Abstract
Retinal dysfunction is the most common cause of vision loss in several retinal disorders. It has been estimated a great increase in these pathologies that are becoming more globally widespread and numerous over time, also supported by the life expectancy increment. Among different types of retinopathies, we can account some that share causes, symptoms, and treatment including diabetic retinopathy, age-related macular degeneration, glaucoma, and retinitis pigmentosa. Molecular changes, environmental factors, and genetic predisposition might be some of the main causes that drive retinal tissue to chronic inflammation and neurodegeneration in these retinopathies. The treatments available on the market contain compounds that efficiently ameliorate some of the important clinical features of these pathologies like stabilization of the intraocular pressure, reduction of eye inflammation, control of eye oxidative stress which are considered the major molecular mechanisms related to retinal dysfunction. Indeed, the most commonly used drugs are anti-inflammatories, such as corticosteroids, antioxidant, hypotonic molecules and natural neuroprotective compounds. Unfortunately, these drugs, which are fundamental to treating disease symptoms, are not capable to cure the pathologies and so they are not life-changing for patients. This review provided an overview of current treatments on the market, but more interestingly, wants to be a quick window on the new treatments that are now in clinical trials. Additionally, it has been here highlighted that the recent technical enhancement of the investigation methods to identify the various retinopathies causes might be used as a sort of “precise medicine” approach to tailor the identification of molecular pathways involved and potentially study a dedicated treatment for each patient. This approach includes the use of cutting-edge technologies like gene therapy and metabolomics.
Key Words: age-related macular degeneration, anti-inflammatory drugs, antioxidants, diabetic retinopathy, glaucoma, neuroprotective compounds, retinitis pigmentosa, retinopathies, vision loss
Introduction
Retinal dysfunction is the result of a complex retinopathy in which a progressive death of retinal cellular layers leads to tissue malfunction and ultimately to vision loss. These are common features of several ocular pathologies with a consequence of multifactorial molecular changes that are triggered by a wide variety of factors ranging from genetic to environmental (Kaur and Singh, 2022).
The World Health Organization estimates that at least 2.2 billion people suffer from visual impairment or blindness. As part of the central nervous system, degenerative diseases affecting the retina are considered forms of neurodegeneration. The visual impairment, resulting from this neurodegeneration, exhibits different accompanying phenotypes that result in the differential diagnosis of retinal dysfunction. The most prominent pathologies include age-related macular degeneration (AMD), glaucoma, diabetic retinopathy (DR) and to a lesser extent, retinitis pigmentosa. Although the primary etiological factors in each of these well-known ocular pathologies are heterogeneous, several molecular mechanisms are shared among them, suggesting a common path to retinal dysfunction that is independent of etiological triggers (Micera et al., 2021). Thus, it is possible that developing one pathology rather than another could be basically due to individual differences in susceptibility, such as those arising from genetic predisposition (i.e., retinitis pigmentosa) or environmental exposures (i.e., AMD) (Shughoury et al., 2022).
Molecular modifications shared by all the prominent ocular pathologies include increased oxidative stress, glutamate excitotoxicity, and metabolic changes. This suggests that targeting these molecular modifications could produce a broad-spectrum therapeutic for retinal dysfunction across ocular disorders (Marchesi et al., 2021; Kaur and Singh, 2022).
Search Strategy
Studies cited in this review published from 2008 to 2022 were searched on the PMC - NCBI - National Center for Biotechnology Information database using the following keywords: retinal degeneration, eye pathologies, retinal glutamate, excitotoxicity, current therapies for eye pathologies, future approaches for eye pathologies.
Current Pharmacological Treatments on the Market
To date, the research for new ophthalmic treatments is a growing business to incentivize the development of effective retinal disease modifiers. As with pharmacological interventions for nearly all types of pathology, ophthalmic pharmaceuticals target reduced progression by contrasting the symptoms of the disease rather than targeting the retinal dysfunction directly. For example, current treatments for glaucoma, such as prostaglandin analogs, β-adrenergic blocking agents, α-2 adrenergic agonists, and carbonic anhydrase inhibitors, target lowering of intraocular pressure by increasing drainage of aqueous humor through the uveo-scleral pathway. Elevated intraocular pressure is a risk factor for glaucoma, and likely a trigger for retinal dysfunction in these patients (Ruan et al., 2020).
Despite the focus on risk factors and disease-specific etiology, there are several pharmaceutical agents currently on the market that could act as a broad-spectrum neuroprotectant that target shared mechanisms of retinal dysfunction, including oxidative stress, inflammation, and cellular triggers of progressive cell loss for different retinal diseases (Wang et al., 2021; Pani et al., 2022).
Oxidative stress therapeutics
Oxidative stress is described as the results of several cellular events that are activated by the intracellular formation of reactive oxidative species. It is known that reactive oxidative species can play a dual role- beneficial and harmful in biological systems, especially depending on the reactive oxidative species concentration, their type, and localization in the cell.
The eyes, because their direct contact to exogenous factors recognized as contributing factors to oxidative damage, like non-ionizing and ionizing radiation (ultraviolet, X-ray, γ-rays, and α particles), high pressure of molecular oxygen, pollutants (industrial smoke, driving fumes, and other oxidizing chemicals), irritants (temperature, wind), and pathogenic microbes, are highly exposed to risk factors for the shift of the redox balance with the involvement of DNA damages, failure of signaling and gene expression and activation of different apoptotic signaling pathways. Therefore, oxidative stress contributes to a harmful pathological development (Wang et al., 2022).
Few compounds have been seen as efficient to limit oxidative stress damage. Docosahexaenoic acid is an antioxidant compound that maintains mitochondrial membrane integrity and selectively reduces photoreceptor apoptosis after oxidative damage (Lafuente et al., 2021). A study showed different potentially positive effects of citicoline (Hassanzadeh et al., 2022), vitamin B3, and CAVAQ10® (Coenzyme Q10) on neurons exposed to oxidative stress. These compounds together could limit neuronal cell damage or neural loss (Mastropasqua et al., 2022).
Ginkgo biloba has powerful antioxidant properties that have been shown to help with a variety of degenerative processes, including retinal diseases associated with neurodegeneration, and its neuroprotective effects are shown both in vitro and in vivo (Martínez-Solís et al., 2019).
Several compounds currently available on the market can simultaneously influence more than one shared mechanism of retinal dysfunction. Lutein is an anti-oxidation compound that affects the pathological pathways of inflammatory cytokines like interleukin-6 and angiotensin II signaling and prevents progressive neurodegeneration (Ahn and Kim, 2021). Similarly, valproic acid reduces neuroinflammation by inhibiting the action of histone deacetylases, which leads to transcriptional activation of anti-apoptotic and neuronal survival pathways in the retina (Tribble et al., 2022). Saffron also has both antioxidant and anti-inflammatory properties, suggesting that it could be used to treat eye diseases; moreover, its antioxidant activity may be useful in alleviating retinal dysfunction symptoms and slowing disease progression (Fernández-Albarral et al., 2020).
Inflammation therapeutics
The eyes are considered an immune-privileged site being relatively a closed anatomic system and having the privilege of the protection of the blood-retinal barrier. Blood-retinal barrier is both an inner barrier formed by a strong network of retinal vascular endothelial cells and an outer barrier formed by the retinal pigment epithelium cells.
However, any process which significantly disrupts retinal architecture can exert a profound impact on vision. The immune response can be controlled, so has an adaptive response to restore homeostasis, or can be altered as the response to secondary factors like aging, metabolic abnormalities, altered vascular perfusion, or degenerative genetic conditions may initiate various inflammatory cascades (Whitcup et al., 2013).
Inflammatory pathways impact vascular functions and result in progressive retinal neurodegeneration. Retinal dysfunction is likewise associated with changes in vascular integrity and function that can both arise from inflammation and promote inflammation. Intravitreal anti-vascular endothelial growth factor agents are the primary treatment for wet AMD and DR, where edema and neovascularization are common clinical phenotypes. Anti-vascular endothelial growth factor therapies, which have the effect to contrast the burden of pathology due to uncontrolled retinal neovascularization, are known to increase visual acuity, decrease retinal edema, and limit neovascularisation (Xu et al., 2022). Corticosteroids are anti-inflammatory compounds that are broadly used in ocular disease to locally suppress inflammation, reduce the proliferation of cells, and contrast neovascularization (Fung et al., 2020).
Neuroprotection therapeutics
Neurotrophic factors, which innately support neuron health and survival, are ideal targets for neuroprotection therapeutics. Targeting of neurotrophic pathways can be accomplished by using compounds, which stimulate neurothopic factor synthesis or by injecting neurotrophic factors. ACT-01, well known as OCS-05, is a first-in-class small molecule with a neuroprotective activity that prevents neuroinflammation and neurodegeneration of optic nerve and retina in animal models (currently in trial, phase 2a) (de Lima Serafim et al., 2018). Its action is linked to the activation of trophic factor pathways, such as insulin-like growth factor-1 and brain-derived neurotrophic factor (Boia et al., 2020). In in vivo studies, the administration of ciliary neurotrophic factor promotes neuronal survival of both photoreceptors and retinal ganglion cells through the activation of Janus kinase (Jak)-signal transducer and activator of transcription (STAT) and Ras-mitogen-activated protein kinase signaling cascades (Zhang et al., 2021). Based on cell type-specific assessments, Jak-STAT signaling appears to promote neuronal survival via both direct and indirect mechanisms. A ciliary neurotrophic factor-based therapy has been created and evaluated in several clinical trials (Chew et al., 2019). Early in vitro studies also indicate that the use of glial cell-derived neurotrophic factor may improve photoreceptor survival via transcriptional upregulation of basic fibroblast growth factor-2, suggesting potential utility for both trophic factors as therapeutic agents (Mallone et al., 2020; Lambuk et al., 2022).
Antioxidant therapeutics
Both current molecules present on-market and those in clinical trials indicate that neuroprotective activity could be a solution for preventing retinal dysfunction and resulting visual impairment. New classes of molecules that can affect common mechanisms of retinal dysfunction must be identified as attractive candidate therapies for use in the clinic.
Several innovative studies have lately emerged indicating that neuropeptides and their receptors could be viable targets for pharmacological research and for drug delivery. Here, we outline new and innovative pharmacological therapies that are now being tested (pre-clinical trial, phase I, II, or III) and may be promising for the treatment of retinal diseases.
As indicated in the diagram (Figure 1), there are a few current trials to test new molecules:
Figure 1.
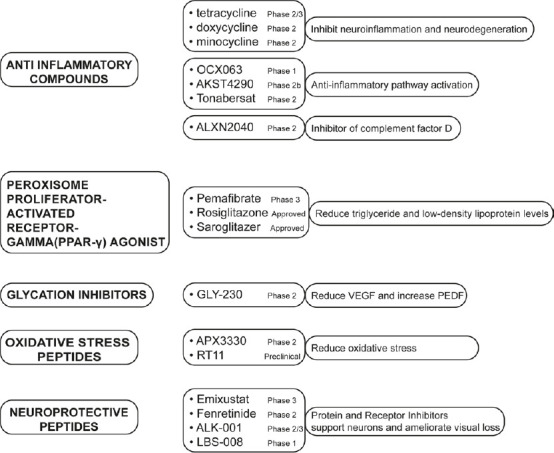
Schematic diagram of new and innovative pharmacological therapies on clinical trial.
List of promising compounds in clinical trial categorized by classes of drugs, clinical trial phase and mechanism of actions. AKST4290: Alkahest; ALK-001: Alkeus Pharmaceuticals; ALXN2040: Danicopan; APX3330: Ocuphire Pharma; GLY-230: Glycadia Pharmaceuticals; LBS-008: BeliteBio; OCX063: Occurx; PEDF: pigment epithelium-derived factor; RT11: Retrotope; VEGF: anti-vascular endothelial growth factor.
(a) Anti-inflammatory molecules
Current anti-inflammatory molecules with neuroprotective properties in AMD and DR include tetracycline, doxycycline, and minocycline that inhibit neuroinflammation and neurodegeneration in the central nervous system (Terauchi et al., 2021; DeBoer et al., 2022). Three additional peptides, OCX063 (Occurx) (Kong et al., 2020), AKST4290 (Alkahest) (currently in trial, phase 2b) (Samanta et al., 2020), and Tonabersat (Xiflam; Ocunexus) (Mat Nor et al., 2020), regulate anti-inflammatory pathway activation. Moreover, ALXN2040 (Danicopan; Alexion Pharmaceuticals) is an inhibitor of complement factor D and is implicated in the treatment of AMD (Boyer et al., 2022).
Peroxisome proliferator-activated receptor alpha (PPARα) agonists: A class of PPARα agonists, like Pemafibrate (Kowa Pharmaceuticals; phase III) (Fujita et al., 2021), Saroglitazar (Lipaglyn; Zydus Cadila; preclinical trial) (Joharapurkar et al., 2021), and Rosiglitazone (Avandia; GlaxoSmithKline) (Shen, 2008; Shen et al., 2008), developed for DR, inducing the reduction of triglyceride levels and low-density lipoprotein levels.
Glycation inhibitor: GLY-230 (glycadia pharmaceuticals) is a glycation inhibitor that could reduce the vascular endothelial growth factor and increase the pigment epithelium-derived factor, and it is in the trial (phase II) for DR (Kennedy et al., 2010).
(b) Oxidative stress targeting compounds
APX3330 (Ocuphire Pharma) is a molecule that targets apurinic/apyrimidinic endonuclease 1/redox effector factor-1 with the result to contrast oxidative stress (Heisel et al., 2021).
RT11 (Retrotope) is a synthetic molecule, docosahexaenoic acid, in preclinical phase development that can reduce oxidative stress in dry AMD and geographic atrophy (Zesiewicz et al., 2018).
(c) Neuroprotection peptides
Visual cycle modulators are inhibitors of some proteins and receptors that could support neurons and ameliorate visual loss. Examples of these compounds are Emixustat (Kubota Pharmaceutical) that inhibits the retinal pigment epithelium 65 protein. In animal models of Stargardt disease and retinal degeneration, emixustat was found to decrease the accumulation of A2E and to protect the retina from light-induced damage (Kubota et al., 2022). Fenretinide (Revision) is a synthetic drug with antagonism activity on serum retinol-binding protein to reduce the high level of vitamin A; it is now under clinical trial for the management of geographic atrophy (Miller et al., 2022). ALK-001 (Alkeus Pharmaceuticals) deuterated vitamin A that is under investigation to inhibit the formation of toxic vitamin A aggregates in Stargardt disease and AMD acting with a competitive mechanism in which ALK-001 is substituting vitamin A to slow the rate of formation of vitamin A dimmers (Hussain et al., 2018). LBS-008 (Belite Bio) is a small molecule with antagonist activity on retinol-binding protein 4. The final effect is to reduce the delivery of retinol to the eye reducing the accumulation of cytotoxic bisretinoids. This approach seems to preserve the integrity of retinal tissues, and ultimately slow or prevent vision loss (currently in trial, phase 3) (Kim and Priefer, 2021).
Future Pharmacological Research in Retinal Dysfunction
The percentage of the human population affected by Retinal dysfunctions has daily increased in the last decades and it raises some challenging work to meet the need of innovative approaches to investigate new biomarkers but also of the discovery of new therapeutics and delivery systems to have effective treatments for retinal diseases (Figure 2).
Figure 2.
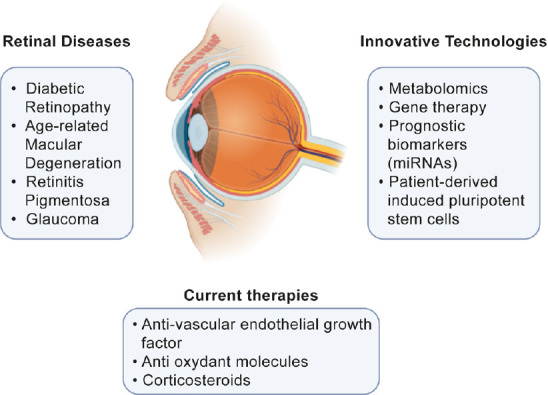
Summary diagram for eye diseases with current and innovative technologies.
The diagram summarizes several pathologies characterized by retinal degeneration and indicates what are the current therapies and potential innovative technologies of the near future.
Anti-vascular endothelial growth factor, anti-inflammatories compounds, and corticosteroids are the drug categories mainly present on the market for retinopathies. These therapies stabilize the disease and also improve vision loss, ameliorating patient’s life quality but on the other side show several disadvantages that limit their uses such as the difficult administration of treatment at the eye level and side effects due to their low safety. Several current clinical trials have been early interrupted due to extremely negative side effects.
This fact, although negative, it is of great stimulus for the research to better and finely characterize the molecular causes of specific retinal dysfunction in order to develop more efficient and safe treatment.
Indeed, it should be important to investigate new therapeutic approaches, such as the identification of new therapeutic targets, and pharmacological combinations of different drugs to improve the treatment efficacy. On the other side, also the identification of different technologies to detect retinal degradation and biomarkers to help the preliminary diagnosis of retinal diseases could be useful to select the most appropriate therapy but also to drive the new research in the right direction.
Metabolomic studies represent, for instance, a potent technology that might improve our understanding of pathogenic mechanisms and molecular processes, discover new pathways, and develop biomarkers for diagnosis and prognosis (Li et al., 2021).
Gene therapy potentially could be another innovative approach to correct genetic defects of retinal diseases. One of the advantages of this technology would be the treatment frequency, in fact, it could be studied as single administration without the requirement for repeated procedures. Additionally, the approach with a gene-specific vector could be more specific compared with local drug treatment (Sinclair et al., 2018).
A recent study showed a direct correlation between specific circulating miRNAs and intra-retinal hyper-reflective spots, indicating that these miRNAs could be validated as prognostic biomarkers as well as potential pharmacological targets (Wu et al., 2022).
Moreover, the use of patient-derived induced pluripotent stem cells could be a good approach to optimize the therapies before treating patients (Mustafi et al., 2022).
According to the new advanced neuroprotective therapies for the treatment of retinopathies, and the new technology under development, it is essential also to determine the subset of patients that could find benefit in the neuroprotective treatments.
Understanding how neuroprotective compounds work will help scientists to use these molecules in combination with other protective agents to benefit from additive or synergistic effects in treatment approaches.
In this review, the old and the new approaches for the treatment of retinopathies have been addressed. Nowadays, it’s needed the development of novel technologies/tools in the field of ophthalmology healthcare.
A synergistic strategy involving multiple interdisciplinary fields like nanotechnology, drug sustained delivery systems, biomechanical engineering, and biotechnology, together with better clinical patient stratification are required to drive future medicine research and application, also in the ophthalmic field, towards a precise medicine to better treat retinal dysfunction.
Footnotes
Funding: This work is partially supported by BrightFocus Glaucoma grant No. G2022015S (to MF).
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Ahn YJ, Kim H. Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants (Basel) 2021;13:10, 1448. doi: 10.3390/antiox10091448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, Santiago AR. Neuroprotective strategies for retinal ganglion cell degeneration:Current status and challenges ahead. Int J Mol Sci. 2020;21:2262. doi: 10.3390/ijms21072262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer DD, Ko YP, Podos SD, Cartwright ME, Gao X, Wiles JA, Huang M. Danicopan, an oral complement factor D inhibitor, exhibits high and sustained exposure in ocular tissues in preclinical studies. Transl Vis Sci Technol. 2022;11:37. doi: 10.1167/tvst.11.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lima Serafim V, Félix MB, Frade Silva DK, Rodrigues KADF, Andrade PN, de Almeida SMV, de Albuquerque dos Santos S, de Oliveira JF, de Lima MDCA, Mendonça-Junior FJB, Scotti MT, de Oliveira MR, de Moura RO. New thiophene–acridine compounds:synthesis, antileishmanial activity, DNA binding, chemometric, and molecular docking studies. ChemBiol Drug Des. 2018;91:1141–1155. doi: 10.1111/cbdd.13176. [DOI] [PubMed] [Google Scholar]
- 5.Chew EY, Clemons TE, Jaffe GJ, Johnson CA, Farsiu S, Lad EM, Guymer R, Rosenfeld P, Hubschman JP, Constable I, Wiley H, Singerman LJ, Gillies M, Comer G, Blodi B, Eliott D, Yan J, Bird A, Friedlander M. Macular Telangiectasia Type 2-Phase 2 CNTF Research Group (2019) Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2:a randomized clinical trial. Ophthalmology. 126:540–549. doi: 10.1016/j.ophtha.2018.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBoer C, Michalak S, Rahimy E. Oral drug pipeline for retinal disease. Retinal Physician. 2022;19:27–28. 30, 32. [Google Scholar]
- 7.Fernández-Albarral JA, de Hoz R, Ramírez AI, López-Cuenca I, Salobrar-García E, Pinazo-Durán MD, Ramírez JM, Salazar JJ. Beneficial effects of saffron (Crocus sativus L.). in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen Res. 2020;15:1408–1416. doi: 10.4103/1673-5374.274325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita N, Sase K, Tsukahara C, Arizono I, Takagi H, Kitaoka Y. Pemafibrate prevents retinal neuronal cell death in NMDA-induced excitotoxicity via inhibition of p-c-Jun expression. Mol Biol Rep. 2021;48:195–202. doi: 10.1007/s11033-020-06032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung AT, Tran T, Lim LL, Samarawickrama C, Arnold J, Gillies M, Catt C, Mitchell L, Symons A, Buttery R, Cottee L, Tumuluri K, Beaumont P. Local delivery of corticosteroids in clinical ophthalmology:a review. Clin Exp Ophthalmol. 2020;48:366–401. doi: 10.1111/ceo.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassanzadeh K, Vahabzadeh Z, Bucarello L, Dragotto J, Corbo M, Maccarone R, Feligioni M. Protective effect of curcuma extract in an ex vivo model of retinal degeneration via antioxidant activity and targeting the SUMOylation. Oxid Med Cell Longev. 20222022:8923615. doi: 10.1155/2022/8923615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heisel C, Jonah Yousif J, Mijiti M, Charizanis K, Brigell M, Corson TW, Kelley MR. APE1/Ref-1 as a novel target for retinal diseases. J Cell Signal. 2021;2:133–138. doi: 10.33696/Signaling.2.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain RM, Gregori NZ, Ciulla TA, Lam BL. Pharmacotherapy of retinal disease with visual cycle modulators. Expert Opin Pharmacother. 2018;19:471–481. doi: 10.1080/14656566.2018.1448060. [DOI] [PubMed] [Google Scholar]
- 13.Joharapurkar A, Patel V, Kshirsagar S, Patel MS, Savsani H, Jain M. Effect of dual PPAR-α/γagonist saroglitazar on diabetic retinopathy and oxygen-induced retinopathy. Eur J Pharmacol. 2021;899:174032. doi: 10.1016/j.ejphar.2021.174032. [DOI] [PubMed] [Google Scholar]
- 14.Kaur G, Singh NK. The role of inflammation in retinal neurodegeneration and degenerative diseases. Int J Mol Sci. 2022;23:386. doi: 10.3390/ijms23010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy L, Pilar Solano M, Meneghini L, Lo M, Cohen MP. Anti-glycation and anti-albuminuric effects of GLY-230 in human diabetes. Am J Nephrol. 2010;31:110–116. doi: 10.1159/000259897. [DOI] [PubMed] [Google Scholar]
- 16.Kim N, Priefer R. Retinol binding protein 4 antagonists and protein synthesis inhibitors:potential for therapeutic development. Eur J Med Chem. 2021;226:113856. doi: 10.1016/j.ejmech.2021.113856. [DOI] [PubMed] [Google Scholar]
- 17.Kong RCK, Chan E, Cox AJ, Glowacka S, Khong FL, Papadimitriou MP, Kelly D. OCX063, a novel small molecule drug, is protective against inflammatory, fibrotic and angiogenic phenotypes in retinal cultures. Invest Ophthalmol Vis Sci. 2020;61:4281. [Google Scholar]
- 18.Kubota R, Birch DG, Gregory JK, Koester JM. Randomised study evaluating the pharmacodynamics of emixustat hydrochloride in subjects with macular atrophy secondary to Stargardt disease. Br J Ophthalmol. 2022;106:403–408. doi: 10.1136/bjophthalmol-2020-317712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafuente M, González-Herrero MER, Villadóniga SR, Domingo JC. Antioxidant activity and neuroprotective role of docosahexaenoic acid (Dha) supplementation in eye diseases that can lead to blindness:a narrative review. Antioxidants. 2021;10:1–13. doi: 10.3390/antiox10030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambuk L, Mohd Lazaldin MA, Ahmad S, Iezhitsa I, Agarwal R, Uskoković V, Mohamud R. Brain-derived neurotrophic factor-mediated neuroprotection in glaucoma:a review of current state of the art. Front Pharmacol. 2022;13:875662. doi: 10.3389/fphar.2022.875662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Cai S, He Z, Reilly J, Zeng Z, Strang N, Shu X. Metabolomics in retinal diseases:an update. Biology (Basel) 2021;10:944. doi: 10.3390/biology10100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallone F, Sacchetti M, Bruscolini A, Scuderi L, Marenco M, Lambiase A. Neurotrophic factors in glaucoma and innovative delivery systems. Applied Sciences (Switzerland) 2020;10:1–22. [Google Scholar]
- 23.Marchesi N, Fahmideh F, Boschi F, Pascale A, Barbieri A. Ocular neurodegenerative diseases:Interconnection between retina and cortical areas. Cells. 2021;10:2394. doi: 10.3390/cells10092394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Solís I, Acero N, Bosch-Morell F, Castillo E, González-Rosende ME, Muñoz-Mingarro D, Ortega T, Sanahuja MA, Villagrasa V. Neuroprotective potential of ginkgo biloba in retinal diseases. Planta Med. 2019;85:1292–1303. doi: 10.1055/a-0947-5712. [DOI] [PubMed] [Google Scholar]
- 25.Mastropasqua L, Agnifili L, Ferrante C, Sacchi M, Figus M, Rossi GCM, Brescia L, Aloia R, Orlando G. Citicoline/coenzyme Q10/vitamin B3 fixed combination exerts synergistic protective effects on neuronal cells exposed to oxidative stress. Nutrients. 2022;14:2963. doi: 10.3390/nu14142963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mat Nor MN, Rupenthal ID, Green CR, Acosta ML. Connexin hemichannel block using orally delivered tonabersat improves outcomes in animal models of retinal disease. Neurotherapeutics. 2020;17:371–387. doi: 10.1007/s13311-019-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micera A, Balzamino BO, di Zazzo A, Dinice L, Bonini S, Coassin M. Biomarkers of neurodegeneration and precision therapy in retinal disease. Front Pharmacol. 2021;11:601647. doi: 10.3389/fphar.2020.601647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller AP, Black M, Amengual J. Fenretinide inhibits vitamin A formation from β-carotene and regulates carotenoid levels in mice. Biochim Biophys Acta Mol Cell Biol Lipids. 20221867:159070. doi: 10.1016/j.bbalip.2021.159070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustafi D, Bharathan SP, Calderon R, Nagiel A. Human cellular models for retinal disease:from induced pluripotent stem cells to organoids. Retina. 2022;42:1829–1835. doi: 10.1097/IAE.0000000000003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pani L, Cicchetti A, de Luca A, Mennini FS, Mini E, Nocentini G, Racagni G, Jommi C. Pricing for multi indication medicines:a discussion with italian experts. Pharmadvances. 2022 doi:10.36118/pharmadvances.2022.27. [Google Scholar]
- 31.Ruan Y, Jiang S, Musayeva A, Gericke A. Oxidative stress and vascular dysfunction in the retina:therapeutic strategies. Antioxidants. 2020;9:1–30. doi: 10.3390/antiox9080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samanta A, Aziz AA, Jhingan M, Singh SR, Khanani AM, Chhablani J. Emerging therapies in neovascular age-related macular degeneration in 2020. Asia Pac J Ophthalmol (Phila) 2020;9:250–259. doi: 10.1097/APO.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen LQ. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126:793. doi: 10.1001/archopht.126.6.793. [DOI] [PubMed] [Google Scholar]
- 34.Shen LQ, Child A, Weber GM, Folkman J, Aiello LP. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126:793–799. doi: 10.1001/archopht.126.6.793. [DOI] [PubMed] [Google Scholar]
- 35.Shughoury A, Sevgi DD, Ciulla TA. Molecular genetic mechanisms in age-related macular degeneration. Genes (Basel) 2022;13:1233. doi: 10.3390/genes13071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinclair A, Islam S, Jones S. CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018. Gene therapy:an overview of approved and pipeline technologies. CADTH Issues in Emerging Health Technologies. [PubMed] [Google Scholar]
- 37.Terauchi R, Kohno H, Watanabe S, Saito S, Watanabe A, Nakano T. Minocycline decreases CCR2-positive monocytes in the retina and ameliorates photoreceptor degeneration in a mouse model of retinitis pigmentosa. PLoS One. 2021;16:e0239108. doi: 10.1371/journal.pone.0239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tribble JR, Kastanaki E, Uslular AB, Rutigliani C, Enz TJ, Williams PA. Valproic acid reduces neuroinflammation to provide retinal ganglion cell neuroprotection in the retina axotomy model. Front Cell Dev Biol. 2022;10:903436. doi: 10.3389/fcell.2022.903436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Li M, Geng Z, Khattak S, Ji X, Wu D, Dang Y. Role of oxidative stress in retinal disease and the early intervention strategies:a review. Oxid Med Cell Longev. 20222022:7836828. doi: 10.1155/2022/7836828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Cao L, Jiang Q, Zhang T. Topical medication therapy for glaucoma and ocular hypertension. Front Pharmacol. 2021;12:749858. doi: 10.3389/fphar.2021.749858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitcup SM, Nussenblatt RB, Lightman SL, Hollander DA. Inflammation in retinal disease. Int J Inflam. 20132013:724648. doi: 10.1155/2013/724648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu HL, Shao Y, Chen ZN, Zhang H, Zhang XM, Li XR. miRNA-451 regulates rhesus choroid-retinal endothelial cell function and proteome profile. Int J Ophthalmol. 2022;15:894–904. doi: 10.18240/ijo.2022.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, Fan R, Fan X, Shao Y, Li X. Progress and challenges of anti-VEGF agents and their sustained-release strategies for retinal angiogenesis. Drug Des Devel Ther. 2022;16:3241–3262. doi: 10.2147/DDDT.S383101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zesiewicz T, Heerinckx F, de Jager R, Omidvar O, Kilpatrick M, Shaw J, Shchepinov MS. Randomized, clinical trial of RT001:Early signals of efficacy in Friedreich's ataxia. Mov Disord. 2018;33:1000–1005. doi: 10.1002/mds.27353. [DOI] [PubMed] [Google Scholar]
- 45.Zhang KY, Aguzzi EA, Johnson TV. Retinal ganglion cell transplantation:approaches for overcoming challenges to functional integration. Cells. 2021;10:1426. doi: 10.3390/cells10061426. [DOI] [PMC free article] [PubMed] [Google Scholar]


