Abstract
A cell envelope-associated proteinase gene (prtH) was identified in Lactobacillus helveticus CNRZ32. The prtH gene encodes a protein of 1,849 amino acids and with a predicted molecular mass of 204 kDa. The deduced amino acid sequence of the prtH product has significant identity (45%) to that of the lactococcal PrtP proteinases. Southern blot analysis indicates that prtH is not broadly distributed within L. helveticus. A prtH deletion mutant of CNRZ32 was constructed to evaluate the physiological role of PrtH. PrtH is not required for rapid growth or fast acid production in milk by CNRZ32. Cell surface proteinase activity and specificity were determined by hydrolysis of αs1-casein fragment 1-23 by whole cells. A comparison of CNRZ32 and its prtH deletion mutant indicates that CNRZ32 has at least two cell surface proteinases that differ in substrate specificity.
Lactic acid bacteria (LAB) are essential for the manufacture of a variety of dairy products, such as cheese and yogurt. Because they are auxotrophic for a number of amino acids, LAB depend upon a complex proteolytic system to obtain essential amino acids from caseins during growth in milk (23). This proteolytic system also plays an important role in cheese flavor development (20). The hydrolysis of casein into amino acids for use by LAB is initiated by a cell envelope proteinase (CEP) which hydrolyzes casein into oligopeptides (23). Oligopeptides are then transported into the bacterial cell via an oligopeptide transport system (Opp) (17, 42). Once the oligopeptides are inside the cell, intracellular peptidases hydrolyze them to free amino acids (25, 27).
Several CEPs (or PrtP proteinases [PrtPs]) from various lactococcal strains have been characterized both biochemically and genetically (23). PrtP is synthesized as a pre-pro-protein of approximately 200 kDa. Autocatalytic cleavage of the pro-region results in a mature, active protein with a molecular mass of approximately 180 to 190 kDa (23). The genes encoding PrtPs have been sequenced from a number of different Lactococcus lactis strains (8, 18, 22, 24, 44, 46). The lactococcal PrtPs are more than 98% identical at the amino acid level (21). Despite this high degree of sequence identity, PrtPs can be classified into at least eight different groups based on substrate specificity by use of αs1-casein fragment 1-23 [αs1-CN (f1-23)] (4, 10, 11). Protein engineering studies have shown that a small number of amino acid substitutions can result in changes in substrate specificity (5, 35, 36, 43).
Much less is known about the CEPs of lactobacilli. The genes encoding CEPs have been cloned from Lactobacillus paracasei subsp. paracasei and Lactobacillus delbrueckii subsp. bulgaricus (13, 15). The deduced CEP amino acid sequences are 95 and 27% identical, respectively, to those of the lactococcal PrtPs. Comparisons of different lactobacilli have indicated heterogeneity of cell surface proteinase activity within the genus Lactobacillus (14, 19). Recent studies have indicated that Lactobacillus helveticus may contain two proteinases with different substrate specificities (14). In addition, a zinc-dependent cell surface proteinase has been purified from L. delbrueckii subsp. bulgaricus (39). This paper describes the genetic and physiological characterization of a CEP from L. helveticus CNRZ32.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All cultures were maintained at −80°C in 11% nonfat dry milk–10% glycerol. Escherichia coli DH5α (Gibco-BRL Life Technologies Inc., Gaithersburg, Md.) was grown in Luria-Bertani medium (32). L. helveticus CNRZ32 was propagated in MRS medium (Difco Laboratories, Detroit, Mich.) without shaking at 42°C. Strains for Southern hybridization were obtained from the American Type Culture Collection (Rockville, Md.). L. helveticus L89 was kindly provided by Fred A. Exterkate from The Netherlands Institute for Dairy Research collection. Growth studies with milk were performed by use of twice-steamed, pasteurized skim milk (pasteurized skim milk was steamed for 20 min, kept at 42°C for 2 h, and then steamed for another 20 min) as described previously (6).
Molecular cloning techniques.
Recombinant DNA techniques were essentially those described by Sambrook et al. (32). Restriction enzymes and T4 DNA ligase were purchased from Gibco-BRL Life Technologies and were used according to the manufacturer’s instructions. E. coli transformation was performed with a Gene Pulser by following the instructions recommended by the manufacturer (Bio-Rad Laboratories, Richmond, Calif.). Transformation of L. helveticus was performed essentially as described previously (6). All antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.).
PCR.
All primers were synthesized by Gibco-BRL Custom Primers (Grand Island, N.Y.). PCR amplifications were performed with a Perkin-Elmer (Norwalk, Conn.) model 480 thermal cycler. Two primers were designed from an alignment of the conserved regions surrounding the active-site residues of the proteinase genes (Asn196 and Ser433; numbering is that of L. lactis subsp. cremoris SK11 CEP) from various LAB. The sequences of the primers were as follows: Jp1, 5′GTTATCTCTGCTGGGAAC3′; and Jp2, 5′GTGAAGCCATTGAAGTCC3′. An inverse PCR strategy was used to identify adjacent DNA regions (32). CNRZ32 chromosomal DNA (1 to 5 μg) was digested with an appropriate restriction enzyme. The digested DNA was incubated at 65°C for 20 min to inactivate the restriction enzyme, precipitated in ethanol, and resuspended in 15 μl of deionized H2O. The digested DNA was self-ligated overnight at 15°C in a 20-μl reaction mixture. A 1-μl sample of the overnight ligation mixture was used as a template for PCR. As the known sequenced progressed, new primers were designed accordingly.
The L. helveticus L89 prt gene was amplified by PCR with primers Jp6 (5′TGGCAGAACCTGTGCCTA3′) (Fig. 1, nucleotides 1522 to 1505) and Jp17 (5′CGATGATAATCCTAGCGAGC3′) (Fig. 1, nucleotides 903 to 922).
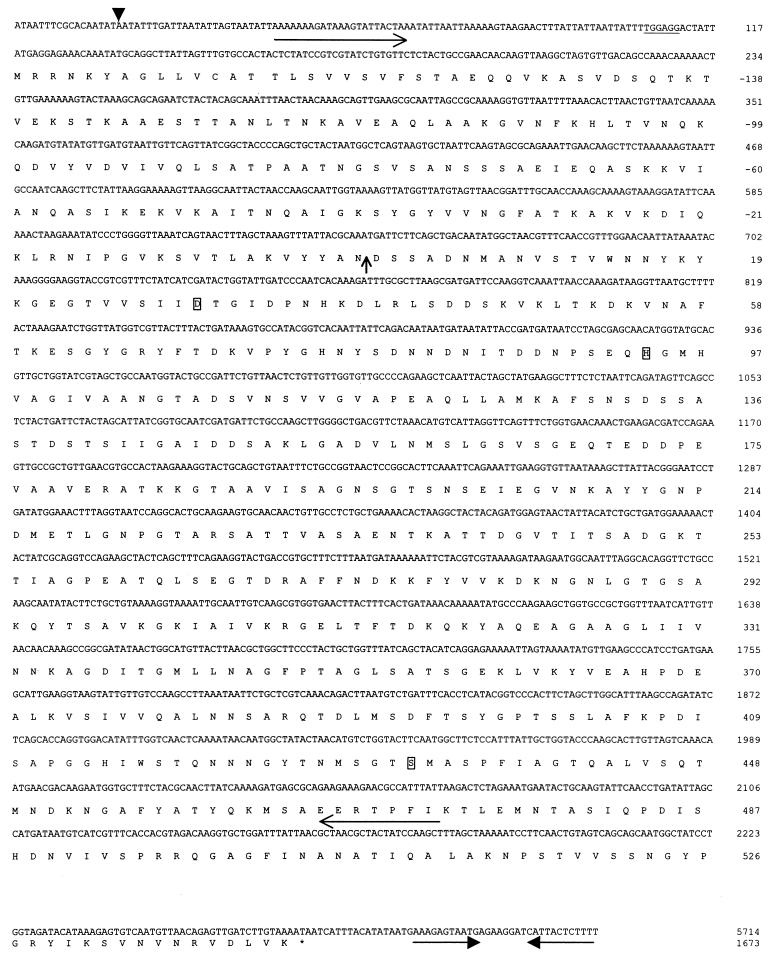
FIG. 1.
Partial nucleotide and deduced amino acid sequence of the L. helveticus CNRZ32 prtH gene. The arrowhead indicates the start of transcription. A putative Shine-Delgarno sequence is underlined. The putative cleavage site for the pre-pro-region is marked with a vertical arrow. The putative active-site residues are boxed. Long horizontal arrows indicate primers used for DNA probe synthesis. The stop codon is indicated with an asterisk. Short horizontal arrows indicate the putative transcriptional terminator.
DNA sequence analysis.
PCR products were purified with a Qiagen Inc. (Hilden, Germany) PCR purification kit. DNA sequencing reactions were performed with a Perkin-Elmer model 480 thermal cycler and a Prism Ready Reaction DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Inc., Foster City, Calif.). The DNA sequence determination was conducted at the Nucleic Acid and Protein Facility of the University of Wisconsin—Madison Biotechnology Center with an ABI Prism model 377XL DNA automated sequencer. Sequences were analyzed with the Genetics Computer Group (Madison, Wis.) sequence analysis program. Protein homology searches were performed by use of the BLAST network service (1). All reported DNA sequence data was confirmed by sequencing both DNA strands from at least two independent PCR products.
RNA methods.
Total RNA was isolated by use of an SV Total RNA Isolation System (Promega Corporation, Madison, Wis.). Mapping of the 5′ end of the prtH transcript was conducted by use of a 5′ rapid amplification of cDNA ends (5′ RACE) kit (version 2.0; Gibco-BRL). The nucleotide sequences of the three prtH-specific primers were 5′ATGATAGAAACGACGGTACC3′ (Jp16), 5′AACGGTTGAAACGTTAGC3′ (Jp43), and 5′GCTTGGTTAGTAATTGCC3′ (Jp45).
Southern blot analysis.
Chromosomal DNA isolation and Southern hybridization procedures were performed as described previously (9). Probe synthesis was performed as described for a Genius kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) with a 2.0-kb internal fragment from the catalytic domain of prtH. The template used for probe synthesis was made by PCR amplification with primers Jp22 (5′CTCTATCCGTCGTATCTGTG3′) and Jp23 (5′GCTTGGATAGTAGCGTTAGC3′). Hybridizations were carried out at 42°C. Low-stringency conditions were achieved by use of 10% formamide in the hybridization buffer.
Construction of a prtH deletion mutant of CNRZ32.
Primers Jp22bam (primer Jp22 with a BamHI extension at the 5′ end) and Jp23bam (primer Jp23 with a BamHI extension at the 5′ end) were synthesized. The Jp22bam-Jp23bam PCR product (described above) was digested with BamHI and ligated into pTRKL2. The resulting plasmid was used as a template for reverse PCR with primers Jp25 (5′GGTGAACAAACTGAAGACG3′) (nucleotides 1144 to 1162) and Jp26 (5′ATTGTGACCGTATGGCACT3′) (nucleotides 876 to 858). The PCR product was self-ligated and transformed into E. coli DH5α. The construct that was created contained a 270-bp internal in-frame deletion subcloned into pTRKL2. An integration vector was constructed by subcloning the deletion fragment into pSA3. The deletion was confirmed by PCR and DNA sequencing. The resulting construct was used to construct a prtH deletion derivative of CNRZ32 by gene replacement as described previously (2) with modifications described by Christensen and Steele (7). Transformation of CNRZ32 was performed at 37°C. Six transformants were chosen at random for spread plating on MRS medium plates containing 50 ng of erythromycin per ml at 44°C (nonpermissive temperature for pSA3). Integrants were grown in MRS broth at 37°C without erythromycin, and erythromycin-sensitive colonies were selected for further characterization.
Proteinase activity.
Cells were grown from frozen stocks in MRS broth to the logarithmic growth phase and then were transferred to citrate-milk medium that had been steamed for 30 min. Cells were grown to an optical density of 0.7 absorbance unit at 590 nm, harvested by centrifugation, washed twice with 50 mM Na2PO4 buffer (pH 6.8), and resuspended in Jennes-Koops buffer (16). Isolation of αs1-CN (f1-23) was performed as described by Exterkate and Alting (12). L. helveticus whole cells were incubated in Jennes-Koops buffer with the αs1-CN (f1-23) fragment at 30°C for 5 min, 15 min, 30 min, or 1 h at pH 6.5 (16). Samples from the reaction mixture were analyzed by high-performance liquid chromatography (HPLC) as described previously (4). Several hydrolysis products [αs1-CN (f1-6), αs1-CN (f18-23), αs1-CN (f9-23), and αs1-CN (f10-23)] were identified by mass spectrometry at the Nucleic Acid and Protein Facility of the University of Wisconsin—Madison Biotechnology Center. All other peptides were identified by use of standards as described previously (4).
Nucleotide sequence accession number.
The GenBank accession no. for the nucleotide sequence reported in this paper is AF133727.
RESULTS
Identification and sequencing of a cell envelope-associated serine proteinase gene (prtH) from L. helveticus CNRZ32.
PCR amplification was used to identify a CEP gene from L. helveticus CNRZ32. A single 700-bp PCR product was obtained and sequenced (data not shown). New primers were designed based on the sequence of this product, and inverse PCR was used to identify the adjacent DNA regions.
By sequencing 6.2 kb of contiguous DNA, an open reading frame that has significant DNA sequence similarity to known lactococcal prtP genes was identified. This L. helveticus cell envelope-associated proteinase is referred to as PrtH, and its corresponding gene is referred to as prtH. prtH encodes a putative protein of 1,849 amino acids and with a deduced molecular mass of 204 kDa (Fig. 1). The start of transcription, as determined by 5′ RACE, is 90 nucleotides upstream of the start codon. A putative ribosome-binding site (TGGAGG) is found at position −7 from the start codon. Downstream of prtH is a putative rho-independent transcriptional terminator (AAAGAGTAATGAGAAGATCATTACTCTTT) with a change in free energy of −14.4 kcal/mol (41).
Comparison with other cell surface proteinases.
A comparison of the N-terminal region of PrtH with those of other CEPs indicates that PrtH may be synthesized as a pre-pro-protein. The N-terminal segment of the deduced PrtH is positively charged and is followed by a putative membrane-spanning domain. This region closely resembles the signal peptide sequence for gram-positive bacteria (33, 38). The predicted cleavage site for the signal peptide is at Ala−152-Glu−151 (29). Adjacent to the signal peptide is a region that has 51% amino acid identity with the pro-region of the PrtPs. This finding suggests that PrtH will be processed similarly. Such processing would result in a mature PrtH of 1,673 amino acids and having 45% identity with the lactococcal PrtPs.
The N-terminal region of the mature proteinase (∼500 amino acids) is referred to as the catalytic domain. This region has similarity to the subtilisin-like serine proteinases (subtilases); therefore, PrtH can be classified within this family (34, 36). The subtilase family of proteins is characterized by a catalytic triad, Asp-His-Ser. The putative catalytic residues of PrtH are at positions Asp30, His94, and Ser432 (Fig. 1). The catalytic sites, as well as adjacent residues, are very well conserved among PrtH, PrtB, PrtP, and other members of the subtilase family (data not shown).
A number of residues in the lactococcal PrtPs have been implicated in substrate specificity by homology modeling, sequence alignments, and protein engineering studies (5, 35, 36, 43). The substrate binding residues are divergent among PrtH, PrtP, PrtB, subtilisin BPN′, and thermitase (Table 1). L. lactis subsp. cremoris SK11 PrtP residues 137 to 139, 166, and 748 have been demonstrated to effect substrate specificity (35). PrtH has a unique amino acid substitution at position 138 (Ser) compared to all other CEPs from LAB. Position 166 is occupied by Val in both PrtH and PrtB, while Asn and Asp are found at this position in PrtP from SK11 and Wg2, respectively. Thr occupies position 748 in PrtH, PrtP from Wg2, and PrtB. Because of the unique combination of amino acids at residues thought to be involved in substrate specificity, PrtH cannot be classified in any of the previously described groups of CEPs. Therefore, PrtH is classified as a new group, designated group I.
TABLE 1.
Sequence alignment of the substrate binding regions of PrtH and other members of the subtilase family
| Protein | Sequence of substrate binding site and adjacent residues
|
||||
|---|---|---|---|---|---|
| 137–139a | 166a | 156b | 217b | 747–748a | |
| PrtH | FSNSDSSASTDSTSII | SLGSVS | SAGNS | MSGTS | SLSATKTYYN |
| PrtP (SK11) | FSNSTDSAKTGSATVV | SLGSNS | SAGNS | MSGTS | STNRKKTYYN |
| PrtP (Wg2) | FTNSDTSATTGSSTLV | SLGSDS | SAGNS | MSGTS | STNLTKTYYN |
| PrtB | FSNNAKNSGAYDDDII | SLGSVS | SAGNS | MSGTS | GKEGTKDYYS |
| Thermitasec | LDN---SGSGTWTAVA | SLGGTV | AAGNA | LSGTS | |
| Subtilisin BPN′c | LGA---DGSGQYSWII | SLGGPS | AAGNE | YNGTS | |
| ↑ ↑↑↑↑↑ ↑ | ↑↑↑↑↑↑ | ↑ ↑↑↑ | ↑↑↑↑↑ | ||
Numbering corresponds to that of L. lactis subsp. cremoris SK11 proteinase.
Numbering corresponds to that of subtilisin BPN′.
Alignment is from reference 37, and arrows indicate subtilisin BPN′ and thermitase substrate binding residues.
The C-terminal region of PrtP has a conserved LPxTG motif, which is found in many cell surface proteins (28). The LPxTG motif functions as an anchor to the cell membrane. This motif is not found in PrtH, although PrtH is most likely located on the cell surface. A 101-amino-acid region at the C terminus of PrtH (amino acid residues 1538 to 1639 of the mature proteinase) has 32% identity with the C terminus of the surface-layer (S-layer) protein (amino acid residues 314 to 415) from Lactobacillus acidophilus (data not shown) (3).
Distribution of prtH within L. helveticus.
Southern blot analysis was used to determine the distribution of prtH among various strains of L. helveticus (CNRZ32, ATCC 15009, ATCC 10797, ATCC 12046, ATCC 8018, ATCC 15807, ATCC 10386, and L89). Under low-stringency conditions (10% formamide and 42°C), a prtH DNA probe hybridized only to a 4.1-kb DNA fragment from L. helveticus CNRZ32 and L89 (data not shown). Because the hybridization patterns were identical for CNRZ32 and L89, we compared the DNA sequences of the substrate binding regions for these two proteinases. The L89 substrate binding region was amplified by PCR with primers specific for the CNRZ32 prtH gene. Sequence analysis revealed that the L89 subtilase-like substrate binding region is 100% identical at the nucleotide level to prtH (data not shown). Although L. helveticus ATCC 15009, ATCC 10797, ATCC 12046, ATCC 8018, ATCC 15807, and ATCC 10386 have cell surface proteinase activity, as measured by the hydrolysis of αs1-CN (f1-23) (data not shown), Southern blot analysis indicates that they do not contain a prtH-like gene.
Physiological role of PrtH.
To determine the physiological role of PrtH, an in-frame deletion was constructed in prtH. A 1.7-kb DNA fragment internal to prtH was constructed to contain a deletion of 270 bp. This deletion removed the active-site residue, His94, and the subtilase-like substrate binding region. The 1.7-kb prtH deletion construct was subcloned into the plasmid vector pSA3 and used to create a prtH deletion mutant of CNRZ32 (data not shown). Growth in milk of CNRZ32 and the prtH deletion mutant was examined. No difference in acidification rate or maximum specific growth rate was observed between CNRZ32 and the prtH deletion mutant (data not shown).
Characterization of cell surface proteinase activity and specificity with αs1-CN (f1-23) as a substrate.
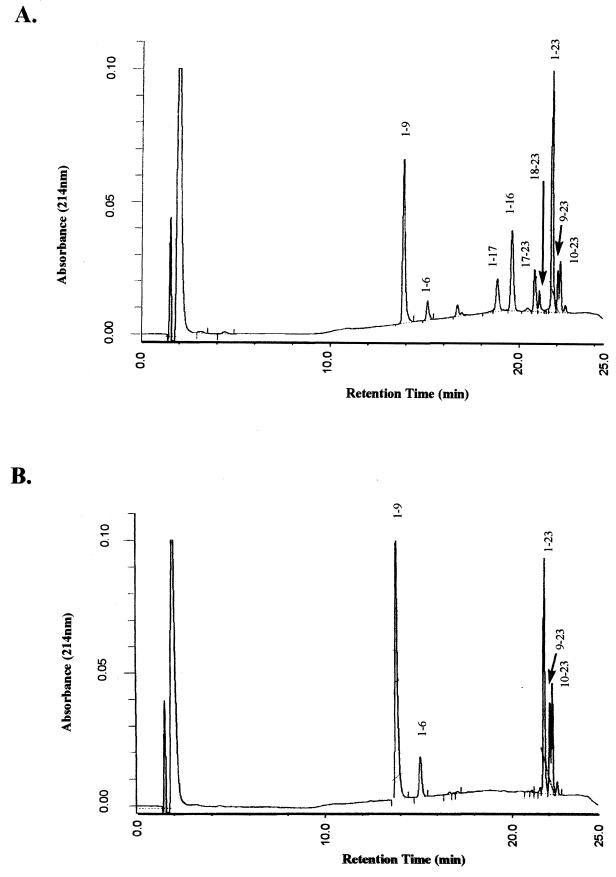
To determine the cell surface proteinase activity and specificity of CNRZ32 whole cells, αs1-CN (f1-23) was used as a substrate for hydrolysis. The hydrolysis products were analyzed by reverse-phase HPLC (Fig. 2A). Brief incubations (5 to 15 min) with CNRZ32 whole cells results in the formation of eight peptides: αs1-CN (f1-9), αs1-CN (f1-6), αs1-CN (f1-17), αs1-CN (f1-16), αs1-CN (f17-23), αs1-CN (f18-23), αs1-CN (f9-23), and αs1-CN (f10-23). This finding indicates that several bonds are preferentially hydrolyzed. Hydrolysis of the Leu16—Asn17 and Asn17—Glu18 bonds results in the formation of four peptides: αs1-CN (f1-16), αs1-CN (f17-23), αs1-CN (f1-17), and αs1-CN (f18-23). Hydrolysis of the Gln9—Gly10 bond results in the formation of two peptides: αs1-CN (f1-9) and αs1-CN (f10-23). Other bonds that appear to be hydrolyzed are the Ile6—Lys7 and His8—Gln9 bonds.
FIG. 2.
Reverse-phase HPLC patterns of the hydrolysis products from αs1-CN (f1-23) after 15 min of incubation with wild-type CNRZ32 whole cells (A) and CNRZ32 prtH deletion mutant whole cells (B).
The cell surface proteinase activity of the prtH deletion mutant was also analyzed (Fig. 2B). Incubation of αs1-CN (f1-23) with whole cells resulted in a pattern of hydrolysis different from that of the wild type. The αs1-CN (f1-9), αs1-CN (f1-6), αs1-CN (f9-23), and αs1-CN (f10-23) peptides are still formed at approximately the same rates. However, the αs1-CN (f1-16), αs1-CN (f17-23), αs1-CN (f1-17), and αs1-CN (f18-23) peptides are not detected.
DISCUSSION
A CEP that has 45% identity to the lactococcal PrtPs was identified in L. helveticus CNRZ32. The highest sequence identity (65%) is within the N-terminal catalytic domain. The substrate binding region of PrtH is distinct from those of all previously identified CEPs; thus, PrtH is classified as a new group, designated group I (Table 1).
Much is known concerning structure-function relationships in the subtilase family (30). An alignment of subtilisin BPN′, thermitase, and the CEPs from LAB reveal regions that are highly conserved (Table 1). Gln156 and Tyr217 have been shown to effect substrate specificity in subtilisins (45). All identified CEPs from LAB contain Ser and Met at the corresponding positions. Interestingly, amino acid substitutions at position 156 in the subtilisins can alter the pH profile by affecting the pKa of the active-site His (31). Comparisons such as this can lead to protein engineering strategies to change the substrate specificity and pH profile for CEPs.
These studies reveal significant differences in the proteolytic systems of CNRZ32 and lactococci. First, CNRZ32 appears to have at least two proteinases present at the cell surface. A prtH deletion mutant of CNRZ32 is indistinguishable from wild-type CNRZ32 in growth rate and acid production in milk. This finding is in contrast to the requirement of PrtP for rapid growth and fast acid production in lactococci. The most probable explanation is the presence in CNRZ32 of a second proteinase that is sufficient for rapid growth and fast acid production in milk. Recent studies support the hypothesis of at least two proteinases present at the cell surface of some lactobacilli (14, 40). In addition to a serine proteinase, L. delbrueckii subsp. bulgaricus ACA DC235 has a zinc-dependent cell surface proteinase (39).
Characterization of cell surface proteinase activity further supports the hypothesis that CNRZ32 has at least two cell surface proteinases. It appears that PrtH hydrolyzes the Leu16—Asn17 and Asn17—Glu18 bonds of αs1-CN (f1-23), resulting in the formation of peptides αs1-CN (f1-16), αs1-CN (f17-23), αs1-CN (f1-17), and αs1-CN (f18-23). These peptides are not detected from hydrolysis of αs1-CN (f1-23) in the prtH deletion mutant of CNRZ32. However, peptides αs1-CN (f1-9), αs1-CN (f10-23), αs1-CN (f1-6), and αs1-CN (f9-23) are detected in approximately equal quantities in both wild-type CNRZ32 and the prtH deletion mutant. Therefore, a second proteinase on the CNRZ32 cell surface is likely responsible for the formation of these peptides. These findings demonstrate that CNRZ32 has at least two cell surface proteinases that differ in substrate specificity.
Cell surface proteinase activity was detected in all L. helveticus strains tested (data not shown). However, Southern blot analysis indicates that prtH is not broadly distributed within the species. A prtH DNA probe hybridized to only L. helveticus CNRZ32 and L89. Sequence analysis of the L89 CEP substrate binding region revealed 100% identity at the nucleotide level to prtH. The L89 proteinase has been purified, and its substrate specificity has been characterized (26). Like PrtH, many CEPs, including the L89 CEP, are able to hydrolyze the Leu16—Asn17 and Asn17—Glu18 bonds (23). Although we expect PrtH and the L89 CEP to have identical substrate specificities, further comparisons are not possible because PrtH has not yet been purified and CNRZ32 has at least two cell surface proteinases. The proteinase activity detected in the other L. helveticus strains examined is most likely due to an unknown cell surface proteinase that does not have significant sequence similarity to PrtH.
Neither PrtH nor PrtB has the cell membrane anchor motif LPxTG, which has been found in many cell surface proteins, including the lactococcal PrtPs (28). However, both PrtH and PrtB have C-terminal regions similar to those of S-layer proteins from lactobacilli (3). The C-terminal region of PrtB (amino acid residues 1743 to 1938) has up to 25% identity to the C-terminal region of the S-layer protein from L. acidophilus (3). These results suggest that PrtH and PrtB are anchored to the cell envelope in a manner similar to that of S-layer proteins.
ACKNOWLEDGMENTS
This project was supported by the Center for Dairy Research through funding from the National Dairy Promotion and Research Board and the College of Agriculture and Life Science at the University of Wisconsin—Madison.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bhowmik T, Fernandez L, Steele J L. Gene replacement in Lactobacillus helveticus. J Bacteriol. 1993;175:6341–6344. doi: 10.1128/jb.175.19.6341-6344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boot H J, Kolen C P, van Noort J M, Pouwels P H. S-layer protein of Lactobacillus acidophilus ATCC 4356: purification, expression in Escherichia coli, and nucleotide sequence of the corresponding gene. J Bacteriol. 1993;175:6089–6096. doi: 10.1128/jb.175.19.6089-6096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broadbent J R, Strickland M, Weimer B C, Johnson M E, Steele J L. Peptide accumulation and bitterness in cheddar cheese using single-strain Lactococcus lactis starters with distinct proteinase specificities. J Dairy Sci. 1998;81:327–337. [Google Scholar]
- 5.Bruinenberg P G, Doesburg P, Alting A C, Exterkate F A, de Vos W M, Siezen R J. Evidence for a large dispensable segment in the subtilisin-like catalytic domain of the Lactococcus lactis cell-envelope proteinase. Protein Eng. 1994;7:991–996. doi: 10.1093/protein/7.8.991. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Steele J L. Genetic characterization and physiological role of endopeptidase O from Lactobacillus helveticus CNRZ32. Appl Environ Microbiol. 1998;64:3411–3415. doi: 10.1128/aem.64.9.3411-3415.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen, J. C., and J. L. Steele. Unpublished data.
- 8.de Vos W M, Vos P, de Haard H, Boerrigter I. Cloning and expression of the Lactococcus lactis subsp. cremoris SK11 gene encoding an extracellular serine proteinase. Gene. 1989;85:169–176. doi: 10.1016/0378-1119(89)90477-0. [DOI] [PubMed] [Google Scholar]
- 9.Dudley E G, Steele J L. Nucleotide sequence and distribution of the pepPN gene from Lactobacillus helveticus CNRZ32. FEMS Microbiol Lett. 1994;119:41–46. doi: 10.1111/j.1574-6968.1994.tb06864.x. [DOI] [PubMed] [Google Scholar]
- 10.Exterkate F A, Alting A C, Slangen C J. Specificity of two genetically related cell-envelope proteinases of Lactococcus lactis subsp. cremoris towards alpha s1-casein-(1-23)-fragment. Biochem J. 1991;273:135–139. doi: 10.1042/bj2730135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Exterkate F A, Alting A C, Bruinenberg P G. Diversity of cell envelope proteinase specificity among strains of Lactococcus lactis and its relationship to charge characteristics of the substrate-binding region. Appl Environ Microbiol. 1993;59:3640–3647. doi: 10.1128/aem.59.11.3640-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exterkate F A, Alting A C. The conversion of the αs1-casein-(1-23)-fragment by the free and bound forms of the cell envelope proteinase of Lactococcus lactis subsp. cremoris under conditions prevailing in cheese. Syst Appl Microbiol. 1993;16:1–8. [Google Scholar]
- 13.Gilbert C, Atlan D, Blanc B, Portailer R, Germond J E, Lapierre L, Mollet B. A new cell surface proteinase: sequencing and analysis of the prtB gene from Lactobacillus delbrueckii subsp. bulgaricus. J Bacteriol. 1996;178:3059–3065. doi: 10.1128/jb.178.11.3059-3065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert C, Blanc B, Frot-Coutez J, Portalier R, Atlan D. Comparison of cell surface proteinase activities within the Lactobacillus genus. J Dairy Res. 1997;64:561–571. [Google Scholar]
- 15.Holck A, Naes H. Cloning, sequencing and expression of the gene encoding the cell-envelope-associated proteinase from Lactobacillus paracasei subsp. paracasei NCDO 151. J Gen Microbiol. 1992;138:1353–1364. doi: 10.1099/00221287-138-7-1353. [DOI] [PubMed] [Google Scholar]
- 16.Jennes R, Koops J. Preparation and properties of a salt solution which simulates milk ultrafiltrate. Neth Milk Dairy J. 1962;16:153–164. [Google Scholar]
- 17.Juillard V, Laan H, Kunji E R, Jeronimus-Stratingh C M, Bruins A P, Konings W N. The extracellular PI-type proteinase of Lactococcus lactis hydrolyzes beta-casein into more than one hundred different oligopeptides. J Bacteriol. 1995;177:3472–3478. doi: 10.1128/jb.177.12.3472-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiwaki M, Ikemura H, Shimizu-Kadota M, Hirashima A. Molecular characterization of a cell wall-associated proteinase gene from Streptococcus lactis NCDO763. Mol Microbiol. 1989;3:359–369. doi: 10.1111/j.1365-2958.1989.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 19.Kojic M, Fira D, Bojovic B, Banina A, Topisirovic L. Comparative study on cell-envelope associated proteinases in natural isolates of mesophilic lactobacilli. J Appl Bacteriol. 1995;79:61–68. [Google Scholar]
- 20.Kok J. Genetics of proteolytic enzymes of lactococci and their role in cheese flavor development. J Dairy Sci. 1993;76:2056–2064. [Google Scholar]
- 21.Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990;87:15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 22.Kok J, Leenhouts K J, Haandrikman A J, Ledeboer A M, Venema G. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988;54:231–238. doi: 10.1128/aem.54.1.231-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunji E R, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 24.Law J, Vos P, Hayes F, Daly C, de Vos W M, Fitzgerald G. Cloning and partial sequencing of the proteinase gene complex from Lactococcus lactis subsp. lactis UC317. J Gen Microbiol. 1992;138:709–718. doi: 10.1099/00221287-138-4-709. [DOI] [PubMed] [Google Scholar]
- 25.Law J, Haandrikman A. Proteolytic enzymes of lactic acid bacteria. Int Dairy J. 1997;7:1–11. [Google Scholar]
- 26.Martin-Hernandez M C, Alting A C, Exterkate F A. Purification and characterization of the mature, membrane-associated cell-envelope proteinase of Lactobacillus helveticus L89. Appl Microbiol Biotechnol. 1994;40:828–834. [Google Scholar]
- 27.Mierau I, Kunji E R, Venema G, Kok J. Casein and peptide degradation in lactic acid bacteria. Biotechnol Genet Eng Rev. 1997;14:279–301. doi: 10.1080/02648725.1997.10647945. [DOI] [PubMed] [Google Scholar]
- 28.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Perona J J, Craik C S. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell A J, Fersht A R. Rational modification of enzyme catalysis by engineering surface charge. Nature. 1987;328:496–500. doi: 10.1038/328496a0. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Schneewind O, Mihaylova-Petkov D, Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siezen R J, Leunissen J A. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6:501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siezen R J, Bruinenberg P G, Vos P, van Alen-Boerrigter I, Nijhuis M, Alting A C, Exterkate F A, de Vos W M. Engineering of the substrate-binding region of the subtilisin-like, cell-envelope proteinase of Lactococcus lactis. Protein Eng. 1993;6:927–937. doi: 10.1093/protein/6.8.927. [DOI] [PubMed] [Google Scholar]
- 36.Siezen R J, de Vos W M, Leunissen J A, Dijkstra B W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991;4:719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- 37.Siezen, R. J. 1999. Personal communication.
- 38.Simonen M, Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993;57:109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefanitsi D, Garel J R. A zinc-dependent proteinase from the cell wall of Lactobacillus delbrueckii subsp. bulgaricus. Lett Appl Microbiol. 1997;24:180–184. doi: 10.1046/j.1472-765x.1997.00376.x. [DOI] [PubMed] [Google Scholar]
- 40.Stefanitsi D, Sakellaris G, Garel J R. The presence of two proteinases associated with the cell wall of Lactobacillus bulgaricus. FEMS Microbiol Lett. 1995;128:53–58. [Google Scholar]
- 41.Tinococ I J, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 42.Tynkkynen S, Buist G, Kunji E, Kok J, Poolman B, Venema G, Haandrikman A. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol. 1993;175:7523–7532. doi: 10.1128/jb.175.23.7523-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vos P, Boerrigter I J, Buist G, Haandrikman A J, Nijhuis M, de Reuver M B, Siezen R J, Venema G, de Vos W M, Kok J. Engineering of the Lactococcus lactis serine proteinase by construction of hybrid enzymes. Protein Eng. 1991;4:479–484. doi: 10.1093/protein/4.4.479. [DOI] [PubMed] [Google Scholar]
- 44.Vos P, Simons G, Siezen R J, de Vos W M. Primary structure and organization of the gene for a procaryotic, cell envelope-located serine proteinase. J Biol Chem. 1989;264:13579–13585. [PubMed] [Google Scholar]
- 45.Wells J A, Estell D A. Subtilisin—an enzyme designed to be engineered. Trends Biochem Sci. 1988;13:291–297. doi: 10.1016/0968-0004(88)90121-1. [DOI] [PubMed] [Google Scholar]
- 46.Xu F F, Pearce L E, Yu P L. Molecular cloning and expression of a proteinase gene from Lactococcus lactis subsp. cremoris H2 and construction of a new lactococcal vector pFX1. Arch Microbiol. 1990;154:99–104. doi: 10.1007/BF00249185. [DOI] [PubMed] [Google Scholar]