Abstract
Objectives/Hypothesis:
One potential treatment for vocal fold injury or neoplasia is to replace the entire vocal fold with a tissue-engineered scaffold. This scaffold should ideally have similar mechanical properties and extracellular matrix composition as the native vocal fold. As one approach toward this goal, we decellularized human vocal folds and characterized their mechanical properties and extracellular matrix microstructure.
Study Design:
Basic science investigation.
Methods:
Human vocal folds were dissected from the laryngeal framework and treated with sodium dodecyl sulfate (SDS) to remove all cells. Mechanical properties were measured by indentation before and after SDS treatment. The extracellular matrix components of collagen, laminin, elastin, and hyaluronic acid were also characterized before and after decellularization using histology and immunofluorescence.
Results:
After 4 days of SDS treatment, we obtained a scaffold that retained the original geometry of the vocal fold but was devoid of cells. The elastic modulus of the vocal folds did not change significantly before and after decellularization. Upon qualitative inspection, the decellularized vocal folds retained the original collagen, elastin, and laminin content and orientation but lost the original hyaluronic acid.
Conclusions:
Vocal folds can be decellularized using SDS without adversely affecting its mechanical stiffness and fibrous extracellular matrix. This preliminary study demonstrates the potential of a decellularized scaffold to serve as a tissue-engineered construct for vocal fold replacement.
Level of Evidence:
NA
Keywords: Vocal folds, Young’s modulus, scaffolds, tissue engineering, decellularized
INTRODUCTION
Dysphonia due to vocal fold scarring is one of the most difficult voice disorders to treat. The scar tissue forms after injury, neoplasm, or inflammation and alters the extracellular matrix (ECM) composition to increase the elastic modulus, or stiffness, of the vocal fold.1–3 ECM changes include increased total procollagen, collagen, and fibronectin; decreased elastin and hyaluronic acid; and decreased fiber organization.1,4–6 The increased stiffness impairs phonation by raising the phonation threshold pressure (i.e., the subglottic pressure needed to initiate self-sustained vocal fold oscillation).7,8 In addition to determining tissue stiffness, proper ECM composition and organization maintain optimal tissue vibration and cell homeostasis. For example, elastin provides the bulk of the vocal fold’s elastic recoil properties, whereas collagen transmits tension from the muscle to the lamina propria and protects elastin from overextension.9–12 Hyaluronic acid, on the other hand, maintains adequate tissue hydration and modulates the wound-healing response.6,8,13 As a result, many investigational therapies to treat vocal fold scarring have focused on restoring the vocal fold’s ECM composition and mechanical properties.
Currently, the most common treatments for vocal fold scarring are voice therapy and medialization laryngoplasty. However, the success of these materials is limited and inconsistent among patients. Neither voice therapy nor medialization restores the vocal fold’s original ECM composition over the long term, and thus does not adequately treat the dysphonia of vocal fold scar.2,3,14
More radical approaches, such as a tissue-engineered vocal fold, may successfully rehabilitate the voice by completely replacing the scarred tissue. In particular, engineered scaffolds created from decellularized tissues have been increasingly promising. Decellularized scaffolds can preserve the native tissue’s microarchitecture and ECM composition, which are important physical cues utilized by seeded cells for infiltration, growth, and differentiation.15 These scaffolds can also preserve native mechanical properties such as elastic modulus or viscosity, which are critical for a tissue undergoing repeated cycles of strain. Finally, decellularized scaffolds have high potential among other tissue-engineered therapies for translation to the patient. Decellularization techniques are relatively simple and produce scaffolds that retain the original geometry of the tissue, allowing for reimplantation. Moreover, if the scaffolds are derived from allogeneic sources, there would be little concern about biocompatibility.15 Several decellularized scaffolds are already mainstream treatments for skin and heart valve replacement, whereas more complex tissues, such as veins and tracheas, have shown preliminary success in individual human case studies.16,17
In the present study, we decellularized human vocal folds and assessed key features of the resulting cell-free ECM: matrix composition and organization, epithelial basement membrane preservation, and overall tissue integrity as measured by elastic modulus. Our goal was to produce an acellular scaffold with similar properties to the native vocal fold cover layer that may serve as a cell substrate for future implantation.
MATERIALS AND METHODS
Human Vocal Folds Collection and Dissection
Five adult larynges were harvested from autopsy within 48 hours of death and were kept frozen at −80°C until use. Ages ranged from 56 to 85 years old. All causes of death were unrelated to any laryngeal pathology. The samples were thawed at 4°C overnight prior to dissection. Both the left and right true vocal folds, consisting of thyroarytenoid muscle with overlying cover layer, were isolated from the surrounding cartilage by sharp scissor dissection. For each larynx, one vocal fold was randomly selected as a control and was fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS). The other vocal fold was used immediately for mechanical characterization and subsequent decellularization.
Mechanical Characterization of Vocal Folds
The elastic modulus E of the vocal folds was measured before and after decellularization by indentation techniques previously described.18 A 1-mm cylindrical indenter tip was positioned to contact the epithelial surface in the center of the excised vocal fold, corresponding to approximately the middle one-third or “striking zone.” The tissues were maintained at 37°C using a temperature-controlled base, and were kept hydrated by periodically adding drops of PBS. Indentation proceeded in 25-micron steps until reading a maximum depth of 0.6 mm; unloading also used 25-micron steps. Force was recorded at each step. Each tissue underwent five loading-unloading cycles. The elastic modulus was calculated from the average of the five initial unloading slopes of the force-distance curves using the Hertzian model for a cylindrical contact, which is given as:
| 1 |
where is the Poisson’s ratio (assumed to be 0.47 for vocal fold tissues, per previous described methods18), is the indenter radius (0.5 mm), and is the slope of the force-distance curve at the point of initial unloading.
For statistical analysis, a paired test was used to compare the vocal folds’ elastic modulus before and after decellularization.
Decellularization of Vocal Folds
After indentation, the vocal folds were immersed in a solution of 0.1% sodium dodecyl sulfate (SDS; Sigma-Aldrich, St. Louis, MO) in and then placed onto a rocker under constant agitation at room temperature for 96 hours. The SDS was exchanged everyday with fresh solutions. After 96 hours of SDS treatment, the decellularized vocal folds were removed from the solution and rinsed under for 15 minutes. The decellularized vocal folds were indented again to measure the elastic modulus, and were then fixed with 3.7% formaldehyde in PBS.
Histology and Immunofluorescence
To prepare the samples for histology and immunofluorescence (IF) analysis, both the control and decellularized vocal folds were fixed with 3.7% formaldehyde in PBS for 24 to 48 hours under constant agitation. The vocal folds were then dehydrated by immersing them in solutions of ethanol of increasing concentrations under constant agitation for at least 1 hour each: 35%, 50%, 70%, 80%, 95%, and 100% ethanol solutions. The ethanol was cleared from the tissue samples by immersing them in xylene for 30 minutes. Then, the tissues were placed into a plastic cassette with paraffin wax warmed to 50°C. The paraffin wax was exchanged twice to remove as much xylene as possible and then allowed to solidify at room temperature prior to sectioning. Sections were cut at a thickness between 5 and 10 μm with a microtome, and the vocal folds were cut at the coronal plane to expose all three vocal fold layers—the epithelium, lamina propria, and thyroarytenoid muscle.
Standard histological staining was performed with solutions for Masson’s trichrome, hematoxylin and eosin (H&E), Alcian Blue pH 2.5, and Verhoeff’s van Gieson. For IF staining, slides were first rehydrated by immersing the samples for 5 minutes each under the following solutions: 100% xylene, 100% ethanol, 70% ethanol, and . Slides were blocked with 2% goat serum in PBS for 1 hour and then stained with monoclonal anti-laminin immunoglobulin G antibody (DSHB, Iowa City, IA) at a 1:20 dilution for 72 hours at 4°C. After rinsing with PBS, rhodamine-conjugated goat anti-mouse secondary antibody (Thermo Fisher Scientific Inc., Waltham, MA) was applied for 1 hour at room temperature. Sections were rinsed again, and coverslips were then mounted with VectaShield Mounting Media with 4’,6-diamidino-2-phenylindole (Vector Laboratories Inc., Burlingame, CA).
RESULTS
Decellularization of the Human Vocal Folds
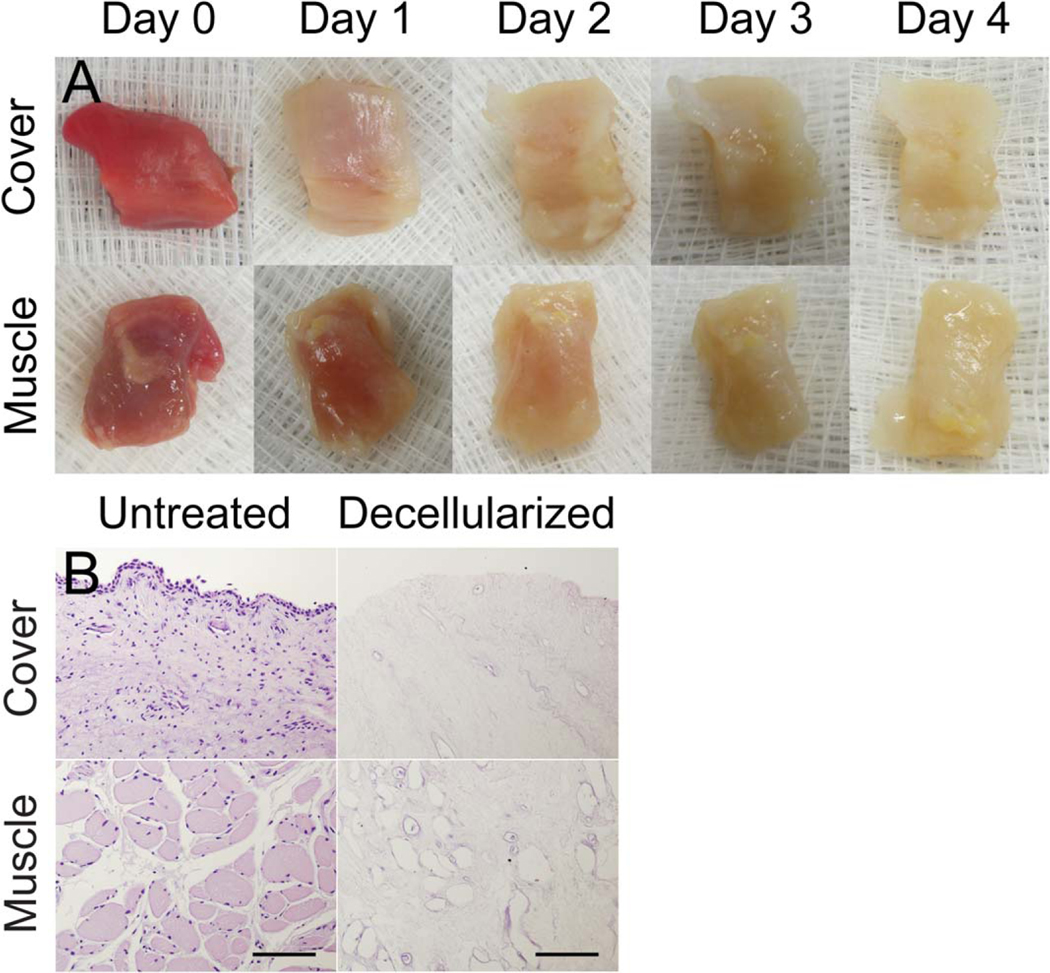
After 4 days of SDS treatment, we obtained a translucent scaffold that preserved the original geometry and dimensions of the vocal fold (Fig. 1A). The scaffold was sectioned and stained with H&E. For each decellularized scaffold, a section was obtained in the middle of the vocal fold to ensure decellularization throughout the entire tissue. H&E staining revealed the remaining intact connective tissue and an absence of cells throughout all three layers of the vocal fold compared to the control side (Fig. 1B). A total of five vocal folds were decellularized, and the subject information for these larynges can be found in Figure 2.
Fig. 1.
(A) Panel of gross specimens showing day-by-day progress in decellularization with both the mucosa and thyroarytenoid muscle layers oriented to camera. (B) Hematoxylin and eosin staining of coronal sections of the vocal folds before and after decellularization. Rows labeled “Cover” show the epithelium and underlying lamina propria, whereas rows labeled “Muscle” show the thyroarytenoid muscle layers.
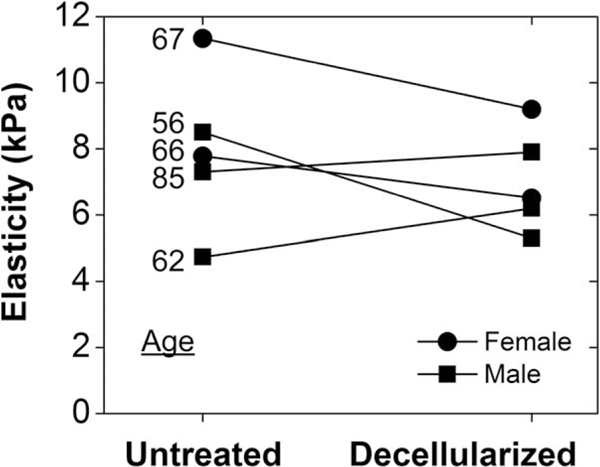
Fig. 2.
Elasticity (kPa) for each of the five subjects was measured before and after decellularization. Subjects’ ages ranged from 56 to 85 years old, and elasticity changed from 7.9 ± 2.4 kPa to 7.0 ± 2.3 kPa (P =.54 using paired t test).
Mechanical Characterization
The vocal folds were indented before and after decellularization to determine how SDS treatment affected their elastic moduli. Prior to decellularization, the vocal folds had an average elastic modulus of 7.9 ± 2.4kPa (Fig. 2). This elastic modulus is within the range of previously published values using indentation techniques.18,19 After decellularization, the vocal folds had an average elastic modulus of 7.0 ± 2.3kPa. A paired test was performed to compare the elastic moduli before and after treatment, and a value of 0.54 was obtained. The statistical power to detect a significant difference was calculated to be >80%.
Characterization of Fibrous Proteins
To qualitatively assess collagen and elastin content, the vocal folds were stained with Masson’s trichrome and Verhoeff’s van Gieson solutions, respectively, with and without decellularization. In both decellularized and control vocal folds, the (blue) collagen bundles were organized longitudinally along the length of the vocal fold, distributed throughout the lamina propria, and lightly interspersed with (red) elastin fibers (Fig. 3, left). Although Masson’s trichrome typically stains keratin red, it can also be used to highlight elastin fibers the same color.20 The basement membrane collagen was also preserved during the treatment process and can be seen as a denser, blue outline along the epithelial border in decellularized tissues. Verhoeff’s van Gieson staining demonstrated mature elastin fibers distributed throughout the lamina propria and were most abundant in the intermediate layer of the lamina propria, consistent with previous observations of untreated vocal folds (Fig. 3, right). Like collagen, the elastin fibers were oriented longitudinally along the length of the vocal fold, and the gross elastin content did not appear to change with the treatment process. These observations were consistent among the five subjects analyzed.
Fig. 3.
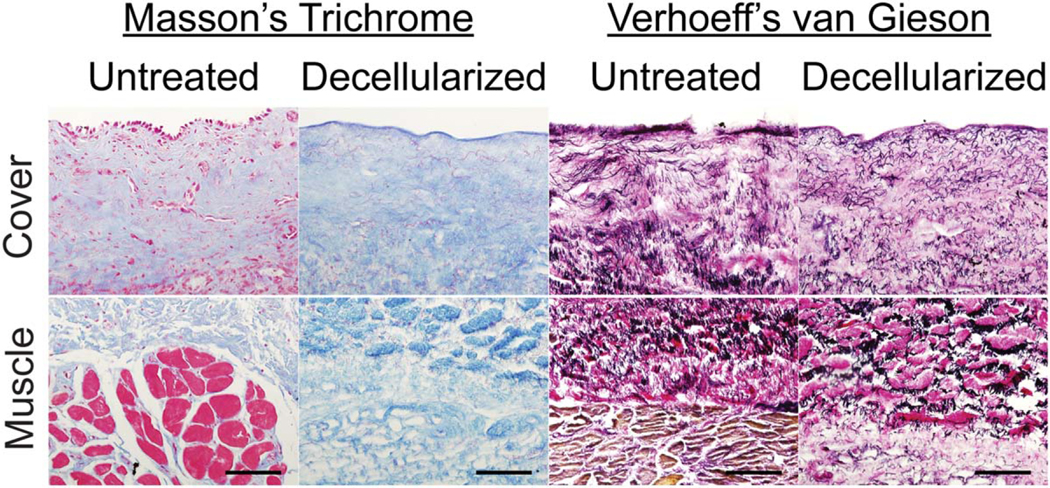
(Left) Masson’s trichrome staining of coronal vocal fold sections before and after decellularization. Collagen is stained blue, and keratin, elastin, and cytoplasm are stained red. Scale bar = 100 μm. (Right) Verhoeff’s van Gieson staining of coronal sections of the vocal folds before and after decellularization. Elastin and cell nuclei are stained black, cytoplasm is stained yellow, and collagen is stained red. Scale bar = 100 μm.
Characterization of Hyaluronic Acid and Laminin
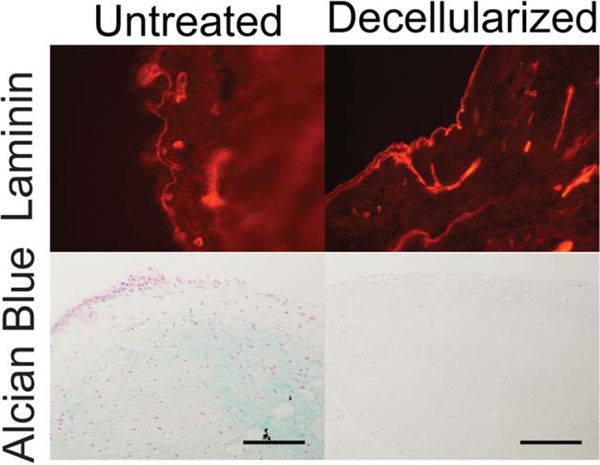
The decellularized vocal folds were also assessed for their retention of hyaluronic acid and laminin via Alcian Blue pH 2.5 and immunofluorescence, respectively. Alcian Blue pH 2.5 staining of control vocal folds showed diffuse, light-blue stain throughout the lamina propria (Fig. 4). However, the Alcian Blue pH 2.5 stain was negative in the decellularized vocal folds, which indicates the removal of mucins, hyaluronic acid, and other acidic mucopolysaccharides. On the other hand, laminin, a basement membrane protein, was preserved throughout the treatment process and can be seen as a highlighted red outline along the epithelial border. These observations were consistent among the five subjects analyzed.
Fig. 4.
Laminin staining (red, top) of vocal fold basement membranes before and after decellularization. Alcian Blue pH 2.5 staining of the vocal fold lamina propria (blue, bottom) shows absent hyaluronic acid after decellularization. Scale bar = 100 μm.
DISCUSSION
Vocal fold scarring is difficult to treat because the ECM changes during remodeling are largely irreversible. As in most adult wounds, severe vocal fold injury results in a permanent loss of elastin and hyaluronic acid, with a concomitant overproduction of procollagen, collagen, and fibronectin.1,4,5 These changes increase the tissue stiffness and decrease the fiber organization, impairing phonation. Thus, any attempt to replace the scarred vocal fold should recapitulate healthy tissue stiffness and fiber organization. Decellularized tissues have recently been sought as a potential vocal fold replacement to meet those goals. Potential sources of vocal folds for decellularization and implantation include numerous animal species (bovine, porcine, ovine) and cadaveric humans. There is precedent for decellularized cadaveric human tissue as a commercially available tissue implant (namely Alloderm, an acellular human dermis manufactured by LifeCell Corp., Branchburg, NJ).
In one of the first investigations of vocal fold decellularization, Xu et al. used hypertonic salt solution to decellularize bovine vocal fold lamina propria. Vocal fold fibroblasts repopulated only the surface of the scaffold but did synthesize ECM proteins, and the resulting rheologic properties of a single tested sample were similar to the untreated human vocal fold cover.21 More recently, proteome analysis of a salt-decellularized human vocal fold cover layer identified numerous bioactive proteins, including persistent antigenic cellular proteins that could induce host reaction after allotransplantation.22
This study more thoroughly investigates the microstructure and mechanical properties of human vocal folds after a detergent-based decellularization process. In our study, we did not observe any changes in collagen or elastin microstructure after decellularization (i.e., the fibers continued to run parallel and longitudinally along the length of the vocal fold with no gross content change). Hyaluronic acid was undetectable after this ionic detergent decellularization, as expected based on its chemical solubility in SDS. However, because its organization within the lamina propria is gelatinous, rather than the cross-linked fibers of collagen or elastin, hyaluronic acid could potentially be restored during cell seeding or after implantation. This question will be addressed in future cell-seeding studies. Basement membrane laminin was preserved, and we hypothesize that this may facilitate cell attachment and homeostasis in future cell-culture–based studies. Given the importance of the epithelium in phonation and the role of the basement membrane in epithelial homeostasis,23,24 an intact basement membrane may be necessary for proper phonation. As a result, both the decellularized epithelium remnant (i.e., basement membrane) and lamina propria ECM would be used for future implant studies.
In concordance with our histologic findings of preserved collagen and elastin architecture, the elastic modulus on indentation was unchanged by this decellularization process. If substantial disruption of the load-bearing ECM network had occurred, tissue stiffness would decrease. The quantitatively similar elastic modulus to native tissue also supports the applicability of this decellularized ECM for vocal fold implantation. Actual tissue stiffness in vivo is expected to differ from these cadaveric measurements due to muscle activation and tissue attachment constraints, but those differences would apply to both the native vocal fold and the processed cell-free scaffold. Other more complex measures of vibratory function, such as ex vivo vibration or highfrequency straining, may be utilized in future studies after cell seeding. Because it is not expected that a cell-free and hyaluronan-free ECM would have identical vibratory capabilities as an intact human vocal fold, those methods were not used for this study.
For small strains (<10%), soft tissues exhibit a linear stress-strain response, though at larger strains, the tissues become hyperelastic, with stiffness increasing with strain.25 Our indentation depth of 0.6 mm was below the generally accepted threshold of 10% of the average tissue thickness of ~1 cm. We confirmed that our stress-strain curves were linear (see Supporting Information, Figure S1, in the online version of this article).
Previous animal models highlight the difficulties of translating an acellular scaffold into a functioning vocal fold. In a canine model, acellular liver ECM was unable to restore the native histologic appearance of the lamina propria 3 months after vocal fold implant surgery.26 In a rat model, acellular bovine lamina propria both with and without hepatocyte growth factor were implanted into wounded vocal folds. After 3 months, both types of acellular scaffolds were completely degraded.27,28 One possible explanation for these disappointing results may be limited cell migration. Long-term implant stability will require infiltration by host cells, either via in vitro seeding before implant or via in vivo cell migration into the scaffold. More recent efforts in other organ systems, such as the trachea or portal veins, indicate that seeding autologous cells before implantation improves outcomes.16,17 Thus, our next step will be introducing cells into the scaffold in preparation for animal implantation experiments.
CONCLUSION
This detergent-based decellularization method for human vocal folds retains native fibrous ECM microstructure and mechanical stiffness. The resulting scaffold meets ideal characteristics for a potential vocal fold replacement and warrants future studies with cell infiltration and animal implantation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Melody Xuan, Zhaoyan Zhang, and Ivan Lopez for their technical support. The laminin B2 monoclonal antibody developed by J. R. Sanes was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biology, Iowa City, Iowa.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Contributor Information
Justin R. Tse, David Geffen School of Medicine, University of California–Los Angeles, Los Angeles, California.
Jennifer L. Long, Department of Head and Neck Surgery, University of California David Geffen School of Medicine, University of California–Los Angeles; VA Greater Los Angeles Medical Center, Los Angeles, California, U.S.A..
BIBLIOGRAPHY
- 1.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic andrheologic characterization of vocal fold scarring. J Voice 2002;16:96–104. [DOI] [PubMed] [Google Scholar]
- 2.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg 2005;13:143–147. [DOI] [PubMed] [Google Scholar]
- 3.Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg 1996;115:474–482. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau B, Hirano S, Scheidt TD, et al. Characterization of vocal foldscarring in a canine model. Laryngoscope 2003;113:620–627. [DOI] [PubMed] [Google Scholar]
- 5.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocalfold scarring in a rabbit model. J Voice 2004;18:116–124. [DOI] [PubMed] [Google Scholar]
- 6.Thibeault SL, Rousseau B, Welham NV, Hirano S, Bless DM. Hyaluronanlevels in acute vocal fold scar. Laryngoscope 2004;114:760–764. [DOI] [PubMed] [Google Scholar]
- 7.Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am 1988;83:1536–1552. [DOI] [PubMed] [Google Scholar]
- 8.Gray SD, Titze IR, Chan R, Hammond TH. Vocal fold proteoglycans andtheir influence on biomechanics. Laryngoscope 1999;109:845–854. [DOI] [PubMed] [Google Scholar]
- 9.Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparativestudies of the vocal fold extracellular matrix II: collagen. Ann Otol Rhinol Laryngol 2006;115:225–232. [DOI] [PubMed] [Google Scholar]
- 10.Moore J, Thibeault S. Insights into the role of elastin in vocal fold healthand disease. J Voice 2012;26:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitativeand comparative studies of the vocal fold extracellular matrix. I: Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol 2006;115:156–164. [DOI] [PubMed] [Google Scholar]
- 12.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elasticproteins: biological roles and mechanical properties. Philos Trans R Soc Lond B Biol Sci 2002;357:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan RW, Tayama N. Biomechanical effects of hydration in vocal fold tissues. Otolaryngol Head Neck Surg 2002;126:528–537. [DOI] [PubMed] [Google Scholar]
- 14.Welham NV, Choi SH, Dailey SH, Ford CN, Jiang JJ, Bless DM. Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. Laryngoscope 2011;121:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006;27:3675–3683. [DOI] [PubMed] [Google Scholar]
- 16.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of atissue-engineered airway. Lancet 2008;372:2023–2030. [DOI] [PubMed] [Google Scholar]
- 17.Olausson M, Patil PB, Kuna VK, et al. Transplantation of an allogeneicvein bioengineered with autologous stem cells: a proof-of-concept study. Lancet 2012;380:230–237. [DOI] [PubMed] [Google Scholar]
- 18.Chhetri DK, Zhang Z, Neubauer J. Measurement of Young’s modulus of vocal folds by indentation. J Voice 2011;25:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodyer E, Muller F, Bramer B, Chauhan D, Hess M. In vivo measurement of the elastic properties of the human vocal fold. Eur Arch Otorhinolaryngol 2006;263:455–462. [DOI] [PubMed] [Google Scholar]
- 20.Simionescu DT, Lu Q, Song Y, et al. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials 2006;27:702–713. [DOI] [PubMed] [Google Scholar]
- 21.Xu CC, Chan RW, Tirunagari N. A biodegradable, acellular xenogeneicscaffold for regeneration of the vocal fold lamina propria. Tissue Eng 2007;13:551–566. [DOI] [PubMed] [Google Scholar]
- 22.WelhaV, ChanZ, Smith LM, Frey BL. Proteomic analysis of a decellularized human vocal fold mucosa scaffold using 2D electrophoresis and high-resolution mass spectrometry. Biomaterials 2013;34:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xuan Y, Zhang Z. Influence of embedded fibers and an epithelium layer onglottal closure pattern in a physical vocal fold model [published online ahead of print October 28, 2013]. J Speech Lang Hear Res. doi: 10.1044/2013_JSLHR-S-13-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope 2010;120:125–131. [DOI] [PubMed] [Google Scholar]
- 25.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. NewYork, NY: Springer-Verlag; 1993. [Google Scholar]
- 26.Gilbert TW, Agrawal V, Gilbert MR, Povirk KM, Badylak SF, Rosen CA. Liver-derived extracellular matrix as a biologic scaffold for acute vocal fold repair in a canine model. Laryngoscope 2009;119:1856–1863. [DOI] [PubMed] [Google Scholar]
- 27.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction. J Biomed Mater Res A 2010;93:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. A bovine acellular scaffold for vocal fold reconstruction in a rat model. J Biomed Mater Res A 2010;92:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.