Abstract
Background:
This phase III, controlled, patient-blinded, multicentre study in two parallel, equal-sized treatment groups compared the efficacy and safety of TISSEEL Lyo, fibrin sealant versus Manual Compression (MC) with surgical gauze pads for use as a haemostatic agent in patients who underwent vascular surgery in Russia.
Methods:
Adult patients, both genders, who received peripheral vascular expanded polytetrafluoroethylene conduits and had suture line bleeding after surgical haemostasis were enrolled. Patients were randomized to be treated with TISSEEL Lyo or MC. The bleeding needed additional treatment and had to be assessed as grade 1 or 2 bleeding according to the Validated Intraoperative Bleeding scale. The primary efficacy endpoint was the proportion of patients achieving haemostasis at 4 min after treatment application (T4) at the study suture line, which was maintained until the closure of the surgical wound. The secondary efficacy endpoints included the proportion of patients achieving haemostasis at 6 min (T6) and 10 min (T10) after treatment application at the study suture line, which was maintained until closure of the surgical wound, as well as the proportion of patients with intraoperative and postoperative rebleeding. Safety outcomes included incidence of adverse events (AEs), surgical site infections and graft occlusions.
Results:
A total of 110 patients were screened; 104 patients were randomized: (TISSEEL Lyo: 51 [49%] patients; MC: 53 [51%] patients). T4 haemostasis was achieved in 43 (84.3%) patients in the TISSEEL Lyo group and in 11 (20.8%) patients in the MC group (p < 0.001). Significantly more patients in TISSEEL Lyo group achieved the haemostasis at T6 (relative risk (RR) of achieving haemostasis 1.74 [95% confidence interval (CI) 1.37; 2.35]) and T10 (RR 1.18 [95% CI 1.05; 1.38]) versus MC. No one had intraoperative rebleeding. Postoperative rebleeding was reported only in one patient in the MC group. No treatment-emergent serious AEs (TESAEs) related to TISSEEL Lyo/MC, TESAEs leading to withdrawal and TESAEs leading to death were reported in patients during the study.
Conclusions:
Data demonstrated TISSEEL Lyo had clinically and statistically significant superiority to MC as a haemostatic agent in vascular surgery at all measured time points including 4, 6 and 10 min and had proven to be safe.
Keywords: Fibrin sealant, surgery, thrombin, TISSEEL Lyo
Introduction
Haemostasis is an essential element in a successful surgical outcome, especially in vascular surgery. Surgical techniques have improved the ability to achieve and maintain haemostasis in vascular surgery; however, this has not entirely solved the problem of bleeding that is difficult to control using primary haemostatic methods. The application of topical haemostats and sealants, including different forms of collagen, oxidized cellulose or gelatine, alone or in combination with thrombin, polyethylene glycol-based glues, gelatine and cyanoacrylate glue have been used with varying success.1–4
Baxter's fibrin sealants (FSs) have been used for more than 30 years in multiple surgical disciplines and surgical procedures for haemostasis, sealing and adherence of tissue, and suture support.
The use of FSs was pioneered in the mid-1970s in the field of cardiothoracic surgery. Fibrin sealants were shown to significantly reduce perioperative blood loss in many examples of coronary surgery, including bypass operations, vascular grafts, repair of septal defects, ventricular ruptures, sealing of prosthetic patches and repair of acute aortic insufficiency associated with aortic dissection.5–7 Bleeding at suture lines was also reduced following the use of FSs during peripheral vascular surgery. Other surgical disciplines in which FSs have been used include gastrointestinal and thoracic surgery, neurosurgery, urology and otolaryngology. 8
In May 1998, FS Vapor Heated (VH) was approved by the United States (US) Food and Drug Administration (FDA), and FS VH Solvent/Detergent (S/D) received licensure in the US in July 2006. 5 As of 30 November 2019, FS VH S/D has been approved in 51 countries.
This study was conducted as part of the registration process for TISSEEL Lyo in the Russian Federation.
The study aimed to prove the superiority of TISSEEL Lyo for haemostasis in patients receiving peripheral vascular prosthetic expanded polytetrafluoroethylene (ePTFE) conduits, as compared to a control arm treated with Manual Compression (MC) with surgical gauze pads.
Materials and methods
This was a prospective, controlled, randomized, patient-blinded, multicentre phase III study in two parallel, equal-sized treatment arms to compare the efficacy and safety of TISSEEL Lyo versus MC with surgical gauze pads in a total of 104 evaluable patients undergoing elective vascular surgery in Russia. The study was conducted at six centres in accordance with the International Conference on Harmonization Harmonized Guideline Good Clinical Practice (ICH GCP), the World Medical Association Declaration of Helsinki (of 1964, as subsequently amended) and applicable international and local law and regulations. Before initiation, the study conducted was authorized by the Ministry of Healthcare of the Russian Federation. The protocol and Patient Information Sheet with the ICF were approved by the Ethics Council of the Ministry of Healthcare of the Russian Federation and by the Local Ethics Committee (LEC) of each study site. Patients provided written informed consent before any study-specific procedures were carried out. The information about the study was published on ClinicalTrials.gov (NCT04083807).
Subject population
The adult patients, both genders, aged 18 years and older, who underwent primary elective vascular surgery with peripheral vascular ePTFE conduits, and signed the informed consent form (ICF) were eligible for the study. Their eligibility for surgery was evaluated based on the routine medical work-up (medical history, physical examination/review of systems, laboratory and imaging) and the screening results for general inclusion and exclusion criteria during the 14 days before surgery. The main criteria for exclusion were emergency surgery, other vascular procedures during the same surgical session, arterio-arterial bypass with more than two anastomoses, severe local inflammation at the operating field, prior radiation therapy to the operating field, known hypersensitivity to aprotinin, heparin, blood products or other components of the investigational product, known exposure to aprotinin within the last 12 months, haemoglobin <9.0 g/dL at screening.
Eligibility according to general and intraoperative criteria led to the final selection of the patient into the study, which was determined intraoperatively by the presence of any suture line bleeding that would prevent immediate closure of the wound and required treatment of the bleeding first, and was assessed as a grade 1 or 2 bleeding according to the Validated Intraoperative Bleeding (VIBe) scale. 9 If the bleeding was greater than grade 2, it could be reduced to grade 2 or 1 by additional sutures. In such a case patient could be randomized.
Surgical procedure
An arterio-arterial bypass was performed according to hospital standards.
Randomized treatment
Upon detection of suture line bleeding, the patients were randomized 1:1 to either TISSEEL Lyo or MC in a stratified manner based on a 1:1 ratio using a study site as a stratification factor. The allocation was conducted using a dynamic randomization approach. A randomization scheme was dynamically generated using validated software by independent statisticians.
Investigators didn’t provide information about the allocation and actual treatment of the patients.
After completion of anastomotic suturing, clamps were opened to determine if eligible suture line bleeding met the inclusion criterion. Upon completion of the bleeding assessment, the vessel was reclamped to ensure there was no active blood flow from the target application site, which could have washed away TISSEEL before the fibrin clot could form.
The surface area of the wound was dried using standard techniques. In the TISSEEL Lyo group, the FS (4 ml total volume) was applied once intraoperatively to the study suture line using the DUPLOJECT Fibrin Sealant Preparation and Application System. In the MC group, a dry surgical, 4 × 4-inch, gauze pad was positioned to completely cover the suture line. The arterial flow was re-established after 2 min of setting TISSEEL Lyo or gauze pad placement. If the haemostatic effect was incomplete once clamps were released after the initial application of the TISSEEL Lyo or gauze pads, the investigator was responsible for deciding on the course of action depending on the severity of the bleeding. In the event of small bleeds or oozing, the main assessment at 4 min could be done without reclamping of the vessel. In a case where there was no bleeding observed at 4 min, the surgeon waited up to 10 min to check the next landmark points until wound closure. The study suture line was inspected for bleeding at 4, 6 and 10 min after the start of the application of the FS/MC. During the observational period, additional haemostatic treatments on the study suture line were used only in cases of severe bleeding that jeopardized patient safety.
The investigators were trained to prepare and use the TISSEEl Lyo appropriately to ensure consistent application across study sites.
Study endpoints
The primary efficacy endpoint was the proportion of patients achieving haemostasis at 4 min after treatment application at the study suture line, which was maintained until closure of the surgical wound. The secondary efficacy endpoints included the proportions of patients achieving haemostasis at 6 and 10 min after treatment application at the study suture line, which was maintained until closure of the surgical wound; the proportion of patients with intraoperative rebleeding after haemostasis at the study suture line, and the proportion of patients with postoperative rebleeding after haemostasis at the study suture line, which required surgical re-exploration.
Safety endpoints included the incidence of the following events: (a) adverse events (AEs) up to 14 (± 3) days postoperatively, (b) abnormal laboratory values and vital signs, (c) surgical site infections and (d) graft occlusions. Safety assessment included assessment of AEs, clinical laboratory tests, and vital signs (systolic and diastolic blood pressure [BP], heart rate [HR] and respiratory rate [RR]).
Investigators evaluated AEs for seriousness, severity, causal relationship and expectedness. Surgical site infections were evaluated by the surgeon according to the national clinical guideline at the follow-up visit (day 14 ± 3) and were recorded according to the following scale:
Grade I: only the dermis affected
Grade II: infections invaded the subcutaneous region but not the arterial implant
Grade III: the arterial implant was infected.
Graft occlusion was determined by the surgeon clinically and defined as an absence of blood flow through the graft.
Statistics
Based on previous studies6,10 expected efficacy was set to 60% in the TISSEEL Lyo group and 33.4% in the control group.
The sample size was calculated using Microsoft R Open software version 3.4.3, package TrialSize for the proportion of patients achieving haemostasis at 4 min after treatment application at the study suture line, which was maintained until closure of the surgical wound for testing the superiority hypothesis.
To achieve 80% power of test at 0.025 level of type I error (one-sided), randomization to treatment groups at a 1:1 ratio, at least 52 evaluable subjects were required in each group.
A blind data review (BDR) was conducted in order to review the data, programmed analysis datasets and outputs. The BDR was conducted at a pre-determined date cut-off (23 September 2019) before the final database lock (DBL).
All analyses were performed using SAS/Graph® 9.4 software, SAS/STAT® 14.1 software, and BaseSAS® 9.4.
The primary efficacy endpoint was investigated by a one-sided test for superiority using a likelihood-ratio chi-square test with a 0.025 one-sided significance level. Proportions, relative risk (RR) and corresponding 95% two-sided CIs (based on the likelihood-ratio chi-square test) for RR were calculated for the primary efficacy endpoint.
A logistic regression analysis was planned on the modified intent-to-treat (mITT) analysis set with haemostasis at 4 min (success/failure) as the dependent variable and the study site, gender, age (≤ 65 or > 65 years), VIBe score, and treatment as the independent variables. A model could also include other important factors which were evaluated at the time of the statistical analysis. Interactions with the treatment variable were explored. Factors that were not statistically significant were dropped from the model (stay level for type III effect is 0.15). Model fit was tested using the Hosmer-Lemeshow test.
Proportions, RR and corresponding 95% two-sided CIs (based on the likelihood-ratio chi-square test) were calculated for each study arm for all secondary efficacy endpoints. Analyses were similar to the primary efficacy endpoint, but p-values were interpreted in a descriptive manner.
AEs were classified as non-treatment AEs and TEAEs. A treatment-emergent AE (TEAE) was defined as an AE that started at or after the time of application of randomized treatment. Fisher's exact test was used to compare the proportion of patients with TEAEs and TESAEs (Treatment-Emergent Serious Adverse Events) between the two treatment groups. No corrections for multiple comparisons were made.
Frequencies and grades of surgical site infections were described by treatment groups and time points. Between-group comparisons were performed for general frequencies (without taking severity grades into account) using Fisher's exact test. For between-group comparisons with grades, the Mann-Whitney test (ordered data) was used.
No adjustments for multiple comparisons were applied.
Frequencies of graft occlusions were described by treatment groups and time points. Comparisons between groups were performed using Fisher's exact test.
No adjustments for multiple comparisons were applied.
Results
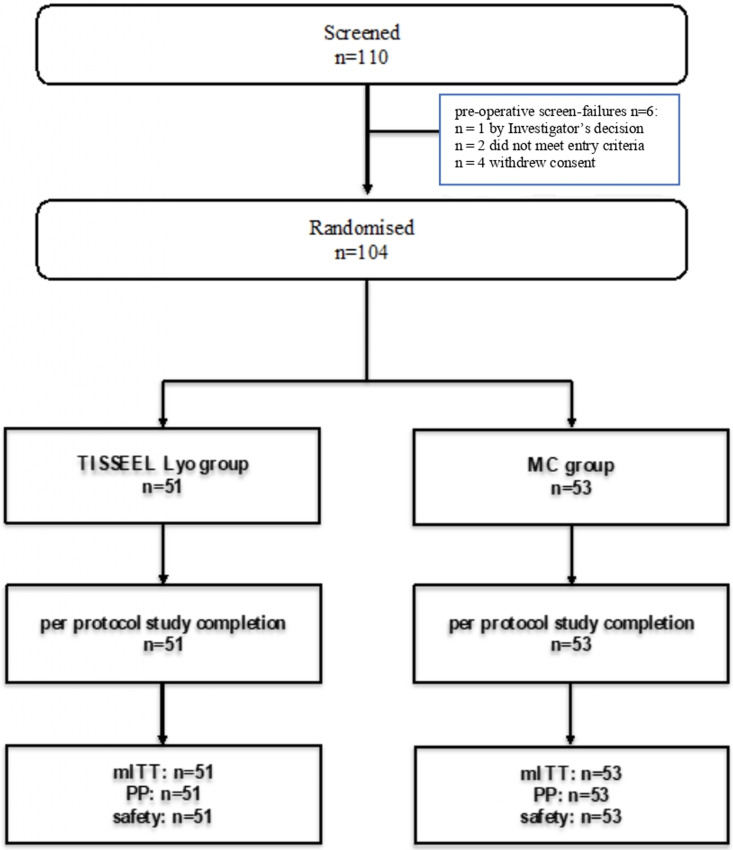
The study was conducted between 09 July 2019 (first patient, first visit) and 22 October 2019 (last patient, last visit). The study was stopped as it was planned. A total of 110 patients were screened in the study; 104 patients were randomized: (TISSEEL Lyo: 51 [49%] patients; MC: 53 [51%] patients). All randomized patients completed the study according to the protocol. There were no early discontinuations from the study (Figure 1).
Figure 1.
Patient disposition flowchart.
Notes: MC—manual compression, mITT—modified intent-to-treat (population), PP—per protocol (population), safety—safety set
Demographic and other baseline characteristics, including the type of elective vascular surgery, the proportion of patients with different grades of bleeding and the median time to surgical closure of the wound were similar between treatment groups (Table 1).
Table 1.
Demographic and other baseline characteristics.
| Parameter | TISSEEL Lyo (N = 51) | MC (N = 53) |
|---|---|---|
| Age | ||
| N | 51 | 53 |
| Mean (SD) | 64.9 (7.4) | 64.6 (6.9) |
| Median | 65.0 | 64.0 |
| Min; Max | 51.0; 84.0 | 44.0; 81.0 |
| p-ANOVA | 0.817 | |
| Sex | ||
| Female | 4 (7.8%) | 9 (17.0%) |
| Male | 47 (92.2%) | 44 (83.0%) |
| p-Fisher's | 0.236 | |
| Race | ||
| Asian | 1 (2.0%) | 0 (0.0%) |
| White | 50 (98.0%) | 53 (100.0%) |
| p-Fisher's | 0.490 | |
| BMI (kg/m2) | ||
| N | 51 | 53 |
| Mean (SD) | 27.2 (5.0) | 25.9 (4.2) |
| Median | 27.4 | 25.3 |
| Min; Max | 18.4; 44.8 | 18.8; 45.0 |
| Planned vascular surgery | ||
| Arterio-arterial bypass | 51 (100.0%) | 53 (100.0%) |
| Ilio-femoral bypass | 5 (9.8%) | 8 (15.1%) |
| Femoro-femoral bypass | 1 (2.0%) | 1 (1.9%) |
| Ilio-popliteal bypass | 2 (3.9%) | 1 (1.9%) |
| Femoro-popliteal bypass | 40 (78.4%) | 37 (69.8%) |
| Femoro-tibial vessel bypass | 3 (5.9%) | 6 (11.3%) |
| VIBe scale | ||
| Grade 1 (mild) | 11 (21.6%) | 19 (35.8%) |
| Grade 2 (moderate) | 40 (78.4%) | 34 (64.2%) |
| Time to surgical closure of the wound (minutes) | ||
| Median (Min–Max) (minutes) | 30 (10–80) | 35 (17–130) |
| Intraoperative complications | ||
| Yes | 2 (3.9%) | 0 (0.0%) |
| No | 49 (96.1%) | 53 (100.0%) |
MC: manual compression; SD: standard deviation; Min: minimum; Max: maximum; BMI: body mass index; VIBe (scale): Validated Intraoperative Bleeding (scale); N: patient number in the group.
Primary efficacy analyses
In the modified intent-to-treat (mITT) population, which included all eligible patients who were randomized into the study and received test product/comparator, haemostasis was achieved within 4 min in 43 (84.3%) patients in the TISSEEL Lyo group versus 11 (20.8%) patients in the MC group (RR with 95% CI = 4.06 [2.51;7.49], p < 0.001) (Table 2).
Table 2.
Primary efficacy analysis – T4 haemostasis.
| Number of patients | Relative risk with 95% CI | p-value | |
|---|---|---|---|
| TISSEEL Lyo (N = 51) | MC (N = 53) | ||
| Primary analysis (mITT population) | |||
| 43 (84.3%) | 11 (20.8%) | 4.06 [2.51; 7.49] | <0.001 |
| Supportive analysis (mITT population) | |||
| 43 (84.3%) | 11 (20.8%) | 20.87 [7.57; 57.52] | <0.001 |
MC: manual compression; T4: haemostasis assessment at 4 min following the start of treatment application; mITT: modified intent-to-treat; CI: confidence interval; N: patient number in the group.
The per-protocol population had no patient exclusion and was identical to the mITT, so the results in the PP population were identical.
A supportive analysis based on logistic regression with T4 haemostasis (success/failure) as the dependent variable and age (≤ 65 or > 65 years) and treatment as the independent variable confirmed the results of the primary analysis. The odds ratio (TISSEEL Lyo vs. MС) along with its 95% CI was 20.87 [7.57; 57.52] (p < 0.001) and indicated superior performance for TISSEEL Lyo.
Secondary efficacy analyses
Results of secondary efficacy endpoints showed the superiority of TISSEEL Lyo versus MC regarding the proportions of patients who achieved haemostasis within 6 min (92.2% vs. 52.8%, p < 0.001) and 10 min (98.0% vs. 83.0%, p = 0.003). No one had intraoperative rebleeding. Postoperative rebleeding was reported only in one patient in the MC group (p = 0.878) (Table 3).
Table 3.
Secondary efficacy analysis.
| Endpoint | Number of patients | Relative risk with 95% CI | p-value | |
|---|---|---|---|---|
| TISSEEL Lyo (N = 51) | MC (N = 53) | |||
| T6 haemostasis achieved | 47 (92.2%) | 28 (52.8%) | 1.74 [1.37; 2.35] | <0.001 |
| T10 haemostasis achieved | 50 (98.0%) | 44 (83.0%) | 1.18 [1.05; 1.38] | 0.003 |
| Intraoperative rebleeding | 0 (0.0%) | 0 (0.0%) | Not estimable | |
| Postoperative rebleeding | 0 (0.0%) | 1 (1.9%) | 0.00 [0.00; 5.96] | 0.878 |
MC: manual compression; T6: haemostasis assessment at 6 min following the start of treatment application; T10: haemostasis assessment at 10 min following start of treatment application; CI: confidence interval; N: patient number in the group.
Safety analyses
Overall, 104 patients (51 in the TISSEEL Lyo group and 53 in the MC group) were included in the safety set.
A total of 153 TEAEs occurred in 92 (88.5%) patients (TISSEEL Lyo: 70 TEAEs in 45 patients; MC: 83 TEAEs in 47 patients).
The system organ classes (SOCs) with the highest number of TEAEs were Injury, Poisoning and Procedural Complications (96 TEAEs in 81 [77.9%] patients), General Disorders and Administration Site Conditions (21 TEAEs in 20 [19.2%] patients) and Blood and Lymphatic System Disorders (19 TEAEs in 18 [17.3%] patients).
Most TEAEs were mild, not related to the investigational therapy (one TEAE in one patient in the TISSEEL Lyo group was probably related to the IP [pain postoperative wound]) and were expected.
The occurrence of TESAEs was 3.9% in the TISSEEL Lyo group and 7.5% in the MC group. A total of 10 TESAEs were reported in 6 (5.8%) patients (TISSEEL Lyo: 2 TESAEs in two patients; MC: 8 TESAEs in four patients). Among them, the most common TESAE was vascular graft thrombosis (Table 4).
Table 4.
TESAEs (safety population).
| System organ class Preferred term | TISSEEL Lyo (N = 51) | MC (N = 53) | Total (N = 104) | |||
|---|---|---|---|---|---|---|
| Number of patients n (%) | Number of events | Number of patients n (%) | Number of events | Number of patients n (%) | Number of events | |
| Patients with SAEs | ||||||
| Total | 2 (3.9%) | 2 | 4 (7.5%) | 8 | 6 (5.8%) | 10 |
| Cardiac disorders | ||||||
| Total | 1 (2.0%) | 1 | 0 (0.0%) | 0 | 1 (1.0%) | 1 |
| Atrial fibrillation | 1 (2.0%) | 1 | 0 (0.0%) | 0 | 1 (1.0%) | 1 |
| Injury, poisoning and procedural complications | ||||||
| Total | 1 (2.0%) | 1 | 4 (7.5%) | 8 | 5 (4.8%) | 9 |
| Anaemia postoperative | 0 (0.0%) | 0 | 1 (1.9%) | 1 | 1 (1.0%) | 1 |
| Arterial bypass thrombosis | 1 (2.0%) | 1 | 0 (0.0%) | 0 | 1 (1.0%) | 1 |
| Limb traumatic amputation | 0 (0.0%) | 0 | 1 (1.9%) | 1 | 1 (1.0%) | 1 |
| Postprocedural haemorrhage | 0 (0.0%) | 0 | 1 (1.9%) | 1 | 1 (1.0%) | 1 |
| Vascular graft thrombosis | 0 (0.0%) | 0 | 3 (5.7%) | 5 | 3 (2.9%) | 5 |
MC: manual compression; TESAE: treatment-emergent serious adverse events; SAE: serious adverse event; N: patient number in the group.
No TESAEs related to IP/comparator, TESAEs leading to withdrawal and TESAEs leading to death were reported in patients during the study.
None of the patients experienced surgical site infection.
Six (6) patients experienced graft occlusions (TISSEEL Lyo: 1; MC: 5).
Evaluation of laboratory parameters showed no significant difference at baseline and during follow-up between treatment groups.
Discussion
Despite the relatively wide availability of various FSs in the world and their active use in almost all areas of surgery, in Russia, their use remains limited, primarily due to the shortage of this kind of product on the market.
Due to this fact, this study was conducted as part of the registration procedure for TISSEEL Lyo under Russian legislation.
A controlled parallel design is the standard design to evaluate the efficacy of pharmaceutical formulations. The study was single-blinded due to its surgical nature, however, it is considered a limitation of the study. TISSEEL Lyo was compared to MC with gauze pads, the valid standard of surgical technique.
There are no specific guidelines on clinical development or clinical investigation of FSs in Russia; while the FDA Guidance for Industry ‘Efficacy studies to support the marketing of fibrin sealant products manufactured for commercial use’ (1999) 11 and European Medicines Agency (EMA) ‘Guideline on the clinical investigation of plasma-derived fibrin sealant/haemostatic products’ (2004), 12 only general recommendations are given. Thus, endpoints were chosen based on previous clinical trials of TISSEEL and similar agents.6,7
All randomized patients (n = 104) underwent arterio-arterial bypass, and there were no patients who underwent arteriovenous shunting for dialysis access.
The preponderance of males included in the study, which might be seen as another limitation of the study, likely reflects the common distribution. According to existing data, lower extremity artery disease, especially when symptomatic, is overall more frequent in men, although the prevalence may vary between countries with different income rates and regions. 13
The efficacy results showed that TISSEEL Lyo therapy is superior to the control arm treated with MC for haemostasis in patients undergoing vascular surgery. T4 haemostasis was achieved in 43 (84.3%) patients in the TISSEEL Lyo group and in 11 (20.8%) patients in the MC group (p < 0.001). Overall, data demonstrated TISSEEL Lyo has superior performance when compared to MC in terms of successful haemostasis at all measured time-points including 4, 6 and 10 min on bleedings representing grades 1 and 2 on the VIBe scale. 9
These results are in line with previously published data, which showed the advantages of different fibrin sealants. The results of a study by Saha et al., 6 2012, showed that TISSEEL is safe and superior to MC for haemostasis in patients with peripheral vascular ePTFE grafts. The haemostasis rate at 4 min was 62.9% in the TISSEEL group and 31.4% in the MC group (p < 0.0001). In another prospective randomized multicentre trial, fibrin sealant Beriplast P (FSBP, Aventis-Behring) was superior to thrombin-soaked gelatin sponge (haemostasis rate at 4 min: 63% vs. 40%, respectively; p = 0.0018) in patients with PTFE graft. 14 In addition, the benefits of fibrin-based and non-fibrin-based sealants in vascular surgery were demonstrated in clinical trials of aprotinin-free FS (EVICEL™ FS [Human]), 15 TachoSil® (a collagen sponge coated with fibrinogen and thrombin),1,16 Coseal, 17 BioGlue18,19 and Floseal Matrix. 20 In general, all types of sealants led to a higher haemostasis rate at prespecified time points, and their effects were achieved in a shorter time frame.
Thus, the available data support the benefit of different types of sealants that are used in vascular surgery. The haemostasis results in published studies are aligned with TISSEEL Lyo haemostasis results from the current study.
Safety was analysed by measuring the incidence of TEAEs, abnormal laboratory values and vital signs, surgical site infections, and graft occlusions.
Regarding cost-effectiveness, TISSEEL Lyo, already showing a high rate of haemostasis after 4 min (84.3%) can save time in the OR, as only after 10 min did MC reach a comparable haemostasis rate (83.0%). The cost of 6 min in the OR can be estimated to be $372, based on an average cost of $62/min. 21 On the other hand, if TISSEEL Lyo could additionally contribute to lowering the number of graft occlusions, treatment costs could be reduced even more significantly, justifying the investment of approx. $287 per unit 4 mL TISSEEL.22
During the study, there were no new findings regarding the safety profile of TISSEEL Lyo. The safety of TISSEEL Lyo and MC were similar, with no significant differences in the assessed parameters. The occurrence of TESAEs was 3.9% in the TISSEEL Lyo group and 7.5% in the MC group. No TESAEs led to death or withdrawal. None of the TEASEs were related to the investigational therapy. 2
Based on the above results, it can be concluded that TISSEEL Lyo has an acceptable safety profile when used for elective arterio-arterial bypass surgery.
Conclusions
The results of this study support previous data that TISSEEL Lyo has a good overall efficacy profile in terms of haemostasis in patients receiving peripheral vascular prosthetic ePTFE conduits and shows the significantly superior performance when compared to manual compression. Furthermore, it can be concluded that TISSEEL Lyo has a strong safety profile.
Acknowledgements
The authors acknowledge Cromos Pharma (Moscow, Russia) for medical writing support of the manuscript and Gerhard Moersdorf (Baxter Healthcare) for proofreading services.
Author biographies
Andrey A Karpenko, professor, doctor of science, head of center vascular and hybrid surgery in national research center named by E.N. Meshalkin, His area of research is artificial intelligence.
Alexey V Cheban, researcher, science center vascular and hybrid surgery in national research center named by E.N. Meshalkin, His area of research is revascularisation of femoropopliteal lesions.
Artem A Rabtsun, PhD, researcher, science center vascular and hybrid surgery in national research center named by E.N. Meshalkin, His area of research is endovascular recanalization of the superficial femoral artery total occlusive lesions.
German Y Sokurenko, professor, doctor of science, head of center cardio-vascular surgery in A.M. Nikiforov All-Russian Center of Emergency and Radiation Medicine. His area of research is treatment of venous thromboembolic complications.
Konstantin A Andreychuk, candidate of medical sciences, researcher, FSBI All-Russian Center for Emergency and Radiation Medicine named by A.M. Nikiforov. His area of research is Thoracic and abdominal aortic aneurysms.
Igor N Kim, candidate of medical sciences, researcher, head of center cardio-vascular surgery in State Novosibirsk Regional Clinical Hospital. His area of research is сritical ischemia of the arteries of the lower extremities.
Valentin A Volf, researcher, State Novosibirsk Regional Clinical Hospital. His area of research is ischemia of the lower limbs.
Maksim R Kuznetsov, professor, doctor of medical science, City Clinical Hospital named by S.S. Yudin. His area of research is сhronic and acute arterial disease of the extremities.
Igor I Prostov, candidate of medical sciences, researcher, Regional Consultative and Diagnostic Center, Rostov-on-Don. His area of research is сhronic and acute arterial disease of the extremities.
Natalia G Sapronova, assistant professor (доцент), doctor of medical science, head of the department of surgical diseases No. 1, Rostov State Medical University. Her area of research is portal hypertension.
Nina D Mzhavanadze, professor, doctor of medical sciences, department of cardiovascular, endovascular, operative surgery and topographic anatomy in Ryazan State Medical University named after academician I.P. Pavlov. Her area of research is сhronic and acute arterial disease of the extremities.
Alexey A Kamaev, candidate of medical sciences, researcher, assistant professor of the department of cardiovascular, endovascular, operative surgery and topographic anatomy in Ryazan State Medical University named after academician I.P. Pavlov. His area of research is сhronic and acute arterial disease of the extremities.
Igor A Suchkov. professor, doctor of medical sciences, assistant professor of the department of cardiovascular, endovascular, operative surgery and topographic anatomy in Ryazan State Medical University named after academician I.P. Pavlov. His area of research is treatment of venous thromboembolism.
Footnotes
Authors’ contributions: Author GYS has made substantial contributions to the conception or the design of the manuscript, and all authors have contributed to the acquisition, analysis and interpretation of the data. All authors have participated in drafting the manuscript, author GYS revised it critically. All authors read and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: The protocol and Patient Information Sheet with the ICF were approved by the Ethics Council of the Ministry of Healthcare of the Russian Federation (№. 4094591-20-I/ЭС from 04.03.2019) and by the Local Ethics Committee (LEC) of each study site (№. 25 from 03.06.19*).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a grant from Baxter Healthcare Corporation (USA). Fibrin Sealant TISSEEL Lyo as a haemostatic agent in vascular surgery: a randomized, multicenter clinical study in the Russian population (grant number BXU529732_Tisseel Russia).
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Alexey V Cheban https://orcid.org/0000-0002-6094-4607
Nina D Mzhavanadze https://orcid.org/0000-0001-5437-1112
Igor A Suchkov https://orcid.org/0000-0002-1292-5452
References
- 1.Spotnitz WD. Fibrin sealant: the only approved hemostat, sealant, and adhesive – a laboratory and clinical perspective. ISRN Surg 2014; 2014: 203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickman DA, Pawlowski CL, Sekhon UDS, et al. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv Mater 2018; 30: 1700859. Epub 2017 Nov 22. PMID: 29164804; PMCID: PMC5831165. DOI: 10.1002/adma.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiara O, Cimbanassi S, Bellanova G, et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg 2018; 18: 68. PMID: 30157821; PMCID: PMC6116382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards SJ, Crawford F, van Velthoven MH, et al. The use of fibrin sealant during non-emergency surgery: a systematic review of evidence of benefits and harms. Health Technol Assess 2016; 20: 1–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjaergard HK, Fairbrother JE. Controlled clinical studies of fibrin sealant in cardiothoracic surgery – a review. Eur J Cardiothorac Surg 1996; 10: 727–733. [DOI] [PubMed] [Google Scholar]
- 6.Saha SP, Muluk S, Schenk W, 3rd, et al. A prospective randomized study comparing fibrin sealant to manual compression for the treatment of anastomotic suture-hole bleeding in expanded polytetrafluoroethylene grafts. J Vasc Surg 2012; 56: 134–141. [DOI] [PubMed] [Google Scholar]
- 7.Chetter I, Stansby G, Sarralde JA, et al. Investigators of the Fibrin Sealant Grifols Study Group. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Ann Vasc Surg 2017; 45: 127–137. [DOI] [PubMed] [Google Scholar]
- 8.Albala DM. Fibrin sealants in clinical practice. Cardiovasc Surg 2003; 11: 5–11. [DOI] [PubMed] [Google Scholar]
- 9.Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery 2017; 161: 771–781. [DOI] [PubMed] [Google Scholar]
- 10.Bektas H, Nadalin S, Szabo I, et al. Hemostatic efficacy of latest-generation fibrin sealant after hepatic resection: a randomized controlled clinical study. Langenbecks Arch Surg 2014; 399: 837–847. [DOI] [PubMed] [Google Scholar]
- 11.FDA Guidance for Industry. Efficacy studies to support marketing of fibrin sealant products manufactured for commercial use. 1999.
- 12.EMA. Guideline on the clinical investigation of plasma derived fibrin sealant/haemostatic products. CPMP/BPWG/1089/00. 2004.
- 13.Pokrovskiy AV. National guidelines on the management of patients with lower extremities arterial disease. Angiology and Vascular Surgery 2013; 19: 7–22.24429554 [Google Scholar]
- 14.Taylor LM, Jr, Mueller-Velten G, Koslow Aet al. et al. The Beriplast P Investigators. Prospective randomized multicenter trial of fibrin sealant versus thrombin-soaked gelatin sponge for suture- or needle-hole bleeding from polytetrafluoroethylene femoral artery grafts. J Vasc Surg 2003; 38: 766–771. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers RT, Darling Iii RC, Wingard JT, et al. Randomized clinical trial of tranexamic acid-free fibrin sealant during vascular surgical procedures. Br J Surg 2010; 97: 1784–1789. [DOI] [PubMed] [Google Scholar]
- 16.Rickenbacher A, Breitenstein S, Lesurtel Met al. et al. Efficacy of TachoSil a fibrin-based haemostat in different fields of surgery–a systematic review. Expert Opin Biol Ther 2009; 9: 897–907. [DOI] [PubMed] [Google Scholar]
- 17.Glickman M, Gheissari A, Money Set al. et al. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gel foam/thrombin: results of a randomized controlled trial. Arch Surg 2002; 137: 326–331; discussion 332. [DOI] [PubMed] [Google Scholar]
- 18.Coselli JS, Bavaria JE, Fehrenbacher J, et al. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg 2003; 197: 243–252; discussion 252-3. [DOI] [PubMed] [Google Scholar]
- 19.Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert Opin Biol Ther 2013; 13: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver FA, Hood DB, Zatina M, et al. Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Ann Vasc Surg 2002; 16: 286–293. [DOI] [PubMed] [Google Scholar]
- 21.Shippert RD. A study of time-dependent operating room fees and how to save $100 000 by using time-saving products. Am J Cosmet Surg 2005; 22: 25–34. 23. [Google Scholar]
- 22.Zhao Z, Ma X, Ma J, et al. A systematic review and meta-analysis of the topical administration of fibrin sealant in total hip arthroplasty. Sci Rep 2018; 8: 78. PMID: 29311570; PMCID: PMC5758515. DOI: 10.1038/s41598-017-16779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]