Abstract
Biomarkers are molecules of biological processes that help in both the diagnosis of human diseases and in follow-up assessments of therapeutic responses. Biomarkers can be measured in many human fluids, such as blood, cerebrospinal fluid, urine, and saliva. The -omics methods (genomics, RNomics, proteomics, and metabolomics) are useful at measuring thousands of markers in a small volume. Saliva is a human fluid that is easily accessible, without any ethical concerns. Yet, saliva remains unexplored in regard to many human disease biomarkers. In this review, we will give an overview on saliva and how it can be influenced by exogenous factors. As we focus on the potential use of saliva as a diagnostic tool in brain disorders (especially Alzheimer’s disease), we will cover how saliva is linked to the brain. We will discuss that saliva is a heterogeneous human fluid, yet useful for the discovery of biomarkers in human disorders. However, a procedure and consensus that is controlled, validated, and standardized for the collection and processing of saliva is required, followed by a highly sensitive diagnostic approach.
Key Words: Alzheimer’s disease, biomarkers, diagnosis, oral-gut-brain axis, periodontitis, saliva
Saliva
Saliva is a human fluid that is easily accessible, without any ethical concerns. Yet, saliva remains unexplored regarding many human disease biomarkers. The saliva proteome contains approximately 2300 proteins, 27% of which are identical to plasma proteins (Loo et al., 2010). Saliva is the fluid that bathes the mouth and oral cavity. It is made up of secretions from the salivary glands (parotid, submandibular, and sublingual), gingival cervicular fluid, nasal and bronchial secretions, and various other secreted molecules (e.g., hormones, amino acids, and metabolites) and components (e.g., food and microorganisms) (Kaufman and Lambster, 2002; Loo et al., 2010). Saliva is colorless, odorless, and has a pH of 6.6–7.1. A normal person produces 0.7–1.5 L saliva per day with a normal salivary flow of approximately 0.6–1 mL/min. Saliva is composed of water (99%) and includes many peptides, proteins, hormones, enzymes, sugars, lipids, and electrolytes. The saliva metabolome appears to be comparable to the metabolomes of human serum and cerebrospinal fluid (CSF) in terms of chemical complexity and the number of compounds (Dame et al., 2015). This is consistent with the data from a previous study, which showed that compounds found in human saliva are usually found in human blood, albeit at different concentrations (Takeda et al. 2009). The function of saliva is manifold. It eliminates bacteria, catalyzes the hydrolysis of sugars, and contributes to immune responses.
Salivary Flow and Composition
The composition and flow of saliva are affected by numerous factors (see Figure 1), including natural conditions, human diseases, and exogenous causes such as medication, therapy, lifestyle, and habits. Age has a significant effect on several salivary parameters. While salivary flow, calcium, and mucin content decrease in the saliva of older adult subjects, protein concentration, amylolytic activity, lipolytic activity, and ionic concentration increase. Regarding gender, females show markedly lower flow rates of both unstimulated and stimulated saliva than males (Percival et al., 1994; Mosca et al., 2019). Saliva is affected by various illnesses, such as Alzheimer’s disease (AD), in which the salivary flow and buffering capacity are decreased (Aragón et al., 2018). Severe periodontitis is strongly correlated with low salivary pH values and flow rate (Lăzureanu et al., 2021). Even in symptom-free periods, children with juvenile recurrent parotitis show decreased salivary flow rates, buffering capacity, and phosphate concentration, while chloride, sodium, and copper concentrations, as well as albumin, IgA, lactoferrin, and kallikrein levels, are raised. Additionally, children with chronic tonsillitis present high levels of cytokine tumor necrosis factor-alpha, and interleukin-1 and -6 in saliva (Ericson and Sjöbäck, 1996). Salivary flow is decreased in type 1 diabetes mellitus in children/adolescents (Liu et al., 2021), and also in adults with lower salivary pH and higher urea levels (Marques et al., 2022). Adult patients with rheumatic diseases exhibit reduced oral health-related quality of life, especially in diseases with oral manifestations caused by salivary flow and composition changes, like Sjögren syndrome (Schmalz et al., 2020). Autoimmune diseases are also associated with salivary gland dysfunctions (Moosavi and Barati, 2020). Reduced salivary flow rate and increased pH and phosphorus concentration in the saliva have been reported in chronic kidney disease patients (Rodrigues et al., 2022). Low salivary flow rates were demonstrated among stroke patients (Dai et al., 2015), whereas a markedly lower salivary pH can be found in individuals diagnosed with autism spectrum disorder (Lam et al., 2020). Children with growth stunting tend to have decreased salivary flow rate and composition, and among bulimics, the unstimulated whole salivary flow rate is reduced, while the stimulated whole salivary flow rate is not affected (Sadida et al., 2022).
Figure 1.
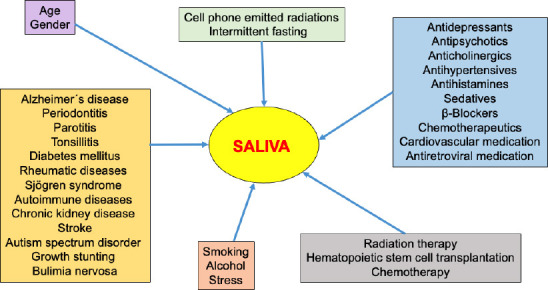
Endogenous and exogenous factors influence saliva production, secretion, and composition.
These external factors must be considered for the discovery of saliva biomarkers.
More than 100 chemical substances have been found to cause medication-induced salivary gland dysfunction, xerostomia, and subjective sialorrhea (Wolff et al., 2017). Among that, a great variety of drugs, such as antidepressants, antipsychotics, anticholinergics, antihypertensives, antihistamines, and sedatives, are reported to be the main medications that cause hyposalivation or xerostomia. Chemotherapeutic substances, antihistamines, antidepressants, and β-blockers are associated with a change in salivary composition. The prevalence of medication-induced salivary gland dysfunction caused by frequently prescribed antihypertensives, antidepressants, cardiovascular and antiretroviral medication ranges from 8% to 71% (Villa et al., 2015). Altered salivary flow is frequently observed in patients with head and neck cancer after treatment with radiation therapy. Most frequently, patients suffer from decreased salivation or xerostomia, but excessive salivary flow or sialorrhea can be a burden as well (Bomeli et al., 2008). After hematopoietic stem cell transplantation, the salivary flow rate is decreased; after 1 week, salivary cytokines represent an inflammatory response, and 6 months later, higher levels of antimicrobial salivary proteins can be detected (van Leeuwen et al., 2019). Decreased salivary flow rate and many more oral manifestations are reported in children after chemotherapy (Busenhart et al., 2018).
In alcohol- and tobacco-dependent individuals, the salivary protein profile exhibits strong changes (Batista et al., 2019). Smoking additionally increases salivary flow rate, calcium concentration, and pH value (Macgregor, 1988). Furthermore, smoking e-cigarettes—alone or in combination with conventional smoking—increases levels of proinflammatory cytokines, tumor necrosis factor-alpha, and interleukin-1β, and decreases the level of anti-inflammatory cytokine interleukin-1RA in saliva (Verma et al., 2021). After alcohol consumption, there is a significant decrease in stimulated whole-saliva flow rate, amylase activity, total protein concentration, and electrolyte concentrations (Enberg et al., 2001). As a reaction to acute stress, inflammatory markers increase in the saliva (Slavish and Szabo, 2019). Even lifestyle may influence saliva secretion. Radiations emitted by cell phones have adverse effects on salivary glands, causing changes in the salivary flow rate and the cytokine expression profile (Mishra et al., 2017). Intermittent fasting, like in Ramadan, results in decreased salivary flow rate, salivary melatonin, glucose, aspartate aminotransferase, and IgA levels, but increases salivary levels of uric acid and alkaline phosphatase (Besbes et al., 2022).
Saliva and the Brain
There seems to be a bidirectional relationship between saliva and the human brain (Sansores-España et al., 2021). The oral-brain axis contains various possible pathways: (1) the neural pathway, with direct anatomic routes along several cranial nerves, (2) the intranasal pathway, (3) the lymphatic pathway, (4) the sublingual route, and (5) the transport of bacteria and inflammatory cytokines to the brain via the peripheral bloodstream. Additionally, (6) the oral-gut-brain axis via the vagal nerve must be considered (Figure 2).
Figure 2.
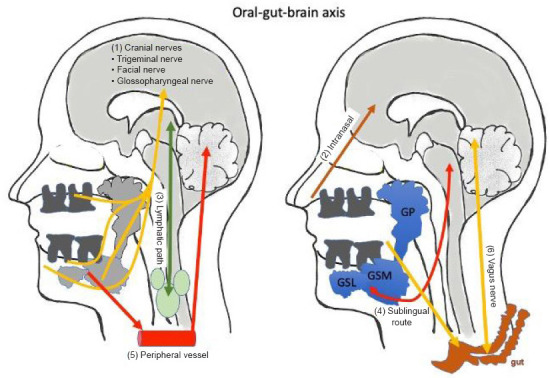
Saliva is connected to the brain via the oral-gut-brain axis.
Saliva is secreted from three glands: the parotid gland (GP), the submandibular gland (GSM) and the sublingual gland (GSL). Molecules from the brain can communicate with the saliva or vice versa via six different routes: (1) the cranial nerves, (2) the intranasal pathway, (3) the lymphatic pathway, (4) the sublingual route, (5) the peripheral bloodstream, or (6) the oral-gut-brain axis and the vagus nerve. This figure was created with PowerPoint.
The trigeminal nerve, which courses through the parotid gland, is a possible route for the transport of oral microorganisms into the brain. A study reported that oral Treponema spp. was found in the trigeminal ganglia, pons, and hippocampus of AD patients (Riviere et al., 2002). In the hippocampus, oral Treponema denticola and Porphyromonas gingivalis can induce inflammation and beta-amyloid (Aβ) accumulation (Ilievski et al., 2018; Su et al., 2021). Treponema pallidum was identified in the axons of peripheral nerves (Sell and Salman, 1992). Further anatomical neural pathways include the facial nerve, via the nervus intermedius, with its sensory fibers for the anterior tongue and soft palate; and the chorda tympani, the parasympathetic innervation to the submandibular and sublingual salivary glands, which induces salivary secretion (Monkhouse, 1990). The glossopharyngeal nerve—the parasympathetic innervation of the parotid gland—is another anatomic connection to the brain. Additionally, the glossopharyngeal nerve seems to have a special role as a neural pathway for immune-to-brain communication (Romeo et al., 2001). The sympathetic and parasympathetic innervation of the three pairs of major salivary glands has been reviewed by Toan and Ahn (2021).
Furthermore, salivary molecules can reach the nasal cavity and migrate along the nasal-olfactory pathway into the brain (see review Humpel, 2022). The finding of a lymphatic connection between the central nervous system (CNS) and the deep cervical lymph nodes (Louveau et al., 2015) represents a potentially new pathway for the dissemination of oral pathogens—and their metabolites and cytokines—to the brain, and vice versa.
The highly vascularized sublingual mucosa is a favored method of drug administration due to its avoidance of the first-pass effect (Assinder et al., 1977). In the same way, salivary components can be transferred into the brain via the sublingual route. In rats, lactoferrin levels in the brain significantly decreased after a sialoadenectomy, but significantly increased 5 minutes after sublingual administration of bovine lactoferrin (Hayashi et al., 2017).
Transient bacteremia happens frequently in everyday life during tooth brushing, flossing, and chewing (Forner et al., 2006), allowing oral bacteria to migrate to other tissues, and organs, and also enter the brain (Li et al., 2000; Poole et al., 2015). Porphyromonas gingivalis is even capable of actively entering endothelial cells (Deshpande et al., 1998). The bacteria-induced, pro-inflammatory cytokines result in systemic low-grade chronic inflammation and can induce neuroinflammation and influence brain function (Ishida et al., 2017; Ilievski et al., 2018; Zhang et al., 2018).
The gut-brain axis is a bidirectional pathway, linking the gut with the brain via the brainstem (Rhee et al., 2009; Carabotti et al., 2015). Oral bacteria swallowed with saliva can induce gut dysbiosis, alteration of intestinal integrity, and gut inflammation, which can further trigger neuroinflammation through communication via different neurotransmitters and the vagus nerve (Arimatsu et al., 2014; Feng et al., 2020; Xue et al., 2020). This pathway also seems to be of importance in prion diseases, as mutated prion-like protein pathogens from the gut can spread via the vagus nerve to the brainstem. This is of interest for Creutzfeldt-Jakob disease, Parkinson’s disease (mutated synuclein), and also AD (mutated Aβ).
The pre-condition for the last two pathways is a leaky blood-brain barrier (BBB). The leaky BBB might be induced by systemic inflammatory mediators in the bloodstream (Abbott, 2000), which pass from the periphery across the BBB into the brain. This creates a vicious cycle of neuroinflammation, BBB permeability, and cognitive decline, which is common in neurodegenerative disorders, such as AD (Pan and Kastin, 1999; Jaeger et al., 2009; Bowman et al., 2018).
Diagnostic Biomarkers in Saliva
Human saliva is an accessible and powerful diagnostic fluid for different diseases, including AD (Zhang et al., 2012; Huan et al., 2018; Gleerup et al., 2019; Liang and Lu, 2019; Marksteiner et al., 2019, 2022; François et al., 2021). Saliva contains many different molecules, and thus, alterations of these compounds may help in the diagnosis of different diseases. It is out of the focus of this review to accurately describe all the diagnostic salivary biomarkers, so we refer to a review by Zhang et al. (2016) on this issue. Briefly, saliva may be useful for the diagnosis of (a) oral diseases, (b) systematic diseases, and (c) brain diseases. The diagnosis of oral diseases includes Sjögren’s syndrome, oral cancer, caries, and periodontal disease (Zhang et al., 2016). The analysis of salivary microorganisms, such as Streptococcus mutans, Lactobacillus, and Porphyromonas gingivalis, may give insights into the microbiome’s role in brain disorder progression. Furthermore, adrenomedullin, nitric oxide, superoxide dismutase, and several inflammatory proteins (matrix metalloproteinases; interleukin-1, -6, and -18; tumor necrosis factor-alpha; and Toll-like receptors) can be useful for diagnosis. Regarding oral cancer, tumor-specific DNA (HIV, herpes virus), different micro RNAs (miR-125a, miR-200a), varying inflammatory proteins, and antibodies can be detected in saliva. The diagnosis of systemic diseases in the saliva is focused on (a) diabetes mellitus, (b) cardiovascular diseases, (c) viral infections, (d) pancreatic, lung, prostate, and breast cancer, or (e) liver and renal diseases. The levels of alpha-2-macroglobulin in the saliva could reflect the glycaemic situation in type 2 diabetes mellitus. In addition, salivary melatonin is decreased in diabetes and periodontal diseases. Furthermore, Barnes et al. (2014) identified 475 specific metabolites in the saliva of patients with diabetes mellitus and periodontal diseases. The diagnostic property for brain diseases is not well explored, and most saliva papers are focused on AD (as reviewed below), but other neurological diseases (see paragraph below) are also under investigation (Cabras et al., 2015).
Saliva-Based Diagnosis, Exemplified in Alzheimer’s Disease
AD is characterized by severe Aβ deposition in the brain (extracellular plaques), tau pathology (hyperphosphorylated tau causes neurofibrillary tangles), cell death of cholinergic neurons (loss of the neurotransmitter acetylcholine), astroglial and microglial activation, inflammation, and cerebrovascular damage. A valid and easily accessible diagnostic procedure should be the basis for treatment. Definitive diagnosis of AD requires both a clinical diagnosis of the disease and post-mortem detection of AD pathologies. A probable diagnosis of AD can be established with the confidence of approximately > 90%, based on clinical criteria such as medical history, physical examination, laboratory tests, neuroimaging, and neuropsychological evaluation. A promising area of research for AD’s laboratory diagnosis is the analysis of CSF, where measurement of Aβ-42 and -40, total tau, and phospho-tau-181 can distinguish AD patients from healthy subjects, with high specificity and sensitivity (Blennow, 2005; Blennow et al., 2010; Humpel, 2011). Unfortunately, the use of CSF biomarkers is limited by CSF’s invasive collection. Thus, there is a need to discover biomarkers in other human biological fluids, such as blood, urine, and saliva, which allow the collection of a high number of samples. Reviews on this issue have been published by Zhang et al. (2016) and Gleerup et al. (2019).
A systemic review of salivary biomarkers associated with AD (see Table 1 for a summary) has been written by Wolgin et al. (2022). A January 2023 PubMed literature retrieval gave only 19 hits when “Alzheimer[title] AND saliva[title]” were used as keywords. In 2008, Li et al. showed the relationship between saliva and mental health in the older adult general population. In the same year, Boston et al. (2008) developed a simple laboratory test for AD by measuring acetylcholinesterase in saliva. A pilot study reported the discovery of diagnostic biomarkers in AD patients’ saliva using 1H NMR-based metabolomics (Yilmaz et al., 2017; Tsuruoka et al., 2011). In 2010, Bermejo-Pareja et al. reported for the first time that salivary Aβ42 could become a potential biomarker, the findings of which have recently been reproduced by others (Lee et al., 2017; Sabbagh et al., 2018; Gleerup et al., 2019). We have published recently (Marksteiner et al., 2022) that tau and phospho-tau181 can be measured in saliva, and can distinguish AD and mild cognitive impairment (MCI) from healthy controls. Finally, lactoferrin and the neurofilament light chain, both neurodegenerative markers, have been investigated and ruled out as useful diagnostic markers for brain diseases (Gleerup et al., 2021a, b).
Table 1.
Saliva biomarkers for diagnosis of Alzheimer´s disease
| Biomarker | Changes between healthy controls and AD patients | References |
|---|---|---|
| 3-Methoxy-4-hydroxyphenylglycol | Correlated with mental health, gender dependent | Li et al., 2008 |
| Acetylcholinesterase | Unaltered or increased or decreased | Bosten et al., 2008; see also review Gleerup et al., 2019 |
| 1H-NMR metabolomics and LC-MS and FUPLC-MS | Several metabolites altered; (arginine and tyrosine; methylguanosine, proprionate, ornithine; spinganine-1-P) | Tsuruoka et al, 2011; see also review Gleerup et al., 2016; Yilmaz et al., 2017 |
| Beta-amyloid42 | Increased | Bermejo-Pareja et al., 2010; Kim et al., 2014; Lee et al., 2017; McGeer et al., 2018; Sabbagh et al., 2018; Cui et al., 2022 |
| Beta-amyloid42 | Undetectable | Shi et al., 2011; Lau et al., 2015; Marksteiner et al., 2022 |
| Beta-amyloid42 | Decreased | Tvarijonaviciute et al., 2020 |
| Beta-amyloid40 | Unaltered | Bermejo-Pareja et al., 2010; Kim et al., 2014; Tvarijonaviciute et al., 2020 |
| Beta-amyloid40 | Undetectable | Marksteiner et al., 2022 |
| Tau | Decreased | Marksteiner et al., 2022 |
| Tau | Unaltered | Shi et al., 2011; Lau et al., 2015; Ashton et al., 2018; Tvarijonaviciute et al, 2020; Cui et al., 2022 |
| pTau181 | Increased | Shi et al., 2011; Lau et al., 2015; Marksteiner et al., 2022 |
| pTau181 | Unaltered | Tvarijonaviciute et al, 2020; Cui et al., 2022 |
| Lipidomics | A pattern of 5 Acyl-Alkyl-phosphatidlycholine lipids are decreased | Marksteiner et al., 2021 |
| Neurofilament light chain | Unaltered | Gleerup et al., 2021a |
| Lactoferrin | Unaltered or decreased | see also review Gleerup et al., 2016; Gleerup et al., 2021b; |
| SIRT-1, -3, -6 | Reduced | Pukhalskaia et al., 2020 |
| Proteomics by RP-HPLC-ESI-IT-MS | 56 proteins altered: α-defensins, S100A8 and A9, Tb4, Hst-1, statherins, cystatin-B | Contini et al., 2021 |
Note that there is a strong diverseness amongst the biomarkers, which are either unaltered, changed, or undetectable. This clearly shows the heterogeneity of saliva and the need for a standardised collection and processing procedure. Also note that collection devices, such as the cotton in Salivettes®, may not recover the proteins or peptides of interest. We thus suggest spitting into a 50 mL tube for exactly 2 minutes and processing the sample within 2–3 hours.
The idea that periodontitis is linked to human brain disorders, such as AD, has been hypothesized (Ishida et al., 2017; Dioguardi et al., 2020), which has been recently searched in PubMed using the terms periodontitis AND saliva AND/OR Alzheimer. The salivary proteome seems to be directly associated with periodontitis (Haigh et al., 2010). In addition, an infection with the asaccharolytic, gram-negative, anaerobic bacterium Porphyromonas gingivialis plays a key role in the development of chronic periodontitis, and thus has been considered a risk factor for AD progression (Stein et al, 2007; Kaye et al., 2010; Darveau et al., 2012; Olsen et al., 2016; Dominy et al., 2019; Singhrao et al., 2019; Fu et al., 2022).
Regarding saliva and other neurological diseases, there has been, so far, not much published. A March 2023 PubMed literature retrieval gave only 2–3 hits when “saliva[title] AND “biomarker[title]” AND neurological[title] OR neurodegenerative[title]” were used as keywords. The potential use of saliva as a diagnostic tool has been reviewed by Farah et al. (2018) and others (Orive et al., 2022; Walton, 2018; Hyvärinen et al., 2023; Schepici et al., 2020). Briefly, several promising salivary biomarkers have been reported for Parkinson’s disease (e.g., alpha-synuclein), multiple sclerosis (e.g., soluble HLA class II), Huntington’s disease (e.g., huntingtin protein), autism spectrum disorder (e.g., some micro RNAs), and amyotrophic lateral sclerosis (e.g., chromogranin A).
Saliva Collection and Analysis
Saliva is a heterogeneous human fluid that is influenced by many exogenous factors. We have extensive experience in processing saliva and found that saliva must be handled in a reproducible way. We discovered that salivary flow and protein can markedly differ between humans, even if collected early in the morning. Our data also showed that the chromatographic pattern, and subsequent detection, of norepinephrine can vary dramatically. Last but not least, we also found that small peptides (e.g. Aβ) are not fully recovered from collection devices such as Salivettes® (Marksteiner et al., 2022). These factors all limit saliva for diagnostic purposes, if not processed consistently. We thus suggest the following procedure. Saliva should be collected by spitting directly into a 50 mL polypropylene tube for exactly 2 minutes. This ensures that all molecules are spat into the tube and do not bind to cotton collection devices. It is also helpful to count the number of blood cells directly in the fresh saliva, to get an impression of bleeding. This sample should be further processed within 2–3 hours, analyzed, and/or frozen at –80°C. We also favor measuring the saliva’s weight and then subjecting the sample to centrifugation for 5 minutes at 3000 × g. Afterward, the supernatant is collected and the volume measured, which represents salivary flow in mL/minute. This also gives additional information on the salivary production of humans. We suggest excluding samples with ≤ 100 µL/min salivary flow. The analysis of control proteins and total mean protein via the Bradford protein assay is recommended to exclude bloody saliva. We also further suggest the involvement of several specialists for saliva collection and the observation of any pathological issues. A dentist should evaluate the dental status, gingival bleeding, the extent of periodontitis, and gingival loss, and ought to also collect the saliva. A doctor for oral and maxillofacial surgery—or an otorhinolaryngologist—may evaluate the parotid glands and tonsil pathologies. There should be a consensus that individuals do not eat, drink, smoke, or brush their teeth for at least 2 hours before the collection of saliva.
Connection to the Patients
All biomarker studies ought to be connected to the patient advantage. These biomarkers will be useful for four aims. (1) We will determine the biomarkers of aging and show the age-dependent changes of healthy controls. This is important to reveal biomarker variation and whether older adult people differ from younger individuals. (2) We will determine the biomarkers of disease, to show if individuals with a specific brain disorder are different from healthy controls of the same age. This will allow us to diagnose brain disorders from human fluids (e.g. CSF, blood, saliva) in the laboratory; the non-invasive collection of saliva may markedly improve the patient’s life quality. (3) We aim to find biomarkers that show us the transformation of a disease, from a mild to a severe form, e.g. from mild cognitive impairment to severe AD. This will help us to adapt the therapeutic medication. (4) Finally, the biomarkers will show the efficacy of a particular therapeutic route, as they ought to be influenced by the disease’s stage. Effective therapeutic medication should revert the biomarkers to control levels.
Conclusion
We are confident that human saliva could be a potent, alternative fluid for the diagnosis of many illnesses, including oral diseases, cancer, diabetes, and brain disorders. Saliva is easy to collect, and spitting directly into a tube seems to be the optimal method of collection, with a fast (2–3 hours) processing window. Thus, saliva is an exceptional alternative to invasive collections, such as a lumbar or venous puncture. So far, the collection of saliva is not validated, and the extremely high variability of saliva must be considered. As saliva is influenced by so many exogenous factors, the diagnosis of a specific brain-related disease must always be controlled for comorbidity. The analysis of, for example, inflammatory markers may be influenced by many different simultaneous disorders. It aims to identify brain-specific diagnostic markers for high sensitivity and specificity. In all cases, it will be important to consult a dentist to exclude oral diseases that may dramatically influence salivary levels. In agreement with Ashton et al. (2021), a procedure and consensus that is controlled, validated, and standardized for collection and processing of saliva is required, followed by a highly-sensitive diagnostic approach.
Acknowledgments:
We thank Joshua Wieser-Insole (Psychiatry I, Medical University of Innsbruck, Austria) for his English-language corrections. We also thank Prof. Ines Kapferer-Seebacher (University Hospital for Restorative Dentistry and Periodontology, Medical University of Innsbruck, Innsbruck, Austria) for her input on this topic.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Song LP; T-Editor: Jia Y
References
- 1.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragón F, Zea-Sevilla MA, Montero J, Sancho P, Corral R, Tejedor C, Frades-Payo B, Paredes-Gallardo V, Albaladejo A. Oral health in Alzheimer's disease:a multicenter case-control study. Clin Oral Investig. 2018;22:3061–3070. doi: 10.1007/s00784-018-2396-z. [DOI] [PubMed] [Google Scholar]
- 3.Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashton NJ, Ide M, Schöll M, Blenoww k, Lovestone S, Hye A, Zetterberg H. No association of salivary total tau concentration with Alzheimer's disease Neurobiol Aging. 2018;70:125–127. doi: 10.1016/j.neurobiolaging.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Ashton NJ, Blennow K, Zetterberg H. Spitting image:can saliva biomarkers reflect Alzheimer's disease? EBioMedicine. 2021;68:103437. doi: 10.1016/j.ebiom.2021.103437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assinder DF, Chasseaud LF, Taylor T. Plasma isosorbide dinitrate concentrations in human subjects after administration of standard and sustained-release formulations. J Pharm Sci. 1977;66:775–778. doi: 10.1002/jps.2600660607. [DOI] [PubMed] [Google Scholar]
- 7.Barnes VM, Kennedy AD, Panagakos F, Devizio W, Trivedi HM, Jönsson T, Guo L, Cervi S, Scannapieco FA. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS One. 2014;9:e105181. doi: 10.1371/journal.pone.0105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista TBD, Chaiben CL, Penteado CAS, Nascimento JMC, Ventura TMO, Dionizio A, Rosa EAR, Buzalaf MAR, Azevedo-Alanis LR. Salivary proteome characterization of alcohol and tobacco dependents. Drug Alcohol Depend. 2019;204:107510. doi: 10.1016/j.drugalcdep.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Bermejo-Pareja F, Antequera D, Vargas T, Molina JA, Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer's disease:a pilot study. BMC Neurol. 2010;10:108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besbes A, Khemiss M, Bragazzi N, Ben Saad H. The impacts of ramadan intermittent fasting on saliva flow-rate and metabolic data:a systematic review. Front Nutr. 2022;9:873502. doi: 10.3389/fnut.2022.873502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blennow K. CSF biomarkers for Alzheimer´s disease:use in early diagnosis and evaluation of drug treatment. Expert Rev Mol Diagn. 2005;5:661–672. doi: 10.1586/14737159.5.5.661. [DOI] [PubMed] [Google Scholar]
- 12.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 13.Bomeli SR, Desai SC, Johnson JT, Walvekar RR. Management of salivary flow in head and neck cancer patients--a systematic review. Oral Oncol. 2008;44:1000–1008. doi: 10.1016/j.oraloncology.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Boston PF, Gopalkaje K, Manning L, Middleton L, Loxley M. Developing a simple laboratory test for Alzheimer's disease:measuring acetylcholinesterase in saliva - a pilot study. Int J Geriatr Psychiatry. 2008;23:439–440. doi: 10.1002/gps.1882. [DOI] [PubMed] [Google Scholar]
- 15.Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, Henry H, Oikonomidi A, Migliavacca E, Bacher M, Popp J. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14:1640–1650. doi: 10.1016/j.jalz.2018.06.2857. [DOI] [PubMed] [Google Scholar]
- 16.Busenhart DM, Erb J, Rigakos G, Eliades T, Papageorgiou SN. Adverse effects of chemotherapy on the teeth and surrounding tissues of children with cancer:A systematic review with meta-analysis. Oral Oncol. 2018;83:64–72. doi: 10.1016/j.oraloncology.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Cabras T, Sanna M, Manconi B, Fanni D, Demelia L, Sorbello O, Iavarone F, Castagnola M, Faa G, Messana I. Proteomic investigation of whole saliva in Wilson's disease. J Proteomics. 2015;128:154–163. doi: 10.1016/j.jprot.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis:interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 19.Contini C, Olianas A, Serrao S, Deriu C, Iavarone F, Boroumand M, Bizzarro A, Lauria A, Faa G, Castagnola M, Messana I, Manconi B, Masullo C, Cabras T. Top-down proteomics of human saliva highlights anti-inflammatory, antioxidant, and antimicrobial defense responses in alzheimer disease. Front Neurosci. 2021;15:668852. doi: 10.3389/fnins.2021.668852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Y, Zhang H, Zhu J, Liao Z, Wang S, Liu W. Investigation of whole and glandular saliva as a biomarker for Alzheimer's disease diagnosis. Brain Sci. 2022;12:595. doi: 10.3390/brainsci12050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai R, Lam OL, Lo EC, Li LS, Wen Y, McGrath C. Orofacial functional impairments among patients following stroke:a systematic review. Oral Dis. 2015;21:836–849. doi: 10.1111/odi.12274. [DOI] [PubMed] [Google Scholar]
- 22.Dame ZT, Aziat F, Mandal R, Krishnanurthy R, Bouatra S, Borzouie S, Guo AC, Sajed T, Deng L, Lin H, Liu P, Dong E, Wishart DS. The human saliva metabolome. Metabolomics. 2015;11:1864–1883. [Google Scholar]
- 23.Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012;91:816–820. doi: 10.1177/0022034512453589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dioguardi M, Crincoli V, Laino L, Alovisi M, Sovereto D, Mastrangelo F, Russo LL, Muzio LL. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer's disease:a systematic review. J Clin Med. 2020;9:495. doi: 10.3390/jcm9020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J. Porphyromonas gingivalis in Alzheimer's disease brains:evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5:eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enberg N, Alho H, Loimaranta V, Lenander-Lumikari M. Saliva flow rate, amylase activity, and protein and electrolyte concentrations in saliva after acute alcohol consumption. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:292–298. doi: 10.1067/moe.2001.116814. [DOI] [PubMed] [Google Scholar]
- 28.Ericson S, Sjöbäck I. Salivary factors in children with recurrent parotitis. Part 2:Protein, albumin, amylase, IgA, lactoferrin lysozyme and kallikrein concentrations. Swed Dent J. 1996;20:199–207. [PubMed] [Google Scholar]
- 29.Farah R, Haraty H, Salame Z, Fares Y, Ojcius DM, Said Sadier N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed J. 2018;41:63–87. doi: 10.1016/j.bj.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng YK, Wu QL, Peng YW, Liang FY, You HJ, Feng YW, Li G, Li XJ, Liu SH, Li YC, Zhang Y, Pei Z, Oral P. Gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J Neuroinflammation. 2020;17:347. doi: 10.1186/s12974-020-02027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 32.François M, Karpe A, Liu JW, Beale D, Hor M, Hecker J, Faunt J, Maddison J, Johns S, Doecke J, Rose S, Leifert WR. Salivaomics as a potential tool for predicting Alzheimers disease during the early stages of neurodegeneration. J Alzheimers Dis. 2021;82:1301–1313. doi: 10.3233/JAD-210283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu KL, Chiu MJ, Wara-Aswapati N, Yang CN, Chang LC, Guo YL, Ni YH, Chen YW. Oral microbiome and serological analyses on association of Alzheimer's disease and periodontitis. Oral Dis. 2022 doi: 10.1111/odi.14348. doi:10.1111/odi.14348. [DOI] [PubMed] [Google Scholar]
- 34.Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer's disease in saliva:a systematic review. Dis Markers. 20192019:4761054. doi: 10.1155/2019/4761054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleerup HS, Sanna F, Høgh P, Simrén J, Blennow K, Zetterberg H, Hasselbalch SG, Ashton NJ, Simonsen AH. Saliva neurofilament light chain is not a diagnostic biomarker for neurodegeneration in a mixed memory clinic population. Front Aging Neurosci. 2021a;13:659898. doi: 10.3389/fnagi.2021.659898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gleerup HS, Jensen CS, Høgh P, Hasselbalch SG, Simonsen AH. Lactoferrin incerebrospinal fluid and saliva is not a diagnostic biomarker for Alzheimer'sdisease in a mixed memory clinic population. EBioMedicine. 2021b;67:103361. doi: 10.1016/j.ebiom.2021.103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haigh BJ, Stewwart KW, Whelan JRK, Barnett MOG, Smolenski GA, Wheeler TT. Alterations in the salivary proteome associated with periodontitis. J Clin Periodontol. 2010;37:241–247. doi: 10.1111/j.1600-051X.2009.01525.x. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi T, To M, Saruta J, Sato C, Yamamoto Y, Kondo Y, Shimizu T, Kamata Y, Tsukinoki K. Salivary lactoferrin is transferred into the brain via the sublingual route. Biosci Biotechnol Biochem. 2017;81:1300–1304. doi: 10.1080/09168451.2017.1308241. [DOI] [PubMed] [Google Scholar]
- 39.Huan T, Tran T, Zheng J, Sapkota S, MacDonald SW, Camicioli R, Dixon RA, Li L. Metabolomics analyses of saliva detect novel biomarkers of Alzheimer's disease. J Alzheimers Dis. 2018;65:1401–1416. doi: 10.3233/JAD-180711. [DOI] [PubMed] [Google Scholar]
- 40.Humpel C. Identifying and validating biomarkers for Alzheimer's disease. Trends Biotechnol. 2011;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humpel C. Intranasal neprilysin rapidly eliminates amyloid-beta plaques, but causes plaque compensations:the explanation why the amyloid-beta cascade may fail? Neural Regen Res. 2022;17:1881–1884. doi: 10.4103/1673-5374.335138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyvärinen E, Solje E, Vepsäläinen J, Kullaa A, Tynkkynen T. Salivary metabolomics in the diagnosis and monitoring of neurodegenerative dementia. Metabolites. 2023;13:233. doi: 10.3390/metabo13020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O'Brien-Simpson NM, Reynolds EC, Watanabe K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 2018;13:e0204941. doi: 10.1371/journal.pone.0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishida N, Ishihara Y, Ishida K, Tada H, Funaki-Kato Y, Hagiwara M, Ferdous T, Abdullah M, Mitani A, Michikawa M, Matsushita K. Periodontitis induced by bacterial infection exacerbates features of Alzheimer's disease in transgenic mice. NPJ Aging Mech Dis. 2017;3:15. doi: 10.1038/s41514-017-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, Banks WA. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein:a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav Immun. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman E, Lamster IB. The diagnostic applications of saliva--a review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 47.Kaye EK, Valencia A, Baba N, Spiro A, 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713–718. doi: 10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CB, Choi YY, Song WK, Song KB. Antibody-based magnetic nanoparticle immunoassay for quantification of Alzheimer's disease pathogenic factor. J Biomed Opt. 2014;19:051205. doi: 10.1117/1.JBO.19.5.051205. [DOI] [PubMed] [Google Scholar]
- 49.Lam PP, Du R, Peng S, McGrath CP, Yiu CK. Oral health status of children and adolescents with autism spectrum disorder:a systematic review of case-control studies and meta-analysis. Autism. 2020;24:1047–1066. doi: 10.1177/1362361319877337. [DOI] [PubMed] [Google Scholar]
- 50.Lau HC, Lee IK, Ko PW, Lee HW, Huh JS, Cho WJ, Lim JO. Non-invasive screening for Alzheimer's disease by sensing salivary sugar using Drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EG-ISFET) biosensor. PLoS One. 2015;10:e0117810. doi: 10.1371/journal.pone.0117810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lăzureanu PC, Popescu F, Tudor A, Stef L, Negru AG, Mihăilă R. Saliva pH and flow rate in patients with periodontal disease and associated cardiovascular disease. Med Sci Monit. 2021;27:e931362. doi: 10.12659/MSM.931362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee M, Guo JP, Kennedy K, McGeer EG, McGeer PL. A method for diagnosing Alzheimer's disease based on salivary amyloid-βprotein 42 levels. J Alzheimers Dis. 2017;55:1175–1182. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- 53.Li GY, Watanabe I, Kunitake Y, Sugataka K, Muraoka T, Kojima N, Kawashima T, Yamada S. Relationship between saliva level of 3-methoxy-4-hydroxyphenylglycol and mental health in the elderly general population. Psychiatry Clin Neurosci. 2008;62:562–567. doi: 10.1111/j.1440-1819.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang D, Hao Lu. Salivary biological biomarkers for Alzheimer's disease. Arch Oral Biol. 2019;105:5–12. doi: 10.1016/j.archoralbio.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Liu T, Wei Y, Zhu Y, Yang W. Caries status and salivary alterations of type-1 diabetes mellitus in children and adolescents:a systematic review and meta-analysis. J Evid Based Dent Pract. 2020;21:101496. doi: 10.1016/j.jebdp.2020.101496. [DOI] [PubMed] [Google Scholar]
- 57.Loo JA, Yan W, Ramachandran P, Wong DT. Comparative human salivary and plasma proteomes. J Dent Res. 2010;89:1016–1023. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macgregor ID. Smoking, saliva and salivation. J Dent. 1988;16:14–17. doi: 10.1016/0300-5712(88)90096-6. [DOI] [PubMed] [Google Scholar]
- 60.Marksteiner J, Oberacher H, Humpel C. Acyl-alkyl phosphatidlycholines are decreased in saliva of patients with Alzheimer´s disease as identified by targeted metabolomics. J Alzheimers Dis. 2019;68:583–589. doi: 10.3233/JAD-181278. [DOI] [PubMed] [Google Scholar]
- 61.Marksteiner J, Defrancesco M, Humpel C. Saliva tau and phospho-tau-181 measured by Lumipulse in patients with Alzheimer´s disease. Front Aging Neurosci. 2022;14:1014305. doi: 10.3389/fnagi.2022.1014305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marques RCR, da Silva JR, Vieira Lima CP, Stefani CM, Damé-Teixeira N. Salivary parameters of adults with diabetes mellitus:a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134:176–189. doi: 10.1016/j.oooo.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 63.McGeer PL, Guo JP, Lee M, Kennedy K, McGeer EG. Alzheimer's disease can be spared by nonsteroidal anti-inflammatory drugs. J Alzheimers Dis. 2018;62:1219–1222. doi: 10.3233/JAD-170706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishra SK, Chowdhary R, Kumari S, Rao SB. Effect of cell phone radiations on orofacial structures:a systematic review. J Clin Diagn Res. 2017;11:ZE01–05. doi: 10.7860/JCDR/2017/26547.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monkhouse WS. The anatomy of the facial nerve. Ear Nose Throat J. 1990;69:677–683. 686-687. [PubMed] [Google Scholar]
- 66.Moosavi MS, Barati H. Salivary gland performance in autoimmune diseases:review and meta-analysis. Acta Clin Belg. 2018;75:19–25. doi: 10.1080/17843286.2018.1540164. [DOI] [PubMed] [Google Scholar]
- 67.Mosca AC, Stieger M, Neyraud E, Brignot H, van de Wiel A, Chen J. How are macronutrient intake, BMI, ethnicity, age, and gender related to the composition of unstimulated saliva?A case study. J Texture Stud. 2019;50:53–61. doi: 10.1111/jtxs.12362. [DOI] [PubMed] [Google Scholar]
- 68.Olsen I, Taubman MA, Singhrao SK. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer's disease. J Oral Microbiol. 2016;8:33029. doi: 10.3402/jom.v8.33029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orive G, Lopera F, Carro E. Saliva is a good candidate to be the next gold-standard sample for neurodegenerative diseases. J Alzheimers Dis. 2022;87:1497–1501. doi: 10.3233/JAD-220144. [DOI] [PubMed] [Google Scholar]
- 70.Pan W, Kastin AJ. Penetration of neurotrophins and cytokines across the blood-brain/blood-spinal cord barrier. Adv Drug Deliv Rev. 1999;36:291–298. doi: 10.1016/s0169-409x(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 71.Percival RS, Challacombe SJ, Marsh PD. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994;73:1416–1420. doi: 10.1177/00220345940730080401. [DOI] [PubMed] [Google Scholar]
- 72.Poole S, Singhrao SK, Chukkapalli S, Rivera M, Velsko I, Kesavalu L, Crean S. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE-/- mice brains. J Alzheimers Dis. 2015;43:67–80. doi: 10.3233/JAD-140315. [DOI] [PubMed] [Google Scholar]
- 73.Pukhalskaia AE, Dyatlova AS, Linkova NS, Kozlov KL, Kvetnaia TV, Koroleva MV, Kvetnoy IM. Sirtuins as possible predictors of aging and Alzheimer's disease development:verification in the hippocampus and saliva. Bull Exp Biol Med. 2020;169:821–824. doi: 10.1007/s10517-020-04986-4. [DOI] [PubMed] [Google Scholar]
- 74.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol Immunol. 2002;17:113–118. doi: 10.1046/j.0902-0055.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 76.Rodrigues RPCB, Vidigal MTC, Vieira WA, Nascimento GG, Sabino-Silva R, Blumenberg C, Siqueira MF, Siqueira WL, Paranhos LR. Salivary changes in chronic kidney disease and in patients undergoing hemodialysis:a systematic review and meta-analysis. J Nephrol. 2022;35:1339–1367. doi: 10.1007/s40620-022-01274-4. [DOI] [PubMed] [Google Scholar]
- 77.Romeo HE, Tio DL, Rahman SU, Chiappelli F, Taylor AN. The glossopharyngeal nerve as a novel pathway in immune-to-brain communication:relevance to neuroimmune surveillance of the oral cavity. J Neuroimmunol. 2001;115:91–100. doi: 10.1016/s0165-5728(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 78.Sabbagh MN, Shi J, Lee M, Arnold L, Al-Hasan Y, Heim J, McGeer P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer's disease dementia from normal controls:preliminary findings. BMC Neurol. 2018;18:155. doi: 10.1186/s12883-018-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sadida ZJ, Indriyanti R, Setiawan AS. Does growth stunting correlate with oral health in children:a systematic review. Eur J Dent. 2022;16:32–40. doi: 10.1055/s-0041-1731887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sansores-España LD, Melgar-Rodríguez S, Olivares-Sagredo K, Cafferata EA, Martínez-Aguilar VM, Vernal R, Paula-Lima AC, Díaz-Zúñiga J. Oral-gut-brain axis in experimental models of periodontitis:associating gut dysbiosis with neurodegenerative diseases. Front Aging. 2021;2:781582. doi: 10.3389/fragi.2021.781582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schepici G, Silvestro S, Trubiani O, Bramanti P, Mazzon E. Salivary biomarkers:future approaches for early diagnosis of neurodegenerative diseases. Brain Sci. 2020;10:245. doi: 10.3390/brainsci10040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmalz G, Patschan S, Patschan D, Ziebolz D. Oral-health-related quality of life in adult patients with rheumatic diseases-a systematic review. J Clin Med. 2020;9:1172. doi: 10.3390/jcm9041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sell S, Salman J. Demonstration of Treponema pallidum in axons of cutaneous nerves in experimental chancres of rabbits. Sex Transm Dis. 1992;19:1–6. doi: 10.1097/00007435-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Shi M, Sui YT, Peskind ER, Li G, Hwang H, Devic I, Ginghina C, Edgar JS, Pan C, Goodlett DR, Furay AR, Gonzalez-Cuyar LF, Zhang J. Salivary tau species are potential biomarkers of Alzheimer's disease. J Alzheimers Dis. 2011;27:299–305. doi: 10.3233/JAD-2011-110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer's disease. J Oral Microbiol. 2019;11:1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slavish DC, Szabo YZ. The effect of acute stress on salivary markers of inflammation:a systematic review protocol. Syst Rev. 2019;8:108. doi: 10.1186/s13643-019-1026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138:1314–1322. doi: 10.14219/jada.archive.2007.0046. quiz 1381-1382. [DOI] [PubMed] [Google Scholar]
- 88.Su X, Tang Z, Lu Z, Liu Y, He W, Jiang J, Zhang Y, Wu H. Oral treponema denticola infection induces Aβ1-40 and Aβ1-42 accumulation in the hippocampus of C57BL/6 mice. J Mol Neurosci. 2021;71:1506–1514. doi: 10.1007/s12031-021-01827-5. [DOI] [PubMed] [Google Scholar]
- 89.Takeda I, Stretch C, Barnaby P, Bhatnager K, Rankin K, Fu H, Weljie A, Jha N, Slupsky C. Understanding the human salivary metabolome. NMR Biomed. 2009;22:577–584. doi: 10.1002/nbm.1369. [DOI] [PubMed] [Google Scholar]
- 90.Toan NK, Ahn SG. Aging-related metabolic dysfunction in the salivary gland:a review of the literature. Int J Mol Sci. 2021;22:5835. doi: 10.3390/ijms22115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsuruoka M, Hara J, Hirayama A, Sugimoto M, Soga T, Shankle WR, Tomita M. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis. 2013;34:2865–2872. doi: 10.1002/elps.201300019. [DOI] [PubMed] [Google Scholar]
- 92.Tvarijonaviciute A, Zamora C, Ceron JJ, Bravo-Cantero AF, Pardo-Marin L, Valverde S, Lopez-Jornet P. Salivary biomarkers in Alzheimer's disease. Clin Oral Investig. 2020;24:3437–3444. doi: 10.1007/s00784-020-03214-7. [DOI] [PubMed] [Google Scholar]
- 93.van Leeuwen SJM, Potting CMJ, Huysmans MDNJM, Blijlevens NMA. Salivary changes before and after hematopoietic stem cell transplantation:a systematic review. Biol Blood Marrow Transplant. 2019;25:1055–1061. doi: 10.1016/j.bbmt.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 94.Verma A, Anand K, Bhargava M, Kolluri A, Kumar M, Palve DH. Comparative evaluation of salivary biomarker levels in e-cigarette smokers and conventional smokers. J Pharm Bioallied Sci. 2021;13:S1642–1645. doi: 10.4103/jpbs.jpbs_393_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villa A, Wolff A, Aframian D, Vissink A, Ekström J, Proctor G, McGowan R, Narayana N, Aliko A, Sia YW, Joshi RK, Jensen SB, Kerr AR, Dawes C, Pedersen AM. World workshop on oral medicine VI:a systematic review of medication-induced salivary gland dysfunction:prevalence, diagnosis, and treatment. Clin Oral Investig. 2015;19:1563–1580. doi: 10.1007/s00784-015-1488-2. [DOI] [PubMed] [Google Scholar]
- 96.Walton EL. Saliva biomarkers in neurological disorders:a “spitting image”of brain health? Biomed J. 2018;41:59–62. doi: 10.1016/j.bj.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolff A, Joshi RK, Ekström J, Aframian D, Pedersen AM, Proctor G, Narayana N, Villa A, Sia YW, Aliko A, McGowan R, Kerr AR, Jensen SB, Vissink A, Dawes C. A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea:a systematic review sponsored by the world workshop on oral medicine VI. Drugs R D. 2017;17:1–28. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolgin M, Zobernig M, Dvornyk V, Braun RJ, Kielbassa AM. Systematic review on saliva biomarkers in patients diagnosed with morbus Alzheimer and morbus Parkinson. Biomedicines. 2022;10:1702. doi: 10.3390/biomedicines10071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xue L, Zou X, Yang XQ, Peng F, Yu DK, Du JR. Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp Neurol. 2020;326:113176. doi: 10.1016/j.expneurol.2020.113176. [DOI] [PubMed] [Google Scholar]
- 100.Yilmaz A, Geddes T, Han B, Bahado-Singh RO, Wilson GD, Imam K, Maddens M, Graham SF. Diagnostic biomarkers of Alzheimer's disease as identified in saliva using 1H NMR-based metabolomics. J Alzheimers Dis. 2017;58:355–359. doi: 10.3233/JAD-161226. [DOI] [PubMed] [Google Scholar]
- 101.Zhang A, Sun H, Wang X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl Biochem Biotechnol. 2012;168:1718–1727. doi: 10.1007/s12010-012-9891-5. [DOI] [PubMed] [Google Scholar]
- 102.Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X, Zhou XD. Saliva in the diagnosis of diseases. Int J Oral Sci. 2016;8:133–137. doi: 10.1038/ijos.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Yu C, Zhang X, Chen H, Dong J, Lu W, Song Z, Zhou W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 2018;15:37. doi: 10.1186/s12974-017-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


