Abstract
This critical review of the literature shows that there is a close link between the microbiome, the gut, and the brain in Parkinson’s disease. The vagus nerve, the main component of the parasympathetic nervous system, is involved in the regulation of immune response, digestion, heart rate, and control of mood. It can detect microbiota metabolites through its afferents, transferring this gut information to the central nervous system. Preclinical and clinical studies have shown the important role played by the gut microbiome and gut-related factors in disease development and progression, as well as treatment responses. These findings suggest that the gut microbiome may be a valuable target for new therapeutic strategies for Parkinson’s disease. More studies are needed to better understand the underlying biology and how this axis can be modulated for the patient’s benefit.
Key Words: alpha-synuclein, enteric microbiota, gastrointestinal tract, Parkinson’s disease, vagus nerve
Introduction
Parkinson’s disease (PD) is a chronic, progressive disorder characterized by the degeneration of midbrain dopaminergic neurons, leading to widespread alpha-synuclein (α-syn) accumulation. The α-syn is a soluble protein found in physiologic conditions in the brain and it is involved in neurotransmission, participating in synaptic vesicle exocytosis, regulation of synaptic plasticity, and maintenance of the synaptic cytoskeleton (Mavroeidi and Xilouri, 2021). However, misfolded α-syn forms aggregates and deposits typically in the substantia nigra and related neurocircuits, in the form of Lewy bodies and Lewy neurites, leading to neuronal loss and resulting in motor symptoms such as bradykinesia, resting tremor, rigidity, and postural instability (Warnecke et al., 2022). Additionally, α-syn aggregates have been found in the enteric nervous system of PD patients contributing to the development of non-motor symptoms notably gastrointestinal issues (Pfeiffer, 2018; Bloem et al., 2021; Klann et al., 2022).
The microbiome-intestine-brain axis is a two-way communication pathway between the gut microbiome, the gastrointestinal tract, and the peripheral and central nervous systems (CNS) (Mörkl et al., 2020). The means they interact include the vagus nerve, the endocrine, and the immune systems (Arneth, 2018). It has been acknowledged that the gut microbiota affects the production of neurotrophic factors, modulates inflammation, and the production of pro-inflammatory cytokines, T cells, and B cells, therefore playing a role in the myelination process, and microglial activation (Lobo et al., 2023). For this reason, it might influence behavior and cognition and is becoming increasingly linked to the risk and advancement of diseases related to the nervous system and mental health (Cryan et al., 2020; Fang et al., 2020).
The non-motor symptoms, in particular the gastrointestinal ones, have been found to come before the movement symptoms that support the diagnosis of the disease, implying the involvement of the microbiome-intestine-brain axis in the mechanisms that lead to PD (Chao et al., 2020). Studies have indicated that synucleinopathy may begin in enteric tissue and reach the brain through innervating autonomic fibers (Challis et al., 2020).
The communication between gut-brain during the development of PD is believed to be largely impacted by the changes in the activity of bacterial metabolites and the disruption of microbial balance (dysbiosis) in the gut (Tan et al., 2021; Willyard, 2021). Recently, increasing evidence suggests a significant role for gut-related processes in PD. Here, we provide a critical evaluation of the evidence from clinical and preclinical studies that implicate gut-related processes in the development, progression, and treatment of PD.
Search Strategy and Selection Criteria
A narrative literature review was conducted according to a systematic review method based on PubMed (Medline) Database. The English-language-based studies were retrieved using the following medical subject headings (MeSH): “Parkinson’s disease” AND “gut-brain axis” “gastrointestinal dysfunction” AND “gut microbiota”. The authors undertook a systematic search of PubMed/Medline peer-reviewed studies (impact factor greater than or equal to two) published in the last 10 years (2015 to 2023). Only studies that exclusively evaluated the role of the gut-brain axis in PD with a focus on the gut microbiota and spreading of misfolded alpha-synuclein were included in this review. Review studies, comments, perspectives, editorials, or other research that did not provide original or unpublished results were excluded.
How the Microbiome-Gut-Brain Axis Influences Parkinson’s Disease
The gut harbors a high diversity of microorganisms, encompassing beneficial and pathogenic species, which interact with the enteric nervous system (Boyajian et al., 2021). It is the commensal bacterial richness and diversity that favors physiological processes and contributes to overall host health (Belizário et al., 2018).
The gut microbiota is thought to facilitate human metabolism through its enzymatic activity and metabolic pathways, assisting in digestion, synthesis of vitamins and other nutrients, and elimination of toxic substances (Belizário et al., 2018). It can also help to maintain the integrity of the intestinal barrier, inhibit pathogens, and support the metabolism of drugs and toxins (Fan and Pedersen, 2021). Moreover, the gut microbiome can regulate the host immune response by producing metabolites, such as short-chain fatty acids, which modulate the activity of the immune system, including microglia, either suppressing or stimulating its response (Erny et al., 2015).
An imbalance in the gut microbial flora, characterized by a disruption of beneficial and harmful bacteria as well as a decrease in bacterial diversity, can result in dysbiosis and subsequent illness (Wallen et al., 2022). Dysbiosis can lead to a disruption of tight junction proteins, increasing the permeability of the intestinal wall, as well as an overproduction of pro-inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6. Furthermore, bacterial endotoxins, such as lipopolysaccharide, can activate Toll-like receptors and trigger inflammatory responses (Yang et al., 2022). Additionally, it has been linked to impaired nutrition and immune responses (Srivastava et al., 2021).
It is noteworthy that dysbiosis may also influence the onset and progression of several diseases, such as diabetes, inflammatory bowel disease, autism, and PD through evidence from a variety of studies in both human and animal models (Fan and Pedersen, 2021; Fritsch et al., 2021; Tan et al., 2021; Wang et al., 2021). Recent research has highlighted changes in the gut microbiota in various animal models of PD, including neurotoxin models, such as that using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administered intraperitoneally (Pu et al., 2019; Xie et al., 2020) or subcutaneously (Lai et al., 2018; Zhu et al., 2020); rotenone administered orally (Bhattarai et al., 2021) or by intraperitoneal injection (Johnson et al., 2018). Those models have been demonstrated to produce changes in gastrointestinal tract physiology, including disruption, local neuronal loss, and classical motor symptoms, as well as dopaminergic cell death in sick rodents compared to the control group (Perez-Pardo et al., 2018; Xie et al., 2020). In several of them, dysbiosis and gastrointestinal symptoms have been observed prior to the onset of motor features (Lai et al., 2018; Ghaisas et al., 2019; Baizabal-Carvallo and Alonso-Juarez, 2020).
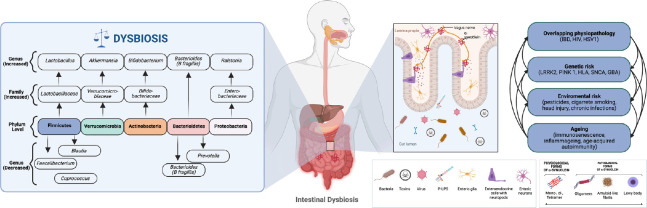
In a meta-analysis conducted by Gerhardt and colleagues (Gerhardt and Mohajeri, 2018) that included 642 PD patients and 531 healthy controls, it was found that alterations in the colonic composition were associated with PD and other neurodegenerative diseases (Figure 1). A number of studies have reported consistently higher abundances of phyla Actinobacteria and Verrucomicrobia, and lower abundances of phyla Firmicutes and Bacteroides, as being of importance for PD (Scheperjans et al., 2015; Bedarf et al., 2017; Petrov et al., 2017; Gerhardt and Mohajeri, 2018). Despite large individual differences in microbiota composition, a microbiota with a stable composition consisting of high levels of Bifidobacteria and Bacteroidides, and low levels of Firmicutes and Proteobacteria, is typically associated with low levels of lipopolysaccharide and is considered to be indicative of a healthy gut epithelium. However, the microbiota of PD patients often differs from this, with similarities to that found in patients with inflammatory bowel disease (Baizabal-Carvallo and Alonso-Juarez 2020).
Figure 1.
Major changes observed in the gut microbiota in patients with Parkinson’s disease (Adapted from Gerhardt and Mohajeri, 2018).
Several processes may predispose to alpha-synuclein misfolding: environmental influences, interactions between the epithelial-mucosa, neuroglia, immune interactions, immunosenescence, and genetic predisposition. Environmental and genetic factors interact in a bidirectional manner, although the exact role of each remains unclear as they tend to overlap and progress in molecular “loops” (adapted from Claudino dos Santos et al., 2023). GBA: Beta-glucocerebrosidase producing gene; HIV: human immunodeficiency; HLA: human leukocyte antigen; HSV1: human herpes virus; IBD: intestinal bowel disease; LRKK2: leucine-rich repeat kinase 2; PINK-1: PTEN- induced kinase 1; SNCA: α-synuclein producing gene. Created using BioRender.com.
Patients with REM sleep behavior disorder or depression, who are at risk of developing PD, have been observed to have a dysbiotic gut microbiota, similar to that seen in PD patients (Heintz-Buschart et al., 2018; Eicher and Mohajeri, 2022; Mitrea et al., 2022). Furthermore, individuals with diabetes, which is also a risk factor for PD, have been found to have an altered gut microbiome relative to healthy individuals (Manos, 2022). This implies a potential association between changes in the intestinal microbiota and the emergence of motor signs in PD. However, the association between the gut microbiota composition and motor symptoms in PD remains to be further explored, and there is currently no conclusive evidence (Tetz et al., 2018; Chiang and Lin, 2019). Recent studies indicate a relationship between PD and a decrease in the diversity and richness of gut bacteria (Tetz et al., 2018). Moreover, a preliminary study suggests that the worsening of motor and non-motor symptoms may be associated with a rapid decrease in gut microbiota variability (Minato et al., 2017).
The Roles of Vagus Nerve in Parkinson’s Disease
The vagus nerve, as part of the parasympathetic nervous system, serves several physiologic functions, including regulating immune responses, digestive processes, heart rate, and more recently, mood control (Breit et al., 2018). It can detect microbiota metabolites via its afferents, transferring this gut information to the CNS. A cholinergic anti-inflammatory pathway has been identified through the vagus nerve, which is capable of reducing peripheral inflammation and decreasing intestinal permeability, potentially altering the composition of the microbiota (Bonaz et al., 2018).
The human body hosts a large and varied microbiota in the gut, and the vagus nerve is its most extensive and intricate nerve; both are essential for maintaining homeostasis (Han et al., 2022). The Braak hypothesis proposes that α-syn pathology could be propagated from the gastrointestinal tract to the brain via the vagus nerve. This idea is corroborated by a mouse model of gut-to-brain α-syn transmission, wherein preformed fibrillar forms of α-syn were injected into the duodenal and pyloric muscularis. Additionally, clinical studies conducted in vivo have provided evidence to support further the Braak hypothesis, expanding its implications beyond idiopathic PD (Kim et al., 2019; Donlon et al., 2021).
Nevertheless, this hypothesis still lacks corroborative evidence. Autopsy studies have suggested the absence of pathologic forms of α-syn in the stomach or other GI locations without it being present in the brain. Furthermore, these studies have not focused on the proposed gut-to-brain conduit itself, the vagus nerve, but on the GI tract. These authors postulate that pathology appears in the CNS first, with a subsequent spread to the PNS at a premotor stage (Beach et al., 2021). Borghammer et al. (2022) propose that there is more than one prodromal subtype of α-synucleinopathy, including a clinical subtype that tends to manifest parkinsonism prior to REM sleep behavior disorder and constipation.
Alpha-synuclein is a vital protein for synaptic plasticity, promoting synaptic-vesicle fusion and trafficking on cellular pathways. It is primarily a monomer located in the cell cytosol that, under pathological conditions, forms neurotoxic aggregates (Riederer et al., 2019; Schaeffer et al., 2020). Abnormal α-syn inclusions are seen in peripheral organs such as the GI tract, the heart, and the adrenal gland up to two decades before the appearance of motor signs (van den Berge et al., 2021). Consequently, non-motor symptoms have been linked to the preclinical stage of PD, including fatigue, depression, sleep disturbances, olfactory dysfunction, and cognitive deficits (Zesiewicz, 2019; Hustad and Aasly, 2020).
It has been postulated that misfolded alpha-synuclein can propagate in a prior-like manner through the enteric nervous system and the autonomic nervous system to the CNS (Braak et al., 2003). In rodent studies, the spread of preformed α-syn fibrils injected into gastric muscle layers to the brain was prevented by truncal vagotomy (Jo et al., 2022). Supporting this hypothesis, vagotomy has been associated with a diminished risk of developing PD (Svensson et al., 2015). Recent in vivo imaging research has shown that patients in the prodromal phase of PD exhibit initial damage to the peripheral autonomic nerves, before propagating rostrally toward the brainstem (Knudsen et al., 2018). Accordingly, new studies have linked inflammatory bowel diseases and PD supporting the idea that gastrointestinal inflammation may play a role in the development of PD (Villumsen et al., 2019; Rolli-Derkinderen et al., 2020).
Alpha-synuclein aggregates accumulating in the pars compacta of the substantia nigra are seen to accompany the emergence of cardinal motor symptoms in PD, and thus, these protein aggregates are thought to play a key role in the pathophysiology of PD. However, the disease also manifests several non-motor symptoms, one of the most frequent being gastrointestinal symptoms experienced by around 80% of PD patients showing evidence of further alpha-synuclein pathology in the GI tract (Schaeffer et al., 2020; Terenzi et al., 2022). Pre-motor symptoms such as constipation not only play an important role in the patient’s quality of life, but also compromise the treatment and prognosis of the disease, hindering medication absorption.
It is of great importance to be able to identify α-syn aggregates in the gastrointestinal tract and vagus nerve, such as through biopsies, in order to further elucidate the pathophysiology of PD, distinguish subgroups of patients, and propose more precise and early treatments. For this, it is necessary to gain a more extensive understanding of the physiological and pathological forms of α-syn, to devise reliable methods to identify them, including its aggregation pathways and predisposing environmental and individual factors (Schaeffer et al., 2020) .
Therapeutic Strategies in Parkinson’s Disease with a Focus on the Gut-Brain Axis
The absence of modifying therapeutic approaches for PD is likely due to its multifaceted etiology that has yet to be fully elucidated (Zhu et al., 2022). Existing PD interventions are mainly centered on symptom relief, such as levodopa, which acts by replenishing dopamine, yet may result in a variety of side effects ranging from nausea, vomiting, constipation, headache, and somnolence to hallucinations, agitation, restlessness, and involuntary movements (Cenci et al., 2022). Considering that over 80% of those diagnosed with PD have a 10-year prognosis of death or severe disability restrictions, it is essential to find curative treatments that surpass symptom management. Currently, there is an urgent need for research into ways to stop, prevent, or reduce the progression of PD (Zhu et al., 2022).
Impairments caused by dysbiosis, such as increased intestinal permeability, altered immune responses, and inflammation, are theorized to play an important role in the development of PD pathology. The interplay between the microbiota, the intestine, and the brain highlights the importance of microbiome modulation in future therapeutic approaches (Metta et al., 2022; Tansey et al., 2022).
For this reason, modulating the gut microbiota has been proposed by the current research as a novel therapeutic strategy, enlightened by new findings from studies in animal models and human subjects about the pathogenesis of neurodegenerative disorders. There is growing interest in the potential role of changes in gut microbiota composition in the onset of alpha-synuclein pathology in PD (Keshavarzian et al., 2020). Various approaches such as simple dietary modifications, use of probiotics, as well as psychobiotics, prebiotics, synbiotics, and postbiotics, consumption of Chinese herbs, and even fecal microbiota transplantation have been proposed as an effort to counteract the negative effects of dysbiosis and slow the progression of PD (Zhu et al., 2022).
The consumption of probiotics containing Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus fermentum, and Lactobacillus reuteri has been proposed to improve some symptoms in PD patients (Tamtaji et al., 2019). A study conducted by Ibrahim et al. (2020) demonstrated that, following 8 weeks of probiotic administration, PD patients with constipation experienced an improved bowel transit time and an increased frequency of bowel opening. This finding was corroborated by Tan et al. (2021), who showed that spontaneous bowel movements escalated following probiotic treatment. Moreover, Alipour et al. (2020) discussed a probiotic cocktail composed of Bifidobacterium bifidum, Lactobacillus fermentum, Lactobacillus acidophilus, and Lactobacillus reuteri, which was found to enhance apomorphine-induced rotational behavior and spatial memory in a 6-hydroxydopamine-induced animal model PD (Alipour Nosrani et al., 2021).
Prebiotics, mainly non-digestible fibers, have been employed to reduce the severity of gastrointestinal dysfunction and allergic disorders, among others (Manzoor et al., 2022). A recent study showed that supplementation of a fiber-rich diet attenuates motor deficits and reduces alpha-synuclein aggregation in the substantia nigra of mice. At the same time, the gut microbiome of PD mice reshapes to a healthier profile after prebiotic treatment, which also reduces microglial activation and, consequently, neuroinflammation (Abdel-Haq et al., 2022).
The progression of PD has been suggested to be associated with gut dysbiosis, and restoration of the gut microbiota has been proposed as a potentially effective therapeutic option. Restoring the gut microbiota might be addressed by fecal microbiota transplantation that is the transplantation of gut microbiota from healthy donors into the intestines of PD patients (Chen et al., 2021). Given the potential connection between changes in gut microbiota composition and different clinical phenotypes of PD, interventions designed to alter the gut microbiota, like probiotics and fecal microbiota transplantation, have the potential to recover gut dysbiosis, reduce inflammation, and may modify the clinical phenotype of PD (Metta et al., 2022).
Conclusion
The gut microbiota has been demonstrated to be a pivotal contributor to the promotion of health. Emerging data has indicated that, up to 20 years before the onset of motor symptoms, an alteration in the gut microbiome may be present in PD patients. This dysbiosis of the gut may lead to increased intestinal permeability and inflammation, as well as Lewis body formation, and can also cause neuroinflammation and decreased neurotransmitter production in the central nervous system. Results from animal studies and investigations of human subjects with PD suggest that an imbalance of the gut microbiota may exacerbate the pathology of PD, whereas restorative strategies, such as dietary alterations, probiotic supplementation, and fecal microbiota transplantation, may impede or prevent the progression of PD. Nevertheless, the relationships between the gut microbiome and PD are still unclear, prompting further efforts to gain a more comprehensive understanding of the mechanisms discussed here and to explore their clinical implications.
Footnotes
Funding: This work was supported by Medical School of the Christus University Center (UNICHRISTUS), Federal University of Ceará (UFC), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2021/06473-4) (to JCCS).
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Abdel-Haq R, Schlachetzki JCM, Boktor JC, Cantu-Jungles TM, Thron T, Zhang M, Bostick JW, Khazaei T, Chilakala S, Morais LH, Humphrey G, Keshavarzian A, Katz JE, Thomson M, Knight R, Gradinaru V, Hamaker BR, Glass CK, Mazmanian SK. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. Elife. 2022;11:e81453. doi: 10.7554/eLife.81453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alipour Nosrani E, Tamtaji OR, Alibolandi Z, Sarkar P, Ghazanfari M, Azami Tameh A, Taghizadeh M, Banikazemi Z, Hadavi R, Naderi Taheri M. Neuroprotective effects of probiotics bacteria on animal model of Parkinson's disease induced by 6-hydroxydopamine:A behavioral, biochemical, and histological study. J Immunoassay Immunochem. 2021;42:106–120. doi: 10.1080/15321819.2020.1833917. [DOI] [PubMed] [Google Scholar]
- 3.Arneth BM. Gut–brain axis biochemical signalling from the gastrointestinal tract to the central nervous system:gut dysbiosis and altered brain function. Postgrad Med J. 2018;94:446–452. doi: 10.1136/postgradmedj-2017-135424. [DOI] [PubMed] [Google Scholar]
- 4.Baizabal-Carvallo JF, Alonso-Juarez M. The link between gut dysbiosis and neuroinflammation in Parkinson's disease. Neuroscience. 2020;432:160–173. doi: 10.1016/j.neuroscience.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Beach TG, Adler CH, Sue LI, Shill HA, Driver-Dunckley E, Mehta SH, Intorcia AJ, Glass MJ, Walker JE, Arce R, Nelson CM, Serrano GE. Vagus nerve and stomach synucleinopathy in Parkinson's disease, incidental Lewy body disease, and normal elderly subjects:evidence against the “Body-First”Hypothesis. J Parkinsons Dis. 2021;11:1833–1843. doi: 10.3233/JPD-212733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med. 2017;9:39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belizário JE, Faintuch J, Garay-Malpartida M. Gut microbiome dysbiosis and immunometabolism:new frontiers for treatment of metabolic diseases. Mediators Inflamm. 2018;2018:1–12. doi: 10.1155/2018/2037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattarai Y, Si J, Pu M, Ross OA, McLean PJ, Till L, Moor W, Grover M, Kandimalla KK, Margolis KG, Farrugia G, Kashyap PC. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson's disease. Gut Microbes. 2021;13:1866974. doi: 10.1080/19490976.2020.1866974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 10.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghammer P, Just MK, Horsager J, Skjærbæk C, Raunio A, Kok EH, Savola S, Murayama S, Saito Y, Myllykangas L, van den Berge N. A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson's disease. NPJ Parkinsons Dis. 2022;8:166. doi: 10.1038/s41531-022-00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyajian JL, Ghebretatios M, Schaly S, Islam P, Prakash S. Microbiome and human aging:probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients. 2021;13:4550. doi: 10.3390/nu13124550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 14.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cenci MA, Skovgård K, Odin P. Non-dopaminergic approaches to the treatment of motor complications in Parkinson's disease. Neuropharmacology. 2022;210:109027. doi: 10.1016/j.neuropharm.2022.109027. [DOI] [PubMed] [Google Scholar]
- 16.Challis C, Hori A, Sampson TR, Yoo BB, Challis RC, Hamilton AM, Mazmanian SK, Volpicelli-Daley LA, Gradinaru V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci. 2020;23:327–336. doi: 10.1038/s41593-020-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao YX, Gulam MY, Chia NSJ, Feng L, Rotzschke O, Tan EK. Gut-brain axis:potential factors involved in the pathogenesis of Parkinson's disease. Front Neurol. 2020;11:625446. doi: 10.3389/fneur.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen PC, Yu CC, Chen YS, Lu CH, Chan SH, Chou KH, Chen MH, Chen MH, Lin WC. The potential effects of oxidative stress-related plasma abnormal protein aggregate levels on brain volume and its neuropsychiatric consequences in Parkinson's disease. Oxid Med Cell Longev. 2021;2021:1–10. doi: 10.1155/2021/3666327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang HL, Lin CH. Altered gut microbiome and intestinal pathology in Parkinson's disease. J Mov Disord. 2019;12:67–83. doi: 10.14802/jmd.18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claudino Dos Santos JC, Lima MPP, Brito GAC, Viana GSB. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res Rev. 2023;84:101812. doi: 10.1016/j.arr.2022.101812. [DOI] [PubMed] [Google Scholar]
- 21.Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 22.Donlon E, Lynch R, Murphy OC, Farrell M, Noel J, Keogan M, O'Connell M, Lynch T. Braak's unfinished hypothesis:a clinicopathological case report of α-synuclein peripheral neuropathy preceding parkinsonism by 20 years. Mov Disord Clin Pract. 2021;8:1129–1133. doi: 10.1002/mdc3.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eicher TP, Mohajeri MH. Overlapping mechanisms of action of brain-active bacteria and bacterial metabolites in the pathogenesis of common brain diseases. Nutrients. 2022;14:2661. doi: 10.3390/nu14132661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erny D, Hraběde Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 26.Fang P, Kazmi SA, Jameson KG, Hsiao EY. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe. 2020;28:201–222. doi: 10.1016/j.chom.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritsch J, Garces L, Quintero MA, Pignac-Kobinger J, Santander AM, Fernández I, Ban YJ, Kwon D, Phillips MC, Knight K, Mao Q, Santaolalla R, Chen XS, Maruthamuthu M, Solis N, Damas OM, Kerman DH, Deshpande AR, Lewis JE, Chen C, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19:1189–1199. doi: 10.1016/j.cgh.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Gerhardt S, Mohajeri M. Changes of colonic bacterial composition in Parkinson's disease and other neurodegenerative diseases. Nutrients. 2018;10:708. doi: 10.3390/nu10060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaisas S, Langley MR, Palanisamy BN, Dutta S, Narayanaswamy K, Plummer PJ, Sarkar S, Ay M, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson's disease. Neurotoxicology. 2019;75:186–199. doi: 10.1016/j.neuro.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Y, Wang B, Gao H, He C, Hua R, Liang C, Zhang S, Wang Y, Xin S, Xu J. Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J Inflamm Res. 2022;15:6213–6230. doi: 10.2147/JIR.S384949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hustad E, Aasly JO. Clinical and imaging markers of prodromal Parkinson's disease. Front Neurol. 2020;11:395. doi: 10.3389/fneur.2020.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim A, Ali RAR, Manaf MRA, Ahmad N, Tajurruddin FW, Qin WZ, Desa SHM, Ibrahim NM. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson's disease:a randomised controlled trial. PLoS One. 2020;15:e0244680. doi: 10.1371/journal.pone.0244680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jo S, Kang W, Hwang YS, Lee SH, Park KW, Kim MS, Lee H, Yoon HJ, Park YK, Chalita M, Lee JH, Sung H, Lee JY, Bae JW, Chung SJ. Oral and gut dysbiosis leads to functional alterations in Parkinson's disease. NPJ Parkinsons Dis. 2022;8:87. doi: 10.1038/s41531-022-00351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson ME, Stringer A, Bobrovskaya L. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson's disease. Neurotoxicology. 2018;65:174–185. doi: 10.1016/j.neuro.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Keshavarzian A, Engen P, Bonvegna S, Cilia R. The gut microbiome in Parkinson's disease:a culprit or a bystander? Prog Brain Res. 2020;252:357–450. doi: 10.1016/bs.pbr.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klann EM, Dissanayake U, Gurrala A, Farrer M, Shukla AW, Ramirez-Zamora A, Mai V, Vedam-Mai V. The gut–brain axis and its relation to Parkinson's disease:a review. Front Aging Neurosci. 2022;13:782082. doi: 10.3389/fnagi.2021.782082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, Nahimi A, Stokholm MG, Pavese N, Beier CP, Brooks DJ, Borghammer P. In-vivo staging of pathology in REM sleep behaviour disorder:a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–628. doi: 10.1016/S1474-4422(18)30162-5. [DOI] [PubMed] [Google Scholar]
- 40.Lai F, Jiang R, Xie W, Liu X, Tang Y, Xiao H, Gao J, Jia Y, Bai Q. Intestinal pathology and gut microbiota alterations in a methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Neurochem Res. 2018;43:1986–1999. doi: 10.1007/s11064-018-2620-x. [DOI] [PubMed] [Google Scholar]
- 41.Lobo B, Tramullas M, Finger BC, Lomasney KW, Beltran C, Clarke G, Santos J, Hyland NP, Dinan TG, Cryan JF. The stressed gut:region-specific immune and neuroplasticity changes in response to chronic psychosocial stress. J Neurogastroenterol Motil. 2023;29:72–84. doi: 10.5056/jnm22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manos J. The human microbiome in disease and pathology. APMIS. 2022;130:690–705. doi: 10.1111/apm.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manzoor S, Wani SM, Ahmad Mir S, Rizwan D. Role of probiotics and prebiotics in mitigation of different diseases. Nutrition. 2022;96:111602. doi: 10.1016/j.nut.2022.111602. [DOI] [PubMed] [Google Scholar]
- 44.Mavroeidi P, Xilouri M. Neurons and glia interplay in α-synucleinopathies. Int J Mol Sci. 2021;22:4994. doi: 10.3390/ijms22094994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metta V, Leta V, Mrudula KR, Prashanth LK, Goyal V, Borgohain R, Chung-Faye G, Chaudhuri KR. Gastrointestinal dysfunction in Parkinson's disease:molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J Neurol. 2022;269:1154–1163. doi: 10.1007/s00415-021-10567-w. [DOI] [PubMed] [Google Scholar]
- 46.Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, Hirayama M. Progression of Parkinson's disease is associated with gut dysbiosis:two-year follow-up study. PLoS One. 2017;12:e0187307. doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitrea L, Nemeş SA, Szabo K, Teleky BE, Vodnar DC. Guts imbalance imbalances the brain:a review of gut microbiota association with neurological and psychiatric disorders. Front Med (Lausanne) 2022;9:813204. doi: 10.3389/fmed.2022.813204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mörkl S, Butler MI, Holl A, Cryan JF, Dinan TG. Probiotics and the microbiota-gut-brain axis:focus on psychiatry. Curr Nutr Rep. 2020;9:171–182. doi: 10.1007/s13668-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Pardo P, Dodiya HB, Engen PA, Naqib A, Forsyth CB, Green SJ, Garssen J, Keshavarzian A, Kraneveld AD. Gut bacterial composition in a mouse model of Parkinson's disease. Benef Microbes. 2018;9:799–814. doi: 10.3920/BM2017.0202. [DOI] [PubMed] [Google Scholar]
- 50.Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, Perevozchikova TV, Fait EA, Babenko VV, Vakhitova MT, Govorun VM, Sazonov AE. Analysis of gut microbiota in patients with Parkinson's disease. Bull Exp Biol Med. 2017;162:734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 51.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Curr Treat Options Neurol. 2018;20:54. doi: 10.1007/s11940-018-0539-9. [DOI] [PubMed] [Google Scholar]
- 52.Pu Y, Chang L, Qu Y, Wang S, Zhang K, Hashimoto K. Antibiotic-induced microbiome depletion protects against MPTP-induced dopaminergic neurotoxicity in the brain. Aging. 2019;11:6915–6929. doi: 10.18632/aging.102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riederer P, Berg D, Casadei N, Cheng F, Classen J, Dresel C, Jost W, Krüger R, Müller T, Reichmann H, Rieß O, Storch A, Strobel S, van Eimeren T, Völker HU, Winkler J, Winklhofer KF, Wüllner U, Zunke F, Monoranu CM. α-Synuclein in Parkinson's disease:causal or bystander? J Neural Transm. 2019;126:815–840. doi: 10.1007/s00702-019-02025-9. [DOI] [PubMed] [Google Scholar]
- 54.Rolli-Derkinderen M, Leclair-Visonneau L, Bourreille A, Coron E, Neunlist M, Derkinderen P. Is Parkinson's disease a chronic low-grade inflammatory bowel disease? J Neurol. 2020;267:2207–2213. doi: 10.1007/s00415-019-09321-0. [DOI] [PubMed] [Google Scholar]
- 55.Schaeffer E, Kluge A, Böttner M, Zunke F, Cossais F, Berg D, Arnold P. Alpha synuclein connects the gut-brain axis in Parkinson's disease patients –a view on clinical aspects, cellular pathology and analytical methodology. Front Cell Dev Biol. 2020;8:573696. doi: 10.3389/fcell.2020.573696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava S, Singh A, Sandeep K, Yadav D. Epigenetic regulation of gut microbial dysbiosis. Indian J Microbiol. 2021;61:125–129. doi: 10.1007/s12088-021-00920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 59.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson's disease:A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Tan AH, Lim SY, Chong KK, A Manap MAA, Hor JW, Lim JL, Low SC, Chong CW, Mahadeva S, Lang AE. Probiotics for constipation in Parkinson's disease:a randomized placebo-controlled study. Neurology. 2020;96:e772–782. doi: 10.1212/WNL.0000000000010998. [DOI] [PubMed] [Google Scholar]
- 61.Tan AH, Chong CW, Lim SY, Yap IKS, Teh CSJ, Loke MF, Song SL, Tan JY, Ang BH, Tan YQ, Kho MT, Bowman J, Mahadeva S, Yong HS, Lang AE. Gut microbial ecosystem in Parkinson disease:new clinicobiological insights from multi-omics. Ann Neurol. 2021;89:546–559. doi: 10.1002/ana.25982. [DOI] [PubMed] [Google Scholar]
- 62.Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. 2022;22:657–673. doi: 10.1038/s41577-022-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terenzi D, Muth AK, Park SQ. Nutrition and gut–brain pathways impacting the onset of Parkinson's disease. Nutrients. 2022;14:2781. doi: 10.3390/nu14142781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tetz G, Brown SM, Hao Y, Tetz V. Parkinson's disease and bacteriophages as its overlooked contributors. Sci Rep. 2018;8:10812. doi: 10.1038/s41598-018-29173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Den Berge N, Ferreira N, Mikkelsen TW, Alstrup AKO, Tamgüney G, Karlsson P, Terkelsen AJ, Nyengaard JR, Jensen PH, Borghammer P. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain. 2021;144:1853–1868. doi: 10.1093/brain/awab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinson's disease:a Danish nationwide cohort study 1977–2014. Gut. 2019;68:18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 67.Wallen ZD, Demirkan A, Twa G, Cohen G, Dean MN, Standaert DG, Sampson TR, Payami H. Metagenomics of Parkinson's disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun. 2022;13:6958. doi: 10.1038/s41467-022-34667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Q, Luo Y, Ray Chaudhuri K, Reynolds R, Tan EK, Pettersson S. The role of gut dysbiosis in Parkinson's disease:mechanistic insights and therapeutic options. Brain. 2021;144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 69.Warnecke T, Schäfer KH, Claus I, del Tredici K, Jost WH. Gastrointestinal involvement in Parkinson's disease:pathophysiology, diagnosis, and management. NPJ Parkinsons Dis. 2022;8:31. doi: 10.1038/s41531-022-00295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willyard C. How gut microbes could drive brain disorders. Nature. 2021;590:22–25. doi: 10.1038/d41586-021-00260-3. [DOI] [PubMed] [Google Scholar]
- 71.Xie W, Gao J, Jiang R, Liu X, Lai F, Tang Y, Xiao H, Jia Y, Bai Q. Twice subacute MPTP administrations induced time-dependent dopaminergic neurodegeneration and inflammation in midbrain and ileum, as well as gut microbiota disorders in PD mice. Neurotoxicology. 2020;76:200–212. doi: 10.1016/j.neuro.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Li S, Le W. Intestinal permeability, dysbiosis, inflammation and enteric glia cells:the intestinal etiology of Parkinson's disease. Aging Dis. 2022;13:1381. doi: 10.14336/AD.2022.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zesiewicz TA. Parkinson disease. Continuum. 2019;25:896–918. doi: 10.1212/CON.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 74.Zhu M, Liu X, Ye Y, Yan X, Cheng Y, Zhao L, Chen F, Ling Z. Gut microbiota:a novel therapeutic target for Parkinson's disease. Front Immunol. 2022;13:937555. doi: 10.3389/fimmu.2022.937555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Huan F, Wang J, Xie X, Yu G, Wang X, Jiang L, Gao R, Xiao H, Ding H, Wang J. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's disease in mouse:potential association between neurotransmitter disturbance and gut microbiota dysbiosis. ACS Chem Neurosci. 2020;11:3366–3376. doi: 10.1021/acschemneuro.0c00475. [DOI] [PubMed] [Google Scholar]