Abstract
Stroke-induced immunosuppression is a process that leads to peripheral suppression of the immune system after a stroke and belongs to the central nervous system injury-induced immunosuppressive syndrome. Stroke-induced immunosuppression leads to increased susceptibility to post-stroke infections, such as urinary tract infections and stroke-associated pneumonia, worsening prognosis. Molecular chaperones are a large class of proteins that are able to maintain proteostasis by directing the folding of nascent polypeptide chains, refolding misfolded proteins, and targeting misfolded proteins for degradation. Various molecular chaperones have been shown to play roles in stroke-induced immunosuppression by modulating the activity of other molecular chaperones, cochaperones, and their associated pathways. This review summarizes the role of molecular chaperones in stroke-induced immunosuppression and discusses new approaches to restore host immune defense after stroke.
Key Words: Hsp70, Hsp72, Hsp90, HspB5, hypothalamic-pituitary-adrenal axis, molecular chaperones, neuroprotection, stroke, stroke-induced immunosuppression, sympathetic nervous system
Introduction
Stroke is a fairly common and fatal cerebrovascular disease, affecting 13.7 million people worldwide, and approximately 5.5 million people die each year from stroke (GBD 2016 Stroke Collaborators, 2019). One out of every four adults is at risk of stroke (GBD 2016 Stroke Collaborators, 2019). With its high incidence and mortality, stroke has become the second leading cause of death worldwide.
The immune and nervous systems communicate with each other via many pathways to maintain the homeostasis of the two systems under physiological conditions. Severe brain damage, such as stroke, can upset this balance and cause a series of changes in both systems. Stroke-induced immunosuppression (SIIS) is a process that leads to peripheral suppression of the immune system after stroke and is part of central nervous system (CNS) injury-induced immunosuppressive syndrome (Chamorro et al., 2007). Acute brain injury caused by stroke as well as subsequent repair involve a large number of immune responses (Woiciechowsky et al., 1999). During a stroke, the damaged brain weakens the function of immune cells, eventually leading to suppression of the immune system (Qin et al., 2020). A considerable number of studies have shown that systemic immunosuppression predisposes patients to infection, thereby contributing to mortality and morbidity from related diseases after stroke (Meisel and Meisel, 2011; Wong et al., 2011). Stroke-associated infections, including pneumonia and urinary tract infections, are complications of stroke, and stroke-associated infections worsen patients’ conditions and increase mortality (Eltringham et al., 2018). In particular, lung infections are a major complication of stroke and have a profound effect on clinical outcomes (Suda et al., 2018).
Molecular chaperones are a family of proteins that mediate the proper assembly of other proteins but are not themselves components of the final functional structure (Pipaón et al., 2021). They are broadly defined as proteins that interact with the unnatural state of other protein molecules. Chaperones can be divided into groups based on sequence homology, with the heat shock proteins (Hsps) being one such well-known group. Hsps are so named because they are induced and synthesized under stressful conditions such as heat shock or oxidative stress, and the stability of some cellular proteins is disrupted during this synthesis (Albakova and Mangasarova, 2021). The chaperones in each group are named according to their molecular weights, and, based on this nomenclature, there are Hsp110s, Hsp90s, Hsp70s, Hsp60s, and small Hsps (sHsps), among others. Molecular chaperones have multiple functions; in addition to protein folding, chaperones play an important role in assembling macromolecular complexes, facilitating protein transport and degradation, and promoting the dissociation and refolding aggregation of denatured proteins (Burston and Clarke, 1995; Kim et al., 2013). More importantly, emerging evidence has suggested that chaperones, such as Hsps, also act on the immune system; for example, some have anti-inflammatory effects (Jeffery, 2009; Henderson and Pockley, 2010). This review specifically addresses the roles that molecular chaperones play in SIIS.
Search Strategy
An electronic search of the PubMed database was performed for articles published between 1974 and 2022. Search terms included “(molecular chaperones) AND (stroke)”; “(stroke-induced immunosuppression) AND (molecular chaperones)”; and “(heat shock protein) AND (stroke)”. Results were further filtered by title and abstract and selected based on their relevance.
Mechanisms and Characteristics of Stroke-Induced Immunosuppression
The presence of peripheral immunosuppression in patients after a stroke is thought to be an important cause of increased susceptibility after a stroke (Faura et al., 2021). First, brain-derived neurogenic innervation controls systemic immunity (Shim and Wong, 2016). The CNS includes the brain and spinal cord, and the peripheral nervous system includes cranial nerves, spinal nerves, and autonomic nerves. Via the hypothalamic-pituitary-adrenal (HPA) axis, the sympathetic nervous system (SNS), and the parasympathetic nervous system (PSNS), the CNS releases various soluble molecules such as cytokines, hormones, neurotransmitters, and neuropeptides (Procaccini et al., 2014). In this way, the CNS precisely regulates the body’s immune response to neutralize invading pathogens, repair damaged tissue, and modulate the host’s response to pathogens (Shim and Wong, 2016).
SNS activation eventually leads to the rapid release of catecholamines from sympathetic nerve endings and the adrenal medulla (Madden, 2017), which causes a rapid and transient increase in granulocytes and blood lymphocytes. However, long-term exposure to catecholamines reduces the number of circulating lymphocytes (Ince et al., 2018). The PSNS induces rapid, timely, and local regulation of immune function through the cholinergic anti-inflammatory pathway. The anti-inflammatory roles of glucocorticoids, the final products of the HPA axis, are well established, and they also play a role in immunosuppression. This prevents inflammation by inhibiting the production of many pro-inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), and IL-8. Conversely, it enhances the release of anti-inflammatory cytokines such as IL-10 and TNF-α (Kokubo et al., 2022).
When stroke occurs, brain damage induces the activation of neurogenic pathways including the SNS, HPA axis, and PSNS. Noradrenaline, acetylcholine, and glucocorticoids are mediators in these systems (Felten et al., 1987; Liu et al., 2017). Together, they affect the magnitude and intensity of the systemic immune response. Severe brain damage resulting from a stroke can disrupt the homeostasis of the immune and nervous systems and lead to a number of related changes in the two systems (Liu et al., 2016). Many studies have investigated how strokes affect the immune system. SIIS is a very easily recognized phenomenon, and there are two main explanations for its causes. First, the brain attempts to suppress the immune system to prevent further brain inflammation (Han et al., 2021), and this systemic immunosuppression occurs as a compensatory mechanism for brain injury, which is a defense mechanism; however, this also makes the host more susceptible to infection. This claim is based on the fact that stroke damages the blood-brain barrier (BBB), increasing its permeability, in which case many peripheral immune cells cross the BBB into the brain parenchyma (Pluta et al., 2022). This theory is also supported by a study showing that post-stroke infections predispose patients to autoimmunity against brain antigens (Becker, 2012). The second theory is that persistent inflammation early after a stroke may deplete the immune system, and immunosuppression is the result of this process (Becker, 2012).
Recent research has indicated that there may be two main mechanisms behind SIIS—a humoral pathway and a neural pathway. The humoral pathway gets its name because it is mechanistically regulated by changes in humoral immunity. Neurotransmitters and neuropeptides are all chemicals in this pathway. Following a stroke, an inflammatory cascade is activated by the release of damage-associated molecules by damaged and dying cells, resulting in neuroinflammation and damage to the ischemic area and surrounding areas of brain tissue. Damaged brain tissue produces the proinflammatory cytokines IL-1, TNF-α, and IL-6, among others. These inflammatory factors are characterized by their neurotoxicity, and, in addition to this neurotoxicity, they induce the production of additional cytokines and chemokines by other brain cells. Chemokines, like CXC chemokine receptor 3, are produced after stroke and can induce neuronal death. Chemokines do this by directly inducing neuronal death through neuronal receptors and can also indirectly induce neuronal death by microglial activation (Altara et al., 2016). In the damaged brain, IL-1 and TNF-α are produced primarily by activated microglia but may also be secreted by other brain cells, including astrocytes, endothelial cells, and neurons (Shi et al., 2019). These inflammatory factors reach areas of the brain that are chemically sensitive to immune control, such as the hypothalamus, where they can, in turn, activate the SNS, PSNS, and HPA axis (Rockstrom et al., 2018). Through such stimulation, peripheral levels of corticotropins, glucocorticoids, and catecholamines are elevated. Adrenergic receptors are widely distributed among immune cells, almost all of which contain adrenergic receptors. Based on the widespread distribution of adrenergic receptors and its pleiotropic effects, noradrenaline can reduce the number and activity of immune cells. The glucocorticoids produced by post-stroke stress-induced stimulation of the HPA axis are proven immunosuppressants. They cause a reduction in peripheral lymphocytes and produce immunosuppression. In addition, increased permeability of the BBB is part of the humoral pathway that leads to SIIS, due to the release of brain inflammatory mediators, oxidative stress, and other factors (Pluta et al., 2022). The increased permeability of the BBB allows many peripheral immune cells to infiltrate the brain parenchyma. At different times, this infiltration plays different roles, damaging the brain’s structure or promoting tissue repair (Iadecola and Anrather, 2011; Chamorro et al., 2012).
Unlike the humoral pathway, the neural pathway concerns the disruption of the CNS and its hard-wired circuits to the secondary lymphatic system caused by a stroke or other brain injuries, leading to immunosuppression. The neural pathways that lead to SIIS are not dependent on cytokine signaling in the brain. Without the initial involvement of any immune mechanism, injury to the part of the nervous system that controls nerve-immune interactions (such as the hypothalamus) can lead to neurogenic anti-inflammatory signals (Lehmann et al., 2014). Meyer et al. (2004) reported that stroke could lead to autonomic dysfunction in patients. Studies of lateralization of the autonomic nervous system in the brain have also demonstrated the neural pathways underlying SIIS, the severity of which depends on the location of the stroke. Compared with patients with left-sided hemiplegia, skin T-lymphocyte activity was markedly reduced ipsilateral to the affected cerebral hemisphere in patients with right hemiplegia, suggesting that stroke leads to stronger immunosuppression when the right side is involved (Honda and Kabashima, 2016). Research has also shown that neural pathways work primarily via SNS regulation of the immune system. Recent studies have shown that SNS fibers were distributed in all primary and secondary immune organs, such as bone marrow and the thymus, spleen, and lymph nodes. The SNS regulates immune function by releasing catecholamines from efferent nerves (Ahmari et al., 2020). Stroke increases SNS discharges, which increases levels of catecholamine neurotransmitters. The number of circulating lymphocytes may decrease as the catecholamine level continues to increase, leading to atrophy of immune organs and reductions in immunity, ultimately greatly increasing the chances of infection (Vogelgesang et al., 2014). However, far less research has been done on the neural pathways after stroke than on the humoral pathways.
Combining the neural and humoral pathways, there is a positive correlation between the severity of brain injury, infarct volume, and severity of neurological deficits and the degree of immunosuppression after stroke (Westendorp et al., 2022). Neural pathways play crucial roles in SIIS, and the degree of nerve damage is a key factor; however, the degree of nerve damage caused by brain inflammation after stroke has important effects on humoral pathway-mediated immunosuppression after a stroke (Westendorp et al., 2022). As a result, neuroprotection during stroke can affect post-stroke immunosuppression by affecting both the neural and humoral pathways (Shi et al., 2018). Investigators have demonstrated that immunosuppression is more pronounced in patients with severe infarction and severe neurological deficiency (Iadecola et al., 2020). Reducing brain damage after a stroke or taking neuroprotective measures can be effective in relieving immunosuppression. For example, when administered after surgical induction of a rat model of middle cerebral artery occlusion (MCAO), β-adrenergic receptor antagonists (such as propranolol) showed neuroprotective effects, reducing infarct volume and improving neurological scores (Goyagi et al., 2010). Stroke mortality and stroke-associated pneumonia rates were also reduced in patients with stroke who received β-blockers, and stroke-associated pneumonia rates were also reduced in patients who received β-blockers before a stroke (Sykora et al., 2015).
Molecular Chaperones
Molecular chaperones are a large class of proteins that assist in the assembly and disassembly of other macromolecular structures; the molecular chaperones themselves are not part of the final functional structure (Freilich et al., 2018). Molecular chaperones can be divided into different groups according to their sequence homology. At present, most attention is paid to the Hsp family, such as Hsp40s, Hsp60s, Hsp70s, Hsp90s, and Hsp100s, among others. The primary function of molecular chaperones is mediating the proper assembly of other proteins and maintaining protein homeostasis by balancing protein folding, quality control, and turnover (Ellis, 1990; Freilich et al., 2018). Here, we focus on the role of chaperones in immunity and stroke.
Since Campbell and Scanes (1995) introduced the term “protein moonlighting” to describe the immune function of “endocrine peptides”, an increasing number of proteins from prokaryotes and eukaryotes have been shown to have moonlighting functions. The part-time function of chaperones has also been increasingly studied. For example, molecular chaperones play roles in cell death (Hoter et al., 2018). Studies have shown that some Hsps are effective inducers of innate and adaptive immunity, acting through Toll-like receptors, dendritic cells, and natural killer cells, and play important roles in major histocompatibility complex antigen processing and presentation (Lahaye et al., 2012; Stepp and Menko, 2021). Furthermore, Hsps exert cytoprotective effects by modulating inflammatory cascades, preventing inflammatory responses that activate proinflammatory cytokines such as TNF-α and, thereby, attenuating chronic inflammation (Ikwegbue et al., 2019).
The Hsp family of molecular chaperones plays an important role in neuroprotection by maintaining protein homeostasis and inhibiting apoptosis and the nuclear factor kappa B (NF-κB) pathway (Penke et al., 2018). Hsps could also prevent neurodegeneration and promote memory recovery in an animal model of Alzheimer’s disease (Zatsepina et al., 2021). In brief, Hsps can exert neuroprotective effects in a variety of ways.
Molecular chaperones also play crucial roles in stroke. van der Weerd et al. (2010) demonstrated that overexpression of Hsp27 played an important neuroprotective role in a mouse model of permanent MCAO. Findings from a study by (Liebelt et al., 2010) suggested that exercise reduced neuronal damage in stroke by upregulating Hsp70.
The Role of Chaperones in Stroke-Induced Immunosuppression
Sigma-1 receptors
Sigma-1 receptors were originally thought by (Martin et al., 1976) to be a subtype of opioid receptors but the well-defined sigma-1 receptor is now seen as a unique membrane-associated protein. It is mainly enriched in the endoplasmic reticulum and is a ligand-regulated chaperone. This review focuses on the neuroprotective roles of the sigma-1 receptor in stroke and its possible association with SIIS.
Ooi et al. (2021) reported that sigma-1 receptors attenuated SNS over-activation in stressed rats. Ajmo et al. (2006) conducted a study of 1,3-di-o-tolylguanidine, which is a non-selective sigma-1/sigma-2 receptor ligand. Their results indicated that subcutaneous injection of 15 mg/kg 1,3-di-o-tolylguanidine 24 hours after MCAO model induction in rats significantly reduced the infarct size in the striatum and hippocampus. They, therefore, hypothesized that 1,3-di-o-tolylguanidine may exert its neuroprotective effects during stroke by acting as a sigma-1 receptor agonist (Ajmo et al., 2006). In addition, many other sigma-1 receptor ligands exert neuroprotection by affecting sigma-1 receptors, such as antitussive agents and dimethyl morphine, which showed neuroprotective effects in a rat MCAO model (Shen et al., 2008). Sigma-1 receptor ligands exert neuroprotective effects by affecting sigma-1 receptors and dehydroepiandrosterone (Albayrak and Hashimoto, 2017). In the above studies, sigma-1 receptors attenuated SNS over-activation, and many sigma-1 receptor ligands exerted neuroprotective effects in stroke by influencing sigma-1. Neuroprotection has a strong effect on the degree of SIIS, so sigma-1 receptors may be a promising research direction.
Hsp70
Hsp70 is a widely studied protein in the Hsp family that can promote the folding or utilization of proteins, especially those damaged by stress factors (Morán Luengo et al., 2019). Hsp70 can affect the CNS, which is strongly involved in the development of SIIS (Ekimova et al., 2010). In stroke, overexpression of Hsp70 protected brain cells from ischemic injury (Kim et al., 2018), and inhibition of Hsp70 synthesis was shown to increase the severity of cerebral infarction. As shown in cultured neurons and in vivo animal models, secreted and recombined Hsp70 had neuroprotective effects (Evgen’ev et al., 2017).
Hsp70 can affect the CNS by antagonizing γ-aminobutyric acid (GABA) receptors. The most important function of GABA is as an inhibitory neurotransmitter in the CNS, but it is also present in the pancreatic islets and blood, with similar inhibitory effects on immune cells. In cerebral ischemia, the imbalance between excitatory and inhibitory postsynaptic potentials is considered to be one of the major factors leading to neuronal death, while the disruption of GABA-mediated inhibition is the major cause of neuronal hyperexcitability (Ahnaou and Drinkenburg, 2021). In studies of chemically induced epilepsy, the GABA-synthesizing enzyme glutamic acid decarboxylase 67 (GAD67) helped maintain GABA levels in the CNS. Furthermore, co-immunoprecipitation analysis showed a physical association between Hsp70 and GAD67 in the bodies and terminals of GABA-ergic neurons, with Hsp70 colocalizing with GAD67. An in-depth study found that Hsp70 could regulate the function of GAD67 (Ekimova et al., 2010). Furthermore, by studying the anatomical connections between CNS nerves and the peripheral immune organs via the HPA axis, researchers have demonstrated a link between GABA-induced CNS and peripheral inflammatory responses (Reyes-García et al., 2007). Innate and adaptive immune cells such as microglia, macrophages, and T-cells all express functional GABA receptors, and these cells have enzymes that synthesize or decompose GABA. For example, GABA was found to regulate the release of pro- and anti-inflammatory cytokines from CD4+ T-cells (Bhandage et al., 2018). GABA also has been shown to alter cell proliferation and cytokine secretion in a concentration-dependent manner and reduce the release of most inflammatory cytokines (Abg Abd Wahab et al., 2019). Thus, Hsp70 can affect the CNS by regulating the inhibitory function of GABA and can affect the peripheral immune system.
The induction of Hsp70 is mediated by heat shock factor (HSF) 1. Under steady-state conditions, Hsps are normally bound to HSFs and are located within cells (van Eden et al., 2017). When denaturation stimuli such as ischemia occur, cellular Hsps detach from HSFs and bind to denatured or aggregated proteins (van Eden et al., 2017). During stroke, Hsp70 is synthesized in large amounts to restore the denatured proteins to their tertiary structure, a process that requires Hsp70, Hsp40, Hsp90, and adenosine triphosphate. These proteins form complexes that bind denatured proteins, enabling metamorphic protein repair and refolding through chaperone functions that can ultimately maintain protein homeostasis (Zhao et al., 2012).
During a stroke, cerebral blood flow ceases or decreases, and subsequent reperfusion of nerve tissue initiates a series of signaling pathways that ultimately lead to cell damage and death. In non-stressed and steady-state conditions, Hsp70 in the brain acts like other Hsps mentioned above, with very little inducible Hsp70 being present (also known as Hsp72). However, Hsp72 increases sharply after injury, and it may be the most highly induced protein expressed in response to stress. In earlier studies, investigators observed initial Hsp70 induction in penumbra neurons as well as in astrocytes in an experimental stroke model (del Zoppo et al., 2011).
As an inducible form of Hsp70, the neuroprotective role of Hsp72 in stroke has been demonstrated (Wang et al., 2019). It belongs to the extracellular Hsps, which can be used as “danger signals” for immune cells to promote immune responses and improve host defense (Campisi et al., 2012). The expression of Hsp72 in the brain of healthy mice has been shown to be low (Sharp et al., 2013), and it is upregulated in the brain after stroke. Hsp72 is induced in large amounts in neurons of the penumbra or ischemic core, and there is an inverse correlation between the percentage of old and newly formed Hsp72-containing neurons and prognosis after ischemic brain injury (Nowak and Jacewicz, 1994; Kim and Lee, 2007). Furthermore, Hsp72 is downregulated in peripheral blood, and SIIS may be associated with Hsp72 based on its immunosuppressive activity (Gruden et al., 2013).
Wang et al. (2019) studied the effects of Hsp72 on neurons in a rat MCAO model using motor preconditioning, showing that motor preconditioning retained old Hsp72-containing neurons and newly formed Hsp72-containing neurons, therefore playing a marked neuroprotective role in ischemic brain injury. Many other studies have yielded similar results; transfection of Hsp72 and viral overexpression ameliorated the damage caused by cerebral ischemia in cellular models; the use of geldanamycin, an inducer of Hsp72, reduced ischemic brain damage in animal models (Kwon et al., 2008); and knockout of Hsp72 aggravated ischemic brain damage in animal models (Lee et al., 2004). Therefore, the neuroprotective effects of Hsp72 after a stroke may be a promising research direction for the treatment of SIIS.
When using viral vectors to overexpress Hsp70 in neurons and astrocytes, (Giffard and Yenari, 2004) found that neurons and astrocytes overexpressing Hsp70 were more resistant to ischemia and ischemic-like injury. Similarly, experiments in animal models have found that transgenic mice overexpressing Hsp70 in the brain had better neurological performance after a stroke, and a lack of Hsp70 worsened the results in a similar model (Lee et al., 2001). The neuroglia (microglia and astrocytes) are essential components of the CNS. Thus, the protective effects of Hsp70 on neuroglia is a promising research direction for SIIS.
The mechanisms underlying the protective effect of Hsp70 on neurons and astrocytes involved Hsp70 inhibition of apoptosis. Ischemic injury preferentially induces cell death through apoptotic mechanisms, which can be divided into intrinsic pathways that occur at the level of mitochondria within the cell and extrinsic pathways that are triggered by cell surface receptors (Parsons and Green, 2010). Hsp70 affects both pathways. In the intrinsic pathway, Hsp70 plays a role in the apoptosis mechanisms in both upstream and downstream of mitochondria. In the upstream pathway, Hsp70 could interrupt cytochrome C release in an experimental stroke model and prevented apoptosis-inducing factor translocation to the nucleus, thereby inhibiting apoptosis (Tsuchiya et al., 2003). In the downstream pathway, translocation of Bax, a pro-apoptotic B-cell lymphoma-2 family member, led to heat-induced apoptosis, and Hsp70 could block the translocation of Bax to suppress this apoptosis (Stankiewicz et al., 2005). In extrinsic pathways, recent studies have shown that Hsp70 could prevent transport of Fas (also known as CD95) to the cell surface by interacting with dynamin (which transports Fas to the cell surface), thus preventing binding to Fas; as a result, caspase-8 activation and cell death were inhibited (Kim et al., 2016).
Studies have shown that after stroke, as an anti-inflammatory factor, Hsp70 could regulate inflammation through pro-inflammatory or anti-inflammatory mechanisms based on its intracellular or extracellular status (Manaenko et al., 2010; Guo et al., 2018). When Hsp70 is inside cells, it suppresses the immune response; while outside the cell, it seems to enhance the immune response (Martin et al., 1976; Kim et al., 2018). Manaenko et al. (2010) found that Hsp70 induction in a model of cerebral hemorrhage reduced TNF-α expression, maintaining the integrity of the BBB. It alleviated brain edema and reduced neurological functional deficits. Further, other studies have shown that Hsp70 could directly bind to the pro-inflammatory transcription factor NF-κB and its regulatory protein, and, in this way, Hsp70 could inhibit the NF-κB pathway in stroke models (Ran et al., 2004). This inhibition resulted in reduced transcription and translation of downstream NF-κB target genes, with Hsp70 playing a neuroprotective role in this process. Moreover, other studies have found that Hsp70 exerted neuroprotection through the microRNA (miR)-122 pathway (Guo et al., 2018).
A study by Ren et al. (2004) found that valproic acid (VPA) played a role in brain damage caused by transient focal cerebral ischemia in rats. One mechanism of action was the up-regulation of Hsp70 expression in ischemic brain regions. VPA is an inhibitor of histone deacetylase; one study showed that histone deacetylase inhibitors trichostatin A and that sodium butyrate could increase the transcription of Hsp70 in Drosophila melanogaster (Chen et al., 2002). Some studies have shown that VPA could induce inactivation of glycogen synthase kinase 3β (De Sarno et al., 2002), while glycogen synthase kinase 3β inactivation, with cytoprotective effects, could activate the transcription of Hsp70 (Bijur and Jope, 2000). Taken together, the induction of Hsp70 transcription by VPA and other histone deacetylase inhibitors appears to be associated with glycogen synthase kinase 3 inhibition, but the mechanism by which VPA upregulates the expression of Hsp70 in ischemic regions of the brain by inducing glycogen synthase kinase 3β inactivation in stroke needs to be investigated. By studying the expression of Hsp70 in stroke, we can more completely elucidate the involvement of Hsp70 in SIIS. Hsp70 can also exert neuroprotection through the ubiquitin-proteasome system (UPS). The UPS is a protein degradation system in cells, maintaining the normal function of cells, tissues, and organs by breaking down abnormal or damaged proteins and breaking down proteins with specific functions. Disruption of the UPS leads to disruptions in cell homeostasis, leading to a variety of diseases, including neurological disorders (Wang and Le, 2019). The proteasome is a multi-catalytic protease complex and the major site of protein turnover in eukaryotic cells (Inobe and Matouschek, 2014). The main function of the proteasome is to degrade proteins, control protein abundance, and degrade misfolded proteins through the UPS (Jankowska et al., 2013). Many studies have shown that proteasome inhibitors can play neuroprotective roles in stroke and have therapeutic effects on SIIS (Awasthi and Wagner, 2005; Tamura et al., 2012; Doeppner et al., 2016). For example, after timed and multiple systemic administration of the proteasome inhibitor BSC2118, the peripheral immune response changed markedly to prevent stroke-induced acute leukocytosis and to reverse subsequent peripheral blood immunosuppression (Doeppner et al., 2016). One study showed that proteasome inhibitors could upregulate the expression of Hsps by inhibiting the proteasome expression (Awasthi and Wagner, 2005). Hsp70 acts as a physiological ligand of Toll-like receptors (Tamura et al., 2012). After stroke, Hsp70 can reduce proteasome activity and inflammation to participate in the pathophysiological cellular cascades of cerebral ischemia. Through this process, Hsp70 plays a neuroprotective role after stroke (Doeppner et al., 2018).
In SIIS, both an increase in regulatory T-cells and a decrease in the cytokines Th1/Th2 are important features, whereas elevated levels of autologous Hsp70 in the circulation were shown to activate antigen-specific regulatory T-cells and lead to a decrease in Th1/Th2 (van Eden et al., 2017). Moreover, circulating Hsp70 is increased during stroke, so circulating Hsp70 may also affect SIIS.
In conclusion, Hsp70 can affect the CNS, the system involved in the development of SIIS (Ekimova et al., 2010), and the peripheral immune system (Ekimova et al., 2010; Abg Abd Wahab et al., 2019). Further, in stroke, Hsp70 may play a neuroprotective role at multiple levels, and in combination with the tremendous neuroprotective effects in SIIS mentioned above. The role of Hsp70 in SIIS has great research potential, and it is also involved in many therapeutic methods (Doeppner et al., 2016, 2018; Figure 1).
Figure 1.
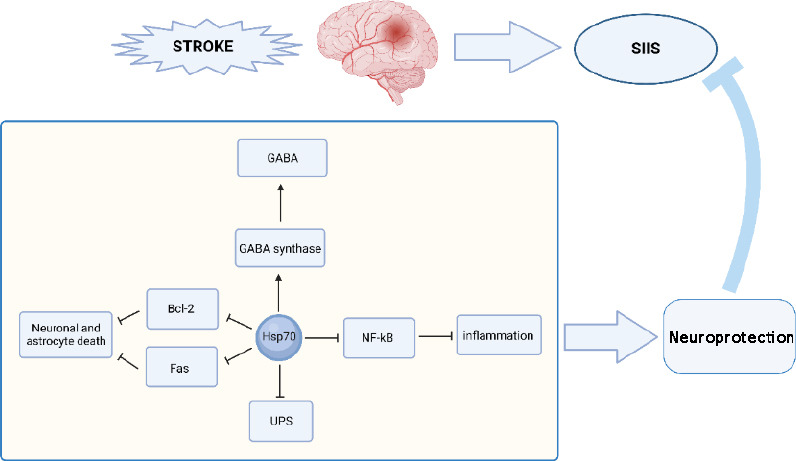
Hsp70 can exert neuroprotective effects at the time of stroke onset and thereby affect SIIS in a variety of ways.
Hsp70 can regulate the function of GABA synthetase, as Hsp70 can maintain GABA-mediated inhibition; Hsp70 directly binds to NF-kB and its regulatory protein to inhibit the NF-κB pathway, which plays an anti-inflammatory role, thus acting as a neuroprotector. Hsp70 can also inhibit neuron and astrocyte apoptosis at the ischemic site of stroke by inhibiting the translocation of Bcl-2 and preventing Fas expression on the cell surface; Hsp70 can also inhibit UPS activity by decreasing the activity of proteasome and exerting neuroprotective effects. Bcl-2: B-cell lymphoma-2; Fas: CD95; GABA: gamma-aminobutyric acid; Hsp70: 70-kDa heat shock protein; NF-κB: nuclear factor kappa B; SIIS: stroke-induced immunosuppression; UPS: ubiquitin-proteasome system.
Hsp90
As an important member of the Hsp family, Hsp90 plays a role in regulating hormone signaling cascades and stress responses. The association between Hsp90 and SIIS is supported by the fact that its multimeric chaperone complex regulates the HPA axis, the system involved in the development of SIIS. Hsp90 binds to FK506-binding protein 52, Hsp70, and P23 to form a polymeric chaperone complex that regulates glucocorticoid receptor activity through several feedback loops (Riggs et al., 2003). Specifically, the multimeric chaperone complex of FK506-binding protein 52 with Hsp90 can enhance glucocorticoid receptor activity, whereas FK506-binding protein 51 can inhibit this enhancement by competing for Hsp90, which enables the HPA axis to regain homeostasis after stress (Riggs et al., 2003). This process has been shown to inhibit the enzymatic activity of Hsp90, resulting in a decrease in glucocorticoid receptor activity (Galigniana et al., 1998). Based on the regulatory role of Hsp90 in the HPA axis, it is a potential therapeutic target for SIIS.
Hsp90 is overexpressed in animal models of cerebral ischemia and has a marked correlation with matrix metalloproteinase 9 (Qi et al., 2015). Qi et al. (2015) found that inhibition of Hsp90 protected the integrity of the BBB in an animal model of ischemic stroke by inhibiting the degradation of tight junction proteins and inhibiting the inflammatory response and subsequent expression of matrix metalloproteinase 9 after ischemic stroke. As mentioned above, BBB disruption can lead to angiogenic edema and further brain damage, which can affect the immune system. Therefore, protecting the integrity of the BBB by inhibiting Hsp90 expression is a therapeutic direction that deserves investigation (Zhou et al., 2021).
In ischemic stroke, ferroptosis also leads to BBB injury and neurological damage (Bu et al., 2021). Hsp90 has been shown to promote the degradation of glutathione peroxidase 4, leading to ferroptosis. One study showed that Hsp90-associated chaperone-mediated autophagy promoted the degradation of glutathione peroxidase 4 and led to ferroptosis by regulating the stability of lysosome-associated membrane protein 2 isoform A (Zhou et al., 2021). Based on the promoting effects of Hsp90 on ferroptosis in stroke, Hsp90 inhibitors could play a neuroprotective role in the BBB by inhibiting ferroptosis.
In addition, Hsp90 can affect the induction of Hsp70, as Hsp90 binds to HSF1. After dissociation from Hsp90, HSF1 is released and then binds to heat shock elements, leading to an increase in Hsp70. This process also occurs in the brain of patients with stroke, allowing Hsp70 to better exert its neuroprotective effects, thereby affecting SIIS (Chaudhury et al., 2021; Figure 2).
Figure 2.
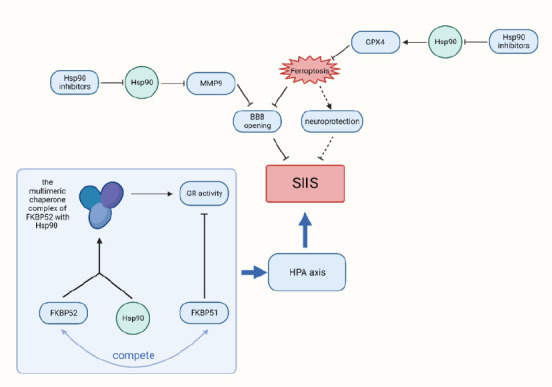
Hsp90 plays multiple roles in SIIS.
When stroke occurs, the inhibition of Hsp90 can protect BBB integrity by inhibiting tight junction protein degradation by inhibiting MMP9 expression; the inhibition of Hsp90 by Hsp90 inhibitors can also reduce the degradation of GPX4 in the brain during stroke, thus reducing ferroptosis. In addition, FKBP52, a polymeric chaperone complex with Hsp90, can enhance GR activity, FKBP51 can inhibit this enhancement by competing for Hsp90, which plays a role in regulating the HPA axis and, thus, plays a role in SIIS. BBB: Blood-brain barrier; FKBP51: FK506-binding protein 51; FKBP52: FK506-binding protein 52; GPX4: glutathione peroxidase 4; GR: glucocorticoid receptor; HPA: hypothalamic-pituitary-adrenal; Hsp90: 70-kDa heat shock protein; MMP9: matrix metallopeptidase 9; SIIS: stroke-induced immunosuppression.
sHsps
sHsps are a large family of molecular chaperones, and they help maintain protein homeostasis under stress conditions such as high temperatures, oxidation, and infection (Kurop et al., 2021). Hsp27 and HspB5 are sHsps that are promising targets for SIIS. The expression of Hsp27 is very low in the healthy brain, but it is highly expressed in a subset of normal motor neurons, medial preoptic neurons, and Purkinje fibers. In healthy adult rats, Hsp27 is also expressed in a variety of peripheral neurons in SNS and PSNS ganglia. Hsp27 is also induced in astrocytes after cerebral ischemia (Behdarvandy et al., 2020). Viral transfection of Hsp27 has been shown to prevent cerebral ischemia and kainic acid-induced neuronal cell death in vivo and in vitro (Stetler et al., 2009). The neuroprotective effects of Hsp27 may have an effect on SIIS.
In a study on the cardioprotective effects of substance abuse-induced stress, selectively blocking the receptor for the corticotropin factor-1 and gene deletion of corticotropin factor-1 receptor antagonized adaptive changes in the heart (Martínez-Laorden et al., 2020). These changes included an increase in noradrenaline turnover, activation of the HPA axis, and a decrease in Hsp27 expression and phosphorylation. This indicated a connection between the HPA axis and Hsp27 (Martínez-Laorden et al., 2020). At present, Hsp27 is an attractive direction for the study of neuroprotection in stroke, as it is not dependent on adenosine triphosphate and can affect the HPA axis, which is highly involved in SIIS. Meanwhile, one study did not find that it aggravated the adverse effects of ischemic brain damage (Martínez-Laorden et al., 2020).
HspB5 is one of the most widely distributed and typical human sHsps, expressed mainly in the lens of the eye and induced during various types of brain damage (Tedesco et al., 2022). It is one of the most abundant inducible transcripts in patients with multiple sclerosis, especially in inflammatory regions (Islam et al., 2020). HspB5 has anti-apoptotic and immunomodulatory properties and is a negative regulator of inflammation. It has been shown that HspB5 could affect the PSNS and had neuroprotective effects (Terrell and Morrison, 2019).
The nicotinic acetylcholine receptors (nAchRs) are unique among more than 20 known amyloid fibrillary receptors and plays a central role in the inflammatory reflex, which is a physiological mechanism involving the vagus nerve (Pavlov and Tracey, 2017). Among the amyloid receptors, the α7nAchR is unique in the endogenous immune anti-inflammatory pathway associated with the vagus nerve (Pavlov and Tracey, 2012). Researchers identified HspB5 and amyloid fibrils as agonists of α7nAchR, which induce a common signaling pathway leading to increased oxidative phosphorylation and autophagy (inhibition of apoptosis) and induction of regulatory macrophages (Shao et al., 2017; Rothbard et al., 2018). This could restrict T-lymphocyte proliferation and cause proinflammatory cytokine reduction (Jiang et al., 2016; Shao et al., 2017; Rothbard et al., 2018). Injection of HspB5 or amyloid fibrils could also induce immunosuppressive pathways through direct binding of α7nAchR; based on the role of the PSNS in SIIS, HspB5 has an effect on SIIS.
In a mouse model of MCAO, Arac et al. (2011) studied the specific effects of HspB5 deficiency. They found that HspB5–/– mice had more brain damage and immune cell infiltration in the brain. Further, the number of different cell populations in the spleens of HspB5–/– mice was also significantly reduced 2 days after model induction. These inflammatory markers suggested that a lack of HspB5 may result in a more robust inflammatory response in MCAO model mice (Urra et al., 2009; Arac et al., 2011). Through experiments in bone marrow chimeras, Arac et al. (2011) also demonstrated that lesions in HspB5–/– MCAO mice were larger due to HspB5 deficiency. These studies suggested that HspB5 had an effect on brain damage after stroke and neuroprotective effects. Further, Arac et al. (2011) evaluated the effects of treatment with HspB5 on splenocyte cytokines 7 days after stroke in wild-type mice and found that the total number of splenocytes did not differ between controls and HspB5-treated mice. When stimulated with concanavalin A, splenocytes from HspB5-treated mice released lower levels of proinflammatory IL-2, IL-17, interferon-γ, and IL-6 compared with control mice. However, the levels of anti-inflammatory IL-10 released from HspB5-treated mice were higher. These findings demonstrated that HspB5 plays an essential role in SIIS by influencing the severity of brain damage, exerting neuroprotective effects, and affecting peripheral immunity after stroke (Urra et al., 2009; Arac et al., 2011).
Kurnellas et al. (2012) showed a unique correlation between HspB5 chaperone function, amyloid formation, and immunosuppressive activity. Moreover, the formation of amyloid appeared to be at the core of its immunosuppressive effects. They demonstrated that the endocytosis of amyloid fibrils by peritoneal B-cells and macrophages stimulated their migration to secondary lymphoid organs (Kurnellas et al., 2015). They also proposed two ways in which HspB5 could act to suppress immunity by reducing the production of proinflammatory cytokines IL-6, TNF-α, and interferon-γ, and via neutrophil activation and induction of type-1 interferons (Kurnellas et al., 2014).
HspB5 can affect SIIS via α7nAchR and can also provide neuroprotection after stroke (Arac et al., 2011). This is of great clinical significance for the treatment of SIIS and subsequent infections.
Other Molecular Chaperones
A previous study on periodontal disease showed that Hsp10 is a cellular stress protein with immunosuppressive activity (Fucarino and Pitruzzella, 2020), and Hsp10 also has anti-apoptotic and anti-inflammatory functions (David et al., 2013). Moreover, the expression of Hsp10 in the brain is upregulated after stroke, and the induction of Hsp10 in the brain after ischemia may have neuroprotective effects (Kim and Lee, 2007), but the effects and mechanisms of this in SIIS have not been reported.
Hsp60 is involved in the regulation of many cellular events including apoptosis, cell proliferation, inflammation, and immunity (Tang et al., 2022). It was found that nitric oxide could downregulate the expression of Hsp60 in the brain after cerebral ischemia, making nerve injury more severe (Bross et al., 2012). It has been hypothesized that Hsp60 may affect SIIS through neuroprotective mechanisms.
40 kDa catecholamine-regulated protein is a molecular chaperone that has been little studied, but it has been shown to be associated with CNS diseases. It may affect SIIS from multiple dimensions (Gabriele et al., 2009).
Conclusions
SIIS has a significant effect on the outcome of stroke, and SIIS-induced systemic infections are conducive to the high mortality of stroke. In addition to assisting in the non-covalent assembly and disassembly of other macromolecular structures, molecular chaperones play an important role in SIIS, with each type of molecular chaperone playing different roles. For example, sHsps can affect SIIS via α7nAchR. Hsp70 can affect SIIS in many ways, such as via neuroprotection. Hsp90 can affect SIIS by reducing brain injury and protecting the BBB in other ways. In addition, some molecular chaperones such as sigma-1 receptors also play roles in SIIS by exerting neuroprotective functions. Molecular chaperones have considerable clinical value and significance in the treatment and prognosis of SIIS. Currently, there are still large gaps in the research on the role of chaperones in SIIS, and this still needs study to fully realize the clinical value of this field.
Additional file: Open peer review report 1 (78.2KB, pdf) .
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 82172147 (to YL), 81571880 (to YL), 81373147 (to YL), 30901555 (to JZ), 30972870 (to YL) and the Natural Science Foundation of Hunan Province, Nos. 2021JJ30900, 2016JJ2157 (both to YL).
Conflicts of interest: The authors declare that they have no conflict of interests.
Data availability statement: The data are available from the corresponding author on reasonable request.
P-Reviewers: Bustamante A, Pluta R; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editor: Song LP; T-Editor: Jia Y
Open peer reviewers: Alejandro Bustamante, Hospital Universitari Germans Trias i Pujol, Spain; Ryszard Pluta, Polish Academy of Sciences, Poland.
References
- 1.Abg Abd Wahab DY, Gau CH, Zakaria R, Muthu Karuppan MK, BS AR, Abdullah Z, Alrafiah A, Abdullah JM, Muthuraju S. Review on cross talk between neurotransmitters and neuroinflammation in striatum and cerebellum in the mediation of motor behaviour. Biomed Res Int. 20192019:1767203. doi: 10.1155/2019/1767203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmari N, Hayward LF, Zubcevic J. The importance of bone marrow and the immune system in driving increases in blood pressure and sympathetic nerve activity in hypertension. Exp Physiol. 2020;105:1815–1826. doi: 10.1113/EP088247. [DOI] [PubMed] [Google Scholar]
- 3.Ahnaou A, Drinkenburg W. Sleep, neuronal hyperexcitability, inflammation and neurodegeneration:Does early chronic short sleep trigger and is it the key to overcoming Alzheimer's disease? Neurosci Biobehav Rev. 2021;129:157–179. doi: 10.1016/j.neubiorev.2021.06.039. [DOI] [PubMed] [Google Scholar]
- 4.Ajmo CT, Jr, Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- 5.Albakova Z, Mangasarova Y. The HSP immune network in cancer. Front Immunol. 2021;12:796493. doi: 10.3389/fimmu.2021.796493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albayrak Y, Hashimoto K. Sigma-1 receptor agonists and their clinical implications in neuropsychiatric disorders. Adv Exp Med Biol. 2017;964:153–161. doi: 10.1007/978-3-319-50174-1_11. [DOI] [PubMed] [Google Scholar]
- 7.Altara R, Manca M, Brandão RD, Zeidan A, Booz GW, Zouein FA. Emerging importance of chemokine receptor CXCR3 and its ligands in cardiovascular diseases. Clin Sci (Lond) 2016;130:463–478. doi: 10.1042/CS20150666. [DOI] [PubMed] [Google Scholar]
- 8.Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awasthi N, Wagner BJ. Upregulation of heat shock protein expression by proteasome inhibition:an antiapoptotic mechanism in the lens. Invest Ophthalmol Vis Sci. 2005;46:2082–2091. doi: 10.1167/iovs.05-0002. [DOI] [PubMed] [Google Scholar]
- 10.Becker K. Autoimmune responses to brain following stroke. Transl Stroke Res. 2012;3:310–317. doi: 10.1007/s12975-012-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behdarvandy M, Karimian M, Atlasi MA, Azami Tameh A. Heat shock protein 27 as a neuroprotective biomarker and a suitable target for stem cell therapy and pharmacotherapy in ischemic stroke. Cell Biol Int. 2020;44:356–367. doi: 10.1002/cbin.11237. [DOI] [PubMed] [Google Scholar]
- 12.Bhandage AK, Jin Z, Korol SV, Shen Q, Pei Y, Deng Q, Espes D, Carlsson PO, Kamali-Moghaddam M, Birnir B. GABA regulates release of inflammatory cytokines from peripheral blood mononuclear cells and CD4(+) T cells and is immunosuppressive in type 1 diabetes. EBioMedicine. 2018;30:283–294. doi: 10.1016/j.ebiom.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J Neurochem. 2000;75:2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- 14.Bross P, Magnoni R, Bie AS. Molecular chaperone disorders:defective Hsp60 in neurodegeneration. Curr Top Med Chem. 2012;12:2491–2503. doi: 10.2174/1568026611212220005. [DOI] [PubMed] [Google Scholar]
- 15.Bu ZQ, Yu HY, Wang J, He X, Cui YR, Feng JC, Feng J. Emerging role of ferroptosis in the pathogenesis of ischemic stroke:a new therapeutic target? ASN Neuro. 2021;13:17590914211037505. doi: 10.1177/17590914211037505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burston SG, Clarke AR. Molecular chaperones:physical and mechanistic properties. Essays Biochem. 1995;29:125–136. [PubMed] [Google Scholar]
- 17.Campbell RM, Scanes CG. Endocrine peptides 'moonlighting'as immune modulators:roles for somatostatin and GH-releasing factor. J Endocrinol. 1995;147:383–396. doi: 10.1677/joe.0.1470383. [DOI] [PubMed] [Google Scholar]
- 18.Campisi J, Sharkey C, Johnson JD, Asea A, Maslanik T, Bernstein-Hanley I, Fleshner M. Stress-induced facilitation of host response to bacterial challenge in F344 rats is dependent on extracellular heat shock protein 72 and independent of alpha beta T cells. Stress. 2012;15:637–646. doi: 10.3109/10253890.2011.653596. [DOI] [PubMed] [Google Scholar]
- 19.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke:a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 20.Chamorro Á, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhury S, Keegan BM, Blagg BSJ. The role and therapeutic potential of Hsp90, Hsp70, and smaller heat shock proteins in peripheral and central neuropathies. Med Res Rev. 2021;41:202–222. doi: 10.1002/med.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T, Sun H, Lu J, Zhao Y, Tao D, Li X, Huang B. Histone acetylation is involved in hsp70 gene transcription regulation in Drosophila melanogaster. Arch Biochem Biophys. 2002;408:171–176. doi: 10.1016/s0003-9861(02)00564-7. [DOI] [PubMed] [Google Scholar]
- 23.David S, Bucchieri F, Corrao S, Czarnecka AM, Campanella C, Farina F, Peri G, Tomasello G, Sciumè C, Modica G, La Rocca G, Anzalone R, Giuffrè M, Conway De Macario E, Macario AJ, Cappello F, Zummo G. Hsp10:anatomic distribution, functions, and involvement in human disease. Front Biosci (Elite Ed) 2013;5:768–778. doi: 10.2741/e657. [DOI] [PubMed] [Google Scholar]
- 24.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 25.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doeppner TR, Kaltwasser B, Kuckelkorn U, Henkelein P, Bretschneider E, Kilic E, Hermann DM. Systemic proteasome inhibition induces sustained post-stroke neurological recovery and neuroprotection via mechanisms involving reversal of peripheral immunosuppression and preservation of blood-brain-barrier integrity. Mol Neurobiol. 2016;53:6332–6341. doi: 10.1007/s12035-015-9533-3. [DOI] [PubMed] [Google Scholar]
- 27.Doeppner TR, Zechmeister B, Kaltwasser B, Jin F, Zheng X, Majid A, Venkataramani V, Bähr M, Hermann DM. Very delayed remote ischemic post-conditioning induces sustained neurological recovery by mechanisms involving enhanced angioneurogenesis and peripheral immunosuppression reversal. Front Cell Neurosci. 2018;12:383. doi: 10.3389/fncel.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekimova IV, Nitsinskaya LE, Romanova IV, Pastukhov YF, Margulis BA, Guzhova IV. Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J Neurochem. 2010;115:1035–1044. doi: 10.1111/j.1471-4159.2010.06989.x. [DOI] [PubMed] [Google Scholar]
- 29.Ellis RJ. The molecular chaperone concept. Semin Cell Biol. 1990;1:1–9. [PubMed] [Google Scholar]
- 30.Eltringham SA, Kilner K, Gee M, Sage K, Bray BD, Pownall S, Smith CJ. Impact of dysphagia assessment and management on risk of stroke-associated pneumonia:a systematic review. Cerebrovasc Dis. 2018;46:99–107. doi: 10.1159/000492730. [DOI] [PubMed] [Google Scholar]
- 31.Evgen'ev MB, Krasnov GS, Nesterova IV, Garbuz DG, Karpov VL, Morozov AV, Snezhkina AV, Samokhin AN, Sergeev A, Kulikov AM, Bobkova NV. Molecular mechanisms underlying neuroprotective effect of intranasal administration of human hsp70 in mouse model of Alzheimer's disease. J Alzheimers Dis. 2017;59:1415–1426. doi: 10.3233/JAD-170398. [DOI] [PubMed] [Google Scholar]
- 32.Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-induced immunosuppression:implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. 2021;18:127. doi: 10.1186/s12974-021-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S. Noradrenergic sympathetic neural interactions with the immune system:structure and function. Immunol Rev. 1987;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 34.Freilich R, Arhar T, Abrams JL, Gestwicki JE. Protein-protein interactions in the molecular chaperone network. Acc Chem Res. 2018;51:940–949. doi: 10.1021/acs.accounts.8b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fucarino A, Pitruzzella A. Role of HSP60/HSP10 in lung cancer:simple biomarkers or leading actors? J Oncol. 20202020:4701868. doi: 10.1155/2020/4701868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriele J, Pontoriero GF, Thomas N, Thomson CA, Skoblenick K, Pristupa ZB, Mishra RK. Cloning, characterization, and functional studies of a human 40-kDa catecholamine-regulated protein:implications in central nervous system disorders. Cell Stress Chaperones. 2009;14:555–567. doi: 10.1007/s12192-009-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- 38.GBD 2016 Stroke Collaborators (2019) Global, regional, and national burden of stroke 1990-2016:a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Goyagi T, Horiguchi T, Nishikawa T, Tobe Y. Post-treatment with selective β1 adrenoceptor antagonists provides neuroprotection against transient focal ischemia in rats. Brain Res. 2010;1343:213–217. doi: 10.1016/j.brainres.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 41.Gruden G, Barutta F, Catto I, Bosco G, Caprioli MG, Pinach S, Fornengo P, Cavallo-Perin P, Davini O, Cerrato P, Bruno G. Serum levels of heat shock protein 27 in patients with acute ischemic stroke. Cell Stress Chaperones. 2013;18:531–533. doi: 10.1007/s12192-013-0403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo D, Ma J, Li T, Yan L. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp Cell Res. 2018;369:34–42. doi: 10.1016/j.yexcr.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Guo L, Zhen X. Sigma-2 receptor ligands:neurobiological effects. Curr Med Chem. 2015;22:989–1003. doi: 10.2174/0929867322666150114163607. [DOI] [PubMed] [Google Scholar]
- 44.Han D, Liu H, Gao Y, Feng J. Targeting brain-spleen crosstalk after stroke:new insights into stroke pathology and treatment. Curr Neuropharmacol. 2021;19:1590–1605. doi: 10.2174/1570159X19666210316092225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson B, Pockley AG. Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol. 2010;88:445–462. doi: 10.1189/jlb.1209779. [DOI] [PubMed] [Google Scholar]
- 46.Honda T, Kabashima K. Novel concept of iSALT (inducible skin-associated lymphoid tissue) in the elicitation of allergic contact dermatitis. Proc Jpn Acad Ser B Phys Biol Sci. 2016;92:20–28. doi: 10.2183/pjab.92.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoter A, El-Sabban ME, Naim HY. The HSP90 family:structure, regulation, function, and implications in health and disease. Int J Mol Sci. 2018;19:2560. doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iadecola C, Anrather J. The immunology of stroke:from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke:mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130:2777–2788. doi: 10.1172/JCI135530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikwegbue PC, Masamba P, Mbatha LS, Oyinloye BE, Kappo AP. Interplay between heat shock proteins, inflammation and cancer:a potential cancer therapeutic target. Am J Cancer Res. 2019;9:242–249. [PMC free article] [PubMed] [Google Scholar]
- 51.Ince LM, Weber J, Scheiermann C. Control of leukocyte trafficking by stress-associated hormones. Front Immunol. 2018;9:3143. doi: 10.3389/fimmu.2018.03143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inobe T, Matouschek A. Paradigms of protein degradation by the proteasome. Curr Opin Struct Biol. 2014;24:156–164. doi: 10.1016/j.sbi.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam MA, Kundu S, Hassan R. Gene therapy approaches in an autoimmune demyelinating disease:multiple sclerosis. Curr Gene Ther. 2020;19:376–385. doi: 10.2174/1566523220666200306092556. [DOI] [PubMed] [Google Scholar]
- 54.Jankowska E, Stoj J, Karpowicz P, Osmulski PA, Gaczynska M. The proteasome in health and disease. Curr Pharm Des. 2013;19:1010–1028. [PubMed] [Google Scholar]
- 55.Jeffery CJ. Moonlighting proteins--an update. Mol Biosyst. 2009;5:345–350. doi: 10.1039/b900658n. [DOI] [PubMed] [Google Scholar]
- 56.Jiang W, St-Pierre S, Roy P, Morley BJ, Hao J, Simard AR. Infiltration of CCR2+Ly6Chigh proinflammatory monocytes and neutrophils into the central nervous system is modulated by nicotinic acetylcholine receptors in a model of multiple sclerosis. J Immunol. 2016;196:2095–2108. doi: 10.4049/jimmunol.1501613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. 70-kDa heat shock protein downregulates dynamin in experimental stroke:a new therapeutic target? Stroke. 2016;47:2103–2111. doi: 10.1161/STROKEAHA.116.012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JY, Han Y, Lee JE, Yenari MA. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin Ther Targets. 2018;22:191–199. doi: 10.1080/14728222.2018.1439477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SW, Lee JK. NO-induced downregulation of HSP10 and HSP60 expression in the postischemic brain. J Neurosci Res. 2007;85:1252–1259. doi: 10.1002/jnr.21236. [DOI] [PubMed] [Google Scholar]
- 60.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 61.Kokubo K, Onodera A, Kiuchi M, Tsuji K, Hirahara K, Nakayama T. Conventional and pathogenic Th2 cells in inflammation, tissue repair, and fibrosis. Front Immunol. 2022;13:945063. doi: 10.3389/fimmu.2022.945063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman L, Rothbard JB. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:36423–36434. doi: 10.1074/jbc.M112.371229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurnellas MP, Schartner JM, Fathman CG, Jagger A, Steinman L, Rothbard JB. Mechanisms of action of therapeutic amyloidogenic hexapeptides in amelioration of inflammatory brain disease. J Exp Med. 2014;211:1847–1856. doi: 10.1084/jem.20140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurnellas MP, Ghosn EE, Schartner JM, Baker J, Rothbard JJ, Negrin RS, Herzenberg LA, Fathman CG, Steinman L, Rothbard JB. Amyloid fibrils activate B-1a lymphocytes to ameliorate inflammatory brain disease. Proc Natl Acad Sci U S A. 2015;112:15016–15023. doi: 10.1073/pnas.1521206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurop MK, Huyen CM, Kelly JH, Blagg BSJ. The heat shock response and small molecule regulators. Eur J Med Chem. 2021;226:113846. doi: 10.1016/j.ejmech.2021.113846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwon HM, Kim Y, Yang SI, Kim YJ, Lee SH, Yoon BW. Geldanamycin protects rat brain through overexpression of HSP70 and reducing brain edema after cerebral focal ischemia. Neurol Res. 2008;30:740–745. doi: 10.1179/174313208X289615. [DOI] [PubMed] [Google Scholar]
- 67.Lahaye X, Vidy A, Fouquet B, Blondel D. Hsp70 protein positively regulates rabies virus infection. J Virol. 2012;86:4743–4751. doi: 10.1128/JVI.06501-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32:2905–2912. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- 69.Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35:2195–2199. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- 70.Lehmann C, Burkovskiy I, Kuethe J, Zhou J, Caldwell C, Kelly ME. Inhibition of the cannabinoid 2 receptor in CNS-injury induced immunodeficiency syndrome. Med Hypotheses. 2014;82:736–739. doi: 10.1016/j.mehy.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 72.Liu Q, Sanai N, Jin WN, La Cava A, Van Kaer L, Shi FD. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci. 2016;19:243–252. doi: 10.1038/nn.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Q, Jin WN, Liu Y, Shi K, Sun H, Zhang F, Zhang C, Gonzales RJ, Sheth KN, La Cava A, Shi FD. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity. 2017;46:474–487. doi: 10.1016/j.immuni.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 74.Madden KS. Sympathetic neural-immune interactions regulate hematopoiesis, thermoregulation and inflammation in mammals. Dev Comp Immunol. 2017;66:92–97. doi: 10.1016/j.dci.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 75.Manaenko A, Fathali N, Chen H, Suzuki H, Williams S, Zhang JH, Tang J. Heat shock protein 70 upregulation by geldanamycin reduces brain injury in a mouse model of intracerebral hemorrhage. Neurochem Int. 2010;57:844–850. doi: 10.1016/j.neuint.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 77.Martínez-Laorden E, Navarro-Zaragoza J, Milanés MV, Laorden ML, Almela P. Cardiac protective role of heat shock protein 27 in the stress induced by drugs of abuse. Int J Mol Sci. 2020;21:3623. doi: 10.3390/ijms21103623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meisel C, Meisel A. Suppressing immunosuppression after stroke. N Engl J Med. 2011;365:2134–2136. doi: 10.1056/NEJMcibr1112454. [DOI] [PubMed] [Google Scholar]
- 79.Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15:357–361. doi: 10.1097/00001756-200402090-00029. [DOI] [PubMed] [Google Scholar]
- 80.Morán Luengo T, Mayer MP, Rüdiger SGD. The Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell Biol. 2019;29:164–177. doi: 10.1016/j.tcb.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Nowak TS, Jr, Jacewicz M. The heat shock/stress response in focal cerebral ischemia. Brain Pathol. 1994;4:67–76. doi: 10.1111/j.1750-3639.1994.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 82.Ooi K, Hu L, Feng Y, Han C, Ren X, Qian X, Huang H, Chen S, Shi Q, Lin H, Wang J, Zhu D, Wang R, Xia C. Sigma-1 receptor activation suppresses microglia m1 polarization via regulating endoplasmic reticulum-mitochondria contact and mitochondrial functions in stress-induced hypertension rats. Mol Neurobiol. 2021;58:6625–6646. doi: 10.1007/s12035-021-02488-6. [DOI] [PubMed] [Google Scholar]
- 83.Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem. 2010;47:99–114. doi: 10.1042/bse0470099. [DOI] [PubMed] [Google Scholar]
- 84.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pavlov VA, Tracey KJ. Neural regulation of immunity:molecular mechanisms and clinical translation. Nat Neurosci. 2017;20:156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 86.Penke B, Bogár F, Crul T, Sántha M, Tóth ME, Vígh L. Heat shock proteins and autophagy pathways in neuroprotection:from molecular bases to pharmacological interventions. Int J Mol Sci. 2018;19:325. doi: 10.3390/ijms19010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pipaón S, Gragera M, Bueno-Carrasco MT, García-Bernalt Diego J, Cantero M, Cuéllar J, Fernández-Fernández MR, Valpuesta JM. Chaperonins:nanocarriers with biotechnological applications. Nanomaterials (Basel) 2021;11:503. doi: 10.3390/nano11020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pluta R, Kiś J, Januszewski S, Jabłoński M, Czuczwar SJ. Cross-talk between amyloid, tau protein and free radicals in post-ischemic brain neurodegeneration in the form of alzheimer's disease proteinopathy. Antioxidants (Basel) 2022;11:146. doi: 10.3390/antiox11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Procaccini C, Pucino V, De Rosa V, Marone G, Matarese G. Neuro-endocrine networks controlling immune system in health and disease. Front Immunol. 2014;5:143. doi: 10.3389/fimmu.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qi J, Liu Y, Yang P, Chen T, Liu XZ, Yin Y, Zhang J, Wang F. Heat shock protein 90 inhibition by 17-Dimethylaminoethylamino-17-demethoxygeldanamycin protects blood-brain barrier integrity in cerebral ischemic stroke. Am J Transl Res. 2015;7:1826–1837. [PMC free article] [PubMed] [Google Scholar]
- 91.Qin X, Akter F, Qin L, Cheng J, Guo M, Yao S, Jian Z, Liu R, Wu S. Adaptive immunity regulation and cerebral ischemia. Front Immunol. 2020;11:689. doi: 10.3389/fimmu.2020.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ran R, Zhou G, Lu A, Zhang L, Tang Y, Rigby AC, Sharp FR. Hsp70 mutant proteins modulate additional apoptotic pathways and improve cell survival. Cell Stress Chaperones. 2004;9:229–242. doi: 10.1379/CSC-19R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats:potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- 94.Reyes-García MG, Hernández-Hernández F, Hernández-Téllez B, García-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 95.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rockstrom MD, Chen L, Taishi P, Nguyen JT, Gibbons CM, Veasey SC, Krueger JM. Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev. 2018;40:69–78. doi: 10.1016/j.smrv.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rothbard JB, Rothbard JJ, Soares L, Fathman CG, Steinman L. Identification of a common immune regulatory pathway induced by small heat shock proteins, amyloid fibrils, and nicotine. Proc Natl Acad Sci U S A. 2018;115:7081–7086. doi: 10.1073/pnas.1804599115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shao BZ, Ke P, Xu ZQ, Wei W, Cheng MH, Han BZ, Chen XW, Su DF, Liu C. Autophagy plays an important role in anti-inflammatory mechanisms stimulated by alpha7 nicotinic acetylcholine receptor. Front Immunol. 2017;8:553. doi: 10.3389/fimmu.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain:role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4:685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen YC, Wang YH, Chou YC, Liou KT, Yen JC, Wang WY, Liao JF. Dimemorfan protects rats against ischemic stroke through activation of sigma-1 receptor-mediated mechanisms by decreasing glutamate accumulation. J Neurochem. 2008;104:558–572. doi: 10.1111/j.1471-4159.2007.05058.x. [DOI] [PubMed] [Google Scholar]
- 101.Shi K, Wood K, Shi FD, Wang X, Liu Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol. 2018;3:34–41. doi: 10.1136/svn-2017-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. doi: 10.1016/S1474-4422(19)30078-X. [DOI] [PubMed] [Google Scholar]
- 103.Shim R, Wong CH. Ischemia, immunosuppression and infection--tackling the predicaments of post-stroke complications. Int J Mol Sci. 2016;17:64. doi: 10.3390/ijms17010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 105.Stepp MA, Menko AS. Immune responses to injury and their links to eye disease. Transl Res. 2021;236:52–71. doi: 10.1016/j.trsl.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27:mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9:863–872. doi: 10.2174/156652409789105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suda S, Aoki J, Shimoyama T, Suzuki K, Sakamoto Y, Katano T, Okubo S, Nito C, Nishiyama Y, Mishina M, Kimura K. Stroke-associated infection independently predicts 3-month poor functional outcome and mortality. J Neurol. 2018;265:370–375. doi: 10.1007/s00415-017-8714-6. [DOI] [PubMed] [Google Scholar]
- 108.Sykora M, Siarnik P, Diedler J. β-Blockers, pneumonia, and outcome after ischemic stroke:evidence from virtual international stroke trials archive. Stroke. 2015;46:1269–1274. doi: 10.1161/STROKEAHA.114.008260. [DOI] [PubMed] [Google Scholar]
- 109.Tamura Y, Torigoe T, Kutomi G, Hirata K, Sato N. New paradigm for intrinsic function of heat shock proteins as endogenous ligands in inflammation and innate immunity. Curr Mol Med. 2012;12:1198–1206. doi: 10.2174/156652412803306710. [DOI] [PubMed] [Google Scholar]
- 110.Tang Y, Zhou Y, Fan S, Wen Q. The multiple roles and therapeutic potential of HSP60 in cancer. Biochem Pharmacol. 2022;201:115096. doi: 10.1016/j.bcp.2022.115096. [DOI] [PubMed] [Google Scholar]
- 111.Tedesco B, Cristofani R, Ferrari V, Cozzi M, Rusmini P, Casarotto E, Chierichetti M, Mina F, Galbiati M, Piccolella M, Crippa V, Poletti A. Insights on human small heat shock proteins and their alterations in diseases. Front Mol Biosci. 2022;9:842149. doi: 10.3389/fmolb.2022.842149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terrell EM, Morrison DK. Ras-mediated activation of the raf family kinases. Cold Spring Harb Perspect Med. 2019;9:a033746. doi: 10.1101/cshperspect.a033746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsuchiya D, Hong S, Matsumori Y, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery. 2003;53:1179–1188. doi: 10.1227/01.neu.0000090341.38659.cf. [DOI] [PubMed] [Google Scholar]
- 114.Urra X, Villamor N, Amaro S, Gómez-Choco M, Obach V, Oleaga L, Planas AM, Chamorro A. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 115.van der Weerd L, Tariq Akbar M, Aron Badin R, Valentim LM, Thomas DL, Wells DJ, Latchman DS, Gadian DG, Lythgoe MF, de Belleroche JS. Overexpression of heat shock protein 27 reduces cortical damage after cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:849–856. doi: 10.1038/jcbfm.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Eden W, Jansen MAA, Ludwig I, van Kooten P, van der Zee R, Broere F. The enigma of heat shock proteins in immune tolerance. Front Immunol. 2017;8:1599. doi: 10.3389/fimmu.2017.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vogelgesang A, Becker KJ, Dressel A. Immunological consequences of ischemic stroke. Acta Neurol Scand. 2014;129:1–12. doi: 10.1111/ane.12165. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Le WD. Autophagy and ubiquitin-proteasome system. Adv Exp Med Biol. 2019;1206:527–550. doi: 10.1007/978-981-15-0602-4_25. [DOI] [PubMed] [Google Scholar]
- 119.Wang YL, Lin CH, Chen CC, Chang CP, Lin KC, Su FC, Chou W. Exercise preconditioning attenuates neurological injury by preserving old and newly formed HSP72-containing neurons in focal brain ischemia rats. Int J Med Sci. 2019;16:675–685. doi: 10.7150/ijms.32962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Westendorp WF, Dames C, Nederkoorn PJ, Meisel A. Immunodepression, infections, and functional outcome in ischemic stroke. Stroke. 2022;53:1438–1448. doi: 10.1161/STROKEAHA.122.038867. [DOI] [PubMed] [Google Scholar]
- 121.Woiciechowsky C, Schöning B, Lanksch WR, Volk HD, Döcke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med (Berl) 1999;77:769–780. doi: 10.1007/s001099900051. [DOI] [PubMed] [Google Scholar]
- 122.Wong CH, Jenne CN, Lee WY, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 123.Zatsepina OG, Evgen'ev MB, Garbuz DG. Role of a heat shock transcription factor and the major heat shock protein Hsp70 in memory formation and neuroprotection. Cells. 2021;10:1638. doi: 10.3390/cells10071638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao H, Michaelis ML, Blagg BS. Hsp90 modulation for the treatment of Alzheimer's disease. Adv Pharmacol. 2012;64:1–25. doi: 10.1016/B978-0-12-394816-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 125.Zhou Y, Liao J, Mei Z, Liu X, Ge J. Insight into crosstalk between ferroptosis and necroptosis:novel therapeutics in ischemic stroke. Oxid Med Cell Longev. 20212021:9991001. doi: 10.1155/2021/9991001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


