Abstract
Pulmonary arteriovenous malformations are abnormal connections between a pulmonary artery and a pulmonary vein that can lead to ischemic stroke and brain abscess due to right-to-left shunting of blood. Embolization is currently considered the first treatment option for pulmonary arteriovenous malformations owing to its minimal invasiveness. This review updates the indications and techniques for the embolization of pulmonary arteriovenous malformations and determines the persistence of pulmonary arteriovenous malformations following embolization based on the most recent literature.
Keywords: pulmonary arteriovenous malformation, embolization, persistence
Clinical Features of Pulmonary Arteriovenous Malformations (PAVMs)
Pulmonary arteriovenous malformations (PAVMs) are abnormal connections between a pulmonary artery and a pulmonary vein and usually bypass the capillary bed to induce right-to-left shunting of blood. This can lead to symptoms depending on the degree of blood shunting [1-3]. PAVMs are often asymptomatic, but they can also be associated with various clinical symptoms. When left untreated, PAVMs can result in serious complications. The risk of symptoms is considered related to the correlation between the size of the PAVM and the degree of right-to-left shunting, which can limit oxygenation and natural filtration in the lung. Consequently, patients may present with varying degrees of dyspnea, cyanosis, clubbing, or chest pain [2, 3]. Furthermore, migraines are recognized as a common neurologic symptom of PAVMs [1]. More serious recognized complications include brain abscess, paradoxical embolism resulting in stroke or transient ischemic attack, and, less frequently, hemoptysis or intrapulmonary hemorrhage [4, 5]. While uncommon, massive hemoptysis or hemothorax has also been reported [6]. In patients with PAVM, pregnancy may be a risk factor for hemoptysis; three of the seven women described in the literature as having massive hemoptysis were pregnant [6]. Pregnancy can expand PAVMs due to increased cardiac output, blood volume, and hormone-related changes in the vasculature.
Most PAVMs are congenital, and a tight association (47%-90%) with hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, has been described in the literature [7-12]. HHT is an autosomal dominant disorder associated with the mutation of one of the following proteins: endoglin (HHT1 subtype) or activin A receptor type II-like 1 (HHT2 subtype) [13, 14]. Among the recognized subtypes of HHT, HHT1 is most strongly associated with PAVMs [15]. In a small percentage, a mutation in SMAD4 is also observed [16].
Prior to 1977, surgery was the only method for pulmonary arteriovenous malformation treatment, including ligation, excision, lobectomy, segmentectomy, and pneumonectomy [17]. Video-assisted thoracic surgery has been reported for PAVMs [18] and involves a short procedural time with neither irradiation nor the requirement for life-long follow-up. Meanwhile, embolization is currently considered the standard of care for PAVM treatment owing to its minimal invasiveness [19].
Indication of Embolization for PAVMs
Embolization should be performed for PAVMs in patients that exhibit symptoms such as ischemic stroke. The indications for embolization of asymptomatic PAVMs have been a contentious issue since the description of the so-called “3-mm guideline.” In 1992, Rosenblatt et al. [20] presented an abstract that described 17 patients each with a single dominant PAVM. Eight of these patients had evidence of stroke on brain magnetic resonance imaging, and four had clinically evident stroke and a feeding artery measuring from 2.9 to 4.5 mm. The 3-mm guideline was established based on these cases and has subsequently been cited in numerous articles. Dramatic technological advances since the establishment of the 3-mm guideline, including refinements in digital subtraction angiography (DSA), catheter systems, microcatheters, and coils, have made it possible to treat PAVMs with feeder vessels ≤ 1.5 mm [21]. Furthermore, reports of symptomatic paradoxical embolization in patients with <3-mm feeding arteries have been published, and paradoxical embolization has been found to occur independent of the feeding artery diameter [22, 23]. Consequently, the potential need to treat PAVMs in the sub-3-mm feeder range was acknowledged by the originators of the 3-mm guideline in 2006 [24]. Furthermore, the 2009 HHT treatment guidelines recognized that it is appropriate to treat PAVM with feeders <3 mm [25]. Because a 3-Fr microcatheter has a diameter of 1 mm, it is theoretically possible to treat PAVMs with a feeding artery of 1 mm [26]. Although embolization is technically possible, even for small PAVMs, catheterization through a small and tortuous feeding artery is challenging and includes the risk of vessel injury during catheter and microguidewire manipulation. The persistence rate following embolization is reportedly high for small PAVMs [27]; therefore, the indication of embolization for small PAVMs should be carefully considered.
Embolic Materials
The choice of embolic material is an important issue. Coils and plugs are the most common embolic materials for PAVM embolization. Gelatin sponge, particles, and liquid embolic materials are not used for the embolization of PAVMs due to the high risk of migration to the pulmonary vein.
Coils are permanent embolic agents that cause vessel occlusion by inducing thrombosis. They can be divided into pushable and detachable coils. The advantages of pushable coils include their ready availability, reasonable cost, and ease of use. However, one disadvantage of pushable coils is that it is not possible to reposition them once they have been deployed, and coil migration to the pulmonary vein is thus possible in PAVM embolization. Detachable coils are deployed through a variety of mechanisms, including mechanically, via electric heating, electrolysis, and hydrostatic means. They offer the advantage of being repositionable before a successful deployment of the coils. However, their disadvantages include high cost and a long time requirement for setup. Furthermore, in the use of detachable coils, continuous saline flushing of the microcatheter lumen is necessary to prevent thrombus formation. Pushable coils can be safely deployed via an anchor or scaffold technique using detachable coils. In the anchor technique, the coil tip is purposely anchored in a small side branch proximal to the fistulous point of the PAVM, and the body of the coil then prolapses into the feeding artery. This securing of the tip in a side branch minimizes the risk of inadvertent coil dislodgment [28]. In the scaffold technique, the first positioned coil creates a scaffold that blocks other devices. The optimal occlusion of the feeding artery can thus be achieved by packing devices with decreasing diameters [29].
Various coil types have been developed, including bare platinum coils, fibered coils, and hydrogel-coated coils. Bare platinum coils can only partially embolize vessels via pure mechanical occlusion. Thus, although all coil embolization depends on the ability of the patient to form a thrombus or any coagulopathic status, bare platinum coils in particular depend on this mechanism more. However, if it was possible to tightly embolize the vessel, there would be less thrombosis formation. Fibered coils have been shown to induce thrombosis more effectively than bare coils [30], but it means that they are more dependent on thrombus formation to occlude the vessel, and it may be a risk of recanalization following PAVM embolization. Hydrogel-coated coils have a layer of hydrogel polymer that surrounds the platinum metal core and can fully expand within 20 min of contact with blood [31]. Hydrogel-coated 0.018-in. coils have a nearly fivefold higher filling volume than platinum coils of the same size. They thus provide a greater volume of occlusion than regular coils and are not dependent on thrombus formation [32]. Hydrogel-coated coils have been suggested to be useful for preventing recanalization following PAVM embolization because they can tightly embolize the malformations with less thrombus formation [33]. However, hydrogel-coated coils have some disadvantages, such as the rigidity of the coil, limitation of repositioning time, and need for a relatively large microcatheter.
Amplatzer Vascular Plugs (AVPs) (St. Jude Medical, Minneapolis, MN) include a family of expandable nitinol mesh vascular occlusion devices [32] and widely used for PAVM embolization [34, 35]. Although AVPs are relatively expensive, they may reduce the need for multiple individual coils and hence are potentially more cost effective. The MVP Micro Vascular Plug (Covidien, Irvine, CA) is a new detachable nitinol skeleton plug that is partially coated with polytetrafluoroethylene, and thus, they can induce immediate occlusion despite procedural anticoagulation. These plugs have been shown to be useful for PAVM embolization [36, 37]. However, the use of plugs requires advancing a relatively large delivery catheter into the feeding artery of the PAVM, which is difficult in cases with a tortuous feeding artery. In addition, they cannot embolize the sac of PAVMs. In our opinion, plugs may be appropriate when embolization of the feeding artery is performed and the feeding artery is large and straight, but coils should be selected when embolization from the sac is performed or the feeding artery is small or tortuous. However, the choice of embolic material remains a controversial issue, and further research that compares coils and plugs is therefore required.
Complications and Persistence after Embolization
Complications of embolization
Pleurisy is the most common complication of embolization, occurring in 14%-31% of patients [38, 39], but most cases improve with conservative management. The most feared complication of embolization is migration of air, thrombus, or embolic material into the systemic arterial circulation, which can result in ischemic stroke or myocardial infarction [28]. Pulmonary hypertension rarely develops in patients who have undergone PAVM embolization, so cardiac failure can develop in patients with pulmonary hypertension [28]. As PAVM rupture can occur during embolization due to injury of the PAVM during catheterization, careful manipulation is essential.
Pattern of persistence
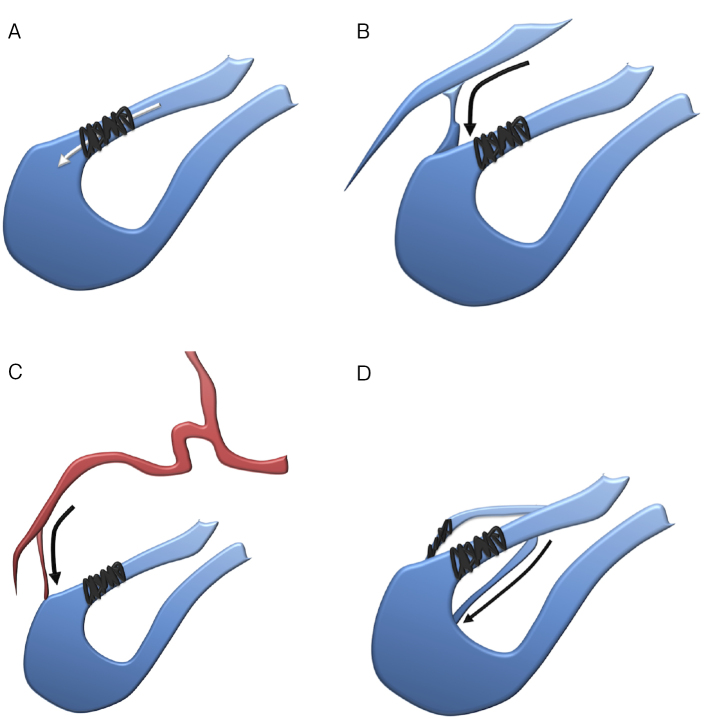
After successful embolization, the persistence of PAVMs is an important concern. Persistence can be categorized into the following patterns (Fig. 1): 1) recanalization (PAVMs are perfused through previously placed embolic materials), 2) pulmonary-to-pulmonary reperfusion (the embolized feeding artery remains occluded, but small feeding arteries from the adjacent normal pulmonary arteries are present), 3) systemic-to-pulmonary reperfusion (PAVMs persist via a systemic arterial feeder), and 4) incomplete primary treatment (there are previously untreated feeders of a PAVM with multiple feeders) [40]. A recent report described recanalization as the most frequent pattern of persistence, occurring in 98% (60/61) of persistent PAVMs diagnosed via angiography [41].
Figure 1.
Pattern of persistence.
A) Recanalization; PAVMs are perfused through previously placed embolic materials. Preventing this requires tighter PAVM embolization.
B) Pulmonary-to-pulmonary reperfusion; the embolized feeding artery remains occluded, but small feeding arteries from the adjacent normal pulmonary arteries are present. Preventing this may require sac embolization or distal feeding artery embolization.
C) Systemic-to-pulmonary reperfusion; PAVMs persist via a systemic arterial feeder. Preventing this may require sac embolization or distal feeding artery embolization.
D) Incomplete primary treatment; for a PAVM with multiple feeders, some feeders remain untreated. Preventing this requires careful interpretation of CT images before embolization to identify all feeding arteries.
The cause of recanalization is considered to be the resolution of thrombus in the embolized PAVM. The pulmonary vasculature is more compliant than the systemic vasculature, and this compliance has been attributed to arterial wall elasticity and vessel size [42]. The pulmonary artery may be distended following coil embolization, leading to coil elongation. In addition, the thrombus can spontaneously resolve in the pulmonary artery owing to its potent fibrinolytic activity [43]. PAVMs therefore need to be embolized more tightly to prevent recanalization. On the other hand, the cause of pulmonary-to-pulmonary reperfusion and systemic-to-pulmonary reperfusion may be related to embolization of the feeding artery at a proximal site [44]. Sac embolization or distal feeding artery embolization may thus be required to prevent such a reperfusion. Incomplete primary treatment can occur in a PAVM with multiple feeders; thus, careful interpretation of images from computed tomography (CT) before embolization is important to identify all feeding arteries.
Diagnosis of persistence and persistence rate
Because persistent PAVMs can potentially cause neurologic complications [45], diagnosis of the persistence of PAVMs during follow-up is crucial. To accurately diagnose persistence, DSA is considered the most sensitive modality for examining blood flow through PAVMs [38]. However, DSA is an invasive procedure and is thus not commonly performed in clinical practice. CT; therefore, is routinely performed after embolization to diagnose persistence based on the size reduction of the sac or draining vein [44-51]. As an issue of CT diagnosis, evaluating size reductions is often difficult due to metal artifacts from coils and the fact that the size of the draining veins does not reflect blood flow through the embolized PAVM, because the vein can be drained from the normal pulmonary parenchyma. In the literature, persistence has been defined as <70% reduction of the sac or the draining vein of a PAVM [45, 49, 50]. However, it has recently been reported that the diagnostic accuracy of CT was 82%, when the cutoff value of the reduction rate was set to 55%, which produced the highest accuracy, and the diagnostic accuracy of CT was 70%, when the cutoff value of the reduction rate was set to 70% [41]. The results of this receiver operating characteristic analysis were similar to those in recent studies [52, 53], which suggests that a cutoff value of 55% may be appropriate for CT diagnosis.
As an alternative to DSA for screening after coil embolization, time-resolved magnetic resonance angiography (TR-MRA) with gadolinium contrast medium has become a valuable option owing to its high sensitivity for detecting blood flow (Fig. 2) [54]. In magnetic resonance (MR) imaging, platinum coils, which have relatively low paramagnetic characteristics, are known to produce very few artifacts (Fig. 3) [55-57]. TR-MRA has been reported to be more useful than CT for diagnosing the persistence of PAVM following coil embolization, with higher diagnostic specificity, positive predictive values, and sensitivity [55]. In addition, a recent article reported that TR-MRA has high accuracy (93%) for diagnosing persistence after PAVM embolization when using DSA as the reference standard [41].
Figure 2.
A 55-year-old woman presented with untreated PAVM in the right middle lobe.
A) CT shows the PAVM in the right middle lobe (arrow).
B) Pulmonary angiography shows the PAVM (arrow).
C) After coil embolization, complete occlusion of the PAVM is confirmed.
D) CT at 42 months following coil embolization shows reduction of the sac, but the reduction rate is 36%, and persistence is suspected (arrow).
E) TR-MRA at 44 months following coil embolization obviously shows persistence.
F) Pulmonary angiography at 44 months following coil embolization confirms persistence.
Figure 3.
A 67-year-old woman presented with untreated PAVM in the right middle lobe.
A) CT shows the PAVM in the right middle lobe (arrow).
B) TR-MRA shows the PAVM (arrow).
C) Pulmonary angiography shows the PAVM (arrow).
D) Complete cessation of blood flow in the PAVM is confirmed following coil embolization.
E) CT at 41 months following coil embolization shows an artifact from coils, making persistence determination difficult.
F) TR-MRA at 41 months following coil embolization shows disappearance of the PAVM without any coil artifacts.
Historically, the persistence rate was usually evaluated via CT, with persistence rates reportedly up to 19% [44-47]. Recently, using TR-MRA, the persistence rate was reported to be 49% at 24 months following embolization [58]. However, this result was from a study with a small number of cases. On the other hand, a recent study evaluated the persistence rate in a large number of cases via CT, TR-MRA, and DSA [41]. For CT with a cutoff value of 70%, the 12-, 24-, 48-, and 96-month persistence rates were 29%, 46%, 66%, and 86%, respectively, and for CT with a cutoff value of 55%, the persistence rates were 23%, 33%, 50%, and 62%, respectively. For TR-MRA, the 12-, 24-, 48-, and 96-month persistence rates were 8%, 12%, 26%, and 53%, respectively. For DSA, the 12-, 24-, 48-, and 96-month persistence rates were 10%, 25%, 31%, and 53%, respectively.
Factors of persistence
A recent study examined factors affecting persistence, including sex, age, presence of HHT, number of PAVMs (single or multiple), location of PAVM, type of PAVM (simple or complex), size of feeding artery, size of sac, and location of embolization (feeding artery or sac, proximal or distal to the last normal branch of the pulmonary artery from the feeding artery) [41]. The authors concluded that embolization of the distal position to the last normal branch of the pulmonary artery was a significant factor for preventing persistence. “Shunting point” occlusion is generally recognized as important when embolizing AVMs in various organs. In PAVMs, the location of the last normal branch of the pulmonary artery is used as a landmark to determine the “shunting point.” The last normal branch of the pulmonary artery is reported to arise from the sac, the junction of the sac and feeding artery, and the feeding artery in 39%, 51%, and 10% of cases, respectively [59]. Therefore, sac embolization is required to occlude the shunting point in most PAVMs, in which the last normal branch of the pulmonary artery is in the sac or junction. However, in the remaining PAVMs, in which the last normal branch of the pulmonary artery is in the feeding artery, sac embolization may not be necessary, which can decrease the cost and duration of the procedure.
Technique of Embolization for PAVMs in Our Institution
The embolization technique for PAVMs in our institution is presented in Fig. 4. An 8-Fr sheath (SuperSheath; Medikit, Tokyo, Japan) is first introduced into the femoral vein. To prevent the formation of a thrombus during the procedure, 3000 units (1000 units/mL) of heparin are intravenously administered, with an additional 1000 units added every hour, to achieve an activated clotting time of >200 s. An 8-Fr balloon catheter (Optimo; Tokai Medical Products, Aichi, Japan, or FlowGate; Stryker Neurovascular, Fremont, CA) is placed at the segmental pulmonary artery, followed by a 4-Fr catheter (C2; Medikit, or Cerulean G; Medikit) and a 2.2-Fr microcatheter (Progreat β3; Terumo, Tokyo, Japan). An 8-Fr balloon catheter is then used to occlude the flow of blood from the PAVM to prevent paradoxical embolization and migration of coils to the venous side. This also acts as a backup in case of perforation. Deep advancement of the 4-Fr catheter can also be attempted to provide additional support for the microcatheter. We usually embolize the PAVM from the sac to the feeding artery beyond the last normal branch of the pulmonary artery and always use coils (AZUR; Terumo, IDC; Boston Scientific Corporation, Natick, MA, Target; Stryker Neurovascular, Interlock; Boston Scientific Corporation, or Trufill; Codman Neurovascular, Johnson & Johnson Medical, Raynham, MA, or Tornado; Cook, Inc., Bloomington, IN) instead of plugs because plugs need a relatively large delivery catheter and cannot embolize the sac. First, the microcatheter is advanced to the sac, and several bare coils are placed until the operator can feel slight resistance, creating a scaffold within the sac. The microcatheter is then pulled back to the distal feeding artery, and embolization is continued using hydrogel-coated or fibered coils to ensure tight packing to prevent persistence. Continuous saline flushing is performed for each lumen during the procedure to prevent thrombus formation.
Figure 4.
A 24-year-old man presented with untreated PAVM in the left upper lobe.
A) CT shows the PAVM in the left upper lobe (arrow) and the last normal branch of the pulmonary artery (arrow head).
B) Pulmonary angiography shows the PAVM (arrow). The microcatheter should be attempted to advance beyond the last normal branch of the pulmonary artery (arrow head).
C) Then, the microcatheter (small arrow) is then advanced to the sac of the PAVM through a 4-Fr catheter (large arrow) under inflation of an 8-Fr balloon catheter (arrowhead).
D) Coil embolization is then performed from the sac to the feeding artery of the PAVM.
E) Complete occlusion of the PAVM is confirmed, and no persistence is evident on follow-up after 36 months.
Summary
Embolization is an effective treatment for PAVMs, but it is important to monitor persistence after the procedure. TR-MRA appears to be an appropriate method for diagnosing persistence. Moreover, embolization of the distal position to the last normal branch of the pulmonary artery is crucial for the prevention of persistence.
Conflict of Interest
None
Disclaimer
Masashi Shimohira is one of the Editorial Board members of Interventional Radiology. This author was not involved in the peer-review or decision-making process for this paper.
References
- 1.Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ. British Thoracic Society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017; 72: 1154-1163. [DOI] [PubMed] [Google Scholar]
- 2.Saboo SS, Chamarthy M, Bhalla S, et al. Pulmonary arteriovenous malformations: diagnosis. Cardiovasc Diagn Ther. 2018; 8: 325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contegiacomo A, Del Ciello A, Rella R, et al. Pulmonary arteriovenous malformations: what the interventional radiologist needs to know. Radiol Med. 2019; 124: 973-988. [DOI] [PubMed] [Google Scholar]
- 4.Circo S, Gossage JR. Pulmonary vascular complications of hereditary haemorrhagic telangiectasia. Curr Opin Pulm Med. 2014; 20: 421-428. [DOI] [PubMed] [Google Scholar]
- 5.Alicea-Guevara R, Cruz Caliz M, Adorno J, et al. Life-threatening hemoptysis: case of Osler-Weber-Rendu Syndrome. Oxf Med Case Rep. 2018; 2018: omx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ference BA, Shannon TM, White RI Jr, Zawin M, Burdge CM. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest. 1994; 106: 1387-1390. [DOI] [PubMed] [Google Scholar]
- 7.Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J. 2002; 78: 191-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzer RJ, Cua CL. Pulmonary arteriovenous malformations and risk of stroke. Cardiol Clin. 2016; 34: 241-246. [DOI] [PubMed] [Google Scholar]
- 9.Naito J, Kasai H, Suga M, Sugiura T, Tanabe N, Tatsumi K. Pulmonary arteriovenous malformations complicated by splenic infarction and abscess. Respirol Case Rep. 2017; 5: e00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brothers M, Peeler B, Paolillo J. Renal thromboembolism from a large pulmonary artery to a pulmonary vein fistula in an asymptomatic adolescent. Cardiol Young. 2017; 27: 199-201. [DOI] [PubMed] [Google Scholar]
- 11.Elmali M, Akan H, Findik S, Kale M, Celenk C. Hereditary hemorrhagic telangiectasia associated with pulmonary arteriovenous malformations presenting as hemothorax. J Thorac Imaging. 2008; 23: 295-297. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal M, Rossoff LJ, Steinberg HN, Marzouk KA, Siegel DN. Pulmonary arteriovenous malformations: a clinical review. Postgrad Med J. 2000; 76: 390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Gussem EM, Lausman AY, Beder AJ, et al. Outcomes of pregnancy in women with hereditary hemorrhagic telangiectasia. Obstet Gynecol. 2014; 123: 514-520. [DOI] [PubMed] [Google Scholar]
- 14.Carette MF, Nedelcu C, Tassart M, Grange JD, Wislez M, Khalil A. Imaging of hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol. 2009; 32: 745-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gent MW, Post MC, Snijder RJ, Westermann CJ, Plokker HW, Mager JJ. Real prevalence of pulmonary right-to-left shunt according to genotype in patients with hereditary hemorrhagic telangiectasia: a transthoracic contrast echocardiography study. Chest. 2010; 138: 833-839. [DOI] [PubMed] [Google Scholar]
- 16.Gallione C, Aylsworth AS, Beis J, et al. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am J Med Genet A. 2010; 152A: 333-339. [DOI] [PubMed] [Google Scholar]
- 17.Puskas JD, Allen MS, Moncure AC, et al. Pulmonary arteriovenous malformations: therapeutic options. Ann Thorac Surg. 1993; 56: 253-257; discussion 257-258 [DOI] [PubMed] [Google Scholar]
- 18.Bakhos CT, Wang SC, Rosen JM. Contemporary role of minimally invasive thoracic surgery in the management of pulmonary arteriovenous malformations: report of two cases and review of the literature. J Thorac Dis. 2016; 8: 195-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamarthy MR, Park H, Sutphin P, et al. Pulmonary arteriovenous malformations: endovascular therapy. Cardiovasc Diagn Ther. 2018; 8: 338-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenblatt M, Pollak J, Fayad P, Egglin T, White RI Jr. Pulmonary arteriovenous malformations: what size should be treated to prevent embolic stroke? (Abstract) Radiology. 1992; 185: 134. [Google Scholar]
- 21.Trerotola SO, Pyeritz RE, Bernhardt BA. Outpatient single-session pulmonary arteriovenous malformation embolization. J Vasc Interv Radiol. 2009; 20: 1287-1291. [DOI] [PubMed] [Google Scholar]
- 22.Todo K, Moriwaki H, Higashi M, Kimura K, Naritomi H. A small pulmonary arteriovenous malformation as a cause of recurrent brain embolism. AJNR Am J Neuroradiol. 2004; 25: 428-430. [PMC free article] [PubMed] [Google Scholar]
- 23.Shovlin CL, Jackson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008; 63: 259-266. [DOI] [PubMed] [Google Scholar]
- 24.Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI Jr. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2006; 17: 35-44; quiz 45. [DOI] [PubMed] [Google Scholar]
- 25.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011; 48: 73-87. [DOI] [PubMed] [Google Scholar]
- 26.Trerotola SO, Pyeritz RE. PAVM embolization: an update. AJR Am J Roentgenol. 2010; 195: 837-845. [DOI] [PubMed] [Google Scholar]
- 27.Stein EJ, Chittams JL, Miller M, Trerotola SO. Persistence in coil-embolized pulmonary arteriovenous malformations with feeding artery diameters of 3 mm or less: a retrospective single-center observational study. J Vasc Interv Radiol. 2017; 28: 442-449. [DOI] [PubMed] [Google Scholar]
- 28.Meek ME, Meek JC, Beheshti MV. Management of pulmonary arteriovenous malformations. Semin Intervent Radiol. 2011; 28: 24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacombe P, Lacout A, Marcy PY, et al. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: an overview. Diagn Interv Imaging. 2013; 94: 835-848. [DOI] [PubMed] [Google Scholar]
- 30.Graves VB, Partington CR, Rüfenacht DA, Rappe AH, Strother CM. Treatment of carotid artery aneurysms with platinum coils: an experimental study in dogs. AJNR Am J Neuroradiol. 1990; 11: 249-252. [PMC free article] [PubMed] [Google Scholar]
- 31.Cantón G, Levy DI, Lasheras JC. Changes in the intraaneurysmal pressure due to HydroCoil embolization. AJNR Am J Neuroradiol. 2005; 26: 904-907. [PMC free article] [PubMed] [Google Scholar]
- 32.Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol. 2008; 25: 204-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimohira M, Kawai T, Hashizume T, Muto M, Kitase M, Shibamoto Y. Usefulness of hydrogel-coated coils in embolization of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. 2018; 41: 848-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tau N, Atar E, Mei-Zahav M, et al. Amplatzer vascular plugs versus coils for embolization of pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol. 2016; 39: 1110-1114. [DOI] [PubMed] [Google Scholar]
- 35.Fidelman N, Gordon RL, Bloom AI, LaBerge JM, Kerlan RK Jr. Reperfusion of pulmonary arteriovenous malformations after successful embolotherapy with vascular plugs. J Vasc Interv Radiol. 2008; 19: 1246-1250. [DOI] [PubMed] [Google Scholar]
- 36.Andersen PE, Duvnjak S, Gerke O, Kjeldsen AD. Long-term single-center retrospective follow-up after embolization of pulmonary arteriovenous malformations treated over a 20-year period: frequency of re-canalization with various embolization materials and clinical outcome. Cardiovasc Intervent Radiol. 2019; 42: 1102-1109. [DOI] [PubMed] [Google Scholar]
- 37.Ratnani R, Sutphin PD, Koshti V, et al. Retrospective comparison of pulmonary arteriovenous malformation embolization with the polytetrafluoroethylene-covered nitinol microvascular plug, AMPLATZER plug, and coils in patients with hereditary hemorrhagic telangiectasia. J Vasc Interv Radiol. 2019; 30: 1089-1097. [DOI] [PubMed] [Google Scholar]
- 38.Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998; 158: 643-661. [DOI] [PubMed] [Google Scholar]
- 39.White RI Jr, Pollak JS, Wirth JA. Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Interv Radiol. 1996; 7: 787-804. [DOI] [PubMed] [Google Scholar]
- 40.Woodward CS, Pyeritz RE, Chittams JL, Trerotola SO. Treated pulmonary arteriovenous malformations: patterns of persistence and associated retreatment success. Radiology. 2013; 269: 919-926. [DOI] [PubMed] [Google Scholar]
- 41.Shimohira M, Kiyosue H, Osuga K, et al. Location of embolization affects patency after coil embolization for pulmonary arteriovenous malformations: importance of time-resolved magnetic resonance angiography for diagnosis of patency. Eur Radiol. 2021; 31: 5409-5420. [DOI] [PubMed] [Google Scholar]
- 42.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev. 2010; 19: 197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenhek R, Korschineck I, Gharehbaghi-Schnell E, et al. Fibrinolytic balance of the arterial wall: pulmonary artery displays increased fibrinolytic potential compared with aorta. Lab Invest. 2003; 83: 871-876. [DOI] [PubMed] [Google Scholar]
- 44.Milic A, Chan RP, Cohen JH, Faughnan ME. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J Vasc Interv Radiol. 2005; 16: 1675-1683 [DOI] [PubMed] [Google Scholar]
- 45.Lee DW, White RI Jr, Egglin TK, et al. Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg. 1997; 64: 930-939; discussion 939-940. [DOI] [PubMed] [Google Scholar]
- 46.Prasad V, Chan RP, Faughnan ME. Embolotherapy of pulmonary arteriovenous malformations: efficacy of platinum versus stainless steel coils. J Vasc Interv Radiol. 2004; 15: 153-160. [DOI] [PubMed] [Google Scholar]
- 47.Remy-Jardin M, Dumont P, Brillet PY, Dupuis P, Duhamel A, Remy J. Pulmonary arteriovenous malformations treated with embolotherapy: helical CT evaluation of long-term effectiveness after 2-21-year follow-up. Radiology. 2006; 239: 576-585. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi S, Baba Y, Senokuchi T, Nakajo M. Efficacy of venous sac embolization for pulmonary arteriovenous malformations: comparison with feeding artery embolization. J Vasc Interv Radiol. 2012; 23: 1566-1577; quiz p. 1581 [DOI] [PubMed] [Google Scholar]
- 49.Letourneau-Guillon L, Faughnan ME, Soulez G, et al. Embolization of pulmonary arteriovenous malformations with Amplatzer vascular plugs: safety and midterm effectiveness. J Vasc Interv Radiol. 2010; 21: 649-656. [DOI] [PubMed] [Google Scholar]
- 50.Kajiwara K, Urashima M, Yamagami T, et al. Venous sac embolization of pulmonary arteriovenous malformation: safety and effectiveness at mid-term follow-up. Acta Radiol. 2014; 55: 1093-1098. [DOI] [PubMed] [Google Scholar]
- 51.Gamondès D, Si-Mohamed S, Cottin V, et al. Vein diameter on unenhanced multidetector CT predicts reperfusion of pulmonary arteriovenous malformation after embolotherapy. Eur Radiol. 2016; 26: 2723-2729. [DOI] [PubMed] [Google Scholar]
- 52.Bélanger C, Chartrand-Lefebvre C, Soulez G, et al. Pulmonary arteriovenous malformation (PAVM) reperfusion after percutaneous embolization: sensitivity and specificity of non-enhanced CT. Eur J Radiol. 2016; 85: 150-157. [DOI] [PubMed] [Google Scholar]
- 53.Makimoto S, Hiraki T, Gobara H, et al. Association between reperfusion and shrinkage percentage of the aneurysmal sac after embolization of pulmonary arteriovenous malformation: evaluation based on contrast-enhanced thin-section CT images. Jpn J Radiol. 2014; 32: 266-273. [DOI] [PubMed] [Google Scholar]
- 54.Spilberg G, Carniato SL, King RM, et al. Temporal evolution of susceptibility artifacts from coiled aneurysms on MR angiography: an in vivo canine study. AJNR Am J Neuroradiol. 2012; 33: 655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai T, Shimohira M, Kan H, et al. Feasibility of time-resolved MR angiography for detecting recanalization of pulmonary arteriovenous malformations treated with embolization with platinum coils. J Vasc Interv Radiol. 2014; 25: 1339-1347. [DOI] [PubMed] [Google Scholar]
- 56.Koganemaru M, Abe T, Uchiyama D, et al. Detection of neck recanalization with follow-up contrast-enhanced MR angiography after renal artery aneurysm coil embolization. J Vasc Interv Radiol. 2010; 21: 298-300. [DOI] [PubMed] [Google Scholar]
- 57.Koganemaru M, Abe T, Nonoshita M, et al. Follow-up of true visceral artery aneurysm after coil embolization by three-dimensional contrast-enhanced MR angiography. Diagn Interv Radiol. 2014; 20: 129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimohira M, Kawai T, Hashizume T, et al. Reperfusion rates of pulmonary arteriovenous malformations after coil embolization: evaluation with time-resolved MR angiography or pulmonary angiography. J Vasc Interv Radiol. 2015; 26: 856-864.e1. [DOI] [PubMed] [Google Scholar]
- 59.Maruno M, Kiyosue H, Hongo N, Matsumoto S, Mori H. Where is the origin of the last normal branch from feeding artery of pulmonary arteriovenous malformations? Cardiovasc Intervent Radiol. 2018; 41: 1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]