Abstract
The cooCTJ gene products are coexpressed with CO-dehydrogenase (CODH) and facilitate in vivo nickel insertion into CODH. A Ni2+ transport assay was used to monitor uptake and accumulation of 63Ni2+ into R. rubrum and to observe the effect of mutations in the cooC, cooT, and cooJ genes on 63Ni2+ transport and accumulation. Cells grown either in the presence or absence of CO transported Ni2+ with a Km of 19 ± 4 μM and a Vmax of 310 ± 22 pmol of Ni/min/mg of total protein. Insertional mutations disrupting the reading frame of the cooCTJ genes, either individually or all three genes simultaneously, transported Ni2+ the same as wild-type cells. The nickel specificity for transport was tested by conducting the transport assay in the presence of other divalent metal ions. At a 17-fold excess Mn2+, Mg2+, Ca2+, and Zn2+ showed no inhibition of 63Ni2+ transport but Co2+, Cd2+, and Cu2+ inhibited transport 35, 58, and 66%, respectively. Nickel transport was inhibited by cold (50% at 4°C), by protonophores (carbonyl cyanide m-chlorophenylhydrazone, 44%, and 2,4-dinitrophenol, 26%), by sodium azide (25%), and hydroxyl amine (33%). Inhibitors of ATP synthase (N,N′-dicyclohexylcarbodiimide and oligomycin) and incubation of cells in the dark stimulated Ni2+ transport. 63Ni accumulation after 2 h was four times greater in CO-induced cells than in cells not exposed to CO. The CO-stimulated 63Ni2+ accumulation coincided with the appearance of CODH activity in the culture, suggesting that the 63Ni2+ was accumulating in CODH. The cooC, cooT, and cooJ genes are required for the increased 63Ni2+ accumulation observed upon CO exposure because cells containing mutations disrupting any or all of these genes accumulated 63Ni2+ like cells unexposed to CO.
Rhodospirillum rubrum expresses a CO oxidation system in response to CO exposure. The CO oxidation system catalyzes the reaction shown in equation 1 (10, 12).
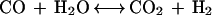 |
1 |
This system contains CO-dehydrogenase (CODH) and a CO-tolerant hydrogenase (4, 12). CODH contains two metal clusters: the active site, Ni-X-Fe4S4, and a second Fe4S4 cluster involved in electron transfer (19). The CO-tolerant hydrogenase contains a nickel-binding motif conserved in other nickel hydrogenases and requires Ni2+ in the growth medium for activity (11). The presence of nickel is essential for the function of the CO oxidation system (4, 12).
The gene encoding CODH (cooS) has been cloned, and the operon containing cooS has been characterized (21). The CO-oxidation operon (coo operon) contains five open reading frames, cooFSCTJ. The cooC and cooJ genes show similarity to genes required for nickel-processing for hydrogenase and urease in other organisms (23). CooC is analogous to the HypB and UreG proteins that have been proposed to hydrolyze nucleotides in order to insert nickel into hydrogenase and urease, respectively (28, 29). CooJ contains a histidine-rich nickel-binding motif that binds four Ni2+ atoms per monomer and is similar to UreE and HypB (45). The requirement for CooC and CooJ as nickel-processing proteins was confirmed by mutations that disrupted the reading frames of cooC and cooJ, resulting in the production of a nickel-deficient CODH (22, 23). The gene products of cooCTJ appear to function specifically for nickel insertion into CODH. A mutation disrupting the cooCTJ genes exhibits normal levels of the CO-tolerant hydrogenase activity (12).
The mechanism of Ni2+ transport and accumulation prior to the insertion of nickel into CODH has not been characterized in R. rubrum. To better understand the specific functions of the CooC, CooT, and CooJ proteins, the process of Ni2+ transport and accumulation needs to be defined. This includes understanding the mechanism of Ni2+ transport and accumulation in the absence of CO when the cooCTJ genes are not expressed and in the presence of CO when the cooCTJ genes are expressed.
Ni2+ transport has been studied in other bacteria, and four classes of Ni2+ transport have been reported. The first class is single-component, energy-dependent, high-affinity Ni2+ transport system where the Km for Ni2+ is low (17 nM to 5 μM) (7, 20, 25, 47) and Ni2+ transport is not inhibited by the presence of other divalent metals (8). The Ni2+ transport proteins HoxN, from Alcaligenes eutrophus (46), HupN, from Bradyrhizobium japonicum (13), UreH, from Bacillus TB90 (26), and NixA, from Helicobacter pylori (14), have been characterized and have conserved sequence motifs proposed to function in Ni2+ binding (14). The second class is the Nik ABCD multicomponent system/transport system from H. pylori. This system couples ATP hydrolysis to transport Ni2+ (16). The transport systems of Azotobacter chroococcum and B. japonicum represent a third class of Ni2+ transport that requires little or no energy (33, 42). The fourth class of Ni2+ transport occurs adventitiously through the Mg2+ transport systems of several organisms (8). Maguire and coworkers have characterized three Mg2+ transport systems (CorA, MgtA, and MgtB) in Salmonella typhimurium (17, 18, 41, 43) and a fourth distinct Mg2+ transporter (MgtE) from Providencia stuartii and Bacillus firmus OF4 (37, 44). CorA, MgtA, and MgtB display different Km and Vmax values for transport, are inhibited differently by temperature and divalent metals, and have different mechanisms for transcriptional regulation (34, 38–40). Each Mg2+ transport system transports Ni2+, and CorA also transports Co2+. Similar work has been performed with Escherichia coli (32). Ni2+ transported by Mg2+ transport systems generally has a much higher Km for Ni2+ than the nickel-specific transport systems and is competitively inhibited by Mg2+ and other divalent metals (8).
The majority of accumulated Ni2+ is found in the form of protein-bound nickel with very little free intracellular nickel (20, 33). The nickel enzymes hydrogenase, urease, CODH, and superoxide dismutase and the F430 cofactor of methyl CoM reductase accumulate nickel, as do several accessory proteins required for nickel insertion into these enzymes. Several of these accessory nickel-binding proteins have been purified and characterized. The HypB protein is expressed under hydrogenase-derepressing conditions (30). HypB binds Ni2+ and is proposed to assist in the induction of the hydrogenase structural genes (30). Nickel accumulated by HypB is also used for the activation of hydrogenase (30). Maier et al. showed that nickel accumulates in a nickel storage protein and that nickel stored during heterotrophic growth could be used for hydrogenase when cells were derepressed in medium lacking Ni2+ (27). A nickel storage role has been proposed for the UreE protein that functions as a nickel-processing protein for urease (5).
The present study demonstrates that Ni2+ transport occurs identically in R. rubrum grown in the presence or absence of CO. 63Ni2+ accumulates more rapidly in CO-induced cells due to the accumulation of 63Ni in CODH. CooC, CooT, and CooJ appear to have no role in Ni2+ transport but are required for the accumulation of 63Ni in CODH.
MATERIALS AND METHODS
Strains and cultivation.
R. rubrum UR2 (a spontaneous streptomycin-resistant mutant of R. rubrum UR1 [ATCC 11170]) was used as the wild-type strain. The following strains were as described previously and contain insertions into the cooCTJ genes that created either polar or nonpolar knockouts of the downstream genes (23). UR469 (UR2 Smr Nxr Gmr cooC16::aacC1Ωlinker) contains an insertion disrupting cooC that is polar onto cooTJ. UR495 (UR2 Smr Nxr cooC20::linker) and UR479 (UR2 Smr Nxr cooT19::linker) contain nonpolar insertions into cooC and cooT. UR500 (UR2 Smr Nxr Gmr cooJ22::aacC1Ωlinker) contains an insertion into cooJ.
R. rubrum strains were cultured in malate ammonium medium with no added nickel (31) in 100-ml vials with rubber stoppers and a gas phase of N2 at 30°C for 12 to 18 h. Cells were grown in a 30°C warm room 4 in. from a 15-W incandescent light bulb and 6-in. from a 20-W fluorescent bulb. Induction with CO was initiated by adding 20 ml of 99.5% CO gas into the gas phase of the 100-ml vial.
Ni2+ transport assay.
The Ni2+ transport assay used in these studies was a modified version of several previously published Ni2+ transport assays (6, 15, 42). R. rubrum cell optical density was measured spectrophotometrically at 600 nm (OD600). When cultures reached OD600 of 2.0 to 2.5, the culture was collected by centrifugation at 6,000 × g for 15 min. The cell pellet was resuspended in 45 ml of anaerobic 100 mM MOPS (morpholinepropanesulfonic acid) buffer at pH 7.5. Cells were transferred (3.0 ml) into anaerobic 20-ml vials with a gas phase of N2. The cells were incubated at 30°C in an illuminated water bath until Ni2+ was added. Controls showed that incubation in MOPS buffer for up to 2 h did not affect the rate of Ni2+ transport.
The assay began when 2.8 μCi of 63Ni2+ (63NiCl2 at 615 mCi/mmol; DuPont NEN Research Products, Inc.) and the indicated amount of unlabeled nickel were added to the cells followed by rapid mixing of the culture (a maximum of 50 μl was added). After being mixed, a sample was removed and vacuum filtered onto a GN-6 Metricell membrane filter (pore size, 0.45 μm; Gelman Sciences, Inc., Ann Arbor, Mich.) at 15 s after Ni2+ addition. The GN-6 Metricell membrane filters were previously washed twice with 5.0-ml aliquots of 10 mM NiCl2 in 100 mM MOPS at pH 6.5 to reduce nonspecific binding of 63Ni2+ to the filter. After the cells were collected on the filter, the cells were washed three times with 10 mM EDTA in 100 mM Tris buffer (pH 8.0) (5.0 ml per wash). With the assistance of the vacuum filtration system, the washing procedure took less than 5 s. The 15-s sample represents rapid binding of 63Ni2+ to the cell surface and some transport (15) (see also results shown in Fig. 1). The 15-s time point is subtracted from the subsequent time points to calculate the net uptake by the cells. Controls were prepared for each nickel concentration with identical contents but lacking cells to correct for 63Ni binding to the filters.
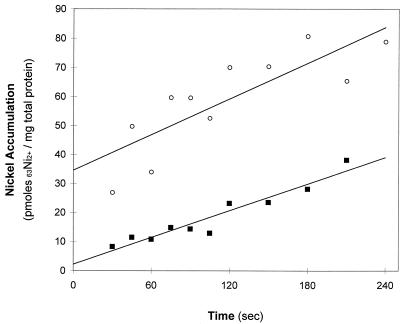
FIG. 1.
Initial rate of 63Ni2+ accumulation by R. rubrum. Cells were grown in malate ammonium medium as described in Materials and Methods. Cells were harvested by centrifugation and resuspended in 100 mM MOPS buffer (pH 7.5) (○) or 100 mM MOPS buffer (pH 7.5) containing 1.0 mM MgCl2 (■). The cells were incubated for 30 min at 30°C before the assay was initiated by the addition of 1.0 μM 63NiCl2. Nickel accumulated by the cells was measured at the indicated time point by collecting the cells by vacuum filtration, followed by three 5.0-ml washes with 10 mM EDTA in 100 mM Tris buffer (pH 8.0). The filtration assay was as described in Materials and Methods. Each data point represents a single measurement.
Additional Ni2+ transport experiments were performed with cells that were resuspended in buffer containing 1.0 mM MgCl2 or cells grown in the presence of CO to induce the coo operon.
For the studies with inhibitors of Ni2+ transport, solvent controls were performed to account for solvents used to solubilize the inhibitors. Filters were transferred to scintillation vials, and Biosafe II scintillation fluid was added (10 ml). 63Ni was detected by liquid scintillation counting by using a 14C setting in a Packard Minaxi Tricarb 4000 series scintillation counter. The protein content of the samples was measured by the method of Lowry et al. (24). Data are expressed as picomoles of Ni2+ transported per minute per milligram of total protein.
Nickel accumulation.
Cells were grown with illumination, either in the presence or the absence of 20% CO, in a 25-ml volume in 100-ml anaerobic vials with an N2 gas phase to an OD600 of 2.0 to 2.5 in malate ammonium medium. 63Ni2+ was added to cell cultures to a final concentration of either 100 nM or 1.0 μM (63Ni at 615 mCi/mmol). Samples of the culture were removed at the indicated time points, and cells were collected on a GN-6 Metricell membrane filter as described above for the Ni2+ transport assay. CODH activity was monitored by adding cells (1.0 ml) from the indicated time point into a degassed vial with a CO atmosphere (99.5%) containing 10 μl of 0.1 M EDTA to chelate nickel not associated with CODH and 10 μl of 0.1 M methyl viologen. Methyl viologen was added as an electron acceptor for monitoring CODH activity and, in the reduced form, also functioned as an oxygen scavenger. Toluene (20 μl) was added to permeabilize the cells, and the vial containing the sample was placed in a shaking 30°C water bath for 15 min. The sample was then assayed for CODH activity spectrophotometrically by the reported method (9).
Effect of divalent metal ions on Ni2+ uptake.
Cell culture preparation was identical to that described in the nickel transport assay. The competing divalent metal ion and 63Ni2+ were added simultaneously to final concentrations of 25 and 1.5 μM (615 mCi/mmol), respectively. The cells were collected and assayed for 63Ni2+ uptake as described above. A control sample was prepared by adding 63Ni (1.5 μM; 615 mCi/mmol) with no competitive metal.
Effect of metabolic inhibitors on Ni2+ uptake.
Cell culture preparation was identical to that described in the Ni2+ transport assay. The indicated inhibitor was added to the cells, and the cells were incubated for 30 min with illumination at 30°C. The assay was initiated by the addition of 63NiCl2 (1.5 μM; 615 μCi/μmol) to the cells. The cells were collected and assayed for 63Ni2+ uptake as described above. A control sample was prepared by adding 63NiCl2 (1.5 μM; 615 mCi/mmol) with no inhibitors present.
RESULTS
Nickel accumulation.
The addition of 63NiCl2 (1.0 μM, final concentration; 615 mCi/mmol) to R. rubrum resulted in the rapid binding of 63Ni2+ to the cell surface, followed by a linear accumulation of 63Ni2+. Figure 1 shows the accumulation of 63Ni2+ in R. rubrum in 100 mM MOPS buffer (pH 7.5). The best-fit line represents the accumulation of 63Ni2+ over a period of 4 min. The line intercepts the y axis at 36 pmol of 63Ni2+/mg of total protein, indicating that a rapid 63Ni2+ binding phase occurred before the fastest time point measured by this assay (15 s after 63Ni2+ addition). The rapid binding to the cells occurred when the cells were in buffer or in growth medium, although the binding in growth medium is less due to the presence of molecules that chelate Ni2+ (EDTA, phosphate, and malate). Cells that have been killed by heat treatment also exhibit this rapid binding but do not exhibit the linear accumulation (data not shown). The rapid binding of 63Ni2+ did not occur when 63Ni2+ was added to R. rubrum that had been incubated for 30 min in 100 mM MOPS buffer (pH 7.5) containing 1.0 mM MgCl2. The presence of 1.0 mM MgCl2 did not alter the linear accumulation that follows the rapid binding phase (Fig. 1). A similar experiment was conducted by adding 1.0 μM 63Ni2+ and 99 μM unlabeled Ni2+ to cells and then monitoring the 63Ni2+ accumulation in the cells. 63Ni2+ accumulation occurred at a much slower linear rate, and the slope crossed the y axis at the zero point, indicating that the unlabeled Ni2+ competed with the 63Ni2+ for both surface binding and for Ni2+ transport into the cell (data not shown). In order to distinguish 63Ni2+ accumulation from surface binding in later experiments, a control sample for rapid binding was used in each assay and subtracted from the subsequent time points to obtain the values representing 63Ni2+ accumulation.
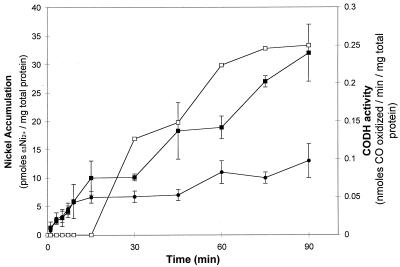
To determine if exposing the cells to CO at the time of 63Ni2+ addition induced the production of proteins required for Ni2+ transport or accumulation, 63Ni2+ accumulation experiments were conducted either in the absence of CO or with the simultaneous addition of CO and 63Ni2+ to a culture. A linear 63Ni2+ accumulation phase occurred during the first 10 min in the presence or absence of CO (Fig. 2). The first linear accumulation phase is followed by a slower accumulation phase in cells not exposed to CO (Fig. 2). The addition of CO (20%) at the same time as 63NiCl2 (1.0 μM, final concentration; 615 mCi/mmol) did not alter the accumulation of 63Ni2+ during the first 30 min compared to the control. After 30 min of exposure to CO, the accumulation of 63Ni increases dramatically when compared with cells not exposed to CO (Fig. 2). The increase in 63Ni accumulation in the CO-induced culture coincided with the appearance of CODH activity in the culture (Fig. 2). Therefore, the increased accumulation of 63Ni appears to result from the insertion of 63Ni into CODH and perhaps into the CooJ and CooC proteins.
FIG. 2.
63Ni2+ accumulation in CO-induced R. rubrum. CO (20% gas phase) and 63Ni (1 μM; 615 mCi/mmol) were added at the zero time point to R. rubrum cells (OD600 = 2.0) in malate ammonium medium (■). The control (no CO added) contains 63Ni (1 μM; 615 mCi/mmol) added to R. rubrum cells (OD600 = 2.0) in malate ammonium medium (●). CODH activity in the cells treated with CO is also indicated (□). The in vitro CODH activity assay is described in the text. Each data point represents the average of two independent measurements. The nickel bound at 15 s was subtracted from the data.
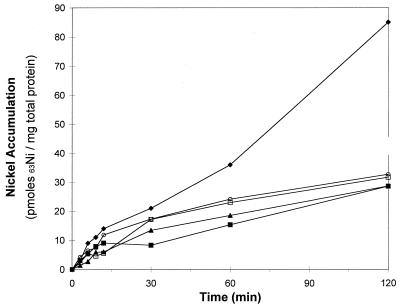
Since apo-CODH the CO-tolerant hydrogenase and CooC, CooT, and CooJ are expressed in CO-induced cells grown on Ni-depleted medium (3, 4, 12, 23), it was possible to examine the accumulation of 63Ni into cells containing apo-CODH and to test the hypothesis that the increased accumulation of 63Ni in CO-treated cultures (Fig. 2) was due to the accumulation of 63Ni into apo-CODH. Apo-CODH can be activated in vivo (in wild-type cells) or in vitro by the addition of nickel (4, 9). All cells in this experiment were grown with nickel-depleted medium and were exposed to CO for 12 h. All cultures expressed similar amounts of apo-CODH, as evidenced by the in vitro activation assay (data not shown). The addition of 63NiCl2 to a wild-type culture containing apo-CODH was the positive control for 63Ni accumulation into apo-CODH and other nickel-containing proteins expressed by the CO oxidation system (CO-tolerant hydrogenase and CooJ). The addition of 63Ni2+ resulted in a linear 63Ni2+ accumulation phase during the first 15 min. After 15 min a slower, but still significant 63Ni2+ accumulation phase occurred (Fig. 3). A negative control was the addition of 63Ni to a wild-type culture that was not exposed to CO (Fig. 3). The negative control accounts for the accumulation of 63Ni2+ by cells not expressing the CO oxidation system. The initial 63Ni2+ accumulation by the negative control was similar to the CO-induced culture, but the amount of 63Ni2+ that accumulated after 15 min was much lower (Fig. 3). Mutant strains lacking a functional cooC gene are deficient in nickel processing and could not insert nickel into CODH unless a 500 μM nickel concentration was added to the medium (22, 23). The 63Ni2+ concentration chosen for this experiment (100 nM 63NiCl2) was chosen because a mutant in cooT grew similarly to wild type in CO-dependent growth experiments (23). At this 63Ni2+ concentration, no 63Ni2+ accumulation into apo-CODH will occur in whole cells because Kerby demonstrated that when 63Ni2+ was added to the cooC mutant strain (UR495), no CODH activity was observed (23). A 63Ni accumulation experiment with a cooC mutant strain provided a control that accounted for 63Ni that accumulated in proteins known to bind nickel from the CO oxidation system other than CODH (i.e., CO-tolerant hydrogenase and CooJ). The results in Fig. 2 show that 63Ni accumulation in a cooC mutant was similar to the negative control that expressed no CO oxidation proteins. This result indicates that 63Ni accumulation in other CO-induced proteins (i.e., CO-tolerant hydrogenase and CooJ) is very small. 63Ni accumulation assays with mutant strains containing linker mutations disrupting the reading frames of the cooT (strain UR479) or cooJ (strain UR500) genes also showed 63Ni accumulation that was similar to the negative control (Fig. 3). These data indicate that the observed increase in 63Ni accumulation in the positive control is due to 63Ni associated with CODH. These data also verify that all three of the cooC, cooT, and cooJ gene products must be present for in vivo nickel insertion into CODH to occur under the conditions of this assay.
FIG. 3.
Nickel accumulation in CO-induced cooC, cooT, and cooJ mutant strains of R. rubrum. The cells were grown in malate ammonium medium lacking Ni2+ for 12 h with a gas phase of 20% CO–80% N2 except as indicated. Symbols: ⧫, UR2; ■, UR2 without CO (negative control); ▴, nonpolar linker mutation in cooC (strain UR495); ○, nonpolar linker mutation in cooT (strain UR479); □, mutation in cooJ (strain UR500). All cultures except the negative control, which was not CO treated, had similar CODH activities when analyzed by the in vitro Ni2+ activation assay. The apo-CODH activation assay was performed on an aliquot of cell culture removed from the culture just prior to the 63Ni2+ accumulation assay. The nickel bound at time zero was subtracted from the data.
Kinetics of nickel transport.
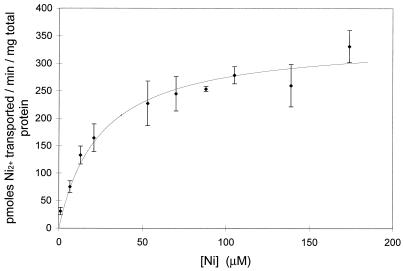
The linear accumulation of Ni2+ during the first 10 min of exposure to 63Ni2+ shown in Fig. 2 showed saturable kinetics with respect to nickel concentration (Fig. 4). This observation is consistent with Ni2+ entering the cell by a transport system. The kinetic data for Ni2+ transport by R. rubrum grown in the absence of CO was obtained by measuring Ni2+ transported at various Ni2+ concentrations. The data are consistent with a kinetic uptake system that is saturable with increased Ni2+ (Fig. 4). Best-fit calculations with a nonlinear computer fit (Prism Program; Graphpad Graphics, Inc.) showed the Km for Ni2+ to be 19 ± 4 μM with a Vmax of 310 ± 22 pmol/min/mg of total protein. Similar data were observed when cells were grown in the presence of CO or with a CO-induced mutant strain (UR469) that has a mutation disrupting the cooCTJ reading frames. This indicates that the increased accumulation of 63Ni2+ observed when cells were induced with CO does not occur from the production of a CO-induced Ni2+ transport system. Ni2+ transport studies were conducted on cells resuspended in buffer containing 1.0 mM MgCl2. The kinetics of Ni2+ transport in the presence of MgCl2 are very similar to those without MgCl2. The Kd of 21 ± 4 μM is within the error expected for the transport without MgCl2, and the Vmax of 252 ± 21 is only 19% lower than the curve without MgCl2. These results indicate that the kinetics of Ni2+ transport into the cell in the absence of MgCl2 are a combination of Ni2+ being transported by both a Mg2+ transporter and a Ni2+ transporter. The data also suggest the Ni2+ transporter does not require induction by CO or the presence of the CooC, CooT, and CooJ proteins.
FIG. 4.
Kinetics of Ni2+ transport by R. rubrum. Cells grown in the absence of CO were harvested and resuspended in 100 mM MOPS (pH 7.5) and divided into 12 vials. 63Ni (1.5 μM; 615 mCi/mmol) was added to each vial with the appropriate amount of unlabeled nickel to reach the indicated final nickel concentration. A sample was immediately removed and analyzed for the initial binding of nickel to the cells. Additional samples were removed at 60 and 120 s and then collected by vacuum filtration followed by three 5.0-ml washes of 10 mM EDTA in 100 mM Tris (pH 8.0). The curve represents the mean of data from three independent experiments. Cultures grown in the presence of CO or strains with cooCTJ mutationally inactivated and grown with or without CO were also tested for Ni2+ transport and produced curves with the same kinetic profiles within the error bars shown (data not shown).
Specificity of nickel transport.
Mg2+ transport systems have been demonstrated to transport Ni2+ with lower affinity than Mg2+ (8, 34). Ni2+-specific, high-affinity transport systems transport only Ni2+ and show very little inhibition from the presence of other divalent metals (8). Table 1 shows the results of nickel transport assays where 63Ni2+ (1.5 μM) was added simultaneously with 25 μM concentrations of competing divalent metals. The divalent metals commonly tested to determine the specificity of Ni2+ transport systems include Mg2+, Mn2+, Zn2+, and Ca2+, and some reports show competition with Co2+, Cu2+, and Cd2+. The results show that Mg2+, Ca2+, Mn2+, and Zn2+ did not inhibit Ni2+ transport at a 17-fold excess. Mg2+ showed only 15% inhibition of 63Ni2+ transport at a 1 mM concentration (1,000-fold excess; Table 1, Fig. 1, and Fig. 4). The addition of Ca2+ actually stimulated 63Ni2+ transport to a small degree. Co2+ inhibited 63Ni2+ transport 36% at a 17-fold excess (25 μM). Co2+ has previously been shown to inhibit nickel-specific transport systems to a slight degree (20). Cu2+ and Cd2+ showed the most significant inhibition of Ni transport by inhibiting at levels of 66 and 58%, respectively.
TABLE 1.
Effect of divalent metals on nickel transporta
| Metalb | Concn (μM) | % of Ni transportc |
|---|---|---|
| Control (Ni2+) | 1 | 100 |
| Ca2+ | 25 | 120 |
| Mn2+ | 25 | 108 |
| Mg2+ | 25 | 95 |
| 1,000 | 85 | |
| Zn2+ | 25 | 87 |
| Co2+ | 25 | 64 |
| Cd2+ | 25 | 42 |
| Cu2+ | 25 | 34 |
The transport assay was performed as described in Materials and Methods. The concentration of 63Ni added to each assay was 1.5 μM (615 mCi/mmol). A data point was taken at 15 s (control for surface binding), 60 s, and 120 s. Cells were collected by filtration, and radioactivity was detected by liquid scintillation counting.
All divalent metal ions were chloride salts.
Percentage of control (no competing metal ion). A 100% value for 1.5 μM 63Ni2+ is equal to 25 pmol of Ni2+/min/mg of total protein.
Effects of metabolic inhibitors and ionophores.
The source of energy required to transport Ni2+ can be identified by using inhibitors of metabolic processes and correlating the inhibition of Ni2+ transport to the mechanism of the inhibitor. The results for inhibition of Ni2+ transport by R. rubrum are shown in Table 2. Ni2+ transport was completely inhibited by the electron transport inhibitor CN−. Although no 63Ni2+ transport occurred in the presence of CN−, the binding of 63Ni2+ to the surface of the cells was also decreased fivefold in the presence of CN− (data not shown). The inhibitory effects of CN− are most certainly caused by the formation of Ni(CN)42−. Other electron transport inhibitors, hydroxyl amine and azide, inhibited 63Ni2+ transport 33 and 25%, respectively. The protonophores CCCP (carbonyl cyanide m-chlorphenhydrazone) and DNP (2,4-dinitrophenol) inhibited 63Ni2+ transport 44 and 26%, respectively. DNP functions poorly in R. rubrum (1), so the effect of protonophores is better represented by the inhibition of CCCP. Incubating the culture in ice for 2 min before the addition of 63Ni2+ resulted in 50% inhibition of 63Ni2+ transport. Metal selective ionophores such as valinomycin, nigericin, and gramicidin D did not inhibit 63Ni2+ transport and, in some cases, stimulated transport. The inhibitors DCCD (N,N′-dicyclohexyl carbodiimide) and oligomycin also stimulated 63Ni2+ transport, as did incubating the cells in the dark.
TABLE 2.
Effect of metabolic inhibitors on nickel transporta
| Inhibitor | % of Ni transportb |
|---|---|
| Control (no addition) | 100 |
| ATPase inhibitors | |
| DCCD (200 μM) | 220 |
| Oligomycin (50 μg/ml) | 142 |
| Electron sink/transport | |
| Sodium azide (1,000 μM) | 75 |
| Potassium cyanide (200 μM) | 0 |
| Hydroxyl amine (1,000 μM) | 67 |
| Protonophores | |
| CCCP (200 μM) | 56 |
| DNP (200 μM) | 74 |
| Metal ionophores | |
| Valinomycin (50 μg/ml) | 109 |
| Valinomycin (50 μg/ml) plus 50 mM KCl | 154 |
| Nigericin (50 μg/ml) | 96 |
| Gramicidin D (50 μg/ml) | 165 |
| Miscellaneous | |
| COc | 102 |
| O2d | 48 |
| Darke | 187 |
| Cold (4°C) | 50 |
Transport assays were performed as described in Materials and Methods. The indicated inhibitor was added to the cells and incubated for 30 min in a shaking 30°C water bath with illumination. Ni2+ was added to a final concentration of 1.5 μM, and then the sample was assayed as described in the text.
Percentage of control (100% = 25 pmol of Ni2+/min/mg of protein).
Gas phase was 20% CO and 80% N2.
Gas phase was air.
Vials containing cells were covered with aluminum foil.
DISCUSSION
This study was undertaken to learn how Ni2+ is transported into the cell, to compare Ni2+ accumulation in CO-induced cells versus non-CO-induced cells, and to correlate variations in Ni2+ transport and accumulation with mutations in the cooCTJ genes. The experimental design was intended to increase the understanding of the function of the CooCTJ gene products in R. rubrum and to gain an understanding of where nickel processing breaks down in mutants of cooC, cooT, and cooJ.
The initial association of Ni2+ with R. rubrum occurs as Ni2+ binds rapidly to the cell surface (Fig. 1). Incubating the cells in 100 mM MOPS buffer at pH 7.5 containing 1.0 mM MgCl2 for 30 min before the addition of Ni2+ stopped the rapid binding of 63Ni2+ to the cell surface but did not affect Ni2+ accumulation by the cells (Fig. 1). The addition of excess unlabeled Ni2+ also abolished the rapid binding of 63Ni2+ to the cells but also significantly slowed the accumulation of 63Ni2+ in the cells. The binding of 63Ni2+ to the cell surface has been observed in Methanobacterium bryantii (20) and Methanothrix concilii (2). Adsorption of Ni2+ to M. concilli occurs to preparations of purified cell sheaths, and the kinetic data for Ni2+ accumulation with purified cell sheath preparations were similar to data obtained for intact cells. These studies concluded that the semispecific cell-surface adsorption was the first step in nickel accumulation by this organism (2).
Nickel transport by R. rubrum was a saturable process with Michaelis-Menten-type kinetics over the concentration range of from 1 to 140 μM Ni2+. Analysis of the data by nonlinear regression computer fits indicated that the Km was 19 ± 4 μM with a Vmax of 310 ± 22 pmol/min/mg of total protein. The kinetics of Ni2+ transport did not change when cells were grown in the presence of CO (20%) or when CO (20%) was added to cells at the time of Ni2+ addition to the cells. Therefore, no evidence for a CO-induced nickel uptake system was observed. Ni2+ transport was also identical in a CO-induced mutant strain (UR469) that has a mutation disrupting the cooCTJ reading frames. This indicates that CooC, CooT, and CooJ are not required for Ni2+ transport. Similar Michaelis-Menten saturation kinetics were observed when identical experimental conditions were used with 1.0 mM MgCl2. The data in the absence of Mg2+ represents Ni2+ transported by the Mg2+ transport system, as well as a transport system that is selective for Ni2+ versus Mg2+. The kinetic parameters of the Ni2+ selective system are better described by the values of Km = 21 ± 4 and of Vmax = 252 ± 21 μmol of Ni2+/min/mg of total protein.
Although the Ni2+ transport studies reported in the literature differ in assay conditions, some rough comparisons may be made. The Km of 19 μM for R. rubrum is higher than most nickel-specific transport systems reported. The following Km values have been reported with the following organisms: M. bryantii, 3.1 μM (20); A. kivui, 2.3 μM (47); C. thermoaceticum, 3.2 μM (25), and A. cylindrica, 17 nM (7). B. japonicum with a Km of 26 and 50 μM for strains SR and SR470, respectively, has the Km that most closely approximates the transport by R. rubrum (42).
The Ni2+ transport system of R. rubrum shows selectivity for Ni2+ versus the divalent metals Mg2+, Mn2+, Ca2+, and Zn2+. These data are similar to those reported for Ni2+ transport in the organisms Anabaena cylindrica, M. bryantii, A. kivui, C. thermoaceticum, and B. japonicum (7, 20, 25, 42, 47). Co2+ and Zn2+ show some competitive inhibition to systems labeled as nickel-specific, since these metals inhibited Ni2+ transport in M. bryantii (Co2+, ∼50%) and B. japonicum (Zn2+, 46%) (20, 42). Although the Cu2+ ion did not affect Ni2+ transport in A. cylindrica (7), it inhibited Ni2+ transport 46% in B. japonicum (42) and 64% in R. rubrum. Cu2+ and Co2+ showed levels of inhibition similar to that of the NixA transport system (14).
The energy dependence of Ni2+ transport may be established by the use of metabolic inhibitors. Nickel transport has been suggested to occur by energy-independent processes in Azotobacter chroococcum (33) and perhaps in B. japonicum (42). Ni2+ transport required energy in M. bryantii and appeared to be coupled to proton movement (20). In A. cylindrica, the energy-dependent transport of Ni2+ was concluded to rely on the membrane potential (7).
Unfortunately, with R. rubrum the effects of metabolic inhibitors were weak and in some cases stimulated Ni2+ transport instead of inhibiting it. The electron transfer inhibitors azide and hydroxyl amine showed slight inhibitions of 25 and 33%, respectively. NaN3 has no effect on photosynthetic phosphorylation, so inhibition by this inhibitor functions at another site (36). The protonophores CCCP and DNP showed mild inhibition of Ni2+ transport. The concentration of CCCP used here completely abolishes photophosphorylation in R. rubrum, indicating that the effect of CCCP on Ni2+ transport is not as drastic as it is for photophosphorylation (36). These data indicate that a proton gradient may be important in Ni2+ transport. Incubation of cells on ice for 2 min inhibited transport 50%, indicating a possible requirement for energy. Metal ionophores that disrupt the membrane potential did not inhibit Ni2+ transport, and valinomycin and gramicidin D stimulated transport. The ATP synthase inhibitors DCCD and oligomycin also stimulated Ni2+ transport. The stimulation of Ni2+ transport by ATPase inhibitors was observed in A. kivui grown in the presence of H2 (47). A possible explanation for the effect of DCCD and oligomycin may be derived from the mechanism of inhibition of these two inhibitors. Both block the flow of protons through the ATP synthase. If protons cannot flow, then the proton gradient increases. This information, coupled with the inhibitory effect of CCCP, indicates that a proton gradient may be involved in Ni2+ transport. The stimulation in A. kivui was also concluded to result from the effects of the proton motive force (47). Cells that were incubated in the dark also showed stimulated Ni2+ transport. Cells that were incubated in the dark and then returned to the light for 1 to 10 min prior to 63Ni2+ addition also exhibited stimulated Ni2+ transport (data not shown). This observation may indicate that incubation in the dark altered the metabolic state of the cells. Since the cells did not return to the original state when exposed to light, it is assumed that an alternate metabolic system is functioning that does not respond like that observed under the original conditions. One possible explanation is that in the absence of light or an energy source, the cells begin to metabolize stored energy sources. Poly-β-hydroxybutyrate is a stored metabolite (35), and the metabolic pathways that convert poly-β-hydroxybutyrate into energy may produce conditions that stimulate Ni2+ transport.
The observation that CO-induced 63Ni2+ accumulation coincided with the appearance of CODH activity in the culture (Fig. 2) suggested that this Ni2+ accumulation could function as an assay to monitor Ni2+ insertion into CODH. The results show that the majority of the accumulated 63Ni is in CODH and that cooC, cooT, and cooJ gene products are required for in vivo insertion of nickel into CODH (Fig. 3). These results are consistent with CO-dependent growth studies showing that mutants lacking cooC or cooJ required an increased nickel concentration (100-fold and 10-fold, respectively) before active CODH was produced, allowing CO-dependent growth (23). The CO-dependent growth studies showed that mutants lacking a functional cooT gene grew slightly better than the wild type, suggesting that nickel was inserted into CODH in this strain (UR479) (23). No observable nickel accumulation occurred in the cooT mutant strain (UR479) under the conditions used in this study. These studies differ in the growth conditions, since the CO-dependent growth studies required growth on CO and were conducted on plates with rich medium. The 63Ni2+ accumulation studies reported here used a minimal liquid medium and did not require CO-dependent growth.
In conclusion, Ni2+ transport occurs by a nickel-selective transport system that can distinguish Ni2+ from Mg2+, Mn2+, and Zn2+, and the transport does not depend on the presence of CO. Ni2+ transport appears to require energy in the form of a proton gradient. Nickel accumulates both in the absence or presence of CO, and the increased accumulation of nickel is proposed to be from the insertion of 63Ni into CODH. Mutant strains containing mutations disrupting the cooC, cooT, or cooJ reading frames do not show a CO-dependent increase in 63Ni accumulation. The increased accumulation of 63Ni is therefore attributed to 63Ni insertion into CODH, confirming the previous observation that the cooCTJ genes are required for nickel insertion into CODH (23).
ACKNOWLEDGMENTS
This work was supported by Department of Energy grant DE-FGO2-87ER13891 to P.W.L.
We thank Robert L. Kerby and Gary P. Roberts for the use of cooCTJ mutants constructed by Robert L. Kerby in the lab of Gary P. Roberts.
REFERENCES
- 1.Baltscheffsky M. Photosynthetic phosphorylation. In: Clayton R K, Sistrom W R, editors. The photosynthetic bacteria. New York, N.Y: Plenum Press, Inc.; 1978. pp. 595–613. [Google Scholar]
- 2.Baudet C, Sprott G D, Patel G B. Adsorption and uptake of nickel in Methanothrix concilii. Arch Microbiol. 1988;150:338–342. [Google Scholar]
- 3.Bonam D, Lehman L, Roberts G P, Ludden P W. Regulation of carbon monoxide dehydrogenase and hydrogenase in Rhodospirillum rubrum: the effects of CO and oxygen on synthesis and activity. J Bacteriol. 1989;171:3102–3107. doi: 10.1128/jb.171.6.3102-3107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonam D, McKenna M C, Stephens P J, Ludden P W. Nickel-deficient carbon monoxide dehydrogenase from Rhodospirillum rubrum: in vivo and in vitro activation by exogenous nickel. Proc Natl Acad Sci USA. 1988;85:31–35. doi: 10.1073/pnas.85.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brayman T G, Hausinger R P. Purification, characterization, and functional analysis of a truncated Klebsiella aerogenes UreE urease accessory protein lacking the histidine-rich carboxyl terminus. J Bacteriol. 1996;178:5410–5416. doi: 10.1128/jb.178.18.5410-5416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryson M F, Drake H L. Energy-dependent transport of nickel by Clostridium pasteurianum. J Bacteriol. 1988;170:234–238. doi: 10.1128/jb.170.1.234-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell P M, Smith G D. Transport and accumulation of nickel ions in the cyanobacterium Anabaena cylindrica. Arch Biochem Biophys. 1986;244:470–477. doi: 10.1016/0003-9861(86)90615-6. [DOI] [PubMed] [Google Scholar]
- 8.Drake H L. Biological transport of nickel. In: Lancaster J Jr, editor. The Bioinorganic chemistry of nickel. New York, N.Y: VCH Publishers, Inc.; 1988. pp. 111–139. [Google Scholar]
- 9.Ensign S A, Campbell M J, Ludden P W. Activation of the nickel-deficient carbon monoxide dehydrogenase from Rhodospirillum rubrum: kinetic characterization and reductant requirement. Biochemistry. 1990;29:2162–2168. doi: 10.1021/bi00460a029. [DOI] [PubMed] [Google Scholar]
- 10.Ensign S A, Ludden P W. Characterization of the CO oxidation/H2 evolution system of Rhodospirillum rubrum. Role of a 22-kDa iron-sulfur protein in mediating electron transfer between carbon monoxide dehydrogenase and hydrogenase. J Biol Chem. 1991;266:18395–18403. [PubMed] [Google Scholar]
- 11.Fox J D, He Y, Shelver D, Roberts G P, Ludden P W. Characterization of the region encoding the CO-induced hydrogenase of Rhodospirillum rubrum. J Bacteriol. 1996;178:6200–6208. doi: 10.1128/jb.178.21.6200-6208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox J D, Kerby R L, Roberts G P, Ludden P W. Characterization of the CO-induced, CO-tolerant hydrogenase from Rhodospirillum rubrum and the gene encoding the large subunit of the enzyme. J Bacteriol. 1996;178:1515–1524. doi: 10.1128/jb.178.6.1515-1524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu C, Javedan S, Moshiri F, Maier R J. Bacterial genes involved in incorporation of nickel into a hydrogenase enzyme. Proc Natl Acad Sci USA. 1994;91:5099–5103. doi: 10.1073/pnas.91.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulkerson J F, Jr, Garner R M, Mobley H L. Conserved residues and motifs in the NixA protein of Helicobacter pylori are critical for the high affinity transport of nickel ions. J Biol Chem. 1998;273:235–241. doi: 10.1074/jbc.273.1.235. [DOI] [PubMed] [Google Scholar]
- 15.Grubbs R D, Snavely M D, Hmiel S P, Maguire M E. Magnesium transport in eukaryotic and prokaryotic cells using magnesium-28 ion. Methods Enzymol. 1989;173:546–563. doi: 10.1016/s0076-6879(89)73038-x. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks J K, Mobley H L. Helicobacter pylori ABC transporter: effect of allelic exchange mutagenesis on urease activity. J Bacteriol. 1997;179:5892–5902. doi: 10.1128/jb.179.18.5892-5902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hmiel S P, Snavely M D, Florer J B, Maguire M E, Miller C G. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989;171:4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hmiel S P, Snavely M D, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986;168:1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z, Spangler N J, Anderson M E, Xia J, Ludden P W, Lindahl P A, Münck E. Nature of the C-cluster in Ni-containing carbon monoxide dehydrogenases. J Am Chem Soc. 1996;118:830–845. [Google Scholar]
- 20.Jarrell K F, Sprott G D. Nickel transport in Methanobacterium bryantii. J Bacteriol. 1982;151:1195–1203. doi: 10.1128/jb.151.3.1195-1203.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerby R L, Hong S S, Ensign S A, Coppoc L J, Ludden P W, Roberts G P. Genetic and physiological characterization of the Rhodospirillum rubrum carbon monoxide dehydrogenase system. J Bacteriol. 1992;174:5284–5294. doi: 10.1128/jb.174.16.5284-5294.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerby R L, Ludden P W, Roberts G P. Carbon monoxide-dependent growth of Rhodospirillum rubrum. J Bacteriol. 1995;177:221–2244. doi: 10.1128/jb.177.8.2241-2244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerby R L, Ludden P W, Roberts G P. In vivo nickel insertion into the carbon monoxide dehydrogenase of Rhodospirillum rubrum: molecular and physiological characterization of cooCTJ. J Bacteriol. 1997;179:2259–2266. doi: 10.1128/jb.179.7.2259-2266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Lundie L L, Jr, Yang H C, Heinonen J K, Dean S I, Drake H L. Energy-dependent, high-affinity transport of nickel by the acetogen Clostridium thermoaceticum. J Bacteriol. 1988;170:5705–5708. doi: 10.1128/jb.170.12.5705-5708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda M, Hidaka M, Nakamura A, Masaki H, Ouzumi T. Cloning, sequencing, and expression of thermophilic Bacillus sp. strain TP-90 urease complex in Escherichia coli. J Bacteriol. 1994;176:432–442. doi: 10.1128/jb.176.2.432-442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maier R J, Pihl T D, Stults L, Sray W. Nickel accumulation and storage in Bradyrhizobium japonicum. Appl Environ Microbiol. 1990;56:1905–1911. doi: 10.1128/aem.56.6.1905-1911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier T, Jacobi A, Sauter M, Böck A. The product of the hypB gene, which is required for nickel incorporation into hydrogenases, is a novel guanine nucleotide-binding protein. J Bacteriol. 1993;175:630–635. doi: 10.1128/jb.175.3.630-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moncrief M B, Hausinger R P. Characterization of UreG, identification of a UreD-UreF-UreG complex, and evidence suggesting that a nucleotide-binding site in UreG is required for in vivo metallocenter assembly of Klebsiella aerogenes urease. J Bacteriol. 1997;179:4081–4086. doi: 10.1128/jb.179.13.4081-4086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson J W, Fu C, Maier R J. The HypB protein from Bradyrhizobium japonicum can store nickel and is required for the nickel-dependent transcriptional regulation of hydrogenase. Mol Microbiol. 1997;24:119–128. doi: 10.1046/j.1365-2958.1997.3251690.x. [DOI] [PubMed] [Google Scholar]
- 31.Ormerod J G, Ormerod K S, Gest H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria: relationships with nitrogen metabolism. Arch Biochem Biophys. 1961;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- 32.Park M H, Wong B B, Lusk J E. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol. 1976;126:1096–1103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge C D, Yates M G. Effect of chelating agents on hydrogenase in Azotobacter chroococcum. Evidence that nickel is required for hydrogenase synthesis. Biochem J. 1982;204:339–344. doi: 10.1042/bj2040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roof S K, Maguire M E. Magnesium transport systems: genetics and protein structure. J Am Coll Nutr. 1994;13:424–428. doi: 10.1080/07315724.1994.10718431. [DOI] [PubMed] [Google Scholar]
- 35.Sirevåg R. Carbon metabolism in green bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Klewer Academic Publishers; 1995. pp. 871–883. [Google Scholar]
- 36.Smith L, Pinder P B. Oxygen-linked electron transport and energy conservation. In: Clayton R K, Sistrom W R, editors. The photosynthetic bacteria. New York, N.Y: Plenum Press, Inc.; 1978. pp. 641–654. [Google Scholar]
- 37.Smith R L, Thompson L J, Maguire M E. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J Bacteriol. 1995;177:1233–1238. doi: 10.1128/jb.177.5.1233-1238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snavely M D, Florer J B, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol. 1989;171:4761–4766. doi: 10.1128/jb.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snavely M D, Florer J B, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems. J Bacteriol. 1989;171:4752–4760. doi: 10.1128/jb.171.9.4752-4760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snavely M D, Gravina S A, Cheung T T, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression. J Biol Chem. 1991;266:824–829. [PubMed] [Google Scholar]
- 41.Snavely M D, Miller C G, Maguire M E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266:815–823. [PubMed] [Google Scholar]
- 42.Stults L W, Mallick S, Maier R J. Nickel uptake in Bradyrhizobium japonicum. J Bacteriol. 1987;169:1398–1402. doi: 10.1128/jb.169.4.1398-1402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao T, Snavely M D, Farr S G, Maguire M E. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J Bacteriol. 1995;177:2654–2662. doi: 10.1128/jb.177.10.2654-2662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend D E, Esenwine A J, George J, Bross D, Maguire M E, Smith R L. Cloning of the mgtE Mg2+ transporter from Providencia stuartii and the distribution of mgtE in gram-negative and gram-positive bacteria. J Bacteriol. 1995;177:5350–5354. doi: 10.1128/jb.177.18.5350-5354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt R K, Ludden P W. The identification, purification, and characterization of CooJ. J Biol Chem. 1998;273:10019–10025. doi: 10.1074/jbc.273.16.10019. [DOI] [PubMed] [Google Scholar]
- 46.Wolfram L, Friedrich B, Eitinger T. The Alcaligenes eutrophus protein HoxN mediates nickel transport in Escherichia coli. J Bacteriol. 1995;177:1840–1843. doi: 10.1128/jb.177.7.1840-1843.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang H C, Daniel S L, Hsu T D, Drake H L. Nickel transport by the thermophilic acetogen Acetogenium kivui. Appl Environ Microbiol. 1989;55:1078–1081. doi: 10.1128/aem.55.5.1078-1081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]