This work |
| pAG (G) | comP (F396) cloned in pUCCMPHOA | This work |
| pAY (Y) | comP (L500) cloned in pUCCMPHOA | This work |
| JF751 | Integrative vector for construction of in-frame fusions to lacZ | 46 |
| pQR (R) | comP (K272) cloned in JF751 | This work |
| pQS (S) | comP (Y299) cloned in JF751 | This work |
| pQW (W) | comP (P314) cloned in JF751 | This work |
| pQT (T) | comP (Y338) cloned in JF751 | This work |
| pQU (U) | comP (I362) cloned in JF751 | This work |
| pQV (V) | comP (F396) cloned in JF751 | This work |
| pQX (X) | comP (L500) cloned in JF751 | This work |
The letters in parentheses designate the fusion proteins encoded by the plasmid.
The last aminoacyl residue of ComP included in the fusion is given in parentheses.
DNA manipulations.
Standard procedures were used to prepare and handle recombinant DNA, for the dideoxy sequencing, and to transform the E. coli cells (2). PCRs were carried out with Vent DNA polymerase (N. E. Biolabs) or with AmpliTaq DNA polymerase (Perkin-Elmer).
Construction of comP-phoA and comP-lacZ fusions.
Fusions of comP to either phoA or lacZ were generated by cloning fragments of comP, amplified by PCR, in frame with the phoA reporter gene of plasmid pUCCMPHOA or with the lacZ reporter gene of plasmid pJF751 (13). Plasmid pUCCMPHOA was constructed by cloning a PstI fragment carrying the E. coli phoA gene, lacking its promoter and the first 14 codons of its sequence, into the PstI site of the pUCCM18 vector (21). Hybrid genes coding for different fusions of the ComP amino-terminal region to the 15th residue of PhoA were constructed by designing PCR primers that placed sections of ComP in frame with PhoA. The upstream primer contained a KpnI site, and the downstream primers each contained SalI sites, which were used for cloning. As a result, the fragments of comP (Table 1) from the start codon to the most distal comP codons (located between D106 and L500) were separated from phoA by four new codons introduced by cloning. These encoded the amino acid sequence SRPA, joined to the first residue (A15) of the PhoA moiety. Similarly, constructs containing several of the same fragments of comP fused to the eight codon of lacZ were generated with primers containing EcoRI and BamHI sites that were then used for cloning. The lacZ fusions each contained codons for the amino acids AD, introduced by cloning, preceding the first residue (P8) of the LacZ moiety. Restriction analysis and DNA sequencing verified the junction of each fusion construct. In all constructs the hybrid genes were located downstream from the lacZ promoter, which was used to drive expression in E. coli. In B. subtilis the fusion constructs were chromosomally integrated at the comP locus and transcribed from the comP promoter (see above).
Construction of B. subtilis strains expressing mutant ComP proteins.
Plasmids allowing inducible expression at the amy locus of ComP derivatives lacking loops a, b, and c (Fig. 1) were generated as follows. A PCR fragment carrying the 545 C-terminal codons of comP (starting from the F225 codon) and flanked by XbaI and ClaI restriction sites, was cloned into pDR67 (22), giving pDR67-′comP. Then, four fragments, flanked by SmaI and XbaI restriction sites and carrying 42, 93, 167 or 224 N-terminal codons of comP were obtained by PCR and cloned into pDR67-′comP to give, respectively, pDR67-ΔLabc-comP, pDR67-ΔLbc-comP, pDR67-ΔLc-comP, and pDR67-Xba-comP plasmids. The pDR67-ΔLabc-comP, pDR67-ΔLbc-comP, and pDR67-ΔLc-comP plasmids contain the sequences encoding ComP derivative proteins missing loops a, b, and c (ΔLabc-ComP), loops b and c (ΔLbc-ComP), or only loop c (ΔLc-ComP). The pDR67-Xba-comP plasmid encodes a ComP protein, derived from the wild type by insertion of S and R residues, in order to introduce XbaI termini for ligation. This construct serves as a control for the others, which also contain an XbaI site at the same position. All of the cloned fragments derived by PCR were completely verified by sequencing. All of these plasmids were used to transform IS75, and chloramphenicol-resistant transformants that had integrated the mutant comP genes at the amyE locus were identified. The resulting strains were then transformed by congression with chromosomal DNA prepared from BD1890 (srfA-lacZ) together with DNA from either BD1853 (comPΔBclI) or BD2356 (comXP::spc), in order to introduce a srfA-lacZ transcriptional fusion while simultaneously inactivating either the comP gene alone or both the comX and comP genes.
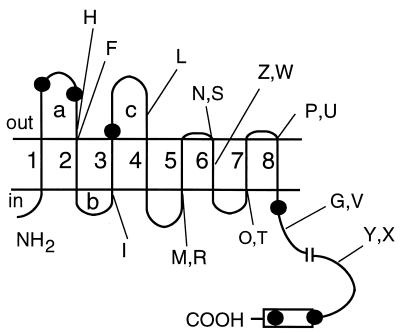
FIG. 1.
Model for ComP topology. The eight putative membrane-spanning segments are (counting from the amino terminus) are as follows: 1, I10 to I33; 2, Y114 to F134; 3, S145 to G167; 4, L236 to L257; 5, K272 to F295; 6, I300 to A323; 7, Y338 to F357; and 8, I362 to F383. Sites of fusion with alkaline phosphatase (H, F, I, L, M, N, Z, P, G, and Y) and with β-galactosidase (R, S, W, T, U, V, and X) are indicated. The conserved histidine kinase domain is shown as a rectangle. The predicted kallikrein cleavage sites are indicated by dots. The transmembrane segments are numbered, and the cytoplasmic and extracellular loops referred to in the text are labeled for convenience.
Assay of alkaline phosphatase and β-galactosidase activities.
Alkaline phosphatase activity in E. coli, conferred by the comP-phoA fusions, was determined in liquid cultures by using p-nitrophenyl phosphate (Sigma) as described previously (36). The same assay was used for B. subtilis cultures grown in competence medium to T0, except that cell permeabilization with detergent and chloroform was omitted and the B. subtilis cells were concentrated fivefold by centrifugation prior to assay. In B. subtilis, alkaline phosphatase activity values were corrected for the low background level exhibited by the control strain IS75. DH5α was used as the host for alkaline phosphatase assays in E. coli, since we detected no background alkaline phosphatase activity in this strain. The alkaline phosphatase activity determinations presented in this study were performed on aliquots of the same samples that were worked up for Western blot analysis. For assay of the comP-lacZ fusion strains, the β-galactosidase specific activity was determined in B. subtilis and E. coli as described earlier (1, 40). The E. coli host strain used for the β-galactosidase assays was JM109. For assay of srfA-lacZ expression in the comP deletion mutants of B. subtilis, strains were grown in competence medium with or without IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM), and 500-μl samples were collected every hour. β-Galactosidase activities were measured in the kinetic mode on a Spectra Rainbow (Tecan) microplate reader. For the calculation of specific activity, the total protein was estimated from Klett readings on culture samples by using an experimentally determined calibration curve.
Preparation of protoplasts and right-side-out membrane vesicles from B. subtilis.
Protoplasts were prepared as described previously (6), and protoplast formation was monitored by microscopic examination. For the preparation of membrane vesicles, hypotonically lysed protoplasts were incubated in the presence of DNase I (10 μg/ml; Sigma) at room temperature for 10 min, and the Mg2+ concentration was subsequently adjusted to 5 mM. Membranes were purified by isopycnic centrifugation through a discontinuous sucrose gradient as described earlier (34, 58), washed in 0.1 M sodium phosphate buffer (pH 7.2)–1 mM phenylmethylsulfonyl fluoride, and stored at −20°C in the same buffer.
Proteolysis with kallikrein.
B. subtilis protoplasts were resuspended in 33 mM Tris-HCl (pH 8.0)–100 mM KCl–5 mM MgSO4–0.5 M sucrose at a protein concentration of 0.5 mg/ml. Triton X-100 (0.5%) was added to some of the samples as noted. Samples (0.5 mg/ml) were incubated with kallikrein (Sigma) at a final concentration of 100 or 200 μg/ml. In some cases Triton X-100 (0.5%) was added to permeabilize the protoplasts. Proteolysis was carried out by incubation for 3 h at 37°C. The protease inhibitor leupeptin (1 μM; Sigma) was added to stop the reaction. Samples were centrifuged, and the pellet (membrane fraction) was resuspended in sample buffer (28). From each sample, 30 μg of protein per lane was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel. The proteins were transferred to nitrocellulose and analyzed by immunoblotting with anti-ComP antisera.
SDS-PAGE and immunoblotting.
Proteins from B. subtilis membrane preparations or from E. coli whole-cell extracts were resolved on 10% gels by SDS-PAGE (28) without previous boiling of the samples. Gels were equilibrated in transfer buffer (48 mM Tris-HCl, pH 9.2; 29 mM glycine; 0.05% SDS; 20% methanol) for 10 to 15 min and then electrophoretically transferred to pre-wetted nitrocellulose membranes (0.45-μm pore size; Schleicher & Schuell) for 15 min at 15 V in a semidry transfer apparatus (Bio-Rad). Nitrocellulose membranes were incubated with monoclonal anti-PhoA antibody (Boehringer) or with a guinea pig anti-ComP antiserum directed against a synthetic peptide corresponding to the last 16 aminoacyl residues of ComP and then with a peroxidase-conjugated secondary antibody, which was detected by using a Chemiluminescent Substrate Kit (Kirkegaard & Perry Laboratories) as recommended by the supplier. The protein concentration was estimated by using the Bio-Rad protein assay reagent.
RESULTS
Based on hydropathy analysis of ComP, eight hydrophobic segments were predicted to be membrane spanning (64), leaving two large regions each consisting of approximately 80 amino acids outside the cell membrane and the C-terminal histidine kinase domain in the cytoplasm (Fig. 1). We have used Western blotting on fractionated lysates to confirm that ComP is localized exclusively in the cell membrane of B. subtilis and behaves as an integral membrane protein with respect to NaOH solubility (not shown) (51).
Deletion of the large external loops of ComP relieves it from ComX control.
Based on the hypothetical topology displayed in Fig. 1, we tested the involvement of the most N-terminal loops (a, b, and c) of ComP in the ComX quorum sensing pathway (see Fig. 1 for the identification of these loops). The expression of a srfA-lacZ transcriptional fusion was measured as an indication of activation of the quorum sensing pathway. We constructed three isogenic B. subtilis strains, all with an inactivating deletion of comP and expressing, from a pSpac (IPTG-inducible) promoter, three ComP derivative proteins with deletions of either loop c (in BD2759), loops b and c (in BD2758), or loops a, b, and c (in BD2757). These constructions were designed so that 13 residues remained between the C terminus of the deleted segment and the N terminus of the following transmembrane segment. These residues were intended to serve as an extracytoplasmic linker connecting the up- and downstream portions of the protein in order to minimize the disruption of the topology of the mutant ComP proteins. The constructions introduced an XbaI restriction site, adding S and R residues after position 224 in all the ComP derivative proteins. We also constructed the control strain BD2760, which expressed an otherwise wild-type ComP but contained the S and R insertions.
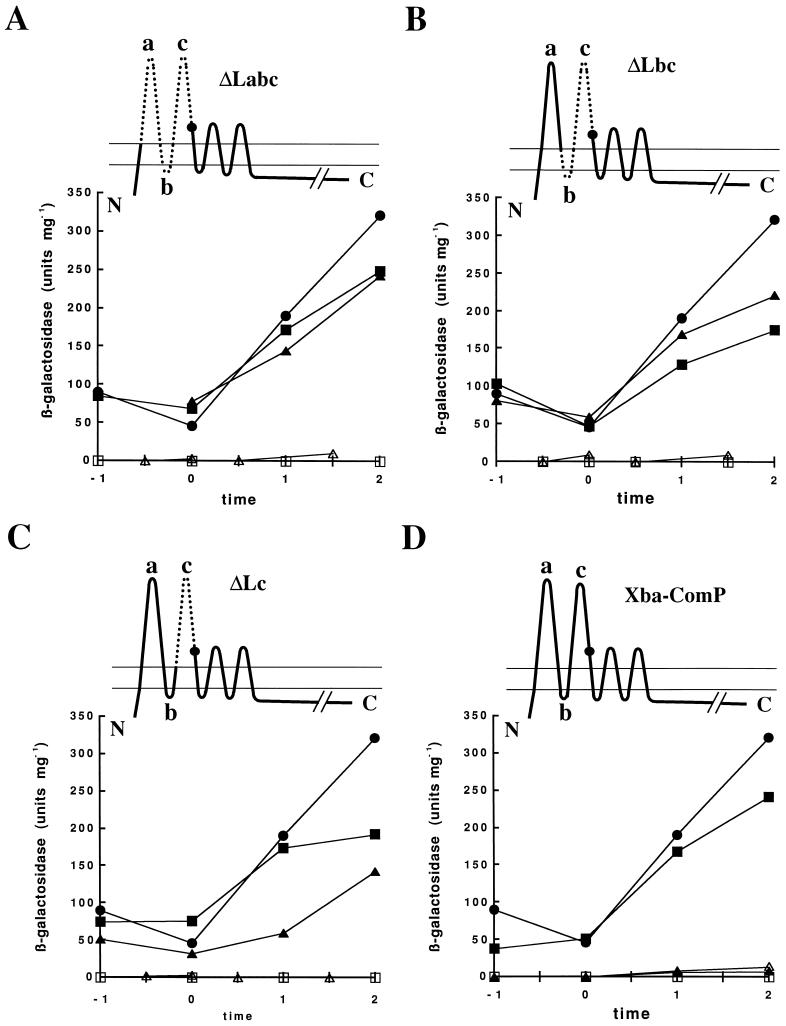
These strains were grown in competence medium with or without 1 mM IPTG, and their β-galactosidase activities were measured during growth (Fig. 2). Little or no β-galactosidase activity was detected after growth in the absence of IPTG, as expected. When expression of the comP derivative genes was induced by the addition of IPTG, srfA-lacZ was expressed in all the strains at levels comparable to that in the wild-type strain (BD1890). Thus, all of these mutant ComP proteins can replace wild-type ComP for the induction of srfA expression in vivo.
FIG. 2.
srfA-lacZ expression in comP mutants. In each panel the diagram at the top presents the extent and position of the relevant deletion. The dots on these diagrams indicate the positions of the S and R insertions introduced during construction of the deletions. In each panel, β-galactosidase activities are presented for the strains grown in the absence of IPTG (▵, □); the solid symbols are for strains grown in the presence of IPTG. Strains are designated as follows: The comP wild-type strain (BD1890) in a comX+ background (●) and the comP mutant strains in comX+ (□, ■) and comX backgrounds (▵, ▴) are as indicated. (A) ΔLabc comP BD2757 (comX+) and BD2744 (comX). (B) ΔLbc comP BD2758 (comX+) and BD2745 (comX). (C) ΔLc comP BD2759 (comX+) and BD2746 (comX). (D) XbaI comP mutant strains BD2760 (comX+) and BD2747 (comX). Time is given in hours before or after T0, the point of transition from exponential to stationary phase.
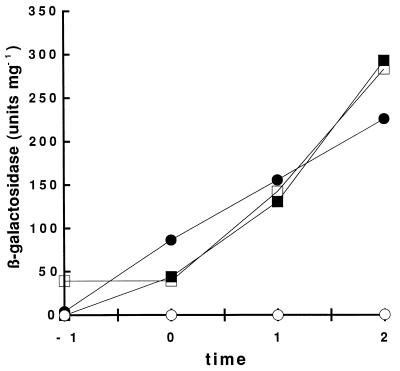
To determine the dependence of the activities associated with the mutant ComP proteins on ComX, we performed the same experiment with these comP derivatives in a comX comP background. Very little activity was detected in the absence of IPTG (Fig. 2). The control strain (BD2747), expressing ComP with the S and R insertions in the comX comP background, did not express detectable β-galactosidase activity even when grown in the presence of IPTG (Fig. 2D). This phenotype could be fully complemented by growth in ComX-containing conditioned medium prepared from the wild-type strain BD1890 (Fig. 3), demonstrating that ComP with the S and R insertions responded to the ComX pheromone, as well as the wild-type protein. However, all strains carrying deletions in the N-terminal loops no longer required ComX for srfA-lacZ expression (Fig. 2). comX inactivation did not affect the activities of the ComP derivative proteins lacking either loops b and c (in BD2745) or loops a, b, and c (in BD2744) (Fig. 2A and B). BD2746, expressing a ComP protein lacking only loop c, exhibited approximately a twofold-lower level of srfA-lacZ activity in the absence of endogenous ComX than did the other deletion strains (Fig. 2C). This was observed in three independent experiments. In spite of this difference, the loop c deletion strain did not respond to conditioned medium (Fig. 3).
FIG. 3.
Response of srfA-lacZ expression to conditioned medium in strains carrying wild-type and mutant comP. The open symbols indicate β-galactosidase activities for strains grown with conditioned medium obtained from a comX strain (BD2356). The solid symbols indicate β-galactosidase activities for strains grown with conditioned medium from a comX+ strain (IS75). The strains grown in these conditioned media carried the XbaI comP (●, ○; BD2747) or the ΔLc comP (■, □; BD2746) mutations. Time is given in hours before or after T0.
Based on the topological model shown in Fig. 1, we may conclude that deletion of loop c relieved the protein from ComX control, at least partially, while deletion of loops b and c completely eliminated ComX dependence. Interpretation of the deletion results obviously depends on the topology of the three most N-terminal loops. However, computer modeling cannot be regarded as definitive. For example we cannot be confident that loops a and c are extracellular, since the polarity of the proposed structure was based solely on the distribution of positive charges (61, 62), and this portion of the structure might actually be reversed. Therefore, we have tested the model by constructing in-frame fusions of comP to a deleted version of the E. coli phoA gene, encoding mature alkaline phosphatase.
Analysis of ComP-PhoA fusions: topology of loops a, b, and c.
Alkaline phosphatase has been used as a reporter for the subcellular localization of different portions of a protein (reviewed in references 37 and 38) by exploiting the fact that it is active only when translocated across the membrane (8). Eleven 3′-deleted fragments of comP were cloned in frame with phoA in the pUCCMPHOA vector. Nearly all of the fusions contained junction points at the C termini of each of the predicted hydrophilic regions of ComP. This strategy was intended to minimize the disruption of topological signals (e.g., membrane-spanning segments and hydrophilic regions with positive net charges) (5). Fusion points are listed in Table 2, and their positions are indicated schematically in Fig. 1. Since the principal aim of these experiments was to test the topological model for the predicted loops a, b, and c, we will first discuss the fusions that are relevant to this purpose.
TABLE 2.
Activities associated with PhoA and LacZ fusions to ComP
| Fusion | Fusion positiona | Relative activitiesb
|
|||
|---|---|---|---|---|---|
|
E. coli
|
B. subtilis
|
||||
| AP | β-Gal | AP | β-Gal | ||
| H | D106 | 100 | 100 | ||
| F | R112 | 98 | 41 | ||
| I | R143 | 0 | 0 | ||
| L | E223 | 1.6 | 37 | ||
| M/R | K272 | 0 | 13 | 0 | 77 |
| N/S | Y299 | 0 | 0 | 0 | 15 |
| Z/W | P314 | 1.2 | 4 | 1.1 | 10 |
| O/T | Y338 | 0 | 100 | 0 | 45 |
| P/U | I362 | 33 | 3.4 | 13 | 3.2 |
| G/V | F396 | 10 | 20 | 5 | 3 |
| Y/X | L500 | 0 | 46 | 0 | 100 |
The positions of the fusions are indicated diagrammatically in Fig. 1. Where pairs of fusion names are given, the first refers to the PhoA fusion and the second refers to the corresponding lacZ fusion.
The phoA and lacZ fusions are to the residues listed in the table. Alkaline phosphatase (AP) activities are normalized relative to that of fusion H. The β-galactosidase (β-Gal) activities for E. coli are normalized relative to that of fusion T; those for B. subtilis are normalized relative to that of fusion X.
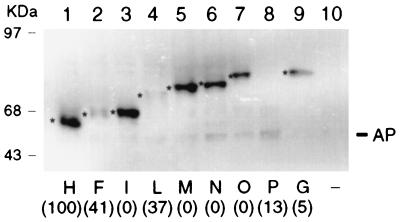
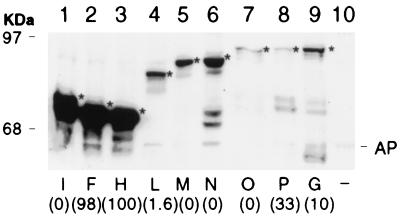
The recombinant phoA fusion plasmids were tested in B. subtilis after chromosomal integration in single copy. Alkaline phosphatase activity was measured in cells grown in competence medium (1). Expression of the endogenous B. subtilis vegetative alkaline phosphatase was repressed by the high concentration of phosphate in this medium (20). The alkaline phosphatase activities (Table 2) were normalized to the highest activity (fusion H). Fusions H, F, and L were associated with high activity and fusion I failed to exhibit any detectable activity above the background. Western blot analysis of purified membrane vesicles from B. subtilis was carried out on these fusion strains by using anti-PhoA antibody. All the fusion proteins were detected in the cell membrane fraction (Fig. 4). A product with an electrophoretic mobility similar to that of mature alkaline phosphatase, presumably formed by degradation, was detected for some fusions and no signal was detectable in the control strain with no fusion (Fig. 4, lane 10). Strong signals were associated with fusions H, I, and M, and weak signals were associated with fusions F and L. It is noteworthy that, with the exception of H, the fusions that place alkaline phosphatase outside the membrane exhibited relatively high alkaline phosphatase activity but weak Western blot signals. Possibly, PhoA tends to be unstable in B. subtilis either in vivo or in the extracts, when exposed on the exterior face of the membrane. However, since low alkaline phosphatase activity was not associated with weak Western blot signals, the B. subtilis results provide strong support for the topological model concerning loops a, b, and c and buttress our interpretation of the structures affected by our deletion mutations.
FIG. 4.
Detection of ComP-PhoA hybrid proteins in B. subtilis membranes. Membrane vesicles were prepared from B. subtilis strains carrying comP-phoA hybrid genes in a single copy on the chromosome. Equal amounts (10 μg of protein/lane) of membrane vesicles were separated by SDS-PAGE on a 10% gel, and monoclonal anti-alkaline phosphatase antibody was used for the immunoblotting. Fusion proteins are indicated by letters, and their positions are denoted by asterisks. The numbers in parentheses represent alkaline phosphatase values normalized to that associated with fusion H (Table 2). Strain BD630, which lacks a comP-phoA fusion, was used as a negative control (−). The positions of molecular size standards (in kilodaltons) are shown, as is the position of mature alkaline phosphatase (AP).
The recombinant phoA fusion plasmids were also tested in E. coli DH5α because the relative paucity of published reports on the use of PhoA fusions in B. subtilis made it advisable to compare results obtained in the two organisms. Alkaline phosphatase activities obtained in E. coli, normalized to that of fusion H which exhibited the highest activity, are shown in Table 2. Fusion proteins H and F exhibited high alkaline phosphatase activity, suggesting that residues D106 and R112 of ComP (Fig. 1 and Table 2) were extracytoplasmic. Fusion L was associated with low but measurable activity, suggesting that residue E223 was possibly extracytoplasmic as well. In contrast, no detectable alkaline phosphatase activity was exhibited by fusions I and M. Residues R143 and K272 were therefore judged to be cytoplasmic. Since the alkaline phosphatase activity will depend on the abundance of each fusion protein in addition to the location of the PhoA moiety, PhoA antiserum was used to detect the fusion proteins in Western blot analysis (Fig. 5). Strong signals were detected for fusions I, F and H. The abundance of the fusion I protein, together with the absence of detectable activity, provided strong support for the cytoplasmic location of R143. Fusion L was evidently much less abundant than F and H, probably due to lower stability. This provided a reasonable explanation for the relatively low activity exhibited by fusion L. Fusion M was at least as abundant as several others that were associated with readily detectable alkaline phosphatase activities. Taken together, the phoA fusion results from E. coli strongly support the predicted ComP topology for loops a, b, and c, and are consistent with the results obtained in B. subtilis.
FIG. 5.
Detection of ComP-PhoA hybrid proteins in E. coli by Western blotting. Total cell extracts containing equal amounts of protein were separated by SDS-PAGE on a 10% gel and visualized by immunoblotting with monoclonal anti-alkaline phosphatase antibody. Fusion proteins are indicated by letters, and their positions are denoted by arrows. The numbers in parentheses represent alkaline phosphatase values normalized to that associated with fusion H (Table 2). A strain carrying the phoA vector with no comP insert was used as a negative control (−). The positions of molecular size standards (in kilodaltons) are shown, as is the position of mature alkaline phosphatase (AP).
Analysis of ComP-PhoA fusions: topology of the distal portions of the ComP N-terminal domain.
PhoA fusions were also used to test the more distal portion of the model, beyond fusion point M (Fig. 1 and Table 2). In both B. subtilis and E. coli, fusion P (I362) exhibited relatively high activity and fusion Z (P314) was associated with low but detectable activity. No activity was detected in the cases of fusions O (Y338) and Y (L500). The behavior of these fusions was consistent with the model. However, the absence of activity with fusion N and the activity associated with fusion G do not support the model presented in Fig. 1. The C terminus of ComP is predicted to be located in the cytoplasm since its histidine kinase domain must be accessible to ATP and to the response regulator ComA. The behavior of fusion G was at variance with this expectation, since it exhibited a higher activity than other fusions with junctions located in the cytoplasm. This discrepancy may be due to the absence in fusion G of an important determinant of cytoplasmic localization. Fusion Y (L500) exhibited no detectable AP activity, suggesting that this putative topogenic signal is located between the junctions of fusions G and Y. In fact, between positions 383 and 400 there are six positively charged residues, not included in fusion G, which may comprise such a cytoplasmic determinant (61, 62). The behavior of fusion N is more difficult to interpret. The absence of fusion N-associated activity was not explicable by a low level of synthesis or the instability of the fusion protein. Western blots revealed a clear signal for the fusion N protein, one considerably stronger than that of fusion P, which had a relatively high AP activity (Fig. 4, 5, and 6). The weak Western blot signals for fusion P in both hosts (and for F and L in B. subtilis) indicate either protein instability in vivo, associated with high specific activities, or more likely instability in extracts.
FIG. 6.
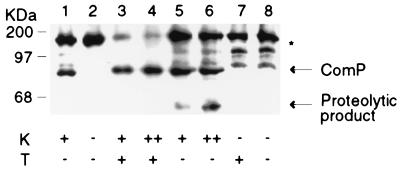
Proteolysis of ComP with kallikrein. Protoplasts of B. subtilis were prepared from strain BD2358 (lanes 3 to 8), which carries comP on a multicopy plasmid, and from strain BD2362 (lanes 1 and 2), which carries a complete deletion of comP. Samples were incubated at 37°C with the protease kallikrein (K) at a final concentration of 100 μg/ml (+) or 200 μg/ml (++). Triton X-100 (T) was added to some of the samples to permeabilize the protoplasts. Proteins were transferred to nitrocellulose and analyzed by immunoblotting with anti-ComP antisera. Arrows indicate the positions of ComP and of the main proteolytic product derived from ComP. The positions of molecular size standards (in kilodaltons) are shown.
Analysis of ComP-LacZ fusions.
The data obtained with the ComP-PhoA fusions described above were generally in agreement with the topology proposed for ComP, especially for the most relevant region encompassing loops a, b, and c. However, the behavior of fusion N in particular failed to support the model. An alternative would hold that the region including membrane-spanning segments 5 and 6, between K272 (fusion M junction) and Y338 (fusion O junction), is located in the cytoplasm.
To further test the model, we constructed comP fusions to lacZ and determined their β-galactosidase activities in E. coli and B. subtilis. When LacZ is used as a reporter protein for membrane topology, a reversal of relative enzymatic activities compared to that of PhoA fusions is observed, since fusion proteins between the amino-terminal segments of membrane proteins and LacZ are active enzymatically only when the LacZ moiety of the hybrid is localized in the cytoplasm (15, 31, 54). The comP-lacZ gene fusions were generated with a strategy similar to that used for cloning the comP-phoA fusions (see Materials and Methods). Plasmid derivatives of JF751 carrying fusions to lacZ are listed in Table 1. The seven fusion points, indicated in Table 2 and Fig. 1, were identical to those in the corresponding PhoA fusions.
In B. subtilis, the LacZ constructs were again inserted chromosomally in single copy. The ambiguity with the phoA fusion N was reflected with fusion S. Although lower than the activities of fusion R, T, and X, which clearly place the lacZ moiety in the cytoplasm, fusion S displays a higher activity than the extracellular fusion U. The low activity of fusion V may once more be explained by the argument that this fusion, as in the case of the corresponding fusion to PhoA (fusion G), excluded important downstream topological determinants for cytoplasmic localization. In summary, the lacZ fusion experiments did not lift the uncertainty associated with segments 5 and 6, and so we cannot clearly exclude the possibility that these segments are located in the cytoplasm.
E. coli strains carrying the comP-lacZ gene fusions were also tested for β-galactosidase activity (Table 2). The activities associated with fusions R, T, and X, predicted to place the LacZ moiety in the cytoplasm, are generally higher than those of fusions S, W, and U, which should place LacZ outside the membrane. These data support the original model.
Protease susceptibility of ComP.
Additional evidence that the amino-terminal part of ComP contains regions exposed on the outer surface of the membrane was obtained from protease susceptibility studies with protoplasts. We used the protease kallikrein, which cleaves the peptide bond N-terminal to FK and LR sequences. ComP contains six potential cleavage sites for kallikrein (Fig. 1). Of these, three are predicted to be extracytoplasmic: two in loop a and one in loop c. Only these three sites will be accessible to kallikrein in intact protoplasts. Our ComP antiserum was raised against a synthetic peptide corresponding to the ultimate 16 C-terminal residues. The respective sizes of the C-terminal proteolytic products generated from cleavage at each of the extracytoplasmic sites would be 79.9, 76.0, and 67.3 kDa. In a limit digest only the 67.3-kDa product would be detectable.
Protoplasts from a B. subtilis strain (BD2358) that expresses ComP from a multicopy plasmid were treated with kallikrein for 3 h, and membrane preparations were analyzed by Western blotting (Fig. 6). The integrity of the protoplasts was confirmed in Western blots by the resistance to kallikrein of the cytoplasmic MecA protein (26), which contains a single, centrally located cleavage site. A prominent proteolytic product of approximately 65 kDa was detected when protoplasts from the strain expressing ComP were treated with kallikrein (Fig. 6, lanes 5 and 6). The 65-kDa signal was absent in kallikrein-treated protoplasts from a comP deletion strain (BD2356), confirming that it was derived from ComP (Fig. 6, lane 1). This product was also absent from extracts prepared from protoplasts incubated without kallikrein (Fig. 6, lanes 7 and 8) and from protoplasts treated with Triton X-100 together with kallikrein (Fig. 6, lanes 3 and 4). In the presence of Triton X-100, protoplasts are permeabilized and cytoplasmic cleavage sites become accessible to the protease, as indicated by the degradation of MecA (data not shown). The ComP-derived products generated by cleavage at these sites are too small to be visible in the Western blot shown in Fig. 6. A strain with a total ComP deletion (BD2356) was included as a negative control (Fig. 6, lanes 1 and 2). In extracts from this strain a proteolytic product with a size similar to that of ComP was detected, presumably originating from a protein that cross-reacts with the antiserum. This product obscured the disappearance of ComP in lanes 3 to 6.
The ComP-specific kallikrein degradation product with a nominal molecular size of 65 kDa probably corresponds to the predicted 67.3-kDa product. This is the expected product from a limit digest of the three sites predicted to be extracellular. The presence of a unique ComP-derived cleavage product obtained only when intact protoplasts were treated with kallikrein supported the conclusion that a hydrophilic portion of the N-terminal domain of ComP, most probably corresponding to loop c, is extracytoplasmic, lending further support to our topological model.
DISCUSSION
The phoA and lacZ fusion experiments established that ComP is a polytopic integral membrane protein with two large extracellular segments. The kallikrein accessibility experiment supported these conclusions since it was consistent with extracellular exposure of a portion of the N-terminal domain. The cytoplasmic location assigned to the C-terminal hydrophilic domain is in accord with the putative role of ComP as an autokinase.
There have been relatively few examples of the use of protein fusions to determine membrane protein topology in gram-positive organisms (14, 21). The normalized activities of the phoA and lacZ fusions are similar in B. subtilis and in E. coli, suggesting that in B. subtilis, which lacks a periplasm in the strict sense (but see reference 39), fusions to phoA and lacZ give results similar to those obtained in E. coli.
The greatest uncertainty in our data concerns the location of the N/S fusion junction, raising the possibility that the entire region, which includes the predicted fifth and sixth membrane-spanning segments, is actually cytoplasmic. Contrary to our original prediction, no detectable alkaline phosphatase activity was associated with fusion N. Also, the normalized β-galactosidase activity associated with fusion S in B. subtilis does not unambiguously support either model. However, the possible presence of eight membrane-spanning segments could be rationalized as follows. The alkaline phosphatase activities of fusions N, Z, and P increase with the distance of the fusion junctions from the N terminus of ComP (Table 2). This pattern is consistent with the possibility that the correct membrane insertion of segments 5 and 6 requires the interactions of protein sequences located downstream from the S/N fusion point (Y299) and that the deletion of these sequences results in decreased probabilities that the correct insertion will occur. Perhaps a domain, which includes transmembrane segments 6 to 8 must fold properly before the region from 5 to 6 can undergo membrane insertion. An alternative possibility is that segments 5 and 6 (Fig. 1) are embedded in the membrane but do not completely cross it. In summary, we conclude that ComP contains either six or eight membrane-spanning segments.
The most important conclusion from the topology studies is that it confirms the extracellular locations of loops a and c and the cytoplasmic disposition of loop b. Removal of the extracellular loop c or of a segment including loops b and c or loops a, b, and c confers a striking phenotype, rendering srfA-lacZ expression independent of ComX. These data imply that sequences included within loop c are required to maintain ComP in an inactive state for autophosphorylation or to activate a dephosphorylation activity and that ComX may serve to antagonize this inhibitory state. In the mutant proteins in which loop c is deleted, this state cannot be established and ComP is active and presumably phosphorylated, even in the absence of ComX. The lower srfA-lacZ activity of the ΔLc strain compared to that of the ΔLabc and ΔLbc strains (Fig. 2) was observed reproducibly in the comX background. It may be that removal of just loop c is not sufficient for full srfA expression in the absence of ComX. However, the ΔLc strain does not respond to extracellular ComX by increasing the level of srfA-lacZ expression (Fig. 3). This suggests that loop c is essential for the signal transduction mechanism that detects the presence of ComX (directly or indirectly). If ComP interacts directly with ComX, then loop c may be essential for this binding. Our data in no way rules out the possibility that residues from loops a and b are also required for interaction with ComX.
In additional experiments (50a), three deletion mutations were constructed that lacked the entire N-terminal hydrophobic domain of ComP. The deleted genes were integrated in a single copy in the B. subtilis chromosome, and the activities of the cognate proteins were determined by using a srfA-lacZ reporter in a comP background. Very low or no β-galactosidase activity was detected with the deletions. When these deletion proteins and a wild-type ComP were expressed from identical plasmids in B. subtilis, Western blotting revealed comparable cellular levels of the truncated and wild-type proteins. Removal of the membrane-localized N-terminal domain of ComP resulted in a dramatic decrease in activity, and the deleted constructs could not respond to ComX when tested with conditioned medium. This suggests that the portions of the N-terminal domain downstream from loops a, b, and c are required for the activity of ComP. We propose therefore that loops a, b, and c (notably loop c) play a role in response to ComX, whereas the downstream region comprising membrane-spanning segments 5 to 8 is needed for ComP function, either as an autokinase or to suppress a phosphatase activity.
It is notable that the growth stage regulation of srfA-lacZ expression in the strains carrying the ComX-independent forms of ComP is not altered; induction of srfA-lacZ expression begins at T0 in these strains as it does in the wild type (Fig. 2). This indicates that the mechanism of growth-stage-dependent control of srfA-lacZ expression is redundant and does not depend exclusively on the ComX signaling pathway. Growth stage-specific expression of srfA is known to be regulated negatively by CodY (53) and positively by CSF (30) and SinR (33).
Most membrane-localized histidine kinases possess two membrane-spanning segments and a single extracytoplasmic loop. ComP is a member of a growing family of membrane-localized bacterial histidine kinases that possess more-complex structures containing several membrane-spanning segments. Most of these proteins are involved in quorum-sensing signal transduction mechanisms that use peptide pheromones, often with posttranslational modifications. For instance, a quorum-sensing system, analogous to the ComP-ComA two-component signal transduction pathway, regulates competence in S. pneumoniae (18, 49). In this system a peptide pheromone interacts with the histidine kinase protein ComD (19). ComD is predicted by hydropathy analysis to contain five to seven transmembrane segments (19) but displays no obvious sequence similarity to ComP except in the cytoplasmic histidine kinase domain. The quorum-sensing histidine kinase AgrC, which regulates virulence in S. aureus in response to a peptide pheromone (25, 27), also contains multiple membrane-spanning segments (32). The synthesis of antibiotics in Lactobacillus and Carnobacterium spp. is regulated by the histidine kinases PlnB (9, 10), SapK (3, 4), and SppK (3, 4) that are related to ComD (19) and also appear to sense the presence of peptide pheromones. These kinases possess hydrophobic N-terminal domains, each about 200 residues long, predicted by hydropathy analysis to contain five to eight membrane-spanning segments (4, 10, 19).
Although ComD and AgrC appear to detect their cognate peptide pheromones directly (19, 24), this has not been demonstrated in the cases of other quorum-sensing histidine kinases, including ComP. The similarities in their membrane topologies noted above suggest that signal transduction in these various systems may have common features. Further insight into the mechanism of ComX-ComP interaction may be obtained by the genetic and biochemical analysis of additional ComP mutations specifically affecting the response to ComX. Localization of these mutations with respect to the membrane topology described in this study would help to identify the determinants involved in interaction with ComX and in the response to this interaction.
ACKNOWLEDGMENTS
We thank the members of our laboratory, especially G. Inamine, for useful discussions and advice. We also thank Eugenio Ferrari for contributing JF751 and Manuella Roggiani for constructing three comP deletions.
This work was supported by NIH grant GM57720 and by a Lavoisier Fellowship to P.T. awarded by the French Ministry of Foreign Affairs.
REFERENCES
- 1.Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidam J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates/Wiley Interscience; 1994. [Google Scholar]
- 3.Axelsson L, Holck A. The genes involved in production and immunity to sakacin A, a bacteriocin from Lactobacillus sake Lb706. J Bacteriol. 1995;177:2125–2137. doi: 10.1128/jb.177.8.2125-2137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelsson L, Holck A, Birkeland S-E, Aukrust T, Blom H. Cloning and nucleotide sequence of a gene from Lactobacillus sake Lb706 necessary for sakacin A production and immunity. Appl Environ Microbiol. 1993;59:2868–2875. doi: 10.1128/aem.59.9.2868-2875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd D, Traxler B, Beckwith J. Analysis of the topology of a membrane protein by using a minimum number of alkaline phosphatase fusions. J Bacteriol. 1993;175:553–556. doi: 10.1128/jb.175.2.553-556.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitling R, Dubnau D. A pilin-like membrane protein is essential for DNA binding by competent Bacillus subtilis. J Bacteriol. 1990;172:1499–1508. doi: 10.1128/jb.172.3.1499-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza C, Nakano M M, Zuber P. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:9397–9401. doi: 10.1073/pnas.91.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derman A I, Beckwith J. Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J Bacteriol. 1991;173:7719–7722. doi: 10.1128/jb.173.23.7719-7722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diep D B, Håvarstein L S, Nes I F. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol Microbiol. 1995;18:631–639. doi: 10.1111/j.1365-2958.1995.mmi_18040631.x. [DOI] [PubMed] [Google Scholar]
- 10.Diep D B, Håvarstein L S, Nissen-Meyer J, Nes I F. The gene encoding plantaricin A, a bacteriocin from Lactobacillus plantarum C11, is located on the same transcription unit as an agr-like regulatory system. Appl Environ Microbiol. 1994;60:160–166. doi: 10.1128/aem.60.1.160-166.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubnau D. Genetic exchange and homologous recombination. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 555–584. [Google Scholar]
- 12.Dubnau D, Hahn J, Roggiani M, Piazza F, Weinrauch Y. Two component regulators and genetic competence in Bacillus subtilis. Res Microbiol. 1994;145:403–411. doi: 10.1016/0923-2508(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari E, Howard S M H, Hoch J. Effect of stage 0 sporulation mutations on subtilisin expression. J Bacteriol. 1986;166:173–179. doi: 10.1128/jb.166.1.173-179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke C M, Leenhouts K J, Haandrikman A J, Kok J, Venema G, Venema K. Topology of LcnD, a protein implicated in the transport of bacteriocins from Lactococcus lactis. J Bacteriol. 1996;178:1766–1769. doi: 10.1128/jb.178.6.1766-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froshauer S, Green G N, McGovern D B K, Beckwith J. Genetic analysis of the membrane insertion and topology of MalF, a cytoplasmic membrane protein of Escherichia coli. J Mol Biol. 1988;200:501–511. doi: 10.1016/0022-2836(88)90539-6. [DOI] [PubMed] [Google Scholar]
- 16.Grossman A D. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 17.Hamoen L W, Eshuis H, Jongbloed J, Venema G, van Sinderen D. A small gene, designated comS, located within the coding region of the fourth amino acid-activation domain of srfA, is required for competence development in Bacillus subtilis. Mol Microbiol. 1995;15:55–63. doi: 10.1111/j.1365-2958.1995.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 18.Håvarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Håvarstein L S, Gaustad P, Nes I F, Morrison D A. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 20.Hulett F M, Jensen K. Critical roles of spoOA and spoOH in vegetative alkaline phosphatase production in Bacillus subtilis. J Bacteriol. 1988;170:3765–3768. doi: 10.1128/jb.170.8.3765-3768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inamine G S, Dubnau D. ComEA, a Bacillus subtilis integral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J Bacteriol. 1995;177:3045–3051. doi: 10.1128/jb.177.11.3045-3051.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ireton K, Rudner D Z, Jaacks-Siranosian K, Grossman A D. Integration of multiple developmental signals in Bacillus subtilis through the SpoOA transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 23.Jaacks K J, Healy J, Losick R, Grossman A D. Identification and characterization of genes controlled by the sporulation-regulatory gene spoOH in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji G, Beavis R, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 25.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong L, Dubnau D. Regulation of competence-specific gene expression by Mec-mediated protein-protein interaction in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:5793–5797. doi: 10.1073/pnas.91.13.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornblum J, Kreiswirth B, Projan S J, Ross H, Novick R P. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lazazzera B A, Grossman A D. The ins and outs of peptide signaling. Trends Microbiol. 1998;6:288–294. doi: 10.1016/s0966-842x(98)01313-4. [DOI] [PubMed] [Google Scholar]
- 30.Lazazzera B A, Solomon J M, Grossman A D. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–925. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Li P, Inouye H, Brickman E R, Beckwith J. Genetic studies on the inability of beta-galactosidase to be translocated across the Escherichia coli cytoplasmic membrane. J Bacteriol. 1989;171:4609–4616. doi: 10.1128/jb.171.9.4609-4616.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lina G, Jarraud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick R P, Vandenesch F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol. 1998;28:655–662. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Nakano M, Lee O H, Zuber P. Plasmid-amplified comS enhances genetic competence and suppresses sinR in Bacillus subtilis. J Bacteriol. 1996;178:5144–5152. doi: 10.1128/jb.178.17.5144-5152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Londoño-Vallejo J A, Dubnau D. Membrane association and role in DNA uptake of the Bacillus subtilis PriA analog ComF1. Mol Microbiol. 1994;13:197–205. doi: 10.1111/j.1365-2958.1994.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 35.Magnuson R, Solomon J, Grossman A D. Biochemical and genetic characterization of a competence pheromone. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 36.Manoil C. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase gene fusions. Methods Cell Biol. 1991;34:61–75. doi: 10.1016/s0091-679x(08)61676-3. [DOI] [PubMed] [Google Scholar]
- 37.Manoil C, Beckwith J. A genetic approach to analyze membrane proteins topology. Science. 1986;233:1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- 38.Manoil C, Mekalanos J J, Beckwith J. Alkaline phosphatase fusions: sensors of subcellular localization. J Bacteriol. 1990;172:515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merchante R, Pooley H M, Karamata D. A periplasm in Bacillus subtilis. J Bacteriol. 1995;177:6176–6183. doi: 10.1128/jb.177.21.6176-6183.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 41.Msadek T, Kunst F, Klier A, Rapoport G. DegS-DegU and ComP-ComA modulator-effector pairs control expression of the Bacillus subtilis pleiotropic regulatory gene degQ. J Bacteriol. 1991;173:2366–2377. doi: 10.1128/jb.173.7.2366-2377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller J P, Bukusoglu G, Sonenshein A L. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992;174:4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakano M M, Xia L, Zuber P. Transcription initiation region of the srfA operon which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991;173:5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakano M M, Zuber P. Mutational analysis of the regulatory region of the srfA operon in Bacillus subtilis. J Bacteriol. 1993;175:3188–3191. doi: 10.1128/jb.175.10.3188-3191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 46.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 47.Perego M, Glaser P, Hoch J A. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol. 1996;19:1151–1157. doi: 10.1111/j.1365-2958.1996.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 48.Perego M, Higgins C F, Pearce S R, Gallagher M P, Hoch J A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991;5:173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 49.Pestova E V, Håvarstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 50.Roggiani M, Dubnau D. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J Bacteriol. 1993;175:3182–3187. doi: 10.1128/jb.175.10.3182-3187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a.Roggiani, M., F. Piazza, and D. Dubnau. Unpublished results.
- 51.Russel M, Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982;28:177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Serror P, Sonenshein A L. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol. 1996;178:5910–5915. doi: 10.1128/jb.178.20.5910-5915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silhavy T J, Beckwith J R. Uses of lac fusion for study of biological problems. Microbiol Rev. 1885;49:398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomon J, Magnuson R, Srivastava A, Grossman A D. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 56.Solomon J M, Lazazzera B A, Grossman A D. Purification and characterization of an extracellular peptide factor that affects two developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–2024. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 57.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and the regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thom J R, Randall L L. Role of the leader peptide of maltose-binding protein in two steps of the export process. J Bacteriol. 1988;170:5654–5661. doi: 10.1128/jb.170.12.5654-5661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turgay K, Hahn J, Burghoorn J, Dubnau D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turgay K, Hamoen L W, Venema G, Dubnau D. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, that controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- 61.von Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986;5:3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 63.Weinrauch Y, Guillen N, Dubnau D. Sequence and transcription mapping of Bacillus subtilis competence genes comB and comA, one of which is related to a family of bacterial regulatory determinants. J Bacteriol. 1989;171:5362–5375. doi: 10.1128/jb.171.10.5362-5375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 1990;4:860–872. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]