Abstract
Background
Child mortality remains a global public health problem, particularly in Sub-Saharan Africa. After initiating ART, the mortality rate among HIV-infected children in Ethiopia was 12–17 deaths per 1000 child-year.
Objective
To determine the time to death and its predictors among under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia, from April 12, 2017, to May 12, 2022.
Method
An institution-based retrospective follow-up study was conducted among 415 HIV-infected children at selected public hospitals of the Addis Ababa town administration. Computer generated simple random sampling technique was used to select each sampling unit. Data was extracted using a structured data extraction checklist. Data were entered into EPI data 4.2 and analyzed using STATA 14. The child mortality rate was calculated. The Cox proportional hazards regression model was fitted to identify predictor factors. The result of the study was presented using text, tables, graphs, and charts. An adjusted hazard ratio with a 95% confidence interval and a p-value less than 0.05 was used to declare the level of significance.
Result
A total of 415 (97.42%) of the 426 children on ART were included for analysis. Of these, 41(9.88%) children were died during the following period. The study participants were followed for a total of 8237 person- months of risk time. The overall mortality rate was 4.98 (95% CI: 3.67–6.77) per 1000 child-months. The estimated survival after starting ART was 61.42% at 56 months of follow-up. Severe underweight (AHR = 3.19; 95% CI: 1.32–7.71), tuberculosis (AHR = 3.86; CI: 1.76–8.47), low hemoglobin level (AHR = 2.51; CI: 1.02–6.20), and advanced WHO clinical stages at enrolment (AHR = 3.38; CI: 1.08–10.58) were predictors of death among HIV-infected under-five children on ART.
Conclusion
The incidence of mortality was 4.98 per 1000 child-months. Severe underweight, tuberculosis infection, low hemoglobin level, and advanced WHO clinical stages at enrolment were predictors of death among under-five children on ART.
Introduction
The child mortality rate is a significant indicator of social and national development since it reflects health equity and access [1]. AIDS is an infection caused by the Human Immunodeficiency Virus (HIV), which predominantly attacks a person’s immune system. HIV infection in children under five has rapid disease progression and opportunistic infections due to immune weakness, which leads to higher morbidity and mortality [2–4].
Antiretroviral therapy (ART) is an HIV treatment that includes multiple antiretroviral medications taken throughout life to enhance duration and quality of life by lowering viral load and raising CD4 cell count [5, 6]. The advancement of HIV therapy had a positive impact on disease progression and immunological capacity, resulting in higher survival rates [2]. Ethiopia’s government established the first highly active antiretroviral therapy (HAART) guideline in 2003, which was modified in 2021 to scale up the program quickly. According to the 2021 national recommendation, all children should receive HAART [7].
To ensure that simple and effective therapy becomes quickly available and accessible for all under-five children in need, the government, NGO, research partners, health specialists, and civil society vigorously promote 4g = ABC+3TC-LPV/r and 4j = ABC+3TC+DTG for the development of fixed-dose combinations [4].
HIV/AIDS resulted in the death of 328 children daily in 2016, accounting for 1.4% of all under-five deaths globally [8]. The burden is still evident despite a 62% drop in AIDS-related mortality and a 70% drop in new HIV infections since 2000 [9]. In children under the age of five, the condition advances quickly, and if treatment is not initiated, up to 30% of children will die before their first birthday, and up to 50% will die before their second birthday [2]. Without treatment, 35.2% of HIV-infected children in Africa died in their first year, and 52.5% died by the age of 2 (two) [10]. More than half of the deaths (50–68.8%) occurred within 6 (six) months after starting ART [11]. Studies in Africa reported that the incidence of death following ART initiation among children was 18.7% in South Africa [12], Zambia 1.6% [13], Zimbabwe 8.14% [14], Kenya 12.78%, and 2.9% in Cameroon [15].
In Ethiopia, the mortality rate among HIV-infected children in Ethiopia after commencing ART was 12–17 deaths per 1,000 children per year. The length of life of HIV-infected children on ART is mainly affected by clinical factors [16, 17]. A study in Ethiopia’s Oromia Liyu Zone found that 5.9% of people died after starting ART [9].
The effects of HIV/AIDS infection happen at a crucial developmental stage when the immune system is immature [18]. On children, wide-ranging factors negatively impact the quality of care, socialization, health, and emotional and cognitive development of affected children. As a result of their status, they are at a higher risk of mortality, illness, and psychological suffering [19]. The physical well-being of HIV-infected children is likewise heavily reliant on HIV treatment. More than one-third of children do not survive their first birthday if they are not treated [20].
HIV/AIDS has been one of the Ethiopian government’s top priorities in the health sector [21]. To achieve this goal, Ethiopia’s government has adopted strategies to minimize morbidity and mortality by limiting future disease transmission and boosting access to HIV care, treatment, and support for under-five children living with HIV [22, 23]. Efforts to scale up early baby diagnostic programs and early assessment of HIV-infected infants on ART, regardless of WHO clinical stage or CD4 cell count [24]. More should be done to address the unique survival needs of children in Ethiopia, even though visible efforts to improve the health of HIV-infected children have led to significant reductions in mortality levels among under-five children [5].
Early ART initiation in under five HIV-infected children significantly reduces children’s mortality [25]. Even though ART has demonstrated significant clinical importance by achieving the therapy’s goal, children continue to die from a variety of preventable causes that could be avoided with appropriate interventions on socioeconomic, demographic, treatment-related, and health factors such as the child’s age, CD4 count or CD4 percent at ART initiation, WHO stage, hemoglobin level, and ART adherence, by developing the PMCTC service [12, 26].
ART for HIV-infected children leads to immunological reconstitution through decreasing viral load, increasing CD4 cells, preventing opportunistic infection, and a longer survival time [5, 27]. Even though shreds of evidence regarding is needed regarding the survival status of children on ART and its predictors to maximize the benefit by addressing any modifiable variables, information regarding time to death and its predictors among under-five children after antiretroviral treatment in Ethiopia is limited. In addition, up to the researcher’s knowledge, there is a published study in the study setting. Therefore, this study aimed to assess time to death and its predictors among HIV-infected under-five children after initiation of ART treatment at selected public hospitals in Addis Ababa, Ethiopia.
Methods and materials
Study design, setting and period
The retrospective follow-up study was conducted to under five children who initiated ART on the period from April 12, 2017, to May 12, 2022 at public hospitals in Addis Ababa, Ethiopia’s capital city. Addis Ababa is the headquarters of the African Union. According to the 2007 census, it had a total population of 3,384,569 people, but by 2017, it had about 4 million people, with a population growth rate of 2.9% per year [28]. An estimated 300,000 people are infected with HIV/AIDS. Around 12,000 were youngsters, and 30 to 40 percent of HIV-infected people were under-five children [29].
There are 12 public hospitals in the area. The Addis Ababa health bureau is responsible for managing (Six) of them, including Gandhi Memorial Hospital, Yekatit 12 Hospital Medical College, Zewditu Memorial, Ras-Desta Damtew Memorial, Menelik II Referral, and Tirunesh Beijing General Hospital. Amanuel Hospital, Alert Hospital, St. Peter Specialized Hospital, Millennium Medical College Hospital, and Eka Kotebe Hospital are the five (5) hospitals under the federal Ministry of Health’s administration. The other is a teaching hospital called Tikur Anbessa Specialized Hospital, administered by Addis Ababa University. Only eight hospitals provide ART services for under-five children, including Black Lion Hospital, Yekatit 12 Hospitals Medical College, Saint Petrous Specialized Hospital, Alert Hospital, Tirunesh Beijing General Hospital, St. Paulo’s Hospital, Zewditu Memorial Hospital, and Menelik II Referral Hospital, included in the study. The remaining four excluded from the study because they did not provide under five ART services.
Populations
All under-five children with HIV infection and on ART at the public hospital of Addis Ababa were the source population, and HIV-infected under-five children who started antiretroviral therapy (ART) the period from April 12, 2017, to May 12, 2022, at the selected public hospital of Addis Ababa were the study population.
Eligibility criteria
All HIV-infected under-five children on antiretroviral therapy (ART) at the selected public hospital of Addis Ababa, from April 12, 2017, to May 12, 2022, were included. Under-five children with incomplete data on the outcome variables like date of treatment initiation, age of the child and pertinent variables were excluded from the study.
Sample size determination
The study sample size of the study calculated using STATA version 14 statistical software using the formula N = Event(E) /Probability of event (E) by considering the following assumptions; A 95% level of confidence, 5% level of significance, 80% power and 10% assumption of withdrawals [30]. The maximum size of the study was 426 using sex as a predictor(h ratio(2.4), failprob(0.107), wdprob(0.1)) [30].
Sampling procedure
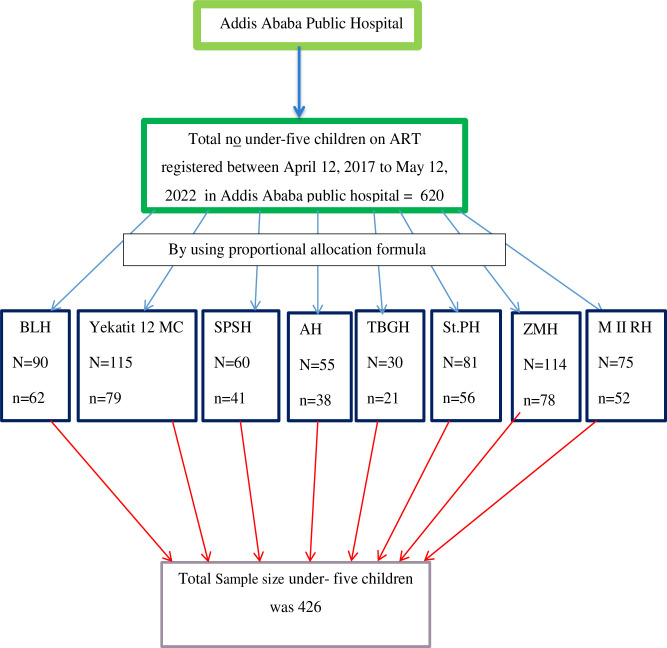
The study covered all public hospitals that delivered ART to under-five children. The sample size for each hospital was determined using proportional allocation based on cases of under-five children on ART services in the previous five years. A random number was generated based on the identification of each medical chart of children using a computer. Then, four hundred twenty-six (426) children’s medical records were selected using a computer-generated random sampling technique. Furthermore, replacing incomplete medical charts was taken into account (Fig 1).
Fig 1. Schematic representation of sampling procedure for time to death and its predictors among under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia 2022.
Study variables
Dependent variable
Time to death.
Independent variables
Socio—demographic characteristics: Age, sex, residence, family status, age of care giver.
Baseline and follow-up clinical and immunologic information: TB infection, TB treatment, WHO clinical stages, Cotrimoxazole prophylaxis use, CD4 count, ART adherence, hemoglobin level, chronic diarrhea, drug regimen, and obstetric/birth history of the child.
Nutritional factors: Weight for age and developmental milestone.
Operational definitions
Time to death: Time at which the children died during the follow up, after initiation Anti-retroviral treatment [31].
Follow up time period: Time from starting of ART up to either the study subjects died or censored.
Survival time: The length of time in months a child was followed from the time the child started ART until death, was lost to follow up, or was still on follow up [32].
Event: The occurrence of death from initiation of ART to the end of the study [33].
Anemia: Is defined as having hemoglobin level is < 11mg/dl [9].
CD4 count below threshold: If CD4 cell count of less than350 cells/mm3 [10].
Underweight: For children under the age of five, weight for age Z-score-2 SD was used [34].
Advanced WHO clinical stages: Under-five children in WHO clinical stages III and IV during ART enrolment. Mild WHO clinical stages: are stage I and II baseline clinical stages of HIV-infected under-five children during antiretroviral therapy (ART) enrolment [35].
Censored: When a child is lost-to-follow-up, transferred out, withdrawn from follow-up, and still on follow up(lives longer than the study period) [32].
Poor adherence to medications: Less than 90% adherence to ART drugs by children under the age of five in the previous three months was termed poor adherence [36].
Fair adherence to medications: Fair adherence was defined as 90–95 percent of children under the age of five who have taken antiretroviral medications in the previous three months [36].
Good adherence: adherence level of under-five children to ART medications within the last three months >95%(7) [37].
Moderate stunting: under five children having Height/ Age Z-score < −2 SD [37].
Moderate underweight: under five children having Weight/Age Z-score < − 2 SD [37].
Moderate wasting: under five children having Weight/ Height Z-score < −2 SD [37].
Severe stunting: under five children having Height/Age Z-score < −3 SD [37].
Severe underweight: under five children having Weight/Age Z-score < −3 SD [37].
Severe wasting: under five children having Weigh/ Height Z-score < −3 SD [37].
Opportunistic infections: During follow-up periods, an HIV-infected under-five child on ART developed one or more reported histories of opportunistic illnesses [9].
Developmental milestone: The child can achieve the four developmental milestones, which are cognitive development, gross motor/fine motor development, language development, and social development, as appropriate for the child’s age [35].
Developmental delay: is the delay of an age-specific capacity for important developmental milestones and without any deformity [35].
Developmental regression: After a time of relatively normal growth, a child loses an already acquired talent or fails to progress beyond a protracted plateau [35].
Data collection procedure & tools
First, the completeness of children’s medical records was checked. Then, a data extraction checklist was prepared. The time from the initiation of ART was the starting point for retrospective follow-up, and the end was the date of death, date of loss to follow-up, or date of transfer out. All HIV-infected children who started ART at the eight hospitals between April 2017 and May 2022 were recruited from the ART follow-up register. Then records were reviewed before the data was collected (both baseline and follow-up records). The death was confirmed by registration follow-up chart, physician recorded, and their medical record number was used to identify them. Eight BSc nurse data collector and Three MSc nurse supervisors were recruited.
Data quality control
To assure the quality of data, data collectors and supervisors were trained. Data quality was also maintained by using a standard pretested data extraction checklist and ongoing supervision. During data analysis, all collected data were checked for completeness and consistency. Before processing, the data was entered and cleaned.
Data analysis
Under-five children’s death was the event of interest coded as “1” and “0” for censored. Data was entered into Epi-Data 4.6 and analyzed with STATA 14. The child mortality rate was estimated and expressed as 1000 children—months. The Kaplan-Meier curve was used to calculate the median survival time. The Schoenfeld residuals test was used to assess model fitness. The model was fitted, and an overall global test result of 0.6434. Bivariable Cox proportional hazards regression was fitted, and those ≤0.25 were fitted for multivariable Cox regression hazard model to identify predictors of the outcome variable. The adjusted hazard ratio (AHR) with a 95% confidence interval (CI) and a P 0.05 was used to determine the strength of association and statistical significance. Variance inflation factor (VIF) was used to test for multicollinearity among variables, and there was no evidence of multicollinearity with a variance inflation factor smaller than 4 for all variables.
Ethical consideration and consent to participants
Ethical approval was sought from the Debre Markos University, College of Health Science Research Ethics Review Committee (Ref. no. HSR/R/C/Ser/PG/Co/175/11/14). A permission letter was secured from each hospital. Moreover, individual consent was not applicable since it is a retrospective study of medical records (record review). The ethics committee waived the requirement for informed consent. Finally, the confidentiality of the information and privacy of study participants was maintained.
Results
Socio demographic characteristics of study participants
Based on the inclusion criteria, 415 (97.42%) of the 426 children on ART were included for analysis. More than half of the 226 children (54.5%) were female. More than ninety percent of the children, 390 (94.0%), resides in urban. Almost two-third (61.2%) of the 254 study participants were under the age of 24 months, with the overall mean and standard deviation of the child’s age at ART initiation being 23.8 and 14.64 months, respectively. More than two-third of the study’s 296 participants (71.3%) were Orthodox Christians. Three hundred and one (72.5%) caregivers were females, and 254 (61.4%) caregivers were between the ages of 20 and 35. More than three-quarters (313) were married, and less than half of caregivers attended primary school (Table 1).
Table 1. Socio-demographic characteristics of under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia 2022.
(n = 415).
| Variables | Categories | Frequency | Percent |
|---|---|---|---|
| Child’s a sex | Male | 189 | 45.5 |
| Female | 226 | 54.5 | |
| age of child | Less than 24 months | 254 | 61.2 |
| 25–59 months | 161 | 38.8 | |
| Residence | Urban | 390 | 94.0 |
| Rural | 25 | 6.0 | |
| Religion | Orthodox | 296 | 71.3 |
| Protestant | 78 | 18.8 | |
| Catholic | 31 | 7.5 | |
| Other | 10 | 2.4 | |
| Sex of care giver | Male | 114 | 27.5 |
| Female | 301 | 72.5 | |
| age of care givers in years | <20 | 6 | 1.4 |
| 20–35 | 254 | 61.4 | |
| >35 | 155 | 37.3 | |
| Marital status of care giver | Married | 313 | 75.4 |
| Unmarried | 102 | 24.6 | |
| Primary care giver | Parents | 385 | 92.8 |
| Relatives | 23 | 5.5 | |
| Guardians | 7 | 1.7 | |
| Educational level of the care giver | No education | 69 | 16.6 |
| Primary | 180 | 43.4 | |
| Secondary | 132 | 31.8 | |
| Tertiary and above | 34 | 8.2 | |
| Family HIV status currently | Both alive | 352 | 84.8 |
| Father died | 30 | 7.2 | |
| Mother died | 17 | 4.1 | |
| Both died | 16 | 3.9 |
Baseline clinical characteristics of children under five years on ART
The majority, 388 (93.5%) of children, had appropriate developmental status, and more than half, 224(54.0%), children started INH prophylaxis. Two hundred forty-three (58.6%) of children started ART in the mild WHO clinical disease stage of HIV (II or I), and 240(57.8%) of children had less than 11 grams per deciliter (g/dl) of hemoglobin. Four hundred (96.9%) of children were using Cotrimoxazole, and less than a quarter of 60(14.5%) children had tuberculosis. Almost two third children, 271 (65.3%), had good ART adherence. Three hundred thirty-three (80.2%) of HIV-infected under-five children were born from mothers who had PMTCT service initiated during pregnancy. More than three-fourth, 341(82.2%) of children had chronic diarrhea, and 388 (93.5%) of children had above the threshold CD4 count (Table 2).
Table 2. Baseline clinical characteristics of under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia 2022.
(n = 415).
| Variables | Categories | Outcome. | Total, n (%) | |
|---|---|---|---|---|
| Died, n (%) | Censored, n (%) | |||
| Developmental status | Appropriate for age | 37(90.2) | 351(93.9) | 388(93.5) |
| Delayed | 4(9.8) | 17(4.5) | 21(5.1) | |
| Regressed | - | 6(1.6) | 6(1.4) | |
| INH prophylaxis | Yes | 15(36.6) | 209(55.9) | 224(54.0) |
| No | 26(63.4) | 165(44.1) | 191(46.0) | |
| WHO Clinical stage | Mild | 9(22.0) | 234(62.6) | 243(58.6) |
| Advanced | 32(78.0) | 140(37.4) | 172(41.4) | |
| Hemoglobin | <11 | 34(82.9) | 206(55.1) | 240(57.8) |
| >11 | 7(17.1) | 168(44.9) | 175(42.2) | |
| Cotrimoxazole use | Yes | 39(95.1) | 363(97.1) | 402(96.9) |
| No | 2(4.9) | 11(2.9) | 13(3.1) | |
| Immunization Status | Immunized | 23(56.1) | 227(60.7) | 250(60.2) |
| Not immunized at all | 18(43.9) | 147(39.3) | 165(39.8) | |
| Tuberculosis | Yes | 19(46.3) | 41(11.0) | 60(14.5) |
| No | 22(53.7) | 333(89.0) | 355(85.5) | |
| Comorbidities other than TB | Yes | 33(80.5) | 228(61.0) | 261(62.9) |
| No | 8(19.5) | 146(39.0) | 154(37.1) | |
| ART adherence | Good | 11(26.8) | 260(69.5) | 271(65.3) |
| Fair | 26(63.4) | 111(29.7) | 137(33.0) | |
| Poor | 4(9.8) | 3(0.8) | 7(1.7) | |
| Maternal history of PMTCT | Yes | 30(73.2) | 303(81.0) | 333(80.2) |
| No | 11(26.8) | 71(19.0) | 82(19.8) | |
| Drugs side effect | Yes | 36(87.8) | 273(73.0) | 309(74.5) |
| No | 5(12.2) | 101(27.0) | 106(25.5) | |
| Chronic Diarrhea | Yes | 37(90.2) | 304(81.3) | 341(82.2) |
| No | 4(9.8) | 70(18.7) | 74(17.8) | |
| Mode of delivery | Cesarean section | 1(2.4) | 67(17.9) | 68(16.4) |
| SVD | 40(97.6) | 307(82.1) | 347(83.6) | |
| Place of delivery | Health facility | 33(80.5) | 335(89.6) | 368(88.7) |
| Home | 8(19.5) | 39(10.4) | 47(11.3) | |
| CD4 count | below threshold | 6(14.6) | 21(5.6) | 27(6.5) |
| above the threshold | 35(85.4) | 353(94.4) | 388(93.5) | |
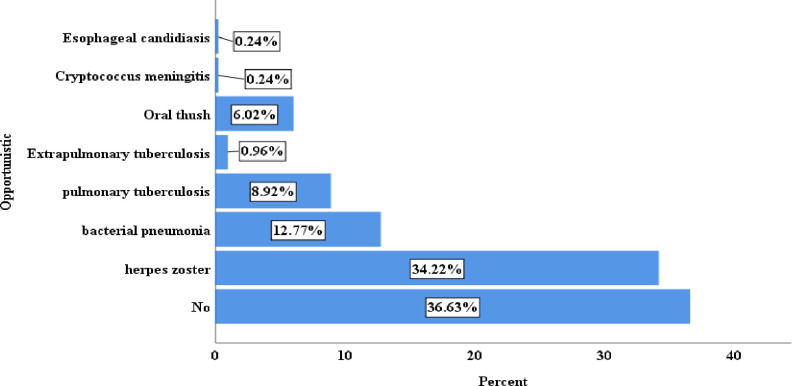
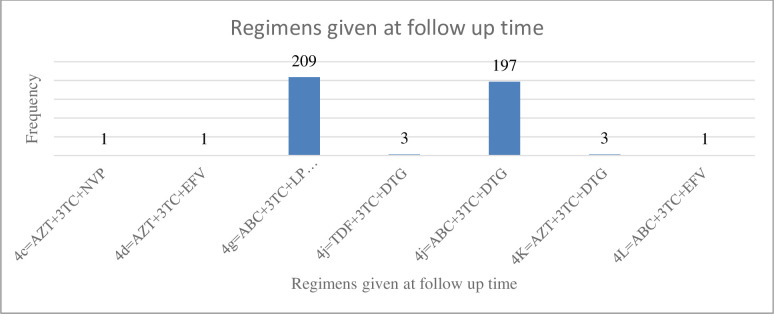
Regarding, opportunistic infection, 152(36.6%) had not developed an opportunistic infection. More than one-fourth (34.22%) of children had herpes zoster, and less than a quarter (12.77%) of children had bacterial pneumonia (Fig 2). More than half, 209 (50.36%) of children have taken a drug of type 4g = ABC+3TC-LPV/r ART regimen followed by 197(47.47%) of children who have taken a drug of type 4j = ABC+3TC+DTG (Fig 3).
Fig 2. Baseline opportunistic infection among under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia, 2022.
Fig 3. ART regimen given at baseline among under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia, 2022.
Nutritional status of children on ART
This study found that 141 (34.4%) of children were severely stunted, whereas 35 (8.4%) were moderately stunted. More than one-fourth of the children, 123(29.6%), were very underweight, and 45(10.8%) were moderately underweight. In addition, 79 (19.0%) and 48 (11.1%) of the children were severely and moderately wasting, respectively (Table 3).
Table 3. Nutritional status of under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia, 2022.
(n = 415).
| Variables | Categories | Outcome | Total, n (%) | |
|---|---|---|---|---|
| Died, n (%) | Censored, n (%) | |||
| Stunting | Normal | 18(43.9) | 221(59.1) | 239(57.6) |
| Moderately stunted | 3(7.3) | 32(8.6) | 35(8.4) | |
| Severely stunted | 20(48.8) | 121(32.4) | 141(34.0) | |
| Underweight | Normal | 18(43.9) | 229(61.2) | 247(59.5) |
| Moderately underweight | 5(12.2) | 40(10.7) | 45(10.8) | |
| Severely underweight | 18(43.9) | 105(28.1) | 123(29.6) | |
| Wasting | Normal | 28(68.3) | 260(69.5) | 288(69.4) |
| Moderately wasting | 5(12.2) | 43(11.5) | 48(11.6) | |
| Severely wasting | 8(19.5) | 71(19.0) | 79(19.0) | |
Kaplan Meier analysis
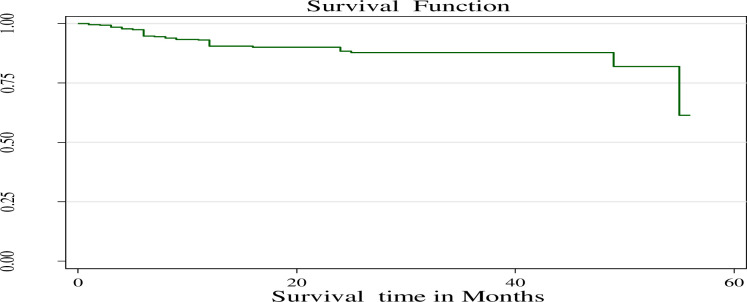
The study reported that 415 children on ART followed up for a minimum of 1 month and a maximum of 56 months, with a median follow-up time of 17 with an interquartile range of (10–29) months. The survival status was estimated by the Kaplan-Meier estimation method. This finding showed that overall estimated survival after starting ART was 61.42% (95%CI: 19.93–86.31) at 56 months of follow-up (Fig 4). The Kaplan-Meier curve also revealed that the survival probability of children declined over time. The highest mortality rate occurred in the first 12 months (Fig 4).
Fig 4. The Kaplan-Meier survival function among under-five children on ART in public hospitals of Addis Ababa, Ethiopia, 2022.
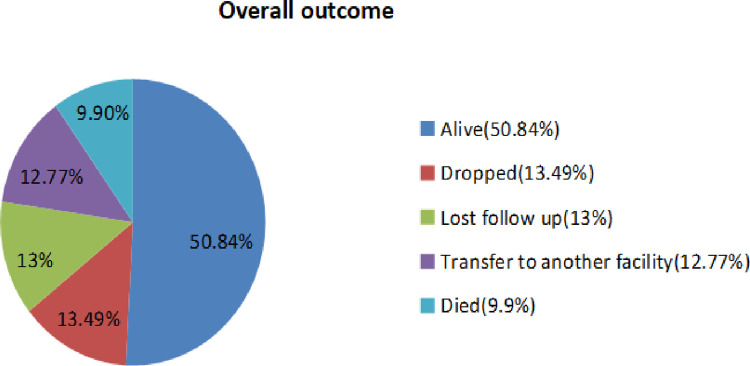
The study reported the incidence of death was 4.98 /1000 person months observations (95% CI: 3.67–6.77). During the follow-up period, 41 were died and the remaining 374 (90.12%) were censored (Fig 5).
Fig 5. Over all outcomes of under five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia, 2022.
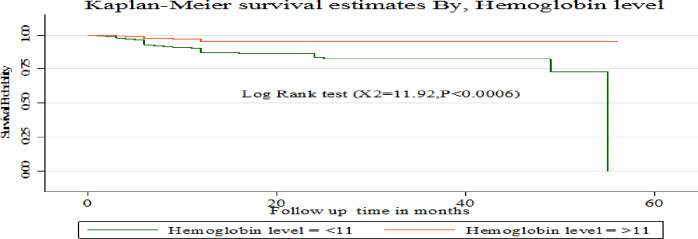
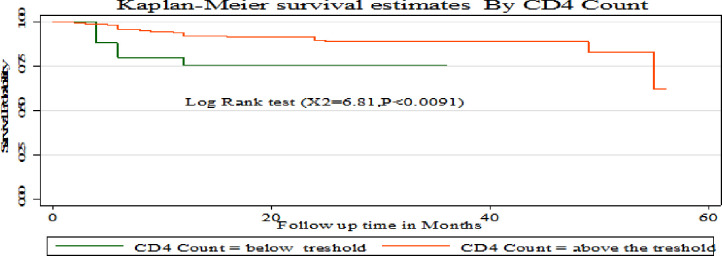
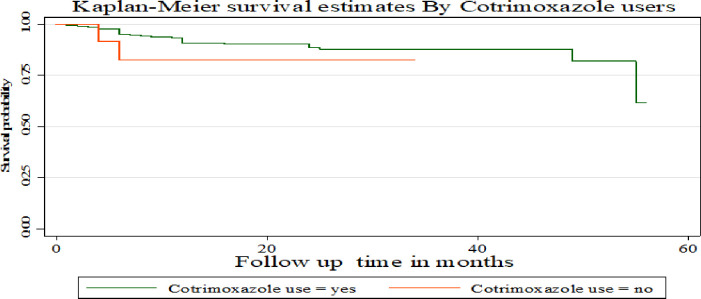
The Kaplan-Meier survival curve showed that children with hemoglobin level > 11 g/L had a higher survival probability compared to children with a hemoglobin level <11 g/L (Fig 6). Compared to children with CD4 counts above the threshold, those with CD4 counts below it had a greater chance of surviving at 2 and 3 months. But starting from 4 months onwards, children with a CD4 level above the threshold had a better chance of surviving than those with a CD4 count below the threshold (Fig 7). This study also showed that children who used Cotrimoxazole for the first two months had a higher survival probability compared to those who did not. However, their chance of survival decreased from the third to the 34th month (Fig 8).
Fig 6. Kaplan-Meier survival estimates based on hemoglobin level among under- five children on ART treatment in public hospitals of Addis Ababa, Ethiopia, 2022.
Fig 7. Kaplan Meier survival estimates based on CD4 count among under- five children on antiretroviral treatment (ART) in public hospitals of Addis Ababa, Ethiopia, 2022.
Fig 8. Kaplan-Meier survival estimates based on cotrimoxazole use under- five children on antiretroviral treatment (ART) in public hospitals of Addis Ababa, Ethiopia, 2022.
Predictors of time to death of children on ART
Bivariable Cox proportional hazard regression revealed that sex, age of a child, sex of caregiver, stunting, underweight, INH prophylaxis, WHO stage, hemoglobin level, tuberculosis, opportunistic infection, maternal history of PMTCT, drug side effects, place of delivery, and CD4 count variables were associated with time to death of children on ART follow up at a P-value less than 0.25. But underweight, having tuberculosis, low hemoglobin level, and WHO clinical stage remained statistically significant with time to death among children on ART in multivariable Cox proportional hazards regression (Table 4).
Table 4. Cox -regression analysis of predictors of death among under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia 2022.
| Variables | Categories | Outcome | CHR (95% CI) | AHR (95.% CI) | P-value | |
|---|---|---|---|---|---|---|
| Event (%) | Censored (%) | |||||
| Sex | Male | 23(12.2) | 166(87.8) | 1.00 | 1.00 | |
| Female | 18(8.0) | 208(92.0) | 0.69(0.37, 1.28) | 0.81(0.40,1.65) | 0.564 | |
| Age of child | < 24 months | 23(9.1) | 231(90.9) | 0.64(0.34, 1.21) | 0.54(0.26,1.11) | 0.093 |
| 25–59 month | 18(11.2) | 143(88.8) | 1.00 | 1.00 | ||
| Sex of caregiver | Male | 8(7.0) | 106(93.0) | 0.58(0.27, 1.26) | 0.51(0.22,1.17) | 0.11 |
| Female | 33(11.0) | 268(89.0) | 1.00 | 1.00 | ||
| Stunting | Normal | 18(7.5) | 221(92.5) | 1.00 | 1.00 | |
| Moderately | 3(8.6) | 32(91.4) | 1.07(0.31, 3.65) | 0.99 (0.25,3.85) | 0.98 | |
| Severely | 20(14.2) | 121(85.8) | 2.28(1.19, 4.35) | 1.32 (0.25, 3.04) | 0.52 | |
| Under Weight | Normal | 18(7.3) | 229(92.7) | 1.00 | 1.00 | |
| Moderately | 5(11.1) | 40(88.9) | 1.65(0.61, 4.47) | 2.24(0.73,6.93) | 0.16 | |
| Severely | 18(14.6) | 105(85.4) | 2.47(1.27, 4.80) | 3.19(1.32,7.71) | 0.01* | |
| INH prophylaxis | Yes | 15(6.7) | 209(93.3) | 0.48(0.25, 0.91) | 0.54(0.26, 1.12) | 0.9 |
| No | 26(13.6) | 165(86.4) | 1.00 | 1.00 | ||
| WHO stage | Mild | 9(3.7) | 234(96.3) | 1.00 | 1.00 | |
| Advanced | 32(18.6) | 140(81.4) | 6.08(2.80,13.21) | 3.38(1.08, 10.58) | 0.037* | |
| Hemoglobin | <11g/dl | 34(14.2) | 206(85.8) | 3.80(1.68, 8.62) | 2.51(1.02,6.20) | 0.046* |
| >11g/dl | 7(4.0) | 168(96.0) | 1.0* | 1.00 | ||
| Tuberculosis | Yes | 19(31.7) | 41(68.3) | 5.56(2.99,10.34) | 3.86(1.76, 8.47) | 0.001* |
| No | 22(6.2) | 333(93.8) | 1.00 | 1.00 | ||
| Opportunistic infection | Yes | 33(12.5) | 230(87.5) | 2.52(1.16, 5.47) | 0.89(0.30, 2.68) | 0.84 |
| No | 8(5.3) | 144(94.7) | 1.00 | 1.00 | ||
| Maternal history of PMTCT | Yes | 30(9.0) | 303(91.0) | 0.62(0.31, 1.24) | 1.73(0.76, 3.94) | 0.20 |
| No | 11(13.4) | 71(86.6) | 1.00 | 1.00 | ||
| Drugs side effect | Yes | 36(11.7) | 273(88.3) | 2.42(0.95, 6.18) | 0.90(0.27, 2.92) | 0.85 |
| No | 5(4.7) | 101(95.3) | 1.00 | 1.00 | ||
| Place of delivery | Health facility | 33(9.0) | 335(91.0) | 1.00 | 1.00 | |
| Home | 8(17.0) | 39(83.0) | 1.98(0.91, 4.30) | 1.35(0.55, 3.32) | 0.508 | |
| CD4 | Below threshold | 6(22.2) | 21(77.8) | 3.00(1.25, 7.17) | 1.71(0.58,5.01) | 0.329 |
| Above the threshold | 35(9.0) | 353(91.0) | 1.00 | 1.00 | ||
Key: 1.00 = Reference; * = statistically significant at a p < 0.05
The study found that severely underweight children on ART are more like to die (AHR = 3.19) than children with normal weight (AHR = 3.19; 95%CI: 1.32–7.71). Children with tuberculosis were 3.86 times more likely to die than their counterparts (AHR = 3.86; CI: 1.76–8.47). And also, children with low hemoglobin levels were 2.51 times more likely to die than children with hemoglobin levels above the threshold (AHR = 2.51; CI: 1.02–6.20). Furthermore, the risk of death among under-five children with advanced WHO clinical stages at enrolment to ART was 3.38 times higher than children with a mild stage (AHR = 3.38; CI:1.08–10.58) (Table 4).
A log-rank test was done to check the existence of significant differences in the survival status among categories. Variables like CD4 count, opportunistic infection, INH prophylaxis, and severely stunted had the statistically significant result of the log-rank test with (P-Value <0.05) (Table 5).
Table 5. Log-rank test for equality of survivor functions among under- five children on ART treatment in public hospitals of Addis Ababa, Ethiopia, 2022.
| Variables | Categories | P-value | Log-rank test (x2) |
|---|---|---|---|
| sex of child | Male | 0.2328 | 1.42 |
| Female | |||
| age of child | < 24 months | 0.1658 | 1.92 |
| 25–59 month | |||
| sex of care giver | Male | 0.1616 | 1.96 |
| Female | |||
| Underweight | Normal | 0.0210 | 7.72 |
| Moderately underweight | |||
| Severely underweight | |||
| Stunting | Normal | 0.0291 | 7.07 |
| Moderately stunted | |||
| Severely stunted | |||
| INH prophylaxis | Yes | 0.0199 | 5.42 |
| No | |||
| WHO clinical Stage | Mild | 0.0000 | 27.40 |
| Advanced | |||
| hemoglobin level | <11g/dl | 0.0006 | 11.92 |
| >11g/dl | |||
| Tuberculosis | Yes | 0.0000 | 37.73 |
| No | _ | _ | |
| opportunistic infection | Yes | 0.0145 | 5.98 |
| No | _ | _ | |
| maternal history of PMTCT | Yes | 0.1690 | 1.89 |
| No | |||
| Drug side effect | Yes | 0.0542 | 3.71 |
| No | _ | _ | |
| place of delivery | Health facility | 0.0757 | 3.16 |
| Home | |||
| CD4 count | Below threshold | 0.0091 | 6.81 |
| Above the threshold | _ | _ |
Test of proportional hazard assumption
The result showed (p-value > 0.05) satisfy the PH assumptions (Table 6).
Table 6. Schoenfeld’s residuals test result among under-five children on antiretroviral treatment in public hospitals of Addis Ababa, Ethiopia 2022.
| Variable | Rho | chi2 | Df | prob>chi2 |
|---|---|---|---|---|
| Sex of child | 0.011 | 0.01 | 1 | 0.944 |
| Age of child | -0.070 | 0.18 | 1 | 0.668 |
| Sex of care giver | -0.158 | 1.10 | 1 | 0.295 |
| Underweight | -0.156 | 1.26 | 1 | 0.261 |
| Stunting | 0.120 | 0.62 | 1 | 0.430 |
| INH prophylaxis | -0.055 | 0.13 | 1 | 0.717 |
| WHO clinical Stage | 0.011 | 0.01 | 1 | 0.932 |
| Hemoglobin level | -0.210 | 1.71 | 1 | 0.191 |
| Tuberculosis | -0.024 | 0.03 | 1 | 0.865 |
| Opportunistic infection | -0.042 | 0.08 | 1 | 0.776 |
| Maternal history of PMTCT | -0.166 | 0.88 | 1 | 0.347 |
| Drug side effect | -0.185 | 1.16 | 1 | 0.282 |
| Place of delivery | 0.162 | 0.89 | 1 | 0.345 |
| CD4 count | -0.003 | 0.00 | 1 | 0.987 |
| Global test | 11.54 | 14 | 0.6434 | |
Discussions
The study aimed to assess time to death and its predictors among under-five children on ART. The study reported that the cumulative incidence rate after starting ART was 4.98 /1000-person month observations with a 95% CI: (3.67–6.77). A study in the Oromiya Liyu zone of Amhara region, Ethiopia is higher than this study (5.9 deaths per 100 child months [9]. This is because of the difference in study participants, which was conducted among TB/AIDS co-infected children and resulted in higher morality than this study. But this result is higher than a study in Malawi, in which the incidence rate was 16.6 per 1000 children years observation [38]. Differences in the quality of ART services between countries may contribute to this discrepancy. And also, this finding is higher than a study done in West Amhara referral hospitals which reported that the overall incidence rate of death was 2.87 deaths per 1000 child months [39]. The difference might be due to the difference in the study setting.
The study found that the overall estimated survival time of children after starting ART was 61.42% at 56 months of follow-up and had a median follow-up time of 17 months. This finding is lower than a study in West Amhara referral hospitals (85%) [39] and the Oromiya Liyu zone (87%) [9]. This might be explained in terms of differences in clinical characteristics of study participants like WHO clinical stage and CD4 level. A difference in the stage of HIV/AIDS at the beginning of ART could be another explanation. While some study participants did not start ART at the WHO clinical stage I&II. Children who began receiving ART earlier had a longer survival time compared counterparts. This finding is also lower than a study done in Europe which showed that the survival rate after starting ART was 97.6 [40]. The discrepancy may be because developed countries like Europe offer high-quality ART care, resulting in a higher survival probability for children compared to developing countries like Ethiopia.
This study revealed that the risk of death among under-five children with severe underweight was 3.19 times higher than those children who had normal weight. Studies in Swaziland and Kenya supported this evidence [41, 42]. In addition, this finding is consistent with studies in Ethiopia [43, 44]. This may be due to severely underweight children may not respond well to treatment because their immunity is weakened due to inadequate nutrient delivery to their cells.
This study also revealed children with tuberculosis had a 3.86 times higher risk of death than children without the disease. This finding was consistent with the study conducted in Tanzania [45] and Jinka Hospital of Ethiopia [46]. This could be due to host reactions to M. tuberculosis that promote HIV replication, speeding up the natural progression of the virus and further suppressing immunity [47]. Another explanation for the increased risk of death among tuberculosis-infected children is the reduced absorption of ART medications, which reduces their efficiency secondary to anti-tuberculosis drugs [48].
In this study, the hazard of death among children on ART with advanced stages (stages III and IV) enrolment was 3.38 times higher than with mild stages (stage I and II) at ART enrolment. Studies Gondar of Ethiopia [49] and Cameroon [15] supported this finding. This could be because children with advanced HIV/AIDS have compromised immune systems and are more vulnerable to opportunistic infections, which can lead to mortality. This reflects the fact that severe immunodeficiency increases the risk of death.
Finally, the study revealed that the risk of death among under-five children with low hemoglobin was 2.51 times higher than compared to counterparts. Studies in Gondar and Addis Ababa, Ethiopia [16, 50] supported this evidence. This could be because low hemoglobin speeds up the progression of HIV infection, which increased the risk of death. Combined effects of anemia and some drugs worsen anemia, and lead to more comorbid conditions and death.
The finding has significant clinical implications for reducing the death rate of under-five children by enhancing nutritional status, increasing hemoglobin level, preventing TB, and preventing advanced HIV infection in under-five children. Additionally, this finding is important for policymakers in developing integrated therapeutic interventions and strategies to prevent tuberculosis, advanced stage of HIV/AIDS, underweight, and low hemoglobin level.
Limitation of the study
Because the study was based on secondary data, some important variables, such as sociodemographic and clinical characteristics, missed due to poor documentation and thus excluded from the study. Additionally, those study participants whose medical chat was not available were not included, in the study, which may undermine the study’s findings.
Conclusion and recommendations
The incidence of mortality was 4.98 per 1000 child-months. Severe underweight, tuberculosis infection, low hemoglobin level, and advanced WHO clinical stages at enrolment were predictors of death among under-five children on ART. Therefore, intervention to reduce the mortality of HIV-infected children should be considered. And also, responsible bodies should implement strategies to improve children’s weight, prevent tuberculosis infection and maintain a high level of hemoglobin.
Supporting information
(SAV)
Acknowledgments
We would like to acknowledge Debre Markos University. We also greatly acknowledge Saint Peter specialized hospital and Addis Ababa public hospital workers especially ART clinic staffs.
Abbreviations and acronyms
- AHR
Adjusted Hazard Ratio
- AIDS
Acquired Immune deficiency Syndrome
- ART
Anti-Retroviral Treatment
- AZT
Zidovudine
- CI
Confidence Interval
- CHR
Crude Hazard Ratio
- EFV
Efavirenz
- HAART
Highly Active Anti-Retroviral Therapy
- HIV
Human Immunodeficiency Virus
- INH
Isoniazid
- NVP
Neverapine
- PH
Proportional Hazard
- 3TC
Lamivudine
- PMTCT
Prevention of Mother-to-Child Transmission
- SPSS
Statistical Product and Service Solutions
- TDF
Tenofovir
- WHO
World Health Organization
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Nasejje JB, Mwambi HG, Achia TN. Understanding the determinants of under-five child mortality in Uganda including the estimation of unobserved household and community effects using both frequentist and Bayesian survival analysis approaches. BMC public health. 2015;15(1):1–12. doi: 10.1186/s12889-015-2332-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chenneville T. A Clinical Guide to Pediatric HIV: Bridging the Gaps Between Research and Practice: Springer; 2017. [Google Scholar]
- 3.P C. CDC P Helping Babies Born To Hiv-Positive Mothers In Sub-Saharan Africa Stay Healthy And Free Of Hiv. 2017. [Google Scholar]
- 4.UNAIDS. Children and HIV: fact sheet. Joint United Nations Programme on HIV/AIDS Geneva, Switzerland; 2016. [Google Scholar]
- 5.Federal H. HIV Prevention in Ethiopia National Road Map 2018–2020. Federal HIV/AIDS Prevention and Control Office Addis Ababa, Ethiopia; 2018. [Google Scholar]
- 6.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS medicine. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FMOH E. National Comprehensive HIV Prevention, Care and Treatment Refresher Training for Health care Providers. 2021. [Google Scholar]
- 8.UNAIDS. Global information and education on HIV and AIDS https://www.avert.org/professionals/hiv-social-issues/key-affected-populations/children. 2020. [Google Scholar]
- 9.Marie BT, Argaw WS, Bazezew BY. Time to death among HIV-infected under-five children after initiation of anti-retroviral therapy and its predictors in Oromiya liyu zone, Amhara region, Ethiopia: a retrospective cohort study. BMC pediatrics. 2022;22(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Organization WH. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach-2010 revision: World Health Organization; 2010. [PubMed] [Google Scholar]
- 11.Biset Ayalew M. Mortality and its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. AIDS research and treatment. 2017;2017. doi: 10.1155/2017/5415298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makatini Z, Blackard J, Mda S, Miles P, Towobola O. High virological failure rates in HIV-1 perinatally infected children in South Africa: A retrospective cohort study. South African Medical Journal. 2021;111(3):255–9. doi: 10.7196/SAMJ.2021.v111i3.15221 [DOI] [PubMed] [Google Scholar]
- 13.Mutanga JN, Mutembo S, Ezeamama AE, Song X, Fubisha RC, Mutesu-Kapembwa K, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015). BMC Public Health. 2019;19(1). doi: 10.1186/s12889-019-6444-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones A, Honermann B, Lankiewicz E, Sherwood J, Millett G. Current allocations and target apportionment for HIV testing and treatment services for marginalized populations: characterizing PEPFAR investment and strategy. Journal of the International AIDS Society. 2021;24(S3). doi: 10.1002/jia2.25753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Njom Nlend AE, Loussikila AB. Predictors of mortality among HIV-infected children receiving highly active antiretroviral therapy. Médecine et Maladies Infectieuses. 2017;47(1). doi: 10.1016/j.medmal.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Mulugeta A, Assefa H, Tewelde T, Dube L. Determinants of survival among HIV positive children on antiretroviral therapy in public hospitals, Addis Ababa, Ethiopia. Quality in Primary Care. 2017;25(4):0–. [Google Scholar]
- 17.Ahmed I, Lemma S. Mortality among pediatric patients on HIV treatment in sub-Saharan African countries: a systematic review and meta-analysis. BMC public health. 2019;19(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernays S, Jarrett P, Kranzer K, Ferrand RA. Children growing up with HIV infection: the responsibility of success. The Lancet. 2014;383(9925):1355–7. doi: 10.1016/S0140-6736(13)62328-4 [DOI] [PubMed] [Google Scholar]
- 19.Victor Minichiello JS. Children Living in HIV Families: A Review. Journal of Child and Adolescent Behaviour. 2014;02(05). [Google Scholar]
- 20.Walakira EJ, Ddumba-Nyanzi I, Kaawa-Mafigiri D. HIV and AIDS and its impact on child well-being. Springer; 2014. [Google Scholar]
- 21.Frehiwot N, Mizan K, Seble M, Fethia K, Tekalign M, Zelalem T. National guidelines for comprehensive HIV prevention, care and treatment. Addis Ababa: Ministry of Health. 2014. [Google Scholar]
- 22.UNAIDS:. FACT SHEET–WORLD AIDS DAY 2021: —latest global and regional statistics on the status of the AIDS epidemic. Geneva: UNAIDS. https://wwwusaidgov/global-health/health-areas/hiv-and-aids/technical-areas/dreams/unaids-fact-sheet-2021. 2021. [Google Scholar]
- 23.Kassaw MW, Matula ST, Abebe AM, Kassie AM, Abate BB. The perceived determinants and recommendations by mothers and healthcare professionals on the loss-to-follow-up in Option B+ program and child mortality in the Amhara region, Ethiopia. BMC infectious diseases. 2020;20:1–13. doi: 10.1186/s12879-020-05583-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organization WH. Guidelines: updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. 2021. [PubMed] [Google Scholar]
- 25.HEALTH FMO. NATIONAL GUIDELINES FOR COMPREHENSIVE HIV PREVENTION, CARE AND TREATMENT. 2017. [Google Scholar]
- 26.Vermund SH, Blevins M, Moon TD, José E, Moiane L, Tique JA, et al. Poor Clinical Outcomes for HIV Infected Children on Antiretroviral Therapy in Rural Mozambique: Need for Program Quality Improvement and Community Engagement. PLoS ONE. 2014;9(10):e110116. doi: 10.1371/journal.pone.0110116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyun V, Technau K-G, Eley B, Rabie H, Boulle A, Fatti G, et al. Earlier Antiretroviral Therapy Initiation and Decreasing Mortality Among HIV-infected Infants Initiating Antiretroviral Therapy Within 3 Months of Age in South Africa, 2006–2017. Pediatric Infectious Disease Journal. 2020;39(2):127–33. doi: 10.1097/INF.0000000000002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wikipedia tfe. Addis Ababa Population https://worldpopulationreview.com/world-cities/addis-ababa-population. 2022.
- 29.Project AACAHBP. HIV/AIDS in Addis Ababa:Background, Projections, Impacts and Interventions http://www.policyproject.com/pubs/countryreports/Ethadhiv.pdf. 2014. [Google Scholar]
- 30.Workie KL, Birhan TY, Angaw DA. Predictors of mortality rate among adult HIV-positive patients on antiretroviral therapy in Metema Hospital, Northwest Ethiopia: a retrospective follow-up study. AIDS research and therapy. 2021;18(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aynalem YA, Mekonen H, Akalu TY, Gebremichael B, Shiferaw WS. Preterm Neonatal Mortality and its predictors in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia: a retrospective cohort study. Ethiopian Journal of Health Sciences. 2021;31(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidamo NB, Hebo SH. Survival time and its predictors among HIV-infected children after antiretroviral therapy in public health facilities of Arba Minch town, Gamo Gofa Zone, Southern Ethiopia. Ethiopian Journal of Health Development. 2018;32(2). [Google Scholar]
- 33.Wondwossen K. SURVIVAL AND PREDICTORS OF MORTALITY AMONG HIV POSITIVE CHILDREN ON ANTIRETROVIRAL THERAPY IN PUBLIC HOSPITALS, EAST GOJJAM, ETHIOPIA, 2019. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Child Growth Standards 1 year 2 years 3 years 4 years 5 years Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age Methods and development. 2006. [Google Scholar]
- 35.Arage G, Assefa M, Worku T, Semahegn A. Survival rate of HIV-infected children after initiation of the antiretroviral therapy and its predictors in Ethiopia: a facility-based retrospective cohort. SAGE open medicine. 2019;7:2050312119838957. doi: 10.1177/2050312119838957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fdro Ethiopiamoh. National consolidated guidelines for comprehensive hiv prevention,care and treatmenT. 2018. [Google Scholar]
- 37.Turck D, Michaelsen KF, Shamir R, Braegger C, Campoy C, Colomb V, et al. World health organization 2006 child growth standards and 2007 growth reference charts: a discussion paper by the committee on nutrition of the European society for pediatric gastroenterology, hepatology, and nutrition. Journal of pediatric gastroenterology and nutrition. 2013;57(2):258–64. doi: 10.1097/MPG.0b013e318298003f [DOI] [PubMed] [Google Scholar]
- 38.Divala O, Michelo C, Ngwira B. Morbidity and mortality in HIV‐exposed under‐five children in a rural Malawi setting: a cohort study. Journal of the International AIDS Society. 2014;17:19696. doi: 10.7448/IAS.17.4.19696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alemu GG, Nigussie ZM, Amlak BT, Achamyeleh AA. Survival time and predictors of death among HIV infected under five children after initiation of anti -retroviral therapy in West Amhara Referral Hospitals, Northwest Ethiopia. BMC Pediatrics. 2022;22(1):670. doi: 10.1186/s12887-022-03693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn D, Woodburn P, Duong T, Peto J, Phillips A, Gibb D, et al. Current CD4 cell count and the short-term risk of AIDS and death before the availability of effective antiretroviral therapy in HIV-infected children and adults. The Journal of infectious diseases. 2008;197(3):398–404. doi: 10.1086/524686 [DOI] [PubMed] [Google Scholar]
- 41.Wamalwa DC, Obimbo EM, Farquhar C, Richardson BA, Mbori-Ngacha DA, Inwani I, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatrics. 2010;10(1):33. doi: 10.1186/1471-2431-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shabangu P, Beke A, Manda S, Mthethwa N. Predictors of survival among HIV-positive children on ART in Swaziland. African Journal of AIDS Research. 2017;16(4). doi: 10.2989/16085906.2017.1386219 [DOI] [PubMed] [Google Scholar]
- 43.Bitew S, Mekonen A, Assegid M. Predictors on mortality of human immunodeficiency virus infected children after initiation of antiretroviral treatment in Wolaita zone health facilities, Ethiopia: retrospective cohort study. Journal of AIDS and HIV Research. 2017;9(4):89–97. [Google Scholar]
- 44.Adem AK, Alem D, Girmatsion F. Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. Journal of AIDS and Clinical Research. 2014;5(3). [Google Scholar]
- 45.Mwiru RS, Spiegelman D, Duggan C, Seage GR, Semu H, Chalamilla G, et al. Nutritional Status and Other Baseline Predictors of Mortality among HIV-Infected Children Initiating Antiretroviral Therapy in Tanzania. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2015;14(2):172–9. doi: 10.1177/2325957413500852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tachbele E, Ameni G. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka Hospital, South Omo, Ethiopia: a six years retrospective cohort study. Epidemiology and health. 2016;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falvo JV, Ranjbar S, Jasenosky LD, Goldfeld AE. Arc of a vicious circle: pathways activated by Mycobacterium tuberculosis that target the HIV-1 long terminal repeat. American journal of respiratory cell and molecular biology. 2011;45(6):1116–24. doi: 10.1165/rcmb.2011-0186TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swaminathan S, Padmapriyadarsini C, Narendran G. HIV-associated tuberculosis: clinical update. Clinical Infectious Diseases. 2010;50(10):1377–86. doi: 10.1086/652147 [DOI] [PubMed] [Google Scholar]
- 49.Andargie AA, Asmleash Y. Survival time of human immunodeficiency virus (HIV) infected children under 15 years of age after initiation of antiretroviral therapy in the University of Gondar Comprehensive Specialized Hospital, Ethiopia. Journal of AIDS and HIV Research. 2018;10(4):49–55. [Google Scholar]
- 50.Atalell KA, Birhan Tebeje N, Ekubagewargies DT. Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study. PloS one. 2018;13(5):e0197145. doi: 10.1371/journal.pone.0197145 [DOI] [PMC free article] [PubMed] [Google Scholar]