Abstract
Illegitimate recombination that usually takes place at a low frequency is greatly enhanced by treatment with DNA-damaging agents. It is thought that DNA double-strand breaks induced by this DNA damage are important for initiation of illegitimate recombination. Here we show that illegitimate recombination is enhanced by overexpression of the DnaB protein in Escherichia coli. The recombination enhanced by DnaB overexpression occurred between short regions of homology. We propose a model for the initiation of illegitimate recombination in which DnaB overexpression may excessively unwind DNA at replication forks and induce double-strand breaks, resulting in illegitimate recombination. The defect in RecQ has a synergistic effect on the increased illegitimate recombination in cells containing the overproduced DnaB protein, implying that DnaB works in the same pathway as RecQ does but that they work at different steps.
Illegitimate recombination takes place between nonhomologous sequences or short homologous sequences at two different sites of DNA(s) and leads to the duplication, deletion, insertion, or translocation of a chromosome. Illegitimate recombination usually occurs at a low frequency and is greatly enhanced by treatment with DNA-damaging agents (8, 11, 18, 21). Illegitimate recombination is also enhanced by mutations of bacterial genes such as polA, sbcB, topB, osmZ, and recQ (3, 5, 6, 14, 25). The mechanisms of illegitimate recombination enhanced by DNA-damaging agents, as well as bacterial mutations, are not well understood.
It has been shown that there are at least two types of illegitimate recombination in Escherichia coli. One type of illegitimate recombination, which is mediated by DNA gyrase, takes place between nonhomologous DNA sequences, while the other type of illegitimate recombination, which occurs spontaneously or is induced by DNA-damaging agents, takes place between short regions of homology (22, 27). To explain the mechanism of short-homology-dependent illegitimate recombination (SHDIR), Ukita and Ikeda (24) have proposed a model in which a DNA lesion that is formed spontaneously or is induced by DNA-damaging agents stalls the progression of replication forks and then double-strand breaks are introduced at the stalled regions. The resulting DNA ends are processed by some exonuclease(s) and are finally joined with each other at complementary single-stranded regions.
Under normal aerobic conditions, an E. coli chromosome is known to suffer many double-strand breaks in each generation, although most of the breaks are repaired by RecBCD-mediated homologous recombination (17). The double-strand breaks are thought to take place at replication forks when they encounter DNA lesions induced spontaneously or by exogenous stresses. Among the functions involved in DNA replication, DnaB protein appears to have an important role in the formation of double-strand breaks in DNA because the DnaB and DnaC proteins form a complex that transfers the DnaB protein to DNA to form a replication fork and to unwind it. The DnaB protein also has the ability to activate the DnaG primase that is included in the primosome protein complex (2, 26). The DnaB protein also can interact with other replication proteins, such as the tau subunit of DNA polymerase III and the λ P protein (10, 13). Inhibition of DnaB helicase by a dnaB(Ts) mutation results in the formation of double-strand breaks (17).
We have developed a system for the analysis of illegitimate recombination during the formation of transducing phage λbio in E. coli (8). Because illegitimate recombination is thought to be initiated by double-strand breaks in this system, we studied the role of the DnaB protein in the initiation of illegitimate recombination by using this assay. Here we show that illegitimate recombination is induced by overproduction of DnaB helicase without any exogenous stress. We discuss a model for the mechanism of involvement of the DnaB protein in illegitimate recombination. In it, the overproduced DnaB helicase is thought to unwind DNA at the replicating forks in the E. coli chromosome, resulting in double-strand breaks and thus increased levels of illegitimate recombination.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All of the strains used in this study are derivatives of E. coli K-12 and are described in Table 1. Ymel was used for plating of total λ phage. WL95 was used for plating of λ Spi− phage. pRLM6 is a pBR322-based plasmid containing the dnaB gene (19). pBRKm was constructed by insertion of the gene for Kmr into the SalI site of pBR322.
TABLE 1.
E. coli strains used in this study
| Strain | Parent | Relevant genotype or phenotype | Source or construction |
|---|---|---|---|
| Ymel | supE supF | Our collection | |
| WL95 | P2 | Amersham | |
| HI2229 | 594 | sup0 (λa)/pBRKm | HI1547 × pBRKm |
| HI2230 | 594 | sup0 (λa)/pRLM6 | HI1547 × pRLM6 |
| HI2245 | 594 | sup0 ΔrecA306::Tetr (λa)/pBRKm | HI2229 × P1 · GY787L |
| HI2246 | 594 | sup0 ΔrecA306::Tetr (λa)/pRLM6 | HI2230 × P1 · GY787L |
| HI2232 | 594 | sup0 recQ1802::Tn3 (λa)/pBRKm | HI2034 × pBRKm |
| HI2233 | 594 | sup0 recQ1802::Tn3 (λa)/pRLM6 | HI2034 × pRLM6 |
All of the λ prophages described here contain a single copy and carry the cI857 mutation.
Media and conditions of growth of bacteria and phages.
λYP broth contained 10 g of Bacto Tryptone (Difco), 1 g of yeast extract, 2.5 g of NaCl, 1.5 g of Na2HPO4, and 0.18 g of MgSO4 in 1 liter of water and was used to grow bacteria and to detect transducing phage λbio. λ Trypticase agar contained 10 g of Trypticase Peptone (Becton Dickinson), 5 g of NaCl, and 12 g of agar per liter of water and was used to titrate Spi− phages. λ agar contained 10 g of Bacto Tryptone (Difco), 2.5 g of NaCl, and 12 g of agar per liter of water and was used to titrate total λ phage.
Determination of Spi− phage frequency during induction of λ prophage.
E. coli λ cI857 lysogen was grown to 2 × 108 cells/ml at 30°C in λ YP broth. Two milliliters of the culture was transferred into a glass dish with a diameter of 8.5 cm by using a 15-W germicidal lamp at a distance of 37.5 cm. Thermal induction of λ phage was carried out by incubation at 42°C for 15 min with aeration. The culture was then incubated at 37°C for 2 h, and the phage lysate was prepared. The titration of λ Spi− phages in a phage lysate was done by spreading 2 × 107 phages on a lawn of WL95 on a λ Trypticase agar plate. The frequency of λ Spi− phage was determined by measuring the phage titer on WL95, representing the λ Spi− phage number in the lysate, and the titer on Ymel that represents the total λ phage number in the lysate. Burst size was determined by measuring the total λ phage number in a lysate and the total cell number in the culture before heat induction.
Localization and sequencing of recombination junctions by PCR.
The locations of recombination junctions in transducing phage λbio were determined by using sets of primers as described by Ukita and Ikeda (24). Phage DNA containing a recombination junction was amplified by PCR with Taq polymerase. The amplified DNA fragment was directly sequenced by an ABI Prism 310 genetic analyzer.
RESULTS
Overexpression of DnaB helicase enhances illegitimate recombination.
We examined the effect of overexpression of DnaB helicase on the formation, by illegitimate recombination, of λbio transducing phage during prophage induction. For selection of λbio transducing phages, we used the Spi− phenotype (29) because most λbio transducing phages have defects involving the λ red and gam genes. These λ Spi− phages are distinguishable from normal λ phage and docL or docR phage by plaque assay on an E. coli P2 lysogen. When the E. coli HI2229 λ lysogen carrying control plasmid pBRKm was induced by high temperature, the frequency of λ Spi− phages was low. On the other hand, in the HI2230 λ lysogen carrying DnaB overproducer plasmid pRLM6, the frequency of λ Spi− phage was 30-fold higher than that in the wild-type strain (Table 2). This result suggests that an excess amount of DnaB helicase may induce double-strand DNA breaks, resulting in the enhancement of illegitimate recombination.
TABLE 2.
Induction of illegitimate recombination by overexpression of the dnaB gene producta
| UV dose (J/m2) | Strain | Plasmid or relevant mutation | Burst sizeb | Mean Spi− phage no. (10−7)/total λ phage no. (SEM) | Frequency relative to control |
|---|---|---|---|---|---|
| 0 | HI2229 | pBRKm | 65 | 0.019 (0.004) | 1 |
| 0 | HI2230 | pRLM6 | 60 | 0.64 (0.27) | 34 |
| 0 | HI2245 | recA306/pBRKm | 65 | 0.024 (0.07) | 1.3 |
| 0 | HI2246 | recA306/pRLM6 | 36 | 0.73 (0.23) | 38 |
| 50 | HI2229 | pBRKm | 59 | 4.0 (0.55) | 1 |
| 50 | HI2230 | pRLM6 | 49 | 5.8 (1.8) | 1.5 |
| 50 | HI2245 | recA306/pBRKm | 32 | 4.2 (1.2) | 1.1 |
| 50 | HI2246 | recA306/pRLM6 | 22 | 9.3 (2.7) | 2.3 |
| 100 | HI2229 | pBRKm | 30 | 5.2 (0.25) | 1 |
| 100 | HI2230 | pRLM6 | 24 | 11 (1.5) | 2.1 |
| 100 | HI2245 | recA306/pBRKm | 34 | 4.6 (2.5) | 0.88 |
| 100 | HI2246 | recA306/pRLM6 | 32 | 12 (2.1) | 2.3 |
E. coli λ cI857 lysogen or its recA derivative carrying dnaB plasmid pRLM6 or control plasmid pBRKm, grown to 2 × 108 cells/ml, was irradiated with UV at several doses. The lysogen was induced by incubation at 42°C for 15 min with shaking and then incubated at 37°C for 2 h. The frequency of Spi− phage per total phage number and burst size were determined by measuring the phage titer on strain WL95 cells, representing the λ Spi− phage number in the lysate, and the titer on Ymel, representing the total λ phage number. The values are averages of four determinations.
Total λ phage number per cell.
The previous study has shown that the formation of λ Spi− phage is enhanced by UV irradiation (8). To compare the action of UV irradiation on illegitimate recombination with that of the overproduced DnaB protein, the strain carrying pRLM6 was irradiated with UV light and the frequency of λ Spi− phage was determined. The result indicates that the effects of the overproduced DnaB protein and UV irradiation on illegitimate recombination were additive but not synergistic, implying that the recombination events promoted by overproduced DnaB and UV irradiation take place independently of each other (Table 2). Table 2 also shows that enhancement of illegitimate recombination by the overproduced DnaB protein takes place independently of the RecA function, implying that induction of illegitimate recombination occurs independently of the SOS response.
Synergistic effect of DnaB overproduction and the RecQ defect on increased illegitimate recombination.
It has been shown that illegitimate recombination is also enhanced by the recQ mutation, whose effect is synergistic with that of UV irradiation (6). To compare the effects of the overproduced DnaB protein and that of the recQ mutation on illegitimate recombination, plasmid pRLM6 was introduced into a recQ mutant and the frequency of λ Spi− phage was determined. The frequency of λ Spi− phage was increased 670-fold compared to that of the wild type without plasmid pRLM6. Therefore, their effects were synergistic (Table 3), suggesting that enhancement of recombination by the overproduced DnaB protein takes place in the same pathway as that influenced by the recQ mutation. The results also imply that the RecQ helicase participates in a step different from that affected by DnaB overproduction and probably acts in the step of broken-DNA joining as a common suppressor of illegitimate recombination induced by overproduced DnaB or UV irradiation.
TABLE 3.
Effect of the recQ mutation on illegitimate recombination induced by overexpression of the dnaB gene producta
| Strain | Plasmid or relevant mutation | Burst sizeb | Mean Spi− phage no. (10−7)/total λ phage no. (SEM) | Frequency relative to control |
|---|---|---|---|---|
| HI2229 | pBRKm | 71 | 0.024 (0.009) | 1 |
| HI2230 | pRLM6 | 52 | 0.57 (0.12) | 24 |
| HI2232 | recQ1802/pBRKm | 15 | 1.3 (0.3) | 54 |
| HI2233 | recQ1802/pRLM6 | 25 | 16 (4.5) | 670 |
E. coli λ cI857 lysogen or its recQ derivative carrying dnaB plasmid pRLM6 or control plasmid pBRKm was grown to 2 × 108 cells/ml. The lysogen was induced by incubation at 42°C for 15 min with shaking and then incubated at 37°C for 2 h. The frequency of Spi− phage per total phage number and burst size were determined as described in the legend to Table 2.
Total λ phage number per cell.
Distributions and nucleotide sequences of junctions of illegitimate recombination induced by overproduced DnaB helicase.
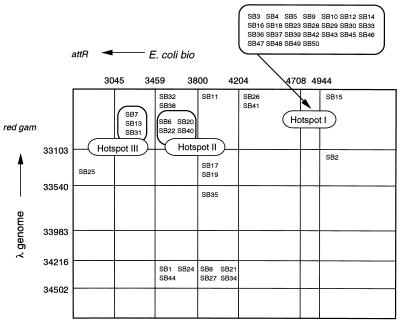
We examined the distribution of recombination junctions of λ Spi− phages induced by overproduced DnaB by using several oligonucleotide primer sets. Many λ Spi− phages were independently isolated from the wild-type bacteria carrying pRLM6. Using PCR, we first confirmed that all of the λ Spi− phages were λbio transducing phages. Next, the distribution of parental recombination sites was estimated from the locations of the junctions. Among the total transducing phages induced by the overproduced DnaB protein, hot spots I, II, and III, which account for 50, 8, and 6%, respectively, were found at the bio gene of E. coli and the gam-git region of λ DNA, whereas the rest were distributed widely (Fig. 1).
FIG. 1.
Distribution of junctions of λbio transducing phages induced by the overproduced DnaB protein. Vertical lines indicate map coordinates of λ DNA, and horizontal lines indicate map coordinates of E. coli bio genes. The box marked hot spot I, II, or III indicates a group of λbio transducing phages that are produced by recombination at the hot spots.
Nucleotide sequences of junctions produced by overproduced DnaB.
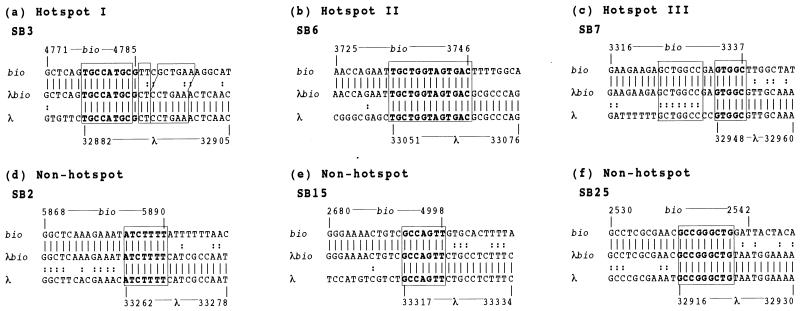
The sequences of recombination junctions of λbio transducing phages SB3, SB6, and SB7, which are formed by recombination at hot spots I, II, and III, respectively, indicated that these recombinations take place between short homologous sequences of 9, 13, and 7 bp, respectively (Fig. 2).
FIG. 2.
Nucleotide sequences of junctions derived from λbio transducing phages induced by the overproduced DnaB protein. (a) Sequences of hot spot I. The junctions of λbio transducing phage SB3 were sequenced, and the sequence of the parental λ and bio recombination sites were then determined. (b) Sequences of hot spot II, which is found in the junctions of λbio transducing phage SB6. (c) Sequences of hot spot III, which is found in the junctions of λbio transducing phage SB7. (d to f) Sequences of non-hot spots detected at the junctions of λbio transducing phages SB2, SB15, and SB25. The sequences in boldface indicate homology at the recombination sites. The enclosed sequences represent short regions and extra short regions of homology between the parental recombination sites. The map coordinates of phage and bacterial sequences are indicated.
Nucleotide sequences of recombination junctions at non-hot spots indicated that λbio transducing phages SB2, SB15, and SB25 are also formed by illegitimate recombination between short regions of homology on the E. coli and λ DNAs (7, 7, and 9 bp, respectively) (Fig. 2). These results indicate that the recombination induced by overproduced DnaB is an SHDIR, which is the same as spontaneous and UV-induced illegitimate recombination (22, 27).
DISCUSSION
Spontaneous illegitimate recombination is enhanced by DnaB overexpression. The recombination enhanced by DnaB overexpression occurs between short regions of homology. These findings indicate that the DnaB protein is involved in SHDIR.
It should be noted that the illegitimate recombination detected in the Spi− assay must be preceded by double-strand breaks because λ prophage needs to be excised before joining of DNA ends. Double-strand breaks of the chromosome are therefore thought to be an essential process in illegitimate recombination. It has been shown that inhibition of DnaB helicase by the dnaB8 mutation induces double-strand breaks in DNA (17). Our study also indicated that the DnaB protein is involved in illegitimate recombination, implying that it participates in double-strand break formation. To explain the mechanism of SHDIR, Ukita and Ikeda (24) proposed a double-strand break-and-join model in which the formation of the transducing phage DNA is initiated by double-strand breaks of the bacterial chromosome. This model assumes that DNA lesions, formed spontaneously or induced by UV irradiation, interfere with the progression of replication forks, leading to the formation of double-strand breaks. The DNA ends thus produced are processed by nucleases and joined to form recombinant DNA molecules. Bierne et al. (4) also proposed that deletion mutations are produced by repair of DNA molecules broken at the blocked replication forks. The fact that the DnaB protein participates in illegitimate recombination is consistent with these models.
The DnaB protein is a DNA helicase essential for DNA replication of bacterial, as well as phage, chromosomes (13). It interacts with many replication proteins, such as DnaC, DnaG, the tau subunit of DNA polymerase III, and the λ P protein (10, 13, 26), forming the replication complex called the replisome. In the replisome, DnaB is thought to play an important role in the unwinding of DNA at the replication forks. Due to these properties of the DnaB protein, double-strand breaks are thought to be produced by the following mechanism. When a DNA lesion is present in a chromosome, the replication forks will be stalled at the DNA lesion but DnaB helicase may continue to unwind the unreplicated region of DNA, thus exposing single-stranded DNA and increasing the possibility of double-strand breaks.
SHDIR has often been observed under spontaneous or UV-induced conditions in many assay systems in E. coli (1, 9, 12, 15, 16, 20, 23, 27, 28). Furthermore, the RecQ function has been shown to participate as a suppressor of illegitimate recombination (6). Our study showed that the effect of the overproduced DnaB on illegitimate recombination is synergistic with that of the recQ mutation. This indicates that the RecQ helicase works as a common suppressor of three kinds of illegitimate recombination, i.e., DnaB-induced recombination, UV-induced recombination, and spontaneous recombination. Hanada et al. (6) proposed that the RecQ helicase may suppress illegitimate recombination by causing the unwinding of a recombination intermediate produced by annealing of complementary single-stranded ends at a late step in recombination. RecQ helicase was also shown to unwind joint molecules formed by the RecA protein (7). The function of the RecQ helicase as a common suppressor of recombination is consistent with Hanada’s model.
ACKNOWLEDGMENTS
We thank H. Ogawa, H. Masai, and the late T. Kogoma for providing bacterial strains and R. Arima and Y. Shobuike for technical assistance.
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas to H.I. from the Ministry of Education, Science, Sports and Culture of Japan and a grant to H.I. from the Uehara Memorial Foundation.
REFERENCES
- 1.Albertini A M, Hofer M, Calos M P, Miller J H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982;29:319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- 2.Allen G C, Kornberg A. Assembly of the primosome of DNA replication in Escherichia coli. J Biol Chem. 1993;268:19204–19209. [PubMed] [Google Scholar]
- 3.Allgood N D, Silhavy T J. Escherichia coli xonA (sbcB) mutant enhances illegitimate recombination. Genetics. 1991;127:671–680. doi: 10.1093/genetics/127.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierne H, Ehrlich S D, Michel B. Deletions at stalled replication forks occur by two different pathways. EMBO J. 1997;16:3332–3340. doi: 10.1093/emboj/16.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coukell M B, Yanofsky C. Increased frequency of deletions in DNA polymerase mutants of Escherichia coli. Nature. 1970;228:633–635. doi: 10.1038/228633a0. [DOI] [PubMed] [Google Scholar]
- 6.Hanada K, Ukita T, Kohno Y, Saito K, Kato J, Ikeda H. RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. Proc Natl Acad Sci USA. 1997;94:3860–3865. doi: 10.1073/pnas.94.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmon F G, Kowalczykowski S C. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 1998;12:1134–1144. doi: 10.1101/gad.12.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda H, Shimizu H, Ukita T, Kumagai M. A novel assay for illegitimate recombination in Escherichia coli: stimulation of λbio transducing phage formation by ultraviolet light and its independence from RecA function. Adv Biophys. 1995;31:197–208. doi: 10.1016/0065-227x(95)99392-3. [DOI] [PubMed] [Google Scholar]
- 9.Jones I M, Primrose S B, Ehrlich S D. Recombination between short direct repeats in a recA host. Mol Gen Genet. 1982;188:486–489. doi: 10.1007/BF00330053. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Dallmann H G, McHenry C S, Marians K J. Tau couples the leading- and lagging-strand polymerases at the Escherichia coli DNA replication fork. J Biol Chem. 1996;271:21406–21412. doi: 10.1074/jbc.271.35.21406. [DOI] [PubMed] [Google Scholar]
- 11.Kokontis J M, Vaughan J, Harvey R G, Weiss S B. Illegitimate recombination induced by benzo[a]pyrene diol epoxide in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:1043–1046. doi: 10.1073/pnas.85.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai M, Ikeda H. Molecular analysis of the recombination junctions of λbio transducing phages. Mol Gen Genet. 1991;230:60–64. doi: 10.1007/BF00290651. [DOI] [PubMed] [Google Scholar]
- 13.LeBowitz J H, McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986;261:4738–4748. [PubMed] [Google Scholar]
- 14.Lejeune P, Danchin A. Mutations in the bglY gene increase the frequency of spontaneous deletions in Escherichia coli K-12. Proc Natl Acad Sci USA. 1990;87:360–363. doi: 10.1073/pnas.87.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez P, Espinosa M, Greenberg B, Lacks S A. Generation of deletions in pneumococcal mal genes cloned in Bacillus subtilis. Proc Natl Acad Sci USA. 1984;81:5189–5193. doi: 10.1073/pnas.81.16.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvo S L, King S R, Jaskunas S R. Role of short regions of homology in intermolecular illegitimate recombination events. Proc Natl Acad Sci USA. 1983;80:2452–2456. doi: 10.1073/pnas.80.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michel B, Ehrlich S D, Uzest M. DNA double-strand breaks caused by replication arrest. EMBO J. 1997;16:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller H J. Artificial transmutation of the gene. Science. 1927;66:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama N, Arai N, Bond M W, Kaziro Y, Arai K. Nucleotide sequence of dnaB and the primary structure of the DnaB protein from Escherichia coli. J Biol Chem. 1984;259:97–101. [PubMed] [Google Scholar]
- 20.Pribnow D, Sigurdson D C, Gold L, Singer B S, Napoli C, Brosius J, Dull T J, Noller H F. rII cistrons of bacteriophage T4. DNA sequence around the intercistronic divide and positions of genetic landmarks. J Mol Biol. 1981;149:337–376. doi: 10.1016/0022-2836(81)90477-0. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz D O, Beckwith J R. Mutagens which cause deletions in Escherichia coli. Genetics. 1969;61:371–376. doi: 10.1093/genetics/61.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu H, Yamaguchi H, Ashizawa Y, Kohno Y, Asami M, Kato J, Ikeda H. Short-homology-independent illegitimate recombination in Escherichia coli: distinct mechanism from short-homology-dependent illegitimate recombination. J Mol Biol. 1997;266:297–305. doi: 10.1006/jmbi.1996.0794. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu H, Yamaguchi H, Ikeda H. Molecular analysis of λbio transducing phage produced by oxolinic acid-induced illegitimate recombination in vivo. Genetics. 1995;140:889–896. doi: 10.1093/genetics/140.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ukita T, Ikeda H. Role of the recJ gene product in UV-induced illegitimate recombination at the hotspot. J Bacteriol. 1996;178:2362–2367. doi: 10.1128/jb.178.8.2362-2367.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whoriskey S K, Schofield M A, Miller J H. Isolation and characterization of Escherichia coli mutants with altered rates of deletion formation. Genetics. 1991;127:21–30. doi: 10.1093/genetics/127.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickner S, Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci USA. 1975;72:921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Yamashita T, Shimizu H, Ikeda H. A hotspot of spontaneous and UV-induced illegitimate recombination during formation of λbio transducing phage. Mol Gen Genet. 1995;248:637–643. doi: 10.1007/BF02191702. [DOI] [PubMed] [Google Scholar]
- 28.Yi T-M, Stearns D, Demple B. Illegitimate recombination in an Escherichia coli plasmid: modulation by DNA damage and a new bacterial gene. J Bacteriol. 1988;170:2898–2903. doi: 10.1128/jb.170.7.2898-2903.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zissler J, Signer E, Schaefer F. The role of recombination in growth of bacteriophage lambda. II. Inhibition of growth by prophage P2. In: Hershey A D, editor. The bacteriophage lambda. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1971. pp. 469–475. [Google Scholar]