Unlocking axon regeneration in the injured central nervous system: In adult mammals, central nervous system (CNS) neurons fail to regenerate after a lesion, whether it is traumatic – after spinal cord injury for example – or in the case of neurodegenerative diseases. This causes axons to degenerate and neurons to die, leading to permanent motor and/or cognitive impairment. One of the reasons behind this regeneration failure lies in the mature CNS environment, where a number of growth-inhibitory factors, at the lesion site, contributes to axon regrowth inhibition (He and Jin, 2016). In this context, removing such extrinsic factors should alleviate the growth-inhibitory barrier. Yet, surprisingly, no robust regeneration is achieved past the lesion site. These results led researchers to investigate the intrinsic regrowth properties of adult neurons themselves. Indeed, adult CNS neurons lose their capacity to grow an axon, not only because of the switch-off of developmental pro-growth programs during maturation, but also in response to the injury itself (Belin et al., 2015; He and Jin, 2016).
The mouse visual system is one of the best models to address the question of CNS regeneration. In the retina, only retinal ganglion cells (RGC) project their axons in the optic nerve and connect well-described brain nuclei to relay visual information. In 2008, mTOR pathway activation in RGC induced robust regeneration in the injured optic nerve, unlocking for the first time the regeneration capacity of adult CNS neurons (Park et al., 2008). This finding clearly demonstrated the importance of neuronal intrinsic contribution to axon regeneration. Since then, a number of pathways have been highlighted, and their activation, alone or in combination, has led to long-distance regeneration. For instance, upon optic nerve injury, the co-activation of mTOR, JAK/STAT, and c-myc pathways induces regeneration over 10 millimeters from the eye to the brain (Belin et al., 2015).
Thus, in the mature CNS, regenerating axons over long distances is not a roadblock anymore. However, one important gap remains: the lack of functional recovery. In particular, in all long-distance regeneration models, no or very little target reinnervation is observed, as regenerative axons are not able to reach properly their initial targets. Indeed, axons get lost on their way, with inappropriate orientations that are either undriven or in response to signals expressed in the adult environment.
Therefore, the field is now facing a new challenge: is there a possibility to guide regenerative axons in the mature CNS in order to reform a functional circuit after injury?
Step 1: Understand why regenerative axons get lost: Axon misguidance in regeneration has been observed for almost ten years. The development of whole-tissue clearing and 3D imaging approaches allowed to highlight such defects in the visual system. For example, Pernet and colleagues observed that, despite robust regenerative effect of Stat3 activation in RGC, axons fail to reach the distal part of the optic nerve, partly due to non-linear trajectories. In particular, regenerative axons exhibit abnormal branching – which is not observed in an intact optic nerve – and a high rate of looping and U-turns back to the eye (Pernet et al., 2013). Among these navigation defects, the most spectacular lie in the optic chiasm, a crucial choice point encountered by RGC axons during development. In the mouse, 95% of RGC decussate in the optic chiasm to project to the contralateral optic tract, whereas the remaining 5% project ipsilaterally. In most long-distance regeneration models (Luo et al., 2013; Belin et al., 2015), many axons reach the chiasm, but get lost in this region, projecting aberrantly in the ventral hypothalamus outside of the optic tracts, or even in the contralateral optic nerve (Figure 1A).
Figure 1.
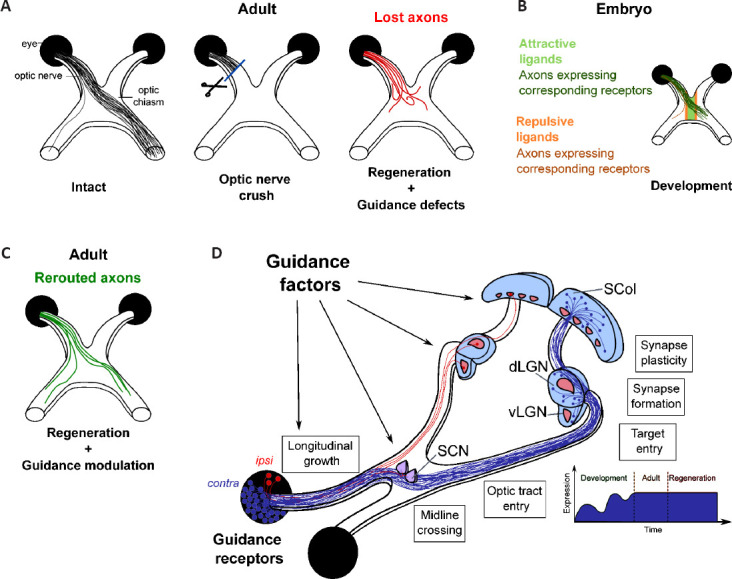
Applying axon guidance principles towards a successful regeneration in the injured central nervous system.
(A) In the adult mouse visual system, 95% retinal ganglion cells normally project to the contralateral side, while 5% project ipsilaterally. In regeneration models, despite long-distance regrowth to the optic chiasm and beyond, regenerative axons fail to resume the correct trajectory, which prevents reinnervation of the initial targets and functional recovery. (B) During development, axons respond to guidance cues in the embryonic environment to extend towards their end targets. Notably, the optic chiasm is a crucial choice point where contra- and ipsi-laterally-projecting axons have to make the decision to cross or not to cross the midline. (C) Combination of guidance modulation and long-distance regeneration allows correct rerouting of regenerative axons on the initial pathway. (D) In regeneration models, guidance factors are integrated at multiple locations of regeneration path. Adult regenerative axons have the molecular tools to respond to guidance cues expressed along their path in the adult environment, in particular, to follow linear tracks and decide to cross or not to cross the midline at the optic chiasm. Guidance factors are also expressed in brain targets, and their expression remains stable upon injury, while during development, they undergo a dynamic regulation that correlates with target innervation and circuit formation. Finally, synapse formation and plasticity rely on expression and activity of guidance factors. dLGN: dorsolateral geniculate nucleus; SCN: suprachiasmatic nucleus; SCol: superior colliculus; vLGN: ventrolateral geniculate nucleus. Created using Inkscape 0.92.3 drawing tool.
Furthermore, even with sufficient growth distance, regenerative axons barely enter in the RGC primary brain targets, including the lateral geniculate nucleus or the superior colliculus. While a small number of studies detected regenerative fibers in the brain targets with partial functional recovery (Lim et al., 2016), this effect is absent in many others (Luo et al., 2013). Instead, these models collectively suffer from striking misguided trajectories that impact successful reinnervation and functional reconnection.
Altogether, the apparent random trajectories taken by regenerative axons may result from their incapacity to sense the environment – due to lack of sensors, e.g. a functional growth cone and/or the expression of appropriate receptors – or rather a capacity to integrate new signaling expressed in the mature CNS.
Step 2: Resume the initial circuit set-up during development: Axon guidance is the developmental process by which growing axons respond to external stimuli (chemical, mechanical) to reach, in a stereotypical manner, their end targets. Axon guidance mechanisms were first proposed by Ramon y Cajal in the early 20th century. The identity of guidance factors has been uncovered since the mid-90s (Stoeckli, 2018). Their description includes their mode of action – repulsive, attractive, contact, short-distance, long-distance – and the molecular mechanisms underlying the conformational change of the growth cone – receptor cleavage or endocytosis, local translation of effectors or cytoskeleton reorganization (Stoeckli, 2018). Moreover, multiple additional mechanisms have been highlighted to finely control neuronal circuit formation, including crosstalk between guidance factors and associated factors, mechanical stiffness of the environment, or the regulation of guidepost cells. Most studies focused on CNS structures where growing neurons make a binary decision: for example, to cross or not to cross the midline at the optic chiasm in the developing visual system (Figure 1B).
Despite this extensive characterization during development, the pattern of guidance cues expression remains elusive in the mature CNS (Crair and Mason, 2016). Indeed, axon guidance is considered of no use in a mature system, when circuits are formed and stabilized. In the injured CNS, many studies have characterized the expression of canonical guidance cues peripheral to the lesion site, which mostly exhibit a growth-inhibitory activity detrimental to regeneration. However, the expression of guidance factors across the path and in targets of regenerative axons is poorly described, and its correlation with misguidance defects is completely unknown. Recent work from our lab proposes such a map based on extensive proteomic characterization of the primary RGC targets in the brain (suprachiasmatic nucleus, ventral and dorsal lateral geniculate nucleus, superior colliculus) and of the critical choice point that is the optic chiasm, where many guidance defects are reported (Vilallongue et al., 2022). Our study highlights a number of canonical guidance ligands and receptors expressed in the different regions, as well as guidance-associated factors such as adhesion molecules and extracellular matrix molecules (ECM).
Late after injury (28 days post-bilateral optic nerve injury), these regions, distal to the optic nerve lesion site, undergo a modification in protein content, including an upregulation of inflammatory molecules, a downregulation of cytoskeletal components, and a remodeling of the ECM (Vilallongue et al., 2022). For example, complement components C1q expression is upregulated in the lateral geniculate nuclei, which may be a consequence of RGC axon degeneration (including glial activation and debris clearing). Conversely, structural components of the axon cytoskeleton are downregulated in these regions after injury (such as the microtubule-associated protein Tau), which correlates with altered axon integrity and function. The remodeling of the ECM observed in all regions is of particular relevance in the guidance map. Indeed, some components of the ECM, such as CSPG, are regulators of the activity of guidance cues themselves. Furthermore, the composition of the ECM directly influences the environmental stiffness, which in turn acts on the guidance path of growing axons (Franze, 2020).
These changes in protein content show that these brain regions integrate the optic nerve injury signals and respond to them. To some extent, they could contribute to the reinnervation failure of these nuclei. Interestingly, the expression of guidance factors is not regulated by axon injury in the target regions, nor in the optic chiasm. Indeed, these structures display dynamic regulation of guidance cues during development, in particular, at key time points of circuit formation: axon guidance at E16–18, synaptogenesis at P0 to P4, synapse refinement at P7–P10 and visually-evoked activity around P14, when the eyes open. These findings suggest that, during maturation, the brain shifts from a pro-innervating state to a refractory state, where it cannot be connected again (Vilallongue et al., 2022). This implies the existence of a tight temporal window that allows circuit connectivity.
Step 3: Define a therapeutic strategy: In our recent work, we demonstrated that inducing growth programs to promote long-distance regeneration is not limiting for guidance. Indeed, the co-activation of the mTOR and the JAK/STAT pathways leads to long-distance regeneration of RGC up to the optic chiasm, but this manipulation does not affect their potential to respond to guidance cues expressed in their environment. We combined this regeneration model with the modulation of guidance molecules to reroute regenerative axons on their correct path (Vilallongue et al., 2022). As a proof-of-concept based on our newly described guidance map, we focused on two guidance cues expressed in the adult optic chiasm and known to be repulsive during development: Ephrin-B3 and Sema4D. We showed that regenerative axons express the corresponding canonical receptors. These cues elicit a repulsive response ex vivo when used in a stripe assay combined with mature retina explant cultures. Furthermore, in vivo modulation of these guidance signals controls axon pathfinding at the optic chiasm to resume the correct trajectory (Vilallongue et al., 2022; Figure 1C). Whether this modulation impacts RGC navigation further on the path remains to be determined, as well as how the ipsi- versus contralateral projections are segregated during regeneration.
These findings show that adult axon guidance is possible and relevant in a context of CNS regeneration. To rebuild a functional neuronal circuit, the upcoming challenge is to resume the initial trajectory for regenerating axons, while addressing an environment completely different from the embryonic one. Future therapeutic strategies will rely on a tight spatiotemporal control of the guidance signaling to allow regenerative axons to (i) grow on the right track, (ii) enter the functional brain target, (iii) form synaptic connections with target neurons, and (iv) (re-)consolidate a functional circuit (Figure 1D). Importantly, these strategies should not interfere with the physiological roles of guidance cues in the mature brain, which are still not well characterized. For example, Eph/Ephrin signaling plays a role in the regulation and maintenance of synaptic activity (Henderson and Dalva, 2018). Therefore, modulation of this guidance signaling may have strong consequences on brain functions. How this type of guidance modulation may affect the capacity of regenerative axons to form and maintain functional synapses remains to be determined. In this context, a transient modulation of such signaling should be considered to avoid such alterations.
Another question that arises is the differential capacity and necessity of the many RGC subpopulations to respond to guidance molecules. With the ever more detailed description of these subpopulations, it is possible that the response to a given guidance cue may differ. Notably, recent studies have described extensively the differential injury response of various RGC subpopulations (Tran et al., 2019), in particular, in their capacity to survive and to regenerate. One feature that is currently uncharacterized is the guidance “identity card” of each RGC subpopulation, i.e. the actual guidance receptors and co-factors expressed in intact and injured conditions. Thus, one could take advantage of the specific RGC subpopulation-tagged mouse lines available to analyze their guidance potential in response to the relevant guidance cues. Indeed, as the topography of RGC projections in different brain nuclei is described in an intact circuit, guiding a specific subpopulation towards its appropriate target is even more meaningful to rebuild the functional connection. In the same line, it is essential to determine whether the modulation of intrinsic growth properties affects the intrinsic mechanisms of segregation of ipsi- versus contralateral-projecting RGC.
Finally, the minimal number of regenerative axons necessary to resume a functional activity remains to be determined. As the system matures, the circuit plasticity decreases, and very little is known about how to refine/strengthen a circuit post-injury. In the case of long-distance regeneration, depending on appropriate guidance of regenerative axons to the correct target, synaptogenesis, and consolidation of the circuit will have to be monitored and possibly unlocked to ensure functional recovery.
Conclusion: While axon guidance is extensively characterized during development, the application of its principles to the injured CNS proves to be highly relevant to unlock the next step of a successful regeneration. In the mature CNS, it is essential to decipher the guidance map to orient regenerative axons towards their appropriate target. Based on these molecular maps, follow-up strategies may focus on the modulation of a particular pair of ligand/receptor to achieve the reinnervation of a particular target region. Not only do axons need to physically enter the target, but they should also form functional synapses to sustain functional recovery. Finally, on top of determining which spatial cues to modulate to resume the correct path, the temporality for guidance modulation is a critical parameter to consider. Altogether, appropriate guidance in an adult system will rely on the opening of this spatiotemporal window of reinnervation necessary for functional reconnection after injury.
This work was supported by the French National Research Agency under the “Investissements d’avenir” program (ANR-17-EURE-0003) (to SB and HN). This work was also supported by ERC-St17-759089-DRIVE and NRJ Foundation to HN and ANR ANR-18-CE16-0007 to SB. JS is supported by a postdoctoral fellowship from Fondation pour la Recherche Médicale (FRM) SPF201909009106. NV is supported by a PhD extension fellowship from Fondation pour la recherche médicale (FRM) – Programme Fin de Thèse FDT202204014716.
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf C, He Z, Steen J. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron. 2015;86:1000–1014. doi: 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crair MC, Mason CA. Reconnecting eye to brain. J Neurosci. 2016;36:10707–10722. doi: 10.1523/JNEUROSCI.1711-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franze K. Integrating chemistry and mechanics:the forces driving axon growth. Annu Rev Cell Dev Biol. 2020;36:61–83. doi: 10.1146/annurev-cellbio-100818-125157. [DOI] [PubMed] [Google Scholar]
- 4.He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Henderson N, Dalva MB. EphBs and ephrin-Bs:trans-synaptic organizers of synapse development and function. Mol Cell Neurosci. 2018;91:108–121. doi: 10.1016/j.mcn.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD. Neural activity promotes long distance, target-specific regeneration of adult retinal axons. Nat Neurosci. 2016;19:1073–1084. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo X, Salgueiro Y, Beckerman SR, Lemmon VP, Tsoulfas P, Park KK. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp Neurol. 2013;247:653–662. doi: 10.1016/j.expneurol.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013;4:e734–734. doi: 10.1038/cddis.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoeckli ET. Understanding axon guidance:are we nearly there yet? Development. 2018;145:dev151415. doi: 10.1242/dev.151415. [DOI] [PubMed] [Google Scholar]
- 11.Tran NM, Shekhar K, Whitney IE, Jacobi A, Benhar I, Hong G, Yan W, Adiconis X, Arnold ME, Lee JM, Levin JZ, Lin D, Wang C, Lieber CM, Regev A, He Z, Sanes JR. Single-cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron. 2019;104:1039–1055. doi: 10.1016/j.neuron.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilallongue N, Schaeffer J, Hesse AM, Delpech C, Blot B, Paccard A, Plissonnier E, Excoffier B, Couté Y, Belin S, Nawabi H. Guidance landscapes unveiled by quantitative proteomics to control reinnervation in adult visual system. Nat Commun. 2022;13:6040. doi: 10.1038/s41467-022-33799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


