Abstract
Objective
The prevalence of obesity and type 2 diabetes is rapidly increasing worldwide, posing serious threats to human health. This study aimed to evaluate the role of FMT in the treatment of obesity and/or metabolic syndrome and its impact on clinically important parameters.
Methods
We searched Medline, Embase, and Cochrane Library databases up to April 31, 2022 and further assessed articles that met the eligibility criteria. Mean differences and 95% confidence intervals were used to analyze continuous data. The I2 statistic was used to measure study heterogeneity. Univariate meta-regression or subgroup analyses were performed to explore the covariates that might contribute to heterogeneity. Potential publication bias was assessed using the Egger’s test. We used the GRADEpro guideline development tool to assess the quality of the evidence.
Results
Nine studies, comprising 303 participants, were included in the meta-analysis. In the short-term outcomes (<6 weeks after FMT), compared with the placebo group, patients in the FMT group had lower FBG (MD = -0.12 mmol/L, 95% Cl: -0.23, -0.01), HbA1c (MD = -0.37 mmol/mol, 95%Cl: -0.73, -0.01), and insulin levels (MD = -24.77 mmol/L, 95% Cl: -37.60, -11.94), and higher HDL cholesterol levels (MD = 0.07 mmol/L, 95% Cl: 0.02, 0.11).
Conclusions
FMT, as an adjunctive therapy, does not produce any serious adverse effects and may be useful in the treatment of metabolic syndrome, especially in improving HbA1c, insulin sensitivity, and HDL cholesterol. However, there was no significant difference between the FMT group and the placebo group in terms of weight reduction.
Introduction
Since the 1970s, obesity has escalated into a global epidemic, with obesity rates tripling worldwide, and affecting approximately one-tenth of the global adult population. With the increased prevalence and severity of the disease, obesity has become a major underlying risk factor for chronic diseases, such as type 2 diabetes mellitus (T2DM), cardiovascular disease, metabolic syndrome (MetS), non-alcoholic fatty liver disease (NAFLD), and cancer, increasing morbidity and mortality [1]. According to statistics released by the International Diabetes Federation (IDF), the number of people with diabetes will increase to 592 million by 2035, the number of adults with diabetes will increase by 55%, and over 80% of people with type 2 diabetes will be obese [2]. Most conventional treatments for obesity and obesity-related diseases are unsuccessful. Therefore, there is an urgent need to develop novel treatment strategies.
In recent years, fecal microbiome transfer (FMT) has been established as an effective treatment for recurrent Clostridioides difficile infection (CDI) because it can re-establish the intestinal microecosystem [3]. FMT has also been shown to be beneficial in the treatment of many other diseases such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and other gastrointestinal disorders [4–10]. In addition to genetic factors and lifestyle, the composition of the gut microbiome plays an important role in obesity and insulin resistance [11–13]. In the last decade, several studies have reported that alterations in gut microbiome composition are associated with obesity, glucose metabolism, and insulin sensitivity [14, 15]. In obese and diabetic patients, insulin sensitivity can be improved by establishing normal gut microbiome ratios, altering low-grade chronic inflammatory responses, correcting disturbances in bile acid metabolism, and interfering with short-chain fatty acid (SCFA) production to modulate the gut microecosystem [16–18]. Animal studies have successfully altered body phenotypes by using FMT. Pioneering experiments in mice have shown that obese and lean phenotypes can be transferred through the fecal microbiome of human donors [19–21]. This evidence highlights the possibility of using FMT as a therapeutic modality for human obesity.
FMT can reverse the pathological microecosystem in the intestinal tract and use the gut microbiome as a new target to treat metabolic diseases, such as diabetes, which will become a unique therapeutic idea. However, the sample sizes in some studies were too small, the statistical power was too low to predict the outcome of a comprehensive study, and there was a lack of research on the long-term effects of FMT. However, the use of FMT to alter the microbiome and improve clinically important parameters remains controversial. We conducted a systematic review and meta-analysis of randomized clinical trials (RCTs). To assess the role of FMT in the treatment of obesity with or without metabolic syndrome and its impact on clinically important parameters.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were adhered to as a methodological template for this review [22] (S1 Table).
Literature search strategy
Two investigators (B. Q and JX. L) independently searched MEDLINE (using PUBMED as the search engine), EMBASE, and Cochrane Library. Databases were used to identify suitable studies published until May 31, 2022. MeSH terms and keywords were used, and the search terms included: “fecal microbiota transplantation,” “fecal microbial transplant,” “fecal microbiota transfer,” “FMT,” “obesity,” “diabetes,” and “metabolic syndrome”. The search was limited to publications on human subjects in English. A manual search was conducted using references listed in the original articles and the review articles retrieved. Two investigators collected results separately.
Inclusion criteria
Randomized clinical trials (RCT);
Diagnosis of obesity and/or metabolic syndrome. (Obesity: defined as BMI ≥ 30 kg/m2)
The following related data were extracted: weight, body mass index (BMI), fasting blood glucose (FBG), hemoglobin A1C (HbA1c), HOMA-IR (Homeostatic Model Assessment for Insulin Resistance), insulin, cholesterol (total/LDL/HDL) and triglyceride,
Exclusion criteria
Duplicate reports
Studies conducted on animals
Systematic reviews or meta-analyses
Case-control and cohort study
Data extraction
For each included study, all data elements uniformly reported across most studies were extracted by two reviewers (B. Q and JX. L) and cross-verified by a third (C. L). When the same population was published in several journals, only the most informative articles or complete studies were retained to avoid duplication. The following information was extracted from each study: first author, publication year, patient characteristics, number of patients, method of FMT/placebo use, preoperative preparation, follow-up, and study results.
Definition of short-term /long-term outcomes
We considered short-term outcomes to be those that occurred within six weeks of intervention. Long-term outcomes were defined as those that occurred ≥ 12 weeks after the intervention. To analyze the effects of the intervention, we divided them into two groups: short-term and long-term effects, and analyzed the means of differences in clinically meaningful parameters separately.
Risk of bias assessment
The Cochrane risk-of-bias tool was used to assess the risk of bias in the randomized trials [23]. The quality of the evidence was assessed using the GRADEpro guideline development tool. Five items were assessed to obtain the quality of evidence: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of evidence can be classified as very low, low, moderate or high. Each included article was independently assessed by two authors using this tool, and disagreements between the two authors were resolved by consensus.
Statistical analysis
Mean differences (MD) and 95% confidence intervals (CIs) were used to analyze continuous data. The methods described by Luo et al. [24] and Wan et al. [25] were used to estimate the mean and standard deviation, with medians and ranges, to make the data suitable for meta-analysis. For the selection of effect sizes, the choice of weight mean differences (WMD) is appropriate if different studies use the same units of measurement for the observed continuous-type metrics, or standardized mean differences (SMD) if the units of measurement differ and/or the mean varies significantly [26].
The I2 statistic was used to measure the study heterogeneity, with I2 ≥ 50% indicating significant heterogeneity. A fixed-effects model was used when heterogeneity was not significant; otherwise, a random-effects model was applied. Univariate meta-regression or subgroup analyses were performed to explore covariates that might contribute to heterogeneity based on the following predetermined characteristics: 1. years of publication (earlier than 2020 vs later than 2020); 2. race (European vs. non-European), and 3. method of FMT intervention (oral vs. non-oral). Sensitivity analysis was performed to determine whether there was an undue influence of a single study on the combined study results [27].
We assessed potential publication bias using Begg’s test and Egger’s test, with P >0.05 indicating no publication bias. All statistical analyses were performed using Stata version 15 (Stata Corp, College Station, Texas, USA) and RevMan 5.4 (The Cochrane Collaboration, Oxford, UK).
Results
Characteristics of the included studies
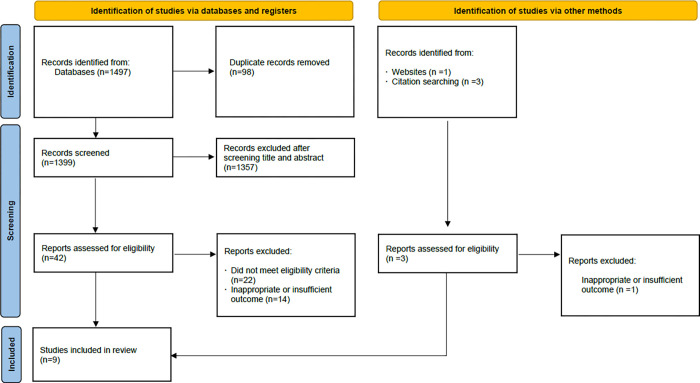
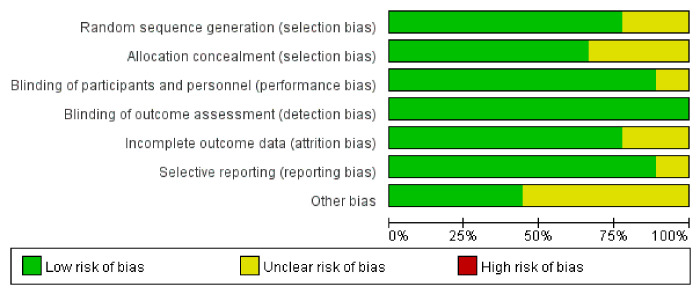
Of the 1428 articles identified through the systematic search, nine studies [28–36] with 303 participants were finally included in our meta-analysis. Fig 1 showed the identification and selection of studies. All the included studies were RCTs. Among these nine studies, two [28, 30] evaluated the effect of FMT in obese patients (BMI ≥ 30 kg/m2) without metabolic syndrome, one [33] only evaluated the effect of FMT in patients with type 2 diabetes without BMI, five studies [29, 31, 32, 34, 35] assessed the role of FMT in patients with metabolic syndrome and obesity, and one study [36] included patients with normal BMI and type I diabetes. Four studies [29, 32, 34, 36] performed FMT using a nasoduodenal tube and five studies [28, 30, 31, 33, 35] used oral capsules. Two of these studies [31, 33] combined FMT with oral capsules in a specific diet. All studies used stool from healthy lean donors for FMT; however, in one study [32] donors were vegetarians. The main characteristics of the included studies are summarized in Table 1. Before data analysis and synthesis, the Cochrane risk of bias tool was used to assess the quality of the studies, as shown in Fig 2. Five studies were at risk of other bias because they did not report the history of cholecystectomy, as animal studies have shown that the composition of the gut microbiota is influenced by a variety of factors, including bile acid composition [28, 31, 33, 35, 36].
Fig 1. Study identification and selection flowchart.
Table 1. Baseline characteristics of the included studies.
| Author (year) | Country | Participants | Case (n) | Age (years) | BMI (kg/m2) | Intervention | Follow up |
|---|---|---|---|---|---|---|---|
| Allegretti (2019) [28] | United Kingdom | Female participants, BMI ≥ 35 kg/m2 without metabolic syndrome | 22: 11 FMT 11 Placebo |
FMT: 44.5±14.4 Placebo: 43.2±13.8 |
FMT: 41.1±5.0 Placebo: 40.4±4.7 |
FMT/ Placebo capsules | 12 weeks |
| Kootte 2017 [29] | Netherlands | Male patients aged 21–69 years with metabolic syndrome | 38: 26 Allogenic FMT 12 Autologous FMT |
Autologous: 54 (49–58) Allogenic: 54 (49–60) |
Autologous: 35.8 (33.1–40.4) Allogenic: 33.8 (32.5–35.7) |
FMT/ autologous FMT was infused through the nasoduodenal tube. | 18 weeks |
| Leong 2020 [30] | New Zealand | Patients aged 14–18 years, BMI ≥ 30 kg/m2 and without chronic diseases. | 87: 42 FMT 45 Placebo |
FMT: 17.3±1.5 Placebo: 17.1±1.4 |
FMT: 38.6±5.9 Placebo: 36.9±4.6 |
FMT/ Placebo capsules | 26 weeks |
| Mocanu 2021 [31] | Canada | Patients aged 18–65 years, BMI ≥ 30 kg/m2 with metabolic syndrome | 61: 29 FMT 32 Placebo |
FMT: 47.3±11.0 Placebo: 48.4±9.6 |
FMT: 46.3±6.6 Placebo: 44.5±7.2 |
FMT/ Placebo capsules coupled with fiber supplementation | 12 weeks |
| Smits (2018) [32] | Netherlands | Male patients aged 21–69 years, BMI ≥ 30 kg/m2 with metabolic syndrome | 20: 10 Allogenic FMT 10 Autologous FMT |
Autologous: 57.7±8.5 Allogenic: 52.3±7.4 |
Autologous: 33.8±4.0 Allogenic: 33.9±3.9 |
FMT/ autologous FMT was infused through the nasoduodenal tube. | 2 weeks |
| Su 2022 [33] | China | Patients aged 41–76 years, with type 2 diabetes | 13: 5 FMT 8 Placebo |
FMT: 57±13.2 Placebo: 60.4±12.0 |
FMT: 25.2±5.0 Placebo: 24.8±3.0 |
received the PPWb formulation only, or coupled with FMT capsules | 90 days |
| Vrieze 2012 [34] | Netherlands | Male patients, BMI ≥ 30 kg/m2 with metabolic syndrome | 18: 9 Allogenic FMT 9 Autologous FMT |
Autologous: 53±3 Allogenic: 47±4 |
Autologous: 35.6±1.5 Allogenic: 35.7±1.5 |
FMT/ autologous FMT was infused through the gastroduodenal tube | 6 weeks |
| Yu 2020 [35] | USA | Patients aged 25–60 years, BMI ≥ 30 kg/m2 and mild to moderate insulin resistancec | 24: 12 FMT 12 Placebo |
FMT: 42.5±8.4 Placebo: 38.5±8.8 |
FMT: 38.8±6.7 Placebo: 41.3±5.1 |
FMT/ Placebo capsules | 12 weeks |
| Groot 2020 [36] | Netherlands | Patients aged 18–35 years with normal BMI, type 1 diabetes | 20: 10 Allogenic FM 10 Autologous FMT |
Autologous: 25.0±3.5 Allogenic: 24.3±5.4 |
Autologous: 23.0±2.0 Allogenic: 21.8±2.5 |
FMT/ autologous FMT was infused through the nasoduodenal tube. | 12 months |
Data are depicted as mean±SD or median (interquartile range), depending on their distribution. BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FMT, fecal microbiota transplantation.
a. FMT-LF group: FMT and low-fermentable fiber.
b. PPW: diet consisting of probiotics, prebiotics and whole grains.
c. mild to moderate insulin resistance: homeostatic model assessment of insulin resistance (HOMA-IR) between 2.0 and 8.0.
Fig 2. Evaluation of study quality.
Short-term outcomes
A total of 303 patients were included and analyzed to determine the significance of FMT in terms of metabolic syndrome-related efficacy outcomes. Statistically significant FBG (MD = -0.12 mmol/L, 95%Cl: -0.23, -0.01, SD: ±0.04, I2 = 7%), HbA1c (MD = -0.37 mmol/mol, 95%Cl: -0.73, -0.01, SD: ±0.13, I2 = 46%), HDL cholesterol (MD = 0.07 mmol/L, 95%Cl: 0.02, 0.11, SD: ±0.02, I2 = 25%), and insulin levels (MD = -24.77 pmol/L, 95%Cl: -37.60, -11.94, SD: ±4.76, I2 = 0%) were achieved with FMT in the short term. Compared with the placebo group, patients in the FMT group had lower FBG, HbA1c, and insulin levels, and higher HDL cholesterol levels. Weight (MD = 2.72 Kg, 95%Cl: -4.74, 10.18, SD: ±11.16, I2 = 50%), BMI (MD = -0.22 Kg/m2, 95%Cl: -1.36, 0.92, SD: ±1.71, I2 = 0%), HOMA-IR (MD = 0.08, 95%Cl: -0.56, 0.73, SD: ±0.96, I2 = 0%), total cholesterol (MD = 0.03 mmol/L, 95%Cl: -0.10, 0.17, SD: ±0.05, I2 = 0%), LDL cholesterol (MD = 0.13 mmol/L, 95%Cl: -0.03, 0.28, SD: ±0.23, I2 = 0%), and triglycerides (MD = -0.03 mmol/L, 95%Cl: -0.13, 0.07, SD: ±0.15, I2 = 17%) did not different between the two groups (Fig 3).
Fig 3. Forest plot of short-term factor results.
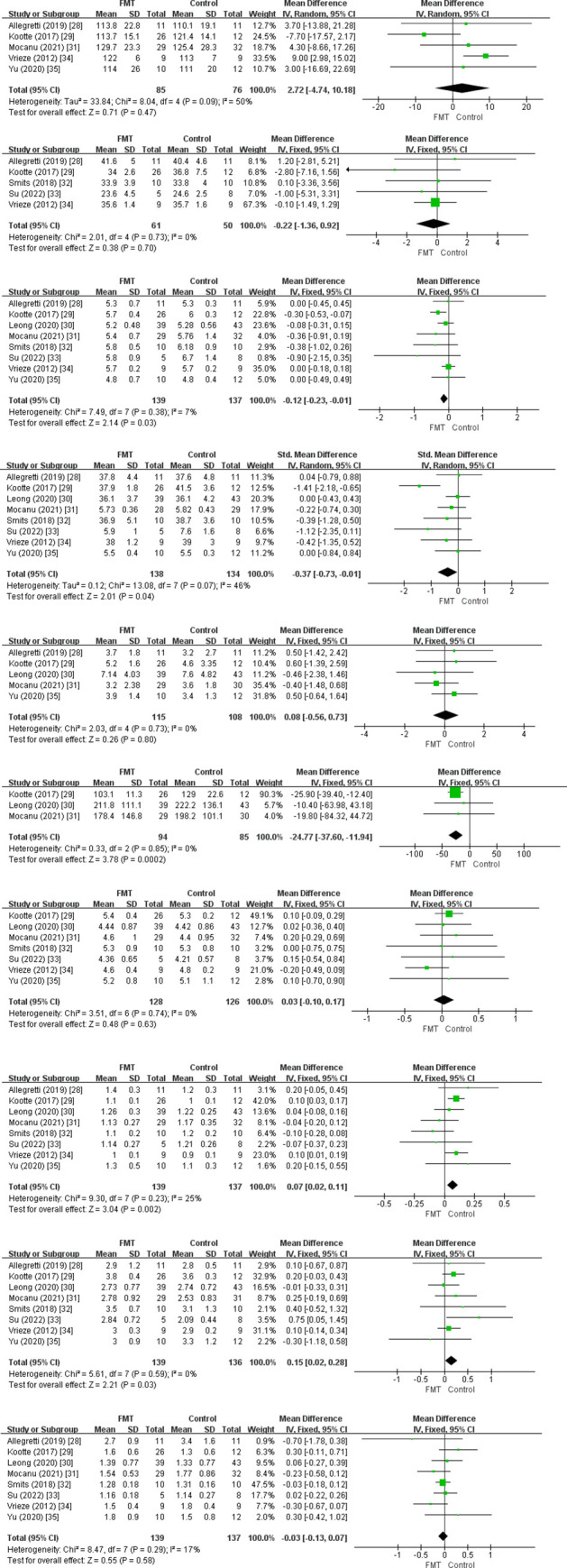
1) Weight (Kg), 2) BMI (Kg/m2), 3) Fasting glucose (mmol/L), 4) HbA1C, 5) HOMA-IR, 6) Insulin (pmol/L), 7) Cholesterol (mmol/L), 8) HDL (mmol/L), 9) LDL (mmol/L), 10) Triglycerides (mmol/L).
Analysis of subgroups based on the method of FMT use
We performed a subgroup analysis of the two different ways of FMT use (Capsules VS. Nasoduodenal tube). There was no significant difference between the FMT group and the placebo group in each parameter by way of oral capsule administration. In contrast, the mean HbA1C and insulin levels were slightly lower and the mean HDL levels were slightly higher in the FMT group by the nasoduodenal tube injection (Table 2).
Table 2. Subgroup analysis based on FMT use method in short-term outcomes.
| Category | Subgroup | MD | 95%Cl | I2 |
|---|---|---|---|---|
| Weight | Capsules | 3.85 | -5.37, 13.07 | 0% |
| Nasoduodenal tube | 1.13 | -15.21, 17.46 | 50% | |
| BMI | Capsules | 0.18 | -2.76, 3.11 | 0% |
| Nasoduodenal tube | -0.29 | -1.53, 0.94 | 0% | |
| Fasting glucose | Capsules | -0.10 | -0.28, 0.07 | 0% |
| Nasoduodenal tube | -0.17 | -0.42, 0.08 | 57% | |
| HbA1C | Capsules | -0.11 | -0.39, 0.17 | 0% |
| Nasoduodenal tube | -0.83 | -1.32, -0.34 | 50% | |
| HOMA-IR | Capsules | 0.02 | -0.65, 0.70 | 0% |
| Nasoduodenal tube | 0.6 | -1.39, 2.59 | ------ | |
| Insulin | Capsules | -14.24 | -55.46, 26.98 | 0% |
| Nasoduodenal tube | -25.90 | -39.40, -12.40 | ------ | |
| Cholesterol | Capsules | 0.10 | -0.16, 0.36 | 0% |
| Nasoduodenal tube | 0.01 | -0.15, 0.17 | 30% | |
| LDLc | Capsules | 0.13 | -0.10, 0.35 | 19% |
| Nasoduodenal tube | 0.13 | -0.09, 0.34 | 0% | |
| HDLc | Capsules | 0.04 | -0.05, 0.12 | 0% |
| Nasoduodenal tube | 0.08 | 0.03, 0.13 | 56% | |
| Triglycerides | Capsules | -0.03 | -0.19, 0.14 | 0% |
| Nasoduodenal tube | -0.03 | -0.16, 0.10 | 56% |
BMI: Body mass index; HbA1c: Hemoglobin A1c (glycated hemoglobin); HOMA-IR: Homeostatic model assessment of insulin resistance; LDL: Low density lipoprotein; HDL: High density lipoprotein
Long-term outcomes
We found no difference between the FMT and control groups through analysis of the mean differences in clinically significant parameters (Fig 4), except for a slight decrease in HbA1c at 12 weeks in the FMT group compared to the placebo group in the study by Yu et al. [35].
Fig 4. Forest plot of long-term factor result.

1) Weight (Kg), 2) BMI (Kg/m2), 3) Fasting glucose (mmol/L), 4) HbA1C, 5) HOMA-IR, 6) Insulin (pmol/L), 7) Cholesterol (mmol/L), 8) HDL (mmol/L), 9) LDL (mmol/L), 10) Triglycerides (mmol/L).
Adverse events
Only minor adverse events (AEs) were reported in the treatment group. Two studies [28, 35] reported fever, headache, nausea/vomiting, diarrhea, bloating, and abdominal pain. No significant differences in AEs were observed and no serious AEs were associated with FMT.
Heterogeneity analysis
Significant heterogeneity was observed in weight (I2 = 50%) in the short-term outcome analyses. Hence, we conducted meta-regression and subgroup analyses to examine the sources of potential heterogeneity, but “Year of publication”, “Race”, and “FMT intervention methods” were not factors for heterogeneity (the p-values were 0.86,0.87 and 0.78, respectively). We then performed a sensitivity analysis by removing one study and recalculating the pooled estimates for the remaining studies, which showed that the pooled results were significantly affected by the Vrieze et al. [34]. Heterogeneity was 0% after its exclusion, but the result was still not significantly different between the two groups (MD = -1.53 Kg, 95%Cl: -8.27, 5.20).
Publication bias
We performed a funnel plot to test for publication bias. Visual inspection of the funnel plot revealed asymmetry, which raises the possibility of publication bias (S1 Fig). Therefore, we used the Begg’s test and Egger’s to detect the risk of bias. All p-values of Begg’s test and Egger’s statistical test were greater than 0.05, and the results indicate that there was no publication bias among the studies included in the meta-analysis (Table 3).
Table 3. Results of Begg’s test and Egger’s statistical test.
| Short-term outcomes | Long-term outcomes | |||
|---|---|---|---|---|
| Category | Begg’s Test | Egger’s test | Begg’s Test | Egger’s test |
| Weight | 0.81 | 0.39 | 1.0 | –– |
| BMI | 0.81 | 0.49 | 1.0 | –– |
| Fasting glucose | 0.27 | 0.65 | 0.30 | 0.19 |
| HbA1C | 0.11 | 0.23 | 0.46 | 0.57 |
| HOMA-IR | 0.46 | 0.76 | 1.0 | –– |
| Insulin | 1.0 | –– | 1.0 | –– |
| Cholesterol | 0.76 | 0.59 | 0.73 | 0.48 |
| LDLc | 0.17 | 0.18 | 0.46 | 0.54 |
| HDLc | 0.99 | 0.52 | 0.81 | 0.69 |
| Triglycerides | 0.71 | 0.83 | 0.81 | 0.38 |
BMI: Body mass index; HbA1c: Hemoglobin A1c (glycated hemoglobin); HOMA-IR: Homeostatic model assessment of insulin resistance; LDL: Low density lipoprotein; HDL: High density lipoprotein
Quality of the evidence for the results
We used the GRADEpro guideline development tool to assess the quality of the evidence (Table 4 and S3 Table).
Table 4. Quality of evidence by Grading of Recommendations Assessment, Development and Evaluation (GRADE).
| Outcome | Studies | Participants | Quality of the evidence | GRADE | Importance | |
|---|---|---|---|---|---|---|
| Short-term effects | Weight | 5 | 161 | ⊕⊝⊝⊝ | very low | IMPORTANT |
| BMI | 5 | 111 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Fasting glucose | 8 | 276 | ⊕⊕⊕⊝ | moderate | IMPORTANT | |
| HbA1C | 8 | 272 | ⊕⊕⊕⊝ | moderate | IMPORTANT | |
| HOMA-IR | 5 | 223 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Insulin | 3 | 179 | ⊕⊕⊕⊝ | moderate | IMPORTANT | |
| Cholesterol | 7 | 254 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| LDLc | 8 | 278 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| HDLc | 8 | 278 | ⊕⊕⊕⊝ | moderate | IMPORTANT | |
| Triglycerides | 8 | 276 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Long-term effects | Weight | 2 | 43 | ⊕⊕⊝⊝ | low | IMPORTANT |
| BMI | 2 | 90 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Fasting glucose | 3 | 111 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| HbA1C | 5 | 149 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| HOMA-IR | 2 | 77 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Insulin | 2 | 96 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Cholesterol | 4 | 166 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| LDLc | 5 | 186 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| HDLc | 5 | 186 | ⊕⊕⊝⊝ | low | IMPORTANT | |
| Triglycerides | 5 | 186 | ⊕⊕⊝⊝ | low | IMPORTANT |
BMI: Body mass index; HbA1c: Hemoglobin A1c (glycated hemoglobin); HOMA-IR: Homeostatic model assessment of insulin resistance; LDL: Low density lipoprotein; HDL: High density lipoprotein
Discussion
Obesity and metabolic syndrome are global health epidemics of the 21st century, and current medical strategies have limited efficacy [37]. Several studies have reported that patients with obesity and metabolic syndrome have abnormal gut microbiota; therefore, the treatment of obesity and diabetes can be initiated by modulating the gut microbiota [38–40]. High-fat and high-sugar diets can lead to a large proliferation of Firmicutes and a decrease in Bacteroidetes; an altered ratio of Firmicutes to Bacteroidetes is associated with metabolic diseases such as obesity [41, 42]. Compared to lean individuals, the gut microbiota of obese individuals is more conducive to the production of energy-related molecules, particularly short-chain fatty acids of resistant starch origin. These molecules can generate additional energy through the citric acid cycle or participate in gluconeogenesis, lipid metabolism, and protein metabolism [43]. FMT transplants the functional gut microbiota from the feces of healthy individuals into the gastrointestinal tract of obese individuals to re-establish functional gut microbiota.
This meta-analysis investigated studies using FMT for the treatment of obesity and metabolic syndrome and basically concluded that the treatment was effective in the short term. At 2 to 6 weeks after the intervention, mean HbA1c and mean fasting glucose were lower in the FMT group than in the placebo group, although this was a small mean difference. However, mean insulin levels were significantly lower in the FMT group, suggesting a significant improvement in insulin sensitivity. One study [31] reported a significant improvement in HOMA2-IR after six weeks of FMT application. There are also studies [29, 34] that reported improved peripheral insulin sensitivity in the FMT group. Moreover, two studies even showed a small decrease in HbA1c after FMT intervention [29, 35]. FMT has been reported to treat obesity in mice [44], and Zhang et al. [45] showed that FMT improved some laboratory parameters (e.g., insulin sensitivity, glycated hemoglobin, etc.) in patients with metabolic syndrome, although none of the weight loss effects were significant. Therefore, these findings could prove that FMT is effective for glycemic control and improves insulin sensitivity, although the improvement is small.
There was epidemiological evidence of an association between systemic and/or local low-grade chronic inflammation and insulin resistance (IR) states. The development of IR is mainly associated with various pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) [46–48]. Additionally, the inflammatory marker C-reactive protein (CRP) is generally elevated in human IR states [49]. Several factors may contribute to the initiation and maintenance of tissue inflammation, such as diet, tissue microenvironment, and gut microbiota [50]. Chronic exposure to pro-inflammatory mediators causes cell-autonomous IR in liver, muscle and adipocytes [48]. Then whether the regulation of gut microbiota can improve the inflammatory response and IR has also become the outcome of interest in current studies. It has been demonstrated in several studies that fasting blood glucose levels were significantly reduced and IR and low inflammatory response were improved after treatment of diabetic patients with gut microbiota improvement. [51, 52].
There was also a small but statistically significant difference in HDL cholesterol levels between the two groups. After the short-term intervention, mean HDL cholesterol levels were higher in the FMT group than in the placebo group. A previous meta-analysis also reported a slightly higher mean HDL in the FMT group compared to the placebo group [53]. Although the quality of the evidence for this finding is low, the evidence accumulated from intervention studies using FMT suggests a possible association between FMT and changes in cholesterol metabolism. Intervention of high-fat diet-induced NAFLD in mice by transplanting fecal bacteria from normal mice in several animal model experiments resulted in lower body weight, lower blood lipids, improved liver function and reduced hepatic steatosis in NAFLD rats [54, 55]. However, there is still a lack of clinical trial results with a strong enough evidence level for evidence-based medicine.
The previously described benefits were not observed in the long-term group, and there were no significant differences between the FMT and placebo groups with respect to important obesity parameters (e.g., weight, BMI) in the short and long term. The small number of patients enrolled in the trial may explain why we did not observe any between-group differences in these parameters. In a study by Kootte et al. [29], long-term clinical effects, such as sensitivity to insulin and plasma metabolites were lacking at 18 weeks after allogeneic FMT. One hypothesis that could explain the return of gut microbiota composition to baseline conditions and the varying degrees of short- and long-term metabolic responses is that the host immune system develops resilience coupled with adherence to its own lifestyle, including diet and exercise [56]. In a study of mice with a tightly controlled diet, weight loss was associated with FMT treatment, and a poor diet may counteract the beneficial effects of FMT [19]. Overall, the findings of these studies and our results suggested that FMT may be effective in alleviating the features of metabolic syndrome. Extensive research is needed to reveal the specific pathophysiological roles of the gut microbiota in obesity and diabetes and to observe the mechanism and clinical efficacy of FMT in the treatment of metabolic-related diseases.
In several studies, the administration of FMT is preferred by using a duodenal infusion [29, 32, 34, 36]. The solution was infused within 6 hours of collection of fecal material through the nasoduodenal tube. In contrast to this method of transplantation, in order to prevent adverse events associated with physical delivery of FMT using nasojejunal tubes or colonoscopy, some trial groups have adopted a non-invasive method of FMT delivered by double wrapping using an encapsulated fecal microbiome to transport the contents to the intestine using a delayed-release hydroxypropyl methylcellulose capsule that remains intact as it passes through the stomach [57]. Notably, in our subgroup analysis, the improvement in HbA1C and HDL was more significant with the method of FMT infusion through the nasal-intestinal tube compared to the oral capsule method This may be due to the fact that fresh fecal suspension can be placed more precisely at the appropriate site by endoscopic injection. Combined with the fact that absorption of sugars and fatty acids is associated with obesity and insulin resistance, and that these substances are mainly absorbed in the small intestine, the best route to achieve FMT is via nasoduodenal tube infusion.
Significant heterogeneities of pooled specificity were still found among studies when we used the random-effects model in the pooling of data. This method might reduce the effect of heterogeneity but not abolish it. To explore the sources of heterogeneity, both the subgroup analyses and meta-regression were performed. They showed that the Year of publication (earlier than 2020 vs later than 2020), Race (European vs. non-European) and FMT intervention methods (oral vs. non-oral) were not associated with the heterogeneity. However, the results of the sensitivity analysis showed the study of Vrieze was the source of heterogeneity. The reason for this analysis may be due to the fact that among the included patients, the mean weight of patients in the allogenic group was significantly higher than that of patients in the autologous group (123kg vs 113kg), and there was no significant change in mean weight in both groups at the end of the 6-week trial (122kg vs 113kg). In the other included studies, there was no significant difference in the mean weight of patients in the two groups. This study was not excluded because of its greater weight and higher quality.
This is a systematic review and meta-analysis to assess the role of FMT in the treatment of obesity and metabolic syndrome. Compared to a previous meta-analysis, we included more studies to comprehensively analyze obesity parameters (e.g., weight, BMI), and metabolic system parameters (e.g., glucose, cholesterol). And we conducted a subgroup analysis of the two ways of using FMT to determine a more effective way of using it. There are several limitations to this systematic review. An important limitation is the small number of studies and patients, which leads to inconsistent and imprecise results, as well as large confidence intervals. In addition, this meta-analysis did not pre-register protocols (as in PROSPERO), which could introduce potential bias. A limitation of some studies is that diet and physical activity have not been tightly controlled. weight loss was associated with FMT treatment, and the beneficial effects of FMT may be negated by poor diet. It requires further study whether the addition of a standard dietary intervention could work synergistically with FMT donors to match host immunology better for optimizing clinical metabolic and immunological responses. A further dose finding study is needed to determine the optimal dose for this particular group of patients.
Conclusion
In conclusion, FMT does not produce any serious adverse effects and may be beneficial as an adjunctive therapy in the treatment of metabolic syndrome, especially in improving blood glucose, increasing insulin sensitivity, and HDL cholesterol. However, due to the small number of relevant studies and the low quality of evidence, we need more high-quality studies on the role of FMT in the metabolic processes of glucose and lipids. Moreover, the fact that the FMT application is associated with changes in obesity-related parameters needs further confirmation when we include diet and lifestyle changes in the design.
Supporting information
(RAR)
(DOC)
(XLSX)
(XLSX)
List of abbreviations
- BMI
body mass index
- CDI
Clostridioides difficile infection
- FBG
fasting blood glucose
- FMT
fecal microbiota transplantation
- HDL
high density lipoprotein
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- IR
insulin resistance
- LDL
low density lipoprotein
- MD
mean differences
- MetS
metabolic syndrome
- NAFLD
non-alcoholic fatty liver disease
- RCT
randomized clinical trial
- SCFA
short-chain fatty acid
- SD
standard deviation
- SMD
standardized mean differences
- T2DM
type 2 diabetes mellitus
- WMD
weight mean differences
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121(6):21–33. doi: 10.3810/pgm.2009.11.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–508. doi: 10.1038/ajg.2013.59 [DOI] [PubMed] [Google Scholar]
- 4.Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3(1):17–24. doi: 10.1016/S2468-1253(17)30338-2 [DOI] [PubMed] [Google Scholar]
- 5.Halkjær SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67(12):2107–2115. doi: 10.1136/gutjnl-2018-316434 [DOI] [PubMed] [Google Scholar]
- 6.Vermeire S, Joossens M, Verbeke K, et al. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(4):387–394. doi: 10.1093/ecco-jcc/jjv203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149(1):102–109.e6. doi: 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015;149(1):110–118.e4. doi: 10.1053/j.gastro.2015.03.045 [DOI] [PubMed] [Google Scholar]
- 9.Tian H, Ge X, Nie Y, et al. Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial. PLoS One. 2017;12(2):e0171308. doi: 10.1371/journal.pone.0171308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66(6):1727–1738. doi: 10.1002/hep.29306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13(6):935–940. doi: 10.1016/j.coph.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Utzschneider KM, Kratz M, Damman CJ, Hullar M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism [published correction appears in J Clin Endocrinol Metab. 2016 Jun;101(6):2622]. J Clin Endocrinol Metab. 2016;101(4):1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong KSW, Derraik JGB, Hofman PL, Cutfield WS. Antibiotics, gut microbiome and obesity. Clin Endocrinol (Oxf). 2018;88(2):185–200. doi: 10.1111/cen.13495 [DOI] [PubMed] [Google Scholar]
- 16.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34(2):392–397. doi: 10.2337/dc10-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014;306(4):G310–G319. doi: 10.1152/ajpgi.00282.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 21.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. doi: 10.1126/scitranslmed.3005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salameh JP, Bossuyt PM, McGrath TA, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 2020;370:m2632. doi: 10.1136/bmj.m2632 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 25.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J Clin Psychiatry. 2020;81(5):20f13681. doi: 10.4088/JCP.20f13681 [DOI] [PubMed] [Google Scholar]
- 27.Copas JB, Shi JQ. A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res. 2001;10(4):251–265. doi: 10.1177/096228020101000402 [DOI] [PubMed] [Google Scholar]
- 28.Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, et al. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol. 2020;18(4):855–863.e2. doi: 10.1016/j.cgh.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 29.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26(4):611–619.e6. doi: 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 30.Leong KSW, Jayasinghe TN, Wilson BC, Derraik JGB, Albert BB, Chiavaroli V, et al. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw Open. 2020;3(12):e2030415. doi: 10.1001/jamanetworkopen.2020.30415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27(7):1272–1279. doi: 10.1038/s41591-021-01399-2 [DOI] [PubMed] [Google Scholar]
- 32.Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018;7(7):e008342. doi: 10.1161/JAHA.117.008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su L, Hong Z, Zhou T, Jian Y, Xu M, Zhang X, et al. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep. 2022;12(1):1152. doi: 10.1038/s41598-022-05127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome [published correction appears in Gastroenterology. 2013 Jan;144(1):250]. Gastroenterology. 2012;143(4):913–6.e7. [DOI] [PubMed] [Google Scholar]
- 35.Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. doi: 10.1371/journal.pmed.1003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. 2021. Jan;70(1):92–105. doi: 10.1136/gutjnl-2020-322630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33(7):673–689. doi: 10.1007/s40273-014-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778 [DOI] [PubMed] [Google Scholar]
- 39.Khan MT, Nieuwdorp M, Bäckhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753–760. doi: 10.1016/j.cmet.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 40.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464 doi: 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Nieuwdorp M, ten Berge IJ, Bemelman FJ, Geerlings SE. The potential beneficial role of faecal microbiota transplantation in diseases other than Clostridium difficile infection. Clin Microbiol Infect. 2014. Nov;20(11):1119–25. doi: 10.1111/1469-0691.12799 [DOI] [PubMed] [Google Scholar]
- 42.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. Jan 23;505(7484):559–63. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010. Jan;18(1):190–5. doi: 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 44.Kurokawa S, Kishimoto T, Mizuno S, Masaoka T, Naganuma M, Liang KC, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J Affect Disord. 2018. Aug 1;235:506–512. doi: 10.1016/j.jad.2018.04.038 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, et al. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients. 2019. Sep 25;11(10):2291. doi: 10.3390/nu11102291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11(6):212–7. doi: 10.1016/s1043-2760(00)00272-1 [DOI] [PubMed] [Google Scholar]
- 47.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S53–55 doi: 10.1038/sj.ijo.0802502 [DOI] [PubMed] [Google Scholar]
- 48.Feve B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(6):305–11. doi: 10.1038/nrendo.2009.62 [DOI] [PubMed] [Google Scholar]
- 49.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Ballantyne C M. Metabolic inflammation and insulin resistance in obesity[J]. Circulation research, 2020, 126(11): 1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, Svendsen KD, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010. Dec;104(12):1831–8. doi: 10.1017/S0007114510002874 [DOI] [PubMed] [Google Scholar]
- 52.Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9. doi: 10.1159/000349922 [DOI] [PubMed] [Google Scholar]
- 53.Proença IM, Allegretti JR, Bernardo WM, de Moura DTH, Ponte Neto AM, Matsubayashi CO, et al. Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials. Nutr Res. 2020. Nov;83:1–14. doi: 10.1016/j.nutres.2020.06.018 [DOI] [PubMed] [Google Scholar]
- 54.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013. Dec;62(12):1787–94. doi: 10.1136/gutjnl-2012-303816 [DOI] [PubMed] [Google Scholar]
- 55.Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017. May 8;7(1):1529. doi: 10.1038/s41598-017-01751-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marques RE, Marques PE, Guabiraba R, Teixeira MM. Exploring the Homeostatic and Sensory Roles of the Immune System. Front Immunol. 2016. Mar 31;7:125. doi: 10.3389/fimmu.2016.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller DS, Parsons AM, Bresland J, et al. A simple and inexpensive enteric-coated capsule for delivery of acidlabile macromolecules to the small intestine. J Zhejiang Univ Sci B. 2015;16(7):586–592. doi: 10.1631/jzus.B1400290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RAR)
(DOC)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.