Multiple sclerosis (MS) is regarded as an immune-mediated, demyelinating disorder of the central nervous system, however neuroaxonal degeneration is accepted as the principal substrate of disability accumulation (Dutta and Trapp, 2011). Neurodegeneration occurs throughout the course of MS and is detectable even in the earliest stages of the disease (Azevedo et al., 2018). Mechanisms of neurodegeneration in MS are complex and not completely understood. Neuro-axonal transection or degeneration can occur within acutely or chronically demyelinated MS lesions, and also within normal-appearing white and gray matter (Dutta and Trapp, 2011). At a tissue level, pathologic contributors to neuro-axonal degeneration may include inflammatory injury, loss of trophic support, retrograde and anterograde degeneration, failure of remyelination, impaired axonal transport, microglial activation, mitochondrial injury, energy failure, oxidative injury, iron accumulation and tissue hypoxia (Dutta and Trapp, 2011; Mahad et al., 2015). One putative mechanism of neurodegeneration is trans-synaptic degeneration – whereby injury to a neuron or axon leads to the degeneration of synaptically-connected neurons. Theoretically, trans-synaptic degeneration may proceed anterogradely (“dying forward”) or retrogradely (“dying back”), ultimately resulting in the loss of neurons in discrete but distant central nervous system locations (Figures 1 and 2). Based on this mechanism, trans-synaptic degeneration could occur in MS both in demyelinated tissue and in normal-appearing tissue. However, while trans-synaptic degeneration is biologically plausible in MS, and supported by indirect evidence (Rocca et al., 2013; Gabilondo et al., 2014), the “real time” documentation of trans-synaptic degeneration has been challenging. Pathological studies of MS are typically not suitable for assessing trans-synaptic changes, since post-mortem studies can only assess a single pathological timepoint, and post-mortem samples of MS brains are usually acquired from older patients – often years after the phase of the disease involving overt inflammatory activity. Furthermore, radiological capturing of trans-synaptic degeneration in vivo has been a major challenge due to the limitations of imaging technology and the complex synaptically-connected central nervous system networks which can be affected by many MS lesions.
Figure 1.
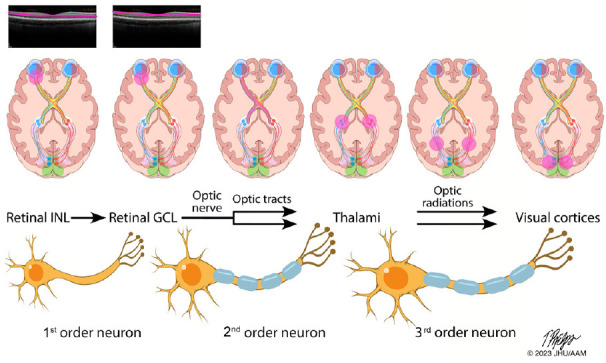
Functionally-eloquent organization of the afferent visual pathway.
The afferent visual pathway is a sensory pathway comprised of three neurons. The 1st order neurons are the shortest neurons in the pathway and are entirely unmyelinated. The cell bodies of the 1st order neurons lie in the retinal inner nuclear layer (INL), with the axons traveling to the retinal ganglion cell layer (GCL) where they synapse with the cell bodies of the 2nd order neurons. In the top row of images, the INL and GCL are highlighted here on a spectral domain optical coherence tomography image of a healthy retina (Spectralis HD-OCT, Heidelberg, Germany). The axons of the 2nd order neuron pass through the peripapillary retinal nerve fiber layer and coalesce to make up the optic nerve, becoming myelinated after they pass through lamina cribrosa. As illustrated in the second row of images, axons from the nasal half of the retina are organized within the optic nerve and cross over to the contralateral cerebral hemisphere at the optic chiasm, while axons from the temporal half of the retina remain uncrossed and continue their path within the ipsilateral cerebral hemisphere. After traversing the optic tracts, the axons of the 2nd order neurons synapse with the cell bodies of the 3rd order neurons in the lateral geniculate nucleus of the thalamus, with each thalamus receiving signals from the right and left eye, due to the crossed and uncrossed nature of the pathway. The axons of the 3rd order neurons then travel within the optic radiations to reach the primary visual cortex of the occipital lobe. From: https://collections.lib.utah.edu/ark:/87278/s6ty03rf.
Figure 2.
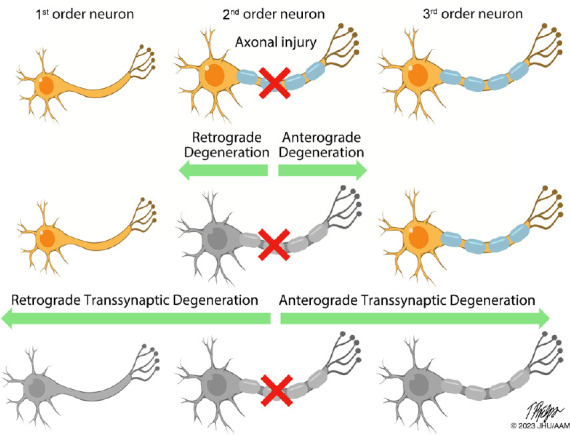
Neuroaxonal degeneration in the afferent visual pathway following axonal injury.
This figure illustrates the potential patterns of neuroaxonal degeneration that may occur after injury to an axon in the afferent visual pathway (e.g., with optic neuritis), or in other synaptically-connected pathways. Following axonal injury, the affected neuron may degenerate in both an anterograde (“dying forward”) or retrograde (“dying back”) direction. Following degeneration of the injured neuron, neurodegeneration may proceed trans-synaptically to the other neurons in the chain, again in an anterograde or retrograde direction, resulting in loss of distant uninjured but synaptically-connected neurons. From: https://collections.lib.utah.edu/ark:/87278/s6ty03rf.
The visual pathway is frequently probed in the examination of neurodegenerative processes in MS, and episodes of acute optic neuritis (AON) represent opportune circumstances for the evaluation of trans-synaptic degeneration for several reasons. The diagnosis of AON can be made quickly and reliably based on clinical and paraclinical findings. AON is the first clinical manifestation of MS in up to 25% of patients and occurs in around 50% of people with MS (PwMS) over the course of the disease. After AON, functional visual outcomes can be sensitively quantified at a monocular level with low-contrast visual acuity (Balcer et al., 2017), while structural tissue outcomes can be reliably measured using retinal optical coherence tomography (OCT) (Al-Louzi et al., 2016). Most importantly, the anterior visual pathway and posterior visual pathways are organized in a functionally eloquent manner, and so the expected consequences of trans-synaptic processes can be localized to distinct structures (Figure 3). Retrograde structural changes in the retina have been well-described during and after AON (Al-Louzi et al., 2016), and there is evidence of long-term structural differences in the posterior visual pathway in people with a history of AON (Rocca et al., 2013; Gabilondo et al., 2014). However, the temporal dynamics, magnitude and clinical relevance of anterograde trans-synaptic degeneration in the cerebral portions of the visual pathway after AON have not been well-elucidated previously.
Figure 3.
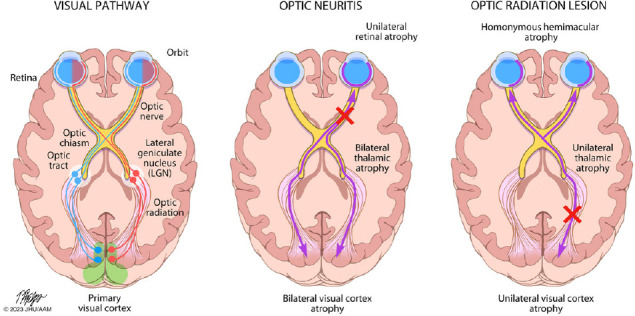
Patterns of trans-synaptic degeneration in the afferent visual pathway in multiple sclerosis.
The visual pathway is a functionally eloquent sensory pathway made up of three neurons, traveling from the retina to the primary visual cortex of the occipital lobe. The axons of the 2nd order neurons (traveling from the retinal ganglion cell layer through the optic nerve and optic tracts to the thalami) are highly organized, with the axons from the nasal half of the retina crossing over to the contralateral cerebral hemisphere within the optic chiasm, while the axons from the temporal half of the retina remain uncrossed and continue their path within the ipsilateral cerebral hemisphere. The crossed and uncrossed nature of the pathway means that each thalamus and visual cortex receives inputs from the right and left eye in a homonymous pattern. In optic neuritis, injury to the axons of the 2nd order neurons (prior to the optic chiasm) can result in anterograde degeneration of affected axons, a process which may then proceed trans-synaptically, resulting in degeneration of the 3rd order neurons traveling from both thalami to both primary visual cortices. Neuroaxonal degeneration after optic neuritis may also proceed in the retrograde direction, resulting in loss of cell bodies in the ipsilateral retinal ganglion cell layer (GCL), and potentially trans-synaptically to the 1st order neurons contained in the ipsilateral retinal inner nuclear layer (INL). On the other hand, if a demyelinating lesion causes axonal injury to the 3rd order neurons (e.g. within the optic radiations), anterograde neuroaxonal degeneration may result in atrophy of the ipsilateral visual cortex, while retrograde degeneration may proceed to the ipsilateral thalamus, and potentially trans-synaptically to the highly-organized crossed and uncrossed 2nd order neurons, resulting in a homonymous pattern of atrophy of the retinal ganglion cell layer (and possibly even trans-synaptically again to the 1st order neurons within the retinal inner nuclear layer). From: https://collections.lib.utah.edu/ark:/87278/s6ty03rf.
In a recent study (Murphy et al., 2022), we probed the process of trans-synaptic degeneration after AON in people with MS or high-risk clinically isolated syndrome. We hypothesized that after AON, neurodegeneration may proceed through the optic tracts, and then trans-synaptically, ultimately resulting in the degeneration of neurons projecting from the thalamus through the optic radiations to the primary visual cortex. Since the visual pathway is both crossed and uncrossed, unilateral AON could result in neuronal loss in both occipital lobes. In addition to documenting “real-time” evidence for trans-synaptic degeneration after AON, we aimed to assess whether the magnitude of retinal tissue injury after AON was related to the magnitude of subsequent atrophy in the brain and relevant brain substructures and whether the extent of trans-synaptic degeneration in the posterior visual pathway is related to post-AON visual outcomes. In this study, 49 participants were recruited within 40 days of AON symptom onset (the AON cohort), and 73 PwMS without a recent episode of AON were recruited for the comparator (non-AON) cohort. Participants were tracked for up to 5 years, undergoing serial retinal OCT imaging with processing, segmentation and quantification of retinal layer thicknesses using techniques developed at Johns Hopkins University that have been shown to perform reliably in cross-sectional and longitudinal studies of PwMS and healthy volunteers. Study participants also underwent annual brain MRI scans with validated techniques employed to segment the brain and brain substructures. Due to the structure of the visual pathway (with approximately half of the optic nerve fibers crossing within the optic chiasm and entering the contralateral optic tract, and the other half remaining uncrossed and entering the ipsilateral optic tract), the analyses utilized summed measures of right and left brain substructure volumes. Statistical analyses accounted for age, sex, time since disease onset, and baseline retinal layer thicknesses or brain substructure volumes.
The primary finding of our study was that the AON cohort exhibited significantly accelerated atrophy of the thalamus and the primary visual cortex (occipital gray matter and calcarine gray matter), as compared to the non-AON cohort over the study period (–1.17% versus –0.67% per year [P = 0.02] for thalamus, –0.76% versus –0.22% per year [P = 0.01] for occipital gray matter, –1.83% versus –0.32% per year [P = 0.008] for calcarine gray matter) (Murphy et al., 2022). In the analysis of changes in the retinal layer thickness, at 1 year post-AON, expected reductions were seen in the ganglion cell-inner plexiform layer (GCIPL – containing the cell bodies of the neurons that make up the optic nerve) and peripapillary retinal nerve fiber layer (containing the axons that make up the optic nerve) thicknesses in the AON cohort. Additionally, reductions in inner nuclear layer (INL) thickness over the first year post-AON (after resolution of bilateral INL and outer nuclear layer swelling which has been well-documented as characteristic in the months after AON) were found to be greater in AON eyes as compared to the non-AON eyes (–3.73% versus –2.34%, P = 0.02), suggesting possible retrograde trans-synaptic changes within the INL, containing the bipolar cells that synapse with the ganglion cell neurons of the optic nerve. Overall, these results offer real-time in vivo evidence for anterograde trans-synaptic degeneration in the posterior visual pathway after AON and possible retrograde trans-synaptic degeneration in the retina after AON. Furthermore, the magnitude of anterograde trans-synaptic degeneration appears to be linked (at least in part) to the magnitude of tissue injury in the inciting inflammatory event, since our results showed a significant relationship between greater reductions in GCIPL thickness over the first year after AON and faster thalamic atrophy over the study period. Finally, significant relationships were also seen between worse visual outcomes after AON, and faster atrophy of the thalamus and subcortical gray matter, supporting the hypothesis that trans-synaptic degeneration is likely to be clinically relevant.
Concomitant bidirectional (anterograde and retrograde) neurodegeneration has been demonstrated in animal studies after retinal ganglion cell axotomy (Kanamori et al., 2012), and in MS, neuroaxonal and trans-synaptic degeneration in both directions may be an important consequence of axonal injury. Interestingly, our study findings support the occurrence of retrograde trans-synaptic degeneration of the INL of the retina after AON, in addition to anterograde trans-synaptic degeneration of the thalamus and primary visual cortex. Along these lines, there is already some evidence to support the occurrence of retrograde trans-synaptic degeneration from the posterior visual pathways to the optic nerve in MS. Studies using retinal OCT have demonstrated that in some patients with an established unilateral demyelinating lesion located in one of the optic radiations of the posterior visual pathway, a hemi-macular pattern of thinning of the GCIPL can be seen, suggesting loss of neurons in areas of the retina topographically related to the posterior visual pathway lesion (Al-Louzi et al., 2017). Quantitative analysis has also suggested that lesion volume in the optic radiation is at least cross-sectionally related to thicknesses of the neuronal retinal layers in PwMS without a history of AON, and diffusion tensor imaging metrics of optic radiation integrity are related to thicknesses of the neuronal retinal layers in PwMS with and without a history of AON (Balk et al., 2015), providing more support for bidirectional trans-synaptic degeneration in MS.
The documentation of trans-synaptic neurodegeneration in PwMS is important from both a research and clinical perspective. Unraveling the contribution of specific pathobiologic mechanisms of neurodegeneration in MS is essential for facilitating the development of the next frontier of MS therapeutics - neuroprotective and neurorestorative agents. Indeed, several clinical trials (either completed or underway) have utilized the anterior visual pathway as an opportune region to test the remyelinating or neuroprotective effect of several novel therapies (Raftopoulos et al., 2016; Cadavid et al., 2017). While GCIPL thinning can be considered the optimal measure of the extent of immediate neuronal injury after AON, this thinning occurs so rapidly (within weeks) that it may be an insensitive measure for the examination of potential neuroprotective effects of a therapy that is not rapidly optimized, or not administered in the hyperacute phase of AON onset. However, it seems likely that an effective remyelinating or neuroprotective therapy would have the ultimate impact of reducing anterograde and retrograde trans-synaptic pathology after AON. Thus, retinal layer changes occurring in the subsequent months (swelling of the INL and outer nuclear layer in the intermediate term, and later thinning of the INL) and brain substructure changes occurring in the subsequent months to years (thalamic and occipital gray matter atrophy) may represent potential novel outcome measures to be explored and tested in future clinical trials of this nature. Based on the reported clinical relationships (Murphy et al., 2022), trans-synaptic degeneration may also represent a possible signature of aggressive MS, with patients who experience poorer clinical outcomes after an inflammatory relapse experiencing a greater extent of subsequent trans-synaptic changes. In the era of the precision medicine approach to MS, the tracking of trans-synaptic degeneration with advanced quantitative imaging techniques represents one potential future biomarker that could contribute to the multimodal clinical, laboratory, and radiological assessment used to inform prognostication, therapeutic decision making, and disease monitoring. However, due to limitations in the precision, reliability and repeatability of the measurements, quantitative retinal OCT and quantitative MRI are both technologies that require further refinement to enable reliable longitudinal tracking of small magnitude changes at an individual level.
Further work is required to elucidate some crucial questions regarding trans-synaptic degeneration in MS. Since evidence for trans-synaptic degeneration comes from cohort studies, it is uncertain if the process is ubiquitous in all PwMS, or may be limited to certain individuals with a propensity to trans-synaptic pathology. Trans-synaptic degeneration could be a marker of more aggressive primary inflammatory demyelinating pathology, poorer tissue repair, or remyelination failure. It is unknown whether the degeneration of synaptically-connected neuronal networks after an inciting inflammatory event may be modified by factors such as disease-modifying treatments, or numerous other modifiers of the MS disease course (e.g., age, sex, metabolic or vascular co-morbidities) that have not been explored in studies of this process to date. The relative contribution of macroscopic inflammatory activity (clinical relapses and new or enhancing MRI lesions) versus microstructural tissue changes to trans-synaptic degeneration of neuronal circuits at a global level is also undetermined. The optimal imaging techniques for the examination of trans-synaptic degeneration in vivo are also unclear. While non-conventional MRI with quantitative metrics has been utilized to study these mechanisms, these techniques have not yet entered routine clinical practice for multiple reasons including the complexity of the image acquisition and processing, the cost and time involved, and the reliability and reproducibility of the metrics at an individual level. On the other hand, retinal OCT is increasingly employed in routine clinical practice as a quantitative imaging tool with high levels of reliability and reproducibility and high sensitivity to capture change at an individual level, which could facilitate lower-cost clinic-based examination of retrograde trans-synaptic changes in the retina (due to either AON or posterior visual pathway pathology). Additionally, it is not yet clear whether clinical markers such as low-contrast letter acuity outcomes could be used as surrogate markers of trans-synaptic degeneration. Further work is needed to examine the occurrence of anterograde and retrograde trans-synaptic degeneration within the visual pathway and beyond the visual pathway in PwMS, to define quantitative markers of trans-synaptic degeneration that could be employed at an individual level, and to address the other important questions raised here.
In conclusion, accumulating evidence supports bidirectional trans-synaptic degeneration as a contributing pathway of neurodegeneration in MS. Further examination of these processes in vivo is likely to yield important information regarding MS pathobiology, offer novel biomarkers for examining the effects of neuroprotective or remyelinating therapies, and potentially lead to the identification of new therapeutic targets.
This work was funded by the National MS Society (RG-1606-08768 & RG-1907-34405 to SS), Race to Erase MS (to SS), and NIH/NINDS (R01NS082347 to PAC).
OCM receives funding from the Race to Erase MS Foundation.
PAC has received consulting fees from Disarm, Nervgen, and Biogen and is Principal Investigator on grants to JHU from Biogen, Genentech, Prinicpia, and Annexon.
SS has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Novartis, Genentech Corporation, TG therapeutics & Bristol Myers Squibb. He has performed consulting for Novartis, Genentech Corporation, JuneBrain LLC, and Lapix therapeutics. He is the Principal Investigator of investigator-initiated studies funded by Genentech Corporation and Biogen. He previously received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC and Lapix therapeutics. He was also the site investigator of trials sponsored by MedDay Pharmaceuticals, Clene Pharmaceuticals, and is the site investigator of a trial sponsored by Novartis.
The authors report no other potential conflicts of interest related to this manuscript.
Additional file: Open peer review report 1 (80.1KB, pdf) .
Footnotes
P-Reviewer: Knier B; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Open peer reviewer: Benjamin Knier, Technical University of Munich, Germany.
References
- 1.Al-Louzi OA, Bhargava P, Newsome SD, Balcer L, Frohman E, Crainiceanu C, Calabresi PA, Saidha S. Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22:362–372. doi: 10.1177/1352458515590646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Louzi O, Button J, Newsome SD, Calabresi PA, Saidha S. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis:a case series. Mult Scler. 2017;23:1035–1039. doi: 10.1177/1352458516679035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo CJ, Cen SY, Khadka S, Liu S, Kornak J, Shi Y, Zheng L, Hauser SL, Pelletier D. Thalamic atrophy in multiple sclerosis:a magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol. 2018;83:223–234. doi: 10.1002/ana.25150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balcer LJ, Raynowska J, Nolan R, Galetta SL, Kapoor R, Benedict R, Phillips G, LaRocca N, Hudson L, Rudick R. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:734–747. doi: 10.1177/1352458517690822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balk LJ, Steenwijk MD, Tewarie P, Daams M, Killestein J, Wattjes MP, Vrenken H, Barkhof F, Polman CH, Uitdehaag BMJ, Petzold A. Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:419–424. doi: 10.1136/jnnp-2014-308189. [DOI] [PubMed] [Google Scholar]
- 6.Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, Frederiksen J, Skeen M, Jaffe GJ, Butzkueven H, Ziemssen F, Massacesi L, Chai Y, Xu L, Freeman S. RENEW Study Investigators (2017) Safety and efficacy of opicinumab in acute optic neuritis (RENEW):a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 16:189–199. doi: 10.1016/S1474-4422(16)30377-5. [DOI] [PubMed] [Google Scholar]
- 7.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, Bullich S, Sepulveda M, Falcon C, Berenguer J, Saiz A, Sanchez-Dalmau B, Villoslada P. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75:98–107. doi: 10.1002/ana.24030. [DOI] [PubMed] [Google Scholar]
- 9.Kanamori A, Catrinescu M, Belisle JM, Costantino S, Levin LA. Retrograde and Wallerian axonal degeneration occur synchronously after retinal ganglion cell axotomy. Am J Pathol. 2012;181:62–73. doi: 10.1016/j.ajpath.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 11.Murphy OC, Sotirchos ES, Kalaitzidis G, Vasileiou E, Ehrhardt H, Lambe J, Kwakyi O, Nguyen J, Zambriczki Lee A, Button J, Dewey BE, Newsome SD, Mowry EM, Fitzgerald KC, Prince JL, Calabresi PA, Saidha S. Trans-synaptic degeneration following acute optic neuritis in multiple sclerosis. Ann Neurol. 2022;93:76–87. doi: 10.1002/ana.26529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raftopoulos R, Hickman SJ, Toosy A, Sharrack B, Mallik S, Paling D, Altmann DR, Yiannakas MC, Malladi P, Sheridan R, Sarrigiannis PG, Hoggard N, Koltzenburg M, Wheeler-Kingshott C, Schmierer K, Giovannoni G, Miller DH, Kapoor R. Phenytoin for neuroprotection in patients with acute optic neuritis:a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:259–269. doi: 10.1016/S1474-4422(16)00004-1. [DOI] [PubMed] [Google Scholar]
- 13.Rocca MA, Mesaros S, Preziosa P, Pagani E, Stosic-Opincal T, Dujmovic-Basuroski I, Drulovic J, Filippi M. Wallerian and trans-synaptic degeneration contribute to optic radiation damage in multiple sclerosis:a diffusion tensor MRI study. Mult Scler. 2013;19:1610–1617. doi: 10.1177/1352458513485146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


