Abstract
Lymphedema is manifested as a chronic swelling arising due to stasis in the lymphatic flow. No cure is currently available. A non-invasive treatment is a 3 week complete decongestive therapy (CDT), including manual lymphatic drainage and compression bandaging to control swelling. As CDT leads to mobilization of several liters of fluid, effects of CDT on hyaluronan clearance (maker for lymphatic outflow), volume regulating hormones, total plasma protein as well as plasma density, osmolality and selected electrolytes were investigated. In this pilot study, we assessed hyaluronan and volume regulating hormone responses from plasma samples of nine patients (three males, six females, aged 55 ± 13 years) with lower limb lymphedema stage II-III, before - and after - CDT. A paired non-parametric test (Wilcoxon) was used to assess hormonal and plasma volume changes. Correlation was tested using Spearman’s correlation. The main findings of this novel study are that lymphedema patients lost volume and weight after therapy. Hyaluronic acid did not significantly change pre- compared to post-CDT. Aldosterone increased significantly after therapy, while plasma renin activity increased, but not significantly. Plasma total protein, density, osmolality and sodium and chloride did not show differences after CDT. To our knowledge, no study has previously investigated the effects of CDT on volume regulating hormones or electrolytes. To identify the time-course of volume regulating hormones and lymphatic flow changes induced by CDT, future studies should assess these parameters serially over 3 weeks of therapy.
Keywords: Hyaluronic acid, lymphatic flow, complete decongestive therapy, volume regulation, leg lymphedema
Introduction
Distinct from the cardiovascular system, the adjunct lymphatic system consists of a second network of vessels that play an important but not well-recognized and poorly understood role in normal physiology. Principal functions of the lymphatic vasculature include the regulation of tissue pressure, immune surveillance, absorption of dietry fat in the intestine, but also the maintainance of fluid and colloid homeostasis through a network of open-ended vessels embedded in the tissue throughout the whole body.1–3 These lymphatic capillaries start blind-ended and are responsible for taking up the lymph, an exudate from the blood vessels. 4 Blood plasma is continuously filtered from blood capillaries into the interstitial space via the dynamics of blood pressure and osmotic pressure, whereas colloid osmotic pressure influences filtration across the endothelial glycocalyx of both, permeable and continuous capillaries. 4 During this process excess fluid and macromolecules from the interstitial are taken up by the lymphatic vessels and returned into the blood stream via an increasingly larger hierarchy of lymphatic vessels, reaching the blood circulation ultimately via the thoracic duct.2,5
Dysfunctional lymphatic vessels can cause accumulation of fluid within the tissues that can lead to a range of pathological conditions such as the appearance of a regional swelling, especially in peripheral extremities. Lymphedema is a chronic, progressive and disabling disease that affects the lymphatic system and is characterized by accumulation of fluid in the tissue resulting from lymphatic system insufficiency and deranged lymphatic transport. 6 Lymphedema arises when filtration of plasma into the interstitium is higher than the drainage capacity of the lymphatic system. 7 Lymphedema is manifested in a chronic severe swelling of various body parts that arises due to stasis in lymphatic outflow and retention of fluid that can reach up to 5–15 liters. Further, lymphedema is characterized by accumulation of fat8,9 and/or transformation of lymphatic fluid toward an increased subcutaneous fat deposition, including fibrosis and inflammation.10–12 Patients suffering from lymphedema have progressive swellings, recurrent infections, pain, and a significantly decreased quality of life. 13 Currently, there is no cure for lymphedema. It, therefore, requires continuous therapy comprising of meticulous care on behalf of the patient, non-invasive treatment, and occasionally, surgery. 14 The non-invasive treatment of this chronic disease is a physical treatment, complete decongestive therapy (CDT), which lasts for 3 weeks. CDT is a multicomponent therapy program aimed at decreasing limb volume and reducing the progression of lymphedema. 15 CDT consists mainly of manual lymphatic drainage (MLD), bandaging and compression of the affected body part to control the swelling together with additional physical exercises. Although MLD alone does not have an effect on fluid reduction, 16 overall CDT has been shown to mobilize the accumulated fluid, and is believed to increase lymphatic flow 17 as well as improve quality of life of patients with lymphedema. 14 This form of non-surgical conservative therapy is rather preventive than curative. 13 To what extent CDT effects the cardiovascular system and volume regulating hormones in lymphedema patients, however, is currently unknown or understudied.
Hyaluronic acid
Hyaluronic acid (HA) is a component of the interstitial matrix in skin. Through its contribution to osmotic pressure and resistance to flow, the macromolecule takes part in the regulation of tissue hydration and the maintenance of water and protein homeostasis. Indeed, HA has strong and important water-retaining properties and appears to be partly responsible for the protein-excluding behavior of the interstitium. Lymphatic HA concentration is several times higher than that in plasma, suggesting that the lymphatic outflow back into the vasculature may account for the majority of HA found in plasma. Therefore, hyaluronic acid can be used as a marker of lymphatic outflow since interaction between lymphatic flow and vascular blood flow can change HA levels in response to different physiological stresses and conditions.18–21 However, the studies just referenced included data collected in healthy participants. The physiological fluid dynamics surrounding lymphedema, as well as how complete decongestive therapy modulates these fluid shifts are currently not well understood. As 3 weeks of complete decongestive therapy leads to mobilization of several liters of fluid from the affected body part (Beix et al.) 22 , we investigated what effect CDT has on lymphatic outflow (assessed using hyaluronic acid levels), volume regulating hormones (plasma renin activity, aldosterone), and total plasma protein as well as plasma density, osmolality and selected plasma electrolytes (chloride and sodium). We hypothesized that lymphatic outflow, as quantified via plasma hyaluronic acid levels, will show increases following 3 weeks of CDT.
Methods
This prospective case-controlled pilot study was conducted at the center for physical therapy and rehabilitation, clinical center for lymphatic disorders, KABEG, Wolfsberg, Austria from March 2017 to April 2017. This was a pilot study, which was aimed at generating data to assess trends in the measured responses, which would then serve as the basis for sample size calculations for future studies. Ethics approval was granted by the Ethics Committee of Klagenfurt (EK: A 03/17) and the Ethics Committee of the Medical University Graz, Austria (EK: 29-090 ex 16/17). The procedures followed were in accordance with the Declaration of Helsinki of 1964 and its most recent revision 2013. Each participant received detailed information and gave written and informed consent prior to participation.
Patients
Nine patients (three male, six female, 55 years ± 13 years, 164.2 ± 6.1 cm height) were recruited prior to undergoing the 3 week complete decongestive therapy at the clinical center for lymphatic disorders, KABEG, Wolfsberg, Austria. Strict inclusion and exclusion criteria were used to enroll patients in the study. Included were patients with stage II or III lower limb lymphedema (defined as pitting edema, irreversible with limb elevation up to fibroadipose deposition and skin changes), in accordance with the 2020 consensus document of the International Society of Lymphology (ISL).23,24 All patients were diagnosed with lymphedema for at least 1 year. Excluded were patients with mental disorders, or those with histories of cardiovascular diseases, syncope or alcoholism as well as those on specific medications that could influence the measured parameters such as beta blockers or diuretics. Pregnant women were also excluded.
CDT protocol
During a 3 week period of complete decongestive therapy, patients received manual lymph drainages every day (except on weekends) for 30 min. Compression bandages were applied by therapists every day and were worn the whole day and overnight. Additionally, the patients took part in physical exercise therapy. Therapy standards that were used in this study were based on the Döller recommendations. 24 These are comprised of: (i) Manual lymphatic drainage (ML): Distinct hand movements performed by lymph therapists during ML contained circulating, rhythmic, pumping and sometimes scrubbing movements with relatively low pressure (30–40 mmHg) together with a slow movement which was adjusted to the spontaneous frequency of the lymphangion (10/min). The skin and subcutaneous tissue was stretched during this procedure. It started in the area of healthy, adjoining body areas. The lymphatic vessels located there react with an increased lympho-angiomotor activity, with a consequent increase in the formation of lymph and lymphatic outflow. Subsequently, the treatment was extended to the blocked trunk quadrants (ventral and dorsal) and further to the affected extremity (proximal before distal); and (ii) application of the compression bandages. Compared to manual lymphatic drainage, which does not add further volume reduction, 16 compression bandaging is the only component for volume reduction in the affected limbs, as well as for improving muscle and joint pump function.
Height, weight and abdominal girth of each patient was assessed at the start and after completion of the 3 weeks of therapy. Body mass index (BMI) was calculated from the weight and height of the patient: (weight in kg)/(height in m) 2 . Abdominal girth was measured at the thickest part of the abdomen (maximum 2 cm below the navel, at the navel or up to 3cm above) using a standard measuring tape.
Perometer measurements for assessing volume changes in affected limb
For measuring leg volume, a perometer typ 550 T (Pero-System Messgeräte, Germany) together with PeroPlus 2000 software was used.
Blood sampling
Ten milliliter venous blood was collected from the ante-cubital vein, pre- and post-therapy. EDTA blood samples were then immediately centrifuged at 1500 G for 15 min at 6°C. Plasma was collected and stored in aliquots at −70°C until used for further measurements, carried out at the Medical University of Graz.
Plasma analyses
Hyaluronic acid levels were measured using a time-resolved fluorescence immunoassay.
Aldosterone was measured using an aldosterone ELISA Kit (Hölzel Disgnostika, Germany), and plasma renin activity (PRA) with an activity ELISA Kit (Hölzel Diagnostika, Germany).
Plasma osmolality was measured with Fiske 2400 Multi-Sample Osmometer and the plasma density was determined with a DMA58 density meter. Total plasma protein was measured using Pierce 600 nm Protein Assay Kit. Electrolytes were measured in a routine laboratory using ionsensitive electrodes.
Statistics
Data are presented as median (interquartile range (IQR); min–max). A paired non-parametric test (Wilcoxon sign rank test) was used to assess hormonal and plasma volume changes. Correlation was tested using Spearman’s correlation. P values <0.05 were considered as statistically significant. All statistics were performed using GraphPad Prism 7.03 (GraphPad Software Inc, La Jolla, CA, USA).
Results
In this pilot study, nine patients (n = 3 males, n = 6 females, aged 55 ± 13 years) diagnosed with lymphedema stage II–III according to the ISL staging system 24 using perometry were included (Table 1). Seven of the nine patients were diagnosed with bilateral leg lymphedema.
Table 1.
Overview of nine lymphedema patients participating in this pilot study.
| ID | Diagnosis | Stage | Sex | Age(years) | Volumereduction (mL) | Height(cm) | Weight (kg) | BMI | Abdominalgirth (cm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Secondary benign leglymphedema left | II–III | f | 59 | 1174 | 156 | 66 (−1) | 27 (−1) | 86 |
| 2 | Primary leg lymphedemaboth sides | III | m | 59 | 554 r, 1083 l,total 1637 | 169 | 94.2 (−1) | 33 | 112 (+1) |
| 3 | Primary leg lymphedema left | II | f | 39 | 1718 | 169 | 65.8 (−2.6) | 23 (−1) | 83 |
| 4 | Primary leg lymphedemaboth sides | III | m | 63 | 3839 r, 4032 l,total 7871 | 162 | 140.4 (−8.4) | 54 (−3) | 138 (−3) |
| 5 | Primary leg lymphedemaboth sides | II | m | 25 | 706 r, 444 l,total 1150 | 168 | 60.4 (+1.2) | 21 | 78 (+1) |
| 6 | Secondary benign leglymphedemaboth sides | II | f | 67 | 483 r, 574 l,total 1057 | 158 | 58.6 (+1.6) | 23 | 85 (+1) |
| 7 | Primary leg lymphedemaboth sides | III | f | 61 | 5110 r, 4113 l,total 9223 | 170 | 104.8 (−9.2) | 26 (−3) | 116 (−11) |
| 8 | Chronic secondary leglymphedemaboth sides | II | f | 58 | 1075 r, 947 l,total 2022 | 171 | 70.4 (−1) | 24 | 87 (−4) |
| 9 | Chronic leg lymphedemaboth sides | II-III | f | 65 | 1937 r, 1331 l,total 3268 | 155 | 107.2 (−4.8) | 45 (−2) | 145 (−2) |
Diagnosis, lymphedema stage, sex, age (in years), height and BMI (body mass index) and was determined for each patient prior to the study. Leg volume (l = left, r = right), weight and abdominal girth was assessed before and after complete decongestive therapy. Absolute changes are displayed in the brackets.
Patients showed a reduction of fluid in the leg from 11.0 (8.4–12.7; min–max: 2.7–16.8) to 10.7 (8.–11.8; min–max: 2.7–13.8) liters (p < 0.001) and a loss of body weight from 70.4 (63.1–106.0; 58.6–140.4) to 69.4 (62.4–99.0; 60.2–132.0) kg after 3 weeks of complete decongestive physical therapy (p = 0.180). Volume loss and weight loss showed a high correlation (rho = 0.926, p < 0.001).
The median basal level of plasma hyaluronic acid was 7.30 (4.85–39.38; 2.73–240.68) ng/mL. Hyaluronic acid concentration did not change due to CDT, with a median of 12.98 (5.60–26.85; 3.98–75.99) (Figure 1) due to high variances between patients (p = 0.333) (Table 2). Analyzing hyaluronic acid changes based on the severity of lymphedema did not show any pattern that could explain the high variances (Table 3).
Figure 1.
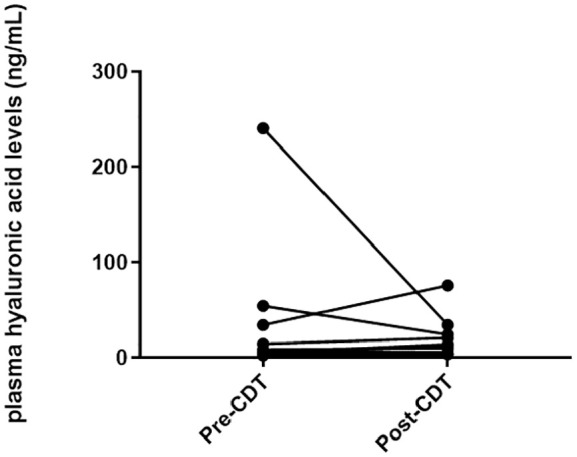
Inidvidual plasma hyaluronic acid levels for each patient (n = 9) before and after 3 weeks of therapy.
Table 2.
Individual hyaluronic acid levels of all patients included in this pilot study (n = 9) showing high inter-individual differences across the 3 weeks of therapy, even at baseline levels.
| ID | Diagnosis | Stage | Plasma hyaluronic acid levels (ng/mL) |
|
|---|---|---|---|---|
| Pre-CDT | Post-CDT | |||
| 1 | Secondary benign leg lymphedema left | II–III | 5.19 | 14.22 |
| 2 | Primary leg lymphedema both sides | III | 34.39 | 75.99 |
| 3 | Primary leg lymphedema left | II | 3.83 | 4.66 |
| 4 | Primary leg lymphedema both sides | III | 240.68 | 34.30 |
| 5 | Primary leg lymphedema both sides | II | 2.73 | 3.98 |
| 6 | Secondary benign leg lymphedemaboth sides | II | 54.37 | 24.36 |
| 7 | Primary leg lymphedema both sides | III | 5.86 | 5.92 |
| 8 | Chronic secondary leg lymphedemaboth sides | II | 5.28 | 11.75 |
| 9 | Chronic leg lymphedema both sides | II–III | 14.51 | 20.88 |
| Median | 7.30 | 12.98 | ||
| IQR | 4.85–39.38 | 5.60–26.85 | ||
| Min-max | 2.73–240.68 | 3.98–75.99 | ||
| p-value | Pre-CDT vs post-CDT: 0.333 | |||
Table 3.
Individual hyaluronic acid levels divided according to the diagnosed stage of lower limb lymphedema (n = 9) showing high inter-individual differences across the 3 weeks of therapy.
| Plasma hyaluronic acid levels (ng/mL) |
||
|---|---|---|
| Pre-CDT | Post-CDT | |
| Stage II | 3.83 | 4.66 |
| 2.73 | 3.98 | |
| 54.37 | 24.36 | |
| 5.28 | 11.75 | |
| Stage II–III | 5.19 | 14.22 |
| 14.51 | 20.88 | |
| Stage III | 34.39 | 75.99 |
| 240.68 | 34.30 | |
| 5.86 | 5.92 | |
| Median | 7.30 | 12.98 |
| IQR | 4.85–39.38 | 5.60–26.85 |
| Min–max | 2.73–240.68 | 3.98–75.99 |
| p-value | Pre-CDT vs post-CDT: 0.333 | |
In comparison to baseline levels, aldosterone levels increased significantly from 66.88 (56.33–92.76; 48.57–98.99) pg/mL to 97.64 (84.55–101.71; 77.69–150.89) pg/mL at the end of therapy (p = 0.005) (Figure 2).
Figure 2.
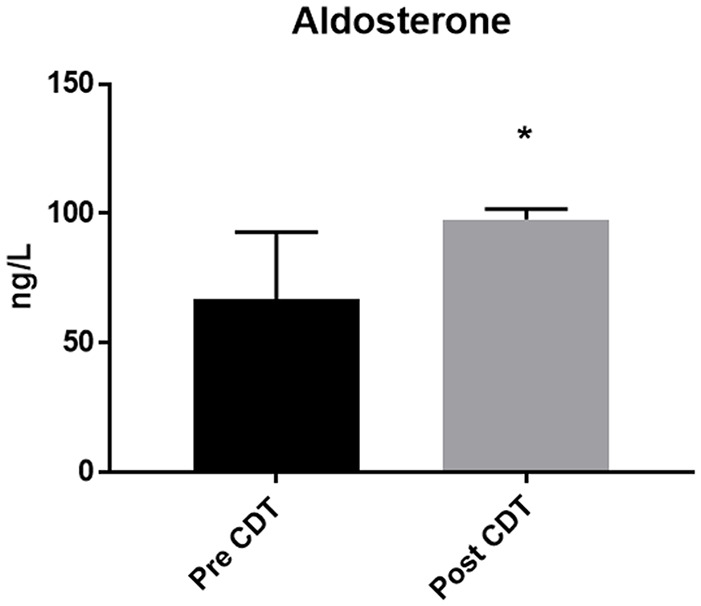
Plasma aldosterone levels increased due to complete decongestive therapy (p = 0.005). Data is presented as median and interquartile range.
Further, a non-significant increase from 0.58 (0.32–2.33; 0.04–5.44) to 0.70 (0.53–1.60; 0.19–19.03) ng/(mL×h) in plasma renin activity (PRA) levels was detected post-CDT (p = 0.721).
Plasma density showed a non-significant decrease from 1.021 (1.020–1.021; 1.018–1.023) g/cm3 to 1.019 (1.019–1.020; 1.018–1.022) g/cm3 after completing CDT (p = 0.093). Similarly, no significant change in plasma total protein (p = 0.646) was found pre- and post-therapy.
No changes were detectable in osmolality (from 313.5 (309.3–317.4; 299.0–324.0) to 311.0 (304.6–318.8; 301.0–320.0) mOsm/k; p = 0.235) as well as in the electrolytes chloride (from 96.45 (93.35–97.38; 93.20–99.40) to 95.65 (92.40–97.10; 91.90–100.00) mmol/l; p = 0.508) and sodium (from 134.50 (129.50–135.50; 126.00–137.00) to 131.50 (131.50–136.50; 125.00–145.00) mmol/l; p = 0.682) levels compared pre- and post-therapy.
Discussion
In this pilot study, we investigated plasma hyaluronic acid levels and volume regulating hormone responses in patients with lymphedema stage II to III before and after 3 weeks of complete decongestive therapy. The main findings, as expected, were that lymphedema patients decrease leg fluid volume as well as body weight due to the complete decongestive therapy. Volume regulating hormones reflected partly the fluid shifts effects of CDT: aldosterone increased significantly after therapy while plasma renin activity increased but not significantly. Hyaluronic acid, used as surrogate marker for lymphatic outflow, increased pre- and post-therapy but again due to the high variance between patients no significance was seen. Furthermore, plasma total protein, density, osmolality and sodium and chloride levels did not show differences before and after complete decongestive therapy.
Fluid and weight reduction
Decongestive physical therapy is known to reduce the volume in lymphedema affected body parts and increase lymphatic flow.25,26 We determined a fluid loss in the affected legs after 3 weeks of therapy (p < 0.001). Additionally, patients lost 1kg of body weight (p = 0.180). Volume loss and weight loss showed a high correlation (rho = 0.926), which suggests that the weight loss could have arisen due to the fluid loss in the legs. An increased capacity for exercise as well as psychological re-engagement with regular activities during therapy could also have positively influenced the fluid and weight loss. 27
Hyaluronic acid
Hyaluronic acid has been suggested to be a marker of lymphatic outflow in healthy subjects.19,21,28 We have previously shown that plasma hyaluronic acid levels increase during exercise 18 or post-prandial in healthy subjects. 20 Roh et al. 29 showed increased level of hyaluronic acid within the lymphedema tissue in an acquired lymphedema mouse model, similar to what was observed in human lymphedema tissue. 30 Using hyaluronan as a surrogate for lymphatic outflow, in our study we observed increases in plasma hyaluronan post-therapy but this was not significant. It is possible that the lack of significance in our hyaluronan results could be due to the high interquartile ranges seen in these values between patients even pre-therapy.
The high variances seen in the hyaluronan data can be seen even in baseline levels, where values from 2.72 ng/mL up to 240.68 ng/mL were measured. As can be seen in Figure 1 and Table 2, there are mainly two patients, ID #4 and #6, which showed a massive reduction in hyaluronic acid levels pre- versus post-therapy. We could not find a common denominator between these two patients. They differ in their lymphedema diagnosis (primary vs secondary lymphedema), and stage (III vs II) as well as sex. Separating the values according to the patients’ diagnosed stage of lower limb lymphedema did not elucidate a pattern in hyaluronic acid plasma level over 3 weeks of therapy that could explain the high inter-individual variances (Table 3). All other patients, however, showed an increase in HA plasma levels after 3 weeks of therapy.
Our results are in contrast our postulated hypothesis that 3 weeks compression therapy along with excercises would increase the lymphatic outflow and be reflected in increases in hyaluronic acid in plasma. Our results also raise the question whether hyaluronan in plasma can act as a realistic indicator of lymphatic outflow in patients, in whom the HA molecule might be retained within the tissue due to it’s large molecular size. It is possible that the decongestive therapy mobilizes fluid from the interstitial space but without accompanying hyaluronic acid molecules in lymphedema patients. Hyaluronic acid is a very large molecule, which may be resistant to breakdown and wash out by local physical pressure application such as during CDT. Indeed, new therapies show that either recombinant hyaluronidase 29 or local heat therapy 31 may be successful in breaking down high molecular weight hyaluronic acid in mouse models.
Several previous studies have reported that exercise therapy enhances lymphatic flow in patients with lymphedema.7,32,33 Our data using plasma hyaluronic acid as a marker for lymphatic outflow, however, do not suggest an increase in lymphatic outflow post-CDT. The discrepancy between our results, which show no changes plasma hyaluronan levels post-therapy, and those of others7,32,33 could be due to different populations of lymphedema patients that were included or different assessment of lymphatic flow used in those studies.
Aldosterone and plasma renin activity
We observed an increase of aldosterone levels pre-and post-therapy (p = 0.05). There are several triggers for aldosterone release, including hypovolemia, changes in plasma electrolytes including sodium and potassium as well as plasma renin activity. As we did not see changes in plasma density and plasma total protein levels post-CDT, hypovolemia following CDT as a trigger of increases in aldosterone can be ruled out. Similarly, as the levels of sodium did not change significantly pre- and post-therapy, hyponatremia could also not have contributed to the increases in aldosterone. Plasma renin activity doubled post-therapy. However, this result was not statistically significant. Again the lack of significance can be due to the higher variance in PRA levels, especially post-CDT. Future studies should examine which physiological alterations during CDT could have led to the tendency of aldosterone and PRA increases.
Limitations
In this pilot study we could only include nine patients. Therefore, the study population in terms of number and homogeneity is limited. However, this study was primarily carried out as a pilot study to (1) allow us to calculate the sample size; (2) to assess whether it was possible to measure all these changes over 3 weeks of treatment; (3) to assess carefully the limitations that could arise from such clinical studies in very sick patients; and (4) to ensure that blood collection to assess plasma hyaluronic acid should be done immediately following CDT, as it has a short plasma half-life. 23
Another limitation of this study is that the time points of blood collection was prior and post 3 weeks of therapy, so the time course of changes in the measured parameters cannot be determined. The reason for the pre- and post- treatment measurements was to, in the first instance, assess whether there are any changes during this period. Now that we know that changes occur over 3 weeks, we are now carefully planning blood collection at different time points during the therapy to assess the time course of the responses.
Finally, as we did not measure plasma potassium levels, a role for electrolytes in elevations of aldosterone levels seen at the end of CDT cannot be entirely ruled out. However, in future studies, we are going to assess levels of different electrolytes.
Conclusions and future directions
The results of this novel pilot study shows that lymphedema patients reduce leg fluid volume as well as body weight due to the decongestive physical therapy. Volume regulating hormones reflected partly the fluid shifts effects of CDT: aldosterone increased significantly after therapy while plasma renin activity increased but not significantly. Hyaluronic acid, used as surrogate marker for lymphatic outflow, did not show significant changes pre- and post-therapy. As the low number of patients analyzed in this pilot study and the inhomogeneity of the results could have led to high variances in our data, it is important to further investigate the effects of decongestive therapy and manual lymphatic drainage in a time course over 3 weeks of therapy. But this pilot study has now provided us with novel data that will be used to plan for sample size calculations for future studies. Our pilot data also suggest that there is a need to carefully study time course of responses and to systematically assess the effect of 3 week therapy on cardiovascular, electrolyte and hormonal responses.
Acknowledgments
We wish to thank the participants of this study.
Author biographies
Bianca Brix completed her PhD at the division of physiology, medical university of Graz, investigating the effects of lymphedema therapy on fluid shifts, cardiovascular system as well as endothelial function.
Gert Apich is a clinician specialized in physical therapy and rehabilitation focusing on the treatment of lymphedema patients.
Andreas Rössler is a professor of physiology, specialized on fluid dynamics, volume regulation and hemodynamic responses.
Sebastian Walbrodt is a medical student focusing on the lymphatic flow assessment via plasma hyaluronic acid measurements.
Nandu Goswami is the acting head of the division of physiology at the medical university of Graz. His research expertises are cardiovascular research, gravitational physiology and aging.
Footnotes
Authors contribution: GA, AR and NG conceived the pilot study and designed the experiment. BB and SW acquired the data. BB preprocessed the data. AR and BB performed the data and statistical analyses. BB, AR, NG wrote the manuscript and created the figures and tables. BB, GA, SW, AR, and NG interpreted the results. All authors contributed to manuscript editing and approved the final version for publication.
Ethics approval: Ethical approval for this study was obtained from the Ethics Committee of Klagenfurt (EK: A 03/17) and the Ethics Committee of the Medical University Graz, Austria (EK: 29-090 ex 16/17).
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: This randomized clinical trial was not registered because it was a pilot study.
ORCID iDs: Bianca Brix  https://orcid.org/0000-0002-7308-5450
https://orcid.org/0000-0002-7308-5450
Nandu Goswami  https://orcid.org/0000-0002-6704-0723
https://orcid.org/0000-0002-6704-0723
Data availability: Data are available from the Medical University of Graz.
References
- 1.Cueni LN, Detmar M, Sc M, et al. The lymphatic system in health and disease. Lymphat Res Biol 2008; 6: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura K, Rockson SG. Biomarkers of lymphatic function and disease: state of the art and future directions. Mol Diagn Ther 2007; 11: 227–238. [DOI] [PubMed] [Google Scholar]
- 3.Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest 2014; 124: 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates DO, Levick JR, Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin Sci (Lond) 1993; 85: 737–746. [DOI] [PubMed] [Google Scholar]
- 5.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 2010; 87: 198–210. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima T, Tsuji T, Sano Y, et al. Immediate effects of active exercise with compression therapy on lower-limb lymphedema. Support Care Cancer 2017; 25: 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen MR, Simonsen L, Karlsmark T, et al. Lymphoedema of the lower extremities – background, pathophysiology and diagnostic considerations. Clin Physiol Funct Imaging 2010; 30: 389–398. [DOI] [PubMed] [Google Scholar]
- 8.Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol 2009; 7: 3–10. [DOI] [PubMed] [Google Scholar]
- 9.Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol 2006; 4: 199–210. [DOI] [PubMed] [Google Scholar]
- 10.Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg 2012; 129: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aschen S, Zampell JC, Elhadad S, et al. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg 2012; 129: 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockson SG. The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphat Res Biol 2013; 11: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg 2011; 213: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herberger K, Blome C, Heyer K, et al. Quality of life in patients with primary and secondary lymphedema in the community. Wound Repair Regen 2017; 25: 466–473. [DOI] [PubMed] [Google Scholar]
- 15.Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol 2008; 52: 799–806. [DOI] [PubMed] [Google Scholar]
- 16.Tambour M, Holt M, Speyer A, et al. Manual lymphatic drainage adds no further volume reduction to Complete Decongestive Therapy on breast cancer-related lymphoedema: a multicentre, randomised, single-blind trial. Br J Cancer 2018; 119: 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Földi E, Sauerwald A, Hennig B. Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 2000; 33: 19–23. [PubMed] [Google Scholar]
- 18.Hinghofer-Szalkay HG, Mekonen W, Rossler A, et al. Post-exercise decrease of plasma hyaluronan: increased clearance or diminished production? Physiol Res 2002; 51: 139–144. [PubMed] [Google Scholar]
- 19.Rössler A, Fink M, Goswami N, et al. Modeling of hyaluronan clearance with application to estimation of lymph flow. Physiol Meas 2011; 32: 1213–1238. [DOI] [PubMed] [Google Scholar]
- 20.Rössler A, László Z, Kvas E, et al. Plasma hyaluronan concentration: no circadian rhythm but large effect of food intake in humans. Eur J Appl Physiol Occup Physiol 1998; 78: 573–577. [DOI] [PubMed] [Google Scholar]
- 21.Goswami N, Roessler A, Haditsch B, et al. Paradoxical clearance of hyaluronan fragments during haemodialysis and haemodiafiltration. Nephrol Dial Transplant 2012; 27: 4420–4422. [DOI] [PubMed] [Google Scholar]
- 22.Brix B, Apich G, Roessler A, Ure C, Schmid-Zalaudek K, Hinghofer-Szalkay H, Goswami N. Fluid Shifts Induced by Physical Therapy in Lower Limb Lymphedema Patients. J Clin Med 2020 Nov 16;9(11):3678. doi: 10.3390/jcm9113678. PMID: 33207688; PMCID: PMC7697258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hespe GE, Nores GG, Huang JJ, Mehrara BJ. Pathophysiology of lymphedema-Is there a chance for medication treatment? J Surg Oncol 2017. Jan;115(1):96–98. DOI: 10.1002/jso.24414. [DOI] [PubMed] [Google Scholar]
- 24.Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020; 53: 3–19. [PubMed] [Google Scholar]
- 25.Döller W. Lymphologie. Wien Med Wochenschr 2013; 163: 153–154. [DOI] [PubMed] [Google Scholar]
- 26.Pereira De, Godoy JM, Gonçalves IP, Barufi S, et al. Large reduction in volume with the intensive treatment of lymphedema: reduction of fluids? Int J Angiol 2012; 21: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukushima T, Tsuji T, Sano Y, et al. Immediate effects of active exercise with compression therapy on lower-limb lymphedema. Support Care Cancer 2017; 25: 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossler A, Hinghofer-Szalkay H. Hyaluronan fragments: an information-carrying system? Horm Metab Res 2003; 35: 67–68. [DOI] [PubMed] [Google Scholar]
- 29.Roh K, Cho S, Park J-H, et al. Therapeutic effects of hyaluronidase on acquired lymphedema using a newly developed mouse limb model. Exp Biol Med 2017; 242: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu N-F, Zhang L. Changes of tissue fluid hyaluronan (hyaluronic acid) in peripheral lymphedema. Lymphology 1998; 31: 173–179. [PubMed] [Google Scholar]
- 31.Liu NF, Olszewski W. The influence of local hyperthermia on lymphedema and lymphedematous skin of the human leg. Lymphology 1993; 26: 28–37. [PubMed] [Google Scholar]
- 32.Olszewski WL, Engeset A, Sokolowski J. Lymph flow and protein in the normal male leg during lying, getting up, and walking. Lymphology 1977; 10: 178–183. [PubMed] [Google Scholar]
- 33.Engeset A, Olszewski W, Jaeger PM, et al. Twenty-four hour variation in flow and composition of leg lymph in normal men. Acta Physiol Scand 1977; 99: 140–148. [DOI] [PubMed] [Google Scholar]


