Abstract
Acinetobacter sp. strain ADP1 is able to grow on a range of esters of aromatic alcohols, converting them to the corresponding aromatic carboxylic acids by the sequential action of three inducible enzymes: an areA-encoded esterase, an areB-encoded benzyl alcohol dehydrogenase, and an areC-encoded benzaldehyde dehydrogenase. The are genes, adjacent to each other on the chromosome and transcribed in the order areCBA, were located 3.5 kbp upstream of benK. benK, encoding a permease implicated in benzoate uptake, is at one end of the ben-cat supraoperonic cluster for benzoate catabolism by the β-ketoadipate pathway. Two open reading frames which may encode a transcriptional regulator, areR, and a porin, benP, separate benK from areC. Each are gene was individually expressed to high specific activity in Escherichia coli. The relative activities against different substrates of the cloned enzymes were, within experimental error, identical to that of wild-type Acinetobacter sp. strain ADP1 grown on either benzyl acetate, benzyl alcohol, or 4-hydroxybenzyl alcohol as the carbon source. The substrate preferences of all three enzymes were broad, encompassing a range of substituted aromatic compounds and in the case of the AreA esterase, different carboxylic acids. The areA, areB, and areC genes were individually disrupted on the chromosome by insertion of a kanamycin resistance cassette, and the rates at which the resultant strains utilized substrates of the aryl ester catabolic pathway were severely reduced as determined by growth competitions between the mutant and wild-type strains.
Acinetobacter sp. strain ADP1, also called BD413 (22), is a saprophytic soil bacterium that has become a focus of research on the organization and evolution of genes involved in aromatic compound degradation (19, 30). ADP1 can degrade a wide variety of aromatic compounds by converting them to either catechol (1,2-dihydroxybenzene) or protocatechuate (3,4-dihydroxybenzoate), compounds whose aromatic rings can be enzymatically cleaved. Following intradiol ring cleavage, metabolites are channelled into the tricarboxylic acid cycle by enzymes encoded by the cat (for catechol) and pca (for protocatechuate) genes. Collectively, this catabolic route is referred to as the β-ketoadipate pathway.
In strain ADP1, the chromosomal cat and pca genes form two distinct supraoperonic clusters of genes each containing functionally related genes involved in funnelling aromatic compounds into the central metabolism. For example, the cat genes are adjacent to the ben genes needed for the conversion of benzoate to catechol (26). Similarly, the pca genes are clustered with pob and qui genes involved in converting 4-hydroxybenzoate and quinate, respectively, to protocatechuate (11, 13). Adjacent to this region are genes needed for the initial degradation of ferulate (29). The ben-cat and pob-qui-pca clusters are separated on the single circular chromosome by approximately 270 kbp (18).
Two exceptions to this clustering have recently been noted. The ant genes, encoding the enzyme that converts anthranilate (2-aminobenzoate) to catechol, lie distant from either cluster (6), and the genes needed for the conversion of vanillate to protocatechuate lie in a fourth distinct region of the chromosome (18a, 29). Despite these observations, the organization, regulation, and integration of the full complement of ADP1 genes for aromatic compound catabolism remain to be fully characterized. In this study, we report the sequence and characterization of an approximately 7-kbp extension of the ben-cat supraoperonic cluster. Included in this region are three contiguous genes whose protein products are capable of channelling the aromatic nucleus of esters of aryl alcohols into the β-ketoadipate pathway. These genes and their products have not previously been reported in strain ADP1.
MATERIALS AND METHODS
Strains and plasmids.
The plasmids and bacterial strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype, phenotype, or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| Acinetobacter | ||
| ADP1 (BD413) | Wild type | 22 |
| ACN73 | areR::Kmr; transformation of ADP1 with pBAC87 | This study |
| ADPW56 | areC::Kmr; transformation of ADP1 with pADPW22 | This study |
| ADPW57 | areB::Kmr; transformation of ADP1 with pADPW26 | This study |
| ADPW58 | areA::Kmr; transformation of ADP1 with pADPW37 | This study |
| ADPW68 | areB::Kmr; transformation of ADP1 with pADPW75 | This study |
| ISA36 | benM::Smr Spr | 9 |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Gibco BRL |
| XL1-Blue MRF′ | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| BL21(DE3)pLysS | F−ompT hsdSB(rB− mB−) dcm gal (DE3) pLysS Cmr | Promega |
| Plasmids | ||
| pET5a | Apr; T7 expression vector | Promega |
| pUC18 and pUC19 | Apr; cloning vector | 40 |
| pUC4K | Apr Kmr; source plasmid for Kmr cassette | 36 |
| pUI1637 | Apr Kmr; source plasmid for Kmr cassette | 14 |
| pBAC68 | 7.0-kbp BglII/NsiI fragment cloned from ISA36 containing Smr Spr cassette and all of benP and part of areR in pUC19 | 8 and this study |
| pBAC78 | 0.86-kbp BglII/KpnI fragment containing part of areR in pUC19 | This study |
| pBAC87 | pBAC78 with Kmr cassette excised from pUI1637 with EcoRV and cloned into SnaBI site in areR | This study |
| pBAC98 | 6.7-kbp NsiI/KpnI fragment cloned from ACN73 containing Kmr cassette, all of areCB, and part of areR and areA | This study |
| pADPW21 | 1.6-kbp BamHI*/SacI fragment containing areC in pUC18 | This study |
| pADPW22 | pADPW21 with Kmr cassette from pUC4K cloned into BglII site in areC | This study |
| pADPW25 | 1.4-kbp BamHI*/EcoRI* fragment containing areB in pUC18 | This study |
| pADPW26 | pADPW25 with Kmr cassette from pUC4K cloned into BclI site in areB | This study |
| pADPW29 | NdeI*/EcoRI* fragment containing areC in pET5a | This study |
| pADPW30 | NdeI*/EcoRI* fragment containing areB in pET5a | This study |
| pADPW31 | 0.65-kbp SacI*/SalI* fragment containing part of areA in pUC18 | This study |
| pADPW32 | 8.0-kbp SacI fragment cloned from ADPW57 containing Kmr cassette and all of areA in pUC18 | This study |
| pADPW33 | 3.6-kbp HindIII subclone of pADPW32 in pUC18 | This study |
| pADPW36 | 1.2-kbp EcoRI* fragment containing areA in pUC18 | This study |
| pADPW37 | pADPW36 with Kmr cassette from pUC4K cloned into NsiI site in areA | This study |
| pADPW40 | NdeI*/EcoRI* fragment containing areA in pET5a | This study |
| pADPW72 | 1.0-kbp EcoRI*/HindIII* fragment containing part of areB in pUC18 | This study |
| pADPW75 | pADPW72 with Kmr cassette from pUI1637 cloned into SacI site in areB | This study |
Restriction sites added by PCR are indicated by superscript asterisks.
Chemicals and media.
Aromatic substrates were obtained from Sigma-Aldrich Co. 2-Hydroxybenzyl acetate and 4-hydroxybenzyl acetate were gifts from A. Boyes, Department of Chemistry, University of Wales Bangor, Bangor, United Kingdom. Luria-Bertani medium (LB) (32) was used to cultivate bacteria unless noted otherwise. For growth on minimal medium, single carbon sources were added to the minimal salts medium (5) at the following concentrations: benzyl acetate, benzyl alcohol, benzaldehyde, and 4-hydroxybenzyl alcohol at 2.5 mM and succinate at 10 mM. Where appropriate, ampicillin at 100 μg/ml and kanamycin at 50 μg/ml for Escherichia coli and at 10 μg/ml for Acinetobacter were used.
DNA manipulations.
Standard methods for DNA manipulations were used (32). Total DNA was prepared from Acinetobacter sp. strain ADP1 by the method of Ausubel et al. (4). Plasmids carrying Acinetobacter DNA were isolated from and maintained in E. coli host XL1-Blue MRF′ or DH5α (Table 1) unless otherwise noted. Plasmid DNA was prepared from E. coli by alkaline lysis miniprep (32) or by Qiaprep columns (Qiagen). DNA fragments were recovered from agarose gels with Qiaquick columns (Qiagen). Southern blots were prepared as described by Sambrook et al. (32), and hybridizations were performed with ECL direct labelling kit (Amersham) according to the manufacturer’s instructions.
PCR amplification.
PCR amplifications were performed in 50-μl reaction mixture volumes containing 10 ng of template DNA, 100 pmol of each primer, 2.5 nmol of each deoxynucleoside triphosphate, 300 nmol of MgSO4, and 1 U of Vent polymerase (New England Biolabs) in the reaction buffer supplied by the manufacturer. In some reaction mixtures, 200 nmol of MgCl2 and 1 U of Taq polymerase were used in lieu of 300 nmol of MgSO4 and 1 U of Vent polymerase. The mixtures were subjected to a 4-min hot start at 94°C and then to 30 cycles, with 1 cycle consisting of 1 min at 94°C, 1 min at 56°C, and 2 min at 74°C.
Cloning of Acinetobacter sp. strain ADP1 DNA.
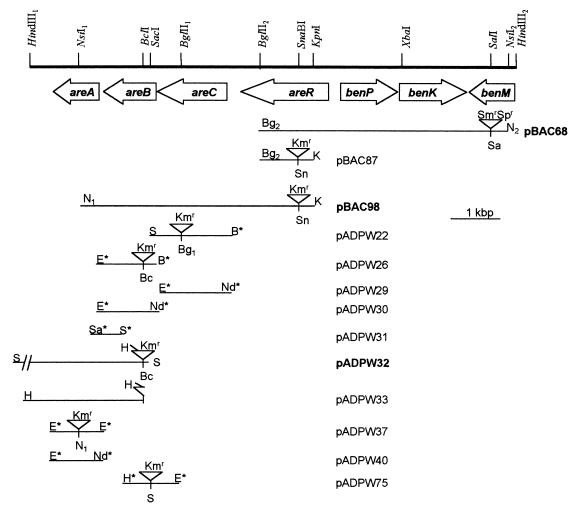
DNA adjacent to the ben-cat cluster was isolated by using the chromosomal drug resistance cassette in benM of strain ISA36 (9). Chromosomal DNA from this strain was used to make a plasmid library in pUC19 in E. coli, and plasmid pBAC68 (Bg2 to N2 in Fig. 1) was selected by appropriate drug resistance. A pBAC68 subclone, pBAC76 (Bg2 to K in Fig. 1), was disrupted by a Kmr cassette to form pBAC87. With previously described methods using the natural transformation system of strain ADP1 (18), the corresponding chromosomal region was replaced by the disrupted Acinetobacter DNA of pBAC87, thereby generating ACN73 (Table 1). A chromosomal fragment from ACN73 was similarly isolated on pBAC98 (N1 to K in Fig. 1).
FIG. 1.
Physical map of the areCBA genes and their locations relative to one end of the supraoperonic ben-cat cluster. The various inserts of the plasmids produced from cloning genomic DNA into vectors are specified in Table 1. Plasmids shown in boldface type contain inserts that were cloned directly from genomic DNA. All other plasmids were produced by PCR from genomic DNA or by subcloning from plasmids containing genomic DNA. Sites at the termini of the inserts marked with an asterisk were incorporated via PCR primers. The Kmr cassette insertions are not drawn to scale. Restriction site abbreviations: B, BamHI; Bc, BclI; Bg, BglII; E, EcoRI; H, HindIII; K, KpnI; N, NsiI; Nd, NdeI; S, SacI; Sa, SalI; Sn, SnaBI.
To clone the additional chromosomal DNA, the areB gene in pADPW25, subcloned from pBAC98, was disrupted by the insertion of a Kmr cassette from pUC4K (36), creating pADPW26 (areB::Kmr). pADPW26 was linearized and transformed into ADP1, creating ADPW57. Chromosomal DNA from ADPW57 was cut with SacI, ligated to SacI-cut pUC18, and transformed into E. coli. Selection for Kmr Apr transformants yielded plasmid pADPW32 with an 8.0-kbp insert. Southern hybridization and DNA sequencing confirmed that pBAC98 and pADPW32 contained overlapping chromosomal DNA fragments. For this purpose, pADPW33 was subcloned from pADPW32 for use as a DNA sequencing template. pADPW31 was designed for use as a hybridization probe for the 5′ end of areA by PCR amplification of pBAC98 with the primer sequences 5′-ATGTTATGTCGACTTTGGTTTC-3′ (forward) and 5′-GCATCATGAGGAGCTCGAGGGTAT-3′ (reverse). The SalI and SacI sites used for cloning the amplified DNA are underlined, and the bases altered from those in the chromosome are in boldface type.
Expression of areC, areB, and areA in E. coli.
Pairs of oligonucleotide primers were designed to produce PCR fragments of each are gene with an NdeI site introduced at the putative ATG start codon of each reading frame and a second constructed restriction site upstream of the NdeI site to allow cloning into pUC18. A natural or constructed restriction site downstream of the gene was used to clone PCR-generated fragments of the expected size first into pUC18. In this way, the areC and areB genes were amplified from pBAC98, and areA was amplified from pADPW32 to create pADPW21, pADPW25, and pADPW36, respectively (Table 1). These three clones were sequenced on one strand to check that mutations had not been incorporated by the PCR. Fragments from each were then excised with NdeI and EcoRI, religated into the expression vector pET5a, and transformed into E. coli BL21(DE3)pLysS to produce plasmids pADPW40 (areA), pADPW30 (areB), and pADPW29 (areC). In the following list of the PCR primers used to make these plasmids, the NdeI site is italicized, the EcoRI or BamHI restriction sites used for cloning into pUC18 are underlined, and the bases that differ from those in the wild-type sequence are in boldface type (roman or italics, as appropriate): areB (forward), 5′-ACAGGATGGATCCATATGACAAAGTTTACC-3′; areB (reverse), 5′-TTCAATGAATTCCCCCGCTACATGAG-3′; areA (forward), 5′-AAAGAATTCTTAACATATGTTACAGAGTTT-3′; and areA (reverse), 5′-GGCATGCAGGGAATTCGAACCAGAGATA-3′. In the case of areC, the reverse primer was chosen from downstream of the native SacI site (see Fig. 1): areC (forward), 5′-AGGACGTTACGGGATCCATATGACATTACT-3′; and areC (reverse), 5′-ATGCCCATCTGGATCTCCACCACTGAAGTT-3′.
The Are proteins encoded on the expression vector pADPW29 (areC), pADPW30 (areB), or pADPW40 (areA) were individually expressed in E. coli BL21(DE3)pLysS by growth in LB to an optical density at 600 nm of 0.6 and then induced for 4 h by the addition of 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a discontinuous gel in a Mini-PROTEAN II Electrophoresis Cell (Bio-Rad) according to the manufacturer’s instructions.
Chromosomal disruption of areA, areB, and areC in Acinetobacter sp. strain ADP1.
Strains ADPW58, ADPW68, and ADPW56 with chromosomal disruptions in areA, areB, and areC, respectively, were constructed by methods similar to that used for ADPW57 (see above). As a first step, pUC18-derived plasmids that each carried one of the are genes disrupted by a Kmr cassette were made. To interrupt areA on pADPW36, the pUC4K cassette was cloned into a unique NsiI site, creating pADPW37. A plasmid-borne areB disruption was made after first creating pADPW72, which has a PCR-generated EcoRI/HindIII insert in pUC18. This 1.0-kbp fragment, flanking the SacI site in areB, was amplified from pBAC98. Primer sequences, with the EcoRI and HindIII sites underlined and the altered bases in boldface type, were as follows: (forward) 5′-TTGAATTCGGCGCGACCCTTGAAATTGGAG-3′ and (reverse) 5′-TTGAAAGCTTCACCTGCACCAGTTTGTATGCC-3′. The central SacI site in the resultant pADPW72 was used as the insertion site for a Kmr cassette from pUI1637 (14) to create pADPW75. The areC gene was disrupted by the insertion of the cassette from pUC4K (36) into the unique BglII site of pADPW21, creating pADPW22. Plasmids pADPW37, pADPW75, and pADPW22 were linearized by digestion with a restriction enzyme, and each was used to transform ADP1. Southern hybridization confirmed that in strains ADPW58 (areA::Kmr), ADPW68 (areB::Kmr), and ADPW56 (areC::Kmr), the altered plasmid-borne allele had replaced the corresponding chromosomal wild-type region.
Competition for growth substrate between wild-type and are mutant Acinetobacter strains.
ADP1 and the gene knockout mutants ADPW56, ADPW58, and ADPW68 were grown overnight in minimal medium containing 10 mM succinate to a cell density of about 109 cells/ml. A 5-μl aliquot of a 10−2 dilution of the wild type and of each of the mutants was inoculated into four tubes containing 990 μl of minimal medium supplemented with either succinate, benzaldehyde, benzyl alcohol, or benzyl acetate as the sole carbon source, respectively. The mixed cultures were grown on each substrate at 37°C for between 12 and 14 generations. Dilutions of the mixed culture were plated on LB agar. A total of 100 colonies were transferred onto LB agar containing 10 μg of kanamycin per ml and onto LB agar as a positive control. The percentage of mutants in the culture was assessed as the number of Kmr colonies. A 10-μl aliquot of each mixed culture was further inoculated into 990 μl of minimal medium containing the corresponding substrate. These cultures were grown for an additional 12 to 14 generations after which the percentage of mutants was again determined.
Preparation of cell extracts.
Cells were harvested by centrifugation, washed with 100 mM phosphate buffer (pH 7.4), and stored as pellets at −20°C. Cell extracts were prepared by suspending frozen pellets in ice-cold 100 mM phosphate buffer, pH 7.4, disrupting by a pass through a French pressure cell (SLM Instruments, Inc., Urbana, Ill.), and centrifuging at 120,000 × g for 30 min at 4°C. The supernatant was stored frozen as 1-ml aliquots at −20°C.
Enzyme assays.
Benzaldehyde dehydrogenase (BZDH) activity was measured in 3-ml assay mixtures containing 100 mM bicine (pH 9.5), 2 mM NAD+, and 100 μM substrate. The reaction was initiated with 10 μl of cell extract, and the production of NADH was monitored spectrophotometrically at 340 nm. All three hydroxybenzaldehydes absorb at 340 nm with the following estimated molar extinction coefficients: 2-hydroxybenzaldehyde, 5,000 mol−1 cm−1; 3-hydroxybenzaldehyde, 1,500 mol−1 cm−1; and 4-hydroxybenzaldehyde, 18,400 mol−1 cm−1. The difference between these values and the molar extinction coefficient of NADH at 340 nm (6,220 mol−1 cm−1) was used to calculate activity with these substrates.
Benzyl alcohol dehydrogenase (BADH) activity was measured in a linked assay in 3 ml containing 100 mM 3-[cyclohexylamino]-1-propanesulfonic acid (CAPS) (pH 10.5), 2 mM NAD+, 200 μM substrate, and 10 μl of undiluted extract of E. coli(pADPW29) overexpressing BZDH. The reaction was initiated with 10 μl of enzyme extract, and the production of NADH was monitored spectrophotometrically at 340 nm. The BZDH was added in excess such that the activity of BADH was rate limiting and the rate of reaction was linear with respect to BADH added. Calculated rates took into account that two NADH molecules are produced per benzyl alcohol molecule oxidized except in the case of 2-methylbenzyl alcohol, since 2-methylbenzaldehyde is not further oxidized by BZDH. A small correction was made to the rate of 2- and 3-hydroxybenzyl alcohol oxidation to allow for the absorbances of 2-hydroxybenzoate (molar extinction coefficient, 40 mol−1 cm−1) and 3-hydroxybenzoate (250 mol−1 cm−1) at 340 nm.
Benzyl esterase was assayed with 4-nitrophenyl ester substrates in 1-ml assay mixtures containing 980 μl of 100 mM phosphate buffer (pH 8) and 10 μl containing various amounts of 4-nitrophenyl ester in methanol, and the reaction was initiated by the addition of 10 μl of enzyme. The activity of the esterase with benzyl ester substrates was determined in a linked assay. The rate of increase of absorbance at 340 nm was measured in 1-ml assay mixtures containing 100 mM phosphate buffer (pH 8), 2 mM NAD+, various amounts of substrate, and 10 μl of BZDH and BADH from extracts of E. coli containing pADPW29 and pADPW30, respectively. The reaction was initiated by the addition of esterase. The fact that the reaction rate was a linear function of esterase concentration verified that the esterase-catalyzed reaction was the rate-limiting step. The assay produces two NADH molecules per molecule of benzyl alcohol produced. This is taken into account when determining Michaelis constant (Km) and maximum velocity (Vmax) values.
Determination of kinetic parameters for benzyl esterase.
To obtain Km and Vmax values, initial velocities were measured at several nonsaturating concentrations of each compound. Preliminary experiments were used to determine the approximate value of Km. Subsequently, accurate rate determinations using from 7 to 10 different substrate concentrations spanning the approximate Km value were performed. Initial velocities were analyzed by Direct Linear analysis using the program EnzPack which calculates the most probable value for the kinetic parameters and the range with 68% confidence (39). Each reaction velocity was determined in triplicate using two separate extract preparations. The concentration of stock solution of substrate was accurately determined enzymatically by making the substrate limiting in the assay, while other components were in excess and the ΔA340 corresponding to the total conversion of added substrate was determined.
Nucleotide sequencing and sequence analysis.
DNA sequences were determined by primer walking of fragments cloned in pUC18 or pUC19. In some cases, sequencing primers (Promega) were used that recognize the pUC18 and pUC19 cloning vectors. Sequencing was done by Alta Biosciences (University of Birmingham, Birmingham, United Kingdom), MWG-Biotech Ltd. (Ebersberg, Germany), and the University of Georgia Molecular Genetics Instrumentation Facility. Searches of the GenBank database were carried out by the BLASTN and BLASTP programs from the National Center for Biotechnology Information, Bethesda, Md. (3). Sequence data were aligned and edited by using the Lasergene software package (DNAStar, Inc., Madison, Wis.). Amino acid sequence alignments were performed by ClustalW (PAM350 matrix) (35). The Wisconsin Genetics Computer Group programs were also used (10).
Nucleotide sequence accession number.
The DNA sequence obtained in this study has been added to GenBank under accession no. AF150928.
RESULTS
Characterization of DNA adjacent to the ben genes.
Plasmid pBAC68 (Fig. 1) was originally isolated during an investigation of benK, the gene for a transport protein involved in the uptake of benzoate, located at one end of the ben-cat supraoperonic cluster for benzoate utilization (8). Nucleotide sequencing of the region immediately upstream of benK revealed an open reading frame, benP (Fig. 1), whose predicted protein product was found to be similar to porins, some of which are associated with genes for aromatic compound degradation (Table 2). The close proximity of benP to benK, with only 37 nucleotides separating the predicted coding regions, indicates that they may be cotranscribed. This possibility, that benP, like benK, is part of a regulon that funnels aromatic compounds through the β-ketoadipate pathway (8), prompted further exploration of the adjacent genetic region.
TABLE 2.
Acinetobacter sp. strain ADP1 genes and gene products
| Gene designation | Putative function of gene product | Size of gene (bp) | % A+T | Size of gene product in:
|
Most similar gene products (species) (% amino acid identity/% amino acid similarity) (accession no.) (reference) | |
|---|---|---|---|---|---|---|
| Residues | kDa | |||||
| benP | Porin | 1,146 | 61 | 381 | 43.8 | PhaK (P. putida) (40/49) (AF029714)a (27) |
| OprD2 (Pseudomonas aeruginosa) (35/43) (X63152)a | ||||||
| areR | Regulatory protein | 1,803 | 60 | 600 | 68.4 | AcoR (P. putida) (25/48) (3688510) |
| AcoR (Clostridium magnum) (21/43) (472325) (24) | ||||||
| areC | BZDH | 1,455 | 53.2 | 484 | 51.9 | XylC (A. calcoaceticus) (77/88) (1408293) (19) |
| XylC (P. putida) (44/64) (D63341) | ||||||
| areB | BADH | 1,116 | 53.1 | 371 | 38.9 | XylB (A. calcoaceticus) (82/91) (1408294) (19) |
| XylB (P. putida) (56/70) (D63341) | ||||||
| areA | Benzyl esterase | 981 | 63 | 326 | 37.1 | Esterase (Archaeoglobus fulgidus) (31/48) (2648837) |
| Esterase (P. putida) (28/50) (2853612) (7) | ||||||
In a selective alignment of 150 residues.
As described in Materials and Methods and illustrated in Fig. 1, plasmids pBAC98 and pADPW32 were isolated and the DNA sequences were determined. An open reading frame, areR, was found upstream of and divergently transcribed from benP, which is homologous to genes encoding XylR-type transcriptional activators (Table 2) (25, 31). The results of sequence analyses were consistent with the possibility that the areR-encoded protein acts in a ς54-dependent fashion to control the expression of the genes immediately downstream (Fig. 1).
Identification of the areCBA genes.
Downstream of areR, three genes whose predicted protein products have homology to bacterial dehydrogenases active against benzaldehyde and benzyl alcohol and to esterases, respectively, were identified. The genes have been designated areCBA in the order of their transcription. From database searches, the deduced amino acid sequence of AreC showed the most similarity to XylC, benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus NCIMB8250 (17), and to a lesser extent to the benzaldehyde dehydrogenase encoded by the xylC gene on the Pseudomonas TOL plasmid pWW0 (20). AreB similarly was most like the corresponding BADHs encoded by the xylB genes from the same sources, A. calcoaceticus NCIMB8250 (17) and the TOL plasmid (34). AreA is similar to a number of esterases, particularly those from Archaeoglobus fulgidus and Pseudomonas putida (7) (Table 2).
Expression of individual are genes.
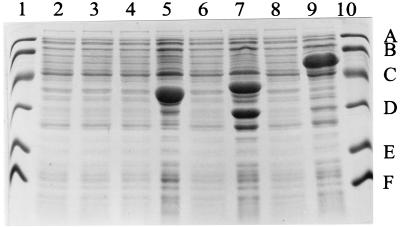
Each of the are genes was inserted into the expression vector pET5a such that gene expression would be optimally controlled by the T7 promoter on the plasmid when carried by an appropriate E. coli host. SDS-PAGE of extracts of the induced E. coli hosts showed high levels of expression of each protein with the predicted size (Fig. 2), whereas these proteins were not observed in either uninduced strains or those lacking the recombinant plasmids. In the case of induced AreB (lane 7), in addition to the expected band, there are also two smaller bands with molecular masses of 32 and 27 kDa: these could be degradation products of AreB but they are both prominent to a lesser extent in lanes 5 (induced AreA) and 9 (induced AreC).
FIG. 2.
SDS-PAGE of overexpressed Are proteins. The lanes contain lysates from E. coli BL21(DE3)pLysS (lane 2) on carrying the following plasmids: pET5a (lane 3), pADPW40 (lane 4), pADPW40 (lane 5), pADPW30 (lane 6), pADPW30 (lane 7), pADPW29 (lane 8), or pADPW29 (lane 9). The proteins were induced (lanes 5, 7, and 9) or not induced (lanes 4, 6, and 8) with IPTG. Lanes 1 and 10 contain the following molecular mass standards (values in kilodaltons): A, 97.4; B, 66.2; C, 45.0; D, 31.0; E, 21.5; and F, 14.5. The estimated molecular masses for the overexpressed bands are as follows: AreA (lane 5), 37 kDa; AreB (lane 7), 39 kDa with minor components at 32 and 27 kDa; and AreC (lane 9), 52 kDa.
Enzyme assays of cloned Are proteins.
The AreC protein, expressed from plasmid pADPW29 (Table 1), showed NAD+-dependent BZDH activity against benzaldehyde and all the methyl- and hydroxy-substituted benzaldehydes except for 2-methylbenzaldehyde, against which there was no measurable activity (Table 3). The best substrate tested was 2-hydroxybenzaldehyde (salicylaldehyde), which was oxidized at a rate sixfold greater than was benzaldehyde or 3-hydroxybenzaldehyde.
TABLE 3.
Relative activities of AreC BZDH against different benzaldehydes in crude extracts of cells grown on different substrates
| Strain | Growth substrate | Relative activitya (sp act [U/mg of protein]) against benzaldehyde with substituent:
|
||||||
|---|---|---|---|---|---|---|---|---|
| None | 2-Methyl | 3-Methyl | 4-Methyl | 2-OH | 3-OH | 4-OH | ||
| E. coli (pADPW29) | LB + Ampb + IPTG | 100 (3.0) | <1 | 67 | 29 | 600 | 102 | 80 |
| ADP1 | Benzyl alcohol | 100 (0.29) | <1 | 80 | 33 | 600 | 101 | 117 |
| ADP1 | Benzyl acetate | 100 (0.16) | <1 | 80 | 29 | 600 | 101 | 96 |
| ADP1 | 4-OH benzyl alcohol | 100 (0.27) | <1 | 80 | 28 | 600 | 105 | 107 |
| ADP1 | Succinate | (<0.001) | ND | ND | ND | ND | ND | ND |
Activity relative to benzaldehyde, which is set at 100. All reaction rates upon which the relative activities were based were the averages of three measurements, none of which varied by >5%. ND, not determined.
Amp, ampicillin.
AreB, expressed from pADPW30 (Table 4), was assayed for NAD+-dependent BADH activity in the presence of excess AreC with compensation made for the twofold production of NADH in all cases except 2-methylbenzyl alcohol: by linking the alcohol dehydrogenase assay to BZDH, the initial rate of NADH production was linear over a much longer time period, expediting accurate rate determination. AreB showed significant activity against benzyl alcohol and the methyl- and hydroxy-substituted benzyl alcohols and, unlike AreC, was able to oxidize the 2-methyl analog at a rate comparable to those of the other substrates.
TABLE 4.
Relative activities of AreB BADH against various benzyl alcohols in crude extracts of cells grown on different substrates
| Strain | Growth substrate | Relative activitya (sp act [U/mg of protein]) against benzyl alcohol with substituent:
|
||||||
|---|---|---|---|---|---|---|---|---|
| None | 2-Methyl | 3-Methyl | 4-Methyl | 2-OH | 3-OH | 4-OH | ||
| E. coli (pADPW30) | LB + Ampb + IPTG | 100 (2.3) | 78 | 105 | 107 | 47 | 41 | 18 |
| ADP1 | Benzyl alcohol | 100 (0.94) | 62 | 99 | 97 | 42 | 32 | 14 |
| ADP1 | Benzyl acetate | 100 (0.90) | 63 | 103 | 106 | 43 | 33 | 11 |
| ADP1 | 4-OH benzyl alcohol | 100 (0.92) | 57 | 106 | 110 | 50 | 51 | 14 |
| ADP1 | Succinate | (<0.001) | ND | ND | ND | ND | ND | ND |
Activity relative to benzyl alcohol, which is set at 100. All reaction rates upon which the relative activities are based were the averages of three measurements none of which varied by >5%. ND, not determined.
Amp, ampicillin.
The esterase AreA, cloned on pADPW40 (Table 1), was assayed in two ways, against 4-nitrophenyl esters by monitoring the absorbance at 405 nm of the 4-nitrophenolate anion product and against benzyl esters by monitoring linkage to NADH production in the presence of excess amounts of both AreB and AreC. With both assays, the results (Table 5) were analyzed to determine the kinetic parameters Km and Vmax. For both sets of substrates, the value of Km dropped as the chain length of the carboxylic acid moiety increased. The acetate esters were the poorest substrates in both classes, as can be judged by the lower relative specificity constants (Vmax/Km) resulting from both higher Km and lower Vmax values. The butyrate esters were the best substrates tested in both classes with higher affinities and higher Vmax values.
TABLE 5.
Kinetic parameters for AreA benzyl esterase
| Substrate | Km (μM) (range)a | Vmax (μmol/min/mg) (range)a | Vmax/Km |
|---|---|---|---|
| 4-Nitrophenyl acetate | 2,410 (2,260–2,720) | 220 (207–236) | 0.11 |
| 4-Nitrophenyl butyrate | 61 (53–68) | 960 (912–1,020) | 16 |
| 4-Nitrophenyl hexanoate | 11 (10.7–12.3) | 430 (424–443) | 39 |
| Benzyl acetate | 227 (213–238) | 690 (670–712) | 3 |
| Benzyl propionate | 67 (63–70) | 2,090 (2,020–2,140) | 31 |
| Benzyl butyrate | 42.4 (40–45) | 2,050 (2,010–2,090) | 47 |
| Benzyl benzoate | 47 (43.6–49) | 1,100 (1,070–1,130) | 23 |
The range in parentheses after each value specifies the 68% confidence limits.
Expression of are genes in strain ADP1.
Wild-type ADP1 is able to utilize the following compounds as growth substrates: benzyl acetate, benzyl propionate, benzyl butyrate, 2-hydroxybenzyl acetate, 4-hydroxybenzyl acetate, benzyl alcohol, 2-hydroxybenzyl alcohol, 4-hydroxybenzyl alcohol, benzaldehyde, 2-hydroxybenzaldehyde, and 4-hydroxybenzaldehyde. It is unable to grow on benzyl benzoate or benzyl alcohols or benzaldehydes with 3-hydroxy or 2-, 3-, or 4-methyl substituents. To test whether the Are enzymes are expressed during growth on some of these substrates, assays of benzyl alcohol and benzaldehyde dehydrogenase activities against the same range of substrates were measured after growth on benzyl acetate, benzyl alcohol, and 4-hydroxybenzyl alcohol (Tables 3 and 4). In all cases, the relative activities against different substrates were essentially identical to those of the overexpressed AreC and AreB activities, although the induced specific activities in ADP1 were lower than in the recombinant E. coli by factors of 10 and 2.5, respectively. AreB and AreC were induced to considerably higher specific activity when grown on benzyl acetate or benzyl alcohol than when grown on succinate (Tables 3 and 4); similarly AreA was induced >500-fold when grown on the aromatic substrates compared with growth on succinate (data not shown).
Insertional inactivation of are genes.
The chromosomal copy of areA, areB, or areC was specifically disrupted by a Kmr cassette in Acinetobacter strains ADPW58, ADPW68, and ADPW56, respectively (Table 1). These strains were tested for plate growth on benzyl acetate, benzyl alcohol, and benzaldehyde. In ADPW56 (areC::Kmr), the rate of growth on all three substrates was severely reduced, but not completely eliminated, and patches took from 2 to 3 days to achieve significant size compared with overnight growth for ADP1. ADPW68 (areB::Kmr) showed a reduced growth rate on benzyl acetate and benzyl alcohol but appeared to grow normally on benzaldehyde. The growth rate of ADPW58 (areA::Kmr) was reduced only on benzyl acetate. The results were therefore consistent with disruption of a pathway involving sequential action of AreA, AreB, and AreC.
To confirm these qualitative assessments of growth rates, growth competition experiments in liquid medium on all three substrates were set up between each of the knockout mutants and ADP1. In these assays, the initial cell densities of wild type and mutants were set to be approximately equal and the proportion of the mutants during two sequential subcultures each of about 12 to 14 generations was determined by plating out serial dilutions and determining the percentage of Kmr colonies. In all cases where the plate growth rate appeared to have been reduced, the percentage of mutant colonies dropped from approximately 50 to below 6 after the two subcultures. This was the case for ADPW56 on all three substrates, for ADPW68 on benzyl acetate and benzyl alcohol and for ADPW58 on benzyl acetate. Neither ADPW68 nor ADPW58 was outcompeted by ADP1 during growth on benzaldehyde nor was the latter outcompeted during growth on benzyl alcohol. Control experiments with succinate as the sole carbon source showed no outcompetition of the mutants by ADP1.
DISCUSSION
Contribution of areABC to the catabolic breadth of Acinetobacter sp. strain ADP1.
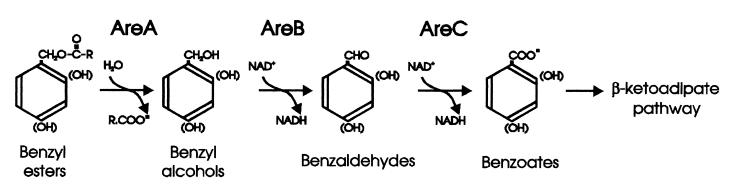
The areABC genes of ADP1, which were identified and characterized in this study, encode enzymes for the sequential conversions of the esters of benzyl alcohol to benzyl alcohol, benzaldehyde, and benzoic acid (Fig. 3). The areABC genes can thereby funnel metabolites into the catechol branch of the β-ketoadipate pathway encoded by the chromosomally adjacent ben and cat genes. The substrate preferences of the Are proteins were tested after individually overexpressing areA, -B, and -C in an E. coli host. The resultant substrate profiles of AreB and AreC, consistent with the proposed enzyme functions, matched those of the wild-type strain grown on benzyl alcohol, benzyl acetate, or 4-OH benzyl alcohol. Although the specific inactivation of each gene on the Acinetobacter chromosome resulted in slow growth on benzyl acetate, it did not completely eliminate the ability to utilize this carbon source. It is likely that other esterases and dehydrogenases within the cell can partially complement the defective are genes. Nevertheless, the catabolic deficiencies of the are mutants were clearly demonstrated by the rapid ability of the wild-type strain to outcompete each mutant during growth on benzyl acetate.
FIG. 3.
Proposed pathway for catabolism of aryl esters by Acinetobacter sp. strain ADP1.
A number of results suggest that the AreCBA proteins are responsible for the catabolism of a wider range of esters than just benzyl esters. First, the substrate ranges of all three enzymes are broad. Both dehydrogenases are able to utilize 2- and 4-hydroxy-substituted substrates, producing salicylate and 4-hydroxybenzoate, respectively. Both of these carboxylic acids are growth substrates for ADP1 and are also metabolized via the β-ketoadipate pathway. Salicylate is converted by salicylate 1-hydroxylase to catechol and thence dissimilated by the cat gene products, whereas 4-hydroxybenzoate is dissimilated by the pca-pob gene products. Growth of ADP1 on 4-hydroxybenzyl alcohol induces both BZDH and BADH activities with the same substrate preference as when grown upon benzyl alcohol or benzyl acetate, indicating that the same genes are expressed and are functional during growth on both substrates. Moreover, the chromosomal disruption of either areA, -B, or -C severely reduces the wild-type ability to grow on the 2-OH- and 4-OH-substituted benzyl acetates as carbon sources.
Given the wide range of natural aromatic acids which ADP1 can utilize (29), ADP1 may degrade a much wider range of aryl esters than those that were tested here. This possibility is difficult to test, since such compounds are not currently commercially available. It is also noteworthy that the esterase itself is nonspecific as far as the acid moiety is concerned and is able to hydrolyze 4-nitrophenyl esters of alkanoic acids with chain lengths of up to 16 carbons (data not presented). Esterase activity on a novel substrate might produce an aromatic alcohol that is not further metabolized, and yet the alkanoic acid itself could serve as a carbon source. In the natural environment, the Are enzymes may therefore be responsible for supporting growth on a wide range of substrates.
Factors beyond substrate specificity clearly contribute to carbon source utilization as well. For example, benzyl benzoate acts as a substrate for AreA to produce two products, benzoic acid and benzyl alcohol, both of which are excellent growth substrates. Despite this, benzyl benzoate is not itself a growth substrate, perhaps because of toxicity or blocks in uptake or regulation.
Comparisons of the Are proteins with similar enzymes.
The deduced amino acid sequence of AreB shows all the characteristics of group 1 long-chain zinc-dependent alcohol dehydrogenases (ADH) as typified by horse liver ADH (12). AreB has the conserved residues which bind the catalytic Zn2+ (Cys44, His65, and Cys173) and the four Cys residues which bind the structural Zn2+ (at positions 94, 97, 100, and 108). It also contains the characteristic motif GHEXXGXXXXXGXXV from residues 64 to 78. Three bacterial sequences that are similar to AreB include two BADHs, both designated XylB, from A. calcoaceticus NCIMB8250 (17) and from the TOL plasmid of P. putida (34), and the XylW protein of unknown function from the TOL plasmid (38). In AreB, as in each of these bacterial sequences, there is a replacement of the His51 (as numbered in the horse liver enzyme) by a hydrophobic residue, in this case Ile49. This His is conserved in almost all of the long-chain zinc-dependent ADHs and is believed to act as a general base during the catalytic reaction. Inoue et al. (21), however, have suggested that in the TOL XylB an adjacent His residue takes this role, and in AreB there is an equivalent adjacent residue (His46).
AreC has an amino acid sequence which is typical of a large family of NAD+-linked aldehyde dehydrogenases (class II). In particular, it contains the consensus sequence FTGSTXVG (residues 228 to 235, FTGSTQVG). Also conserved are the residues Cys285 and Glu251, homologous to Cys302 and Glu268 in the human liver mitochondrial aldehyde dehydrogenase that have been shown to be involved in the catalytic reaction (15, 37).
AreA appears to be a member of the α/β hydrolase fold family of enzymes (2, 28, 33). Despite a characteristic GXSXG consensus sequence, many members of this family display relatively low primary sequence conservation and are identified mainly by a method based on predicted structural similarities (2). In the case of AreA, the primary sequence can be aligned well with two other Acinetobacter esterases in this family. In the Acinetobacter lwoffii RAG-1 esterase, the residues that comprise the catalytic triad were suggested to be Ser149, either Asp196 or Glu244, and either His265, His274, or His298 (1). Three of these residues, Ser149, Glu244, and His274, align with those in AreA, indicating that in AreA, Ser166, Asp266, and His296 may form the catalytic triad. These AreA residues also align, respectively, with Ser201, Asp295, and His325 of a carboxylesterase of Acinetobacter sp. strain BD413 (ADP1) (23).
Genetic organization and regulation.
The areCBA genes are all transcribed in the same direction, raising the possibility that these genes are cotranscribed. However, the order of the genes is opposite to that in which the gene products are needed, making it difficult to draw conclusions about whether the chromosomal insertion of Kmr cassettes in the are genes had polar effects on gene expression. For example, insertion of the cassette in the upstream gene areC, encoding BZDH activity, caused reduced growth on all the ester, alcohol, and aldehyde substrates, as would be expected by a polar effect of the inserted cassette on expression of the downstream genes. Nevertheless, this phenotype would also be expected if the genes were independently expressed, since the AreC gene product is the last of the three Are enzymes used in the formation of benzoic acids (Fig. 3). However, the relatively short intergenic regions between areC and -B (27 bp) and between areB and -A (83 bp) suggest coexpression of areCBA as an operon.
A sequence upstream of areC indicates that areC is at the start of a transcriptional unit. From bases −123 to −109 upstream of the start codon of areC, there is a possible ς54-dependent (−12, −24) promoter sequence (TGGCAC-N5-CTGC) that is well matched to the consensus sequence TGGCAC-N5-TTGC. This putative promoter is of particular interest, since the upstream gene, areR, appears to encode a member of the ς54-dependent XylR transcriptional regulator family. The deduced AreR amino acid sequence contained the conserved regions found in members of this family that function in ATP binding, ς54 interaction, and DNA binding (16, 25, 31).
The xylBC genes of A. calcoaceticus NCIMB8250 (17) are even more similar to areBC of ADP1 than are xylBC of the TOL plasmid. The two A. calcoaceticus genes are in the same order as in ADP1, although no areA homolog has been identified downstream of these xyl genes. In the published NCIMB8250 xylCB sequence, there are 200 bp downstream of xylB which show negligible homology to that downstream of areB. Furthermore, we have probed Southern blots of a plasmid containing 1 to 2 kb downstream of xylBC (a gift of D. J. Gillooly and C. A. Fewson) with an areA probe with negative results (data not shown). In the NCIMB8250 strain, the xylCB genes may therefore be involved in catabolism of only benzyl alcohol and not of benzyl esters.
The aromatic supraoperonic cluster in ADP1.
The known size of the genetic region involved in the catabolism of compounds by the catechol branch of the β-ketoadipate pathway was extended by the identification of the are genes. The characterized region of this supraoperonic cluster now encompasses the region from areA at one end (Fig. 1) to catD (accession number AF00924) at the other, a total distance of 25 kbp. The linkage of genes encoding functionally related Are, Ben, and Cat proteins suggests that AreR and BenP also participate in aromatic compound degradation. The possibilities that areR encodes a XylR-like transcriptional regulator and that benP encodes a porin remain to be tested, however.
ACKNOWLEDGMENTS
This research was funded in part by a BBSRC research studentship (to R.M.J.) and by National Science Foundation grant MCB-9808784 (to E.L.N.).
REFERENCES
- 1.Alon R N, Gutnick D L. Esterase from the oil-degrading Acinetobacter lwoffii RAG-1: sequence analysis and over-expression in Escherichia coli. FEMS Microbiol Lett. 1993;112:275–280. doi: 10.1111/j.1574-6968.1993.tb06462.x. [DOI] [PubMed] [Google Scholar]
- 2.Alon R N, Mirny L, Sussman J L, Gutnick D L. Detection of α/β-hydrolase fold in the cell surface esterases of Acinetobacter species using an analysis of 3D profiles. FEBS Lett. 1995;371:231–235. doi: 10.1016/0014-5793(95)00861-3. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D E, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 5.Bauchop T, Elsden S R. The growth of microorganisms in relation to energy supply. J Gen Microbiol. 1960;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- 6.Bundy B M, Campbell A L, Neidle E L. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol. 1998;180:4466–4474. doi: 10.1128/jb.180.17.4466-4474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo D W, Kurihara T, Suzuki T, Soda K, Esaki N. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol. 1998;64:486–491. doi: 10.1128/aem.64.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier L S, Nichols N N, Neidle E L. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J Bacteriol. 1997;179:5943–5946. doi: 10.1128/jb.179.18.5943-5946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collier L S, Gaines III G L, Neidle E L. Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcriptional activator. J Bacteriol. 1998;180:2493–2501. doi: 10.1128/jb.180.9.2493-2501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devereaux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doten R C, Ngai K-L, Mitchell D J, Ornston L N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987;169:3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eklund H, Samama J-P, Wallen L, Branden C-I. Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase. J Mol Biol. 1981;146:561–587. doi: 10.1016/0022-2836(81)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Elsemore D A, Ornston L N. The pca-pob supraoperonic cluster of Acinetobacter calcoaceticus contains quiA, the structural gene for quinate-shikimate dehydrogenase. J Bacteriol. 1994;176:7659–7666. doi: 10.1128/jb.176.24.7659-7666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthetic gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farres J, Wang T T Y, Cunningham S J, Weiner H. Investigation of the active-site cysteine residue of rat-liver mitochondrial aldehyde dehydrogenase by site-directed mutagenesis. Biochemistry. 1995;34:2592–2598. doi: 10.1021/bi00008a025. [DOI] [PubMed] [Google Scholar]
- 16.Fernández S, Shingler V, De Lorenzo V. Cross-regulation by XylR and DmpR activators of Pseudomonas putida suggests that transcriptional control of biodegradative operons evolves independently of catabolic genes. J Bacteriol. 1994;176:5052–5058. doi: 10.1128/jb.176.16.5052-5058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillooly D J, Robertson A G S, Fewson C A. Molecular characterization of benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II of Acinetobacter calcoaceticus. Biochem J. 1998;330:1375–1381. doi: 10.1042/bj3301375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralton E M, Campbell A L, Neidle E L. Directed introduction of DNA cleavage sites to produce a high-resolution genetic and physical map of the Acinetobacter sp. strain ADP1 (BD413UE) chromosome. Microbiology. 1997;143:1345–1357. doi: 10.1099/00221287-143-4-1345. [DOI] [PubMed] [Google Scholar]
- 18a.Gralton, E. M., and E. L. Neidle. Unpublished observation.
- 19.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 20.Inoue J, Shaw J P, Rekik M, Harayama S. Overlapping substrate specificities of benzaldehyde dehydrogenase (the xylC gene product) and 2-hydroxymuconic semialdehyde dehydrogenase (the xylG gene product) encoded by TOL plasmid pWW0 of Pseudomonas putida. J Bacteriol. 1995;177:1196–1201. doi: 10.1128/jb.177.5.1196-1201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue J, Tomioka N, Itai A, Harayama S. Proton transfer in benzyl alcohol dehydrogenase during catalysis: alternate proton-relay routes. Biochemistry. 1998;37:3305–3311. doi: 10.1021/bi970726g. [DOI] [PubMed] [Google Scholar]
- 22.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kok R G, Christoffels V M, Vosman B, Hellingwerf K J. Growth-phase dependent expression of the lipolytic system of Acinetobacter calcoaceticus BD413: cloning of a gene encoding one of the esterases. J Gen Microbiol. 1993;139:2329–2342. doi: 10.1099/00221287-139-10-2329. [DOI] [PubMed] [Google Scholar]
- 24.Krüger N, Oppermann F B, Lorenzl H, Steinbüchel A. Biochemical and molecular characterization of the Clostridium magnum acetoin dehydrogenase enzyme system. J Bacteriol. 1994;176:3614–3630. doi: 10.1128/jb.176.12.3614-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morett E, Segovia L. The ς54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993;175:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidle E L, Shapiro M K, Ornston L N. Cloning and expression in Escherichia coli of Acinetobacter calcoaceticus genes for benzoate degradation. J Bacteriol. 1987;169:5496–5503. doi: 10.1128/jb.169.12.5496-5503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivera E R, Minambres B, Garcia B, Muniz C, Moreno M A, Ferrandez A, Diaz E, Garcia J L, Luengo J M. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc Natl Acad Sci USA. 1998;95:6419–6424. doi: 10.1073/pnas.95.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ollis D L, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken S M, Harel M, Remington S J, Silman I, Schrag J, Sussman J L, Verschueren K H G, Goldman A. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 29.Ornston, L. N. Personal communication.
- 30.Ornston L N, Neidle E L. Evolution of genes for the β-ketoadipate pathway in Acinetobacter calcoaceticus. In: Towner K, Bergogne-Berezin E, Fewson C A, editors. The biology of Acinetobacter. New York, N.Y: Plenum Press; 1991. pp. 201–237. [Google Scholar]
- 31.Osuna J, Soberon X, Morett E. A proposed architecture for the central domain of the bacterial enhancer-binding proteins based on secondary structure prediction and fold recognition. Protein Sci. 1997;6:543–555. doi: 10.1002/pro.5560060304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schrag J D, Cygler M. Lipases and α/β hydrolase fold. Methods Enzymol. 1997;284:85–107. doi: 10.1016/s0076-6879(97)84006-2. [DOI] [PubMed] [Google Scholar]
- 34.Shaw J P, Rekik M, Shwager F, Harayama S. Kinetic studies on benzyl alcohol dehydrogenase encoded by TOL plasmid pWW0. J Biol Chem. 1993;268:10842–10850. [PubMed] [Google Scholar]
- 35.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W improving the sensitivity of progressive multiple sequence alignment through sequence weights, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 37.Wang X P, Weiner H. Involvement of glutamate-268 in the active-site of human liver mitochondrial (class-2) aldehyde dehydrogenase as probed by site-directed mutagenesis. Biochemistry. 1995;34:237–243. doi: 10.1021/bi00001a028. [DOI] [PubMed] [Google Scholar]
- 38.Williams P A, Shaw L M, Pitt C W, Vrecl M. xylUW, two genes at the start of the upper pathway operon of TOL plasmid pWW0, appear to play no essential part in determining its catabolic phenotype. Microbiology. 1997;143:101–107. doi: 10.1099/00221287-143-1-101. [DOI] [PubMed] [Google Scholar]
- 39.Williams P A, Zaba B N. EnzPack for Windows. Cambridge, United Kingdom: Biosoft; 1997. [Google Scholar]
- 40.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]