Abstract
Spodoptera frugiperda (J. E. Smith) is an important invasive pest that poses a serious threat to global crop production. Both emamectin benzoate (EB) and diamide insecticides are effective insecticides used to protect against S. frugiperda. Here, 16S rRNA sequencing was used to characterize the gut microbiota in S. frugiperda larvae exposed to EB or tetrachlorantraniliprole (TE). Firmicutes and Proteobacteria were found to be the dominant bacterial phyla present in the intestines of S. frugiperda. Following insecticide treatment, larvae were enriched for species involved in the process of insecticide degradation. High-level alpha and beta diversity indices suggested that exposure to TE and EB significantly altered the composition and diversity of the gastrointestinal microbiota in S. frugiperda. At 24 h post-EB treatment, Burkholderia-Caballeronia-Paraburkholderia abundance was significantly increased relative to the control group, with significant increases in Stenotrophobacter, Nitrospira, Blastocatella, Sulfurifustis, and Flavobacterium also being evident in these larvae. These microbes may play a role in the degradation or detoxification of EB and TE, although further work will be needed to explore the mechanisms underlying such activity. Overall, these findings will serve as a theoretical foundation for subsequent studies of the relationship between the gut microbiota and insecticide resistance in S. frugiperda (J. E. Smith) (Lepidoptera: Noctuidae).
Keywords: Spodoptera frugiperda, gut bacteria, 16S rRNA, insecticide resistance
The insect gut is the site of food storage, digestion, and excretion. As in other animal species, the insect gastrointestinal tract is heavily colonized by a diverse range of microbial species that have co-evolved with their hosts (Engel and Moran 2013), establishing a robust symbiotic relationship. The gut offers a stable environment in which these microbes can thrive and obtain essential nutrients (Peterson and Scharf 2016, Pang et al. 2016), with the specific composition of the gut microenvironment ultimately shaping gut microbial species diversity and host specificity (Kim et al. 2013, Kuraishi et al. 2013). In return, these microbes serve as critical regulators of a range of host biological processes including nutritional metabolism, growth, development, and environmental adaptability (Douglas 2015, Hammer and Bowers 2015, Wu et al. 2020). When stable, the intestinal microbiota can support host immunity and enhance resistance against potentially pathogenic microorganisms (Dillon et al. 2005). It also provides critical nutrients required for normal insect physiological activity (Nikoh et al. 2011, Salem et al. 2014), aiding insects in the digestion of complex macromolecular substrates (Lundgren and Lehman 2010). These gut microbes can also shape host movement and migration, environmental adaptability (Tsuchida et al. 2010, Zhang et al. 2021), insecticide resistance (Soltani et al. 2017, Pang et al. 2018), population genetic diversity (Mattila et al. 2012), mating, and reproductive behavior (Zhang et al. 2020).
Several recent studies have outlined the relationship between gastrointestinal microbes and insecticide resistance (Kikuchi and Yumoto 2013, Xia et al. 2013, 2018, Cheng et al. 2017, Zhang et al. 2019, Ishigami et al. 2021, EI Khoury et al. 2022). For example, symbiotic Burkholderia can degrade fenitrothion, thereby increasing stinkbug resistance to this insecticide (Tago et al. 2015). Similarly, symbiotic Citrobacter sp. within the gastrointestinal tract of Bactrocera dorsalis can readily break down trichlorfon, thus detoxifying this insecticide and augmenting host resistance thereto (Cheng et al. 2017). Gut microbes are similarly critical mediators of insecticide resistance in Plutella xylostella (Xia et al. 2013, 2018), and various endogenous bacteria in the gut of honeybees, including Enterobacter sp., Serratia sp., Pantoea sp. Edwardsiella sp., Hafnia sp., and Rahnella sp. can break down the pesticide clothianidin (EI Khoury et al. 2022). The gut microbiota such as Pseudomonas stutzeri, Pseudomonas psychrotolerans, Microbactetium arborescens, and others from the larvae of Spodoptera frugiperda can degrade lambda-cyhalothrin, deltamethrin, spinosad, and lufenuron (de Almeida et al. 2017). Serratia oryzae was the dominant gut bacteria that increased resistance to deltamethrin in Aedes albopictus (Wang et al. 2022).
Spodoptera frugiperda (J. E. Smith) are major migratory pests native to subtropical and tropical regions of the Americas. S. frugiperda was first identified as an invasive species in Yunnan in December 2018, and it has since spread to 26 Chinese provinces, including cities and autonomous regions, posing a major threat to national food security (Sparks 1979, Sun et al. 2019, Zhou et al. 2021, Kenis et al. 2023). Chemical insecticides are among the most effective and direct approaches to controlling S. frugiperda, with insecticides such as diamide insecticides, emamectin, and ethyl spinosad exhibiting particularly good control efficacy (Zhan et al. 2019, Niu et al. 2022). However, the excessive use of these chemical pesticides, together with the high reproductive rate exhibited by S. frugiperda in China, has led to the emergence of resistance to classes of insecticides in this invasive pest species (Liang et al. 2020, Zhao et al. 2020, Guan et al. 2021). The gut microbiota has been shown to play a role in the development of insecticide resistance (Kikuchi and Yumoto 2013, Xia et al. 2013, 2018, Cheng et al. 2017, Zhang et al. 2019, Ishigami et al. 2021, EI Khoury et al. 2022). Prior studies of the S. frugiperda gut microbiome have revealed Proteobacteria and Firmicutes to be the dominant bacterial species therein, whereas Bacteroidetes and Actinobacteria abundance is limited (Gichuhi et al. 2020). Many factors influence the composition of the gastrointestinal microbiota in these armyworms, as evidenced by the pronounced differences in microbial community composition between the S. frugiperda foregut and midgut (Jones et al. 2019). Moreover, the gut microbiota of S. frugiperda varies markedly when comparing different instars and adults, with certain important members of the larval microbiome being absent in adults after being lost over the course of metamorphosis and development (Gichuhi et al. 2020, Mason et al. 2020). The S. frugiperda gut microbiota also varies as a function of geographical distribution, mating state, and host plants (Zhang et al. 2020, Liu et al. 2021, Chen et al. 2022, Lü et al. 2022). In S. frugiperda subject to insecticide selection under field conditions, the composition of the gut microbiome was richer than that in resistant populations screened under laboratory conditions, with some identified microbial species being capable of metabolizing insecticides (Gomes et al. 2020). However, further research is needed to experimentally characterize shifts in the S. frugiperda gastrointestinal microbiota following insecticide exposure.
Given their ability to interact with a range of physiological processes, bacteria within the gastrointestinal tract of insects can influence host metabolic and biochemical activity, and studying these microbial communities represents an important focus for research efforts aimed at understanding immunity and pest management strategies for S. frugiperda and other invasive pest species. Emamectin benzoate (4ʺ-epi-methylamino-4ʺ-deoxyavermectin B1, EB) is a highly efficient, broad-spectrum, semi-synthetic insecticide used for the control of agricultural and forestry insect pests., EB stimulates high-affinity γ-aminobutyric acid receptor (GABA-R) and glutamate-gated chloride channels (GluCls), and produces a consequent increase in membrane chloride ion permeability and disrupts nerve signals within nematodes, arthropods, and platyhelminths, and eventually leading to death (Liu et al. 2022). Spray application of EB can reduce fall armyworm larval populations, lower plant damage, and achieve a higher fodder yield (Liu et al. 2022). Tetrachlorantraniliprole (TE) binds to ryanodine receptors (RyRs) and activates the calcium channel, causing the depletion of internal calcium stores, which leads to uncontrolled muscle contraction, paralysis, and eventually death (Teng et al. 2020). In this study, these 2 kinds of pesticides with good efficacy in the control of S. frugiperda were selected, and 16S rRNA sequencing was used to analyze the gut bacteria in the 4th instar larvae of S. frugiperda after treatment with sublethal doses of TE and EB. The aim of this study was to analyze the effects of these insecticides on gut microbiota abundance, community structure, metabolic functions, and the specific gut bacteria involved in insecticide detoxification activity in S. frugiperda. These findings lay a foundation to strengthen the resistance management of S. frugiperda while also providing a novel approach and theoretical basis for the study and management of pest resistance.
Materials and Methods
Insect Rearing
S. frugiperda larvae were provided by the Plant Protection Institute of Hebei Academy of Agricultural and Forestry Sciences (Baoding, Hebei). Larvae were reared using fresh maize leaves of Guangliangtian 27, and were used for the present study following more than 20 generations of breeding under laboratory conditions. 1st–3rd instar larvae were fed in sterilized glass Petri dishes, while 4th instar larvae were individually placed in finger tubes until pupation. Following emergence, adults were transferred into plastic buckets in which they were fed 10% honey in water until laying eggs. Insects were raised at 25 ± 2°C with a relative humidity of 70% ± 5% and a photoperiod (L:D) of 16 h:8 h.
Pesticide Toxicity Assays
Emamectin benzoate was obtained from Shijiazhuang Huaxing Pesticide Co. Ltd (73. 65%, Shijiazhuang, Heibei), while tetrachlorantraniliprole was from Shenyang Kechuang Chemical Co. Ltd (94.1%, Shenyang, Liaoning). A leaf-dipping method was used to assess the toxicity of both EB and TE. Briefly, the EB and TE active ingredients were dissolved with acetone to produce stock solutions that were then diluted with a 0. 1% (v/v) aqueous Tween-80 to 5–7 different concentrations (EB: 0.40000, 0.20000, 0.10000, 0.05000, 0.02500, 0.01250, and 0.00625 mg/liter; TE: 200.0, 100.0, 50.0, 25.0, and 12.5 mg/liter). Leaves harvested from young maize plants in the bell-mouth stage of growth were cut into 5 cm sections, dipped in the prepared EB or TE solutions (or vehicle control) for 10 s, allowed to air dry, and the lower edges of leaves were then wrapped using moistened cotton, after which they were transferred into a round plastic box (diameter: 10 cm; height: 2 cm). The 4th instar larvae were used as experimental subjects, and were picked up after 4 h of starvation treatment. All treatments were performed with 4 replicates of 20 larvae per replicate. Following a 48 h incubation period, S. frugiperda larval mortality was recorded. Statistics were compiled using Microsoft Excel, and the LC30, LC50, and 95% confidence interval (CI) values were computed with SPSS 20. 0.
Experimental Design and Sample Collection
Sublethal EB and TE treatment groups were used as experimental groups, while larvae in the control group (CK) were instead exposed to a 0.1% (v/v) aqueous Tween-80 solution as the control group. Samples were selected at 24 and 48 h of treatment, with 3 replicates of 30 healthy 4th instar larvae being collected per group for subsequent gut dissection.
Gut Dissection and DNA Extraction
Samples surfaces were disinfected with 75% alcohol and were rinsed 3 times with sterilized water. Larvae were dissected under sterile conditions on an ultra-clean workbench. The gut of each larva was dissected and placed in a centrifuge tube containing PBS, shaken with an oscillator, and mixed to prepare a suspension of intestinal contents. After snap-freezing in liquid nitrogen, samples were quickly stored at −80°C for later use. HiPure Stool DNA kits (Magen, Guangzhou, China) were used based on provided directions to extract DNA from these samples.
Gene Amplification and Sequencing
The V3–V4 regions of the 16S rRNA gene in these gut microbial DNA samples were amplified via PCR (95°C for 5 min, followed by 30 cycles at 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min and a final extension at 72°C for 7 min) with specific primers (341F: CCTACGGGNGGCWGCAG, and 806R: GGACTACHVGGGTATCTAAT). Amplified sequences were then separated via 2% agarose gel electrophoresis and extracted with an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, CA, USA) based on provided directions, followed by quantification with the ABI StepOnePlus Real-Time PCR System (Life Technologies, CA, USA). After purification, these amplicons were pooled in equimolar amounts and subjected to paired-end sequencing (PE250) with an Illumina platform based on standard protocols (Gene Denovo, Guangzhou, Guangdong).
Bioinformatics Analysis
Raw reads were subjected to quality control to remove low-quality reads and adapter sequences, after which the remaining reads were spliced. Clean tags were then clustered into operational taxonomic units (OTUs) at a ≥97% similarity threshold with the UPARSE (v 9. 2. 64) pipeline (Edgar 2013). The UCHIME algorithm (Edgar et al. 2011) was used to remove chimeric tags, with the remaining effective tags being the focus of subsequent analyses. Tag sequences with the highest abundance were selected as representative sequences within each cluster.
A naive Bayesian model was used to classify representative OTU sequences into species using RDP classifier (v 2. 2) (Wang et al. 2007) based on the SILVA database (v 132) (Pruesse et al. 2007), with a confidence threshold value of 0. 8. The R heatmap package (v 1. 0. 12) was used to generate a heatmap demonstrating bacterial abundance (Kolde and Kolde 2015). The R VennDiagram package (v 1. 6. 16) was used to compare overlap in bacterial species among groups (Chen and Boutros 2011), while upset plots were generated with the R UpSet package (v 1. 3. 3) as a means of identifying unique and shared OTUs (Conway et al. 2017). To check the assumptions of normality and homogeneity for parametric analysis, Kolmogorov-Smirnov and Levene’s tests were used, respectively. Welch’s t-test was used to compare species among groups using the R Vegan package (v 2. 5. 3) (Oksanen et al. 2010). Tukey’s HSD test was used to compare alpha diversity indices among groups using the Vegan package, while principal coordinates analysis (PCoA) plots of unweighted unifrac distance values were also generated with the Vegan package and plotted with the R ggplot2 package (v 2. 2. 1) (Wickham 2011). ANOSIM analyses were performed with the Vegan package. KEGG pathway analyses for these OTUs were conducted with PICRUSt (v 2. 1. 4) (Langille et al. 2013). Functional differences among groups were compared in the Vegan package using Welch’s t-test.
Results
Identification of Sublethal Doses of Tetrachlorantraniliprole and Emamectin Benzoate When Used to Treat S. frugiperda
Initially, regression equations were established based on the observed toxicity of a range of TE and EB concentrations to the 4th instar larvae of S. frugiperda (Table 1); The LC30 values for TE and EB were 15. 5 mg/liter and 0. 01 mg/liter, respectively. As such, these 2 concentrations were used for subsequent testing of the impact of TE and EB on the composition of the S. frugiperda gut microbiome.
Table 1.
The toxicity of TE and EB to 4th instar S. frugiperda larvae
| Insecticides | Regression equation | R 2 | Chi-Square (χ2) | df | LC30/(mg/liter) (95% CL) | LC50/(mg/liter) (95% CL) |
|---|---|---|---|---|---|---|
| TE | y = 2.325 + 1.414x | 0.982 | 0.790 | 4 | 15.555 (8.792–22.693) | 41.067 (28.930–58.669) |
| EB | y = -2.007 + 1.244x | 0.974 | 1.289 | 4 | 0.010 (0.006–0.013) | 0.023 (0.017–0.031) |
Sequence Splicing Assembly and Quality Control
After quality control processing of raw sequencing data (filtering, splicing, and clustering), effective tags were obtained. In total, 118,204–129,029 effective tags accounting for 95.03%–98.67% of raw reads were obtained after quality control (Supplementary Table S1), consistent with good sequencing data suitable for downstream experimental use. The total length of effective tags in each sample ranged from 54,908,411 to 59,957,159, with the shortest and longest obtained sequences being 202 bp and 478 bp, respectively (Supplementary Table S2).
Community Composition and Indicator Species Analyses
Species annotations were performed based on the obtained OTU sequence information, after which relative bacterial abundance in these different treatment groups was assessed at the genus level (Fig. 1). Community composition analyses revealed certain differences in gut bacteria species abundance among these different treatment groups. At 24 h post-treatment, significant increases in Akkermansia, Lachnospiraceae_NK4A136_group, Prauserella, and Burkholderia-Caballeronia-
Fig. 1.
Heatmap presentation of the relative abundance of different bacterial genera in individual samples by log10 transformation. Rows and columns respectively correspond to bacterial genera and sample names. Higher and lower levels of relative abundance are respectively marked in red and blue. Samples from the CK, EB, and TE groups were collected at 24 h and 48 h, with individual biological replicates as numbered (1, 2, or 3).
Paraburkholderia abundance was observed in the EB-treated larvae relative to CK controls. While Stenotrophobacter, Nitrospira, and Sulfurifustis were present in the gut microbiota of S. frugiperda at 24 and 48 h after TE treatment, these bacterial genera were absent in the CK and EB groups (Fig. 1 and Supplementary Table S3). However, Firmicutes and Proteobacteria remained the dominant gut bacteria in these larvae at all time points and under all treatment conditions (Supplementary Table S3).
The genus levels of indicator species at each time point in the control and treatment groups were analyzed. Both shared and unique species were observed in all 3 treatment groups at both 24 h (Fig. 2A) and 48 h (Fig. 2B). The number of specific species detected declined as the treatment period increased (Fig. 2). When genus level species comparisons were made among groups using Welch’s t-test, Burkholderia-Caballeronia-Paraburkholderia levels were found to be significantly increased in the EB treatment group at 24 h post-treatment relative to the CK group (Fig. 3A), whereas Psychromonas levels were significantly reduced in the TE treatment group relative to the CK group (Fig. 3B). At 48 h post-treatment, Delftia and Streptococcus levels were significantly lower in the EB treatment group as compared to the CK group (Fig. 3C). In larvae from the TE treatment group, significant increases in Stenotrophobacter, Nitrospira, Blastocatella, Sulfurifustis, and Flavobacterium levels were observed relative to CK controls, whereas Micrococcus abundance was decreased at this time point (Fig. 3D).
Fig. 2.
Venn diagram demonstrating the numbers of genus level indicator species in the different treatment groups. Bacteria were assessed at 24 h (A) and 48 h (B) post-treatment.
Fig. 3.
Analyses of relative abundance (%) and proportions of bacteria in different treatment groups at the genus level. (A) CK vs. EB at 24 h post-treatment. (B) CK vs. TE at 24 h post-treatment. (C) CK vs. EB at 48 h post-treatment. (D) CK vs. TE at 48 h post-treatment.
Analyses of Alpha Diversity in Different Treatment Groups
Alpha diversity analyses of the observed_Species (Sob) index were conducted based on the number of OTUs detected in sequences samples, with Tukey’s HDS test being used to analyze differences among groups. After treatment for 24 h, the highest and lowest levels of diversity were evident in the TE and EB treatment groups, respectively (Fig. 4A). At 48 h post-treatment, no significant differences in alpha diversity were observed among these groups (Fig. 4B). The Sob index in the CK group had decreased at 48 h relative to that of the TE group at 48 h, whereas the Sob indices of the EB and TE groups increased, suggesting that the species in the gut microbiota increase with the prolongation of treatment time in all cases (Fig. 4).
Fig. 4.
Alpha diversity indices for bacterial OTUs in the S. frugiperda gut microbiota in different treatment groups. Alpha diversity indices were calculated in the indicated treatment groups at 24 h (A) and 48 h (B) post-treatment.
Analyses of Bacterial Beta Diversity
OTU sequences were further used to analyze the beta diversity of bacteria in the gastrointestinal tract of S. frugiperda. Principal coordinate analysis (PCoA) plots revealed that while there were differences in the composition of the gastrointestinal microbiota among treatment time points, at any given time point the microbes in these larvae were largely similar to one another, with some limited differences in beta diversity among treatment conditions (Fig. 5A). An analysis of similarity (ANOSIM) of these groups revealed an R-value of 0. 621 at 24 h post-treatment and a P-value of 0. 012, indicating that differences among groups at this time point were more significant than differences within groups (Fig. 5B). In contrast, at 48 h post-treatment, the R-value was 0. 383 and the P-value was 0. 101, indicating a lack of significance consistent with limited differences among groups (Fig. 5C).
Fig. 5.
Beta diversity indices for bacterial OTUs in the S. frugiperda gut microbiota in different treatment groups. (A) PCoA plots were generated based on the unweighted unifrac distances for the indicated samples. ANOSIM tests revealed differences in beta diversity among treatment groups at 24 h (B) and 48 h (C) post-treatment. Potential R values ranged from −1 to 1, with values greater than 0 being indicative of significant differences among groups. P < 0. 05 denotes statistical significance.
Predicted Functions and Pathways Associated With Bacteria Communities From the Gut of S. frugiperda Subjected to Different Treatments
The functions and pathways associated with the gut bacteria of S. frugiperda following these different treatments were next predicted. At 24 h post-treatment, the functions of these gut bacteria were largely comparable in all 3 treatment groups, with enriched functions primarily being associated with S. frugiperda functional activities including membrane transport, energy metabolism, replication and repair, xenobiotic biodegradation and metabolism, lipid metabolism, metabolism of other amino acids, metabolism of terpenoids and polyketides, metabolism of cofactors and vitamins, amino acid metabolism, and carbohydrate metabolism (Fig. 6A). At 48 h post-treatment, these gut microbes were associated with activities including the endocrine system, infectious diseases, immune system, neurodegenerative diseases, digestive system, cardiovascular diseases, development, immune diseases, and signaling molecules and interactions (Fig. 6B). Overall, these findings suggested that at a given time point, the physiological roles of gut microbes in S. frugiperda are largely similar with some differences among groups, whereas more pronounced differences were evident among treatment groups at different time points. For example, at 24 h post-treatment, gut microbes were primarily involved in metabolic pathways (such as amino acid metabolism, terpenoid and polyketone metabolism, and lipid metabolism) whereas at 48 h post-treatment, these microbes were primarily involved in immunological and detoxification activities (including infectious diseases, neurodegenerative diseases, and immune diseases) (Fig. 6).
Fig. 6.
Functional annotation of KEGG pathway using the PICRUSt2 software for gut bacteria in S. frugiperda subjected to different treatments. KEGG functional predictions for the gut bacteria in S. frugiperda were performed at 24 h (A) and 48 h (B) post-treatment.
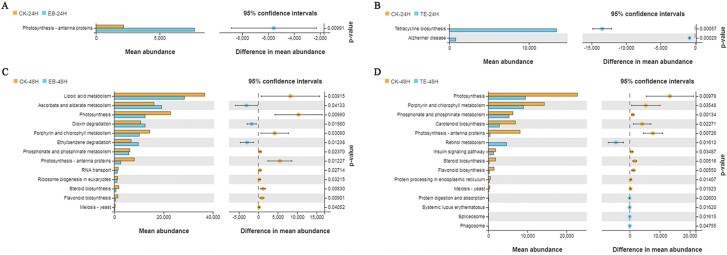
Differences in the function of the gut microbiota in these different treatment groups at a given time point were next detected using Welch’s t-test. At 24 h following treatment with EB, significant increases in the abundance of gut bacteria associated with photosynthesis were observed in EB-treated larvae relative to those in the CK group (Fig. 7A). In contrast, at 24 h post-TE treatment, larvae exhibited significant increases, in bacteria associated with tetracycline biosynthesis and Alzheimer’s disease relative to the CK group (Fig. 7B). Moreover, at 48 h post-EB treatment, larvae exhibited an increased abundance of bacteria involved in ascorbate and aldarate metabolism, dioxin degradation, and ethylbenzene degradation relative to CK group larvae (Fig. 7C), while TE-treated larvae exhibited increases in the abundance of bacteria associated with retinol metabolism, protein digestion and absorption, systemic lupus erythematosus, spliceosome activity, and phagosome activity compared to the CK group at 48 h post-treatment (Fig. 7D).
Fig. 7.
Analyses of gut microbiota functional differences for S. frugiperda in the different treatment groups. EB vs. CK groups at 24 h (A) and 48 h (C). TE vs. CK group after 24 h (B) and 48 h (D).
Discussion
In insects, bacteria in the gut microbiota play critical roles in essential processes during all stages of the life cycle. S. frugiperda are a highly heterotrophic species and an important food crop pest, leading to growing interest in the composition of the gut microbiota in these insects. Here, a high-throughput 16S rRNA sequencing approach was used to characterize the composition of the S. frugiperda gut microbiome at 24 and 48 h post-treatment with sublethal EB and TE concentrations. These data offer valuable insight regarding the association between the gut microbiota and pesticide detoxification, highlighting promising avenues for subsequent research aimed at clarifying how S. frugiperda are able to resist pesticide treatment.
These 16S rRNA sequencing analyses revealed Firmicutes and Proteobacteria to be the dominant phyla in the gut of S. frugiperda, in line with prior works (Gichuhi et al. 2020, Chen et al. 2021, Shu et al. 2022). Firmicutes and Proteobacteria are also reportedly dominant in Spodoptera exigua, Dendrolimus superans, and Lymantria dispar, indicating that these bacteria may be important commensal members of the intestinal microbiota in a range of Noctuidae insects. All S. frugiperda samples in this study were found to harbor Enterococcus species as the most abundant genus. Enterococcus species have been shown to produce a diverse array of bacteriocins, which are active antibacterial compounds that can directly influence the composition of the gastrointestinal microbiota (De Kwaadsteniet et al. 2005, Van Arnam et al. 2018). The gut bacteria of 30 species of Lepidoptera were compared and found that Enterococcus was core microorganisms in the lepidopteran gut (Paniagua Voirol et al. 2018). High levels of Proteobacteria abundance were also evident in these experimental insects, including Burkholderia-Caballeronia-Paraburkholderia and Sulfurifustis species, which were respectively enriched following EB and TE treatment. These microbes may thus play a role in detoxifying these particular insecticides. Indeed, there is prior evidence that Proteobacteria can support the degradation of a range of pesticides and secondary plant metabolites including glycosides, terpenes, alkaloids, and phenolic compounds (Itoh et al. 2018). Consistently, Proteobacteria-mediated detoxification has been observed in Diptera, Coleoptera, and Lepidoptera specimens (Kikuchi et al. 2012, Akami et al. 2019).
Here, Venn diagrams were used to highlight similarities and differences in the gut microbiota of S. frugiperda in different treatment groups. A marked increase in Burkholderia-Caballeronia-
Paraburkholderia abundance was observed following 24 h of EB treatment relative to control treatment. As a Proteobacteria species, Burkholderia-caballeronia-paraburkholderia may play a role in pesticide detoxification as do other members of this phylum (Kikuchi et al. 2012, Itoh et al. 2018, Akami et al. 2019, Gomes et al. 2020), particularly given that there is evidence for the ability of Burkholderia to metabolize the insecticide fenitrothion (Itoh et al. 2018, Zeng et al. 2020a). Burkholderia-Caballeronia-Paraburkholderia may thus be an important mediator of EB detoxification in S. frugiperda. Significant increases in Stenotrophobacter, Nitrospira, Blastocatella, Sulfurifustis, and Flavobacterium abundance were observed following TE treatment for 48 h relative to CK control treatment, indicating a possible role for these microbes in the detoxification of TE although this possibility will require further experimental verification.
Alpha and beta diversity analyses revealed significant differences in the composition of the S. frugiperda gut microbiota at 24 h post-treatment, although no such differences were evident at 48 h post-treatment. This suggested that insecticide exposure can alter the composition of the intestinal microbiome in these agricultural pests, in line with prior data demonstrating the ability of nicotine and aconitine to significantly impact gut bacteria and the abundance of Dendrolimus superans and Lymantria dispar larvae, respectively (Li et al. 2020). Silkworm larvae fed phoxim-treated mulberry leaves also exhibited significant shifts in gut microbial community composition and structure, with significant reductions in the abundance of Methylobacterium and Aurantimonadaceae species in the phoxim treatment group relative to the control group (Zeng et al. 2020b). Abamectin can also markedly alter the diversity and composition of the gastrointestinal microbiota in L. dispar larvae (Lv et al. 2021). Camptothecin can significantly alter the bacteria present in the S. frugiperda larval midgut (Shu et al. 2022). These pesticide-related changes may represent a form of community adaptation such that probiotic species become more abundant to combat the chronic impact of insecticide exposure.
Predictive functional analyses of the potential roles of the dominant microbes in these different S. frugiperda larval treatment groups were also conducted, leading to the identification of distinct functional predictions in the EB and TE treatment groups at 24 and 48 h post-treatment. At 24 h post-treatment with EB, significant increases in the abundance of bacteria involved in the synthesis of antenna proteins were evident relative to the control group. This may be linked to the activity of EB as a disruptor of normal insect nerve conduction, with increases in antenna protein synthesis potentially representing a strategy to compensate for impaired nerve conduction in S. frugiperda. At 48 h following EB treatment, significant increases in bacteria associated with ascorbate and aldarate metabolism, dioxin degradation, and ethylbenzene degradation were evident relative to the CK group. These processes may be linked to the degradation of EB by specific bacterial species. Consistently, shifts in the physiological activity of S. frugiperda larvae were observed following TE treatment. While these bacteria may support TE detoxification and/or degradation, more experimental work will be necessary to test this possibility.
While most members of the intestinal microbiota were unaffected by EB or TE treatment in S. frugiperda larvae, the abundance and diversity of microbes with potentially critical functions were altered. In particular, bacteria with the potential to support the degradation and detoxification of EB and TE were differentially abundant following such treatment. This is consistent with prior evidence showing that bumblebees exposed to imidacloprid and flupyradifurone exhibit a significant decrease in levels of bacteria involved in carbon metabolism with a concomitant increase in energy metabolism-related bacterial species, with researchers having speculated that the observed drop in carbon metabolism was attributable to a shift towards bacteria capable of increasing energy metabolism to facilitate pesticide detoxification (Derecka et al. 2013, Zhang et al. 2022). Indeed, several symbiotic gut microbes in S. frugiperda larvae have been reported to facilitate the degradation of chlorpyrifos, flubendiamid, deltamethrin, lambda-cyhalothrin, spinosad, teflubenzuro, and telufenuron (Almeida et al. 2017, Gomes et al. 2020). Similarly, the results of the present study suggest a role for certain members of the intestinal microbiota of S. frugiperda in the processes of EB and TE detoxification, although further study of the underlying mechanism will be required.
Here, a high-throughput 16S rRNA sequencing strategy was used to conduct the first-ever evaluation of the impact of sublethal emamectin benzoate and tetrachlorantraniliprole concentrations on the intestinal microbiota of S. frugiperda larvae. These analyses revealed marked shifts in the intestinal microbiota present within these S. frugiperda larvae in terms of both diversity and composition following EB and TE treatment. Pronounced differences were also observed among these treatment conditions, potentially as a consequence of the ability of intestinal bacteria to aid in insecticide detoxification processes and general post-treatment adaptation. While these intestinal bacteria were found to play a role in EB and TE detoxification, more work will be necessary to clarify the mechanisms through which they exert this protective activity. Overall, these data provide valuable new insight regarding the intestinal community present within S. frugiperda larvae while offering a robust theoretical foundation for subsequent efforts to understand the link between intestinal bacteria and pesticide resistance in this species.
Supplementary Material
Acknowledgments
This research was funded by Key-Area Research and Development Program of Guangdong Province (2020B020223004) and Youth tutorial program of Guangdong Academy of Agricultural Sciences (R2020QD-021).
Contributor Information
Hong Chang, Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, China.
Jianglong Guo, Key Laboratory of Integrated Pest Management on Crops in Northern Region of North China, Ministry of Agriculture and Rural Affairs, IPM Center of Hebei Province, Plant Protection Institute, Hebei Academy of Agricultural and Forestry Sciences, Baoding 071000, China.
Guojun Qi, Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, China.
Yan Gao, Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, China.
Siwei Wang, Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, China.
Xiaonan Wang, Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, China.
Yanping Liu, Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, China.
Author Contributions
Hong Chang (Data curation-Equal, Formal analysis-Equal, Funding acquisition-Lead, Methodology-Lead, Project administration-Equal, Software-Lead, Writing – original draft-Lead), Jianglong Guo (Software-Supporting, Writing – original draft-Supporting), Guojun Qi (Software-Supporting, Writing – original draft-Supporting), Yan Gao (Conceptualization-Equal, Writing – review & editing-Equal), Siwei Wang (Formal analysis-Supporting, Methodology-Supporting), Xiaonan Wang (Supervision-Equal, Writing – original draft-Supporting), Yanping Liu (Conceptualization-Equal, Investigation-Lead, Resources-Lead, Supervision-Equal, Validation-Lead, Writing – review & editing-Equal)
References
- Akami M, Njintang N, Gbaye O, Andongma AA, Rashid MA, Niu CY, Nukenine EN.. Gut bacteria of the cow-pea beetle mediate its resistance to dichlorvos and susceptibility to Lippia adoensis essential oil. Sci Rep. 2019:.:6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LG, Moraes LA, Trigo JR, Omoto C, Cônsoli FL.. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: a potential source for biotechnological exploitation. PLoS One. 2017:1.:e0174754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Boutros PC.. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinf. 2011:1.:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Li YH, Sun ZX, Du EW, Lu ZH, Li H, Gui FR.. Effects of host plants on bacterial community structure in larvae midgut of Spodoptera frugiperda. Insects. 2022:1.:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Zhou HC, Lai YS, Chen Q, Yu XQ, Wang XY.. Gut microbiota dysbiosis influences metabolic homeostasis in Spodoptera frugiperda. Front Microbiol. 2021:1.:727434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DF, Guo ZJ, Riegler M, Xi ZY, Liang GW, Xu YJ.. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome. 2017:.:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JR, Lex A, Gehlenborg N.. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017:3.:2938–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida LG, de Moraes LAB, Trigo JR, Omoto C, Consoli FL.. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: a potential source for biotechnological exploitation. PLoS One. 2017:1.:e0174754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kwaadsteniet M, Todorov SD, Knoetze H, Dicks LMT.. Characterization of a 3944 Da bacteriocin, produced by Enterococcus mundtii ST15, with activity against gram-positive and gram-negative bacteria. Int J Food Microbiol. 2005:10.:433–444. [DOI] [PubMed] [Google Scholar]
- Derecka K, Blythe MJ, Malla S, Genereux DP, Guffanti A, Pavan P, Moles A, Snart C, Ryder T, Ortori C, et al. Transient exposure to low levels of insecticide affects metabolic networks of honeybee larvae. PLoS One. 2013:.:e68191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RJ, Vennard CT, Buckling A, Charnley AK.. Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett. 2005:.:1291–1298. [Google Scholar]
- Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol. 2015:6.:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013:1.:996–998. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R.. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011:2.:2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EI Khoury S, Giovenazzo P, Derome N.. Endogenous honeybee gut microbiota metabolize the pesticide clothianidin. Microorganisms. 2022:1.:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, Moran NA.. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol Rev. 2013:3.:699–735. [DOI] [PubMed] [Google Scholar]
- Gichuhi J, Sevgan S, Khamis F, Berg JV, Plessis H, Ekesi S, Herren JK.. Diversity of fall armyworm, Spodoptera frugiperda and their gut bacterial community in Kenya. PeerJ. 2020:.:e8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AFF, Omoto C, Cônsoli FL.. Gut bacteria of field-collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory-selected resistant strains. J Pest Sci. 2020:9.(2):833–851. 10.1007/s10340-020-01202-0. [DOI] [Google Scholar]
- Guan F, Zhang JP, Shen HW, Wang XL, Padovan A, Walsh TK, Tay WT, Gordon KHJ, James W, Czepak C, et al. Whole-genome sequencing to detect mutations associated with resistance to insecticides and Bt proteins in Spodoptera frugiperda. Insect Sci. 2021:2.:627–638. [DOI] [PubMed] [Google Scholar]
- Hammer TJ, Bowers MD.. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia. 2015:17.:1–14. [DOI] [PubMed] [Google Scholar]
- Ishigami K, Jang S, Itoh H, Kikuchi Y.. Insecticide resistance governed by gut symbiosis in a rice pest, Cletus punctiger, under laboratory conditions. Biol Lett. 2021:1.:20200780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Hori T, Sato Y, Nagayama A, Tago K, Hayatsu M, Kikuchi Y.. Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J. 2018:1.:909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Tago K, Hayatsu M, Kikuchi Y.. Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat Prod Rep. 2018:3.:434–454. [DOI] [PubMed] [Google Scholar]
- Jones AG, Mason CJ, Felton GW, Hoover K.. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci Rep. 2019:.:2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenis M, Benelli G, Biondi A, Paul-André C, Day R, Desneux N, Harrison RD, Kriticos D, Rwomushana I, van den Berg J., et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol Gen. 2023:4.:187–241. 10.1127/entomologia/2022/1659. [DOI] [Google Scholar]
- Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Fukatsu T.. Symbiont-mediated insecticide resistance. Proc Natl Acad Sci USA. 2012:10.:8618–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Yumoto I.. Efficient colonization of the bean bug Riptortus pedestris by an environmentally transmitted burkholderia symbiont. Appl Environ Microbiol. 2013:7.:2088–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Kim NH, Jang HA, Kikuchi Y, Kim CH, Fukatsu T, Lee BL.. Specific midgut region controlling the symbiont population in an insect-microbe gut symbiotic association. Appl Environ Microbiol. 2013:7.:7229–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde R, Kolde MR.. Package ‘pheatmap’. R Package; 2015. p. 1. [Google Scholar]
- Kuraishi T, Hori A, Kurata S.. Host-microbe interactions in the gut of Drosophila melanogaster. Front Physiol. 2013:.:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RV, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013:3.:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FC, Li MX, Mao TT, Wang H, Chen J, Lu ZT, Qu JW, Fang YL, Gu ZY, Li B.. Effects of phoxim exposure on gut microbial composition in the silkworm, Bombyx mori. Ecotoxicol Environ Saf. 2020:18.:110011. [DOI] [PubMed] [Google Scholar]
- Liang P, Gu SH, Zhang L, Gao XW.. Research status and prospects of Spodoptera frugiperda (Lepidoptera: Noctuidae) in China. Acta Entomol Sin. 2020:6.:624–638. [Google Scholar]
- Liu Y, Zhao XQ, Yin YQ, Li XY, Chen FS, Zhang HM, Wang Y, Chen AD.. Differences in the diversity and community structure of intestinal bacteria in four geographic populations of fall armyworm Spodoptera frugiperda in Yunnan Province. J Plant Prot. 2021:4.:1244–1253. [Google Scholar]
- Liu ZK, Li XL, Tan XF, Yang MF, Idrees A, Liu JF, Song SJ, Shen J.. Sublethal effects of emamectin benzoate on fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Agriculture. 2022:1.(7):959. 10.3390/agriculture12070959. [DOI] [Google Scholar]
- Lundgren JG, Lehman RM.. Bacterial gut symbionts contribute to seed digestion in an omnivorous beetle. PLoS One. 2010:.:e10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv NN, Ma KS, Li R, Liang PZ, Liang P, Gao XW.. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol Environ Saf. 2021:21.:111969. [DOI] [PubMed] [Google Scholar]
- Mason CJ, Clair AS, Peiffer M, Gomez E, Jones AG, Felton GW, Hoover K.. Diet influences proliferation and stability of gut bacterial populations in herbivorous lepidopteran larvae. PLoS One. 2020:1.:e0229848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila HR, Rios D, Walker-Sperling VE, Roeselers G, Newton ILG.. Characterization of the active microbiotas associated with honey bees reveals healthier and broader communities when colonies are genetically diverse. PLoS One. 2012:.(3):e32962. 10.1371/journal.pone.0032962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh N, Hosokawa T, Oshima K, Hattori M, Fukatsu T.. Reductive evolution of bacterial genome in insect gut environment. Genome Biol Evol. 2011:.:702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu DB, Tan CL, Wu YJ, Li XX, Li GT, Sheng CW, Cao HQ.. Detection of insecticides sensitivity and target site mutations in field populations of Spodoptera frugiperda in Anhui province. Plant Prot. 2022:4.:201–207. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Hara RBO, Simpson GL, Solymos P, Stevens H, Wagner HH.. Vegan: community ecology package. R package version 1. 17-8. Acesso Em. 23, 2010; 2010. [Google Scholar]
- Pang R, Chen M, Yue L, Xing K, Li TC, Kang K, Liang ZK, Yuan LY, Zhang WQ.. A distinct strain of Arsenophonus symbiont decreases insecticide resistance in its insect host. PLoS Genet. 2018:1.:e1007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang XJ, Xiao XP, Liu Y, Zhang RD, Liu JY, Liu QY, Wang PH, Cheng G.. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat Microbiol. 2016:.:16023. [DOI] [PubMed] [Google Scholar]
- Paniagua Voirol LR, Frago E, Kaltenpoth M, Hilker M, Fatouros NE.. Bacterial symbionts in Lepidoptera: their diversity, transmission, and impact on the host. Front Microbiol. 2018:.:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BF, Scharf ME.. Lower termite associations with microbes: synergy, protection, and interplay. Front Microbiol. 2016:.:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies JX, Glckner FO.. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007:3.:7188–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H, Bauer E, Strauss A, Vogel H, Marz M, Kaltenpoth M.. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc R Soc B. 2014:28.:20141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu BS, Dai JH, Yu JC, Yang XM, Lin JT.. Effects of camptothecin on the composition and diversity of the bacterial community in the midgut of Spodoptera frugiperda larvae. J. Environ Entomol. 2022:4.:44–54. [Google Scholar]
- Soltani A, Vatandoost H, Oshaghi MA, Enayati AA, Chavshin AR.. The role of midgut symbiotic bacteria in resistance of Anopheles stephensi (Diptera: Culicidae) to organophosphate insecticides. Pathog Glob Health. 2017:11.:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks AN. A review of the biology of the fall armyworm. Fla Entomol. 1979:6.:82–86. [Google Scholar]
- Sun XX, Hu CX, Jia HR, Wu QL, Shen XJ, Zhao SY, Jiang YY, Wu KM.. Case study on the first immigration of fall armyworm Spodoptera frugiperda invading into China. J Integr Agric. 2019:2.:664–672. [Google Scholar]
- Tago K, Kikuchi Y, Nakaoka S, Katsuyama C, Hayatsu M.. Insecticide applications to soil contribute to the development of burkholderia mediating insecticide resistance in stinkbugs. Mol Ecol. 2015:2.:3766–3778. [DOI] [PubMed] [Google Scholar]
- Teng HY, Yuan YD, Zhang TS, Chang XL, Wang DS.. Evaluation of the sublethal effect of tetrachlorantraniliprole on Spodoptera exigua and its potential toxicity to two non-target organisms. PLoS One. 2020:1.:e0242052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T.. Symbiotic bacterium modifies aphid body color. Science. 2010:33.(6007):1102–1104. 10.1126/science.1195463. [DOI] [PubMed] [Google Scholar]
- Van Arnam EB, Currie CR, Clardy J.. Defense contracts: Molecular protection in insect-microbe symbioses. Chem Soc Rev. 2018:4.:1638–1651. [DOI] [PubMed] [Google Scholar]
- Wang HY, Liu HM, Peng H, Wang Y, Zhang CX, Guo XX, Wang HF, Liu LJ, Lv QX, Cheng P, et al. A symbiotic gut bacterium enhances Aedes albopictus resistance to insecticide. PLoS Negl Trop Dis. 2022:1.:e0010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR.. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007:7.:5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2. Wiley interdisciplinary reviews: computational statistics. Rev Comput Stat. 2011:.:180–185. [Google Scholar]
- Wu YQ, Zheng YF, Chen YN, Wang S, Chen YP, Hu FL, Zheng HQ.. Honey bee (Apis mellifera) gut microbiota promotes host endogenous detoxification capability via regulation of P450 gene expression in the digestive tract. Microb Biotechnol. 2020:1.:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XF, Sun B, Gurr GM, Vasseur L, Xue MQ, You MS.. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front Microbiol. 2018:.:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XF, Zheng DD, Zhong HZ, Qin BC, Gurr G, Vasseur L, Lin HL, Bai JL, Hei WY, You MS.. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS One. 2013:.:e68852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng JY, Vuong TMD, Shi JH, Shi ZB, Guo JX, Zhang GC, Bi B.. Avermectin stress varied structure and function of gut microbial community in Lymantria dispar asiatica (Lepidoptera: Lymantriidae) larvae. Pestic Biochem Physiol. 2020a:16.:196–202. [DOI] [PubMed] [Google Scholar]
- Zeng JY, Wu DD, Shi ZB, Yang J, Zhang GC, Zhang J.. Influence of dietary aconitine and nicotine on the gut microbiota of two lepidopteran herbivores. Arch Insect Biochem Physiol. 2020b:10.:e21676. [DOI] [PubMed] [Google Scholar]
- Zhan SY, Sun XX, Zhang HW, Yang XM, Wu KM.. Laboratory test on the control efficacy of common chemical insecticides against Spodoptera frugiperda. Plant Prot. 2019:4.:10–14. [Google Scholar]
- Zhang LY, Yu H, Fu DY, Xu J, Yang S, Ye H.. Mating leads to a decline in the diversity of symbiotic microbiomes and promiscuity increased pathogen abundance in a moth. Front Microbiol. 2020:1.:878856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QC, Wang QL, Zhai YF, Zheng H, Wang XF.. Impacts of imidacloprid and flupyradifurone insecticides on the gut microbiota of Bombus terrestris. Agriculture. 2022:1.:389. [Google Scholar]
- Zhang YY, Li JH, Wan H.. Research progress on the relationship between host detoxification metabolism and insect microbial symbionts. Chin J Pest Sci. 2019:2.:729–735. [Google Scholar]
- Zhang ZJ, Huang MF, Qiu LF, Song RH, Zhang ZX, Ding YW, Zhou X, Zhang X, Zheng H.. Diversity and functional analysis of Chinese bumblebee gut microbiota reveal the metabolic niche and antibiotic resistance variation of Gilliamella. Insect Sci. 2021:2.:13. [DOI] [PubMed] [Google Scholar]
- Zhao YX, Huang JM, Ni H, Guo D, Yang FX, Wang X, Wu SF, Gao CF.. Susceptibility of fall armyworm, Spodoptera frugiperda (J. E. Smmith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic Biochem Physiol. 2020:16.:104623. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wu QL, Zhang HW, Wu KM.. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J Integr Agric. 2021:2.:637–645. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.