Abstract
Zucker diabetic fatty (ZDF) rats that harbor a mutation in the leptin receptor innately develop type 2 diabetes (T2D) with obesity. Transcutaneous auricular vagal nerve stimulation (taVNS) has an antidiabetic effect in ZDF rats. However, the underlying mechanisms of the weight-gain attenuating effect in ZDF rats by taVNS is still unclear. This study aimed to assess whether the weight-gain attenuating effect of taVNS in ZDF rats is associated with changes in the central nervous system (CNS) expression of P2Y1 receptors (P2Y1R). Adult male ZDF rats were subjected to taVNS and transcutaneous non-vagal nerve stimulation (tnVNS). Their food intake and body weight were recorded daily and weekly, respectively. P2Y1R expression in the hypothalamus, amygdala, and hippocampus was evaluated by western blotting. Hypothalamic P2Y1R expressing cells were detected using immunohistochemistry. Naïve ZDF rats were much heavier (p < 0.05) than their lean littermates (ZL rats), with elevated hypothalamic P2Y1R expression (p < 0.05). Further, taVNS but not tnVNS attenuated weight gain (p < 0.05) without decreasing food intake (p > 0.05) and suppressed hypothalamic P2Y1R expression in ZDF rats (p < 0.05). Moreover, P2Y1R showed major expression in astrocytes of ZDF rats’ hypothalamus. ZDF rats innately develop obesity associated with elevated hypothalamic P2Y1R expression. taVNS attenuates weight gain in ZDF rats without changes in food intake, suggesting increased energy expenditure. Whether the reduced hypothalamic P2Y1R expression in response to taVNS is mechanistically linked to the increased energy expenditure remains to be determined.
Keywords: Obesity, hypothalamus, energy expenditure, astrocytes, tanycytes
Introduction
In recent decades, the most dramatic rise of diabetes prevalence has been witnessed in China. 1 At present, approximately 11% of the population (i.e. >110 million) suffers from diabetes, with a significant proportion remaining undiagnosed.1,2 Being overweight, obese, and gaining excessive weight substantially increase diabetes risk; therefore, interventions to reduce diabetes risk are primarily targeted at weight reduction.3,4
Currently, there are multiple pharmacological and surgical management options for obesity.5,6 In recent years, neuromodulation of the vagal nerve for the treatment of obesity has attracted increasing attention. 7 The vagal nerve innervates the gut and plays an essential role in controlling metabolism. Vagal nerve stimulation (VNS) prevents weight gain in response to a high-fat diet. 8 Previous small-sized clinical studies in patients with depression or epilepsy have shown that VNS could promote weight loss.9,10 Vagal blockade (VBLOC), which directly inhibits the gastric vagal trunks, also results in significant weight loss. 11 Although the mechanisms remain poorly understood, its potential merits further investigation.
Both VNS and VBLOC involve surgical procedures. The regulators and the surgeries are expensive. Potential adverse effects, especially infection, are possible. The vagal nerve has a branch of afferent projections in the auricular concha and external ear channels in mammals. 12 Thus, transcutaneous auricular vagal nerve stimulation (taVNS) was developed based on this anatomical advantage. Besides, auricular acupuncture (AA) or stimulation is known to effectively reduce obesity. 13 Animal studies have shown that auricular acupuncture (AA) or auricular stimulation activates the satiety center, 14 which is more likely concerned with satiation development and preservation and is more effective on obese than in normal rats. 15 A recent study also revealed that auricular vagal nerve stimulation (AVNS) effectively reduces body weight and causes a visceral fat loss in high-fat-diet–induced obese rats. 16 Lately, we also found that auricular concha electroacupuncture (ACEA)/taVNS played an essential role in regulating lipid-lipoprotein metabolism in rats submitted to cold stress. 17 Moreover, AA and taVNS share overlapping stimulating areas and mechanisms. 18 Studies have confirmed that taVNS, a novel auricular non-invasive stimulation, produces comparable efficacy with classic VNS.19,20 The underlying mechanisms of taVNS on obesity need to be further investigated.
The vagal nerve carries visceral and somatic efferent and afferent nerve fibers distributed throughout the central nervous system, either via the nucleus of the solitary tract (NTS) or monosynaptically. 21 There are ascending projections from NTS to the hypothalamus, 22 amygdala, 23 and hippocampus 24 that are limbic structures related to emotion, food intake, and body weight, 25 respectively. VNS or taVNS might stimulate these brain regions to affect metabolism and body weight.
Zucker diabetic fatty (ZDF) rats have a mutation in the leptin receptor, which is associated with leptin resistance, insulin resistance, obesity, and increased fat content. 26 These rats innately develop diabetes and obesity. In our previous study, we showed that taVNS has an antidiabetic effect in ZDF rats. 27 The body weight and food intake results pre- and post-taVNS treatment were also recorded, indicating that taVNS plays a role in the weight-gain attenuating effect in ZDF rats. 27 Nevertheless, the mechanisms of this phenomenon were still unclear.
P2Y1R, a purinergic receptor expressed in a wide range of tissues, has been reported to be involved in feeding behavior, 28 obesity, 29 and metabolism. 30 Stimulation of hypothalamic P2Y1R enhances food intake in rats, possibly depending on P2Y1 receptor-mediated nitric oxide production. 28 P2Y1 receptors are also responsible for the extracellular ATP-mediated intracellular triglyceride accumulation in adipocytes. The P2Y1 receptor antagonist—MRS 2500—significantly inhibited triacylglycerol accumulation, suggesting the P2Y1 receptor to be a novel therapeutic target for the treatment of lipid disorders.29,31 A previous study found that P2Y1R was majorly expressed in the hypothalamus, amygdala, and hippocampus of rat brains, 32 the regions involved in food intake, body weight, and metabolism,25,33,34 respectively. Hence, it is worth investigating the connection between the altered P2Y1R expression in these brain regions and the weight-gain attenuating effect in ZDF rats by taVNS.
In this study, we examined whether taVNS is effective to attenuate weight gain in ZDF rats. Moreover, we determined P2Y1R expression in the hypothalamus, amygdala, and hippocampus of these rats to assess a potential association between attenuation of weight gain by taVNS and central nervous system expression of P2Y1R. Furthermore, we identified P2Y1R expressing cell types within the hypothalamus of ZDF rats.
Methods
Animals
This study’s sample size was calculated based on Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals 35 and according to the 3R principle. 36 Five-week-old male ZDF (fa/fa) rats (n = 30) and lean littermates (ZL [fa/+]; n = 8) were purchased from Beijing Vital River Laboratory Animal Technology Co (Beijing, China) and housed four per cage under an artificial 12:12-h light/dark cycle at an ambient temperature of 22°C ± 1°C, with ad libitum access to food (Purina #5008) and water for 1 week. The bedding and cages were changed every other day. The same person handled the animals and conducted the experiments. The rats were not handled during the week of acclimatization. At the age of 6 weeks, the rats were divided into ZL, ZDF, ZDF + taVNS, and ZDF + tnVNS groups (n = 8 per group) according to their genotype and treatment. The remaining ZDF rats (n = 6) were reared without handling for immunohistochemical detection of P2Y1R-expressing cells. The experimental timeline is shown in Figure 1. The average daily food intake was calculated as a whole for each group during the experiment, and the body weight was recorded weekly.
Figure 1.
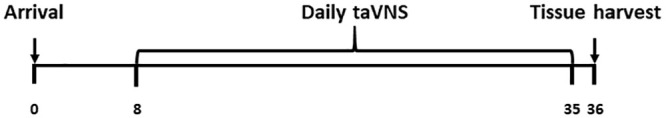
Illustration of experimental time in days. The time points for animal arrival, daily taVNS, and tissue harvest are shown. The daily taVNS administration began 1 week after arrival (on day 8) and continued for 4 weeks (until day 35). On day 36, tissue harvesting was done in the afternoon (between 1400 and 1700 h).
The experimental protocol was approved by the Ethics Committee of the Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, China (Permit No. 20160616). The experiments were carried out according to the Guidelines on the Humane Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China in 2006.
taVNS and tnVNS
Rats in the ZL and ZDF groups received no treatment; those in the ZDF+taVNS and ZDF+tnVNS groups received taVNS and tnVNS, respectively. The procedure was initiated on day 8 (W1) and was continued until day 35 (W5). For taVNS, rats were anesthetized by inhalation of 2% isoflurane, and positive and negative electrodes were placed on the skin inside and outside the auricular concha region of each ear, respectively, 27 so that the electric current would be transmitted through the skin to stimulate the auriculo-vagal nerve fiber. 37 For tnVNS, rats were anesthetized by inhalation of 2% isoflurane, and positive and negative electrodes were placed on the skin inside and outside the auricular margin of each ear, respectively, 27 where few or no vagal nerve fibers are distributed. 37 Both taVNS and tnVNS were applied for 30 min at an intensity of 2 mA, a pulse width of 0.5 ms, and a frequency of 15 Hz once daily using an electrical stimulator (HANS-100). The stimulation was delivered in the afternoon (14:00–17:00) for four consecutive weeks.
Western blotting (WB)
The expression of P2Y1R in the hypothalamus, amygdala, and hippocampus was tested by WB. Rats were decapitated under anesthesia, and brain tissue samples were collected. The segments were homogenized in a lysis sample buffer containing a mixture of proteinase inhibitors (Sigma). Protein samples were separated on SDS-PAGE gel and transferred to polyvinylidene difluoride filters (Millipore, Bedford, MA). The filter was blocked with 5% milk and incubated overnight at 4°C with a primary antibody of P2Y1R (rat monoclonal, 1:100, Santa/Sc-377324) for 1 h at room temperature with HRP-conjugated secondary antibody (Santa/Sc-2005, 1:5000). The blots were visualized in ECL solution (Thermo/34080) for 1 min and exposed onto hyperfilms (Amersham Biosciences) for 1–10 min. The blots were then incubated in a stripping buffer and reprobed with a rabbit polyclonal β-tubulin antibody (Wanleibio/WL01931, 1:2000) as the loading control. The density of specific bands was measured with a computer-assisted imaging analysis system and normalized against the loading controls. We compared the differences using one-way ANOVA.
Immunohistochemistry (IHC)
ZDF rats (n = 6) were anesthetized with sodium pentobarbital and transcardially perfused with 200 mL saline, followed by 200–300 mL cold 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brain sections from the bregma (−1.4 to −4 mm) 38 were dissected, post-fixed for 2 h, and kept in 30% sucrose in 0.1 M PB until they sank to the bottom. Tissues were then mounted in OCT compound and frozen on dry ice.
Brain tissue was sectioned (30-μm thickness) on a cryostat, mounted serially onto microscope slides, and stored at −80°C until further analysis. We used immunohistochemical (IHC) staining to detect P2Y1R (rabbit polyclonal, 1:1000; Abcam, Cambridge, MA); GFAP (astrocyte marker, chicken polyclonal, 1:1000; Abcam, Cambridge, MA); IBA-1 (microglia marker, goats polyclonal, 1:1000; Abcam, Cambridge, MA); and NeuN (neuronal marker, rabbit monoclonal, 1:1000; Abcam, Cambridge, MA). Sections were blocked with 1% goat serum in 0.3% triton for 1 h at room temperature and incubated overnight at 4°C with a primary antibody. For controls, the primary antibody was omitted. The sections were then incubated for 1 h at room temperature with corresponding FITC- or CY3-conjugated secondary antibody (1:200; Jackson ImmunoResearch, West Grove, PA). Four to six nonadjacent brain sections were randomly selected, analyzed using a LEXT OLS4000 3D Laser Measuring Microscope (Olympus), recorded using a digital camera, and processed using Adobe Photoshop.
Statistical analysis
Prism 6 software (GraphPad, La Jolla, CA, USA) was used to analyze the data, which are presented as mean ± SD. Differences between groups were evaluated by one-way analysis of variance followed by the Tukey’s post-hoc test. Differences with p < 0.05 were considered statistically significant.
Results
taVNS attenuates weight gain in ZDF rats without decreasing food intake
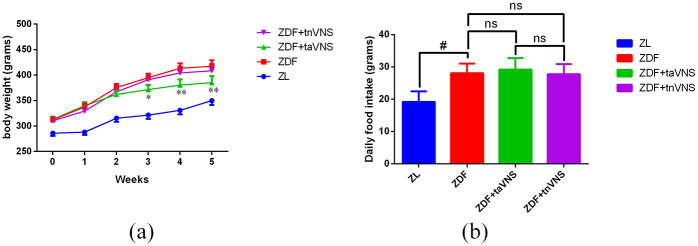
As shown in Figure 2(a), the weight of the naïve ZDF rats was much higher than their lean littermates (ZL rats) at all time points in the study (p < 0.05), and taVNS effectively attenuated the weight gain of ZDF rats 2 weeks after the treatment (p < 0.05 or p < 0.01). However, tnVNS did not bring about a significant weight change in ZDF rats (p > 0.05). This result is consistent with a previous study 27 that showed that taVNS but not tnVNS effectively attenuated weight gain in ZDF rats.
Figure 2.
The weekly body weight of rats and average daily food intake (both in grams) of rats during the experiment: (a) *p < 0.05 ZDF versus ZDF + taVNS or ZDF + tnVNS; **p < 0.01 ZDF versus ZDF + taVNS or ZDF + tnVNS and (b) #p < 0.05 ZL versus ZDF.
As shown in Figure 2(b), the daily food intake of ZDF rats was significantly higher than that of ZL rats (p < 0.05). However, neither taVNS nor tnVNS significantly changed the quantity of food intake of ZDF rats (p > 0.05). This result was also consistent with a previous study, 27 illustrating that taVNS can hardly change the food intake in ZDF rats.
taVNS inhibits hypothalamic P2Y1R expression in ZDF rats
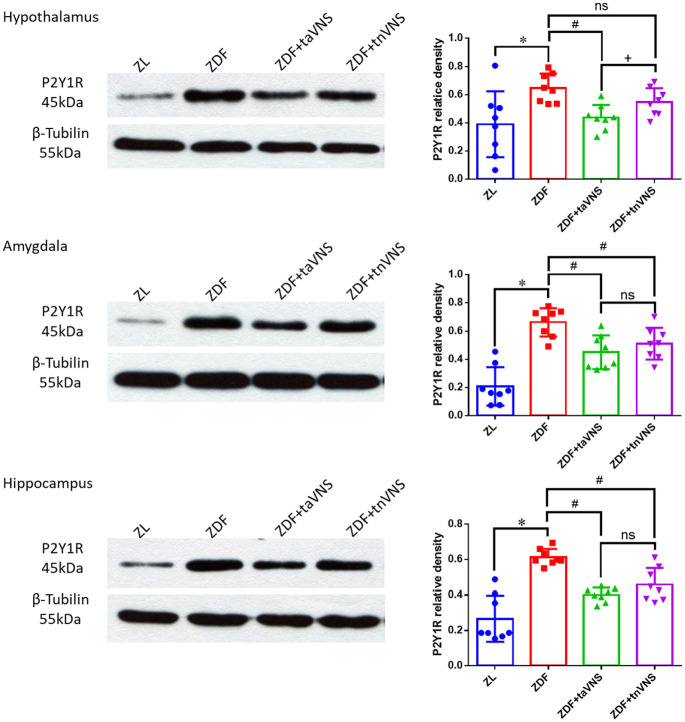
P2Y1R expression in the hypothalamus, amygdala, and hippocampus was detected by WB (Figure 3). The P2Y1R expression in the hypothalamus, amygdala, and hippocampus of the naïve ZDF rats was much higher (p < 0.05) than that in the ZL rats. Compared with those of the naïve ZDF rats, taVNS significantly inhibited the P2Y1R expression in the hypothalamus, the amygdala, and the hippocampus (p < 0.05). tnVNS also inhibited P2Y1R expression in the amygdala and hippocampus of ZDF rats (p < 0.05). However, the P2Y1R expression in the hypothalamus of tnVNS-treated ZDF rats displayed no statistical significance compared with those of the naïve ZDF rats (p > 0.05). At the same time, the hypothalamic P2Y1R expression of taVNS-treated ZDF rats and the hypothalamic P2Y1R expression of tnVNS-treated ZDF rats showed a statistical significance (p = 0.0366 < 0.05).
Figure 3.
Expression of P2Y1R in the hypothalamus, amygdala, and hippocampus of the rats. WB results showing the expression of P2Y1R in the hypothalamus, amygdala, and hippocampus of ZL rats, naïve ZDF rats, ZDF rats treated with taVNS, or ZDF rats treated with tnVNS for 4 consecutive weeks. The ZL group, ZDF group, ZDF + taVNS group, the ZDF + tnVNS group (n = 8 each group). *p < 0.05, ZDF versus ZL; #p < 0.05 ZDF + taVNS or ZDF + tnVNS versus naïve ZDF; +p < 0.05 ZDF + taVNS versus ZDF + tnVNS.
P2Y1R is expressed primarily in hypothalamic astrocytes in ZDF rats
Double-labeling immunofluorescence staining showed that hypothalamic P2Y1R was colocalized with GFAP (Figure 4(a)–(d)) but not IBA-1 (Figure 4(e)–(h)) or NeuN (Figure 4(i)–(l)), indicating that P2Y1R-immunopositive cells in the hypothalamus of ZDF rats have characteristics of astrocytes.
Figure 4.
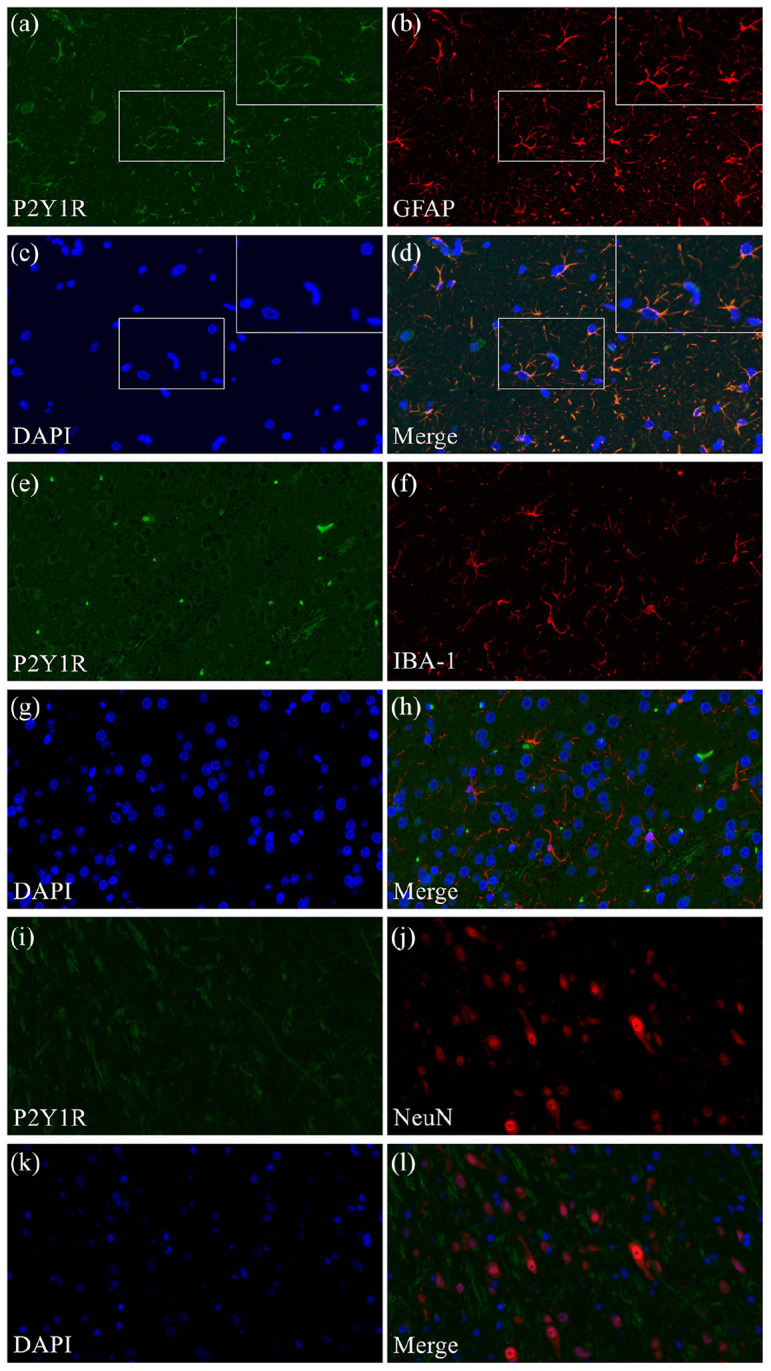
Neurochemical characteristics of P2Y1R-positive cells in the brain. Double-labeling immunofluorescence staining showing P2Y1R colocalization with GFAP (a–d) but not IBA-1 (e–h) or NeuN (i–l).
Discussion
Homozygous for the fa gene mutation, male ZDF rats develop progressively more significant levels of glucose intolerance, insulin resistance, and impaired pancreatic β-cell function with age, leading to frank diabetes. 39 Since these features are consistent with human type 2 diabetes (T2D) associated with obesity, male ZDF rats are widely used as a genetic model for diabetes and obesity.
Previously, we found that taVNS has an antidiabetic effect in ZDF rats, possibly because of the IR expression upregulation and increased melatonin secretion by taVNS.27,40 In the present study, we reconfirmed that only taVNS attenuated weight gain in ZDF rats without affecting their food intake, which is consistent with our previous work. 27 On the other hand, tnVNS, wherein only limited or no vagal nerve stimulation occurs, 37 did not achieve such efficacy. 27
Previous studies have shown that stimulating the cervical vagal nerve (which innervates both the abdominal and auricular branches) affects both food intake and thermogenesis; stimulating the vagal trunks selectively affecting the abdominal organs only decreases food intake; and selectively and transcutaneously stimulating the auriculo-vagal nerve exclusively increases thermogenesis. 7 Thus, taVNS increased thermogenesis in ZDF rats, which resulted in enhanced energy expenditure and attenuated weight gain without affecting the food intake.
Inhibition of hypothalamic P2Y1R expression by taVNS might partially explain the weight-gain attenuating effect in ZDF rats. Compared with naïve ZDF rats, specifically only taVNS-treated rats showed significantly inhibited hypothalamic P2Y1R expression. tnVNS treatment also showed a weak inhibitory effect on P2Y1R expression, but there was no statistical significance in hypothalamic P2Y1R expression between the naïve ZDF rats and tnVNS-treated ZDF rats. Both taVNS and tnVNS significantly inhibited the amygdala-hippocampal P2Y1R expression, which indicated that this inhibited amygdala-hippocampal P2Y1R expression might not be crucial to the weight-gain attenuating effect of taVNS in ZDF rats. 41 Obesity is mostly related to an imbalance between energy intake and expenditure. There is a consensus that energy expenditure is more related to the hypothalamus than the amygdala and hippocampus, 41 with the hypothalamus being the primary focus.41,42
Altered signaling in the hypothalamus is an essential mechanism in obesity. 42 P2Y1R expression in the hypothalamus has previously been identified as a target for food intake. For instance, restricted feeding may enhance the sensitivity of the hypothalamus to extracellular ADP/ATP by regulation of the expression of P2Y1R 43 and stimulation of hypothalamic P2Y1R may increase food intake in rats. 28 However, in this study, inhibition of hypothalamic P2Y1R expression by taVNS did not change daily food intake in ZDF rats. Nevertheless, to the best of our knowledge, no previous research has focused on the hypothalamic P2Y1R expression in energy expenditure.
P2Y1R is responsible for the extracellular ATP-mediated intracellular triglyceride accumulation in adipocytes. 29 MRS 2500, a P2Y1R antagonist, significantly inhibited triacylglycerol accumulation, 44 demonstrating that P2Y1R is a novel therapeutic target for treating lipid disorders and obesity. Hypothalamic P2Y1R in tanycytes has a potential role in the regulation of feeding and energy balance. 45 Thus, the P2Y1R significantly affects energy expenditure, and modulating hypothalamic P2Y1R expression could therefore be a potential therapeutic target for obesity. Besides, we previously found that ACEA/taVNS modulates lipid-lipoprotein metabolism in rats submitted to cold stress, 17 which was considered to have a relation with the altered hypothalamic P2Y1R expression. Thence, inhibiting hypothalamic P2Y1R expression by taVNS might significantly affect triacylglycerol accumulation, which resulted in the weight-gain attenuating effect in ZDF rats. Furthermore, inhibiting hypothalamic P2Y1R expression by taVNS might increase thermogenesis in ZDF rats, which in turn accelerates energy expenditure and attenuates weight gain. Therefore, the results of the present study suggest that hypothalamic P2Y1R expression might affect energy expenditure in ZDF rats.
An immunohistochemical study found that P2Y1R was expressed in neurons and glial cells in several brain regions of SD rats. 46 Another study also found that P2Y1R is located on GFAP-positive astrocytes in male Wistar rats. 47 In this study, we found that P2Y1R was majorly expressed in astrocytes rather than neurons or microglial cells in the hypothalamus of ZDF rats. The neuroendocrine functions of hypothalamic astrocytes were described more than three decades ago. 48 This study has shown that P2Y1R is expressed majorly in hypothalamic astrocytes, which might significantly affect ZDF rats’ energy expenditure. Because tanycytes share some features with astrocytes, we speculated that hypothalamic P2Y1R in tanycytes was the real target. 45 This should be further investigated in future studies.
Our study has some limitations. First, we attempted to perform taVNS in conscious rats in our pre-test, but failed. It seems that for now only big animals or humans can withstand taVNS without anesthesia. A more advanced rodent taVNS equipment should be developed to elucidate the influence of anesthesia in the future. Second, we only tested one frequency (15 Hz) in this study. More frequencies and intensities should be tested in further studies to clarify whether the weight-gain attenuating effect of taVNS in ZDF rats is frequency-dependent or intensity-dependent. Third, the hypothalamus can be subdivided into multiple hypothalamic nuclei; the arcuate nucleus (ARC), paraventricular nucleus (PVN), ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), lateral hypothalamic area (LH), and optic chiasm (OC) are hypothalamic nuclei especially involved in energy homeostasis. 49 Therefore, further studies should elucidate the altered P2Y1R expression in these nuclei, which likely significantly affect body weight and energy expenditure. Last, given the original study design, we did not test whether the blockade of hypothalamic P2Y1R signaling prevents the body weight effect of taVNS; additional studies should clear out this important point.
In summary, taVNS inhibits P2Y1R expression in the hypothalamus of ZDF rats. Whether this effect of taVNS is mechanistically linked to the weight-gain attenuating effect of taVNS in ZDF rats through accelerating energy expenditure remains to be determined. Finally, P2Y1R expression was mainly found in hypothalamic astrocytes of ZDF rats.
Conclusion
Compared to their lean littermates, ZDF rats innately develop obesity with elevated P2Y1R expression in the hypothalamus. taVNS has a weight-gain attenuating effect in ZDF rats that appears to be mediated by increased energy expenditure. Whether the inhibition of hypothalamic P2Y1R expression in astrocytes is mechanistically linked to the increased energy expenditure caused by taVNS remains to be determined. Based on the results of this study, it would be interesting to study the potential role of taVNS as an adjunct treatment modality for obesity along with diet and physical activity.
Acknowledgments
We cordially thank Prof. Lian Zhou of Guangzhou University of Chinese Medicine and Dr. Lingling Yu of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their invaluable guidance throughout this work. We thank Dr. Jing Ling of Shenzhen Traditional Chinese Medicine Hospital for her writing assistance.
Author biographies
Yutian Yu is a medical doctor and researcher working at Beijing Shijitan Hospital, Capital Medical University.
Xun He is a technician working at Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Science.
Jinling Zhang is a technician working at Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Science.
Chunzhi Tang is a researcher working at Guangzhou University of Chinese Medicine.
Peijing Rong is a researcher working at Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Science.
Footnotes
Author contributions: YY and PR designed the experiments. YY and XH performed the experiments. YY, CT, and JZ analyzed the data. YY drafted the manuscript. All the authors discussed the results, reviewed the manuscript, and approved it for publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Joint Sino-German Research Project [GZ1236], National Natural Science Foundation of China [81674072], Beijing Municipal Science and Technology Commission [Z161100002616003], China Scholarship Council [CSC No. 201709920086], Deutscher Akademischer Austauschdienst [91658555], Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme [2016], Beijing Municipal Administration of Hospitals [ZYLX201812], Beijing Administration of Traditional Chinese Medicine ([2019] No.106), Clinical Cooperation Ability Construction Project of Chinese and Western Medicine for Major and Difficult Diseases (Department of Medical Administration, National Administration of Traditional Chinese Medicine [2018] No.3), National Administration of Traditional Chinese Medicine (2019XZZX-JB004), and Youth Project of Beijing Shijitan Hospital (2019-q04).
Ethics approval: Ethical approval for this study was obtained from the Ethics Committee of Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences (Permit No. 20160616).
Animal welfare: The present study followed the Guideline on the Humane Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China in 2006.
ORCID iD: Yutian Yu  https://orcid.org/0000-0002-6399-2807
https://orcid.org/0000-0002-6399-2807
References
- 1.Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018; 61: 1249–1260. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran A, Wan Ma RC, Snehalatha C.Diabetes in Asia. Lancet 2010; 375: 408–418. [DOI] [PubMed] [Google Scholar]
- 3.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29: 2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan KMV, Boyle JP, Thompson TJ, et al. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007; 30: 1562. [DOI] [PubMed] [Google Scholar]
- 5.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015; 100: 342–362. [DOI] [PubMed] [Google Scholar]
- 6.Fisher BL, Schauer P.Medical and surgical options in the treatment of severe obesity. Am J Surg 2002; 184: S9–S16. [DOI] [PubMed] [Google Scholar]
- 7.de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol 2016; 594: 5791–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Królczyk G, Laskiewicz J, Sobocki J, et al. The effects of baclofen on the feeding behaviour and body weight of vagally stimulated rats. J Physiol Pharmacol 2005; 56: 121–131. [PubMed] [Google Scholar]
- 9.Burneo J, Faught E, Knowlton R, et al. Weight loss associated with vagus nerve stimulation. Neurology 2002; 59: 463–464. [DOI] [PubMed] [Google Scholar]
- 10.Pardo J, Sheikh S, Kuskowski M, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes 2007; 31: 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarr MG, Billington CJ, Brancatisano R, et al. The EMPOWER Study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg 2012; 22: 1771–1782. [DOI] [PubMed] [Google Scholar]
- 12.He W, Wang X, Shi H, et al. Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med 2012; 2012: 786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh T-L, Chen H-H, Pai T-P, et al. The effect of auricular acupoint stimulation in overweight and obese adults: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2017; 2017: 3080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asamoto S, Takeshige C.Activation of the satiety center by auricular acupuncture point stimulation. Brain Res Bull 1992; 29: 157–164. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122: 481–486. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zhang J-B, Xu C, et al. Effects and mechanisms of auricular vagus nerve stimulation on high-fat-diet—induced obese rats. Nutrition 2015; 31: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y-t, Xiao G, Zhang J-l, et al. Auricular concha eletroacupuncture modulates lipid-lipoprotein metabolism in rats submitted to cold stress. World J Acupunct Moxibustion 2020; 30: 113–119. [Google Scholar]
- 18.Kong J, Fang J, Park J, et al. Treating depression with transcutaneous auricular vagus nerve stimulation: state of the art and future perspectives. Front Psychiatry 2018; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong P, Liu A, Zhang J, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: a randomized controlled trial. Clin Sci. Epub ahead of print 1April2014. DOI: 10.1042/CS20130518. [DOI] [PubMed] [Google Scholar]
- 20.Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry 2016; 79: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutecki P.Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia 1990; 31: S1–S6. [DOI] [PubMed] [Google Scholar]
- 22.Horst GJT, De Boer P, Luiten PGM, et al. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 1989; 31: 785–797. [DOI] [PubMed] [Google Scholar]
- 23.Ricardo JA, Koh ET.Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 1978; 153: 1–26. [DOI] [PubMed] [Google Scholar]
- 24.Castle M, Comoli E, Loewy A.Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience 2005; 134: 657–669. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud H-R.Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 2002; 26: 393–428. [DOI] [PubMed] [Google Scholar]
- 26.Unger RH.How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab 1997; 8: 276–282. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Zhai X, Li S, et al. Transcutaneous vagus nerve stimulation induces tidal melatonin secretion and has an antidiabetic effect in Zucker fatty rats. PLoS One 2015; 10: e0124195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kittner H, Franke H, Harsch JI, et al. Enhanced food intake after stimulation of hypothalamic P2Y1 receptors in rats: modulation of feeding behaviour by extracellular nucleotides. Eur J Neurosci 2006; 24: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G, Gentile D.The involvement of purinergic signalling in obesity. Purinergic Signall 2018; 14: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Léon C, Freund M, Latchoumanin O, et al. The P2Y 1 receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signall 2005; 1: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laplante M-A, Monassier L, Freund M, et al. The purinergic P2Y1 receptor supports leptin secretion in adipose tissue. Endocrinology 2010; 151: 2060–2070. [DOI] [PubMed] [Google Scholar]
- 32.Kittner H, Franke H, Fischer W, et al. Stimulation of P2Y 1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: implications for the involvement of P2Y 1 receptor-mediated nitric oxide production. Neuropsychopharmacology 2003; 28: 435. [DOI] [PubMed] [Google Scholar]
- 33.Soto M, Cai W, Konishi M, et al. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci U S A 2019; 116: 6379–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luiten P, Ter Horst G, Steffens A.The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol 1987; 28: 1–54. [DOI] [PubMed] [Google Scholar]
- 35.Festing MF, Altman DG.Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 2002; 43: 244–258. [DOI] [PubMed] [Google Scholar]
- 36.Richter V, Muche R, Mayer B.How much confidence do we need in animal experiments? Statistical assumptions in sample size estimation. J Appl Anim Welf Sci 2018; 21: 325–333. [DOI] [PubMed] [Google Scholar]
- 37.He W, Jing X-H, Zhu B, et al. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci 2013; 14: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C.The rat brain in stereotaxic coordinates: compact. 6th ed. New York, NY: Academic Press, 2009. [Google Scholar]
- 39.Kava R, Greenwood M, Johnson P.New rat models of obesity and type II diabetes. ILAR J 1990; 32. [Google Scholar]
- 40.Wachtel LE, Reti IM, Ying H.Stability of intraocular pressure after retinal reattachment surgery during electroconvulsive therapy for intractable self-injury in a 12-year-old autistic boy. J ECT 2014; 30: 73–76. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MW, Porte D.Diabetes, obesity, and the brain. Science 2005; 307: 375–379. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki K, Jayasena CN, Bloom SR.Obesity and appetite control. Exp Diabetes Res 2012; 2012: 824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidel B, Bigl M, Franke H, et al. Expression of purinergic receptors in the hypothalamus of the rat is modified by reduced food availability. Brain Res 2006; 1089: 143–152. [DOI] [PubMed] [Google Scholar]
- 44.Kita T, Arakaki N.Contribution of extracellular ATP on the cell-surface F1F0-ATP synthase-mediated intracellular triacylglycerol accumulation. Biomed Res 2015; 36: 115–120. [DOI] [PubMed] [Google Scholar]
- 45.Bolborea M, Dale N.Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci 2013; 36: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moran-Jimenez M-J, Matute C.Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Mol Brain Res 2000; 78: 50–58. [DOI] [PubMed] [Google Scholar]
- 47.Franke H, Kittner H, Grosche J, et al. Enhanced P2Y 1 receptor expression in the brain after sensitisation with d-amphetamine. Psychopharmacology 2003; 167: 187–194. [DOI] [PubMed] [Google Scholar]
- 48.Salm AK, Smithson KG, Hatton GI.Lactation-associated redistribution of the glial fibrillary acidic protein within the supraoptic nucleus. Cell Tissue Res 1985; 242: 9–15. [DOI] [PubMed] [Google Scholar]
- 49.Neary NM, Goldstone AP, Bloom SR.Appetite regulation: from the gut to the hypothalamus. Clin Endocrinol 2004; 60: 153–160. [DOI] [PubMed] [Google Scholar]