Abstract
In recent years, the pharmacological benefits of herbal extracts have been revisited for their potential neuroprotective effects in glaucoma. The polysaccharides extracted from the fruits of Lycium barbarum L., or Lycium barbarum polysaccharides, exert their anti-aging effect through reducing oxidative stress, modulating the immune response, enhancing neuronal responses, and promoting cytoprotection. The therapeutic efficacy of Lycium barbarum polysaccharides in preserving retinal ganglion cells and their functions was demonstrated in a range of experimental models of optic neuropathies. These include the acute and chronic ocular hypertension models, the partial optic nerve transection model, and the ischemic-reperfusion injuries model. Based on these findings, Lycium barbarum polysaccharides appear to be a good candidate to be developed as a neuroprotective agent for treating multifactorial diseases. This review aims to present a comprehensive review on the latest preclinical evidence on the pre- and post-treatment benefits of Lycium barbarum polysaccharides in retinal ganglion cell neuroprotection. The possible mechanisms of Lycium barbarum polysaccharides mediating retinal ganglion cell neuroprotection will also be described. Moreover, the potential research gaps in the effective translation of Lycium barbarum polysaccharides treatment into clinical glaucoma management will be discussed.
Key Words: animal model, complementary and alternative medicine, glaucoma, retinal ganglion cell, Lycium barbarum polysaccharide, neuroprotection, neuro-rescue, ocular hypertension, post-treatment, pre-treatment
Introduction
Glaucoma is an irreversible blinding disease that causes compartmentalized degeneration of the dendrites, axons and/or soma of retinal ganglion cells (RGC; (Weber et al., 1998; Buckingham et al., 2008; El-Danaf and Huberman, 2015). It typically increases optic nerve cupping causing progressive loss of functional visual field (Weinreb and Khaw, 2004; Weinreb et al., 2014). In 2020, glaucoma was the second major cause of global blindness that affected 3.6 million people and the fourth leading cause of global moderate and severe visual impairment in 4.1 million adults aged 50 years and above (GBD Blindness, 2021). It is estimated that glaucoma would affect 112 million people worldwide by 2040 (Tham et al., 2014). To prevent the onset of severe vision loss or blindness, early diagnosis and effective management are matters of serious concern for glaucoma patients and health care professionals. Even though glaucoma is a multifactorial disease (Salmon, 1999; Omoti and Edema, 2007; Coleman and Miglior, 2008; Rivera et al., 2008), current standard treatments depend largely on altering a single risk factor, which is the elevated intraocular pressure (IOP). Lowering IOP, either by medical, laser, surgery, or a combination of these approaches, remains the mainstay of current standard clinical protocol (Migdal, 2000; Parikh et al., 2008; Berlin, 2009a, b; Stamper et al., 2009; Tataru and Purcarea, 2012; Weinreb et al., 2014). IOP reduction via these approaches has shown to be effective in preventing or delaying the disease onset in glaucoma suspects and reducing the rate of disease progression in glaucoma patients in large, multi-center clinical trials (Collaborative Normal-Tension Glaucoma Study Group, 1998; The Advanced Glaucoma Intervention Study, 2000; Heijl et al., 2002; Kass et al., 2002; Garway-Heath et al., 2015).
On the other hand, a proportion of glaucoma sufferers continued to deteriorate despite well-controlled IOP (Collaborative Normal-Tension Glaucoma Study Group, 1998; The Advanced Glaucoma Intervention Study, 2000; Heijl et al., 2002; Kass et al., 2002; Garway-Heath et al., 2015). This is not surprising as there is mounting evidence from human and experimental glaucoma studies demonstrating the role of various IOP-independent mechanisms on the neuronal microenvironment and RGC survival. To target these mechanisms, adjuvant therapeutic approaches, such as complementary and alternative medicines (CAM), are widely considered to be applied on top of the current standard IOP lowering treatments (Figure 1). In recent years, the pharmacological benefits and neuroprotective effects of ancient herbal extracts on retinal neurons have been revisited. This review describes the beneficial effects of the polysaccharides from the fruits of Lycium barbarum L., or Lycium barbarum polysaccharides (LBP), as a neuroprotective agent for RGC, using preclinical animal models of optic neuropathies. Furthermore, the challenges in effectively translating LBP neuroprotective therapies from preclinical to clinical use in terms of glaucoma management are also discussed.
Figure 1.
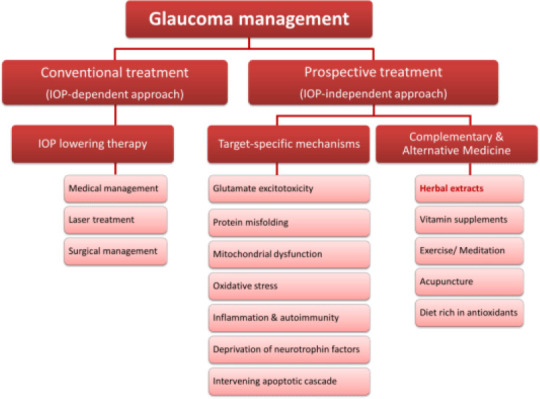
An overview of current and prospective treatment approaches for glaucoma management.
Search Strategy and Selection Criteria
The literature search was performed in PubMed using the advanced search builder. The combinations of search or MeSH (Medical Subject Headings) terms used were Lycium barbarum polysaccharides or wolfberry, neuroprotection, retinal ganglion cell, ocular hypertension, retinal ischemia, glaucoma, animal models, pre-treatment, and post-treatment. An additional filter of English language was included and all the publication dates were considered. Some articles were also considered from the review article or similar articles list. The final list comprised of in vivo studies was prepared after excluding the review articles.
Neuroprotection and Complementary Therapies in Glaucoma
The rationale for applying neuroprotective therapy in glaucoma
The concept of neuroprotection in glaucoma is derived from the field of neurodegenerative disease. This is because some characteristics of glaucoma, including the loss of specific neuronal cells (RGC), the secondary degeneration of healthy cells, the progressive nature of the disease, apoptotic cell death, and senility are frequently observed in other central nervous system (CNS) disorders, such as Alzheimer’s, Parkinson’s and amyotrophic lateral sclerosis (Gupta and Yucel, 2007; Danesh-Meyer and Levin, 2015). Apoptotic cell death in glaucoma is triggered by oxidative stress, glutamate excitotoxicity, tumor necrosis factor-α released from reactive glial cells, and abnormal protein accumulations, similar to other CNS disorders (Gupta and Yucel, 2007). Therefore, glaucoma is now being recognized as a neurodegenerative disease and thus is potentially amenable to neuroprotective therapies. Although the success of neuroprotective therapies in CNS diseases has been limited (Cheng et al., 2004), it can still be an effective approach in glaucoma, management as there is a difference in the site of insult onset between the two. In glaucoma, neurons undergo compartmentalized degeneration, whereby the axons are damaged first, followed by the cell bodies (Weber et al., 1998; Buckingham et al., 2008; El-Danaf and Huberman, 2015). This is different from diseases like stroke, in which cell bodies are being affected prior to the axons (Weinreb and Levin, 1999). The time window between axonal and somatic damages in glaucoma can potentially provide an opportunity for effective neuroprotective interventions.
Earlier in 1885, the possible role of IOP-independent mechanisms in glaucomatous progression was proposed by the eminent ophthalmologist and glaucoma surgeon, Priestley Smith, “The excavation of the disc in glaucoma is not a purely mechanical result of exalted pressure: it is, in part at least, an atrophic condition which, though primarily due to pressure, includes vascular changes and impaired nutrition in the substance of the optic disc… which may probably progress even though all excessive pressure be removed” (Girkin, 2001). However, it was not until 1972 that Becker et al. (1972) first applied non-IOP lowering therapy, in the form of diphenylhydantoin (a sodium channel blocker for treating convulsions) for two months in primary open-angle glaucoma patients, and demonstrated an improvement in visual field sensitivity. Subsequently, significant efforts have been undertaken to understand how IOP-independent disease mechanisms affecting neuronal survival and the methods to alleviate these effects.
Neuroprotective approaches
Neuroprotection in glaucoma is defined as an approach that slows down the functional visual field loss by targeting disease mechanisms that are independent of IOP and is used to supplement IOP-lowering therapies (Levin and Peeples, 2008; Levin et al., 2017). It is intended to directly treat RGC through preserving healthy neurons and rescuing damaged neurons, in addition to treating the primary cause (i.e., IOP elevation). Such interventions may be more appropriate for patients who have developed increasing visual field defects and/or neural rim changes despite well-controlled IOP as well as those who do not respond well to current treatment approaches (Levin, 2003).
Neuroprotective therapies in glaucoma can target different IOP-independent mechanisms involved in neurodegeneration effectively. This includes glutamate excitotoxicity (Weber et al., 1995; Perlman et al., 1996; Lagreze et al., 1998), protein misfolding (McKinnon et al., 2002; Goldblum et al., 2007; Kipfer-Kauer et al., 2010; Ito et al., 2012; Chiasseu et al., 2016), mitochondrial dysfunction (Abu-Amero et al., 2006; Kong et al., 2009; Osborne and del Olmo-Aguado, 2013), oxidative stress (Ferreira et al., 2004; Feilchenfeld et al., 2008; Gherghel et al., 2013; Goyal et al., 2014; Kimura et al., 2017), inflammation and autoimmune disorder (Wax et al., 1994; Tezel et al., 1998; Wax et al., 1998; Kremmer et al., 2004; Bell et al., 2013; Gramlich et al., 2013), and neurotrophic factors deprivation (Johnson et al., 2000; Pease et al., 2000; Quigley et al., 2000; Rudzinski et al., 2004; Knox et al., 2007). They can also be applied to intervene with the apoptotic cascade (Quigley et al., 1995; Song et al., 2015) and modulate glial responses (Tezel, 2009). Currently, none of these interventions have been approved for clinical use.
Complementary and alternative treatments for glaucoma
Aging is one of the major risk factors of glaucoma. The levels of free radicals and oxidative stress in the cells, which can affect the mitochondrial energy production and the normal functioning of the neurons, increase with age (Harman, 1956, 2006). The holistic approaches to improve the energy deprivation in aging or diseased cells, possibly through maintaining normal cell functioning, are collectively known as complementary and alternative treatment (CAM). It is thought that through replenishing the energy deprivation in age-related disorders, including glaucoma, the disease progression can be altered. CAM for glaucoma, including vitamin supplements, herbal medicines, exercise, acupuncture, meditation, therapeutic touch, and antioxidant-rich diets (Rhee et al., 2001; Parikh and Parikh, 2011), are thought to improve the patients’ overall physical and mental health. Although none of these interventions have been approved as a complementary therapy for glaucoma, one in nine patients reportedly used CAM along with their conventional therapy (Wan et al., 2012). Vitamin supplements and herbal medicines are the commonly used CAM among glaucoma patients. In recent years, many herbal extracts have been studied for their neuroprotective properties in slowing down the RGC neurodegeneration and as a complementary treatment. To name a few, Ginkgo biloba extract, LBP, forskolin extracted from Coleus Forskohlii, curcumin, saffron, Bilberry, and Marijuana (Adornetto et al., 2020). This review will focus on the preclinical animal studies that investigated the neuroprotective effects of LBP on RGC survival using rodent models of optic neuropathies.
Lycium Barbarum Polysaccharides and Retinal Ganglion Cell Neuroprotection
Properties of Lycium barbarum polysaccharides
The fruit of Lycium barbarum L., commonly known as Wolfberry, is an ancient anti-aging herbal medicine, that is known for improving functions of liver, kidney, and eye along with enhancing one’s psychological well-being. The small reddish-orange fruit contains multiple bioactive components, such as polysaccharides, carotenoids (especially zeaxanthin), flavonoids, amino acids, vitamins, fatty acids, and other trace elements (Amagase and Farnsworth, 2011; Jin et al., 2013; Cheng et al., 2014). The polysaccharides extracted from the fruit consist of 6 types of monosaccharides, namely glucose, arabinose, galactose, rhamnose, xylose, mannose and galacturonic acid as well as types of amino acids (Yu et al., 2007). LBP is well studied for its pharmacological effects on anti-aging (Li et al., 2007; Tang and He, 2013), cytoprotection (Wu et al., 2006; Yu et al., 2006; Li, 2007; Niu et al., 2008; Cheng and Kong, 2011), immunomodulation (Gan et al., 2004; Cui et al., 2012), and neuromodulation (Yang et al., 2012; Chen et al., 2014; Hu et al., 2018). LBP is also able to combat the effects of fatigue (Zhao et al., 2015), hyperglycemia (Zhu et al., 2013), hyperlipidemia (Luo et al., 2004), and other induced toxicities (Xin et al., 2011; Xiao et al., 2012).
Interestingly, LBP is known to target multiple mechanisms that can also promote neuroprotection in glaucomatous degeneration. LBP has been demonstrated to be effective in combating glutamate excitotoxicity (Ho et al., 2009; Shi et al., 2017), protein misfolding (Yu et al., 2005), mitochondrial dysfunction (Liu et al., 2017), oxidative stress (Wu et al., 2006; Li, 2007; Niu et al., 2008; Cheng and Kong, 2011; He et al., 2014), and autoimmunity (Gan et al., 2004), as well as modulating glial cells (Chiu et al., 2009), apoptosis (Wang et al., 2014b; Xia et al., 2014), and vascular dysregulation (Mi et al., 2012a, b; Yang et al., 2012). Thus, LBP is considered to be a promising candidate for treating multifactorial diseases.
The neuroprotective effects of LBP in preserving RGC were studied using both IOP-dependent and -independent experimental models. These include rodent models of chronic ocular hypertension (OHT; Chan et al., 2007; Chiu et al., 2009, 2010), acute ocular hypertension (AOH; Mi et al., 2012a; He et al., 2014), complete optic nerve transection (CONT; Li et al., 2013), partial optic nerve transection (PONT; Chu et al., 2013; Li et al., 2013), and ischemic reperfusion injuries (Li et al., 2011a). While most of them focused on the prophylactic effects of LBP by pre-treating the animals before the onset of injury, recent studies have also confirmed the benefits of LBP post-treatment that is initiated well after the onset of insult. The following sections will comprehensively review the studies demonstrating the pre-treatment effects of LBP, followed by its post-treatment effects in various animal models of optic neuropathies.
The neuroprotective effects of LBP pre-treatment
The effect of LBP pre-treatment on RGC survival has been studied using both IOP-dependent and -independent rodent models (Table 1). The IOP-dependent models include the chronic ocular hypertension model and the acute ocular hypertension model. The chronic ocular hypertension model involves experimentally inducing a moderate IOP elevation for a long period (2–15 weeks). As for the acute ocular hypertension model, extremely high IOP is being elevated for a short period (60–120 minutes). Both models result in the indirect insult of RGC. The IOP-independent models inflict RGC injuries directly, either by transecting the optic nerve completely or by blocking the vascular supply to the eye through ligating the common carotid artery for a certain period of time (120 minutes). In studies that assessed the pre-treatment benefits, rodents were prophylactically treated with LBP from 7 days before model induction to the end of the study. The LBP solution was prepared by dissolving a desired amount of LBP powder (as per dosage requirement) in a phosphate-buffered solution (PBS). The animals were fed once daily by oral gavage or nasogastric tube.
Table 1.
Summary of LBP pre-treatment outcomes in experimental models of optic nerve insult
| Experimental model / species | LBP treatment (Duration) | Structural outcomes | Functional outcomes | Key findings and possible mechanisms of RGC neuroprotection | Reference |
|---|---|---|---|---|---|
| Chronic ocular hypertension (COH) | |||||
| Laser photocoagulation of episcleral and limbal veins in SD rat | Pre-treatment (–1 to 4 weeks) | LBP preserved RGC at both week 2 and 4 | Not assessed (n/a) | LBP treatment (0.01, 0.1, 1, 10, 100, and 1000 mg/kg) exhibited a U-shaped dose-response on RGC survival. Maximum RGC protection was achieved at 1 and 10 mg/kg LBP LBP did not alter the elevated IOP levels | Chan et al., 2007 |
| Laser photocoagulation of episcleral and limbal veins in SD rat | Pre-treatment (–1 to 4 weeks) | LBP preserved RGC at both week 2 and 4 | n/a | LBP modulated the microglial activation in the inner retinal layers in a dose-response manner. Moderately activated microglia observed at 1 and 10 mg/kg LBP offered maximum RGC protection than highly activated at 1000 mg/kg LBP | Chiu et al., 2009 |
| Laser photocoagulation of episcleral and limbal veins in SD rat | Pre-treatment (–1 to 2 weeks) | LBP preserved RGC | n/a | LBP modulated retinal crystalline proteins levels (mainly βB2), which was otherwise downregulated under IOP stress conditions | Chiu et al., 2010 |
| Laser photocoagulation of episcleral and limbal veins in SD rat | Pre-treatment (–1 to 2 weeks) | LBP preserved RGC | n/a | LBP modulated the expressions of ET-1 and its receptors in both RGCs and retinal vasculature, which improved RGC survival | Mi et al., 2012b |
| Acute ocular hypertension (AOH) | |||||
| AOH of 90 mmHg x 60 minutes in C57 mouse | Pre-treatment (–7 to 7 days) | LBP retained ~88% of IRL thickness and reduced RGC loss (LBP vs. PBS: ~15% vs. ~38%) | n/a | LBP protected blood retinal barrier by increasing the expression of occludin and preserving the tight junctions LBP maintained the blood vessel density by down-regulating the expressions of ET-1, receptors of advanced glycation end products (RAGE), advanced glycation end products, and amyloid beta protein | Mi et al., 2012a |
| AOH of 90 mmHg x 60 minutes in C57 mouse | Pre-treatment (–7 to 4 days) | n/a | n/a | LBP reduced AOH-induced retinal gliosis by decreasing the expressions of glial fibrillary acidic protein (GFAP), glutamine synthetase, aquaporin-4 (AQP-4), S-100 proteins, iba-1, RAGE and amyloid precursor protein | Mi et al., 2020 |
| AOH of 130 mmHg x 60 minutes in SD rat | Pre-treatment (–7 to 7 days) | LBP reduced RGC loss (LBP vs PBS: ~40% vs. ~70%) | n/a | LBP enhanced the adaptive Nrf2/HO-1 anti-oxidant pathway which reduced the rate of ROS generation and improved RGC survival | He et al., 2014 |
| Ischemic-reperfusion retinal injury | |||||
| Surgically occlusion of right internal carotid artery for 2 hours in C57 mouse | Pre-treatment (–7 to 1 days) | LBP prevented retinal swelling, reduced RGC apoptosis, and retained 97% of the GCL cell density | n/a | LBP reduced the level of oxidative stress, the expressions of GFAP and AQP4, and the blood vessel leakages | Li et al., 2011a |
| Partial or complete optic nerve transaction (PONT/CONT) | |||||
| CONT/PONT in SD rat | Pre-treatment (–1 to 2/4 weeks) | LBP did not prevent primary/secondary degeneration of RGC in CONT model, but improved the RGC survival at the site of secondary degeneration in PONT model | n/a | LBP upregulated the expressions of oxidative enzyme and insulin growth factor-1, and downregulated the pro-apoptotic JNK signalling pathway | Li et al., 2013 |
| PONT in SD rat | Pre-treatment (–1 to 4 weeks) | LBP reduced the axonal loss at the site of secondary degeneration | n/a | LBP reduced the activation of microglial/ macrophages in the optic nerve | Li et al., 2015 |
| Temporal ONT in SD rat | Pre-treatment (–1 to 4 weeks) | LBP delayed the secondary degeneration of RGC | n/a | LBP increased the expression of M2 types of microglia/ macrophages, which promoted the levels of anti-inflammatory cytokines and reduced autophagy levels | Li et al., 2019 |
| PONT in SD rat | Pre-treatment (–1 to 4 weeks) | n/a | LBP treatment resulted in the showed a recovery of inner retinal functions by mfERG (P1, PhNR) at both sites of primary and secondary degeneration | Chu et al., 2013 |
AOH: Acute ocular hypertension; AQP: aquaporin; ET: Endothelin; ERG: electroretinogram; GCL: ganglion cell layer; IOP: intraocular pressure; LBP: Lycium barbarum polysaccharides; PBS: phosphate-buffered solution; PhNR: photopic negative response; RGC: retinal ganglion cells; SD: Sprague-Dawley.
Chronic ocular hypertension model
The dose-response effect of LBP on retinal ganglion cell survival
Chan et al. (2007) were the first to demonstrate the in vivo neuroprotective effects of LBP pre-treatment and investigated the dose-dependent efficacy using six LBP concentrations (0.01, 0.1, 1, 10, 100, 1000 mg/kg) in a glaucoma rat model. Before OHT induction, adult female Sprague-Dawley (SD) rats were pre-treated with LBP or the vehicle, PBS, for 1 week. Following this, OHT was induced by laser photocoagulation of the episcleral and limbal veins twice (at days 0 and 7). The increased resistance to aqueous outflow in the lasered eyes led to an IOP elevation, which was ~1.5 times higher than that of the contralateral control that was maintained for at least 4 weeks. LBP treatment did not alter IOP elevation (21.2 ± 0.2 mm Hg) and was found to be similar with vehicle treatment (23.6 ± 0.6 mm Hg). While the vehicle-treated group showed 15 ± 1% and 21 ± 2% of the RGC loss at weeks 2 and 4 respectively, 1 mg/kg LBP pre-treatment preserved RGC with losses of about 1 ± 2% at week 2 and 6 ± 2% at week 4. LBP also showed a U-shaped dose-dependent relationship on RGC survival. Maximum protection was achieved at 10 mg/kg LBP, with almost no RGC loss, and 1 mg/kg LBP. Other LBP dosages, both higher and lower levels, could only alleviate 50% of the RGC loss as compared with the PBS-treated animals. The authors proposed that LBP protected RGC by regulating the c-Jun N-terminal kinase (Yang et al., 2014), which was potentially involved in the apoptotic process. The lower protective effect at higher dosages could have been resulted from the over-inhibition of the c-Jun N-terminal kinase pathway, which is detrimental to RGC survival.
The effects of LBP on the modulation of retinal immune cells
Glaucoma-related stress is known to affect RGC survival mainly through altering neuroinflammation-associated retinal gene expressions (Ahmed et al., 2004; Steele et al., 2006; Piri et al., 2013). Chui et al. (2009) investigated the effect of LBP in modulating the activation of retinal microglia, which are the immune cells of the retina, and subsequent RGC protection. Different LBP dosages (1, 10, 100, and 1000 mg/kg) were administered and were continued for 2 weeks post-OHT induction. Laser photocoagulation of limbal and episcleral veins induced a 1.7 times higher increase in IOP level in this study. PBS-treated animals showed minimally activated microglia in the inner retinal layers (i.e., ganglion cell layer, inner plexiform layer, and inner nuclear layer) but 17% RGC loss at week 2. In LBP-treated animals, a dose-dependent microglial activation was reported. The microglial cells in the inner retinal layers were moderately activated at lower dosages of LBP (1 and 10 mg/kg) and fully activated at higher dosages (1000 mg/kg). However, maximum RGC protection (1% RGC loss at 1 mg/kg LBP, no RGC loss at 10 mg/kg LBP) was only observed in the groups with moderately activated microglia. 10% RGC loss was detected in the LBP 1000 mg/kg group. LBP at 1 mg/kg maintained a moderate activation of microglial for at least 4 weeks post-OHT insult with 6.6% RGC loss as compared with the PBS-treated group that has 21% loss. In the same study, the protective effects of LBP were also examined under simulated retinal conditions, wherein retinal microglial activation was either completely inhibited or maintained in a fully activated state. Animals pre-treated with 10 mg/kg LBP were intravitreally injected with PBS, microglial inhibitor factor, lipopolysaccharides of bacterial endotoxin (microglial activator) immediately after the first laser treatment. RGC loss in the microglial inhibitor factor group was greater (10%) than the PBS group (2%) at week 2. Microglial inhibitor factor might have attenuated the protective effect of LBP by reducing microglial activation. On the other hand, the lipopolysaccharides-treated group revealed that fully activated microglia was associated with a greater RGC loss (28 %) at the end of week 4. The results suggested that LBP treatment could modulate microglial activation, and moderate activation is protective to RGC under OHT induced stress. On the other hand, both minimally and highly reactive retinal microglia did not offer protection to RGC.
The effects of Lycium barbarum polysaccharides on the regulation of crystalline proteins
Crystalline proteins are small heat shock proteins, which offer cellular protection under the conditions of stress. In experimental in vivo models of glaucoma, certain α and β crystalline protein transcriptions and their expression levels were significantly down-regulated during the early stage of IOP elevation (Ahmed et al., 2004; Steele et al., 2006; Piri et al., 2007, 2013). Also, the intravitreal injection of βB2 crystalline protein during the early phase of IOP elevation was found to be neuroprotective that slowed down the retinal nerve fiber layer (RNFL) thinning and RGC loss in a rodent model of cauterization of episcleral veins (Anders et al., 2017). To investigate whether LBP could regulate the crystalline proteins in retinal neurons under IOP stress, the laser OHT model mentioned earlier was applied to induce IOP elevation in SD rats (Chiu et al., 2010). Although the IOP elevation was similar between the 1 mg/kg LBP pre-treatment and PBS vehicle control groups, RGC counts were better preserved in LBP-treated animals (1% RGC loss) than PBS ones (18% RGC loss) at day 14. Proteomic analysis on samples collected on day 2 showed significantly increased levels of both α (A and B) and β (A4 and B2) crystalline proteins in LBP-treated OHT retinas as compared with PBS treatment. However, these changes were not observed in all other normal controls, including LBP, PBS, and no feeding groups using healthy animals. Also, an increased immunoreactivity of βB2 crystalline protein in the ganglion cell layer and the inner nuclear layers on both days 2 and 14 were reported, as compared with the PBS-treated group that showed relatively weaker expressions. The study demonstrated that LBP could modulate the crystalline proteins, mainly βB2, under IOP stress conditions, and this may be one of the mechanisms through which LBP exerts its RGC protection.
The effects of LBP on endothelin-1 expression
Endothelin-1 (ET-1) is a vasoactive peptide ubiquitously found in the eye (MacCumber and D’Anna, 1994; Stitt et al., 1996). In recent years, there is emerging evidence suggesting the potential role of ET-1 in the pathophysiology of glaucoma. ET-1 is involved in the alteration of vasoregulation and the activation of inflammatory responses (Good and Kahook, 2010; Shoshani et al., 2012). The expression levels of ET-1 and its receptors, ET-A or ET-B, are found to be increased in both human (Wang et al., 2006) and animal models of glaucoma (Prasanna et al., 2005; Yang et al., 2007; Minton et al., 2012; McGrady et al., 2017). In experimental models of IOP elevation, RGC loss was associated with an increased immunoreactivity of ET-1, ET-A, and ET-B in the inner retinal layers (including the ganglion cell layer, inner plexiform, and nuclear layers) that were co-localized with glial activation (Prasanna et al., 2005; McGrady et al., 2017). The loss in RGC was reduced in ET-B deficient rats (Minton et al., 2012) or in dual ET-A and B receptor antagonist (Macitentan) treated rats under IOP elevation conditions (Krishnamoorthy et al., 2018). This implies that regulating the levels of ET-1 and its receptors can potentially be another approach in glaucoma management. To understand the effects of LBP on the modulation of the expressions ET-1 and its receptors as well as its impact on RGC rescue, an OHT model of laser photocoagulation in SD rats was used (Mi et al., 2012b). On day 14, increased expressions of ET-1 and ET-B receptors and a decreased level of ET-A receptors in the retina were detected in PBS-treated animals that had elevated IOP. On the other hand, LBP treatment resulted in significantly reduced expressions of ET-1 and ET-B receptors and an increased level of ET-A receptors. Moreover, LBP treatment resulted in reduced ET-1 and ET-A receptor expressions and an increased level of ET-B in the retinal vasculature. This differed from the PBS-treated eyes which showed increased expressions of ET-1 and ET-A receptor and a decreased level of ET-B receptor in the retinal vasculature. These results suggested that LBP could alter expressions of ET-1 and its receptors in RGCs and the vasculature. The combined effects of these mechanisms may lead to the RGC rescue detected. To summarize, LBP protected RGCs in COH retinas through moderate activation of microglial cells, increasing crystalline proteins expression and modulation of the expressions of ET-1 and its receptors.
Acute ocular hypertension model
AOH is one of the well-established models of ischemic-reperfusion injury applied to test the efficacy of neuroprotective agents against ischemia-induced RGC death (Osborne et al., 1999). In this model, the severity of retinal damage can be controlled by the careful selection of peak IOP and the duration of exposure. When the IOP in rodents is raised to 155 mmHg, which is well above the systolic pressure for 60 minutes, irreversible damage to the inner retina will be inflicted. If the duration is prolonged for an additional 30 minutes, the damage will be extended to the outer retina (Hughes, 1991; Selles-Navarro et al., 1996). The efficacy of LBP pre-treatment has been assessed using AOH models by elevating the IOP to about 90 mmHg in C57 mice or 130 mmHg in SD rats for 60 minutes (Mi et al., 2012a, 2020; He et al., 2014). An episode of AOH insult (90 mmHg for 60 minutes) in C57 mice resulted in severely disrupted blood-retinal barrier (BRB), reduced vascular density, and most importantly, 33% loss in RGC density at day 4 and around 50% reduction in inner retinal layer thickness at day 7 (Mi et al., 2012a). Pre-treatment with 1 mg/kg LBP maintained the integrity of BRB by increasing the expression of occludin and preserving the tight junctions. LBP treatment significantly improved the viability of retinal endothelial and pericytes as compared with the PBS-treated controls. As a result, the LBP group showed higher blood vessel density, 88% retention of inner retinal layer thickness, and only 15% loss in RGC. These neuroprotective effects on the retinal blood vessel and neurons were attributable to the downregulation of the expressions of ET-1, receptors of advanced glycation end products, advanced glycation end products, and amyloid-beta protein in the AOH retina. Recently, Mi et al. (2020) also showed that LBP significantly reduced the retinal gliosis in the AOH retina by decreasing the expressions of glial fibrillary acidic protein, glutamine synthetase, aquaporin-4 (AQP-4), S-100 proteins, iba-1, receptors of advanced glycation end products, and amyloid precursor protein. By reducing the expression of glutamine synthetase in astrocytes and AQP-4 around the blood vessels, LBP treatment resulted in the remodeling of astrocytes that were associated with the blood vessels.
Oxidative stress is one of the major pathological processes involved in ischemic-reperfusion retinal injuries which produce excessive reactive oxygen species (ROS) that result in cell damage or death (Wei et al., 2011). Nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor associated with the Nrf2/Heme oxygenase-1 (HO-1) anti-oxidation pathway, is activated by binding to the antioxidant response element. It leads to increased expressions of anti-oxidative proteins and enzymes that inhibit ROS, and hence, promote cell protection and survival (Kensler et al., 2007; Kaspar et al., 2009; Gozzelino et al., 2010). To understand the relationship between LBP and the Nrf2/HO-1 pathway, He et al. (2014) compared the impact of 1 mg/kg LBP and PBS treatments in an AOH rat model. After 24 hours of elevated IOP insult, which was maintained at 130 mmHg for 60 minutes, the PBS-treated animals showed an early and significant RGC loss (50%) that was further dropped to 70% at day 7. Although increased expressions of Nrf2 and HO-1 were detected at 24 hours, they returned to basal levels along with an increase in ROS level at day 7. As for LBP pre-treatment, the reduced rate of ROS generation associated with the increased Nrf2 and HO-1 expressions was maintained up to at least day 7. This delayed the loss of RGC and retained ~60% of the RGC density at day 7. The absence of such protective effects was reported in animals co-pre-treated with the HO-1 inhibitor, zinc protoporphyrin at 20 mg/kg. This is suggestive of the role of LBP in enhancing RGC survival through the adaptive Nrf2/HO-1 antioxidant pathway. In short, LBP protected RGCs in the AOH retina by preserving the BRB, retaining the vascular density, reducing the glial activation, remodeling the astrocytes surrounding the blood vessels, and decreasing the ROS by enhancing the Nrf2/HO-1 oxidative pathway.
Vascular model of ischemic-reperfusion retinal injury
Li and co-workers investigated the neuroprotective benefits of LBP in a vascular model of retinal ischemia through surgically occluding the right internal carotid artery for 2 hours, followed by 22 hours of reperfusion (Li et al., 2011a). Vehicle-treated C57 mice showed a swelling of inner retinal layers, a decrease in ganglion cell layer (GCL) cell density and an increase in the number of apoptotic cells in the GCL. It also exhibited elevated glial fibrillary acidic protein expression in the retina, upregulated AQP4 level in the perivascular region, increased number of leaky blood vessels (identified as IgG positive signals) located external to the endothelial vessel lining, and raised oxidative stress levels in the cell nucleus of GCL as indicated by increased poly (ADP-ribose) and nitrotyrosine expressions. While the GCL cell density of the vehicle control was 54% that of the normal control, pre-treatment with 1 mg/kg LBP prevented retinal swelling and retained 97% of the GCL cell density as compared with the normal control. The treatment significantly reduced the levels of oxidative stress, the expressions of glial fibrillary acidic protein and AQP4 as well as the leakage of blood vessels. The result indicates that LBP has a protective effect on micro-vessels which is vital for the proper provision of nutrients to retinal cells, and thus ensuring the preservation of retinal function.
Optic nerve transection model
In glaucoma, neurons undergo progressive damage and functional loss despite being treated for the primary cause, IOP elevation (Collaborative Normal-Tension Glaucoma Study Group, 1998; The AGIS Investigators, 2000; Heijl et al., 2002; Kass et al., 2002; Garway-Heath et al., 2015). Such secondary degeneration of healthy neurons is thought to result from the toxic substances released from the primary insult (i.e. the damaged neurons; Schwartz et al., 1996; Yoles and Schwartz, 1998). Animal models of optic nerve transection are commonly used to study the effects of neuroprotective agents against secondary degeneration (Schwartz et al., 1996). In this model, the superior part of the optic nerve was partially transected (usually 1 mm behind the eyeball). RGC and axonal loss were not confined only to the superior retina (primary injury), subsequent loss in the inferior retina was also observed due to secondary degeneration (Levkovitch-Verbin et al., 2001, 2003). One study investigated the outcomes of LBP and PBS pre-treatment in CONT or PONT rats (Li et al., 2013). The RGC densities in the superior and inferior retina were compared to assess the protective effects of LBP on secondary degeneration. For CONT animals, an overall decrease in RGC density was detected at week 1, which was further dropped at week 2. Similar outcomes were observed in animals treated with different dosages of LBP (0.1, 1, 10 mg/kg). LBP treatment did not seem to improve RGC survival at both time points. As for PONT rats, PBS-treated animals showed progressive neuronal loss at weeks 1 and 4. The degree of loss, reflected by RGC density, was similar in the superior and inferior retina. LBP treatment at 1 mg/kg preserved ~18% more RGC density in the inferior retina than that in PBS-treated control at week 4. The authors also reported increased expressions of the oxidative enzyme, manganese superoxide dismutase and insulin growth factor-1 as well as the down-regulation of apoptotic signals associated with the c-Jun N-terminal kinase pathway. While LBP was unable to prevent primary or secondary degeneration in the CONT model, it delayed the impact of secondary degeneration in the PONT model. A subsequent study assessed the protective effects of LBP at 1 mg/kg on axonal degeneration and the activation of microglia/macrophages in the PONT model (Li et al., 2015). Transecting the dorsal part of the optic nerve resulted in a severe axonal loss at the primary site (i.e. the dorsal region) and the distant secondary site at the central region, but not in the ventral region. LBP treatment could significantly preserve axons at the secondary site of degeneration, i.e. the central region, but not in the primary site. The morphology at the central region was comparable to the normal optic nerve with a significant reduction in the activation of microglia/macrophages. Another study also evaluated the role of LBP in the polarization of microglia/macrophages (M1 and M2) and autophagy levels (LC3-II) using a temporal ONT model (Li et al., 2019). M1 and M2 types of microglia/macrophages produce pro-inflammatory (inducible nitric oxide synthase) and anti-inflammatory cytokines (Arginase-1) respectively. These cytokines are involved in the process of tissue damage and repair. Higher levels of M1 and M2 were detected in the PBS group at week 1. These levels returned to basal values at week 4, but increased autophagy was observed. LBP treatment could effectively modulate glial activation and delay RGC degeneration at week 4 by increasing the M2 polarization and reducing autophagy.
In terms of retinal functions, which are seldom investigated for LBP treatments, Chu et al. (2013) characterized these properties using a slow paradigm multifocal electroretinogram (mfERG) in a PONT rat model. The first-order kernel response from this mfERG protocol produced three distinct responses; a small trough (N1) at 25 ms, followed by a peak (P1) at 55 ms and a photopic negative response (PhNR) at 75 ms. The long-term effects of PONT on retinal functions were assessed by measuring mfERG at three time points (weeks 1, 2, and 4) after PONT. To examine the effect of PONT on regional specific retinal functions (superior and inferior), the responses were grouped into 5 regions, which were superior, far superior, central, inferior, and far inferior regions. Following dorsal PONT, P1 and PhNR responses of PBS-treated animals were reduced significantly in the superior retina at all-time points. The reductions in P1 responses of the central and inferior regions occurred gradually at week 4. On the other hand, N1 responses remained unaffected. With LBP treatment, reductions in P1 and PhNR responses from the superior regions and enhanced N1 responses were observed at week 1. Most importantly, prolonged LBP treatment for 5 weeks showed a significant functional recovery of all retinal responses, including N1, P1, and PhNR, which were derived from the primary (superior) and secondary sites (central and inferior) of degeneration at week 4, when compared with vehicle-treated animals.
In summary, these studies showed that LBP preserved RGCs in both IOP-dependent and IOP-independent models of optic neuropathy under pre-treatment conditions. Also, LBP might protect RGC through modulating glial activation, crystalline proteins, and ET-1 expressions, preserving BRB and vascular density, and decreasing ROS through enhancing the Nrf2/HO-1 oxidative pathway. These findings demonstrated the potential of LBP as a neuroprotective agent for glaucoma management.
The neuroprotective effects of LBP post-treatment
To investigate the translational potential of LBP therapy, it is crucial to dedicatedly study its efficacy at the level of preclinical research comparable to the clinical trial settings. Most importantly, in clinical trials, therapeutic drugs are tested on subjects with confirmed or established disease conditions of varying severity. Therefore, this raises the critical question of whether the LBP efficacy observed in animals under pre-treatment conditions would yield similar results when applied in clinical trials, where treatments are often initiated after the onset of the disease or insult, in other words, under post-treatment conditions. To mitigate this uncertainty, the LBP post-treatment outcomes should be evaluated using different animal models.
Recently, the neuroprotective benefits of LBP under post-treatment conditions were reported in two different rodent OHT models (Table 2). These included a moderate AOH model (Lakshmanan et al., 2019b) and a mild chronic OHT (Lakshmanan et al., 2019a). To improve its translational value, clinically relevant in vivo techniques were applied to characterize the longitudinal changes in retinal structure and functions upon LBP post-treatment. These included optical coherence tomography (OCT) imaging and flash ERG measurements. The ERG measurements include positive scotopic threshold response (pSTR), scotopic b- and a-wave responses which are surrogate measures of ganglion, bipolar and photoreceptor cell functions respectively.
Table 2.
Summary of LBP post-treatment outcomes in experimental models of optic nerve insult
| Experimental model / Species | LBP treatment (Duration) | Structural outcomes | Functional outcomes | Key findings and possible mechanisms of RGC neuroprotection | Reference |
|---|---|---|---|---|---|
| Acute ocular hypertension (AOH) | |||||
| AOH of 80 mmHg x 120 minutes in SD rats | Pre-treatment (–1 to 4 weeks) | While LBP pre-treatment preserved ~90% of IRL thickness, post-treatment delayed the rate of secondary degeneration and retained ~60% of IRL thickness | Ganglion (pSTR) & bipolar (scotopic b-wave) cell responses were similarly preserved under pre- and post-treatments | Post-treatment with LBP showed a neuronal rescue in preserving both the retinal structure (IRL/RGC) and function | Lakshmanan et al., 2019b |
| Post-treatment (6 hours, post AOH to 4 weeks) | Pre- and post-treatments showed comparable GCL cell density preservation | ||||
| Chronic ocular hypertension | |||||
| Circumlimbal suture model in SD rats | Pre-treatment (–1 to 15 weeks) | Pre-treatment delayed the onset of RNFL thinning and also reduced its subsequent rate of thinning. Post-treatment initiated after the onset of RNFL thinning (from week 5) showed an insignificant gradual increase in RNFL thickness from week 8 onwards | Ganglion (pSTR) & bipolar (scotopic b-wave) cell responses were similarly preserved under pre- and post-treatments | Post-treatment with LBP showed a neuronal rescue in preserving both retinal structure (RGC) and function LBP did not alter the elevated IOP levels | Lakshmanan et al., 2019a |
| Post-treatment (5 to 15 weeks) | Pre- and post-treatments showed comparable RGC density preservation |
IOP: Intraocular pressure; IRL: inner retinal layer; LBP: Lycium barbarum polysaccharides; pSTR: positive scotopic threshold response; RGC: retinal ganglion cells; SD: Sprague-Dawley.
Acute ocular hypertension model
Some studies have evaluated the efficacy of LBP pre-treatment in AOH insults, which were established by elevating IOP to about 90 mmHg in mice or 130 mmHg in rats for 60 minutes (Mi et al., 2012a; He et al., 2014). The maximum follow-up period was 7 days. These induction conditions led to drastic and irreversible damages to the inner retina as the retinal and choroidal blood circulations were completely blocked (Zhi et al., 2012). However, sub-ischemic AOH models in rats can be achieved by choosing IOP above 70 mmHg and below 90 mmHg as previous studies showed that IOP below 70 mmHg (Bui et al., 2013) resulted in reversible retinal dysfunction in pSTR, while IOP of 80 mmHg or above would lead to structural changes (Holcombe et al., 2008). Subsequently, the post-treatment efficacy of LBP over 4 weeks using an AOH model of 80 mmHg for 120 minutes in SD rats was examined (Lakshmanan et al., 2019b). Animals in the pre-treatment groups received LBP administration 1 week before the AOH insult, and those in the post-treatment groups received it in 6 hours post-cannulation. The treatments were continued till the end of the study. While the animals in the PBS vehicle treatment group showed ~50% reduction in inner retinal layer (IRL) thickness (i.e. combined thicknesses of inner plexiform and inner nuclear layer) and ~70% reduction in GCL cell density, LBP pre-treatments at 1 and 10 mg/kg could retain ~80% and ~90% of IRL thickness respectively. Well-preserved GCL cell densities were also observed at week 4. In agreement with the structural findings, ~60% loss in retinal functions associated with ganglion and bipolar cells were detected in the vehicle treatment group, whereas both pre-treatment groups could retain ~80% of the functions. LBP post-treatment at 10 mg/kg showed a delayed but significant preservation of IRL thickness at day 28 (~40% loss) despite a substantial loss at day 10 (~35%), unlike the vehicle treatment group which showed a progressive loss (day 10: ~35% loss; day 28: ~50% loss). This delayed protective effect of LBP is reasonable as treatment was initiated 6 hours after the AOH insult, it may take some time for LBP to reach therapeutic levels at the neural retina and exert its protective effect. Interestingly, LBP post-treatment could preserve retinal functions and GCL cell density at a level comparable with LBP pre-treatment.
Chronic ocular hypertension model
The neuroprotective effects of LBP have been reported in an experimental model using laser photocoagulation of the limbal and episcleral veins for up to 4 weeks (Chan et al., 2007). Although sustained IOP elevation can be achieved through repeated applications of laser, the role of biphasic IOP change and the complications of repeated procedures, including inflammation and corneal changes, may affect the results and complicate the interpretation of the treatment outcomes.
Recently, a minimally invasive, single intervention circumlimbal suture model, which does not involve intraocular manipulations, has been proposed for chronic OHT (Liu et al., 2015). After an initial IOP spike during the induction phase, the model has a moderate IOP elevation that was sustained for 12 to 15 weeks (Liu et al., 2015; Liu and Flanagan, 2017; Zhao et al., 2017). This provides a long study window for the characterization of treatment effects. Moreover, the model appears to mimic many features of the clinical glaucomatous pathology, including progressive RNFL thinning, ganglion cell dysfunction that is more severe than outer retinal dysfunction, and RGCs loss. An advantage of this model is that it allows longitudinal measurements of in vivo retinal structure and function. This can minimize the effects of confounding factors such as inflammation and corneal changes on the outcome measures. Hence, this model has been applied in SD rats to study the neuroprotective effects of LBP pre- and post-treatments (Lakshmanan et al., 2019a). Pre-treatment with 1 mg/kg LBP was initiated 1 week before OHT induction. Post-treatment with 1 mg/kg LBP or the vehicle, PBS, was administered at 5 weeks after the induction and was continued till week 15 (Lakshmanan et al., 2019a). The OHT eyes in all three groups developed a chronic and mild IOP elevation. The average cumulative IOP of these eyes was around 35% greater than the fellow controls. Animals receiving PBS vehicle treatment showed RNFL thinning at week 4 (~23% loss from baseline) that was sustained up to week 15 (~17% loss from baseline), a gradual reduction of retinal functions from week 8 to 12 (pSTR: ~40%; b-wave: ~23% loss at week 15), and ~50% reduction in RGC density at week 15. LBP pre-treatment delayed the onset and reduced the rate of further RNFL thinning (Week 8: ~11%; Week 15: ~10% loss from baseline). The retention of RGC density and retinal functions were also maintained up to the end of the study (week 15). Although significant RNFL thinning was detected at week 4 (~25 % loss from baseline), post-treatment resulted in the retention of RNFL thickness (~3.5 µm, in both OHT and control eyes) from week 8 onwards and was maintained up to week 15. At the same time, retinal functions related to ganglion and photoreceptor cells were improved and RGC preservation was achieved. The level of rescue was comparable with the LBP pre-treatment and sham control groups.
LBP pre- and post-treatments could successfully preserve the neuronal structures and functions in both acute (Lakshmanan et al., 2019b) and chronic OHT (Lakshmanan et al., 2019a) models. The post-treatment approach is highly relevant to the clinical scenario as patients are more likely to start their treatment after the diagnosis of glaucoma. In addition, the studies characterized the longitudinal effects of LBP over a follow-up period up to 15 weeks, which was more extensive than earlier reports on acute (1 week; Mi et al., 2012a; He et al., 2014) and chronic (4 weeks; Chan et al., 2007) OHT models. The fellow, normotensive control eyes of the LBP treatment groups did not show any toxic effect from prolonged treatment over 15 weeks of study. Both retinal structure and functions of LBP treated eyes were similar to naïve eyes of the sham control or the fellow eyes of the vehicle controls (Lakshmanan et al., 2019a).
Effects of LBP pre- and post-treatment on halting the RGC degeneration
RGCs undergo compartmentalized degeneration in which dendritic shrinkage is followed by axonal and somatic degeneration and loss (Weber et al., 1998; Buckingham et al., 2008; El-Danaf and Huberman, 2015). The degeneration of each neuronal compartment followed a distinctive timeline. Under pre-treatment conditions, LBP halted the initial stage of neuronal degeneration, which involved dendritic and axonal loss, as demonstrated by the preservation of IRL thickness in the AOH model and RNFL thickness in the OHT model using OCT. It also arrested the subsequent somatic loss, as shown by the substantially preserved GCL/RGC cell density and retinal functions. Under post-treatment conditions, LBP was effective in arresting the secondary degeneration in AOH (Lakshmanan et al., 2019b). However, it showed limited effects in reversing the primary damages that led to IRL thinning in the AOH model and RNFL thinning in the OHT model. These were related to the reduced rate of progressive IRL loss and the interruption of the degeneration cascade. As a result, GCL cell density and retinal functions were retained (Lakshmanan et al., 2019b). Also, LBP post-treatment improved RNFL thickness, retained RGC density, and improved retinal functions under chronic OHT conditions (Lakshmanan et al., 2019a). In summary, LBP post-treatment can achieve “neuronal rescue” upon both acute and chronic OHT insults. Most importantly, LBP could exert its protective effects in the presence of elevated IOP in a chronic OHT model.
Limitations in Pre-Clinical Research Outcomes of Lycium Barbarum Polysaccharides Treatment
Animal models of OHT cannot replicate all aspects of clinical glaucoma. Therefore, it is important to choose a suitable model for evaluating the outcomes of LBP treatment to replicate the clinical management of the disease. The acute and chronic models adopted in LBP studies mimiced ked one aspect of clinical glaucoma, which is IOP elevation. One limitation of the ischemia-reperfusion injury model, achieved by raising IOP ≥ 80 mmHg, is that such high-pressure levels are rarely seen in clinical chronic glaucoma cases. Although transient IOP spikes as high as 30 or 40 mmHg can occur during saccadic eye movements or blinking (Edmiston et al., 2014; Turner et al., 2019), Valsalva maneuver (Rafuse et al., 1994; Palamar et al., 2015; Sun et al., 2020), swimming goggles usage (Ma et al., 2007; Paula et al., 2016), wind instruments playing (Schuman et al., 2000; Schmidtmann et al., 2011), and certain yoga positions (Baskaran et al., 2006), extremely high pressures are only seen in acute glaucoma. Nevertheless, this model can mimic some retinal and optic nerve characteristics of glaucoma (Osborne et al., 1999). These include hypoxia (Holcombe et al., 2008), glutamate toxicity (Sucher et al., 1997; Nucci et al., 2005), oxidative stress (Liu et al., 2007; Lee et al., 2012), and apoptotic cell death (Rosenbaum et al., 1997). It is noteworthy that IOP elevation to 60 mmHg for 8 hours resulted in anatomical and genetic changes at the optic nerve head that was very similar to the hypertonic saline OHT model (Johnson et al., 2011; Morrison et al., 2016). Despite these limitations, triangulating the findings from different glaucoma models allows researchers to compare the outcomes of LBP treatments. Consistently, LBP post-treatment provides clear benefits in treating OHT induced changes, albeit not as strong as pre-treatment.
As for chronic OHT models, the neuroprotective benefits of LBP pre-treatment and its possible mechanism were mainly reported in an experimental model induced by laser photocoagulation of limbal and episcleral veins (Chan et al., 2007; Chiu et al., 2009, 2010; Mi et al., 2012b). Although this method could sustain moderate IOP elevation with repeated applications of laser, the possible role of biphasic IOP change and the complications of repeated procedures (inflammation and corneal changes) in influencing the study outcomes cannot be excluded. Secondly, the circumlimbal suture model adapted in the post-treatment study involved the physical compression of the globe which results in ocular hypertension, likely through increasing the episcleral venous pressure (Wong et al., 2021). The suture model in albino rats (Lakshmanan et al., 2020) produced milder OHT responses than that in pigmented rats (Liu et al., 2015; Liu and Flanagan, 2017). This suggested the presence of strain-specific susceptibility to IOP stress. Also, further immunohistochemistry evaluation can provide more evidence on the possible role of photoreceptor swelling and inflammatory responses in the thickening of the outer retinal layer. On top of that, in vivo studies of LBP post-treatment can further be benefitted from detailed RGC quantification, evaluation of retinal vasculature/ocular blood flow, and examination of the neuroprotective mechanisms as in the pre-treatment OHT models. Since IOP stress in rodents can induce sectorial RGC loss (Soto et al., 2011), estimating such loss using retinal cross-sections may underestimate the RGC survival rate. While this can be improved by performing retinal whole mounts, the assessment of temporal RGC loss requires the sacrifice of experimental animals at different time points. To overcome this problem, the transgenic mice strain, Thy1-CFP23Jrs, which expresses cyan fluorescent protein under the control of the promoter for Thy-1, a cell-surface glycoprotein specifically expressed in RGC (Murata et al., 2008; Leung et al., 2009), or in vivo confocal neuroimaging (Sabel et al., 1997; Prilloff et al., 2010) can be applied to facilitate longitudinal in vivo evaluation of RGCs. Alternatively, the transgenic mouse strain, Thy1-YFP16Jrs, which expresses yellow fluorescent proteins in RGC under the control of a Thy-1 promoter, can be used to investigate the effect of LBP in preserving RGC dendrites, axons, and cell body through the direct tracking of individual RGCs in a longitudinal fashion (Leung et al., 2011; Li et al., 2011b). Secondly, as ocular blood flow is known to be reduced in glaucoma (Flammer et al., 2002; Grieshaber and Flammer, 2005), it can serve as a potential treatment target. Previously, LBP has been shown to protect RGC by modulating ET-1 expression in the ganglion cell layer and the vasculature in an OHT rat model (Mi et al., 2012b). The improvement in RNFL thickness under post-treatment conditions can potentially be due to the modulatory effects of LBP on ET-1. Considering the role of ET-1 in regulating the ocular blood flow, assessing the retinal vasculature and blood flow using clinical methods, including OCT-microangiography (Zhi et al., 2012) and Doppler (Zhao et al., 2017), can provide further evidence on the protective effects of LBP against vascular dysregulation in glaucoma. Although the benefits of LBP post-treatment intervention have been demonstrated using the AOH and chronic OHT models, further investigations on the mechanisms involved in neuroprotection should be undertaken. For instance, more efforts are needed to understand why there was a rebound in RNFL thickness in the chronic OHT model under LBP post-treatment. Delineating the differences in the protective mechanisms when LBP interventions are offered involved at different time points of disease course will be useful in informing future glaucoma management in clinical settings.
Future Directions
LBP has demonstrated to be a viable candidate for RGC neuroprotection in the clinical management of glaucoma based on the encouraging treatment outcomes in preclinical studies. However, as with other CNS diseases, barriers that hinder the translation of preclinical neuroprotective therapies in glaucoma to clinical trials remain. Although a number of neuroprotective agents and CAM approaches has demonstrated promising outcomes in preclinical glaucoma studies, only a few of them (e.g. Memantine, Brimonidine, and different neurotrophic factors) have been studied in clinical trials and, to date, none of them has been approved for clinical use in glaucoma management (Liu and Pang, 2013). The failure to replicate the animal studies results in human trials can mainly be attributed to the differences in disease models used, the mechanisms of neuronal loss involved, the study designs applied, the outcome measurements (endpoint) considered, the dosages of the drug studied, and the differences in the therapeutic windows involved in preclinical and clinical research (Girkin, 2001; Goldberg, 2007; Danesh-Meyer and Levin, 2009; Levin and Danesh-Meyer, 2010; Ergorul and Levin, 2013; Liu and Pang, 2013).
LBP pre- and post-treatments exhibited neuroprotection in in vivo glaucoma models through RGC structural and functional preservation. Till date, there are no clinical studies demonstrating the efficacy of LBP in glaucoma patients. However, it is encouraging to note that the treatment benefits of LBP reported in the retinitis pigmentosa mouse model, rd10 mice, in preserving the photoreceptor structure and function (Wang et al., 2014a), was later confirmed in a double-masked, 12-months clinical trial (Chan et al., 2019). To enable the successful translation of LBP interventions, it is imperative to identify the potential issues impeding the progress and the possible measures to be undertaken to improve the rate of success (Girkin, 2001; Cheng et al., 2004; Danesh-Meyer and Levin, 2009; Ergorul and Levin, 2013; Liu and Pang, 2013). Hence, to ensure the translational success of LBP, as an adjuvant therapy for glaucoma management in clinical settings, more preclinical studies that employ clinically relevant designs are warranted (Girkin, 2001; Cheng et al., 2004; Danesh-Meyer and Levin, 2009; Ergorul and Levin, 2013; Liu and Pang, 2013). First, it is important to investigate the combined efficacy of LBP and IOP-lowering treatment against the stand-alone effects of individual therapeutic approaches in preclinical settings. This can help to delineate the standalone effect of the neuroprotective agents, which is difficult to be tested in randomized controlled trials as IOP-lowering therapy is considered to be the clinical standard of treatment. Second, as glaucomatous changes are frequently observed in patients with normal IOP, (i.e. normal-tension glaucoma), it is essential to study the neuroprotective effects of LBP using IOP-independent glaucoma models. These may include the glutamate aspartate transporters deficient normal-tension glaucoma mice model and the ET-1 model with induced optic neuropathies. It allows a more comprehensive characterization of LBP treatment across different glaucoma treatment scenarios. Third, the therapeutic effects of LBP should be studied in older animals and in vivo models that represent different comorbidities. This enables researchers to understand how LBP performs in the presence of different glaucoma risk factors such as old age and chronic conditions like hypertension and diabetes mellitus. Fourth, since glaucoma damage can advance to the higher center (Gupta et al., 2006, 2009; Fukuda et al., 2018), it is valuable to investigate whether LBP can extend its neuroprotective effect to the lateral geniculate nucleus and the visual cortex. Fifth, due to the chronic nature of glaucoma and the need for continuous treatment, it is important to document the washout effects of LBP on retinal structure and functions in case of treatment switchover. Sixth, instead of using histological endpoints, which is common in animal studies, clinical research often uses in vivo structural and functional assessment to study the rate of loss. Thus, preclinical studies should be designed to adapt comparable in vivo techniques for the longitudinal assessment of both retinal structure and function. Lastly, studies on the evaluation of potential adverse effects of LB are needed. Currently, our understanding is limited to the interaction of LB with Warfarin (an anticoagulant; Rivera et al., 2012). As LBP may exhibit hypolipidemic and hypoglycaemic effects, it is vital to assess whether this interferes with the medications used in the management of patients with chronic comorbidities. Therefore, integrative animal models displaying multiple glaucoma risk factors should be considered for testing the efficacy of LBP at the preclinical level. Effective scrutiny preclinical level can potentially improve the chances of successful LBP translation into clinical applications.
Conclusion
LBP is a promising candidate for treating glaucoma. It exhibits neuroprotective effects in both IOP-dependent and -independent models of RGC degeneration in preclinical animal studies. The multi-target action of LBP was demonstrated through its ability to rescue differential neuronal compartments and retinal functions. Although LBP pre-treatment achieved better neuroprotection, the beneficial effects offered by post-treatment were remarkable as well. The promising pre-treatment results support the use of LBP as preventive medicine for patients who are at higher risk of developing the disease, such as ocular hypertension or glaucoma suspects. The post-treatment efficacy provides important evidence for the application of LBP as an adjuvant neuroprotective therapy for glaucoma patients who are at risk for progression or show progressive vision loss despite well-controlled IOP. Further preclinical research is warranted to evaluate the efficacy of LBP post-treatment in different animal models. Evaluation methods and study designs closely relevant to clinical settings should be applied. Once LBP proves its efficacy at all the levels of preclinical scrutiny, it can be further evaluated in the clinical trial pipeline leading to bedside applications.
Additional file: Open peer review report 1 (81.2KB, pdf) .
Footnotes
Funding: This work was supported by the PolyU Central Research Grants (No. UAG1 and UAHD, to HHLC).
Conflicts of interest: The authors declare no conflicts of interest.
Editor note: KFS is an Editorial Board member of Neural Regeneration Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and their research groups.
Data availability statement: The data are available from the corresponding author on reasonable request.
Open peer reviewer: Yiqing Li, Sun Yat-Sen University, China.
P-Reviewer: Li Y; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 2.Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- 3.Adornetto A, Rombolà L, Morrone LA, Nucci C, Corasaniti MT, Bagetta G, Russo R. Natural products:evidence for neuroprotection to be exploited in glaucoma. Nutrients. 2020;12:3158. doi: 10.3390/nu12103158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- 5.Amagase H, Farnsworth NR. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji) Food Res Int. 2011;44:1702–1717. [Google Scholar]
- 6.Anders F, Teister J, Liu A, Funke S, Grus FH, Thanos S, von Pein HD, Pfeiffer N, Prokosch V. Intravitreal injection of β-crystallin B2 improves retinal ganglion cell survival in an experimental animal model of glaucoma. PLoS One. 2017;12:e0175451. doi: 10.1371/journal.pone.0175451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskaran M, Raman K, Ramani KK, Roy J, Vijaya L, Badrinath SS. Intraocular pressure changes and ocular biometry during Sirsasana (headstand posture) in yoga practitioners. Ophthalmology. 2006;113:1327–1332. doi: 10.1016/j.ophtha.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Becker B, Stamper RL, Asseff C, Podos SM. Effect of diphenylhydantoin on glaucomatous field loss:a preliminary report. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:412–422. [PubMed] [Google Scholar]
- 9.Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, Beck S, Funke S, Wilding C, Pfeiffer N, Grus FH. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res. 2013;36:199–216. doi: 10.1016/j.preteyeres.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Berlin MS. Laser treatment for outflow obstruction. Stamper RL, Lieberman MF, Drake MV, editors. Becker-Shaffer's diagnosis and therapy of the glaucomas. (eighth edition) 2009a:447–455. Edinburgh:Mosby. [Google Scholar]
- 11.Berlin MS. Laser treatment for internal flow block. In: Stamper RL, Lieberman MF, Drake MV, editors. Becker-Shaffer's eiagnosis and therapy of the glaucomas. eighth edition. Edinburgh: Mosby; 2009b. pp. 439–446. [Google Scholar]
- 12.Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui BV, Batcha AH, Fletcher E, Wong VH, Fortune B. Relationship between the magnitude of intraocular pressure during an episode of acute elevation and retinal damage four weeks later in rats. PLoS One. 2013;8:e70513. doi: 10.1371/journal.pone.0070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Chan HHL, Lam HI, Choi KY, Li SZC, Lakshmanan Y, Yu WY, Chang RCC, Lai J, So KF. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J Ethnopharmacol. 2019;236:336–344. doi: 10.1016/j.jep.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X, Qin J, Tian M, Jin G, Zhang X. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9:e88076. doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng D, Kong H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011;16:2542–2550. doi: 10.3390/molecules16032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, Sun T, Zhang X, Zhao RJ, Gu L, Cao C, Zhou SF. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2014;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke:two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiasseu M, Cueva Vargas JL, Destroismaisons L, Vande Velde C, Leclerc N, Di Polo A. Tau accumulation, altered phosphorylation, and missorting promote neurodegeneration in glaucoma. J Neurosci. 2016;36:5785. doi: 10.1523/JNEUROSCI.3986-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu K, Chan HC, Yeung SC, Yuen WH, Zee SY, Chang RCC, So KF. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J Ocul Biol Dis Infor. 2009;2:47–56. doi: 10.1007/s12177-009-9023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu K, Zhou Y, Yeung SC, Lok CKM, Chan OOC, Chang RCC, So KF, Chiu JF. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. J Cell Biochem. 2010;110:311–320. doi: 10.1002/jcb.22539. [DOI] [PubMed] [Google Scholar]
- 23.Chu PH, Li HY, Chin MP, So KF, Chan HH. Effect of lycium barbarum (wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS One. 2013;8:e81339. doi: 10.1371/journal.pone.0081339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(Suppl1):S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Cui B, Chen Y, Liu S, Wang J, Li S, Wang Q, Li S, Chen M, Lin X. Antitumour activity of Lycium chinensis polysaccharides in liver cancer rats. Int J Biol Macromol. 2012;51:314–318. doi: 10.1016/j.ijbiomac.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Danesh-Meyer HV, Levin LA. Neuroprotection:extrapolating from neurologic diseases to the eye. Am J Ophthalmol. 2009;148:186–191.e182. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Danesh-Meyer HV, Levin LA. Glaucoma as a neurodegenerative disease. J Neuroophthalmol. 2015;351(Suppl):S22–28. doi: 10.1097/WNO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 28.Edmiston A, Turner DC, Hethcox LA, Girkin CA, Downs JCC. The magnitude of IOP spikes associated with blink and saccade. Invest Ophthalmol Vis Sci. 2014;55:149–149. [Google Scholar]
- 29.El-Danaf RN, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma:evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015;35:2329–2343. doi: 10.1523/JNEUROSCI.1419-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ergorul C, Levin LA. Solving the lost in translation problem:improving the effectiveness of translational research. Curr Opin Pharmacol. 2013;13:108–114. doi: 10.1016/j.coph.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feilchenfeld Z, Yücel YH, Gupta N. Oxidative injury to blood vessels and glia of the pre-laminar optic nerve head in human glaucoma. Exp Eye Res. 2008;87:409–414. doi: 10.1016/j.exer.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 33.Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard J-P, Stefánsson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda M, Omodaka K, Tatewaki Y, Himori N, Matsudaira I, Nishiguchi KM, Murata T, Taki Y, Nakazawa T. Quantitative MRI evaluation of glaucomatous changes in the visual pathway. PLoS One. 2018;13:e0197027. doi: 10.1371/journal.pone.0197027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gan L, Zhang SH, Yang XL, Xu HB. Immunomodulation and antitumor activity by a polysaccharide–protein complex from Lycium barbarum. Int Immunopharmacol. 2004;4:563–569. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, McNaught AI, Negi A, Patel K, Russell RA, Shah A, et al. Latanoprost for open-angle glaucoma (UKGTS):a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- 37.GBD 2019 Blindness and Vision Impairment Collaborators;Vision Loss Expert Group of the Global Burden of Disease Study (2021) Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020:the right to sight:an analysis for the Global Burden of Disease Study. Lancet Glob Health. 9:e144–160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gherghel D, Mroczkowska S, Qin L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma reduction in blood glutathione levels in glaucoma. Invest Ophthalmol Vis Sci. 2013;54:3333–3339. doi: 10.1167/iovs.12-11256. [DOI] [PubMed] [Google Scholar]
- 39.Girkin CA. Strategies for neuroprotection. J Glaucoma. 2001;10:S78–80. doi: 10.1097/00061198-200110001-00028. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg I. Is this neuroprotective drug good for my glaucoma patients?Some key factors in clinical decision-making. Can J Ophthalmol. 2007;42:418–420. [PubMed] [Google Scholar]
- 41.Goldblum D, Kipfer-Kauer A, Sarra G-M, Wolf S, Frueh BE. Distribution of amyloid precursor protein and amyloid-βimmunoreactivity in DBA/2J glaucomatous mouse retinas. Invest Ophthalmol Vis Sci. 2007;48:5085–5090. doi: 10.1167/iovs.06-1249. [DOI] [PubMed] [Google Scholar]
- 42.Good TJ, Kahook MY. The role of endothelin in the pathophysiology of glaucoma. Expert Opin Ther Targets. 2010;14:647–654. doi: 10.1517/14728222.2010.487065. [DOI] [PubMed] [Google Scholar]
- 43.Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39:823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- 44.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 45.Gramlich OW, Bell K, von Thun Und Hohenstein-Blaul N, Wilding C, Beck S, Pfeiffer N, Grus FH. Autoimmune biomarkers in glaucoma patients. Curr Opin Pharmacol. 2013;13:90–97. doi: 10.1016/j.coph.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. 2005;16:79–83. doi: 10.1097/01.icu.0000156134.38495.0b. [DOI] [PubMed] [Google Scholar]
- 47.Gupta N, Ang LC, de Tilly LN, Bidaisee L, Yücel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- 49.Gupta N, Greenberg G, de Tilly LN, Gray B, Polemidiotis M, Yücel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol. 2009;93:56–60. doi: 10.1136/bjo.2008.138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harman D. Aging:a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 51.Harman D. Free radical theory of aging:an update:increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 52.He M, Pan H, Chang RC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014;9:e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial G. Reduction of intraocular pressure and glaucoma progression:results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 54.Ho YS, Yu MS, Yik SY, So KF, Yuen WH, Chang RC. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell Mol Neurobiol. 2009;29:1233–1244. doi: 10.1007/s10571-009-9419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holcombe DJ, Lengefeld N, Gole GA, Barnett NL. The effects of acute intraocular pressure elevation on rat retinal glutamate transport. Acta Ophthalmol. 2008;86:408–414. doi: 10.1111/j.1600-0420.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- 56.Hu X, Qu Y, Chu Q, Li W, He J. Investigation of the neuroprotective effects of Lycium barbarum water extract in apoptotic cells and Alzheimer's disease mice. Mol Med Report. 2018;17:3599–3606. doi: 10.3892/mmr.2017.8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes WF. Quantitation of ischemic damage in the rat retina. Exp Eye Res. 1991;53:573–582. doi: 10.1016/0014-4835(91)90215-z. [DOI] [PubMed] [Google Scholar]
- 58.Ito Y, Shimazawa M, Tsuruma K, Mayama C, Ishii K, Onoe H, Aihara M, Araie M, Hara H. Induction of amyloid-β(1-42) in the retina and optic nerve head of chronic ocular hypertensive monkeys. Mol Vis. 2012;18:2647–2657. [PMC free article] [PubMed] [Google Scholar]
- 59.Jin M, Huang Q, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 60.Johnson EC, Deppmeier LMH, Wentzien SKF, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:431–442. [PubMed] [Google Scholar]
- 61.Johnson EC, Doser TA, Cepurna WO, Dyck JA, Jia L, Guo Y, Lambert WS, Morrison JC. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Invest Ophthalmol Vis Sci. 2011;52:504–518. doi: 10.1167/iovs.10-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, Wilson MR, Gordon MO. The ocular hypertension treatment study:a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 64.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 65.Kimura A, Namekata K, Guo X, Noro T, Harada C, Harada T. Targeting oxidative stress for treatment of glaucoma and optic neuritis. Oxid Med Cell Longev. 20172017:2817252. doi: 10.1155/2017/2817252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kipfer-Kauer A, McKinnon SJ, Frueh BE, Goldblum D. Distribution of amyloid precursor protein and amyloid-beta in ocular hypertensive C57BL/6 mouse eyes. Curr Eye Res. 2010;35:828–834. doi: 10.3109/02713683.2010.494240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knox DL, Eagle RC, Jr, Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport:histopathologic features in human high-pressure secondary glaucoma. Arch Ophthalmol. 2007;125:347–353. doi: 10.1001/archopht.125.3.347. [DOI] [PubMed] [Google Scholar]
- 68.Kong GYX, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 69.Kremmer S, Kreuzfelder E, Bachor E, Jahnke K, Selbach JM, Seidahmadi S. Coincidence of normal tension glaucoma, progressive sensorineural hearing loss, and elevated antiphosphatidylserine antibodies. Br J Ophthalmol. 2004;88:1259–1262. doi: 10.1136/bjo.2003.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krishnamoorthy RR, McGrady NR, Jefferies H, He S, Stankowska DL. The endothelin receptor antagonist macitentan attenuates neurodegeneration in a rodent model of glaucoma. Invest Ophthalmol Vis Sci. 2018;59:1593. [Google Scholar]
- 71.Lagreze WA, Knorle R, Bach M, Feuerstein TJ. Memantine is neuroprotective in a rat model of pressure-induced retinal ischemia. Invest Ophthalmol Vis Sci. 1998;39:1063–1066. [PubMed] [Google Scholar]
- 72.Lakshmanan Y, Wong FSY, Zuo B, So KF, Bui BV, Chan HHL. Posttreatment intervention with lycium barbarum polysaccharides is neuroprotective in a rat model of chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2019a;60:4606–4618. doi: 10.1167/iovs.19-27886. [DOI] [PubMed] [Google Scholar]
- 73.Lakshmanan Y, Wong FS, Yu WY, Li SZ, Choi KY, So KF, Chan HH. Lycium barbarum polysaccharides rescue neurodegeneration in an acute ocular hypertension rat model under pre- and posttreatment conditions. Invest Ophthalmol Vis Sci. 2019b;60:2023–2033. doi: 10.1167/iovs.19-26752. [DOI] [PubMed] [Google Scholar]
- 74.Lakshmanan Y, Wong FSY, Zuo B, Bui BV, Chan HH. Longitudinal outcomes of circumlimbal suture model-induced chronic ocular hypertension in Sprague-Dawley albino rats. Graefes Arch Clin Exp Ophthalmol. 2020;25:2715–2728. doi: 10.1007/s00417-020-04820-7. [DOI] [PubMed] [Google Scholar]
- 75.Lee D, Kim KY, Noh YH, Chai S, Lindsey JD, Ellisman MH, Weinreb RN, Ju WK. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. 2012;7:e47098. doi: 10.1371/journal.pone.0047098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung CKS, Lindsey JD, Chen L, Liu Q, Weinreb RN. Longitudinal profile of retinal ganglion cell damage assessed with blue-light confocal scanning laser ophthalmoscopy after ischaemic reperfusion injury. Br J Ophthalmol. 2009;93:964–968. doi: 10.1136/bjo.2008.150482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leung CK, Weinreb RN, Li ZW, Liu S, Lindsey JD, Choi N, Liu L, Cheung CY, Ye C, Qiu K, Chen LJ, Yung WH, Crowston JG, Pu M, So KF, Pang CP, Lam DS. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011;52:1539–1547. doi: 10.1167/iovs.10-6012. [DOI] [PubMed] [Google Scholar]
- 78.Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv Ophthalmol. 2003;48(Suppl 1):S21–24. doi: 10.1016/s0039-6257(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 79.Levin LA, Peeples P. History of neuroprotection and rationale as a therapy for glaucoma. Am J Manag Care. 2008;14:S11–14. [PubMed] [Google Scholar]
- 80.Levin LA, Danesh-Meyer HV. Lost in translation:bumps in the road between bench and bedside. JAMA. 2010;303:1533–1534. doi: 10.1001/jama.2010.463. [DOI] [PubMed] [Google Scholar]
- 81.Levin LA, Crowe ME, Quigley HA, Lasker IIoAoGN, Participants Neuroprotection for glaucoma:Requirements for clinical translation. Exp Eye Res. 2017;157:34–37. doi: 10.1016/j.exer.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D'Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2001;42:975–982. [PubMed] [Google Scholar]
- 83.Levkovitch-Verbin H, Quigley HA, Martin KR, Zack DJ, Pease ME, Valenta DF. A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci. 2003;44:3388–3393. doi: 10.1167/iovs.02-0646. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Liang Y, Chiu K, Yuan Q, Lin B, Chang RCC, So KF. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS One. 2013;8:e68881. doi: 10.1371/journal.pone.0068881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li HY, Ruan YW, Kau PW, Chiu K, Chang RC, Chan HH, So KF. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant. 2015;24:403–417. doi: 10.3727/096368915X686896. [DOI] [PubMed] [Google Scholar]
- 86.Li HY, Huang M, Luo QY, Hong X, Ramakrishna S, So KF. Lycium barbarum (Wolfberry) increases retinal ganglion cell survival and affects both microglia/macrophage polarization and autophagy after rat partial optic nerve transection. Cell Transplant. 2019;28:607–618. doi: 10.1177/0963689719835181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li SY, Yang D, Yeung CM, Yu WY, Chang RC, So KF, Wong D, Lo AC. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One. 2011a;6:e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li XM. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int J Biol Macromol. 2007;40:461–465. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Li X, Ma Y, Liu X. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J Ethnopharmacol. 2007;111:504–511. doi: 10.1016/j.jep.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 90.Li ZW, Liu S, Weinreb RN, Lindsey JD, Yu M, Liu L, Ye C, Cui Q, Yung WH, Pang CP. Tracking dendritic shrinkage of retinal ganglion cells after acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2011b;52:7205–7212. doi: 10.1167/iovs.10-6868. [DOI] [PubMed] [Google Scholar]
- 91.Liu HH, Bui BV, Nguyen CT, Kezic JM, Vingrys AJ, He Z. Chronic ocular hypertension induced by circumlimbal suture in rats. Invest Ophthalmol Vis Sci. 2015;56:2811–2820. doi: 10.1167/iovs.14-16009. [DOI] [PubMed] [Google Scholar]
- 92.Liu HH, Flanagan JG. A mouse model of chronic ocular hypertension induced by circumlimbal suture. Invest Ophthalmol Vis Sci. 2017;58:353–361. doi: 10.1167/iovs.16-20576. [DOI] [PubMed] [Google Scholar]
- 93.Liu Q, Ju WK, Crowston JG, Xie F, Perry G, Smith MA, Lindsey JD, Weinreb RN. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007;48:4580–4589. doi: 10.1167/iovs.07-0170. [DOI] [PubMed] [Google Scholar]
- 94.Liu WJ, Jiang HF, Rehman FU, Zhang JW, Chang Y, Jing L, Zhang JZ. Lycium barbarum polysaccharides decrease hyperglycemia-aggravated ischemic brain injury through maintaining mitochondrial fission and fusion balance. Int J Biol Sci. 2017;13:901–910. doi: 10.7150/ijbs.18404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Pang IH. Challenges in the development of glaucoma neuroprotection therapy. Cell Tissue Res. 2013;353:253–260. doi: 10.1007/s00441-013-1584-z. [DOI] [PubMed] [Google Scholar]
- 96.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 97.Ma KT, Chung WS, Seo KY, Seong GJ, Kim CY. The effect of swimming goggles on intraocular pressure and blood flow within the optic nerve head. Yonsei Med J. 2007;48:807–809. doi: 10.3349/ymj.2007.48.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacCumber MW, D'Anna SA. Endothelin receptor-binding subtypes in the human retina and choroid. Arch Ophthalmol. 1994;112:1231–1235. doi: 10.1001/archopht.1994.01090210119024. [DOI] [PubMed] [Google Scholar]
- 99.McGrady NR, Minton AZ, Stankowska DL, He S, Jefferies HB, Krishnamoorthy RR. Upregulation of the endothelin A (ET(A)) receptor and its association with neurodegeneration in a rodent model of glaucoma. BMC Neurosci. 2017;18:27. doi: 10.1186/s12868-017-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H, Quigley HA, Zack DJ. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002;43:1077–1087. [PubMed] [Google Scholar]
- 101.Mi XS, Feng Q, Lo ACY, Chang RC-C, Lin B, Chung SK, So KF. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS One. 2012a;7:e45469. doi: 10.1371/journal.pone.0045469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mi XS, Chiu K, Van G, Leung JW, Lo AC, Chung SK, Chang RC, So KF. Effect of Lycium barbarum Polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural Regen Res. 2012b;7:645–651. doi: 10.3969/j.issn.1673-5374.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mi XS, Feng Q, Lo ACY, Chang RCC, Chung SK, So KF. Lycium barbarum polysaccharides related RAGE and Aβlevels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural Regen Res. 2020;15:2344–2352. doi: 10.4103/1673-5374.284998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Migdal C. Glaucoma medical treatment:philosophy, principles and practice. Eye (Lond) 2000;14:515–518. doi: 10.1038/eye.2000.138. [DOI] [PubMed] [Google Scholar]
- 105.Minton AZ, Phatak NR, Stankowska DL, He S, Ma HY, Mueller BH, Jiang M, Luedtke R, Yang S, Brownlee C, Krishnamoorthy RR. Endothelin B receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7:e43199. doi: 10.1371/journal.pone.0043199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morrison JC, Cepurna WO, Tehrani S, Choe TE, Jayaram H, Lozano DC, Fortune B, Johnson EC. A period of controlled elevation of IOP (CEI) produces the specific gene expression responses and focal injury pattern of experimental rat glaucoma. Invest Ophthalmol Vis Sci. 2016;57:6700–6711. doi: 10.1167/iovs.16-20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murata H, Aihara M, Chen YN, Ota T, Numaga J, Araie M. Imaging mouse retinal ganglion cells and their loss in vivo by a fundus camera in the normal and ischemia-reperfusion model. Invest Ophthalmol Vis Sci. 2008;49:5546–5552. doi: 10.1167/iovs.07-1211. [DOI] [PubMed] [Google Scholar]
- 108.Niu AJ, Wu JM, Yu DH, Wang R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int J Biol Macromol. 2008;42:447–449. doi: 10.1016/j.ijbiomac.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Nucci C, Tartaglione R, Rombolà L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005;26:935–941. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 110.No authors listed (1998) Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 111.No authors listed (2000) The Advanced Glaucoma Intervention Study (AGIS):7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 112.Omoti AE, Edema OT. A review of the risk factors in primary open angle glaucoma. Niger J Clin Pract. 2007;10:79–82. [PubMed] [Google Scholar]
- 113.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S102–128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 114.Osborne NN, del Olmo-Aguado S. Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr Opin Pharmacol. 2013;13:16–22. doi: 10.1016/j.coph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Palamar M, Dag MY, Yagci A. The effects of Valsalva manoeuvre on ocular response analyzer measurements. Clin Exp Optom. 2015;98:447–450. doi: 10.1111/cxo.12303. [DOI] [PubMed] [Google Scholar]
- 116.Parikh RS, Parikh SR, Navin S, Arun E, Thomas R. Practical approach to medical management of glaucoma. Indian J Ophthalmol. 2008;56:223–230. doi: 10.4103/0301-4738.40362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parikh RS, Parikh SR. Alternative therapy in glaucoma management:is there any role? Indian J Ophthalmol. 2011;59(Suppl):S158–160. doi: 10.4103/0301-4738.73679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paula AP, Paula JS, Silva MJ, Rocha EM, De Moraes CG, Rodrigues ML. Effects of Swimming Goggles wearing on intraocular pressure, ocular perfusion pressure, and ocular pulse amplitude. J Glaucoma. 2016;25:860–864. doi: 10.1097/IJG.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 119.Pease ME, McKinnon SJ, Quigley HA, Kerrigan–Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:764–774. [PubMed] [Google Scholar]
- 120.Perlman JI, McCole SM, Pulluru P, Chang CJ, Lam TT, Tso MO. Disturbances in the distribution of neurotransmitters in the rat retina after ischemia. Curr Eye Res. 1996;15:589–596. doi: 10.3109/02713689609008898. [DOI] [PubMed] [Google Scholar]
- 121.Piri N, Song M, Kwong JMK, Caprioli J. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 2007;1141:1–9. doi: 10.1016/j.brainres.2006.11.095. [DOI] [PubMed] [Google Scholar]
- 122.Piri N, Kwong JMK, Caprioli J. Crystallins in retinal ganglion cell survival and regeneration. Mol Neurobiol. 2013;48:819–828. doi: 10.1007/s12035-013-8470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prasanna G, Hulet C, Desai D, Krishnamoorthy RR, Narayan S, Brun AM, Suburo AM, Yorio T. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51:41–50. doi: 10.1016/j.phrs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 124.Prilloff S, Fan J, Henrich-Noack P, Sabel BA. In vivo confocal neuroimaging (ICON):non-invasive, functional imaging of the mammalian CNS with cellular resolution. Eur J Neurosci. 2010;31:521–528. doi: 10.1111/j.1460-9568.2010.07078.x. [DOI] [PubMed] [Google Scholar]
- 125.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 126.Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 127.Rafuse PE, Mills DW, Hooper PL, Chang TS, Wolf R. Effects of Valsalva's manoeuvre on intraocular pressure. Can J Ophthalmol. 1994;29:73–76. [PubMed] [Google Scholar]
- 128.Rhee DJ, Katz LJ, Spaeth GL, Myers JS. Complementary and alternative medicine for glaucoma. Surv Ophthalmol. 2001;46:43–55. doi: 10.1016/s0039-6257(01)00233-8. [DOI] [PubMed] [Google Scholar]
- 129.Rivera CA, Ferro CL, Bursua AJ, Gerber BS. Probable interaction between Lycium barbarum (Goji) and warfarin. Pharmacotherapy. 2012;32:e50–53. doi: 10.1002/j.1875-9114.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 130.Rivera JL, Bell NP, Feldman RM. Risk factors for primary open angle glaucoma progression:what we know and what we need to know. Curr Opin Ophthalmol. 2008;19:102–106. doi: 10.1097/ICU.0b013e3282f493b3. [DOI] [PubMed] [Google Scholar]
- 131.Rosenbaum DM, Rosenbaum PS, Gupta A, Michaelson MD, Hall DH, Kessler JA. Retinal ischemia leads to apoptosis which is ameliorated by aurintricarboxylic acid. Vision Res. 1997;37:3445–3451. doi: 10.1016/S0042-6989(96)00328-8. [DOI] [PubMed] [Google Scholar]
- 132.Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58:341–354. doi: 10.1002/neu.10293. [DOI] [PubMed] [Google Scholar]
- 133.Sabel BA, Engelmann R, Humphrey MF. In vivo confocal neuroimaging (ICON) of CNS neurons. Nat Med. 1997;3:244–247. doi: 10.1038/nm0297-244. [DOI] [PubMed] [Google Scholar]
- 134.Salmon JF. Predisposing factors for chronic angle-closure glaucoma. Prog Retin Eye Res. 1999;18:121–132. doi: 10.1016/s1350-9462(98)00007-x. [DOI] [PubMed] [Google Scholar]
- 135.Schmidtmann G, Jahnke S, Seidel EJ, Sickenberger W, Grein HJ. Intraocular pressure fluctuations in professional brass and woodwind musicians during common playing conditions. Graefes Arch Clin Exp Ophthalmol. 2011;249:895–901. doi: 10.1007/s00417-010-1600-x. [DOI] [PubMed] [Google Scholar]
- 136.Schuman JS, Massicotte EC, Connolly S, Hertzmark E, Mukherji B, Kunen MZ. Increased intraocular pressure and visual field defects in high resistance wind instrument players. Ophthalmology. 2000;107:127–133. doi: 10.1016/s0161-6420(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 137.Schwartz M, Belkin M, Yoles E, Solomon A. Potential treatment modalities for glaucomatous neuropathy:neuroprotection and neuroregeneration. J Glaucoma. 1996;5:427–432. [PubMed] [Google Scholar]
- 138.Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, Ruiz-Gomez JM, Vidal-Sanz M. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:2002–2014. [PubMed] [Google Scholar]
- 139.Shi Z, Zhu L, Li T, Tang X, Xiang Y, Han X, Xia L, Zeng L, Nie J, Huang Y, Tsang CK, Wang Y, Lei Z, Xu Z, So KF, Ruan Y. Neuroprotective mechanisms of Lycium barbarum polysaccharides against ischemic insults by regulating NR2B and NR2A containing NMDA receptor signaling pathways. Front Cell Neurosci. 2017;11:288. doi: 10.3389/fncel.2017.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shoshani YZ, Harris A, Shoja MM, Rusia D, Siesky B, Arieli Y, Wirostko B. Endothelin and its suspected role in the pathogenesis and possible treatment of glaucoma. Curr Eye Res. 2012;37:1–11. doi: 10.3109/02713683.2011.622849. [DOI] [PubMed] [Google Scholar]
- 141.Song W, Huang P, Zhang C. Neuroprotective therapies for glaucoma. Drug Des Devel Ther. 2015;9:1469–1479. doi: 10.2147/DDDT.S80594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest Ophthalmol Vis Sci. 2011;52:434–441. doi: 10.1167/iovs.10-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stamper RL, Lieberman MF, Drake MV. Glaucoma outflow procedures. Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. (8th ed) 2009:466–490. Elsevier. [Google Scholar]
- 144.Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 145.Stitt AW, Chakravarthy U, Gardiner TA, Archer DB. Endothelin-like immunoreactivity and receptor binding in the choroid and retina. Curr Eye Res. 1996;15:111–117. doi: 10.3109/02713689609017618. [DOI] [PubMed] [Google Scholar]
- 146.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 147.Sun L, Chen W, Chen Z, Xiang Y, Guo J, Hu T, Xu Q, Zhang H, Wang J. Dual effect of the Valsalva maneuver on autonomic nervous system activity, intraocular pressure, Schlemm's canal, and iridocorneal angle morphology. BMC Ophthalmol. 2020;20:5. doi: 10.1186/s12886-019-1275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang T, He B. Treatment of d-galactose induced mouse aging with Lycium barbarum polysaccharides and its mechanism study. Afr J Tradit Complement Altern Med. 2013;10:12–17. doi: 10.4314/ajtcam.v10i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tataru CP, Purcarea VL. Antiglaucoma pharmacotherapy. J Med Life. 2012;5:247–251. [PMC free article] [PubMed] [Google Scholar]
- 150.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–2287. [PubMed] [Google Scholar]
- 151.Tezel G. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1001–1012. doi: 10.1167/iovs.08-2717. [DOI] [PubMed] [Google Scholar]
- 152.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040:a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 153.Turner DC, Edmiston AM, Zohner YE, Byrne KJ, Seigfreid WP, Girkin CA, Morris JS, Downs JC. Transient intraocular pressure fluctuations:source, magnitude, frequency, and associated mechanical energy. Invest Ophthalmol Vis Sci. 2019;60:2572–2582. doi: 10.1167/iovs.19-26600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wan MJ, Daniel S, Kassam F, Mutti G, Butty Z, Kasner O, Trope GE, Buys YM. Survey of complementary and alternative medicine use in glaucoma patients. J Glaucoma. 2012;21:79–82. doi: 10.1097/IJG.0b013e3182027c0c. [DOI] [PubMed] [Google Scholar]
- 155.Wang K, Xiao J, Peng B, Xing F, So KF, Tipoe GL, Lin B. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci Rep. 2014a;4:7601. doi: 10.1038/srep07601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L, Fortune B, Cull G, Dong J, Cioffi GA. Endothelin B receptor in human glaucoma and experimentally induced optic nerve damage. Arch Ophthalmol. 2006;124:717–724. doi: 10.1001/archopht.124.5.717. [DOI] [PubMed] [Google Scholar]
- 157.Wang T, Li Y, Wang Y, Zhou R, Ma L, Hao Y, Jin S, Du J, Zhao C, Sun T, Yu J. Lycium barbarum polysaccharide prevents focal cerebral ischemic injury by inhibiting neuronal apoptosis in mice. PLoS One. 2014b;9:e90780. doi: 10.1371/journal.pone.0090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wax MB, Barrett DA, Pestronk A. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1994;117:561–568. doi: 10.1016/s0002-9394(14)70059-5. [DOI] [PubMed] [Google Scholar]
- 159.Wax MB, Tezel G, Saito I, Gupta RS, Harley JB, Li Z, Romano C. Anti-Ro/SS-a positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1998;125:145–157. doi: 10.1016/s0002-9394(99)80084-1. [DOI] [PubMed] [Google Scholar]
- 160.Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- 161.Weber M, Bonaventure N, Sahel JA. Protective role of excitatory amino acid antagonists in experimental retinal ischemia. Graefes Arch Clin Exp Ophthalmol. 1995;233:360–365. doi: 10.1007/BF00200485. [DOI] [PubMed] [Google Scholar]
- 162.Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011;51:216–224. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weinreb RN, Levin LA. Is neuroprotection a viable therapy for glaucoma? Arch Ophthalmol. 1999;117:1540–1544. doi: 10.1001/archopht.117.11.1540. [DOI] [PubMed] [Google Scholar]
- 164.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 165.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma:a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wong VHY, Zhao D, Bui BV, Millar CJ, Nguyen CTO. Increased episcleral venous pressure in a mouse model of circumlimbal suture induced ocular hypertension. Exp Eye Res. 2021;202:108348. doi: 10.1016/j.exer.2020.108348. [DOI] [PubMed] [Google Scholar]
- 167.Wu H, Guo H, Zhao R. Effect of Lycium barbarum polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM rats. Yakugaku Zasshi. 2006;126:365–371. doi: 10.1248/yakushi.126.365. [DOI] [PubMed] [Google Scholar]
- 168.Xia G, Xin N, Liu W, Yao H, Hou Y, Qi J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol Med Report. 2014;9:1237–1241. doi: 10.3892/mmr.2014.1964. [DOI] [PubMed] [Google Scholar]
- 169.Xiao J, Liong EC, Ching YP, Chang RCC, So KF, Fung ML, Tipoe GL. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 170.Xin Y, Zhang S, Gu L, Liu S, Gao H, You Z, Zhou G, Wen L, Yu J, Xuan Y. Electrocardiographic and biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin-induced cardiotoxicity in the beagle dogs. Biol Pharm Bull. 2011;34:1523–1526. doi: 10.1248/bpb.34.1523. [DOI] [PubMed] [Google Scholar]
- 171.Yang D, Li SY, Yeung CM, Chang RCC, So KF, Wong D, Lo AC. Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS One. 2012;7:e33596. doi: 10.1371/journal.pone.0033596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Y, Li W, Li Y, Wang Q, Gao L, Zhao J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid Med Cell Longev. 2014;2014:145641–145641. doi: 10.1155/2014/145641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ. Changes in gene expression in experimental glaucoma and optic nerve transection:the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48:5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 174.Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion:implications for optic nerve neuropathies. Exp Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 175.Yu MS, Leung SK, Lai SW, Che CM, Zee SY, So KF, Yuen WH, Chang RC. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against beta-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 176.Yu MS, Ho YS, So KF, Yuen WH, Chang RC. Cytoprotective effects of Lycium barbarum against reducing stress on endoplasmic reticulum. Int J Mol Med. 2006;17:1157–1161. [PubMed] [Google Scholar]
- 177.Yu MS, Lai CS, Ho YS, Zee SY, So KF, Yuen WH, Chang RC. Characterization of the effects of anti-aging medicine Fructus lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med. 2007;20:261–268. [PubMed] [Google Scholar]
- 178.Zhao D, Nguyen CT, Wong VH, Lim JK, He Z, Jobling AI, Fletcher EL, Chinnery HR, Vingrys AJ, Bui BV. Characterization of the circumlimbal suture model of chronic IOP elevation in mice and assessment of changes in gene expression of stretch sensitive channels. Front Neurosci. 2017;11:41. doi: 10.3389/fnins.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao R, Cai Y, Shao X, Ma B. Improving the activity of Lycium barbarum polysaccharide on sub-health mice. Food Funct. 2015;6:2033–2040. doi: 10.1039/c4fo01108b. [DOI] [PubMed] [Google Scholar]
- 180.Zhi Z, Cepurna WO, Johnson EC, Morrison JC, Wang RK. Impact of intraocular pressure on changes of blood flow in the retina, choroid, and optic nerve head in rats investigated by optical microangiography. Biomed Opt Express. 2012;3:2220–2233. doi: 10.1364/BOE.3.002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhu J, Liu W, Yu J, Zou S, Wang J, Yao W, Gao X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr Polym. 2013;98:8–16. doi: 10.1016/j.carbpol.2013.04.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


