BACKGROUND:
Despite improvements in acute stroke therapies and rehabilitation strategies, many stroke patients are left with long-term upper limb motor impairment. We assessed whether an inhibitory repetitive transcranial magnetic stimulation treatment paradigm started within 3 weeks after stroke onset promotes upper limb motor recovery.
METHODS:
We performed a single-center randomized, sham-controlled clinical trial. Patients with ischemic stroke or intracerebral hemorrhage and unilateral upper limb motor impairment were randomized to 10 daily sessions of active or sham continuous theta-burst stimulation (cTBS) of the contralesional primary motor cortex combined with standard upper limb therapy, started within 3 weeks after stroke onset. The primary outcome was the change in the Action Research Arm Test score from baseline (pretreatment) at 3 months after stroke. Secondary outcomes included the score on the modified Rankin Scale at 3 months and the length of stay at the rehabilitation center. Statistical analyses were performed using mixed models for repeated measures.
RESULTS:
We enrolled 60 patients between April 2017 and February 2021, of whom 29 were randomized to active cTBS and 31 to sham cTBS. One patient randomized to active cTBS withdrew consent before the intervention and was excluded from the analyses. The mean difference in the change in Action Research Arm Test score from baseline at 3 months poststroke was 9.6 points ([95% CI, 1.2–17.9]; P=0.0244) in favor of active cTBS. Active cTBS was associated with better scores on the modified Rankin Scale at 3 months (OR, 0.2 [95% CI, 0.1–0.8]; P=0.0225) and with an 18 days shorter length of stay at the rehabilitation center than sham cTBS ([95% CI, 0.0–36.4]; P=0.0494). There were no serious adverse events.
CONCLUSIONS:
Ten daily sessions of cTBS of the contralesional primary motor cortex combined with upper limb training, started within 3 weeks after stroke onset, promote recovery of the upper limb, reduce disability and dependence and leads to earlier discharge from the rehabilitation center.
REGISTRATION:
URL: https://trialsearch.who.int/; Unique identifier: NTR6133.
Keywords: arm, brain, ischemic stroke, rehabilitation, stroke
See related article, p 1972
Upper limb motor impairment is one of the most frequent long-term neurological consequences of ischemic stroke or intracerebral haemorrhage.1,2 Despite recent developments in acute stroke treatment and rehabilitative therapy, recovery of upper limb motor function is often incomplete, resulting in limitations in functioning and participation.3–6 More effective rehabilitation strategies that improve stroke recovery and lead to better clinical outcomes are therefore required.7
Spontaneous recovery after stroke is believed to be driven by neurobiological processes that occur mainly during a period of heightened brain plasticity in the first 3 months after stroke.8,9 Conventional rehabilitation strategies focus on regaining function within this period primarily through physical therapy. Previous studies have shown that patients who have recovered from stroke present a more symmetrical inhibitory drive between the primary motor cortices.10–14 An increased and persistent inhibitory drive from the contralesional to the ipsilesional primary motor cortex (M1) has been associated with more severe poststroke motor deficits.10–13 It has been suggested that restoration of the interhemispheric balance can result in a brain state that is more prone to spontaneous recovery and rehabilitation therapy of the affected arm.13 On the contrary, excitability of the contralesional M1 may be within the normal levels after stroke15 and others have suggested that an interhemispheric imbalance might be a consequence of underlying processes of motor recovery.15,16
An interhemispheric imbalance may be restored through excitation of the lesioned M1 or inhibition of the contralesional M1.17 Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation method to upregulate18 or downregulate19 cortical excitability. Inhibitory rTMS of the contralesional M1 combined with upper limb therapy was initially investigated as a treatment for the promotion of upper limb recovery in chronic stroke patients.20 However, a recent guidelines article and a meta-analysis of randomized controlled trials indicate that inhibition of the contralesional M1 using inhibitory low-frequency (LF) rTMS followed by upper limb therapy is more effective in promoting upper limb recovery when started within the first 2 to 3 months poststroke.17,21 This has been shown to be the sensitive period for improvement of motor recovery,22 presumably associated with heightened plasticity, which could potentially also facilitate a therapeutic effect of rTMS. Yet, it remains to be determined whether additional recovery achieved with rTMS treatment reduces disability and dependency and whether the effects persist after the first 3 months poststroke.
Conventional LF rTMS treatment consists of 15-minute sessions, whereas the continuous theta-burst stimulation (cTBS), a novel inhibitory rTMS paradigm, has a much shorter treatment duration of 40 seconds per session. Therefore, the use of cTBS can improve patient comfort and increase cost-effectiveness of the intervention.23,24 So far, the effect of cTBS on upper limb recovery has only been tested in a single randomized trial randomizing only 14 patients to receive active cTBS group and 13 patients to receive sham cTBS.25 This did not reveal the effect of cTBS started within 10 weeks after stroke on the change on a multifaceted upper limb motor function score (obtained from 4 upper limb function tests) within 30 days after treatment with respect to the change in this score (over a period of 7 days) before treatment. We assessed whether 2 weeks of daily contralesional cTBS started within the first 3 weeks after stroke improves the long-term upper limb motor recovery up to 12 months poststroke.
METHODS
Study Design
We performed a single-center, prospective, randomized, sham-controlled clinical trial with a single-blind intervention and a double-blind primary outcome evaluation at rehabilitation center De Hoogstraat (Utrecht, the Netherlands), according to the CONSORT (Consolidated Standards of Reporting Trials) guidelines. Deidentified participant data are available from the corresponding author upon reasonable request. A summary of the trial protocol has been published26 and the full protocol is available in the Supplemental Material. The study was approved by the Medical Research Ethics Committee of the University Medical Center Utrecht.
Participants
We included patients aged ≥18 years with first-ever ischemic stroke or intracerebral hemorrhage and a paresis of 1 arm, as defined by a Motricity Index27 between 9 and 99, in whom treatment could be started within 3 weeks after stroke onset.28 Patients were excluded from participation if they had another disabling medical condition, as determined by the treating physician; could use the hand of the paretic arm (almost) normally (Motricity index pinch grip score of 33); had a severe deficit in communication, memory, or understanding that would impede proper study participation; or had a contraindication to rTMS according to the TMS safety guidelines.29 All patients gave written informed consent.
Randomization and Masking
Patients were randomly assigned to 10 daily sessions of contralesional cTBS or to sham cTBS during 2 weeks, in addition to regular care upper limb therapy, using a secured online allocation system (Research Online V2.0, Julius Centre, the Netherlands) by the investigator performing the treatment. Randomization was stratified according to the ability to extend 1 or more fingers of the paretic arm.30 Sham cTBS was performed at 10% of the resting motor threshold (RMT), which was defined as the minimum machine output at which stimulation evoked at least 5 of 10 MEPs with a peak-to-peak amplitude of over 50 μV,24 with the TMS coil rotated 45° relative to the scalp, and patients were masked to treatment allocation using auditory masking of the TMS coil sound.
Procedures
Treatment was delivered in 10 daily sessions on consecutive working days. cTBS was delivered over the contralesional M1, which was defined as the position on the scalp at which motor-evoked potentials (MEPs) with the largest peak-to-peak amplitude could be evoked in the contralateral first dorsal interosseous (FDI) muscle by delivering TMS pulses and monitoring the electromyogram. A Neuro-MS/D advanced therapeutic magnetic stimulator and an angulated 100 mm figure-of-eight TMS coil (Neurosoft, Ivanovo, Russia) were used for stimulation. Electromyogram was recorded, amplified, and digitized at a sampling frequency of 20 kHz using a 4-channel Neuro-MEP amplifier (Neurosoft, Ivanovo, Russia). cTBS consisted of continuous delivery of 3 stimuli bursts at 50 Hz repeated at 5 bursts per second for a duration of 40 seconds with a biphasic TMS-induced current at 45° to the midline. Stimulation intensity was set at 70% of the RMT. TMS coil placement was recorded using a neuronavigation system (Brain Science Tools BV, De Bilt, the Netherlands) starting from the 16th patient. cTBS or sham cTBS was delivered 15 minutes before standard upper limb therapy, which consisted of a 60-minute group therapy session of individualized upper limb exercises, according to the Concise Arm and Hand Rehabilitation Approach in Stroke (CARAS).31 CARAS consists of a daily training program during which patients perform specific exercises with the paretic arm under guidance of physical or occupational therapists, supplemented with exercises they can perform independently during the rest of the day.
Outcomes
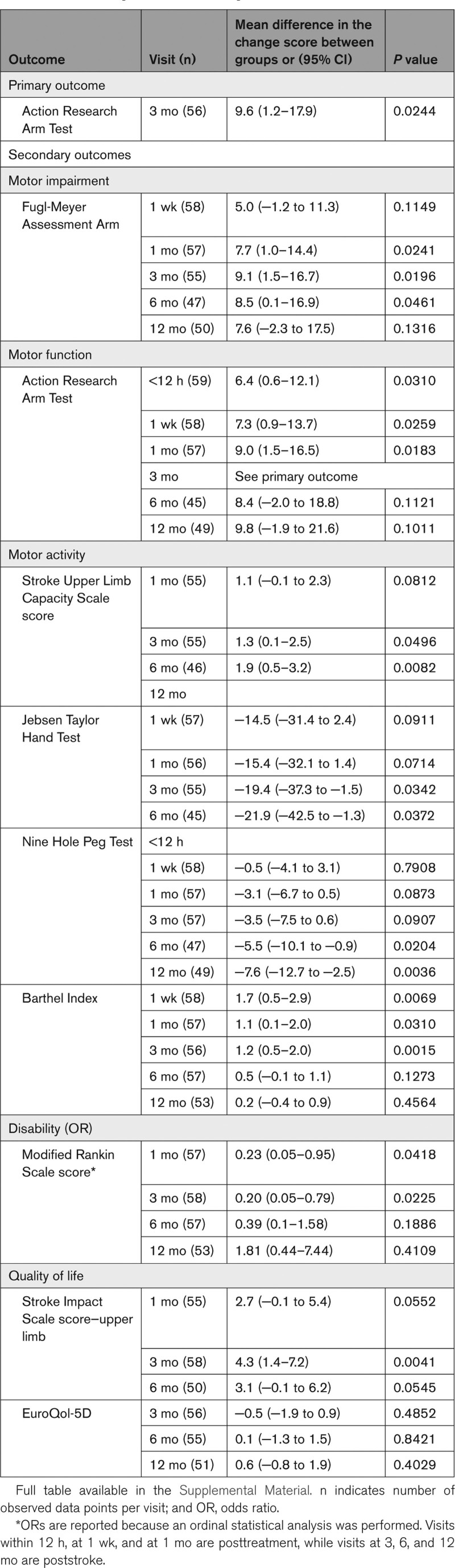
The primary outcome measure was the change in the Action Research Arm Test (ARAT) score from baseline at 3 months after stroke, as recommended by the Stroke Recovery and Rehabilitation Roundtable.32 The ARAT is a performance test that assesses the ability to perform gross movements and to grasp, move, and release objects differing in size, weight, and shape, of which validity and reliability have been demonstrated previously.33,34 The ARAT score ranges from 0 to 57, with higher scores indicating better performance. The ARAT score was measured at 3 months after stroke by an independent trained physician assistant, blinded to treatment allocation.
Predefined secondary outcomes included the change in ARAT score <12 hours, 1 week, and 1 month posttreatment, and 6 and 12 months poststroke. Other predefined secondary outcomes were tests that assess different domains of the international classification of functioning, disability, and health framework.35 These included the upper limb section of the Fugl-Meyer Assessment (FMA) score36 for motor impairment; the Stroke Upper Limb Capacity Scale,37 Jebsen Taylor test38 score, Barthel Index,39 and Nine Hole Peg Test40 for activity; the modified Rankin Scale (mRS)41 for disability; and the upper limb section of the stroke impact scale42 and EuroQol(EQ)-5D43 for quality of life. Secondary outcomes were assessed by an investigator who was aware of the treatment allocation. We also assessed the length of stay in the rehabilitation center, the dose of upper limb therapy, and self-practice during the 2-week treatment period and the week thereafter, and the contralesional RMT before each cTBS session. We monitored and recorded (serious) adverse events that occurred during the 2-week treatment period or 1 week thereafter.
Statistical Analysis
The sample size calculation was based on an effect size of 0.55 on the ARAT score obtained from a meta-analysis.26 To detect the hypothesized effect with 80% power, and a 2-sided alpha of 0.05, a total sample size of 56 patients was required. We included 60 patients, with 30 patients per group, to account for loss to follow-up.
We followed the prespecified statistical analysis plan, which is available in the Supplemental Material and was completed before data-lock. The primary outcome was the change in ARAT score from baseline (pretreatment) at 3 months poststroke.26 The primary analysis was performed using a linear mixed model for repeated measures with an unstructured variance-covariance matrix in the intention-to-treat population, includes all randomized participants irrespective of follow-up. Missing data were assumed to be missing at random. The model included the baseline value of the investigated outcome; the stratification factor (ability versus no ability to extend 1 or more fingers), visit (<12 hours, 1 week, and 1 month posttreatment, and 3, 6, and 12 months poststroke), and the interaction of treatment (sham cTBS; active cTBS) by visit. We performed a sensitivity analysis of the primary outcome in the per-protocol population (defined as those who had an ARAT score assessed at 3 months) and additional sensitivity analyses in which additional covariates (ie, MEP status and dominant hand paresis) were included in the main analysis.
We performed similar analyses for the effect of treatment on the secondary outcomes at all visits except for the mRS and length of stay. The mRS was analyzed using a cumulative link mixed model of the total mRS scores due to the ordinal nature of the data. The effect of treatment on the length of stay and the dose of upper limb therapy and self-practice that patients received were analyzed with independent samples t tests. The effect of treatment on excitability of the contralesional M1 was analyzed using a linear mixed-effects model. The outcome was the contralesional RMT determined before each cTBS session and the model included the baseline RMT, number of cTBS session (1–10), and type of treatment (sham cTBS; active cTBS). Pearson’s correlation was used to calculate the correlation between the change in contralesional RMT during the treatment period and the change in ARAT score between baseline and 3 months poststroke.
Statistical analysis was performed with R 4.1 and SPSS 26.0 (IBM, Chicago, IL). All hypotheses were tested a 2-sided alpha of 0.05.
RESULTS
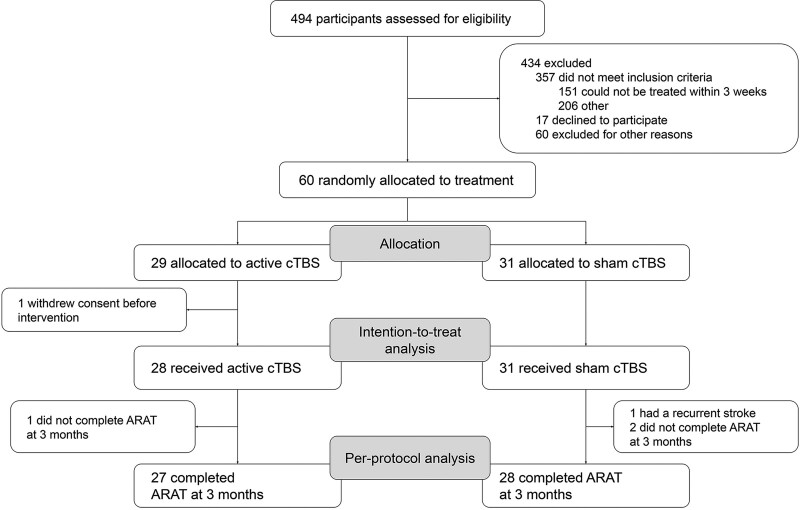
Between April 14, 2017 and February 12, 2021, a total of 494 stroke patients with arm weakness were screened for eligibility, of whom 60 were enrolled (Figure 1). Twenty-nine patients were randomly assigned to receive active cTBS, of whom 1 withdrew consent before starting treatment, leaving 28 patients in the cTBS group, and 31 patients were assigned to receive sham cTBS. Therefore, the intention-to-treat population consisted of 59 patients. The ARAT score could not be assessed at 3 months in 3 patients (1 in the active cTBS group and 2 in the sham cTBS group) due to physical limitations (shoulder pain and tiredness) and 1 patient in the sham cTBS group had a recurrent stroke. These 4 patients were excluded from the per-protocol population (Figure 1).
Figure 1.
Participant flow diagram. ARAT indicates Action Research Arm Test; and cTBS, continuous theta-burst stimulation.
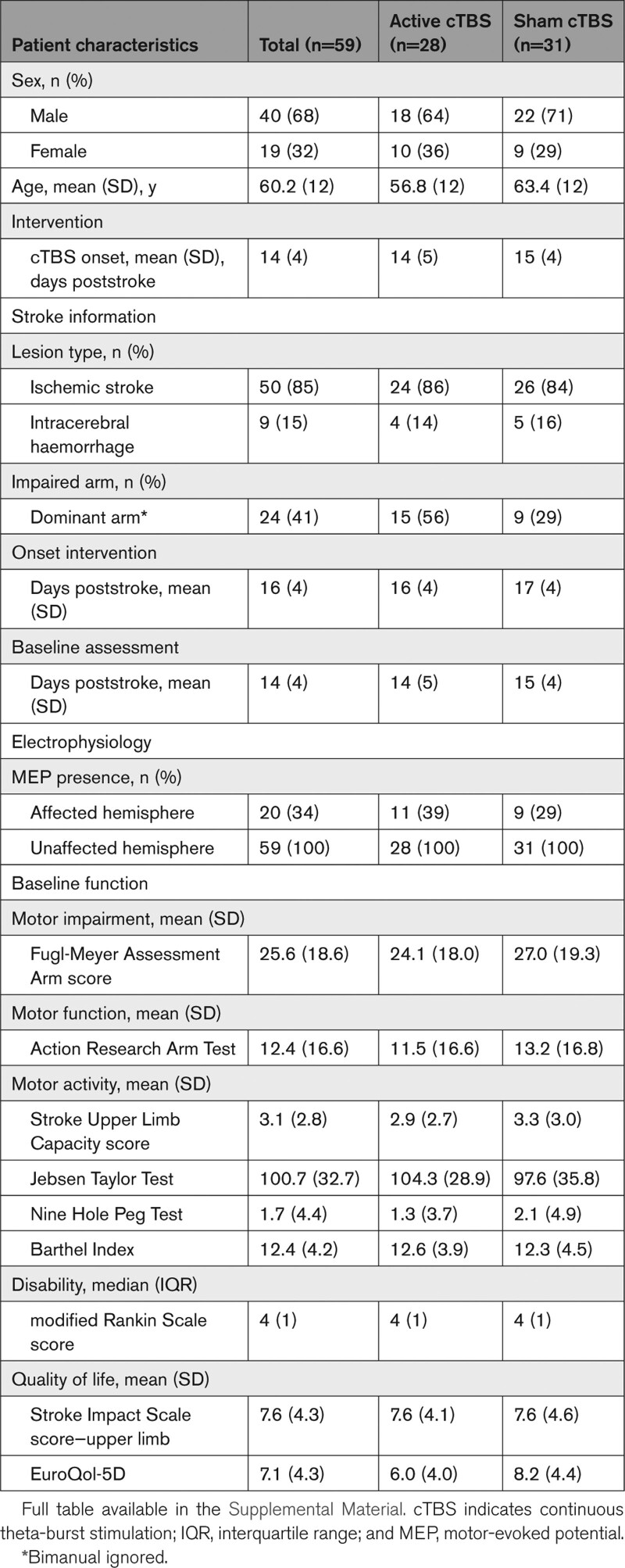
Patient characteristics are shown in Table 1. None of the patients had previously been treated with cTBS.
Table 1.
Baseline Characteristics
The <12 hours, 1 week, and 1 month posttreatment visits were assessed 29 (SD 4), 36 (SD 5), and 59 (SD 7) days poststroke, respectively, and the 3, 6, and 12 months poststroke visits were assessed 90 (SD 4), 184 (SD 11), and 368 (SD 10) days poststroke, respectively.
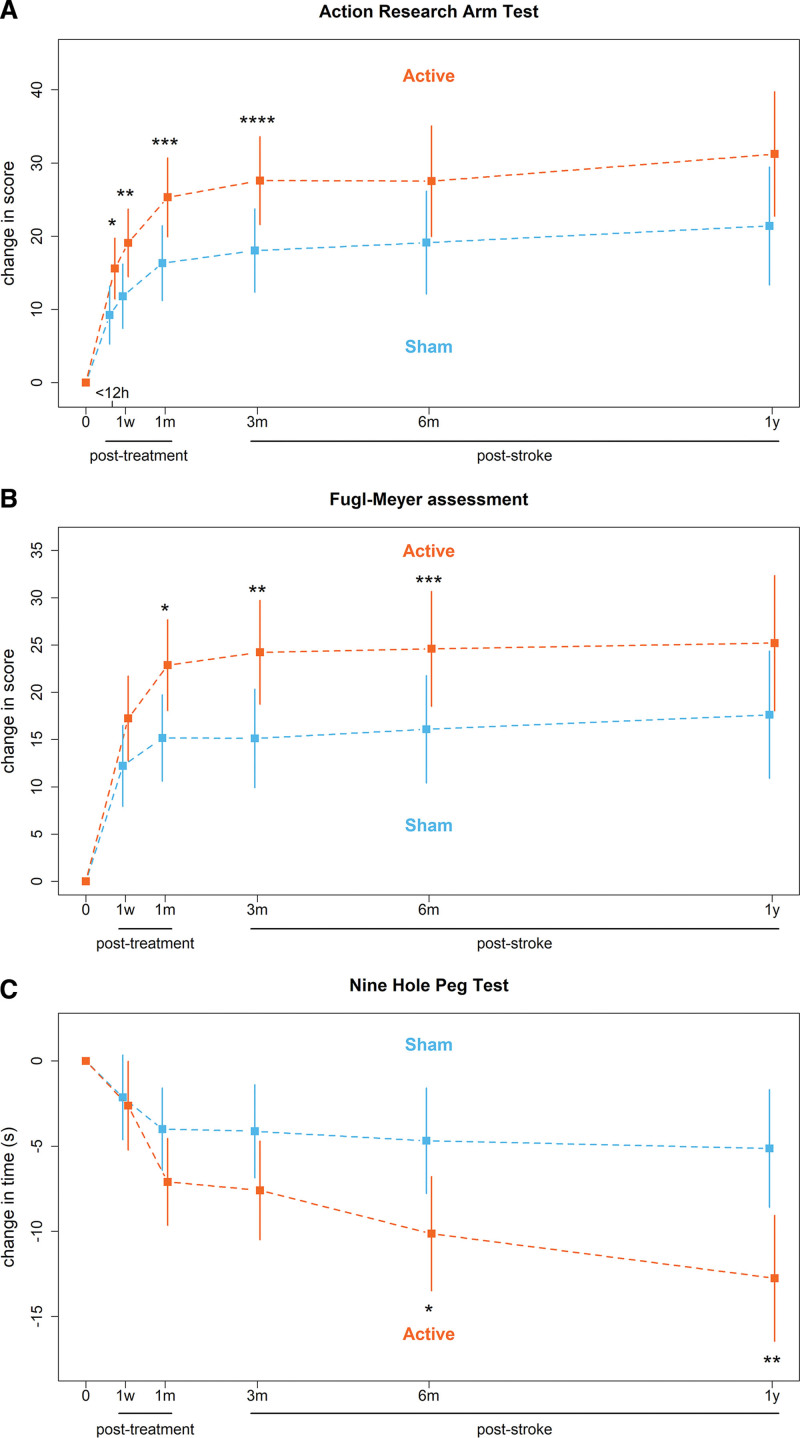
The change in ARAT score from baseline at 3 months poststroke was 27.6 points in the active cTBS group compared with 18.0 points in the sham cTBS group, with a mean difference of 9.6 points ([95% CI, 1.2–17.9]; P=0.0244; Figure 2A). Sensitivity analysis showed a mean difference in ARAT change score of 9.9 points ([95% CI, 1.1–18.7]; P=0.0276) in the per-protocol population in favor of active cTBS. Sensitivity analyses including baseline MEP status or dominant hand paresis as additional covariate showed mean differences in ARAT change score of 8.4 points ([95% CI, 0.3–16.5]; P=0.0414) and 9.2 points ([95% CI, 0.7–17.6]; P=0.0341), respectively, both in favor of active cTBS.
Figure 2.
Mean and 95% CIs of the changes in upper limb outcome measures for the active and sham continuous theta-burst stimulation (cTBS) groups calculated using mixed-effects model for repeated measures. A, Action Research Arm Test (ARAT) scores. B, Fugl-Meyer assessment (FMA) arm scores. C, Nine Hole Peg Test (NHPT) times. 0, baseline; h, hours; w, weeks; m, months; y, years. A, ARAT score ranges from 0 to 57 points and a higher score indicates better outcome. *P=0.0310; **P=0.0259; ***P=0.0183; ****P=0.0244. B, FMA score ranges from 0 to 66 points and a higher score indicates better outcome. *P=0.0241; **P=0.0196; ***P=0.0461. C, Maximum NHPT time is 50 s and a lower time indicates better outcome. *P=0.0204; **P=0.0036.
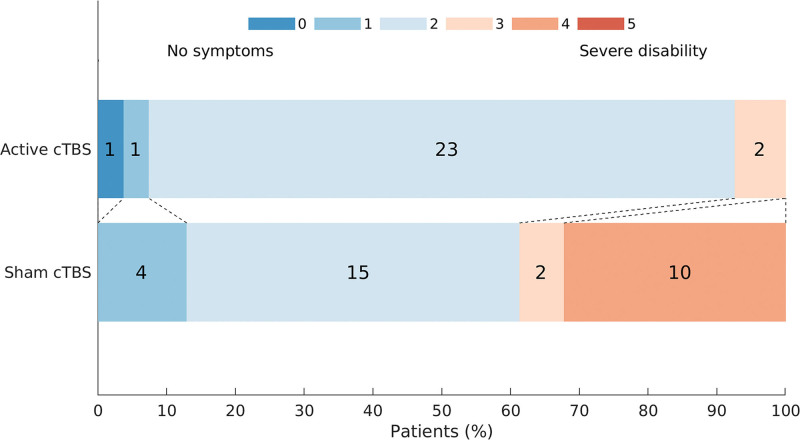
In the secondary analyses, the mean difference at 3 months poststroke between active and sham cTBS groups was 9.1 points on the FMA ([95% CI, 1.5–16.7]; P=0.0196; Figure 2B), 1.3 points on the Stroke Upper Limb Capacity Scale score ([95% CI, 0.1–2.5]; P=0.0496), −19.4 points on the Jebsen Taylor test score ([95% CI, −37.3 to −1.5]; P=0.0342), −3.5 points on the Nine Hole Peg Test ([95% CI, −7.5 to 0.6]; P=0.0907; Figure 2C), 1.2 points on the BI ([95% CI, 0.5–2.0]; P=0.0015), 4.3 points on the upper limb section of the stroke impact scale score ([95% CI, 1.4–7.2]; P=0.0041), and −0.5 on the EuroQol-5D ([95% CI, −1.9 to 0.9]; P=0.4852), all indicating better outcomes except for the Nine Hole Peg Test and EuroQol-5D. Score on the mRS were better with active than with sham cTBS (odd ratio, 0.2 [95% CI, 0.1–0.8]; P=0.0225; Figure 3). Details are presented in Table 2.
Figure 3.
Modified Rankin Scale (mRS) scores at 3 mo poststroke for the active and sham continuous theta-burst stimulation (cTBS) groups. The mRS score ranges from 0 to 5 and a lower score indicates better outcome. P=0.0225.
Table 2.
Primary and Secondary Outcomes
The mean length of stay was 63 days in the active cTBS group compared with 81 days in the sham cTBS group with a mean difference of 18 days ([95% CI, 0.0–36.4]; P=0.0494). Mean duration of upper limb therapy in the first 3 weeks after TMS treatment onset was 17.0 hours in the active cTBS group compared with 17.4 hours in the sham cTBS group ([95% CI, −2.2 to 1.4]; P=0.6628). Mean duration of independent self-practice was 8.1 hours in the active cTBS group compared with 8.5 hours of in the sham cTBS group ([95% CI, −4.9 to 4.1]; P=0.8572). Active cTBS resulted in a mean increase of 1.4% of the contralesional RMT ([95% CI, −0.0 to 2.7]; P=0.0515). The change in contralesional RMT during the treatment was not associated with the change in ARAT score between baseline and 3 months poststroke in the active cTBS group (r=0.02; P=0.9082).
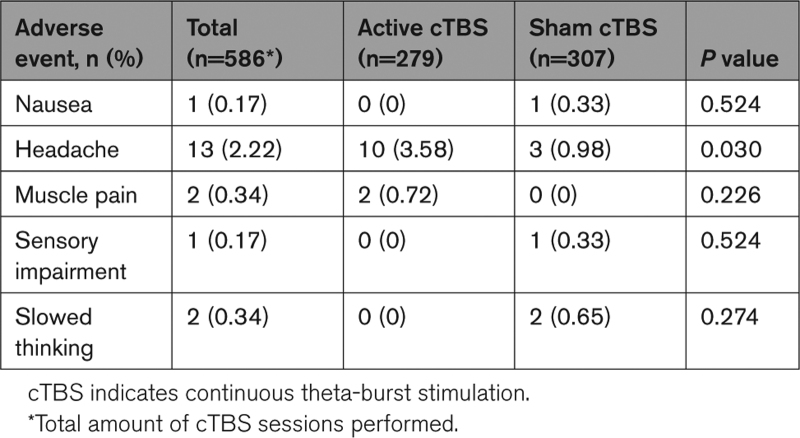
No serious adverse events were reported. Headache was the most prevalent side effect, which occurred more frequently with active cTBS (in 10/279 sessions) than with sham cTBS (in 3/307 sessions). Other side effects (ie, muscle pain and nausea) were rare (Table 3).
Table 3.
Adverse Events Reported During the 2-Weeks Treatment Period and 1 Week Thereafter
DISCUSSION
Patients who received active cTBS showed greater improvement in upper limb recovery as measured with the ARAT at 3 months poststroke compared with patients who received sham cTBS. The mean additional improvement of 9.5 points (17% of the maximum ARAT score) on the ARAT is clinically meaningful and exceeds the previously established minimal clinically important difference of 5.7 points (10% of the maximum ARAT score).44 The treatment benefit was observed directly after the 2-week treatment period and until follow-up at 3 months after stroke. The therapeutic effect could not be established at 6 and 12 months poststroke, possibly due to a plateau effect on the ARAT. A similar treatment effect was observed for the FMA arm score, which was also clinically meaningful,36 and the Barthel Index. However, other tests (Nine Hole Peg Test, Stroke Upper Limb Capacity Scale, and Jebsen Taylor test) in the activity domain did show a treatment benefit up to 12 months poststroke. These tests are more sensitive to improvement in fine motor skills45 and manual dexterity.40 Patients in the sham cTBS group showed little improvement on these test after 3 months, while patients in the active cTBS group showed continued improvement of fine motor skills and manual dexterity up to 12 months poststroke. We hypothesize that additional upper limb recovery in the first months after stroke, which may plateau out on commonly used upper limb motor scales, leads to more frequent use of the affected limb in more complex daily life activities, resulting in increased performance of fine motor skills and manual dexterity that persists over longer times.
Patients who received active cTBS showed more improvement on metrics of disability and dependency (mRS) and quality of life (upper limb section of the stroke impact scale ) at 1 month posttreatment and at 3 months poststroke, compared with patients who were treated with sham stimulation. The EuroQol-5D was a quality of life metric that did not show a treatment effect, presumably due to involvement of factors that are not directly related to an improvement in upper limb recovery, such as pain and anxiety. In addition to a treatment effect on these metrics, the length of stay in the rehabilitation center, an indicator of independency, was 18 days shorter in patients treated with active cTBS compared with patients who received sham cTBS. These findings suggest that patients who received active cTBS were able to independently perform activities of daily living and participate in society at an earlier stage compared with patients who received sham cTBS.
Equally important to efficacy, the investigated cTBS treatment was safe and tolerable, as no serious adverse events were reported. Headache was more prevalent in the active cTBS group, but overall mild side-effects (headache/nausea) were uncommon (<4% of cases).
We did not detect an effect of active cTBS on contralesional M1 excitability, although a trend toward long-lasting (days) inhibition of the contralesional M1 could be observed. We speculate that active cTBS leads to a short-lasting reduction (<2 hours) in contralesional M1 excitability, which has dissipated the next day, but which facilitates the effects of upper limb therapy directly following cTBS. Unfortunately, the acute effect of cTBS could not be measured as the cTBS treatments were directly followed by upper limb therapy. Our findings are in line with a recent meta-analysis that showed that contralesional inhibitory rTMS improves upper limb recovery on the FMA arm score with a similar mean difference of 9 points at 3 months poststroke.21 Our study provides additional evidence that contralesional cTBS started within 3 weeks poststroke effectively promotes upper limb recovery after stroke with a continued benefit until at least 12 months. Furthermore, we also observed a positive effect on scores of activity, disability, and quality of life, which were not identified in aforementioned meta-analysis.21
Earlier meta-analyses of effects of rTMS treatment within the first months poststroke were based (almost) exclusively on inhibition with conventional LF rTMS.17,21 A recently completed trial on contralesional LF rTMS in 77 patients did not find an effect on upper limb recovery.46 Continuous TBS has a substantially shorter treatment duration than LF rTMS, which increases patient comfort, makes it more suitable for use in time-constrained rehabilitation programs, and potentially increases cost-effectiveness. An earlier study that assessed the impact of contralesional cTBS in the subacute poststroke stage did not observe a clinical effect on motor function.25 This apparent discrepancy with our study may be explained by the smaller sample size (14 patients in the cTBS group), the later start of cTBS treatment with respect to stroke onset, the lower frequency of cTBS sessions (3 sessions/week over 3 weeks), or the nonmatching of upper limb physical therapy with cTBS sessions in that study.
A third of the screened patients did not meet the inclusion criteria because the treatment could not be started within 3 weeks after stroke onset. Extension of the treatment time window would have increased the number of eligible patients, but posed the risk of reducing the treatment efficacy, as a previous meta-analysis demonstrated efficacy for treatment only when started within the first month poststroke.21
Limitations
Our study had some limitations. First, the researchers were not blinded to the treatment allocation due to practical reasons concerning the sham condition, what could have introduced bias during the treatment period. In addition, although we performed double-blinded assessment of the primary outcome at 3 months, most secondary outcomes were assessed in a single-blind fashion. However, the considerably shorter length of stay in the rehabilitation center strongly suggests that the benefits observed on the other secondary outcomes are real.
Second, the sham condition did not consist of electrical stimulation to mask sensory sensations on the scalp evoked by peripheral nerve stimulation during TMS, and successful blinding of patients was not verified by asking patients which treatment group they believed they were assigned to. However, all patients were naive to cTBS treatment, which potentially reduces the bias introduced by limitations in the sham condition.
Third, we only measured the duration of upper limb therapy that patients received during the 2-week treatment period and the week thereafter. However, it is unlikely that patients in both groups received different durations of upper limb therapy after this period, as all patients were part of the same treatment schedule. While upper limb therapy content was balanced between groups at onset of treatment, this may have changed based on the additional improvement in upper limb function due to active cTBS, in accordance with the CARAS protocol. However, because both patients and therapists were blinded to treatment allocation, we consider it unlikely that patients in the active cTBS group received different upper limb therapy content unless the change in therapy was the direct result of improved motor function caused by active cTBS.
Fourth, we only scored the upper limb component of the FMA. Assessment of the lower limb component could have provided insights into the specificity of the treatment.
Fifth, the investigated sample consisted predominantly of males, which could limit generalizability of the results to females.
Finally, our treatment paradigm was based on the theoretical interhemispheric imbalance model. However, we did not explicitly evaluate the interhemispheric balance before or after the intervention.
Future Research
The results in this single-center study are promising and provide a strong foundation for future multicenter trials to provide conclusive evidence on the efficacy of cTBS treatment in the promotion of upper limb recovery after stroke. These trials could tailor treatment to individual patients, as recent studies suggest that efficacy of contralesional cTBS may depend on stroke severity47 or stroke type.48
Conclusions
In the present study, treatment with cTBS of the contralesional M1 combined with upper limb training, started within 3 weeks after stroke onset, improved upper limb motor recovery and led to better functional outcomes. Some treatment benefits persisted up to at least 12 months after stroke.
ARTICLE INFORMATION
Acknowledgments
The authors thank all patients and investigators for their contribution to the B-STARS study (Brain Stimulation for Arm Recovery After Stroke).
Sources of Funding
This work was supported by the Netherlands Organization for Scientific Research (VICI 016.130.662) and in part by Brain Science Tools B.V. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
Disclosures
J.J.T. Vink is a part-time employee of and Dr Neggers is the CEO and shareholder of Brain Science Tools B.V. Data analysis was performed during J.J.T. Vink’s part-time employment at Brain Science Tools B.V. Dr van der Worp is consultant for Bayer and receives funding from the Dutch Heart Foundation, Horizon 2020 Framework Programme and Stryker. The other authors report no conflicts.
SUPPLEMENTAL MATERIAL
Tables S1–S2
Trial protocol
Statistical analysis plan
CONSORT checklist
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ARAT
- Action Research Arm Test
- CARAS
- Concise Arm and Hand Rehabilitation Approach in Stroke
- cTBS
- continuous theta-burst stimulation
- FMA
- Fugl-Meyer Assessment
- LF
- low-frequency
- M1
- primary motor cortex
- mRS
- modified Rankin Scale
- RMT
- resting motor threshold
- rTMS
- repetitive transcranial magnetic stimulation
J.J.T. Vink and E.C.C. van Lieshout contributed equally.
For Sources of Funding and Disclosures, see page 1940.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.123.042924.
Contributor Information
Eline C.C. van Lieshout, Email: elinevanl@live.nl.
Willem M. Otte, Email: wmotte@gmail.com.
Ruben P.A. van Eijk, Email: R.P.A.vanEijk-2@umcutrecht.nl.
Mirjam Kouwenhoven, Email: m.kouwenhoven@dehoogstraat.nl.
Sebastiaan F.W. Neggers, Email: basneggers@gmail.com.
H. Bart van der Worp, Email: h.b.vanderworp@umcutrecht.nl.
Johanna M.A. Visser-Meily, Email: J.M.A.Visser-Meilij@umcutrecht.nl.
REFERENCES
- 1.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. The Lancet. 2011;377:1693–1702. doi: 10.1016/s0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3.Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21:357–364. doi: 10.1080/096382899297459 [DOI] [PubMed] [Google Scholar]
- 4.Mayo NE, Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, Salbach N. Disablement following stroke. Disabil Rehabil. 1999;21:258–268. doi: 10.1080/096382899297684 [DOI] [PubMed] [Google Scholar]
- 5.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f [DOI] [PubMed] [Google Scholar]
- 6.van Lieshout ECC, van de Port IG, Dijkhuizen RM, Visser-Meily JMA. Does upper limb strength play a prominent role in health-related quality of life in stroke patients discharged from inpatient rehabilitation? Top Stroke Rehabil. 2020;27:525–533. doi: 10.1080/10749357.2020.1738662 [DOI] [PubMed] [Google Scholar]
- 7.Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, Pennisi M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord. 2019;12:1756286419878317. doi: 10.1177/1756286419878317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923–931. doi: 10.1177/1545968312440745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hordacre B, Austin D, Brown KE, Graetz L, Pareés I, De Trane S, Vallence AM, Koblar S, Kleinig T, McDonnell MN, et al. Evidence for a window of enhanced plasticity in the human motor cortex following ischemic stroke. Neurorehabil Neural Repair. 2021;35:307–320. doi: 10.1177/1545968321992330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 11.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848 [DOI] [PubMed] [Google Scholar]
- 12.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661 [DOI] [PubMed] [Google Scholar]
- 13.Boddington LJ, Reynolds JNJ. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017;10:214–222. doi: 10.1016/j.brs.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Casula EP, Pellicciari MC, Bonnì S, Spanò B, Ponzo V, Salsano I, Giulietti G, Martino Cinnera A, Maiella M, Borghi I, et al. Evidence for interhemispheric imbalance in stroke patients as revealed by combining transcranial magnetic stimulation and electroencephalography. Hum Brain Mapp. 2021;42:1343–1358. doi: 10.1002/hbm.25297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul. 2017;10:721–734. doi: 10.1016/j.brs.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Branscheidt M, Schambra H, Steiner L, Widmer M, Diedrichsen J, Goldsmith J, Lindquist M, Kitago T, Luft AR, et al. ; SMARTS Study Group. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann Neurol. 2019;85:502–513. doi: 10.1002/ana.25452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Arai N, Okabe S, Furubayashi T, Mochizuki H, Iwata NK, Hanajima R, Terao Y, Ugawa Y. Differences in after-effect between monophasic and biphasic high-frequency rTMS of the human motor cortex. Clin Neurophysiol. 2007;118:2227–2233. doi: 10.1016/j.clinph.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Taylor JL, Loo CK. Stimulus waveform influences the efficacy of repetitive transcranial magnetic stimulation. J Affect Disord. 2007;97:271–276. doi: 10.1016/j.jad.2006.06.027 [DOI] [PubMed] [Google Scholar]
- 20.Harvey RL, Edwards D, Dunning K, Fregni F, Stein J, Laine J, Rogers LM, Vox F, Durand-Sanchez A, Bockbrader M, et al. ; NICHE Trial Investigators *. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke: the NICHE trial. Stroke. 2018;49:2138–2146. doi: 10.1161/STROKEAHA.117.020607 [DOI] [PubMed] [Google Scholar]
- 21.van Lieshout ECC, van der Worp HB, Visser-Meily JMA, Dijkhuizen RM. Timing of repetitive transcranial magnetic stimulation onset for upper limb function after stroke: a systematic review and meta-analysis. Front Neurol. 2019;10:1–16. doi: 10.3389/fneur.2019.01269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dromerick AW, Geed S, Barth J, Brady K, Giannetti ML, Mitchell A, Edwardson MA, Tan MT, Zhou Y, Newport EL, et al. Critical Period After Stroke Study (CPASS): a phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc Natl Acad Sci USA. 2021;118:e2026676118. doi: 10.1073/pnas.2026676118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iezzi E, Suppa A, Conte A, Li Voti P, Bologna M, Berardelli A. Short-term and long-term plasticity interaction in human primary motor cortex. Eur J Neurosci. 2011;33:1908–1915. doi: 10.1111/j.1460-9568.2011.07674.x [DOI] [PubMed] [Google Scholar]
- 24.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolo P, Magnin C, Pedrazzini E, Plomp G, Mottaz A, Schnider A, Guggisberg AG. Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Arch Phys Med Rehabil. 2018;99:862–872.e1. doi: 10.1016/j.apmr.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 26.van Lieshout ECC, Visser-Meily JMA, Neggers SFW, van der Worp HB, Dijkhuizen RM. Brain stimulation for arm recovery after stroke (B-STARS): protocol for a randomised controlled trial in subacute stroke patients. BMJ Open. 2017;7:e016566. doi: 10.1136/bmjopen-2017-016566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry. 1990;53:576–579. doi: 10.1136/jnnp.53.7.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19:382–389. doi: 10.1159/000115178 [DOI] [PubMed] [Google Scholar]
- 29.Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwakkel G, Winters C, Van Wegen EEH, Nijland RHM, Van Kuijk AAA, Visser-Meily A, De Groot J, De Vlugt E, Arendzen JH, Geurts ACH, et al. ; EXPLICIT-Stroke Consortium. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: the EXPLICIT-stroke randomized clinical trial. Neurorehabil Neural Repair. 2016;30:804–816. doi: 10.1177/1545968315624784 [DOI] [PubMed] [Google Scholar]
- 31.Franck JA, Halfens J, Smeets R, Seelen H. Concise Arm and hand Rehabilitation Approach in Stroke (CARAS): a practical and evidence-based framework for clinical rehabilitation management. Open J Occup Ther. 2015;3:10. doi: 10.15453/2168-6408.1164 [DOI] [Google Scholar]
- 32.Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, Saposnik G, Winstein C, van Wegen EEH, Wolf SL, et al. Standardised measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable (SRRR). International Journal of Stroke. 2017;12:451–461. doi: 10.1177/1747493017711813 [DOI] [PubMed] [Google Scholar]
- 33.van der Lee JH, Roorda LD, Beckerman H, Lankhorst GJ, Bouter LM. Improving the action research arm test: a unidimensional hierarchical scale. Clin Rehabil. 2002;16:646–653. doi: 10.1191/0269215502cr534oa [DOI] [PubMed] [Google Scholar]
- 34.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19. doi: 10.1053/apmr.2001.18668 [DOI] [PubMed] [Google Scholar]
- 35.Üstün TB, Chatterji S, Bickenbach J, Kostanjsek N, Schneider M. The International Classification of Functioning, Disability and Health: a new tool for understanding disability and health. Disabil Rehabil. 2003;25:565–571. doi: 10.1080/0963828031000137063 [DOI] [PubMed] [Google Scholar]
- 36.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171 [DOI] [PubMed] [Google Scholar]
- 37.Houwink A, Roorda LD, Smits W, Molenaar IW, Geurts AC. Measuring upper limb capacity in patients after stroke: reliability and validity of the stroke upper limb capacity scale. Arch Phys Med Rehabil. 2011;92:1418–1422. doi: 10.1016/j.apmr.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 38.Bovend’Eerdt TJH, Dawes H, Johansen-Berg H, Wade DT. Evaluation of the modified Jebsen test of hand function and the University of Maryland arm questionnaire for stroke. Clin Rehabil. 2004;18:195–202. doi: 10.1191/0269215504cr722oa [DOI] [PubMed] [Google Scholar]
- 39.Collin C, Wade DT, Davies S, Horne V. The barthel ADL index: a reliability study. Disabil Rehabil. 1988;10:61–63. doi: 10.3109/09638288809164103 [DOI] [PubMed] [Google Scholar]
- 40.Grice KO, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available nine hole peg test for finger dexterity. Am J Occup Ther. 2003;57:570–573. doi: 10.5014/ajot.57.5.570 [DOI] [PubMed] [Google Scholar]
- 41.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–612. doi: 10.1016/s1474-4422(06)70495-1 [DOI] [PubMed] [Google Scholar]
- 42.Duncan PW, Bode RK, Lai SM, Perera S. Rasch analysis of a new stroke-specific outcome scale: the stroke impact scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/s0003-9993(03)00035-2 [DOI] [PubMed] [Google Scholar]
- 43.Krabbe PFM, Stouthard MEA, Essink-Bot ML, Bonsel GJ. The effect of adding a cognitive dimension to the EuroQol multiattribute health-status classification system. J Clin Epidemiol. 1999;52:293–301. doi: 10.1016/s0895-4356(98)00163-2 [DOI] [PubMed] [Google Scholar]
- 44.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 46.Kim WS, Kwon BS, Seo HG, Park J, Paik NJ. Low-frequency repetitive transcranial magnetic stimulation over contralesional motor cortex for motor recovery in subacute ischemic stroke: a randomized sham-controlled trial. Neurorehabil Neural Repair. 2020;34:856–867. doi: 10.1177/1545968320948610 [DOI] [PubMed] [Google Scholar]
- 47.Bertolucci F, Chisari C, Fregni F. The potential dual role of transcallosal inhibition in post-stroke motor recovery. Restor Neurol Neurosci. 2018;36:83–97. doi: 10.3233/RNN-170778 [DOI] [PubMed] [Google Scholar]
- 48.Thickbroom GW, Cortes M, Rykman A, Volpe BT, Fregni F, Krebs HI, Pascual-Leone A, Edwards DJ. Stroke subtype and motor impairment influence contralesional excitability. Neurology. 2015;85:517–520. doi: 10.1212/WNL.0000000000001828 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.