Abstract
Male homosexual preference (MHP) is an evolutionary enigma because it is partially heritable and imposes a fertility cost. In occidental societies, homosexual men are feminized at various levels and they have more older brothers than heterosexual men. To evaluate whether femininity and the fraternal birth order (FBO) effect are universal features of MHP or not, we collected original data from homosexual men, heterosexual men, and heterosexual women from Java (Indonesia). Facial photographs were used to test whether homosexual faces are feminized when compared with heterosexual ones. We found that faces manipulated to resemble the average face of homosexual men are perceived as facially feminized, suggesting that homosexual men are facially feminized compared to heterosexual men, although a higher facial femininity was not captured by morphological analyses. Then, family data were used to detect differences in siblings’ composition between homosexuals and heterosexuals. Homosexual men displayed a higher number of older brothers than heterosexual men, even when sibship size was controlled for, suggesting that the FBO effect exists in Indonesian populations. Independent of sexual orientation, men with older brothers seem more feminized than those without older brothers, consistent with the immune origin of the FBO effect. In conclusion, MHP in Indonesia is partially feminized and they have more older brothers. Such features are also associated with MHP in other cultural contexts, suggesting a cross-cultural effect of men homosexual preference. An evolutionary explanation is available for the feminizing effect, although the FBO effect remains unexplained even if proximal mechanisms start to be identified.
Keywords: homosexuality, sexual dimorphism, fraternal birth order effect, sexual orientation, facial morphology
Male homosexual preference (MHP), the preference of males for same-sex mates even if female partners are available, is an evolutionary enigma because, in humans, preference for male–male relationships is partially heritable (Bailey, Dunne, & Martin, 2000; Ganna et al., 2019; Kendler, Thornton, Gilman, & Kessler, 2000; Långstro¨m, Rahman, Carlstro¨m, & Lichtenstein, 2010) and imposes a fertility cost (lower offspring number; Bell & Weinberg, 1978; Iemmola & Camperio-Ciani, 2009; Rieger, Blanchard, Schwartz, Bailey, & Sanders, 2012). In order to better understand the evolutionary trajectory of MHP, it is pivotal to know if the associated biological changes are regional (e.g., restricted to occidental cultures) or universal.
At least two possibly related biological traits are differing between homosexual and heterosexual men in occidental societies. First, homosexual men are feminized at various levels. Anatomically, the hypothalamic structure of homosexual men differs from that of heterosexual men and is typical of that of women (LeVay, 1991), whereas the neural brain response of homosexual men to putative pheromones is more akin to that of heterosexual women than heterosexual men (Savic, Berglund, & Lindstro¨m, 2005). Homosexual men also appear to be slightly feminized in their long bone proportions (Martin & Nguyen, 2004) and facial traits (Hughes & Bremme, 2011; Skorska, Geniole, Vrysen, McCormick, & Bogaert, 2015, but see Valentova, Kleisner, Havlicek, & Neustupa, 2014). Vocally, several dimensions of speech differ between homosexual and heterosexual men (Gaudio, 1994; Munson, McDonald, DeBoe, & White, 2006; Smyth, Jacobs, & Rogers, 2003), with overall feminization of the vocal characteristics of homosexual men by up to 9.4% (on a scale of 0% for heterosexual men to 100% for females; Suire, Tognetti, Durand, Raymond, & Barkat-Defradas, forthcoming). Behaviorally, homosexual men are more likely to recall childhood behaviors typical of the opposite sex than heterosexual men (Alanko et al., 2010; Bailey & Zucker, 1995), and their adult body walk and movements are more swaying than those of heterosexuals, which is perceived as more feminine (Johnson, Gill, Reichman, & Tassinary, 2007). In conclusion, the general pattern is consistent with some feminization of homosexual men (Balthazart & Young, 2015), although a minority of studies report otherwise for some traits (e.g., Valentova et al., 2014, for facial traits).
Second, homosexual men have more older brothers than heterosexual men (Blanchard & Bogaert, 1996). The underlying mechanism of this fraternal birth order (FBO) effect is biological and prenatal, since MHP is predicted by neither the number of nonbiological older brothers nor the amount of time spent with biological or nonbiological older brothers (Bogaert, 2006). The proposed explanation is a maternal immune reaction to successive male pregnancies, with each male fetus increasing the likelihood of an immune response of the mother. This maternal immune reaction would lead to an alteration of the typical development of sexually dimorphic brain structures relevant to the sexual orientation of the fetus and possibly femininity (Bogaert & Skorska, 2011). Recently, possible molecular evidence of this specific immune reaction has been presented (Bogaert et al., 2018). The finding that homosexual men present an increased number of older brothers has been replicated several times in Western societies (e.g., Blanchard, 2004, 2018a, 2018b, 2018c; Bogaert & Skorska, 2011). As most of the relevant studies were performed in individuals from North America or Europe, whether the same conclusion applies to other geographic areas or not remains to be determined. Some anecdotal reports suggest that the feminization of homosexual men could be more general. For example, in Samoa, there is a claim that “As a group, fa’afafine tend to be effeminate both as children and as adults” (Bartlett & Vasey, 2006; Vasey & VanderLaan, 2010). Additionally, in Guatemala, observers assess more overtly effeminate individuals with a higher Kinsey rating (a quantitative measure of sexual orientation from heterosexuality to homosexuality; Whitam & Mathy, 1986, table 2.8). Similarly, few studies have evaluated the existence of the older brother effect in non-Western societies. Considering cases with a sufficient sample size (thus discarding two studies from Polynesia involving 5 and 13 individuals; Poasa, Blanchard, & Zucker, 2004; Zucker & Blanchard, 2003), only data from Brazil, Iran, Samoa, Hong Kong, and Turkey are available (Blanchard, 2018a; Li & Wong, 2018). In Brazil, an older brother effect was initially described in one study (VanderLaan et al., 2017), although in the meta-analysis of Blanchard (2018a), that Brazilian study turned out to be nonsignificant. In other countries, an older brother effect was found. In Samoa, an older sister effect and a younger brother effect were also present (Vasey & VanderLann, 2007). The older sister effect was found again in an independent sample but not the younger brother effect (VanderLaan & Vasey, 2011). As an older sister effect cannot be explained by the maternal immune response, an additional explanation is required, suggesting the possible existence of a different type of MHP biological effect in Samoa. Thus, to better understand biological features of MHP, such as femininity and the FBO effect, on a worldwide basis, more data from non-Western countries will be required.
To this end, we collected original data from homosexual and heterosexual men and heterosexual women from Java (Indonesia). Facial photographs were used to test whether homosexual faces are feminized compared with heterosexual faces. Family data were used to detect differences in sib composition between homosexuals and heterosexuals, particularly for older brothers.
Material and Methods
FBO
Family data collected from a previous study of homosexual (N = 116) and heterosexual men (N = 62) from Western and central Java, Indonesia, were used (see Nila, Barthes, Crochet, Suryobroto, & Raymond, 2018, for details). The data considered for each individual were sexual orientation and the number of biological older brothers, younger brothers, older sisters, and younger sisters. The older brother hypothesis was tested via generalized linear regression using a Poisson error structure with the number of older brothers as a response variable and sexual orientation as the explanatory variable. To conservatively control for possible overdispersion, a quasipoisson distribution was used. This model was also run with sib number as a control variable (excluding the subject himself). The same analysis was performed for the other sib categories (number of younger brothers, number of older sisters, and number of younger sisters). For the younger sib categories, age was added as a control variable, as the number of younger sibs could be influenced by the age of the sampled individuals.
Facial Photographs
Photographs of 74 Indonesian men (45 and 29 homosexual and heterosexual men, respectively, aged 20–59 years, Mage = 30.28 years, SD = 9.18 years) were used to create the stimuli. These men were selected from samples from a previous study (see Nila et al., 2018, for details) in which faces showed a neutral expression and were perfectly oriented toward the camera. Photographs of 38 heterosexual women (aged 20–54 years, Mage = 28.14 years, SD = 8.17 years) were also taken following the same criteria of a neutral expression and straight position of the face. The women were recruited from 2014 to 2017 in Java, Indonesia. For each of these individuals, the following information was collected: date and place of birth, parent and grandparent origins, and sexual orientation. All the digital photographs were aligned to standardize the position of the chin and pupils, and shape information was obtained from salient facial anatomical landmarks and semilandmarks (Mitteroecker & Gunz, 2009). The x–y coordinates of 142 points were obtained by manually delineating all faces using the Psychomorph morphing software (Rowland & Perrett, 1995; Tiddeman, Burt, & Perrett, 2001; Figure S1).
Facial Femininity Scores
To extract shape information from raw facial landmarks and semilandmarks, we conducted a generalized Procrustes analysis (Zelditch, Swiderski, Sheets, & Fink, 2004) on raw x- and y-coordinates for all faces (heterosexual men and women and homosexual men). This procedure removes translation, size, and rotation effects. The coordinates were then transformed into shape variables via principal component analysis (PCA). The first 20 axes were retained (explaining 89.5% of variance) for further analyses. To compute a data-driven single measure of facial masculinity, an LDA was conducted on the PCA coordinates with sex as the grouping variable (female and male), where homosexual and heterosexual males were pooled. The resulting discriminant function correctly classified 85.7% of individuals in the two categories. Each individual coordinate on the male–female axis was used as a facial femininity index.
Femininity/Masculinity Evaluation by Judges
Men’s faces (n = 74) were manipulated to change some of their characteristics in opposite directions, and the resulting pair of images for each man was assessed by raters. This procedure ensured that all the confounding variables that potentially influence the assessment of femininity/masculinity (age, haircut, skin color, etc.) are controlled for. First, an average heterosexual male face and an average female face were created as follows. Fifteen heterosexual men’s faces and 15 female faces were chosen to minimize the difference in mean age and its variance between the two groups. The resulting groups of men’s and women’s faces did not differ in the age (men: Mage = 28.12 years, SD = 8.16 years; females: Mage = 28.14 years, SD = 8.17 years; Wilcoxon–Mann–Whitney test, W = 111, p = 1), ethnicity or education level (Fisher’s exact test for contingency data, p = .43 and p = .25, respectively), or salary (Wilcoxon–Mann–Whitney test, W = 105.5, p = .79) of the individuals in the images. The two groups were used to create an average heterosexual male face and an average female face, respectively, using WebMorph (Figure 1). Second, each of the original male faces was shape transformed into a masculinized version (M+) by 50% warping toward the average male face and a feminized version (F+) by 50% warping toward the average female face. The texture and color of the men’s faces were unchanged. The averaging and transformation of the facial images were performed using the morphing software WebMorph (Version v0.0.0.9001, https://webmorph.org//) (DeBruine & Tiddeman, 2017).
Figure 1.

Heterosexual men’s (left) and women’s (right) facial averages used to generate the M+ and F+ faces. See text for explanations.
The same procedure was used to modify male faces according to sexual orientation. Fifteen heterosexual men’s faces and 15 homosexual men’s faces were chosen to minimize the difference in the mean age and its variance between the two groups. The resulting groups of heterosexual and homosexual male faces did not differ in age (heterosexuals: Mage = 28.99 years, SD = 7.19 years; homosexuals: Mage = 29.83 years, SD = 7.63 years; Wilcoxon–Mann–Whitney test, W = 113, p = 1), ethnicity or education level (Fisher’s exact test for contingency data, p = 1 and p = .24, respectively), or salary (Wilcoxon–Mann–Whitney test, W = 96, p = .51). The two groups were used to create an average heterosexual man face and an average homosexual male face, respectively. Each of the 74 original male faces was shape-transformed toward a 50% heterosexual average (Hetero+) and a 50% homosexual average (Homo+), without changing the texture or color.
Finally, the same procedure was used to modify male faces according to birth order. Fifteen faces of heterosexual men without older brothers and 15 faces of heterosexual men with at least one older brother were chosen to minimize the difference in the mean age and its variance between the two groups. The resulting without-older-brother and with-older-brother groups of men’s faces did not differ in age (without-older-brother: Mage = 33.46 years, SD = 10.18 years; with-older-brothers: Mage = 33.50 years, SD = 10.17 years; Wilcoxon–Mann–Whitney test, W = 113, p = 1), ethnicity or education level (Fisher’s exact test for contingency data, p = .56 and p = .25, respectively), or salary (Wilcoxon–Mann–Whitney test, W = 80.5, p = .19). The two groups were used to create an average without-older-brother male face and an average with-older-brother male face, respectively. Each of the 74 original male faces was shape transformed toward a 50% without-older-brother average (OB−) or a 50% with-older-brothers average (OB+), without changing the texture or color. These three groups resulted in a total of 444 transformed photographs that were used as stimuli (Figure 2).
Figure 2.
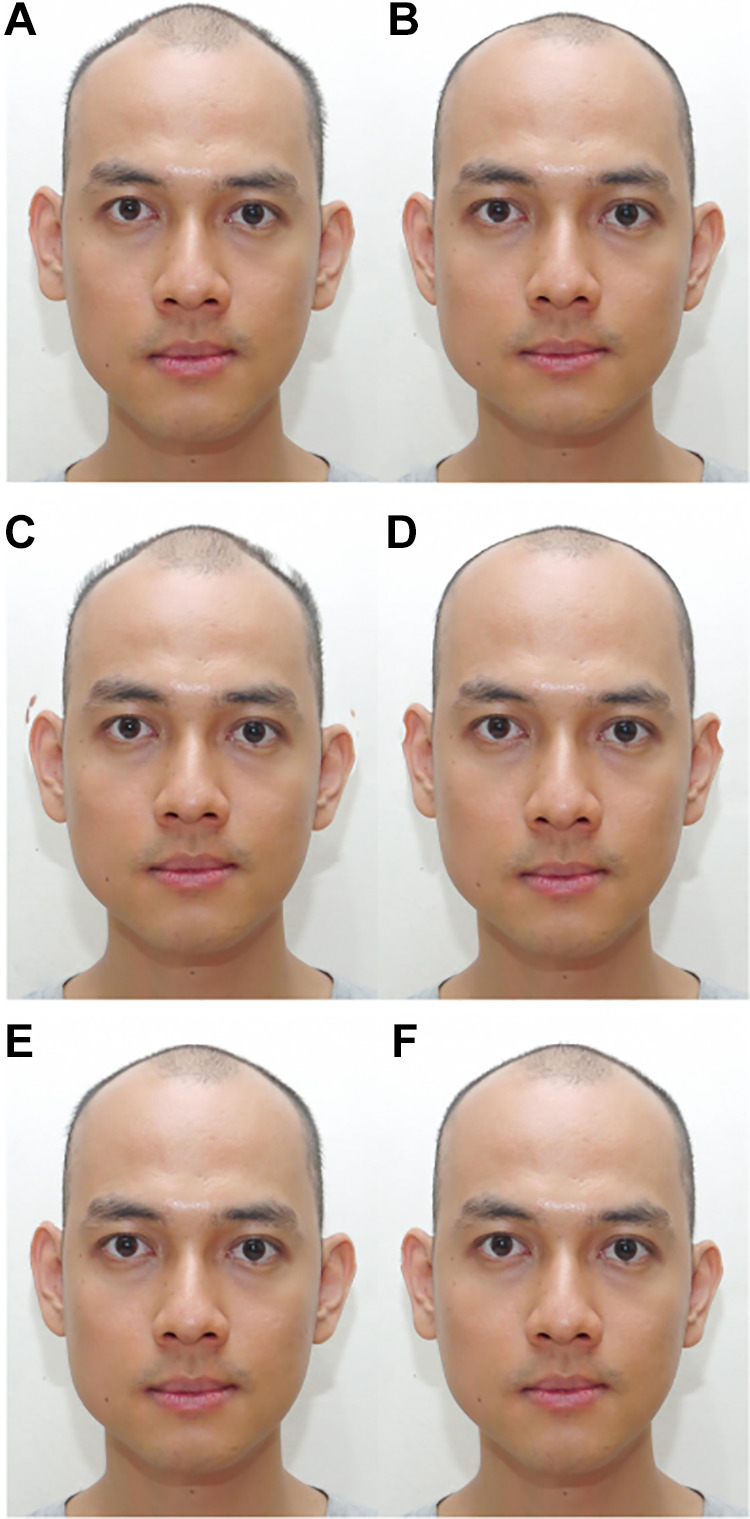
Examples of M+ (A), F+ (B), Hetero+ (C), Homo+ (D), OB− (E), and OB+ (F) transformed photographs of the same male face presented to raters. Reproduced with permission. M+ = masculinized version; F+ = feminized version; Hetero+ = 50% heterosexual average; Homo+ = 50% homosexual average; OB− = 50% without-older-brother average; OB+ = 50% with-older-brothers average.
A computer program was generated to randomly present pairs of modified photographs from the same subject. The two modification types presented in a pair were M+/F+, Hetero+/Homo+, or OB−/OB+, which were presented on a random basis for each pair. For each pair, the rater was instructed to click on the photograph depicting the most masculine subject. Images were presented on a 13.3-inch computer screen with dimensions of 2,560 × 1,600 pixels. The position of the photograph on the screen (left or right) was randomly determined for each pair for each rater. Each rater had 26 distinct pairs of photographs to assess, which were randomly drawn from the set of 74 subjects. If the rater knew the man depicted in the pair that he or she had to judge, the trial was removed from the analysis. Four pairs randomly chosen from among those previously viewed were presented again at the end to estimate judgment reliability.
A total of 350 Indonesian raters (205 males and 149 females) assessed the effects of the three types of facial modifications on masculinity. Their age varied between 18 and 63 years (Mage = 30.92 years, SD = 9.61 years). They were recruited in public places (street and restaurants) in Western Java, Indonesia, and were unaware of the purpose of the study when assessing the pairs of pictures. For each rater, the following information was collected: sex, date of birth, sexual orientation, monthly income, highest level of education, marital status, and ethnic origin. Unreliable raters (i.e., with more than one incorrect answer during the test of judgment reliability) were removed. A total of 273 raters were retained in the final sample. Each subject was observed by 36.8, 36.8, and 33.9 raters on average for M+/F+, Hetero+/Homo+, and OB−/OB+ comparisons, respectively (range: 25–49, 20–46, and 22–70, respectively).
The aim was to examine the influence of the morphological transformation of the subject’s faces on the probability of being judged as more masculine. Three models were generated, one for each type of facial transformation. The binary response variable was whether or not (1 or 0) the focal face (arbitrarily the photograph presented at the left position) was chosen during the presentation of each pair. The variable of interest, Foc_masc, was whether or not (1 or 0) the focal face was an M+ face for M+/F+ pairs, Hetero+ face for the Homo+/Hetero+ pairs, or OB− face for the OB−/OB+ pairs. The subjects and raters were considered random samples from a larger population of interest and were, thus, random-effect variables. Therefore, generalized linear mixed models with binary error structures were used. To control for potential confounding effects, variables concerning the raters’ characteristics were also included in the model as interaction terms with the variable of interest. These variables were the rater’s sex (qualitative: male or female), age (quantitative, standardized), sexual orientation (qualitative: heterosexual or homosexual), marital status (qualitative: in a couple or not), salary (quantitative, standardized), education level (qualitative: primary and secondary, or tertiary), and ethnic origin (qualitative: Javanese, Sundanese, Sumatran, or others). These generalized linear mixed-effects models were performed using the glmer function of the lme4 package (Bates, Ma¨chler, Bolker, & Walker, 2017) or, when singularity prevented convergence, with the Bayesian bglmer function of blme package (Chung, Rabe-Hesketh, Dorie, Gelman, & Liu, 2013), which forces the model fit away from singularity. The significance of each independent variable was calculated by removing it and comparing the resulting variation in deviance using the χ2 test, as done by the function Anova from the car R package. All computations were conducted using the R Version 3.5.1 software (R Core Team, 2018).
Results
Facial Feminization
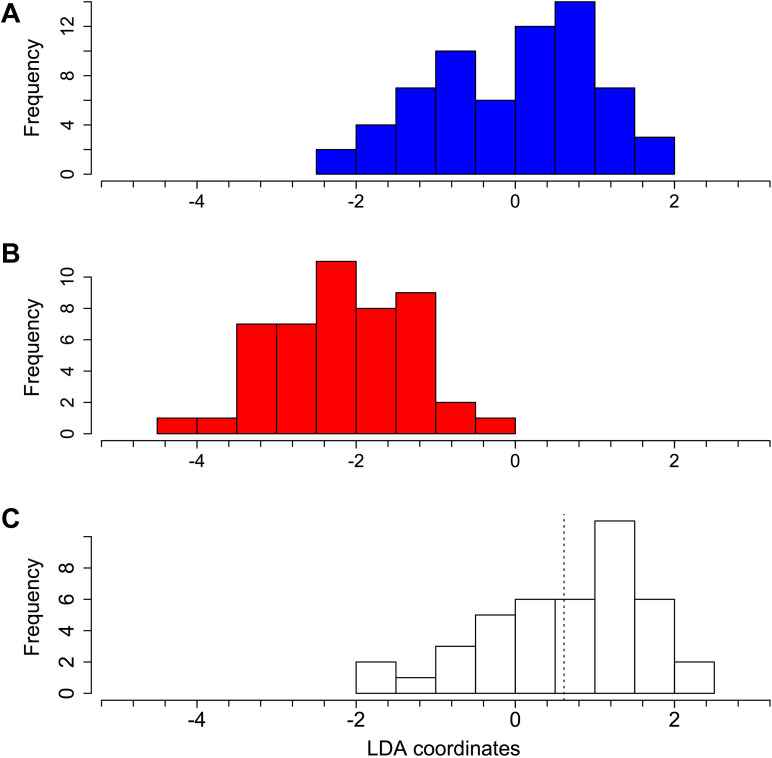
Coordinates on the male–female axis of the discriminant analysis were used as a scale to measure the facial feminization of homosexual men. Overall, the degree of facial feminization of homosexual men was −14.6% (SE = 7.3), although this was not different from 0%, as the facial femininity index was not different between homosexual and heterosexual men (Student’s test, t = 1.56, df = 105, p = .12; Figure 3).
Figure 3.
Distribution of scaled LDA coordinates of each face on the male–female axis for heterosexual men (A), women (B), and homosexual men (C). The LDA coordinates are centered on the mean for heterosexual men. The vertical dotted line indicates the mean value for homosexual men. LDA = linear discriminant-function analysis.
When raters evaluated M+/F+ faces regarding, which was more masculine, M+ faces were chosen significantly more often (p = .002, Tables 1 and S1). The results varied according to the rater’s age (p = .008) and marginally with the rater’s ethnic group (p = .048). The effect of age was negative: Younger individuals choose M+ faces more often than older individuals. When raters evaluated Hetero+/Homo+ faces regarding which was more masculine, Hetero+ faces were chosen significantly more often (p = 2.9 × 10−4; Tables 1 and S2). None of the raters’ characteristics were significant. When raters evaluated OB−/OB+ faces regarding which was more masculine, OB− faces were chosen significantly more often (p = 4.9 × 10−6; Tables 1 and S3). None of the raters’ characteristics were significant.
Table 1.
Effect of each variable on the choice of a face. Foc_masc represents the choice of the most masculinized face in various pairs (M+ for pairs M+/F+, Hetero+ for pairs Hetero+/Homo+, and OB- for pairs OB-/OB+).
| M+/F+ | Hetero+/Homo+ | OB−/OB+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | χ2 | df | p Value | χ2 | df | p Value | χ2 | df | p Value |
| Foc_masc | 9.27 | 1 | .002 | 13.14 | 1 | .0003 | 20.89 | 1 | <10−5 |
| Interaction with | |||||||||
| Sex | 2.98 | 1 | .084 | 1.74 | 1 | .187 | 0.76 | 1 | .383 |
| Age | 7.04 | 1 | .008 | 0.05 | 1 | .825 | 1.50 | 1 | .221 |
| Sexual orientation | 0.74 | 1 | .388 | 0.71 | 1 | .398 | 1.34 | 1 | .248 |
| Study | 1.11 | 1 | .292 | <0.01 | 1 | .987 | 3.15 | 1 | .076 |
| Salary | 2.23 | 1 | .135 | 1.42 | 1 | .233 | 0.05 | 1 | .815 |
| Ethnics | 7.90 | 3 | .048 | 5.65 | 3 | .130 | 4.36 | 3 | .226 |
| Couple | 0.50 | 1 | .479 | 0.01 | 1 | .916 | 3.76 | 1 | .384 |
Note. Bold-faced characters indicate significant (p < .05) values.
Older Brother Effect
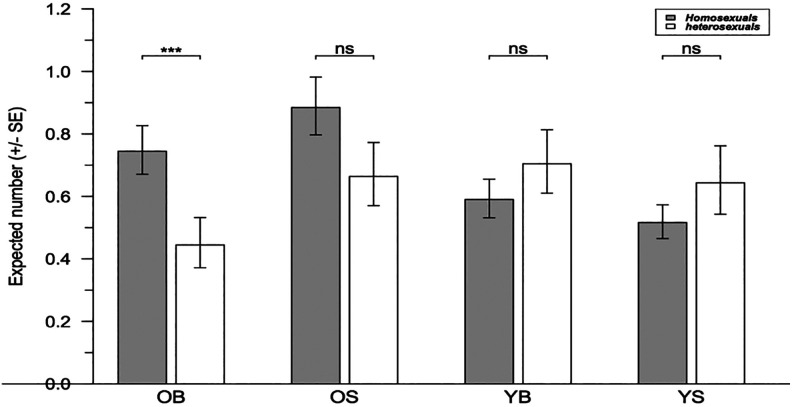
Homosexual men had a significantly greater number of older brothers than heterosexual men (p = .017, Table 2). Similarly, the number of older sisters was significantly greater for homosexual men (p = .027), which was not the case for the number of either younger brothers (p = .14) or younger sisters (p = .23). Homosexual men had a greater number of sibs than heterosexual men (3.22 and 3.03 sibs, respectively), although the difference was not significant (Wilcoxon–Mann–Whitney test, W = 3,933, p = .30). To control for sib number, generalized linear regression using a quasipoisson error structure was performed, with the number of older brothers as a response variable and sibship size as a control variable. Homosexual men displayed a significantly greater number of older brothers than heterosexual men (0.52 additional older brothers, in linear units, χ2 = 8.15, df = 1, p = .004). For 3.16 sibs (the mean number in the sample), homosexual men displayed 0.30 additional older brothers compared to heterosexual men (Figure 4). The numbers of older sisters, younger brothers, or younger sisters were not significantly different between homosexual and heterosexual men when controlled for sibship size (p = .08, p = .32, and p = .31, respectively, Figure 4).
Table 2.
Mean Number of Older and Younger Brothers and Sisters in Heterosexual and Homosexual Men.
| Sib category | Heterosexual Men | Homosexual Men | Test | |||
|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | W | p Value | |
| Brothers | ||||||
| Older | 0.677 | (.191) | 0.957 | (.114) | 4,306.5 | .017 |
| Younger | 0.742 | (.097) | 0.621 | (.079) | 3,157.0 | .139 |
| Sisters | ||||||
| Older | 0.935 | (.219) | 1.095 | (.115) | 4,271.5 | .027 |
| Younger | 0.677 | (.114) | 0.543 | (.079) | 3,252.5 | .231 |
| All | 3.032 | (.328) | 3.224 | (.202) | 3,933.0 | .298 |
Note. The standard error (SE) of the mean is indicated. The statistics (W) and the associated p value of the Wilcoxon–Mann–Whitney test for the difference between heterosexual and homosexual men are indicated. Bold-faced characters indicate significant (p < .05) values.
Figure 4.
Expected number of various sib categories for homosexual and heterosexual sampled men. OB, OS, YB, and YS represent older brother, older sister, younger brother, and younger sister, respectively. Values are derived from the generalized linear regression for sampled individuals with a mean number of sibs and, for YB and YS, with a mean age.
For homosexual men, the degree of facial feminization was not related to the number of older brothers (Pearson’s product–moment correlation = .09, t = 0.47, df = 27, p = .64).
Discussion
Faces manipulated to resemble the average face of homosexual men are perceived as facially feminized, which indicates that homosexual men are facially feminized compared to heterosexual men, although higher facial femininity was not captured by the morphological analyses. Homosexual men displayed a greater number of older brothers, which was also associated with higher perceived femininity. They did not display a greater number of other sib categories.
When the shape of photographed men’s faces was partially transformed using an average homosexual face, the resulting faces appeared more feminized compared to similar transformation using an average heterosexual face. As age and masculinity are positively related in men (Boothroyd et al., 2005), a different mean age between the two groups of men used to build the average faces could trigger an unwanted difference in femininity/masculinity. However, the average faces were constructed with homosexual and heterosexual men sampled to minimize the difference in the age distribution (mean and variance). The resulting difference in mean age was less than 2 months, which is probably too small to generate a perceptible difference in masculinity. It is, however, possible that another variable, independent of sexual orientation, generated masculinity/femininity differences between the two samples, although this variable was not salary, education level, or ethnic origin, as these variables were not significantly different between the two groups. When the morphological difference between males and females was maximized during the discriminant analysis, homosexual men were not distributed differently compared to heterosexual men. Thus, any feminization displayed by homosexual men is not readily captured by the set of point coordinates or by their linear combinations. In this data set, when homosexual men were removed, heterosexual men and females were significantly differentiated by the discriminant analysis, although ∼20% of individuals were not correctly assigned. Other studies in occidental populations using similar morphological procedures to sex assign individuals typically report incorrect assignation within a range of 3–19% (e.g., Lee et al., 2014; Scott, Pound, Stephen, Clark, & Penton-Voak, 2010). This suggests that the present morphological analysis did not fully capture sexual facial differentiation. Alternatively, a real overlap could exist between male and female facial shapes.
Homosexual men displayed a greater number of older brothers than heterosexual men, suggesting that the older brother effect exists in Indonesian populations. The greater number of older brothers was present independently of possible higher fecundity observed in the families of homosexual men, as sib number was controlled for. A greater number of older sisters were also found, although it was no longer significant when sib number was controlled for, suggesting that this older sister effect is possibly driven by higher fecundity associated with the families of homosexual men. However, overall sibship size was not significantly differing between homosexual and heterosexual men. In Samoa, an older sister effect has been reported, although it is unclear if it remains after taking sib number into account. Thus far, the older brother effect has been found in all the populations in which it has been looked for (Western countries, Turkey, Iran, Hong Kong, Samoa, and Indonesia), suggesting that it is a general feature associated with MHP, although there is perhaps one counterexample (Brazil).
Using an index of facial femininity, homosexual men with more older brothers were not more feminized. As this femininity index does not capture differences between homosexual and heterosexual men, this result is preliminary. It has been shown that homosexuals with more older brothers are more feminine, the measure of femininity being a preference for the receptive role in anal intercourse (Blanchard, 2018a, 2018c), although a replication was equivocal (Swift-Gallant, Coome, Monks, & VanderLaan, 2018). The link between number of older brothers and femininity of homosexuals is not settled.
Independent of sexual orientation, men with older brothers seem more feminized than those without older brothers, consistent with the known effect of maternal parity on life-history traits (Skjærvø & Røskaft, 2013) and the hypothesis of the immune origin of the older brother effect. The possible maternal immune reaction primarily alters the development of sexually dimorphic brain structures relevant to sexual orientation, although other direct or indirect feminization effects are possible (Bogaert & Skorska, 2011). For example, birth weight is lower for newborn males with older brothers, but not for newborn females with older brothers or older sisters (Côté, Blanchard, & Lalumière, 2003). The higher influence of previous brothers to reduce male birth-weight, compared to female birth-weight, has been repeated in large samples (e.g., Nielsen et al., 2008) and is consistent with a lower birth weight of homosexual men relatively to heterosexuals (Xu, Norton, & Rahman, 2019).
Taken together, these results suggest the presence of a feminizing factor associated with male homosexuality that is partially determined by male birth order. This is consistent with the findings from Western societies and, thus, argues for a common pathway that could apply to various populations. Indeed, there are several lines of evidence showing that some specific aspects of the MHP are found cross-culturally. For example, early cross-gender or atypical behavior has been retrospectively assessed among men showing MHP in Brazil, Guatemala, Philippines, Thailand, Turkey, and United States (Cardoso, 2005, 2009; Whitam & Mathy, 1986; Whitam & Zent, 1984). These behaviors are displayed early during childhood and are found in distinct cultures; thus, providing another argument for a biological basis of this sexual preference. The developmental pathway of MHP could, therefore, rely on the same biological basis in distinct populations.
A feminizing factor is only a proximate explanation for the presence of MHP, and a more global framework is required to understand why such a feminizing factor exists. Interestingly, a sexually antagonistic gene that favors MHP in males and promotes fecundity (i.e., the ability to have children) in females has been proposed (Camperio-Ciani, Corna, & Capiluppi, 2004; Iemmola & Camperio-Ciani, 2009, but see Blanchard, 2012). Several studies support this hypothesis, and other studies have provided results that are consistent with predictions from this hypothesis (for a review, see Barthes, Crochet, & Raymond, 2015). The nature of the antagonistic factor has not yet been identified, but it has been proposed that it proximally enhances femininity in both sexes, resulting in the opposite effect on expected reproduction in each sex (Barthes, Godelle, & Raymond, 2013). Thus, under the sexually antagonistic gene hypothesis, the higher femininity of homosexual men, including their feminized sexual orientation, is seen as a pleiotropic cost of selection for higher fertility in females. In women, femininity of various traits is associated with fertility and is considered attractive (Hill & Hurtado, 1996; Law-Smith et al., 2006; Manning, Scutt, Whitehouse, & Leinster, 1997; Pawlowski, Boothroyd, Perrett, & Kluska, 2008; Rhodes, Simmons & Peters, 2005; Singh, 1993; Sugiyama, 2005).
Similarly, the immune origin of the older brother effect remains a proximate explanation and a broader context is required to understand why male birth rank interferes with sexual orientation in men. Birth order is obviously not heritable; thus, this trait cannot evolve by natural selection. However, the ability to generate a birth order effect is potentially heritable. Curiously, it is unclear whether the FBO effect should be seen as a feminizing effect that increases with male birth rank or as an anti-feminizing effect that decreases with birth order. The former phenomenon would be consistent with a mechanism that decreases competitive ability in later-born offspring, which would be useful to reduce, for example, the cost of sib competition in males. The latter phenomenon could operate when the firstborn males have special reproductive importance, for example, in societies promoting primogeniture (eldest son as the primary heir). However, the present study does not confirm such an association: Primogeniture has not been described in traditional Indonesian culture, particularly on the island of Java (Gultom, 2017), despite a significant FBO effect. The same situation is found in Turkey, where primogeniture was not traditionally enforced, but a significant FBO effect has been described (Blanchard, 2018a). Whether such a feminizing effect according to FBO exists in other mammals or not has apparently not been investigated (to our knowledge), although this would help to better understand this phenomenon in humans.
Conclusion
Men exhibiting MHP in Indonesia are partially feminized, and they have more older brothers. Such features are also associated with MHP in other cultural contexts, suggesting a cross-cultural effect of men’s homosexual preference. An evolutionary explanation is available for the feminizing effect, whereas the FBO effect remains unexplained, although proximal mechanisms are beginning to be identified.
Supplemental Material
Supplemental Material, MHP_Indonesia_revised_supplementary for Male Homosexual Preference: Femininity and the Older Brother Effect in Indonesia by Sarah Nila, Pierre-Andre Crochet, Julien Barthes, Puji Rianti, Berry Juliandi, Bambang Suryobroto and Michel Raymond in Evolutionary Psychology
Acknowledgments
We are very grateful to Siti Nur Faizah, SKM, Dr Sih Kahono, Andi Pangeran, Rumah Singgah Kebaya Yogyakarta, Suara Kita, Arus Pelangi and the Innovative Program One Stop HIV-AIDS of the Kedung Badak Community Health Center. We would like to thank all the participants, especially Yusin Januar Aditya Maulana, Vinolia Wakijo and Bambang Prayudi. We also acknowledged Valérie Durand for the technical support during the experiment. This is contribution ISEM 2019-186 SUD.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Agence Nationale de la Recherche (Humanway, ANR-12-BSV7-0008-01), and PHC - Nusantara grant #41100 WC. and Beasiswa Unggulan Kemdikbud 2016.
ORCID iD: Michel Raymond  https://orcid.org/0000-0002-1714-6984
https://orcid.org/0000-0002-1714-6984
Supplemental Material: Supplemental material for this article is available online.
References
- Alanko K., Santtila P., Harlaar N., Witting K., Varjonen M., Jern P.…Sandnabba N. K. (2010). Common genetic effects of gender atypical behavior in childhood and sexual orientation in adulthood: A study of Finnish twins. Archives of Sexual Behavior, 39, 81–92. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Dunne M. P., Martin N. G. (2000). Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. Journal of Personality and Social Psychology, 78, 524–536. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Zucker K. J. (1995). Childhood sex-typed behavior and sexual orientation: A conceptual analysis and quantitative review. Developmental Psychology, 31, 43–55. [Google Scholar]
- Balthazart J., Young L. J. (2015). Mate selection, sexual orientation and pair bonding. In Plant T. M., Zelenik A. J. (Eds.), Knobil and Neill’s physiology of reproduction (pp. 2157–2210). Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- Bartlett N. H., Vasey P. L. (2006). A retrospective study of childhood gender-atypical behavior in samoan fa’afafine. Archives of Sexual Behavior, 35, 659–666. [DOI] [PubMed] [Google Scholar]
- Barthes J., Crochet P.-A., Raymond M. (2015). Male homosexual preference: Where, when, why? PLoS One, 10, e0134817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthes J., Godelle B., Raymond M. (2013). Human social stratification and hypergyny: Toward an understanding of male homosexual preference. Evolution and Human Behavior, 34, 155–163. [Google Scholar]
- Bates D., Ma¨chler M., Bolker B. M., Walker S. C. (2017). Fitting linear mixed-effects model using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Bell A. P., Weinberg M. S. (1978). Homosexualities: A study of diversity among men and women. New York, NY: Simon and Schuster. [Google Scholar]
- Blanchard R. (2004). Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. Journal of Theoretical Biology, 230, 173–187. [DOI] [PubMed] [Google Scholar]
- Blanchard R. (2012). Fertility in the mothers of firstborn homosexual and heterosexual men. Archives of Sexual Behavior, 41, 551–556. [DOI] [PubMed] [Google Scholar]
- Blanchard R. (2018. a). Fraternal birth order, family size, and male homosexuality: Meta-analysis of studies spanning 25 years. Archives of Sexual Behavior, 47, 1–15. [DOI] [PubMed] [Google Scholar]
- Blanchard R. (2018. b). Older brothers and older sisters odds ratios in 36 samples of homosexual males. Archives of Sexual Behavior, 47, 829–832. [DOI] [PubMed] [Google Scholar]
- Blanchard R. (2018. c). Response to commentaries: Meta-analysis of probability samples and other new evidence. Archives of sexual behavior, 47, 49–57. [DOI] [PubMed] [Google Scholar]
- Blanchard R., Bogaert A. F. (1996). Homosexuality in men and number of older brothers. American Journal of Psychiatry, 153, 27–31. [DOI] [PubMed] [Google Scholar]
- Bogaert A. F. (2006). Biological versus nonbiological older brothers and men’s sexual orientation. Proceedings of the National Academy of Sciences of United States of America, 103, 10771–10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert A. F., Skorska M. (2011). Sexual orientation, fraternal birth order, and the maternal immune hypothesis: A review. Frontiers in Neuroendocrinology, 32, 247–254. [DOI] [PubMed] [Google Scholar]
- Bogaert A. F., Skorska M. N., Wang C., Gabrie J., MacNeil A. J., Hoffarth M. R. (2018). Male homosexuality and maternal immune responsivity to the Y-linked protein NLGN4Y. Proceedings of the National Academy of Sciences of United States of America, 115, 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd L. G., Jones B. C., Burt D. M., Cornwell R. E., Little A. C., Tiddeman B. P., Perrett D. I. (2005). Facial masculinity is related to perceived age, but not perceived health. Evolution and Human Behavior, 26, 417–431. [Google Scholar]
- Camperio-Ciani A., Corna F., Capiluppi C. (2004). Evidence for maternally inherited factors favoring male homosexuality and promoting female fecundity. Proceeding Royal Social London: B, 271, 2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F. L. (2005). Cultural universal and differences in male homosexuality: The case of a Brazilian fishing village. Archives of Sexual Behavior, 34, 103–109. [DOI] [PubMed] [Google Scholar]
- Cardoso F. L. (2009). Recalled sex-typed behavior in childhood and sports’ preference in adulthood of heterosexual, bisexual, and homosexual men from Brazil, Turkey, and Thailand. Archives of Sexual Behavior, 38, 726–736. [DOI] [PubMed] [Google Scholar]
- Chung Y., Rabe-Hesketh S., Dorie V., Gelman A., Liu J. (2013). A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika, 78, 685–709. [DOI] [PubMed] [Google Scholar]
- Côté K., Blanchard R., Lalumière M. L. (2003). The influence of birth order on birth weight: does the sex of preceding siblings matter?. Journal of Biosocial Science, 35, 455–462. [DOI] [PubMed] [Google Scholar]
- DeBruine L. M., Tiddeman B. P. (2017). WebMorph. Retrieved fromhttp://webmorph.org
- Ganna A., Verweij K. J. H., Nivard M. G., Maier R., Wedow R., Busch A. S.…Zietsch B. P. (2019). Large-scale GWAS reveals insights into the genetic architecture of same-sex sexual behavior. Sciences, 365, eaat7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio R. P. (1994). Sounding gay: Pitch properties in the speech of gay and straight men. American Speech, 69, 30–57. [Google Scholar]
- Gultom E. R. (2017). Development of women position in the patrilineal inheritance of Indonesian society. Jurnal Dinamika Hukum, 17, 195–202. [Google Scholar]
- Hill K., Hurtado A. M. (1996). Ache life history: The ecology and demography of a foraging people. New York, NY: Aldine de Gruyter. [Google Scholar]
- Hughes S. M., Bremme R. (2011). The effects of facial symmetry and sexually-dimorphic facial proportions on assessments of sexual orientation. Journal of Social, Evolutionary, and Cultural Psychology, 5, 214–230. [Google Scholar]
- Iemmola F., Camperio-Ciani A. (2009). New evidence of genetic factors influencing sexual orientation in men: Female fecundity increase in the maternal line. Archives of Sexual Behavior, 38, 393–399. [DOI] [PubMed] [Google Scholar]
- Johnson K. L., Gill S., Reichman V., Tassinary L. G. (2007). Swagger, sway, and sexuality: Judging sexual orientation from body motion and morphology. Journal of Personality and Social Psychology, 93, 321–334. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Thornton L. M., Gilman S. E., Kessler R. C. (2000). Sexual orientation in a US national sample of twin and nontwin sibling pairs. The American Journal of Psychiatry, 157, 1843–1846. [DOI] [PubMed] [Google Scholar]
- Långstro¨m N., Rahman Q., Carlstro¨m E., Lichtenstein P. (2010). Genetic and environmental effects on same-sex sexual behavior: A population study of twins in Sweden. Archives of Sexual Behavior, 39, 75s80. [DOI] [PubMed] [Google Scholar]
- Law-Smith M. J., Perrett D. I., Jones B. C., Cornwell R. E., Moore F. R., Feinberg D. R.…Hillier S. G. (2006). Facial appearance is a cue to oestrogen levels in women. Proceedings Royal Society London: B, 273, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. J., Mitchem D. G., Wright M. J., Martin N. G., Keller M. C., Zietsch B. P. (2014). Genetic factors that increase male facial masculinity decrease facial attractiveness of female relatives. Psychological Science, 25, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S. (1991). A difference in hypothalamic structure between heterosexual and homosexual men. Science, 253, 1034–1037. [DOI] [PubMed] [Google Scholar]
- Li G., Wong W. I. (2018). Single-sex schooling: Friendship, dating, and sexual orientation. Archives of Sexual Behavior, 47, 1025–1039. [DOI] [PubMed] [Google Scholar]
- Manning J. T., Scutt D., Whitehouse G. H., Leinster S. J. (1997). Breast asymmetry and phenotypic quality in women. Evolution and Human Behavior, 18, 223–236. [Google Scholar]
- Martin J. T., Nguyen D. H. (2004). Anthropometric analysis of homosexuals and heterosexuals: Implications for early hormone exposure. Hormones and Behavior, 45, 31–39. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P., Gunz P. (2009). Advances in geometric morphometrics. Evolutionary Biology, 36, 235–247. [Google Scholar]
- Munson B., McDonald E. C., DeBoe N. L., White A. R. (2006). The acoustic and perceptual bases of judgments of women and men’s sexual orientation from read speech. Journal of Phonetics, 34, 202–240. [Google Scholar]
- Nielsen H. S., Mortensen L., Nygaard U., Schnor O., Bjarne C., Andersen A-M. N. (2008). Brothers and reduction of the birth weight of later-born siblings. American Journal of Epidemiology, 167, 480–484. [DOI] [PubMed] [Google Scholar]
- Nila S., Barthes J., Crochet P. A., Suryobroto B., Raymond M. (2018). Kin selection and male homosexual preference in Indonesia. Archives of Sexual Behavior, 47, 2455–2465. [DOI] [PubMed] [Google Scholar]
- Pawlowski B., Boothroyd L. G., Perrett D. I., Kluska S. (2008). Is female attractiveness related to final reproductive success? Collegium Antropologicum, 32, 457–460. [PubMed] [Google Scholar]
- Poasa K. H., Blanchard R., Zucker K. J. (2004). Birth order in transgendered males from Polynesia: A quantitative study of Samoan Fa’afāfine. Journal of Sex & Marital Therapy, 30, 13–23. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved fromhttps://www.R-project.org/ [Google Scholar]
- Rhodes G., Hickford C., Jeåery L. (2000). Sex-typicality and attractiveness: Are supermale and superfemale faces super-attractive? British Journal of Psychology, 91, 125–140. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Simmons L. W., Peters M. (2005). Attractiveness and sexual behavior: Does attractiveness enhance mating success? Evolution and Human Behavior, 26, 186–201. [Google Scholar]
- Rieger G., Blanchard R., Schwartz G., Bailey J. M., Sanders A. R. (2012). Further data concerning Blanchard’s (2011) “Fertility in the Mothers of Firstborn Homosexual and Heterosexual Men.” Archives of Sexual Behavior, 41, 529–531. [DOI] [PubMed] [Google Scholar]
- Rowland D. A., Perrett D. I. (1995). Manipulating facial appearance through shape and color. IEEE Computer Graphics and Applications, 15, 70–76. [Google Scholar]
- Savic I., Berglund H., Lindstro¨m P. (2005). Brain response to putative pheromones in homosexual men. Proceedings of the National Academy of Sciences of United States of America, 102, 7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. M. L., Pound N., Stephen I. D., Clark A. P., Penton-Voak I. S. (2010). Does masculinity matter? The contribution of masculine face shape to male attractiveness in humans. PLoS One, 5, e13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. (1993). Body shape and women’s attractiveness: The critical role of waist-to-hip ratio. Human Nature, 4, 297–321. [DOI] [PubMed] [Google Scholar]
- Skjærvø G. R., Røskaft E. (2013). Early conditions and fitness: Effect of maternal parity on human life-history traits. Behavioral Ecology, 24, 334–341. [Google Scholar]
- Skorska M. N., Geniole S. N., Vrysen B. M., McCormick C. M., Bogaert A. F. (2015). Facial structure predicts sexual orientation in both men and women. Archives of Sexual Behavior, 44, 1377–1394. [DOI] [PubMed] [Google Scholar]
- Smyth R., Jacobs G., Rogers H. (2003). Male voices and perceived sexual orientation: An experimental and theoretical approach. Language in Society, 32, 329–350. [Google Scholar]
- Sugiyama L. S. (2005). Physical attractiveness in adaptationist perspective. In Buss D. M. (Ed.), The handbook of evolutionary psychology (pp. 292–343). Hoboken, NJ: Wiley. [Google Scholar]
- Suire A., Tognetti A., Durand V., Raymond M., Barkat-Defradas M. (forthcoming). The influence of sexual orientation and testosterone levels on speech acoustic features. Archives of Sexual Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift-Gallant A., Coome L. A., Monks D. A., VanderLaan D. P. (2018). Gender nonconformity and birth order in relation to anal sex role among gay men. Archives of Sexual Behavior, 47, 1041–1052. [DOI] [PubMed] [Google Scholar]
- Tiddeman B., Burt M., Perrett D. (2001). Prototyping and transforming facial textures for perception research. IEEE Computer Graphics and Applications, 21, 42–50. [Google Scholar]
- Valentova J. V., Kleisner K., Havlicek J., Neustupa J. (2014). Shape differences between the faces of homosexual and heterosexual men. Archives of Sexual Behavior, 43, 353–361. [DOI] [PubMed] [Google Scholar]
- VanderLaan D. P., Blanchard R., Zucker K. J., Massuda R., Fontanari A. M. V., Borba A. O.…Schwarz K. (2017). Birth order and androphilic male-to-female transsexualism in Brazil. Journal of Biosocial Science, 49, 527–535. [DOI] [PubMed] [Google Scholar]
- VanderLaan D. P., Vasey P. L. (2011). Male sexual orientation in independent Samoa: Evidence for fraternal birth order and maternal fecundity effects. Archives of Sexual Behavior, 40, 495–503. [DOI] [PubMed] [Google Scholar]
- Vasey P. L., VanderLaan D. P. (2007). Birth order and male androphilia in Samoan fa’afafine. Proceedings of the Royal Society B: Biological Sciences, 274, 1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey P. L., VanderLaan D. P. (2010). Monetary exchanges with nieces and nephews: A comparison of Samoan men, women, and fa’afafine. Evolution and Human Behavior, 31, 373–380. [Google Scholar]
- Whitam F. L., Mathy R. M. (1986). Male homosexuality in four societies. Connecticut, CT: Praeger, Wesport. [Google Scholar]
- Whitam F. L., Zent M. (1984). A cross-cultural assessment of early cross-gender behavior and familial factors in male homosexuality. Archives of Sexual Behavior, 13, 427–439. [DOI] [PubMed] [Google Scholar]
- Xu Y., Norton S., Rahman Q. (2019). Early life conditions and adolescent sexual orientation: A prospective birth cohort study. Developmental Psychology, 55, 1226–1243. [DOI] [PubMed] [Google Scholar]
- Zelditch M. L., Swiderski D. L., Sheets H. D., Fink W. L. (2004). Geometric morphometrics for biologists: A primer. New York, NY: Elsevier Academic Press. [Google Scholar]
- Zucker K. J., Blanchard R. (2003). Birth order in the fakafefine. Journal of Sex & Marital Therapy, 29, 251–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, MHP_Indonesia_revised_supplementary for Male Homosexual Preference: Femininity and the Older Brother Effect in Indonesia by Sarah Nila, Pierre-Andre Crochet, Julien Barthes, Puji Rianti, Berry Juliandi, Bambang Suryobroto and Michel Raymond in Evolutionary Psychology