Abstract
This study aimed to review our experience with the clinical characteristics and management of deep neck infections (DNIs) and determine the changing trends of their characteristics over time in southern China. Patients diagnosed with a DNI between January 2009 and December 2018 were screened retrospectively for their demographic characteristics, etiology of infection, site of infection, microbiology, treatment, and complications. In total, 127 patients were included: 41 (32.3%) were treated between 2009 and 2013 (group A), and 86 (67.7%) were treated between 2014 and 2018 (group B). The most common site of infection in group A was the parapharyngeal space (15 patients, 36.6%), while that in group B involved multiple spaces (36 patients, 41.9%). The leucocyte count (×109 cells/L) was 13.23 ± 4.19 in group A and 16.04 ± 4.33 in group B (p < 0.001). Streptococcus viridans was the most common bacteria in both groups. The mean hospital stay was 21.46 ± 33.09 days in group A and 10.44 ± 6.19 days in group B. The rate of diabetes mellitus (DM) in group A was lower than that in group B (8/41 and 33/86, respectively; p = 0.034). Airway obstruction was the most common complication in both groups. DNIs are more likely to show multi-space involvement, affect more DM patients, and be associated with higher leucocyte counts over time. We infer that the duration from morbidity to admission and that from admission to operation play roles in the successful management of DNIs, possibly causing fewer complications, lower mortality rates, and shorter hospital stays. DM patients require increased attention.
Keywords: Deep neck infection, changing trends, complications
Introduction
A deep neck infection (DNI) is the collection of pus within the potential spaces and fascial planes of the head and neck. This condition remains a challenging problem, despite widespread use of antibiotics because it is a potentially life-threatening condition that can progress to descending mediastinitis, pleural empyema, airway compromise, jugular vein thrombosis, pericarditis, septic shock, and disseminated intravascular coagulation.1–4 Treatment of a DNI may be affected by the widespread and inappropriate use of antibiotics, which alter the sites, presenting signs and symptoms, etiology, bacteriology, and associated systemic diseases, making these infections more elusive and less predictable.5–9 The diagnosis and management of DNIs are delayed because of antibiotic factors and the complex anatomy of the head and neck.
Although the inappropriate use of antibiotics remains a common factor in these diseases, 8 according to the literature, increases in the elderly population and diabetes mellitus (DM) patients remain the most common causes in China.10–12 Therefore, we present our experience with the clinical characteristics and management of DNIs along with the changing trends of these diseases over time in southern China.
Materials and methods
Study design and setting
This observational retrospective study was conducted at the Department of Otolaryngology from January 2009 to December 2018 in the Zhongshan City People’s Hospital, Zhongshan Affiliated Hospital of Sun Yat-sen University, which serves a population of 5 million people. The research protocols were approved by the Ethics Committee of the Zhongshan Affiliated Hospital of Sun Yat-sen University and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent was waived because of the retrospective nature of our study.
Study population and procedure
We reviewed the medical records of all the patients who were diagnosed with a DNI and divided the patients into two groups: patients from the first half of the study period (group A; January 2009 to December 2013) and those from the second half of the study period (group B; January 2014 to December 2018). Patients were excluded if they had superficial infections or abscesses, limited intraoral abscesses, limited peritonsillar abscesses, limited cervical necrotizing fasciitis, or infections due to surgical neck trauma. Finally, 127 patients were included in this study. The site of infection was classified according to the previously published literature in the following spaces: submandibular, parapharyngeal, parotid, masticatory, carotid, retropharyngeal, prevertebral, anterior cervical, posterior cervical, and pretracheal space. Patients with an infection in two or more spaces were defined as having multi-space involvement.
Data source
Data sources included the admission registers of the Otolaryngology Department, patient admission files, and clinical examination and imaging data from the information retrieval of our hospital. For each patient included, we recorded the following on a prevalidated form designed for this study: the patient’s demographic characteristics, inpatient month, leucocyte count, etiology of infection, bacteriology, DM status, radiology, duration of hospital stay, treatment, complications, and outcomes.
Statistics
All the descriptive data were reported in percentages. Statistical evaluations were performed with a two-sided t-test corrected for the inequality of variances and degrees of freedom. Fisher’s exact test and χ2 tests were used to compare the categorical variables. Statistical analysis was performed with SPSS v13.0. A p value <0.05 was considered statistically significant.
Results
Patient characteristics
Because of the policies and public availability of medical insurance, patient compliance with treatment has improved significantly since 2014. Therefore, we divided the study population into two groups: group A (2009–2013) and group B (2014–2018).
Our study comprised 80 men and 47 women, ranging in age from 6 to 89 years with a median age (±SD) of 49.3 (±16.7) years. The major age category was from 51 to 60 years (30 patients, 23.6%; Figure 1). In group A, 24 (58.5%) patients were male, and 17 (41.5%) were female. In group B, 56 (65.1%) patients were male, and 30 (34.9%) were female.
Figure 1.
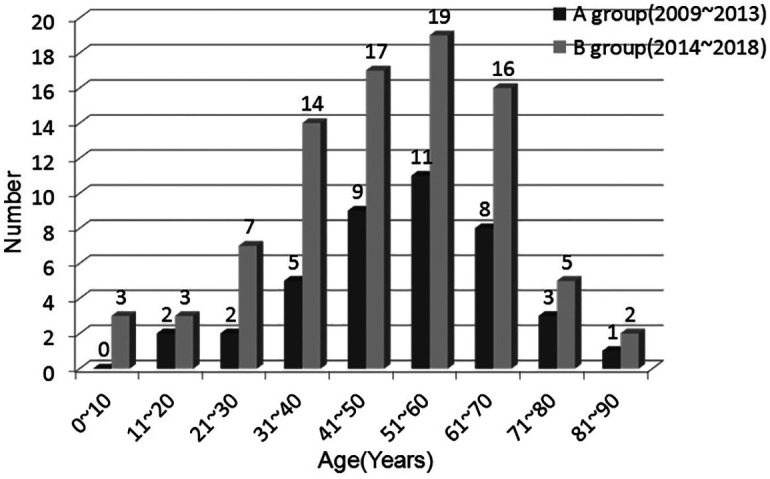
Age distribution of 127 patients with deep neck infections.
Etiology
The causes of DNI were identified in 72 cases (56.7% of all patients), among which an esophageal foreign body (7 patients, 17.1%) and upper airway infections (12 patients, 14.0%) were the most common causes in groups A and B, respectively (Table 1). The significant common causes were upper airway infections, odontogenic abscesses, peritonsillar abscesses, and esophageal foreign bodies, which accounted for 22 patients (53.7%) in group A and 39 (41.8%) in group B, and the cause remained unknown in 15 patients (36.6%) and 40 patients (46.5%), respectively.
Table 1.
Etiology of deep neck infection.
| Etiology | Total n(%) (N = 127) | A group n(%) (N = 41) | B group n(%) (N = 86) |
|---|---|---|---|
| Upper airway infection | 17 (13.4) | 5 (12.2) | 12 (14.0) |
| Odontogenic | 14 (11.0) | 5 (12.2) | 9 (10.5) |
| Peri-tonsillar abscess | 14 (11.0) | 5 (12.2) | 9 (10.5) |
| Esophageal foreign body | 13 (10.2) | 7 (17.1) | 6 (7.0) |
| Skin infection | 8 (6.3) | 3 (7.3) | 5 (5.8) |
| Lymphadenitis | 3 (2.4) | 1 (2.4) | 2 (2.3) |
| Parotitis | 3 (2.4) | 0 | 3 (3.4) |
| Unknown | 55 (43.3) | 15 (36.6) | 40 (46.5) |
Site of infection
Computed tomography scans (CT scans) were included in the routine investigation. MRI scans and sonography of the neck were performed in 4 and 12 patients, respectively. The most common site of infection in group A was the parapharyngeal space (15 patients, 36.6%), followed by multi-space in 9 patients (22.0%) and the submandibular space in 8 patients (19.5%; Table 2). The site most commonly involved in group B was multi-space (36 patients, 41.9%), followed by the parapharyngeal space in 26 patients (30.2%) and the submandibular space in 7 patients (8.1%). The anterior cervical space, prevertebral space and carotid space were less involved in both groups A and B. Group A developed multi-space infections less frequently than group B (9/41 and 36/86 patients; p = 0.028; Table 3).
Table 2.
Distribution of the sites of deep neck infection.
| Site | Total n(%) (N = 127) | A group n(%) (N = 41) | B group n(%) (N = 86) |
|---|---|---|---|
| Multispace | 45 (35.4) | 9 (22.0) | 36 (41.9) |
| Parapharyngeal space | 41 (32.3) | 15 (36.6) | 26 (30.2) |
| Submandibular space | 15 (11.8) | 8 (19.5) | 7 (8.1) |
| Retropharyngeal space | 10 (7.9) | 5 (12.2) | 5 (5.7) |
| Pre-tracheal space | 5 (4.0) | 2 (4.9) | 3 (3.5) |
| Parotid space | 4 (3.1) | 1 (2.4) | 3 (3.5) |
| Masticator space | 4 (3.1) | 1 (2.4) | 3 (3.5) |
| Anterior cervical space | 1 (0.8) | 0 | 1 (1.2) |
| Pre-vertebral space | 1 (0.8) | 0 | 1 (1.2) |
| Carotid space | 1 (0.8) | 0 | 1 (1.2) |
Table 3.
The clinical data of deep neck infection.
| No. of patients | Leucocytecount(×109 cells/L) | Neutrophilcount(×109 cells/L) | No.of multi-space | No. ofdiabetesmellitus | No. of complication | |
|---|---|---|---|---|---|---|
| A group | 41 | 13.23 ± 4.19 | 11.03 ± 4.12 | 9 | 8 | 12 |
| B group | 86 | 16.04 ± 4.33 | 13.69 ± 4.38 | 36 | 33 | 27 |
| p Value | <0.001 | 0.002 | 0.028 | 0.034 | 0.059 |
Laboratory data
The reference range of the leucocyte count was 4.0–10.0 × 109 cells/L. The leucocyte count (×109 cells/L) was 13.23 ± 4.19 in group A and 16.04 ± 4.33 in group B. The neutrophil count (×109 cells/L) was 11.03 ± 4.12 in group A and 13.69 ± 4.38 in group B. The mean leucocyte and neutrophil counts in group A were both significantly lower than those in group B (p < 0.001 and p = 0.002, respectively; Table 3).
Microbiology
Results for the culture of purulent exudates were available for 79 patients (62.2%), and bacteria were found in 52 samples. Streptococcus viridans was the most common bacteria in both groups A and B, accounting for 6 patients (33.3%) and 11 patients (32.4%); of the latter patients, 3 and 8 had DM, respectively. Staphylococcus aureus and K. pneumoniae were common pathogens in both groups. Staphylococcus aureus accounted for 3 (16.7%) and 6 (17.6%) patients, and K. pneumoniae accounted for 3 (16.7%) and 5 (14.7%) patients in groups A and B, respectively. Beta-hemolytic streptococci accounted for 5 (14.7%) in group B. The results for the culture of purulent exudates are shown in Table 4.
Table 4.
Result of positive culture of the purulent exudate in 52 patients with microorganisms growth.
| Organism | Total n(%)(N = 52)* | A group n(%)(N = 18)* | B group n(%)(N = 34)* |
|---|---|---|---|
| Streptococcus viridans | 17 (32.7) | 6 (33.3) | 11 (32.4) |
| Staphylococcus aureus | 9 (17.3) | 3 (16.7) | 6 (17.6) |
| Pneumoniae | 8 (15.4) | 3 (16.7) | 5 (14.7) |
| Beta-hemolyticStreptococcus | 7 (13.5) | 2 (11.1) | 5 (14.7) |
| Neisseriaceae | 5 (9.6) | 2 (11.1) | 3 (8.8) |
| Candida | 3 (5.8) | 1 (5.6) | 2 (5.9) |
| Gram-positive bacilli | 3 (5.8) | 1 (5.6) | 2 (5.9) |
| Gram-negative bacilli | 2 (3.8) | 1 (5.6) | 1 (2.9) |
| Peptostreptococcusmorbillorum | 2 (3.8) | 1 (5.6) | 1 (2.9) |
| Bacteroides | 1 (1.9) | 0 | 1 (2.9) |
The total of the percentages exceeds 100% 5 patients had polymicrobial infections.
Treatment and outcomes
Intravenous antibiotics were part of routine treatment for all 127 patients, among whom 78 (61.4%) had undergone surgical drainage. Surgical drainage was performed in 27 patients (65.9%) in group A and 51 patients (59.3%) in group B. Six and 18 patients had undergone needle aspiration in groups A and B, respectively; 2 and 6 received tooth extractions, and 6 and 11 patients only had conservative antimicrobial management, respectively.
In both groups, the mean 7-day duration from morbidity to admission was determined. Group A had a higher proportion with a longer duration (>7 days; 41.5%) than group B (>7 days; 23.3%) (p = 0.035) (Table 5). The mean duration from admission to operation in the surgical drainage cases was 5.34 ± 6.8 days in group A and 2.61 ± 2.98 days in group B (p = 0.008). The duration of hospital stay ranged from 1 to 213 days, with a mean of 21.46 ± 33.09 days in group A and 10.44 ± 6.19 days in group B (p = 0.004).
Table 5.
The duration of treatment of deep neck infection.
| No. of duration frommorbidity to admission(>7 days) n(%) | Duration fromadmission to operation | Duration of thehospital stay | |
|---|---|---|---|
| A group | 17 (41.5) | 5.34 ± 6.8 | 21.46 ± 33.09 |
| B group | 20 (23.3) | 2.61 ± 2.98 | 10.44 ± 6.19 |
| p Value | 0.035 | 0.008 | 0.004 |
Diabetes mellitus (DM) and complications
All 127 patients with associated DM are shown in Table 3. The rate of DM in group A was lower than that in group B (8/41 and 33/86; p = 0.034). The complications of DNIs are shown in Table 6. Complications were found in 12 patients in group A and 27 patients in group B. Of the 12 patients in group A, 6 had an airway obstruction and 3 had sepsis. Of the 27 patients in group B, 13 had an airway obstruction and 6 had mediastinitis. The incidence of complications was not different between the groups (p = 0.059). Airway obstruction was experienced by 6 and 13 patients, respectively, in group A or B, among whom 3 or 9 had undergone either tracheotomy or endotracheal intubation. One and two patients in groups A and B with DM died of septic shock and multiple organ dysfunction syndromes, respectively.
Table 6.
Complications of deep neck infection.
| Complication | A group n(%) (N = 41)* | B group n(%) (N = 86)* |
|---|---|---|
| Airway obstruction | 6 (14.6) | 13 (15.1) |
| Mediastinitis | 2 (4.9) | 6 (7.0) |
| Pneumonia | 2 (4.9) | 4 (4.7) |
| Sepsis | 3 (7.3) | 3 (3.5) |
| Empyema | 1 (2.4) | 3 (3.5) |
| Deep venous thrombosis | 1 (2.4) | 2 (2.3) |
| Upper gastrointestinal perforation | 2 (4.9) | 2 (2.3) |
| Diabetic ketoacidosis | 0 | 1 (1.2) |
| Skin defect | 0 | 1 (1.2) |
| None | 29 (70.7) | 59 (68.6) |
An individual patient may have two or more complications.
Discussion
The major age category was 51–60 years in both groups (Figure 1). An age-related decline in neutrophil function, including impairment of neutrophil phagocytosis and bactericidal ability in healthy individuals, may lead to increased susceptibility to DNIs. Lee and Kanagalingam 13 also reported that 51–60 years was the most common age group among 131 patients with deep neck abscesses. Our data also showed that season may be an important influencing factor in DNIs. The reason for this may be partly due to a variable environment and the changes in the temperature between summer and autumn in southern China.
The etiology of DNIs had many factors in both groups, consistent with previous literature. Some studies have shown that odontogenic infections are the most common cause. 14 Several other studies have also implicated upper airway infections as the major cause of DNIs. 15 In our data, the significant common causes, namely, upper airway infections, odontogenic abscesses, peritonsillar abscesses, and esophageal foreign bodies, did not change between the groups. The first three causes can easily lead to DNI formation. Meanwhile, the source of infection in 55 patients was unknown.
The most common site of infection in group A was the parapharyngeal space because the parapharyngeal space is often involved with the peritonsillar, submandibular, masticatory, and parotid space. Several other studies have also reported that it was the space most commonly involved.16,17 However, multi-space involvement was more common in group B than group A. The reason may be an increased incidence of systemic diseases, including DM, in these patients who may have worse defenses against infections and thus have higher rates of more severe multi-space infections over time.
Baglam et al. 18 indicated that the neutrophil-to-lymphocyte ratio could be a potential laboratory parameter for diagnosing DNIs. Our data showed that the counts of leucocytes and neutrophils in group A were lower than those in group B. We hypothesized that the higher counts of leucocytes or neutrophils in group B were due to the effects of DM and antibiotic abuse over time.
No bacterial growth was observed in 34.2% of available samples of purulent exudate, likely because high-dose antimicrobials were administered early in the disease course and intravenous antibiotics were used before surgical drainage. Streptococcus viridans was the most common bacteria in both groups. No significant change was found in the bacterial strains over time. In some Western studies, the most common organism cultured was K. pneumoniae. 17 Several other studies have shown that the most common bacterial strains were aerobic S. viridans, β-hemolytic streptococci, Staphylococcus, K. pneumoniae, anaerobic Bacteroides, and Peptostreptococcus. 17 Additionally, Celakovsky et al. 19 indicated that the bacteriology of DNIs is polymicrobial, and infections of dental origin and in nondiabetic patients had a high incidence of anaerobic bacteria in adults. However, the most common bacterial strain in patients with DM was K. pneumoniae in recent studies, resulting in severe infections, including pneumoniae, bacteremia, hepatic abscess, and meningitis. 20 These results could help in choosing an appropriate empirical antibiotic before obtaining a positive bacterial culture in DM patients.
All 127 patients were treated with intravenous antibiotics following admission. However, in our study, 6 and 11 patients only had conservative antimicrobial management in groups A and B, respectively. Many cases were associated with surgical drainage and intravenous antibiotics, but no significant changes were found between the groups (65.9% vs 59.3%).
Early diagnosis and treatment are necessary for DNIs. 21 Cramer et al. 22 indicated that a delay in surgical drainage of DNIs is associated with increased morbidity and mortality in adult patients. Their study showed that a delay in drainage >3 days was associated with a 2.38-fold increase in morbidity and mortality. Das et al. 23 also indicated that for a complete cure and to prevent complications of DNIs, early diagnosis, and management were necessary. We suppose that the key factors of the successful management of DNIs are the duration from morbidity to admission and duration from admission to operation. The reference values of the effect of management may be greater than those of other factors, including the leucocyte counts, DM, and multi-space infection.
Immunosuppressed patients require more attention because they are the group with the most difficult and life-threatening DNIs. Patients with DM are immunosuppressed. Huang et al. 24 compared DNIs in DM patients and non-DM patients. The results showed that the DM group had an older mean age (57.2 vs 46.2), a longer hospital stay (19.7 days vs 10.2 days), more frequent complications (33.9% vs 8.5%), and more frequent tracheostomy or intubation (19.6% vs 6.2%). Lin et al. 25 also reported that the DM group had a significantly higher complication rate, a longer hospital stay, and a higher tracheotomy rate than the non-DM group. A meta-analysis 26 reported that DM was associated with a higher prevalence of multi-space spread of infections, complications, and failure to identify the pathogen, with risk ratios of 1.96, 2.42, and 1.29, respectively, and bacteriological differences from non-DM. Type 1 DM also showed similar features. 27 This study showed that patients with type 1 DM required significantly longer hospitalization for DNI than those without DM (9.0 ± 6.2 vs 4.1 ± 2.0 days). T1DM is associated with a 10-fold increase in DNI risk.
Other diseases with low immunity also need attention, such as human immunodeficiency virus (HIV) patients, end-stage renal disease patients, and injection drug users. Sittitrai et al. 28 reviewed DNI in an HIV group and a non-HIV group. In the HIV group, Ludwig’s angina was common, and upper airway obstruction was more common in the HIV group. These patients also had a higher risk of other complications (sepsis, mediastinitis, jugular vein thrombosis, and pneumonia) (6/31 compared with 12/192), a higher mortality rate (3/31 compared with 2/192), and a longer hospital stay (19 days compared with 16 days). The authors thought that DNIs in these patients were more severe and that the early detection and management of the airway and complications may improve their outcomes. End-stage renal disease (ESRD) is a predisposing factor for DNI, increasing its risk by two-fold. 29 DNIs are not associated with higher rates of surgical debridement, tracheostomy, mediastinal complications, or longer hospital stays in patients with ESRD, but ESRD is associated with worse survival outcomes. However, DNIs in adult intravenous drug users may have a higher rate of comorbidities, abnormal laboratory data, and microbiology cultures, but these patients may achieve good outcomes after reasonable interventions. 30
Despite the widespread use of antibiotics, 9 life-threatening complications, such as descending mediastinitis, airway obstruction, and pleural empyema, can still result from DNIs. In our data, three patients in both groups with DM died of septic shock and multiple organ dysfunction syndromes. Other complications showed no differences between the groups. The reasons may be related to the comprehensive effects of the duration from morbidity to admission and duration from admission to operation, DM, site of infection, and age. Additionally, Hidaka et al. 31 indicated that delayed oral dietary intake in patients with DNIs may be associated with descending necrotizing mediastinitis. Descending mediastinitis as a lethal complication requires early detection and intervention. Alegbeleye 32 reported two cases of DNI and descending mediastinitis as lethal complications of dentoalveolar infections. Both patients were admitted to the intensive care unit for close monitoring. The first patient continued to make satisfactory clinical progress and was discharged by the fourth week of admission, but the patient with human immunodeficiency viral infection died on the fifth postoperative day. Airway obstruction is also a common complication. Assessing the risk of a potentially difficult airway and preparing the most appropriate airway management method is critical, particularly at the level of the epiglottis and aryepiglottic fold. 33 Imaging with computed tomography may be beneficial to airway management under general anesthesia.
Our study was limited by its small sample size and retrospective nature. It is difficult to determine the necessary sample size because we included all the cases from 2009 to 2018; thus, the data may be partially biased. Second, our anaerobic bacteria data were missing. Finally, we have no data on low immunity, including HIV status. Thus, the examination of larger samples and prospective research is necessary.
Conclusions
Deep neck infections are more likely to have parapharyngeal space or multi-space involvement. Successful management of DNIs requires shortening the duration from morbidity to admission and the duration from admission to operation, which may reduce complications, the mortality rate, and the hospital stay. DM patients need more attention.
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author biographies
Jian-Hui Wu Graduated ten years ago and is an attending physician in the Department of otolaryngology. He is good at the diagnosis and treatment of common diseases of ear, nose and throat, especially head and neck surgery.
Xin Li is a deputy chief physician in the Department of otolaryngology. He is good at the diagnosis and treatment of head and neck surgery, allergic diseases.
Guo-Ping Chen is an attending physician in the Department of otolaryngology. He is good at the diagnosis and treatment of head and neck surgery, ear diseases.
Min-Yi Fu is a chief physician in the Department of otolaryngology. He has worked for 30 years and is good at the diagnosis and treatment of head and neck surgery, nasal diseases.
Fei Ye is a Chief physician in the Department of otolaryngology. He has worked for 20 years and is good at the diagnosis and treatment of head and neck surgery.
Footnotes
Author contributions: Jian-Hui Wu wrote the main manuscript text and prepared Figures and statistical analysis. Xin Li revised the article. Min-Yi Fu and Guo-Ping Chen have checked and corrected those in the abstract, Introduction, and Discussion. Min-Yi Fu and Fei Ye have corrected those in the results and participated in polishing this manuscript. All authors reviewed the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jian-Hui Wu  https://orcid.org/0000-0003-2576-1802
https://orcid.org/0000-0003-2576-1802
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Chen MK, Wen YS, Chang CC, et al. Predisposing factors of life-threatening deep neck infection: logistic regression analysis of 214 cases. J Otolaryngol 1998; 27: 141–144. [PubMed] [Google Scholar]
- 2.Ridder GJ, Technau-Ihling K, Sander A, et al. Spectrum and management of deep neck space infections: an 8-year experience of 234 cases. Otolaryngol Head Neck Surg 2005; 133: 709–714. [DOI] [PubMed] [Google Scholar]
- 3.Stalfors J, Adielsson A, Ebenfelt A, et al. Deep neck space infections remain a surgical challenge: a study of 72 patients. Acta Otolaryngol 2004; 124: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 4.Mejzlik J, Celakovsky P, Tucek L, et al. Univariate and multivariate models for the prediction of life-threatening complications in 586 cases of deep neck space infections: retrospective multi-institutional study. J Laryngol Otol 2017; 131: 779–784. [DOI] [PubMed] [Google Scholar]
- 5.Boscolo-Rizzo P, Marchiori C, Montolli F, et al. Deep neck infections: a constant challenge. ORL J Otorhinolaryngol Relat Spec 2006; 68: 259–265. [DOI] [PubMed] [Google Scholar]
- 6.Hvistendahl M. Public health. China takes aim at rampant antibiotic resistance. Science 2012; 336: 795. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Eggleston K, Rotimi V, et al. Antibiotic resistance as a global threat: evidence from China, Kuwait and the United States. Global Health 2006; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds L, McKee M. Factors influencing antibiotic prescribing in China: an exploratory analysis. Health Policy 2009; 90: 32–36. [DOI] [PubMed] [Google Scholar]
- 9.Yezli S, Li H. Antibiotic resistance amongst healthcare-associated pathogens in China. Int J Antimicrob Agents 2012; 40: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang TT, Liu TC, Chen PR, et al. Deep neck infection: analysis of 185 cases. Head Neck 2004; 26: 854–860. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 12.Fu JF, Liang L, Gong CX, et al. Status and trends of diabetes in Chinese children: analysis of data from 14 medical centers. World J Pediatr 2013; 9: 127–134. [DOI] [PubMed] [Google Scholar]
- 13.Lee YQ, Kanagalingam J. Deep neck abscesses: the Singapore experience. Eur Arch Otorhinolaryngol 2011; 268: 609–614. [DOI] [PubMed] [Google Scholar]
- 14.Gujrathi AB, Ambulgekar V, Kathait P. Deep neck space infection – a retrospective study of 270 cases at tertiary care center. World J Otorhinolaryngol 2016; 2: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LF, Kuo WR, Tsai SM, et al. Characterizations of life-threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol 2003; 24: 111–117. [DOI] [PubMed] [Google Scholar]
- 16.Yonetsu K, Izumi M, Nakamura T. Deep facial infections of odontogenic origin: CT assessment of pathways of space involvement. AJNR Am J Neuroradiol 1998; 19: 123–128. [PMC free article] [PubMed] [Google Scholar]
- 17.Har-El G, Aroesty JH, Shaha A, et al. Changing trends in deep neck abscess: a retrospective study of 110 patients. Oral Surg Oral Med Oral Pathol 1994; 77: 446–450. [DOI] [PubMed] [Google Scholar]
- 18.Baglam T, Binnetoglu A, Yumusakhuylu AC, et al. Predictive value of the neutrophil-to-lymphocyte ratio in patients with deep neck space infection secondary to acute bacterial tonsillitis. Int J Pediatr Otorhinolaryngol 2015; 79: 1421–1424. [DOI] [PubMed] [Google Scholar]
- 19.Celakovsky P, Kalfert D, Smatanova K, et al. Bacteriology of deep neck infections: analysis of 634 patients. Aust Dent J 2015; 60: 212–215. [DOI] [PubMed] [Google Scholar]
- 20.Tang LM, Chen ST, Hsu WC, et al. Klebsiella meningitis in Taiwan: an overview. Epidemiol Infect 1997; 119: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirasawa K, Tsukahara K, Motohashi R, et al. Deep neck cellulitis: limitations of conservative treatment with antibiotics. Acta Otolaryngol 2017; 137: 86–89. [DOI] [PubMed] [Google Scholar]
- 22.Cramer JD, Purkey MR, Smith SS, et al. The impact of delayed surgical drainage of deep neck abscesses in adult and pediatric populations. Laryngoscope 2016; 126: 1753–1760. [DOI] [PubMed] [Google Scholar]
- 23.Das R, Nath G, Mishra A. Clinico-pathological profile of deep neck space infection: a prospective study. Indian J Otolaryngol Head Neck Surg 2017; 69: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang TT, Tseng FY, Liu TC, et al. Deep neck infection in diabetic patients: comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol Head Neck Surg 2005; 132: 943–947. [DOI] [PubMed] [Google Scholar]
- 25.Lin HT, Tsai CS, Chen YL, et al. Influence of diabetes mellitus on deep neck infection. J Laryngol Otol 2006; 120: 650–654. [DOI] [PubMed] [Google Scholar]
- 26.Hidaka H, Yamaguchi T, Hasegawa J, et al. Clinical and bacteriological influence of diabetes mellitus on deep neck infection: systematic review and meta-analysis. Head Neck 2015; 37: 1536–1546. [DOI] [PubMed] [Google Scholar]
- 27.Chang GH, Ding MC, Yang YH, et al. High risk of deep neck infection in patients with type 1 diabetes mellitus: a nationwide population-based cohort study. J Clin Med 2018; 7: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sittitrai P, Srivanitchapoom C, Reunmakkaew D. Deep neck infection in patients with and without human immunodeficiency virus: a comparison of clinical features, complications, and outcomes. Br J Oral Maxillofac Surg 2018; 56: 962–967. [DOI] [PubMed] [Google Scholar]
- 29.Chang GH, Tsai MS, Liu CY, et al. End-stage renal disease: a risk factor of deep neck infection – a nationwide follow-up study in Taiwan. BMC Infect Dis 2017; 17: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamir SO, Marom T, Len A, et al. Deep neck infections in cervical injection drug users. Laryngoscope 2015; 125: 1336–1339. [DOI] [PubMed] [Google Scholar]
- 31.Hidaka H, Ozawa D, Kuriyama S, et al. Risk factors for delayed oral dietary intake in patients with deep neck infections including descending necrotizing mediastinitis. Eur Arch Otorhinolaryngol 2017; 274: 3951–3958. [DOI] [PubMed] [Google Scholar]
- 32.Alegbeleye BJ. Deep neck infection and descending mediastinitis as lethal complications of dentoalveolar infection: two rare case reports. J Med Case Rep 2018; 12: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho SY, Woo JH, Kim YJ, et al. Airway management in patients with deep neck infections: a retrospective analysis. Medicine 2016; 95: e4125. [DOI] [PMC free article] [PubMed] [Google Scholar]


