Abstract
SGLT2 (sodium-glucose cotransporter 2) inhibitors interfere with the reabsorption of glucose and sodium in the early proximal renal tubule, but the magnitude and duration of any ensuing natriuretic or diuretic effect are the result of an interplay between the degree of upregulation of SGLT2 and sodium-hydrogen exchanger 3, the extent to which downstream compensatory tubular mechanisms are activated, and (potentially) the volume set point in individual patients. A comprehensive review and synthesis of available studies reveals several renal response patterns with substantial variation across studies and clinical settings. However, the common observation is an absence of a large acute or chronic diuresis or natriuresis with these agents, either when given alone or combined with other diuretics. This limited response results from the fact that renal compensation to these drugs is rapid and nearly complete within a few days or weeks, preventing progressive volume losses. Nevertheless, the finding that fractional excretion of glucose and lithium (the latter being a marker of proximal sodium reabsorption) persists during long-term treatment with SGLT2 inhibitors indicates that pharmacological tolerance to the effects of these drugs at the level of the proximal tubule does not meaningfully occur. This persistent proximal tubular effect of SGLT2 inhibitors can be hypothesized to produce a durable improvement in the internal set point for volume homeostasis, which may become clinically important during times of fluid expansion. However, it is difficult to know whether a treatment-related change in the volume set point actually occurs or contributes to the effect of these drugs to reduce the risk of major heart failure events. SGLT2 inhibitors exert cardioprotective effects by a direct effect on cardiomyocytes that is independent of the presence of or binding to SGLT2 or the actions of these drugs on the proximal renal tubule. Nevertheless, changes in the volume set point mediated by SGLT2 inhibitors might potentially act cooperatively with the direct favorable molecular and cellular effects of these drugs on cardiomyocytes to mediate their benefits on the development and clinical course of heart failure.
Keywords: diuresis, heart failure, natriuresis, proximal renal tubule, SGLT2 inhibitors
SGLT2 (sodium-glucose cotransporter 2) inhibitors reduce the risk of heart failure hospitalizations by ≈30% in large-scale trials of patients with type 2 diabetes, chronic heart failure, and chronic kidney disease, an effect that is independent of glycemic status, ejection fraction, or renal function.1 Although myriad hypotheses have been proposed, 2 broad mechanisms are considered likely to underlie this benefit. First, SGLT2 inhibitors act on the kidney to promote changes in urinary electrolyte and water excretion. The resulting shift in the volume of circulating and noncirculating fluid compartments, in conjunction with potential modulation of renal salt sensing, reduces the likelihood of volume expansion that often precedes hospitalization for heart failure.2 Second, SGLT2 inhibitors act directly on the heart to induce nutrient deprivation signaling and promote autophagy, which reduces oxidative and endoplasmic reticulum stress and enhances cellular function and viability.3,4 This direct action on cardiomyocytes is independent of the presence, binding, or inhibition of SGLT2,3,5 and the effects on cellular health can be negated by genetic or pharmacological interventions that silence sirtuin-1 or AMP-activated protein kinase or interfere with autophagic flux.3
Recent reviews have focused on the direct cardiac effects of SGLT2 inhibitors mediated by the modulation of nutrient transport and nutrient deprivation signaling,3,4 whereas the current overview provides a comprehensive and critical analysis of the direct effects of SGLT2 inhibitors on the urinary handling of electrolytes and water.
EFFECTS OF SGLT2 INHIBITION IN THE PROXIMAL RENAL TUBULE AND IMPORTANCE OF COUNTERBALANCING RESPONSES IN DOWNSTREAM NEPHRON SEGMENTS
Diabetes and heart failure lead to upregulation of SGLT2 in the proximal renal tubule, enhancing its sensitivity to SGLT2 inhibition.6–8 The expression of SGLT2 is enhanced by renal sympathetic hyperactivity;7,9 consequently, renal denervation reduces glycosuria, and it attenuates the glycosuric effect of SGLT2 inhibitors.7,9,10 Renal sympathetic nerve activity is heightened particularly in acutely decompensated heart failure,11 potentially augmenting the glycosuric effect of SGLT2 inhibitors; accordingly, the magnitude of glycosuria appears to wane as the acuity of the episode subsides.12
SGLT2 in the S1 and S2 segments of the proximal tubule is responsible for the overwhelming majority of glucose reabsorption in the kidney (Figure 1).13 In micropuncture studies, SGLT2 inhibition produces a glycosuria-dependent osmotic diuresis, which increases tubular fluid osmolality sufficiently to dilute proximal tubular sodium and induce a back leak of sodium into the lumen by passive paracellular pathways;13,14 thus, an osmotic diuresis yields an increase in urine volume accompanied by a modest reduction in proximal tubular sodium reabsorption.13 The magnitude of SGLT2 inhibitor–mediated glycosuria is reduced in chronic kidney disease.14,15
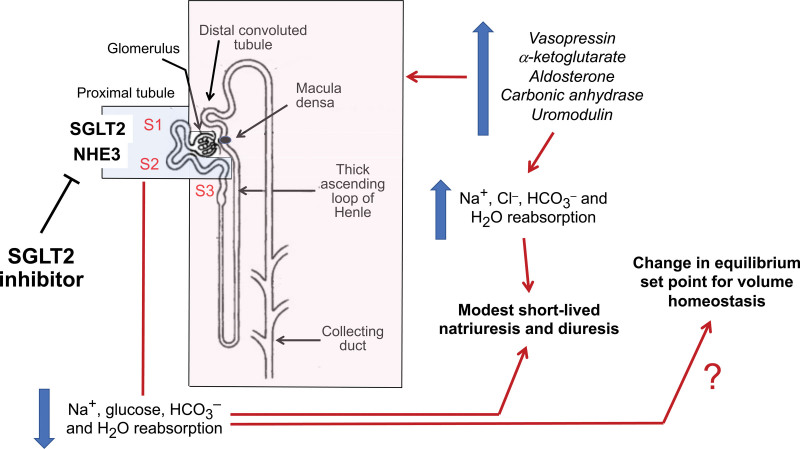
Figure 1.
Direct and compensatory actions of SGLT2 inhibitors on the proximal renal tubule and distal nephron segments. SGLT2 (sodium glucose cotransporter 2) inhibitors directly interfere with the actions of SGLT2 and NHE3 (sodium hydrogen exchanger 3) in the S1 and S2 segments of the proximal tubule, thus inhibiting the reabsorption of sodium, glucose, bicarbonate, and water. These effects are counteracted by upregulation of electrolyte and water reabsorptive pathways in downstream nephron segments, mediated by vasopressin, aldosterone, α-ketoglurate, carbonic anhydrase, and uromodulin. The net result is a modest short-lived natriuresis and diuresis, and potentially, a change in the equilibrium set point for volume homeostasis. Segments in blue are sites of sodium, glucose, bicarbonate and water reabsorption that are attenuated by SGLT2 inhibitors; segments in red are sites of sodium, chloride, bicarbonate, and water reabsorption that are potentiated by SGLT2 inhibitors.
Two-thirds of the filtered sodium is absorbed in the proximal tubule,16 primarily through the action of NHE3 (sodium-hydrogen exchanger 3). NHE3 is colocalized with and structurally interlinked with SGLT2 in the brush border.17,18 As a result, SGLT2 inhibitors modulate the functionality of NHE3 (even in the absence of luminal glucose), and this action likely underlies the effects of these drugs on renal sodium handling. Experimental silencing of NHE3 abrogates the effects of SGLT2 inhibition on proximal tubular sodium reabsorption.8,17,19 Inhibition of NHE3 also promotes bicarbonate excretion,8,16,17,19–21 contributing to the small decrease in serum bicarbonate observed in clinical trials.22,23
Activation of Counterbalancing Renal Tubular Mechanisms
If the effects of SGLT2 inhibitors were determined entirely by their actions on SGLT2 and NHE3 in the proximal renal tubule, their administration would result in a massive loss of sodium, water, and bicarbonate, leading to intravascular and extracellular volume depletion and marked acidosis.24 Although hypovolemic euglycemic acidosis can occur in patients receiving SGLT2 inhibitors, it is typically associated with ketosis resulting from calorie restriction or insulinopenia.25 In most circumstances, the lack of hypovolemia and acidosis is related to compensatory nephron mechanisms that counteract the effects of SGLT2 and NHE3 inhibition.
Specifically, SGLT2 inhibition is followed by marked enhancement of sodium reabsorption in downstream nephron segments, including the S3 segment of the proximal tubule, the loop of Henle, and the distal nephron (Figure 1).13,26,27 Clinical trial proteomic analyses suggest that SGLT2 inhibition is accompanied by upregulation of carbonic anhydrase and uromodulin.28 Activation of carbonic anhydrase promotes sodium and bicarbonate reabsorption in the proximal tubule and in the cortical collecting duct,29,30 and uromodulin activates the Na+,K+,2Cl– cotransporter in the loop of Henle.31 SGLT2 inhibition elevates circulating levels of aldosterone in some studies,32 and increased glucose delivery to the distal convoluted tubule activates sodium reabsorption by the Na-Cl cotransporter.33 In addition, SGLT2 inhibitors increase the proximal tubular levels of α-ketoglutarate,19 which promotes sodium and chloride reabsorption by facilitating chloride-bicarbonate exchanger–related reabsorption pathways, while enhancing ammoniagenesis to achieve renal acid excretion.19,34,35 These compensatory mechanisms, acting in concert, enhance sodium chloride reabsorption in the loop of Henle and more distal segments of the nephron, while simultaneously preventing the development of severe metabolic acidosis.
The mild osmotic diuresis that occurs with the tubular glucose load induced by SGLT2 inhibitors is easily compensated for by normal osmoregulatory mechanisms, primarily vasopressin.36–39 The magnitude of the reactive antiaquaretic effect can be sufficiently marked to paradoxically cause a decrease in free water clearance in clinical studies of SGLT2 inhibitors,37,40 explaining why plasma osmolarity increases only slightly during the water diuresis induced by glycosuria18,39,40 and why serum sodium concentration can decline after initiation of treatment.41 An increase in electrolyte-free water clearance becomes apparent if vasopressin is suppressed by water loading.42 Vasopressin also increases sodium reabsorption in the loop of Henle and distal segments.43
Consequences of SGLT2 Inhibitor–Mediated Increases in Distal Chloride Delivery
In micropuncture studies, inhibition of SGLT2 and NHE3 in the proximal tubule enhances the delivery of chloride to downstream segments,20 with several potential consequences.
Activation of Downstream Sodium Avidity and Mitigation of Hyperkalemia
As noted earlier, after SGLT2 inhibition, the reabsorption of sodium and chloride is enhanced in downstream nephron sites20,33 as a result of upregulation of vasopressin, uromodulin, aldosterone, and α-ketoglutarate.20,28,32,36–38,44–46 Increases in aldosterone may in part explain the effect of SGLT2 inhibitors to mitigate the risk of hyperkalemia (without inducing hypokalemia) in patients with diabetes or heart failure.47,48
Activation of Tubuloglomerular Feedback
Increased delivery of chloride to the macula densa after SGLT2 inhibition activates tubuloglomerular feedback, leading to afferent arteriolar vasoconstriction or efferent arteriolar vasodilation and a decline in glomerular filtration pressure.20,49–51 Tubuloglomerular feedback becomes saturated, and single-nephron glomerular filtration falls dramatically, but with time, there is partial adaptation caused by increased sodium reabsorption in the loop of Henle, in conjunction with resetting of tubuloglomerular feedback.20 Tubuloglomerular feedback has been hypothesized to contribute to the early dip in glomerular filtration rate after initiation of SGLT2 inhibitors, although this link has been challenged.52
Alleviation of Resistance to Loop Diuretics
Hypochloremia is a hallmark of diuretic resistance.53,54 A reduction in filtered chloride combined with cellular chloride depletion activates WNK4 (with no lysine 4), thus increasing the activity of the Na+,K+,2Cl- cotransporter,55 the site of action of loop diuretics. Efforts to enhance chloride delivery (by dietary chloride53 or acetazolamide56) may inhibit WNK4 and restore responsiveness to loop diuretics.53,57 SGLT2 inhibitors can increase serum chloride58–60 and enhance the effects of loop diuretics,61 presumably because the increased delivery of sodium chloride to the loop of Henle enlarges the drug target.62
Therefore, the net effect of SGLT2 inhibitors on the urinary sodium and water excretion may depend on (1) the activation of SGLT2 and NHE3; (2) the magnitude of glycosuria, which is limited in patients with impaired renal function; (3) the effect to promote downstream chloride delivery; and (4) the degree of upregulation of counterregulatory antinatriuretic and antiaquaretic mechanisms at downstream nephron sites.
EVALUATION OF SODIUM AND WATER EXCRETION IN CLINICAL STUDIES OF SGLT2 INHIBITORS
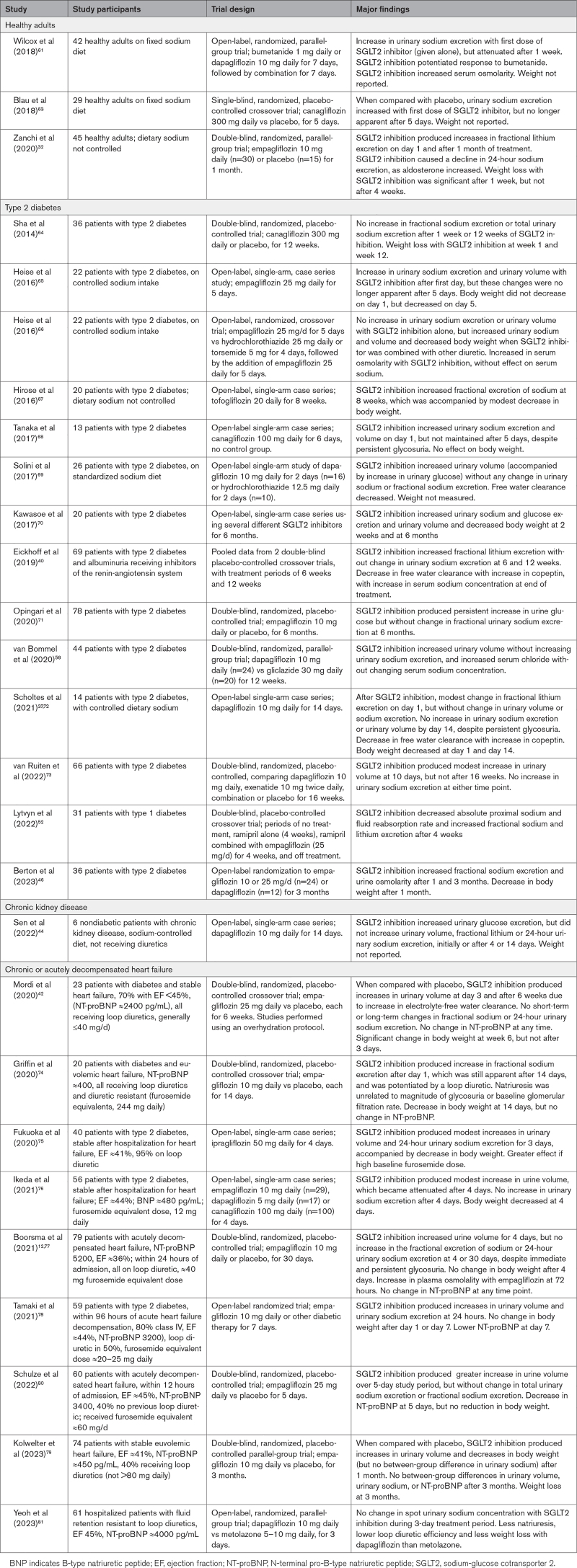
From our comprehensive review, we identified 27 studies that evaluated the effects of SGLT2 inhibitors on urinary sodium and water excretion in healthy volunteers or patients with type 2 diabetes, heart failure, or chronic kidney disease, for periods ranging from 3 days to 3 months (Table 1).12,32,37,39,40,42,44,46,58,61,63–81 Typically, dietary sodium and use of diuretics were not controlled; sodium balance was not achieved; and some studies analyzed spot (rather than 24-hour) urine collections.12,46,67,81 Trials that were double-blind and placebo-controlled are less subject to these confounding factors.
Table 1.
Studies Reporting Effect of SGLT2 Inhibitors on Urinary Sodium and Water Excretion
Body weight was reported in many studies, but decreases in body weight during SGLT2 inhibition can be caused by the excretion of fluid or the loss of calories due to glycosuria.82 It is noteworthy that Zanchi et al32 and Sha et al64 (who studied healthy volunteers and patients with diabetes) reported a reduction in body weight in the absence of a diuresis, but paradoxically, Mordi et al,42 Kolwelter et al,79 Boorsma et al,12,77 Tamaki et al,78 and Schulze et al80 (who studied patients with heart failure) did not observe decreases in weight, despite increased urine volume. These observations suggest that changes in body weight after SGLT2 inhibitors do not reliably reflect pretreatment fluid retention or the diuretic response to these drugs.
As shown in Table 1, the effect of SGLT2 inhibitors on sodium and water excretion varies with the clinical setting or study design (Figure 2).
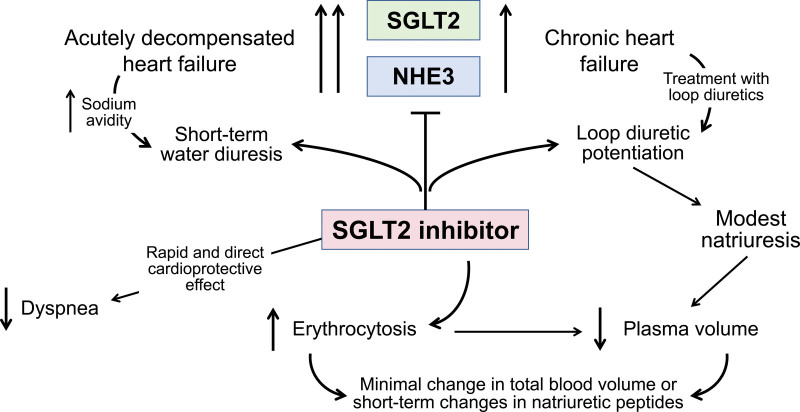
Figure 2.
Effects of SGLT2 inhibitors on urinary sodium and water excretion in chronic heart failure and acutely decompensated heart failure. The administration of an SGLT2 (sodium glucose cotransporter 2) inhibitor to patients with acutely decompensated heart failure is often accompanied by a short-term water diuresis, but clinical improvement may be related primarily to a rapid and direct cardioprotective effect similar to that seen during the first 2 to 4 weeks of treatment in clinical trials of patients with chronic heart failure. The administration of an SGLT2 inhibitor to patients with chronic heart failure who are receiving loop diuretics can be accompanied by potentiation of the effect of the loop diuretic, resulting in a modest diuresis and a reduction in plasma volume. However, because of their erythropoietic effect, these drugs produce minimal changes in total blood volume or decreases in circulating natriuretic peptides, and thus, short-term changes in urinary sodium or water excretion do not explain the short- and long-term decrease in the risk of heart failure events. Intriguingly, these events are not reduced in randomized controlled trials of short-term intensification of other diuretic drugs. NHE3 indicates sodium hydrogen exchanger 3.
Healthy Volunteers and Patients With Type 2 Diabetes
In 18 studies of healthy individuals or patients with type 2 diabetes (none of whom were reportedly receiving diuretics), SGLT2 inhibition was typically (but not invariably) accompanied by immediate increases in the fractional excretion of lithium (indicative of inhibited proximal tubular sodium reabsorption), total urinary sodium excretion, and urinary volume (Table 1). However, after 5 to 7 days, although fractional lithium excretion was maintained and glycosuria persisted, the drugs had no significant effect on total urinary sodium excretion or volume. Several studies37,39,40,44,46,72 have noted a decrease in free water clearance, accompanied by vasopressin activation; such an antiaquaretic counterregulatory response may be particularly likely in patients with diabetes.83
Heart Failure With and Without Recent Acute Decompensation
In patients with chronic heart failure receiving low doses of loop diuretics, the response to SGLT2 inhibition was characterized primarily by an increase in electrolyte-free water clearance. Four double-blind, placebo-controlled trials reported on the effects of SGLT2 inhibitors in patients who were treated with and responsive to furosemide equivalent doses <40 to 80 mg/d;12,42,79,80 2 of the 4 trials evaluated patients with acutely decompensated heart failure. In all 4 trials, SGLT2 inhibition produced an immediate and persistent increase in urinary volume, which was not accompanied by significant changes in urinary sodium excretion and was attributed to an osmotic diuresis produced by glycosuria. Counterregulatory downstream sodium reabsorptive mechanisms may be particularly active in the sodium-avid state of heart failure. A water diuretic effect may be especially evident in patients with acutely decompensated heart failure, possibly because renal sympathetic nerve hyperactivity in this setting enhances the glycosuric effect of SGLT2 inhibitors.7–12 However, any water diuresis that follows a tubular glucose load would be rapidly opposed by normal osmoregulatory mechanisms, such as thirst and the release of vasopressin, thus minimizing the durability and clinical importance of an osmotic effect.
Chronic Heart Failure With Diuretic Resistance
Two studies evaluated in the effect of SGLT2 inhibition in patients with heart failure who were diuretic-resistant. Yeoh et al81 evaluated 61 patients with heart failure who had fluid retention while receiving mean furosemide equivalent doses of ≈250 mg/d, who were randomized to dapagliflozin 10 mg/d or metolazone 5 to 10 mg/d; metolazone (but not dapagliflozin) potentiated the natriuretic effect of loop diuretics. Griffin et al74 randomized 20 patients with diabetes and chronic heart failure who were clinically euvolemic while receiving mean furosemide equivalent doses of 244 mg/d. Patients received empagliflozin 10 mg/d or placebo (double blind), each for 14 days, followed by crossover to the alternative treatment. SGLT2 inhibition produced an immediate increase in the fractional sodium excretion, which was still discerned after 14 days, and the natriuresis was enhanced by bumetanide but was unrelated to the magnitude of glycosuria. Wilcox et al61 and Heise et al66 also observed that SGLT2 inhibitors could potentiate the natriuretic effects of loop diuretics. Such potentiation may be related to the action of SGLT2 inhibitors to increase distal chloride delivery, thus sensitizing the Na+,K+,2Cl- cotransporter to pharmacological antagonism,84 yet potentiation of natriuresis was not observed in the patients with diuretic resistance and fluid retention who were studied by Yeoh et al.81
It is understood that the initial natriuretic response to any diuretic is typically attenuated as euvolemia is achieved as a result of the activation of counterregulatory mechanisms. The finding that fractional lithium clearance and glucose excretion persists during long-term SGLT2 inhibition indicates that pharmacological tolerance to the effects of these drugs on the proximal tubule does not develop.
EVALUATION OF PLASMA AND BLOOD VOLUME IN CLINICAL STUDIES OF SGLT2 INHIBITORS
Loop diuretics produce pulmonary decongestion in patients with heart failure by decreasing circulating plasma and blood volume, thus reducing cardiac filling pressures. Therefore, in characterizing the diuretic properties of SGLT2 inhibitors, studies of their effects on plasma volume are relevant.
Challenges in the Assessment of Plasma Volume During SGLT2 Inhibition
Plasma volumes can be measured using indicator dilution methods based on agents that bind to albumin. However, in most reports, plasma volume has been estimated indirectly, relying on formulae developed by Kaplan and Hakim for single assessments and by Strauss et al for paired assessments.85 The Kaplan formula is based on hematocrit and body weight, whereas the Strauss formula focuses on the ratios of hemoglobin and hematocrit. These formulas yield poor estimates of directly measured values86—often deviating by >1 liter87—and provide unreliable metrics of clinical congestion88 or decongestion.89 Furthermore, the Strauss formula assumes that red blood cell mass in the central circulation does not change between paired assessments.86,87 Because SGLT2 inhibitors stimulate erythropoietin and reticulocytosis within 7 days,90–92 the Strauss formula cannot be applied for the estimation of changes in plasma volume with these drugs. Even if there were no erythropoiesis, estimation of plasma volume by the Strauss formula can be distorted by shifts in the distribution of red blood cells from intravascular pools, as is commonly seen during the clinical course of patients with acutely decompensated heart failure.86–88,93
Even if measured accurately, decreases in plasma volume do not reliably reflect intravascular or total body decongestion. Diuretics may have little effect on or even increase plasma volume in heart failure, if they mobilize fluid from the interstitial space.89,93–95 Furthermore, any expansion of red blood cell mass triggers an adaptive reduction in plasma volume, because if plasma volume were to remain constant, erythrocytosis would result in intolerable hypervolemia. This principle explains why physiological or pharmacologically induced increases in hematocrit (regardless of cause) lead to decreases in plasma volume.96,97
Effect of SGLT2 Inhibitors on Plasma and Blood Volume in Clinical Studies
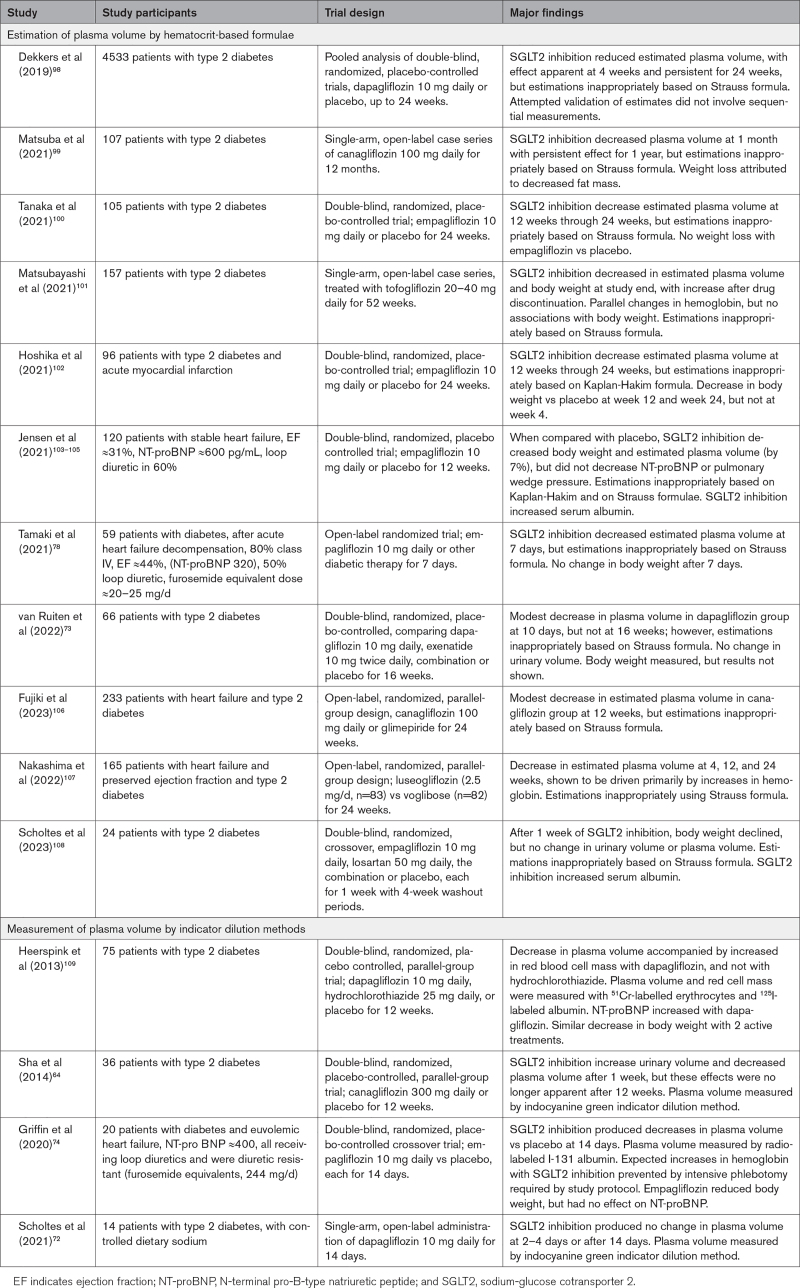
On the basis of a comprehensive review, we identified 15 studies that evaluated the effect of SGLT2 inhibitors on plasma volume (Table 2).64,72–74,78,98–109 Unfortunately, most studies estimated changes in plasma volume using the Kaplan-Hakim and Strauss formulae, which cannot be applied to the evaluation of changes produced by SGLT2 inhibitors. Four reports64,72,74,109 used indicator dilution methods, and of these, 3 were double blind and placebo controlled. In patients with type 2 diabetes, SGLT2 inhibition for 12 weeks reduced plasma volume in 1 trial,109 but not in another.64 In the third trial,74 SGLT2 inhibition decreased plasma volume in diuretic-resistant patients with heart failure after 2 weeks.
Table 2.
Studies Reporting Effect of SGLT2 Inhibitors on Plasma Volume
Decreases in the serum albumin concentration may reflect a dilution that occurs when plasma volume expands during sodium-avid states.110 SGLT2 inhibitors increase serum albumin in mechanistic studies and large-scale trials; the effects are seen after a few weeks and persist for the duration of treatment.23,103,108,110 When SGLT2 inhibitors are discontinued after long-term therapy, serum albumin falls within 7 days.111
These observations indicate that SGLT2 inhibitors likely lead to a short- and long-term decrease in plasma volume (Figure 2). However, it is not clear whether this effect is related to the action of these drugs to promote a diuresis or an erythrocytosis. Regardless, as a result of SGLT2 inhibitor–stimulated erythropoiesis, the increase in red blood cell mass offsets any ability of a decrease in plasma volume to be translated into a change in total circulating blood volume during long-term therapy. SGLT2 inhibition reduced estimated total blood volume by only 1% in the only study in which it was evaluated.112
The lack of a meaningful effect on total blood volume may explain why SGLT2 inhibition did not reduce NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels in the 5 placebo-controlled trials in patients with heart failure summarized in Table 1, whereas NT-proBNP levels are reduced by loop diuretics.113,114 In large-scale heart failure trials, SGLT2 inhibition for 3 months produces only modest changes in natriuretic peptides and left ventricular filling pressures (even in patients with volume overload), with little correlation between changes in natriuretic peptides and metrics of decongestion.105,115–117 Even in acutely decompensated heart failure, changes in NT-proBNP are not apparent79 or are modest and not sustained.118
EVALUATION OF EXTRACELLULAR FLUID IN CLINICAL STUDIES OF SGLT2 INHIBITORS
Most of the excess fluid in sodium-avid states is retained in the interstitial compartment, where it is manifested as edema.119 Diuretics alleviate fluid retention by reducing intravascular volume, which promotes the movement of sodium and water from the interstitial space into the circulation, followed by excretion through the kidneys. Interstitial fluid is mobilized through both plasma transcapillary refill and the action of lymphatics, which drain the interstitial space, moving fluid into the great veins.120 Lymphatic mobilization is gradient-dependent, and increases in right-sided filling pressures can impair both plasma transcapillary refill and lymphatic fluid uptake and duct flow;120,121 lymphatic congestion within the kidney may also impair sodium excretion.122 Therefore, by reducing right-sided filling pressures, loop diuretics not only enhance plasma transcapillary refill but also markedly increase lymphatic flows and drainage of the interstitial space,123 allowing a reduction in total body extracellular water and edema.89
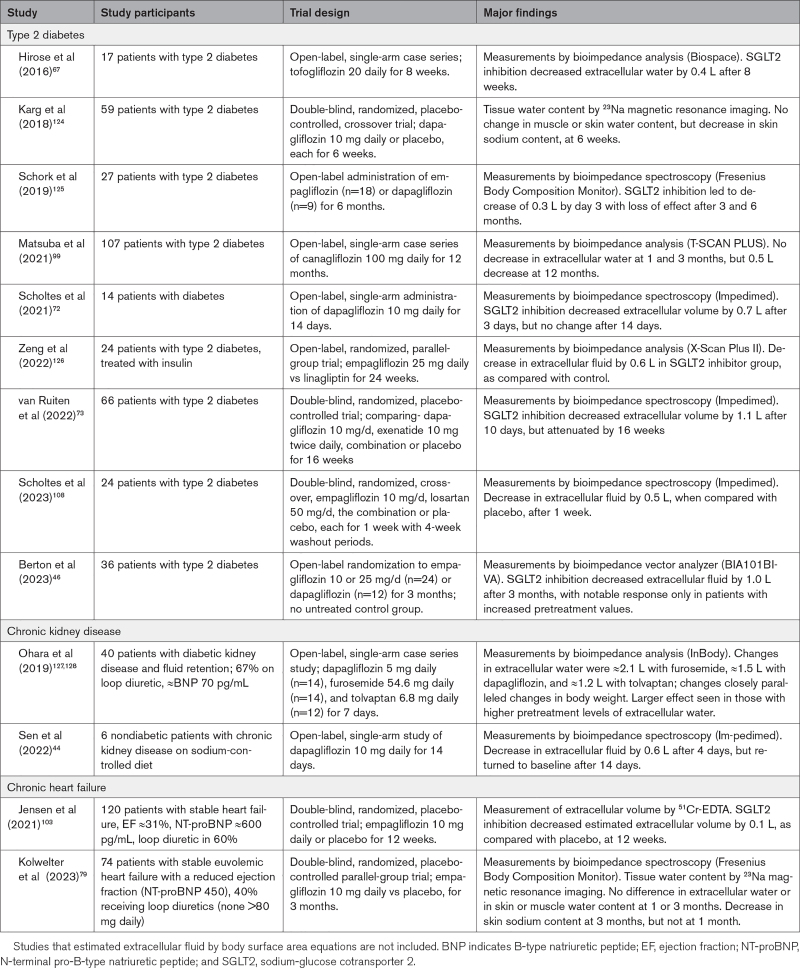
Effect of SGLT2 Inhibitors on Extracellular Fluid in Clinical Studies
On the basis of a comprehensive review, we identified 13 studies that evaluated the effects of SGLT2 inhibitors on extracellular water (Table 3).44,46,67,72,73,79,99,103,108,124–128 These reports performed measurements using diverse methods, typically without a control group, making it difficult to reliably discern a treatment effect. In 5 trials that were double blind and placebo controlled, SGLT2 inhibition modestly decreased extracellular fluid in patients with diabetes or heart failure after 1 to 2 weeks, but with little effect after 1 to 3 months. Meaningful responses were noted only in patients with pretreatment fluid retention,46,127,128 but these reports were not double blind or placebo controlled.
Table 3.
Studies Reporting Effect of SGLT2 Inhibitors on Extracellular Water
Some investigators have used computer modeling to project the effect of SGLT2 inhibitors on interstitial fluid and concluded that these drugs deplete extracellular water more profoundly than conventional diuretics.2 These models assumed that SGLT2 inhibitors act primarily as aquaretics to increase serum osmolarity, a finding not confirmed by clinical studies,2,129 and the models also did not consider the effect of enhanced erythropoiesis to maintain total blood volume. The conclusions of these models are inconsistent with the efficacy of conventional diuretics in edematous states.89,130
It has been hypothesized that the interstitium contains nonosmotically active sodium, which has adverse effects on vascular function.131 SGLT2 inhibitors may reduce skin (but not muscle) sodium content after 1 and 3 months of treatment,79,124 but it is not clear that these measurements reflect nonosmotic stores of the ion.132 Nonosmotic stores of sodium might be a determinant of vascular resistance, but not of edema.132,133
EFFECT OF SGLT2 INHIBITORS ON EDEMA AND CONGESTION
The primary end point for the regulatory approval of diuretics is the alleviation of edema in sodium-avid states. Thiazide and loop diuretics, mineralocorticoid receptor antagonists, and vasopressin antagonists ameliorate fluid retention in heart failure, hepatic cirrhosis, and nephrotic syndrome.134–137 In contrast, there are no reports of a favorable effect of SGLT2 inhibitors to reduce the edema of heart failure or the ascites of hepatic cirrhosis. Observations about decongestion are difficult to interpret when patients are being concurrently treated with an intensified regimen of loop diuretics.81
It is interesting that in patients hospitalized for heart failure, there may be little relationship between the magnitude of natriuresis and the relief of symptoms. Intensive intravenous loop diuretics produce a greater diuresis but not greater effects on pulmonary congestion.138 The addition of hydrochlorothiazide to a loop diuretic yields a modest diuretic effect, but no incremental benefit on dyspnea.139 Acetazolamide potentiates the natriuretic response to loop diuretics but without a reported benefit on symptoms.140 Conventional diuretics may preferentially act to remove excess fluid from the interstitial space, with little change in total blood volume, cardiac filling pressures, or symptoms.89
Effect of SGLT2 Inhibitors on Congestion in Acutely Decompensated Heart Failure
Three double-blind, placebo-controlled trials have evaluated the effects of SGLT2 inhibitors in patients with acutely decompensated heart failure, treated for ≤90 days: EMPAG-HF (Empagliflozin in Acute Decompensated Heart Failure),80 EMPA-RESPONSE-AHF (Empagliflozin Response Acute Heart Failure),12,77 and EMPULSE (Empagliflozin in Patients Hospitalized for Acute Heart Failure).118,141 The largest trial (EMPULSE [Empagliflozin in Patients Hospitalized for Acute Heart Failure]) enrolled 530 patients (NT-proBNP ≈3200 pg/mL) who (after in-hospital stabilization) were randomized to placebo or empagliflozin (10 mg/d) for 3 months. Empagliflozin reduced a composite symptom score, with benefits seen as early as 15 days, but with modest changes in NT-proBNP. Although patients were required to have pulmonary congestion or peripheral edema at randomization, the effects of empagliflozin on signs of fluid retention were not reported. Treatment with empagliflozin did not spare the use of loop diuretics; the number of patients receiving furosemide (>40 mg/d) was numerically more frequent in the empagliflozin group. Changes in body weight and hematocrit could not be ascribed to a diuresis, because they can be influenced by the rapid effects of empagliflozin to induce urinary caloric loss and erythropoiesis.
How did SGLT2 inhibition improve symptoms in patients with acutely decompensated heart failure at 15 days? Although urinary volume and sodium excretion were not measured in the EMPULSE trial, 2 earlier trials performed these assessments in the same clinical setting. In the EMPAG-HF trial,80 60 patients were randomized within 12 hours of admission (NT-proBNP ≈4800 pg/mL) to placebo or empagliflozin for 5 days. In the EMPA-RESPONSE-AHF trial,12,77 79 patients were randomized within 24 hours of admission (NT-proBNP ≈5200 pg/mL) to placebo or empagliflozin for 30 days. In both studies, SGLT2 inhibition increased urinary volume without an effect on fractional sodium excretion, total urinary sodium excretion, body weight, or NT-proBNP. Neither trial reported changes in edema or in signs of pulmonary congestion. These findings suggest that the water diuresis produced by SGLT2 inhibitors in patients with acutely decompensated heart failure is insufficiently durable to reduce body weight or NT-proBNP. Similarly, although urinary assessments were not performed, it seems unlikely that a water diuresis contributed to the findings in the EMPULSE trial, because empagliflozin reduced serum sodium concentration in that study, an effect that is inconsistent with an increase in free water clearance.141
Early Effects of SGLT2 Inhibitors in Chronic Heart Failure
Rather than reflecting a diuresis, the effect of SGLT2 inhibitors to improve symptoms at 15 days in the EMPULSE trial parallels a similar early reduction in symptoms and in the risk of hospitalization for heart failure in large-scale trials, which reaches nominal levels of statistical significance within 2 to 4 weeks.142,143 Some investigators have proposed that this statistical event supports an action of SGLT2 inhibitors to promote a diuresis.143 However, a similar early statistical event on heart failure hospitalizations is seen after the initiation of sacubitril/valsartan,144 although the drug does not produce a natriuretic effect, even when combined with loop diuretics.145,146 Early-onset statistical significance is also seen with β-blockers,147 although these drugs typically promote early sodium retention. Because the benefits of SGLT2 inhibitors in heart failure are not time dependent,148 the achievement of early statistical significance is merely a function of the duration of follow-up required to accrue a sufficient number of events rather than an indicator of a mechanism of action. It is therefore noteworthy that the effect of SGLT2 inhibitors to induce nutrient deprivation signaling and promote autophagy emerges rapidly3,4 and are poised to make a meaningful difference in patients who are acutely ill or on the brink of decompensation at the time of randomization.
Additional analyses of the large-scale trials of SGLT2 inhibitors provide further evidence against a significant early diuretic effect of these drugs. In the EMPEROR-Reduced trial, 40% of the patients had recent volume overload before study enrollment and received larger doses of loop diuretics at baseline.117 However, those with recent volume overload were not more likely to show a reduction in cardiovascular death or hospitalization for heart failure with empagliflozin. The incidence plots separated immediately in patients without recent volume overload, but this separation occurred after 30 to 45 days in those with recent volume overload. Patients with recent volume overload were not more likely to show an improvement in functional class, decreases in body weight or natriuretic peptides, or increases in hematocrit during the first 4 weeks of treatment.
Some might ascribe a diuretic effect to SGLT2 inhibitors if these drugs were to influence the dose requirements of concurrently administered loop diuretics. In a 3-day randomized controlled trial of diuretic-resistant individuals, patients receiving dapagliflozin required more furosemide to achieve a smaller diuresis than those receiving metolazone.81 In the large-scale landmark trials with dapagliflozin and empagliflozin in patients with chronic heart failure, the dose of prescribed diuretics did not change in most patients.117,149 Although SGLT2 inhibition reduced the need for diuretic intensification and increased the likelihood of diuretic dose reduction during long-term therapy,117,149 only ≈5% of randomized patients experienced a change in diuretic dose that was attributable to SGLT2 inhibition, and drug-induced changes in diuretic dosing were not seen during the first 90 days.150 Long-term changes in the doses of prescribed diuretics do not imply a diuretic action, because the use of diuretics (and the prevention of new-onset edema150) is influenced by an effect of SGLT2 inhibitors to slow the progression of heart failure. A similar pattern of reduced intensification and greater dose reduction has been observed in large-scale trials with angiotensin receptor blockers and sacubitril/valsartan, which do not exert a diuretic effect in chronic heart failure.151,152
It is noteworthy that treatments that produce short-term increases in sodium or water excretion do not generally reduce the subsequent risk of cardiovascular death or heart failure hospitalization. Although intensive short-term diuresis to achieve hemoconcentration in hospitalized patients is associated with lower cardiac filling pressures,153 early decongestion has not been associated with improved outcomes in observational studies or clinical trials.138–141,154,155 More intensive treatment with intravenous loop diuretics yields a greater short-term diuresis, but does not reduce heart failure events at 60 days.138 The addition of hydrochlorothiazide to a loop diuretic yields a modest diuretic effect, but no effect on death or rehospitalization at 90 days.139 Acetazolamide potentiates the natriuretic response to loop diuretics, but has no effect on morbidity and mortality at 3 months.141 In a trial comparing short-term therapy with low or high doses of spironolactone, there was no between-group difference in major heart failure events after 30 to 60 days.156 Last, in a large-scale trial in patients hospitalized for heart failure, vasopressin antagonism induced a short-term diuretic effect, but continued treatment for a median of 10 months did not reduce the risk of cardiovascular death or hospitalization for heart failure.136,157
These observations, taken collectively, suggest that a short-term diuretic effect of SGLT2 inhibitors does not contribute meaningfully to the early or long-term ability of these drugs to reduce the risk of major heart failure events.
Effect of SGLT2 Inhibitors on the Risk of Volume Depletion
As a result of their potent natriuretic effects, loop diuretics can produce volume depletion (hypotension and worsening renal function), especially in patients receiving inhibitors of the renin-angiotensin system.158 Because SGLT2 inhibitors are frequently coadministered with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, the risk of volume depletion has been prospectively evaluated in large-scale trials.
In a meta-analysis159 of 8 major cardiovascular outcomes trials, volume depletion was reported in 4.4% of the placebo group and in 4.5% of the SGLT2 inhibitor group. The DAPA-HF trial noted an excess of volume depletion, but only in patients taking furosemide-equivalent doses ≥40 mg daily.149 In patients not taking a loop diuretic, volume depletion was seen in 8.5% in the placebo group and 4.3% in the dapagliflozin group, whereas in patients receiving a furosemide-equivalent dose ≥40 mg daily, volume depletion was seen in 6.8% of placebo-treated patients and 9.0% of dapagliflozin-treated patients (treatment-by-furosemide dose interaction, P=0.012). A similar pattern of diuretic potentiation was seen in a pooled analysis of double-blind trials in type 2 diabetes.160
Summary and Conclusions
SGLT2 inhibitors block proximal tubular sodium and glucose reabsorption, but the nature and duration of any natriuretic or osmotic diuretic effect are the result of an interplay of the degree of SGLT2 and NHE3 upregulation, the extent to which downstream nephron mechanisms are activated in a compensatory manner, and the volume status and set point in individual patients. In euvolemic patients, counterregulatory sodium- and water-retaining downstream mechanisms are activated rapidly, thus truncating the duration of any observable diuresis. In fluid overloaded patients with acutely decompensated heart failure, the net early effect of SGLT2 inhibition is a water diuresis, but this aquaresis does not contribute to the early clinical improvement in these patients. The magnitude of any glycosuria-dependent effect is markedly attenuated in patients with a glomerular filtration rate <45 mL/min per 1.73 m2,15 and yet these patients still show a robust reduction in the risk of heart failure hospitalizations with SGLT2 inhibitors.161,162 In patients with chronic heart failure, SGLT2 inhibitors may potentiate the natriuretic effect of loop diuretics, but in the short term, in patients with fluid retention, the effect of SGLT2 inhibitors to enhance the effect of loop diuretics appears to be smaller than with metolazone.81 There is little evidence that SGLT2 inhibitors alleviate edema or physical signs of congestion.
It should be noted that attenuation of the initial urinary response is an expected finding with any diuretic, because compensatory mechanisms must be activated to prevent volume depletion as patients approach euvolemia. Once volume overload is alleviated by loop diuretics, a new equilibrium is achieved, with sodium excretion matching intake. The goal of diuretic therapy is to change the internal set point, so that salt and water homeostasis is maintained at steady-state conditions characterized by diminished total body sodium and water stores.163 The actions of SGLT2 inhibitors to increase fractional lithium excretion and promote glycosuria persist during long-term therapy, even when the volume status of patients is not changing, suggesting that SGLT2 inhibitors are poised to exert a durable effect to modulate the set point for volume homeostasis. If aberrations of sodium or water intake were to occur during long-term treatment, the activation of downstream counterregulatory mechanisms diminishes, and a net diuretic effect of the drugs would become clinically evident until the set point for volume homeostasis is reachieved.42 Studies evaluating the volume responses after salt loading or discontinuation of long-term therapy with SGLT2 inhibitors are needed to confirm this hypothesis. Yet, assuming that the effect of SGLT2 inhibitor on volume homeostasis occur and are durable, it is difficult to know if treatment-mediated changes in the volume set point (if any) contribute to the effect of these drugs to reduce the risk of major heart failure events. Short- or long-term use of many conventional natriuretic and aquaretic drugs has not reduced cardiovascular death or hospitalizations for heart failure,136,138–140,157 demonstrating that immediate changes in urinary sodium or water excretion do not yield long-term clinical benefits. It is possible that the effects of SGLT2 inhibitors on sodium avidity may differ from other agents, but the volume effect of these drugs is small. In contrast, the cardioprotective effects of SGLT2 inhibitors represent a direct beneficial effect to reduce cardiomyocyte stress and injury, which is independent of the binding to SGLT2 in the heart or the presence or actions of these drugs on SGLT2 in the proximal renal tubule.3–5 Nevertheless, any durable changes in the volume set point produced by SGLT2 inhibitors might potentially act cooperatively with the direct favorable molecular and cellular cardiac effects of these drugs to mediate their benefits on the clinical course of heart failure.
ARTICLE INFORMATION
Sources of Funding
None.
Disclosures
M.P. reports consulting fees from 89Bio, Abbvie, Altimmune, Amgen, Ardelyx, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Lilly, Moderna, Novartis, Reata, Regeneron, Relypsa, and Salamandra. J.M.T. reports grants or personal fees from 3iveLabs, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Astra Zeneca, Novartis, Cardionomic, MagentaMed, Reprieve Inc, FIRE1, W.L. Gore, Sanofi, Sequana Medical, Otsuka, Abbott, Merck, Windtree Therapeutics, Lexicon, Precardia, Relypsa, Regeneron, BD, Edwards Life Sciences, and Lilly. In addition, J.M.T. has a patent for treatment of diuretic resistance issued to Yale University and Corvidia Therapeutics Inc, a patent for methods for measuring renalase issued to Yale University, and a patent for treatment of diuretic resistance pending with Reprieve Inc. The other author reports no conflicts.
Nonstandard Abbreviations and Acronyms
- EMPAG-HF
- Empagliflozin in Acute Decompensated Heart Failure
- EMPA-
- Empagliflozin Response Acute RESPONSE-AHF Heart Failure
- EMPULSE
- Empagliflozin in Patients Hospitalized for Acute Heart Failure
- NHE3
- sodium-hydrogen exchanger isoform 3
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- SGLT2
- sodium-glucose cotransporter 2
- WNK4
- with no lysine 4
For Sources of Funding and Disclosures, see page 367.
Circulation is available at www.ahajournals.org/journal/circ.
Contributor Information
Christopher S. Wilcox, Email: wilcoxch@georgetown.edu.
Jeffrey M. Testani, Email: jeffrey.testani@yale.edu.
REFERENCES
- 1.Giugliano D, Longo M, Scappaticcio L, Bellastella G, Maiorino MI, Esposito K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs. Cardiovasc Diabetol. 2021;20:236. doi: 10.1186/s12933-021-01430-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/dom.13126 [DOI] [PubMed] [Google Scholar]
- 3.Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146:1383–1405. doi: 10.1161/CIRCULATIONAHA.122.061732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer M. SGLT2 inhibitors induce protective reprogramming of cardiac nutrient transport and metabolism. Nat Rev Cardiol. 2023;20:443–462. doi: 10.1038/s41569-022-00824-4 [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Wang Q, Christodoulou A, Mylonas N, Bakker D, Nederlof R, Hollmann MW, Weber NC, Coronel R, Wakker V, et al. Sodium glucose cotransporter-2 inhibitor empagliflozin reduces infarct size independently of sodium glucose cotransporter-2. Circulation. 2023;147:276–279. doi: 10.1161/CIRCULATIONAHA.122.061688 [DOI] [PubMed] [Google Scholar]
- 6.Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, Wang D, Peng Y, Grenz A, Lucia S, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem. 2017;29:5335–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsurada K, Nandi SS, Sharma NM, Patel KP. Enhanced expression and function of renal SGLT2 (sodium-glucose cotransporter 2) in heart failure: role of renal nerves. Circ Heart Fail. 2021;14:e008365. doi: 10.1161/CIRCHEARTFAILURE.121.008365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges-Júnior FA, Silva Dos Santos D, Benetti A, Polidoro JZ, Wisnivesky ACT, Crajoinas RO, Antônio EL, Jensen L, Caramelli B, Malnic G, et al. Empagliflozin inhibits proximal tubule NHE3 activity, preserves GFR, and restores euvolemia in nondiabetic rats with induced heart failure. J Am Soc Nephrol. 2021;32:1616–1629. doi: 10.1681/ASN.2020071029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafiq K, Fujisawa Y, Sherajee SJ, Rahman A, Sufiun A, Kobori H, Koepsell H, Mogi M, Horiuchi M, Nishiyama A. Role of the renal sympathetic nerve in renal glucose metabolism during the development of type 2 diabetes in rats. Diabetologia. 2015;58:2885–2898. doi: 10.1007/s00125-015-3771-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo JY, Jiang WY, Zhang SG, Lyu YT, Geng J, Chen M, Chen YY, Jiang ZX, Shan QJ. Renal denervation ameliorates cardiac metabolic remodeling in diabetic cardiomyopathy rats by suppressing renal SGLT2 expression. Lab Invest. 2022;102:341–351. doi: 10.1038/s41374-021-00696-1 [DOI] [PubMed] [Google Scholar]
- 11.Jönsson S, Agic MB, Narfström F, Melville JM, Hultström M. Renal neurohormonal regulation in heart failure decompensation. Am J Physiol Regul Integr Comp Physiol. 2014;307:R493–R497. doi: 10.1152/ajpregu.00178.2014 [DOI] [PubMed] [Google Scholar]
- 12.Boorsma EM, Beusekamp JC, Ter Maaten JM, Figarska SM, Danser AHJ, van Veldhuisen DJ, van der Meer P, Heerspink HJL, Damman K, Voors AA. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. 2021;23:68–78. doi: 10.1002/ejhf.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol. 2016;310:F1269–F1283. doi: 10.1152/ajprenal.00543.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol. 2018;314:F969–F984. doi: 10.1152/ajprenal.00551.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu S, Lin C, Cai X, Zhu X, Lv F, Nie L, Ji L. The urinary glucose excretion by sodium-glucose cotransporter 2 inhibitor in patients with different levels of renal function: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;12:814074. doi: 10.3389/fendo.2021.814074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969 [DOI] [PubMed] [Google Scholar]
- 17.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol. 2014;25:2028–2039. doi: 10.1681/ASN.2013060588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, Dumayne C, Bichet DG, Lapointe JY. MAP17 Is a necessary activator of renal Na+/glucose cotransporter SGLT2. J Am Soc Nephrol. 2017;28:85–93. doi: 10.1681/ASN.2015111282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2020;319:F712–F728. doi: 10.1152/ajprenal.00264.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl). 2013;91:951–963. doi: 10.1007/s00109-013-1015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrykiv S, Sjöström CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12:751–759. doi: 10.2215/CJN.10180916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segar MW, Kolkailah AA, Frederich R, Pong A, Cannon CP, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley RE, Liu CC, et al. Mediators of ertugliflozin effects on heart failure and kidney outcomes among patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2022;24:1829–1839. doi: 10.1111/dom.14769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol. 2001;281:F718–F727. doi: 10.1152/ajprenal.2001.281.4.F718 [DOI] [PubMed] [Google Scholar]
- 25.Perry RJ, Rabin-Court A, Song JD, Cardone RL, Wang Y, Kibbey RG, Shulman GI. Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun. 2019;10:548. doi: 10.1038/s41467-019-08466-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol. 2015;308:F1343–F1357. doi: 10.1152/ajprenal.00007.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura S, Amlal H, Schultheis PJ, Galla JH, Shull GE, Soleimani M. HCO-3 reabsorption in renal collecting duct of NHE-3-deficient mouse: a compensatory response. Am J Physiol. 1999;276:F914–F921. doi: 10.1152/ajprenal.1999.276.6.F914 [DOI] [PubMed] [Google Scholar]
- 28.Zannad F, Ferreira JP, Butler J, Filippatos G, Januzzi JL, Sumin M, Zwick M, Saadati M, Pocock SJ, Sattar N, et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights from the EMPEROR program. Eur Heart J. 2022;43:4991–5002. doi: 10.1093/eurheartj/ehac495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seki G, Frömter E. Acetazolamide inhibition of basolateral Cl-/HCO3- exchange in rabbit renal proximal tubule S3 segment. Pflugers Arch. 1992;422:55–59. doi: 10.1007/BF00381513 [DOI] [PubMed] [Google Scholar]
- 30.Tsuruoka S, Kittelberger AM, Schwartz GJ. Carbonic anhydrase II and IV mRNA in rabbit nephron segments: stimulation during metabolic acidosis. Am J Physiol. 1998;274:F259–F267. doi: 10.1152/ajprenal.1998.274.2.F259 [DOI] [PubMed] [Google Scholar]
- 31.Mary S, Boder P, Padmanabhan S, McBride MW, Graham D, Delles C, Dominiczak AF. Role of uromodulin in salt-sensitive hypertension. Hypertension. 2022;79:2419–2429. doi: 10.1161/HYPERTENSIONAHA.122.19888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanchi A, Burnier M, Muller ME, Ghajarzadeh-Wurzner A, Maillard M, Loncle N, Milani B, Dufour N, Bonny O, Pruijm M. Acute and chronic effects of SGLT2 inhibitor empagliflozin on renal oxygenation and blood pressure control in nondiabetic normotensive subjects: a randomized, placebo-controlled trial. J Am Heart Assoc. 2020;9:e016173. doi: 10.1161/JAHA.119.016173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahena-Lopez JP, Rojas-Vega L, Chávez-Canales M, Bazua-Valenti S, Bautista-Pérez R, Lee JH, Madero M, Vazquez-Manjarrez N, Alquisiras-Burgos I, Hernandez-Cruz A, et al. Glucose/fructose delivery to the distal nephron activates the sodium-chloride cotransporter via the calcium-sensing receptor. J Am Soc Nephrol. 2023;34:55–72. doi: 10.1681/ASN.2021121544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, et al. α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest. 2013;123:3166–3171. doi: 10.1172/JCI67562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazo-Fernandez Y, Welling PA, Wall SM. α-Ketoglutarate stimulates pendrin-dependent Cl- absorption in the mouse CCD through protein kinase C. Am J Physiol Renal Physiol. 2018;315:F7–F15. doi: 10.1152/ajprenal.00576.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda T, Ohara K, Vallon V, Nagata D. SGLT2 inhibitor and loop diuretic induce different vasopressin and fluid homeostatic responses in nondiabetic rats. Am J Physiol Renal Physiol. 2022;323:F361–F369. doi: 10.1152/ajprenal.00070.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholtes RA, Muskiet MHA, van Baar MJB, Hesp AC, Greasley PJ, Hammarstedt A, Karlsson C, Hallow KM, Danser AHJ, Heerspink HJL, et al. The adaptive renal response for volume homeostasis during 2 weeks of dapagliflozin treatment in people with type 2 diabetes and preserved renal function on a sodium-controlled diet. Kidney Int Rep. 2022;7:1084–1092. doi: 10.1016/j.ekir.2022.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuda T, Muto S, Fukuda K, Watanabe M, Ohara K, Koepsell H, Vallon V, Nagata D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep. 2020;8:e14360. doi: 10.14814/phy2.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lytvyn Y, Bjornstad P, Katz A, Singh SK, Godoy LC, Chung LT, Vinovskis CL, Pyle L, Roussel R, Perkins BA, et al. SGLT2 inhibition increases serum copeptin in young adults with type 1 diabetes. Diabetes Metab. 2020;46:203–209. doi: 10.1016/j.diabet.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eickhoff MK, Dekkers CCJ, Kramers BJ, Laverman GD, Frimodt-Møller M, Jørgensen NR, Faber J, Danser AHJ, Gansevoort RT, Rossing P, et al. Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med. 2019;8:779. doi: 10.3390/jcm8060779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeoh SE, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, et al. Relationship of dapagliflozin with serum sodium: findings from the DAPA-HF trial. JACC Heart Fail. 2022;10:306–318. doi: 10.1016/j.jchf.2022.01.019 [DOI] [PubMed] [Google Scholar]
- 42.Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation. 2020;142:1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachmann S, Mutig K. Regulation of renal Na-(K)-Cl cotransporters by vasopressin. Pflugers Arch. 2017;469:889–897. doi: 10.1007/s00424-017-2002-2 [DOI] [PubMed] [Google Scholar]
- 44.Sen T, Scholtes R, Greasley PJ, Cherney DZI, Dekkers CCJ, Vervloet M, Danser AHJ, Barbour SJ, Karlsson C, Hammarstedt A, et al. Effects of dapagliflozin on volume status and systemic haemodynamics in patients with chronic kidney disease without diabetes: results from DAPASALT and DIAMOND. Diabetes Obes Metab. 2022;24:1578–1587. doi: 10.1111/dom.14729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimm PR, Welling PA. α-Ketoglutarate drives electroneutral NaCl reabsorption in intercalated cells by activating a G-protein coupled receptor, Oxgr1. Curr Opin Nephrol Hypertens. 2017;26:426–433. doi: 10.1097/MNH.0000000000000353 [DOI] [PubMed] [Google Scholar]
- 46.Berton AM, Parasiliti-Caprino M, Prencipe N, Bioletto F, Lopez C, Bona C, Caputo M, Rumbolo F, Ponzetto F, Settanni F, et al. Copeptin adaptive response to SGLT2 inhibitors in patients with type 2 diabetes mellitus: the GliRACo study. Front Neurosci. 2023;17:1098404. doi: 10.3389/fnins.2023.1098404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuen BL, Oshima M, Agarwal R, Arnott C, Cherney DZ, Edwards R, Langkilde AM, Mahaffey KW, McGuire DK, Neal B, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145:1460–1470. doi: 10.1161/CIRCULATIONAHA.121.057736 [DOI] [PubMed] [Google Scholar]
- 48.Ferreira JP, Zannad F, Butler J, Filipattos G, Ritter I, Schüler E, Kraus BJ, Pocock SJ, Anker SD, Packer M. Empagliflozin and serum potassium in heart failure: an analysis from EMPEROR-Pooled. Eur Heart J. 2022;43:2984–2993. doi: 10.1093/eurheartj/ehac306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/jci110820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnermann J, Ploth DW, Hermle M. Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch. 1976;362:229–240. doi: 10.1007/BF00581175 [DOI] [PubMed] [Google Scholar]
- 51.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020;97:202–212. doi: 10.1016/j.kint.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 52.Lytvyn Y, Kimura K, Peter N, Lai V, Tse J, Cham L, Perkins BA, Soleymanlou N, Cherney DZI. Renal and vascular effects of combined SGLT2 and angiotensin-converting enzyme inhibition. Circulation. 2022;146:450–462. doi: 10.1161/CIRCULATIONAHA.122.059150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanberg JS, Rao V, Ter Maaten JM, Laur O, Brisco MA, Perry Wilson F, Grodin JL, Assefa M, Samuel Broughton J, Planavsky NJ, et al. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016;9:1. doi: 0.1161/CIRCHEART FAILURE.116.003180 e003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ter Maaten JM, Damman K, Hanberg JS, Givertz MM, Metra M, O’Connor CM, Teerlink JR, Ponikowski P, Cotter G, Davison B, et al. Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circ Heart Fail. 2016;9:e003109. doi: 10.1161/CIRCHEARTFAILURE.116.003109 [DOI] [PubMed] [Google Scholar]
- 55.Terker AS, Castañeda-Bueno M, Ferdaus MZ, Cornelius RJ, Erspamer KJ, Su XT, Miller LN, McCormick JA, Wang WH, Gamba G, et al. With no lysine kinase 4 modulates sodium potassium 2 chloride cotransporter activity in vivo. Am J Physiol Renal Physiol. 2018;315:F781–F790. doi: 10.1152/ajprenal.00485.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kataoka H. Acetazolamide as a potent chloride-regaining diuretic: short- and long-term effects, and its pharmacologic role under the “chloride theory” for heart failure pathophysiology. Heart Vessels. 2019;34:1952–1960. doi: 10.1007/s00380-019-01433-x [DOI] [PubMed] [Google Scholar]
- 57.Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, Tartaglia K, Chenot F, Moubayed S, Dierckx R, et al. ; ADVOR Study Group. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387:1185–1195. doi: 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 58.van Bommel EJM, Geurts F, Muskiet MHA, Post A, Bakker SJL, Danser AHJ, Touw DJ, van Berkel M, Kramer MHH, Nieuwdorp M, et al. SGLT2 inhibition versus sulfonylurea treatment effects on electrolyte and acid-base balance: secondary analysis of a clinical trial reaching glycemic equipoise: tubular effects of SGLT2 inhibition in type 2 diabetes. Clin Sci (Lond). 2020;134:3107–3118. doi: 10.1042/CS20201274 [DOI] [PubMed] [Google Scholar]
- 59.Kataoka H, Yoshida Y. Enhancement of the serum chloride concentration by administration of sodium-glucose cotransporter-2 inhibitor and its mechanisms and clinical significance in type 2 diabetic patients: a pilot study. Diabetol Metab Syndr. 2020;12:5. doi: 10.1186/s13098-020-0515-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L, LaRocque L, Efe O, Wang J, Sands JM, Klein JD. Effect of dapagliflozin treatment on fluid and electrolyte balance in diabetic rats. Am J Med Sci. 2016;352:517–523. doi: 10.1016/j.amjms.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc. 2018;7:e007046. doi: 10.1161/JAHA.117.007046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilcox CS. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors. Hypertension. 2020;75:894–901. doi: 10.1161/HYPERTENSIONAHA.119.11684 [DOI] [PubMed] [Google Scholar]
- 63.Blau JE, Bauman V, Conway EM, Piaggi P, Walter MF, Wright EC, Bernstein S, Courville AB, Collins MT, Rother KI, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3:e99123. doi: 10.1172/jci.insight.99123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Mörschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–1095. doi: 10.1111/dom.12322 [DOI] [PubMed] [Google Scholar]
- 65.Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, Broedl UC. Acute pharmacodynamic effects of empagliflozin with and without diuretic agents in patients with type 2 diabetes mellitus. Clin Ther. 2016;38:2248–2264.e5. doi: 10.1016/j.clinthera.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 66.Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, Broedl UC. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:2265–2276. doi: 10.1016/j.clinthera.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 67.Hirose S, Nakajima S, Iwahashi Y, Seo A, Takahashi T, Tamori Y. Impact of the 8-week administration of tofogliflozin for glycemic control and body composition in japanese patients with type 2 diabetes mellitus. Intern Med. 2016;55:3239–3245. doi: 10.2169/internalmedicine.55.6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, Arakawa K, Togo M, Inagaki N, Kaku K. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–451. doi: 10.1007/s12325-016-0457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. doi: 10.1186/s12933-017-0621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, Kubozono T, Ohishi M. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18:23. doi: 10.1186/s40360-017-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Opingari E, Verma S, Connelly KA, Mazer CD, Teoh H, Quan A, Zuo F, Pan Y, Bhatt DL, Zinman B, et al. The impact of empagliflozin on kidney injury molecule-1: a subanalysis of the Effects of Empagliflozin on Cardiac Structure, Function, and Circulating Biomarkers in Patients with Type 2 Diabetes CardioLink-6 trial. Nephrol Dial Transplant. 2020;35:895–897. doi: 10.1093/ndt/gfz294 [DOI] [PubMed] [Google Scholar]
- 72.Scholtes RA, Muskiet MHA, van Baar MJB, Hesp AC, Greasley PJ, Karlsson C, Hammarstedt A, Arya N, van Raalte DH, Heerspink HJL. Natriuretic effect of two weeks of dapagliflozin treatment in patients with type 2 diabetes and preserved kidney function during standardized sodium intake: results of the DAPASALT trial. Diabetes Care. 2021;44:440–447. doi: 10.2337/dc20-2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Ruiten CC, Smits MM, Kok MD, Serné EH, van Raalte DH, Kramer MHH, Nieuwdorp M, IJzerman RG. Mechanisms underlying the blood pressure lowering effects of dapagliflozin, exenatide, and their combination in people with type 2 diabetes: a secondary analysis of a randomized trial. Cardiovasc Diabetol. 2022;21:63. doi: 10.1186/s12933-022-01492-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukuoka S, Dohi K, Takeuchi T, Moriwaki K, Ishiyama M, Omori T, Fujimoto N, Ito M. Mechanisms and prediction of short-term natriuretic effect of sodium-glucose cotransporter 2 inhibitor in heart failure patients coexisting type 2 diabetes mellitus. Heart Vessels. 2020;35:1218–1226. doi: 10.1007/s00380-020-01597-x [DOI] [PubMed] [Google Scholar]
- 76.Ikeda Y, Ishii S, Maemura K, Oki T, Yazaki M, Fujita T, Nabeta T, Maekawa E, Koitabashi T, Ako J. Glucose-dependent diuresis in relation to improvements in renal-tubular markers of sodium-glucose cotransporter-2 inhibitors in hospitalized heart failure patients with diabetes. Heart Vessels. 2021;36:978–985. doi: 10.1007/s00380-020-01768-w [DOI] [PubMed] [Google Scholar]
- 77.Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, van Eck JWM, Heerspink HJL, Voors AA. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22:713–722. doi: 10.1002/ejhf.1713 [DOI] [PubMed] [Google Scholar]
- 78.Tamaki S, Yamada T, Watanabe T, Morita T, Furukawa Y, Kawasaki M, Kikuchi A, Kawai T, Seo M, Abe M, et al. Effect of empagliflozin as an add-on therapy on decongestion and renal function in patients with diabetes hospitalized for acute decompensated heart failure: a prospective randomized controlled study. Circ Heart Fail. 2021;14:e007048. doi: 10.1161/CIRCHEARTFAILURE.120.007048 [DOI] [PubMed] [Google Scholar]
- 79.Kolwelter J, Kannenkeril D, Linz P, Jung S, Nagel AM, Bosch A, Ott C, Bramlage P, Nöh L, Schiffer M, et al. The SGLT2 inhibitor empagliflozin reduces tissue sodium content in patients with chronic heart failure: results from a placebo-controlled randomised trial. Clin Res Cardiol. 2023;112:134–144. doi: 10.1007/s00392-022-02119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S, von Haehling S, Schumacher U, Möbius-Winkler S, Busch M. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF). Circulation. 2022;146:289–298. doi: 10.1161/CIRCULATIONAHA.122.059038 [DOI] [PubMed] [Google Scholar]
- 81.Yeoh SE, Osmanska J, Petrie MC, Brooksbank KJM, Clark AL, Docherty KF, Foley PWX, Guha K, Halliday CA, Jhund PS, et al. Dapagliflozin versus metolazone in heart failure resistant to loop diuretics [published online May 21, 2023]. Eur Heart J. 2023;ehad341. doi: 10.1093/eurheartj/ehad341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahloulay M, Schmitt F, Déchaux M, Bankir L. Vasopressin and urinary concentrating activity in diabetes mellitus. Diabetes Metab. 1999;25:213–222. [PubMed] [Google Scholar]
- 84.Wilcox CS, Testani JM, Pitt B. Pathophysiology of diuretic resistance and its implications for the management of chronic heart failure. Hypertension. 2020;76:1045–1054. doi: 10.1161/HYPERTENSIONAHA.120.15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest. 1951;30:862–868. doi: 10.1172/JCI102501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahlgrim C, Birkner P, Seiler F, Grundmann S, Bode C, Pottgiesser T. Estimated plasma volume status is a modest predictor of true plasma volume excess in compensated chronic heart failure patients. Sci Rep. 2021;11:24235. doi: 10.1038/s41598-021-03769-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fudim M, Miller WL. Calculated estimates of plasma volume in patients with chronic heart failure-comparison with measured volumes. J Card Fail. 2018;24:553–560. doi: 10.1016/j.cardfail.2018.07.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuthbert JJ, Pellicori P, Rigby AS, Abel AAI, Kalvickbacka-Bennet A, Shah P, Kearsley JW, Kazmi S, Cleland JGF, Clark AL. Are non-invasive estimations of plasma volume an accurate measure of congestion in patients with chronic heart failure? Eur Heart J Qual Care Clin Outcomes. 2022;20:qcac035. doi: 10.1093/ehjqcco/qcac035 [DOI] [PubMed] [Google Scholar]
- 89.Miller WL, Lobo R, Grill DE, Mullan BP. Diuresis-related weight loss reflects interstitial compartment decongestion with minimal impact on intravascular volume expansion or outcomes in post-acute heart failure: metrics of decongestion and volume status. J Card Fail. 2021;27:445–452. doi: 10.1016/j.cardfail.2020.12.006 [DOI] [PubMed] [Google Scholar]
- 90.Yamada T, Sakaguchi K, Okada Y, Miura H, Otowa-Suematsu N, So A, Komada H, Hirota Y, Ohara T, Kuroki Y, et al. Analysis of time-dependent alterations of parameters related to erythrocytes after ipragliflozin initiation. Diabetol Int. 2020;12:197–206. doi: 10.1007/s13340-020-00474-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuchs Andersen C, Omar M, Glenthøj A, El Fassi D, Møller HJ, Lindholm Kurtzhals JA, Styrishave B, Kistorp C, Tuxen C, Poulsen MK, et al. Effects of empagliflozin on erythropoiesis in heart failure: data from the Empire HF trial. Eur J Heart Fail. 2023;25:226–234. doi: 10.1002/ejhf.2735 [DOI] [PubMed] [Google Scholar]
- 92.Aberle J, Menzen M, Schmid SM, Terkamp C, Jaeckel E, Rohwedder K, Scheerer MF, Xu J, Tang W, Birkenfeld AL. Dapagliflozin effects on haematocrit, red blood cell count and reticulocytes in insulin-treated patients with type 2 diabetes. Sci Rep. 2020;10:22396. doi: 10.1038/s41598-020-78734-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swolinsky JS, Tuvshinbat E, Leistner DM, Edelmann F, Knebel F, Nerger NP, Lemke C, Roehle R, Haase M, Costanzo MR, et al. Discordance between estimated and measured changes in plasma volume among patients with acute heart failure. ESC Heart Fail. 2022;9:66–76. doi: 10.1002/ehf2.13739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schuster CJ, Weil MH, Besso J, Carpio M, Henning RJ. Blood volume following diuresis induced by furosemide. Am J Med. 1984;76:585–592. doi: 10.1016/0002-9343(84)90281-x [DOI] [PubMed] [Google Scholar]
- 95.Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail. 2014;2:298–305. doi: 10.1016/j.jchf.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 96.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne A-S. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a [DOI] [PubMed] [Google Scholar]
- 97.Borovka M, Teruya S, Alvarez J, Helmke S, Maurer MS. Differences in blood volume components between hyporesponders and responders to erythropoietin alfa: the heart failure with preserved ejection fraction (HFPEF) anemia trial. J Card Fail. 2013;19:685–691. doi: 10.1016/j.cardfail.2013.08.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dekkers CCJ, Sjöström CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:2667–2673. doi: 10.1111/dom.13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsuba I, Takihata M, Takai M, Maeda H, Kubota A, Iemitsu K, Umezawa S, Obana M, Kaneshiro M, Kawata T, et al. Effects of 1-year treatment with canagliflozin on body composition and total body water in patients with type 2 diabetes. Diabetes Obes Metab. 2021;23:2614–2622. doi: 10.1111/dom.14508 [DOI] [PubMed] [Google Scholar]
- 100.Tanaka A, Shimabukuro M, Teragawa H, Okada Y, Takamura T, Taguchi I, Toyoda S, Tomiyama H, Ueda S, Higashi Y, et al. ; EMBLEM Investigators. Reduction of estimated fluid volumes following initiation of empagliflozin in patients with type 2 diabetes and cardiovascular disease: a secondary analysis of the placebo-controlled, randomized EMBLEM trial. Cardiovasc Diabetol. 2021;20:105. doi: 10.1186/s12933-021-01295-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsubayashi Y, Yoshida A, Suganami H, Oe M, Sato T, Yaguchi Y, Fujihara K, Yamada T, Tanaka S, Kaku K, et al. Association of estimated plasma volume and weight loss after long-term administration and subsequent discontinuation of the sodium-glucose cotransporter-2 inhibitor tofogliflozin. Diabetes Obes Metab. 2021;23:1660–1665. doi: 10.1111/dom.14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoshika Y, Kubota Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, Tsukada Y, et al. Effect of empagliflozin versus placebo on plasma volume status in patients with acute myocardial infarction and type 2 diabetes mellitus. Diabetes Ther. 2021;12:2241–2248. doi: 10.1007/s13300-021-01103-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jensen J, Omar M, Kistorp C, Tuxen C, Gustafsson I, Køber L, Gustafsson F, Faber J, Malik ME, Fosbøl EL, et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2021;9:106–116. doi: 10.1016/S2213-8587(20)30382-X [DOI] [PubMed] [Google Scholar]
- 104.Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, Køber L, Gustafsson F, Faber J, Fosbøl EL, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020;228:47–56. doi: 10.1016/j.ahj.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 105.Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Möller S, Ali M, Gustafsson F, Køber L, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2020;76:2740–2751. doi: 10.1016/j.jacc.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 106.Fujiki S, Tanaka A, Imai T, Shimabukuro M, Uehara H, Nakamura I, Matsunaga K, Suzuki M, Kashimura T, Minamino T, et al. ; CANDLE Trial Investigators. Body fluid regulation via chronic inhibition of sodium-glucose cotransporter-2 in patients with heart failure: a post hoc analysis of the CANDLE trial. Clin Res Cardiol. 2023;112:87–97. doi: 10.1007/s00392-022-02049-4 [DOI] [PubMed] [Google Scholar]
- 107.Nakashima M, Miyoshi T, Ejiri K, Kihara H, Hata Y, Nagano T, Takaishi A, Toda H, Nanba S, Nakamura Y, et al. ; MUSCAT-HF Study Investigators. Effects of luseogliflozin on estimated plasma volume in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2022;9:712–720. doi: 10.1002/ehf2.13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scholtes RA, Mosterd CM, Hesp AC, Smits MM, Heerspink HJL, van Raalte DH. Mechanisms underlying the blood pressure-lowering effects of empagliflozin, losartan and their combination in people with type 2 diabetes: a secondary analysis of a randomized crossover trial. Diabetes Obes Metab. 2023;25:198–207. doi: 10.1111/dom.14864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heerspink HJL, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cherney DZI, Cosentino F, Pratley RE, Dagogo-Jack S, Frederich R, Maldonado M, Liu J, Pong A, Liu CC, Cannon CP; VERTIS CV Investigators. The differential effects of ertugliflozin on glucosuria and natriuresis biomarkers: prespecified analyses from VERTIS CV. Diabetes Obes Metab. 2022;24:1114–1122. doi: 10.1111/dom.14677 [DOI] [PubMed] [Google Scholar]
- 111.van Raalte DH, Bjornstad P, Persson F, Powell DR, de Cassia Castro R, Wang PS, Liu M, Heerspink HJL, Cherney D. The impact of sotagliflozin on renal function, albuminuria, blood pressure, and hematocrit in adults with type 1 diabetes. Diabetes Care. 2019;42:1921–1929. doi: 10.2337/dc19-0937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Omar M, Jensen J, Burkhoff D, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Gustafsson F, Køber L, Borlaug BA, et al. Effect of empagliflozin on blood volume redistribution in patients with chronic heart failure and reduced ejection fraction: an analysis from the Empire HF randomized clinical trial. Circ Heart Fail. 2022;15:e009156. doi: 10.1161/CIRCHEARTFAILURE.121.009156 [DOI] [PubMed] [Google Scholar]
- 113.Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, Verhaert D, Vandervoort P, Tang WH, Mullens W. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70:265–273. doi: 10.1080/ac.70.3.3080630 [DOI] [PubMed] [Google Scholar]
- 114.Martens P, Chen HH, Verbrugge FH, Testani JT, Mullens W, Tang WHW. Assessing intrinsic renal sodium avidity in acute heart failure: implications in predicting and guiding decongestion. Eur J Heart Fail. 2022;24:1978–1987. doi: 10.1002/ejhf.2662 [DOI] [PubMed] [Google Scholar]
- 115.Januzzi JL, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, Ferreira JP, Sattar N, Verma S, Vedin O, et al. ; EMPEROR-Reduced Trial Committees and Investigators. Prognostic importance of NT-proBNP and effect of empagliflozin in the EMPEROR-Reduced trial. J Am Coll Cardiol. 2021;78:1321–1332. doi: 10.1016/j.jacc.2021.07.046 [DOI] [PubMed] [Google Scholar]
- 116.Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143:1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503 [DOI] [PubMed] [Google Scholar]
- 117.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Brueckmann M, Jamal W, Cotton D, et al. ; EMPEROR-Reduced Trial Committees and Investigators. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-Reduced trial. J Am Coll Cardiol. 2021;77:1381–1392. doi: 10.1016/j.jacc.2021.01.033 [DOI] [PubMed] [Google Scholar]
- 118.Biegus J, Voors AA, Collins SP, Kosiborod MN, Teerlink JR, Angermann CE, Tromp J, Ferreira JP, Nassif ME, Psotka MA, et al. Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Eur Heart J. 2023;44:41–50. doi: 10.1093/eurheartj/ehac530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aronson D. The interstitial compartment as a therapeutic target in heart failure. Front Cardiovasc Med. 2022;9:933384. doi: 10.3389/fcvm.2022.933384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Itkin M, Rockson SG, Burkhoff D. Pathophysiology of the lymphatic system in patients with heart failure. J Am Coll Cardiol. 2021;78:278–290. doi: 10.1016/j.jacc.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 121.Rossitto G, Mary S, McAllister C, Neves KB, Haddow L, Rocchiccioli JP, Lang NN, Murphy CL, Touyz RM, Petrie MC, et al. Reduced lymphatic reserve in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2020;76:2817–2829. doi: 10.1016/j.jacc.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilcox CS, Sterzel RB, Dunckel PT, Mohrmann M, Perfetto M. Renal interstitial pressure and sodium excretion during hilar lymphatic ligation. Am J Physiol. 1984;247:F344–F351. doi: 10.1152/ajprenal.1984.247.2.F344 [DOI] [PubMed] [Google Scholar]
- 123.Szwed JJ, Hamburger RJ, Kleit SA. Effect of ethacrynic acid on thoracic duct lymph flow in the dog. Am J Physiol. 1971;221:544–547. doi: 10.1152/ajplegacy.1971.221.2.544 [DOI] [PubMed] [Google Scholar]
- 124.Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, Boemke-Zelch F, Linz P, Nagel AM, Titze J, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5. doi: 10.1186/s12933-017-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, Häring HU, Stefan N, Fritsche A, Artunc F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18:46. doi: 10.1186/s12933-019-0852-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeng YH, Liu SC, Lee CC, Sun FJ, Liu JJ. Effect of empagliflozin versus linagliptin on body composition in Asian patients with type 2 diabetes treated with premixed insulin. Sci Rep. 2022;12:17065. doi: 10.1038/s41598-022-21486-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohara K, Masuda T, Murakami T, Imai T, Yoshizawa H, Nakagawa S, Okada M, Miki A, Myoga A, Sugase T, et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: a comparison study with furosemide and tolvaptan. Nephrology (Carlton). 2019;24:904–911. doi: 10.1111/nep.13552 [DOI] [PubMed] [Google Scholar]
- 128.Ohara K, Masuda T, Morinari M, Okada M, Miki A, Nakagawa S, Murakami T, Oka K, Asakura M, Miyazawa Y, et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr. 2020;12:37. doi: 10.1186/s13098-020-00545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hallow KM, Greasley PJ, Helmlinger G, Chu L, Heerspink HJ, Boulton DW. Evaluation of renal and cardiovascular protection mechanisms of SGLT2 inhibitors: model-based analysis of clinical data. Am J Physiol Renal Physiol. 2018;315:F1295–F1306. doi: 10.1152/ajprenal.00202.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iwatani H, Yamato M, Bessho S, Mori Y, Notsu S, Asahina Y, Koizumi S, Kimura Y, Shimomura A. Tolvaptan reduces extracellular fluid per amount of body fluid reduction less markedly than conventional diuretics. Intern Med. 2022;61:2561–2565. doi: 10.2169/internalmedicine.8533-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bjornstad P, Greasley PJ, Wheeler DC, Chertow GM, Langkilde AM, Heerspink HJL, Van Raalte DH. The potential roles of osmotic and nonosmotic sodium handling in mediating the effects of sodium-glucose cotransporter 2 inhibitors on heart failure. J Card Fail. 2021;27:1447–1455. doi: 10.1016/j.cardfail.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rossitto G, Mary S, Chen JY, Boder P, Chew KS, Neves KB, Alves RL, Montezano AC, Welsh P, Petrie MC, et al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun. 2020;11:4222. doi: 10.1038/s41467-020-17820-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laffer CL, Scott RC, 3rd, Titze JM, Luft FC, Elijovich F. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension. 2016;68:195–203. doi: 10.1161/HYPERTENSIONAHA.116.07289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patterson JH, Adams KF, Jr, Applefeld MM, Corder CN, Masse BR. Oral torsemide in patients with chronic congestive heart failure: effects on body weight, edema, and electrolyte excretion. Torsemide Investigators Group. Pharmacotherapy. 1994;14:514–521. [PubMed] [Google Scholar]
- 135.Applefeld JJ, Kasmer RJ, Hak LJ, Dukes GE, Wermeling DP, McClain CJ. A dose-response study of orally administered torsemide in patients with ascites due to cirrhosis. Aliment Pharmacol Ther. 1994;8:397–402. doi: 10.1111/j.1365-2036.1994.tb00306.x [DOI] [PubMed] [Google Scholar]
- 136.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, et al. ; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 137.Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K; ASCITES-DOUBLEBLIND Study Group. Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82. doi: 10.1111/hepr.12098 [DOI] [PubMed] [Google Scholar]
- 138.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, et al. ; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trullàs JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Sánchez-Marteles M, Conde-Martel A, Dávila-Ramos MF, Llácer P, Salamanca-Bautista P, Pérez-Silvestre J, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. 2023;44:411–421. doi: 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 140.Martens P, Dauw J, Verbrugge FH, Nijst P, Meekers E, Augusto SN, Jr, Ter Maaten JM, Damman K, Mebazaa A, Filippatos G, et al. Decongestion with acetazolamide in acute decompensated heart failure across the spectrum of left ventricular ejection fraction: a pre-specified analysis from the ADVOR trial. Circulation. 2023;147:201–211. doi: 10.1161/CIRCULATIONAHA.122.062486 [DOI] [PubMed] [Google Scholar]
- 141.Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation. 2021;143:326–336. doi: 10.1161/CIRCULATIONAHA.120.051783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vaduganathan M, Claggett BL, Jhund P, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, et al. Time to clinical benefit of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified secondary analysis of the DELIVER randomized clinical trial. JAMA Cardiol. 2022;7:1259–1263. doi: 10.1001/jamacardio.2022.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. ; PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748 [DOI] [PubMed] [Google Scholar]
- 145.Wang TD, Tan RS, Lee HY, Ihm SH, Rhee MY, Tomlinson B, Pal P, Yang F, Hirschhorn E, Prescott MF, et al. Effects of sacubitril/valsartan (LCZ696) on natriuresis, diuresis, blood pressures, and NT-proBNP in salt-sensitive hypertension. Hypertension. 2017;69:32–41. doi: 10.1161/HYPERTENSIONAHA.116.08484 [DOI] [PubMed] [Google Scholar]
- 146.Ayalasomayajula S, Schuehly U, Pal P, Chen F, Zhou W, Sunkara G, Langenickel TH. Effect of the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan on the pharmacokinetics and pharmacodynamics of a single dose of furosemide. Br J Clin Pharmacol. 2018;84:926–936. doi: 10.1111/bcp.13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krum H, Roecker EB, Mohacsi P, Rouleau JL, Tendera M, Coats AJ, Katus HA, Fowler MB, Packer M; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:712–718. doi: 10.1001/jama.289.6.712 [DOI] [PubMed] [Google Scholar]
- 148.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 149.Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA, Böhm M, Boulton DW, Chopra VK, DeMets DL, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation. 2020;142:1040–1054. doi: 10.1161/CIRCULATIONAHA.120.047077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, Inzucchi SE, Fitchett D, Anker SD, Marx N, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. Eur J Heart Fail. 2020;22:126–135. doi: 10.1002/ejhf.1663 [DOI] [PubMed] [Google Scholar]
- 151.Chatur S, Claggett BL, Vardeny O, Jering K, Desai AS, Pfeffer MA, Lefkowitz M, McMurray JJV, Solomon SD, Vaduganathan M. Sacubitril/valsartan and loop diuretic requirement in heart failure with preserved ejection fraction in the PARAGON-HF trial. Eur J Heart Fail. 2023;25:87–94. doi: 10.1002/ejhf.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21:337–341. doi: 10.1002/ejhf.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Almufleh A, Desai AS, Fay R, Ferreira JP, Buckley LF, Mehra MR, Rossignol P, Zannad F. Correlation of laboratory haemoconcentration measures with filling pressures obtained via pulmonary arterial pressure sensors in ambulatory heart failure patients. Eur J Heart Fail. 2020;22:1907–1911. doi: 10.1002/ejhf.1848 [DOI] [PubMed] [Google Scholar]
- 154.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–524. doi: 10.1016/j.jacc.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, Rubini Gimenez M, Kozhuharov N, Sabti Z, Breitenbücher D, et al. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail. 2017;19:226–236. doi: 10.1002/ejhf.667 [DOI] [PubMed] [Google Scholar]
- 156.Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, et al. ; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol. 2017;2:950–958. doi: 10.1001/jamacardio.2017.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, et al. ; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332 [DOI] [PubMed] [Google Scholar]
- 158.Packer M, Lee WH, Medina N, Yushak M, Kessler PD. Functional renal insufficiency during long-term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med. 1987;106:346–354. doi: 10.7326/0003-4819-106-3-346 [DOI] [PubMed] [Google Scholar]
- 159.Qiu M, Ding LL, Zhang M, Zhou HR. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 2021;18:14791641211011016. doi: 10.1177/14791641211011016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kinduryte Schorling O, Clark D, Zwiener I, Kaspers S, Lee J, Iliev H. Pooled safety and tolerability analysis of empagliflozin in patients with type 2 diabetes mellitus. Adv Ther. 2020;37:3463–3484. doi: 10.1007/s12325-020-01329-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. ; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 162.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021;143:310–321. doi: 10.1161/CIRCULATIONAHA.120.051685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hollenberg NK. Set point for sodium homeostasis: surfeit, deficit, and their implications. Kidney Int. 1980;17:423–429. doi: 10.1038/ki.1980.50 [DOI] [PubMed] [Google Scholar]