Abstract
Radiotheranostics with 177Lu-PSMA have changed the treatment paradigm in patients with prostate cancer, becoming the new standard in certain settings. Terbium-161 (161Tb) has been recently investigated as a potential radionuclide for radiotheranostics in various types of cancer, including metastatic castration-resistant prostate cancer (mCRPC). The nuclear medicine team at King Hussein Cancer Center (KHCC) in Amman, Jordan, recently published the first-in-human SPECT/CT imaging results following a well-tolerated dose of 161Tb-PSMA radioligand therapy with no treatment-related adverse events, adding to the potential of radiotheranostics in prostate cancer. Two clinical trials for 161Tb-PSMA radioligand therapy in prostate cancer are currently underway and will provide valuable insights. This review will shed light on the expanding field of radiotheranostics in prostate cancer, which is not without challenges, and will discuss how the introduction of a new therapeutic option like 161Tb-PSMA may help to combat these challenges and build on the proven success of 177Lu-PSMA-based radiotheranostics for the benefit of prostate cancer patients worldwide.
Keywords: 161Tb-PSMA, Radiotheranostics, Nuclear medicine, Prostate cancer, King Hussein Cancer Center (KHCC), Jordan
Introduction
Theranostics, the combination of targeted diagnostic imaging and therapeutic agents, have become a fundamental setting for precision oncology and have revolutionized the field of nuclear medicine [1, 2]. Radiotheranostic approaches based on nuclear medicine have changed the treatment paradigm in patients with prostate cancer and neuroendocrine tumors over the last decade, becoming the new standard in certain settings with many successful outcomes [3–6]. The breakthrough that a proportion of patients with progressive metastatic burdens on standard treatments respond well to radiotheranostics is fueling the search for novel theranostics approaches focusing on molecular phenotyping, targeting chemical and radioisotopes [7, 8]. Recently, there has been increasing interest in the use of terbium-161 (161Tb) as a potential radionuclide for radiotheranostics in various types of cancer [9, 10]. A recent case report of a heavily pretreated patient with metastatic castration-resistant prostate cancer (mCRPC) provides stimulating preliminary evidence of the potential therapeutic capability of 161Tb-labeled prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) in patients with progressive metastatic disease after extensive 177Lu-PSMA-617 RLT [10].
161Tb-PSMA: First-in-Human SPECT/CT Post-RLT
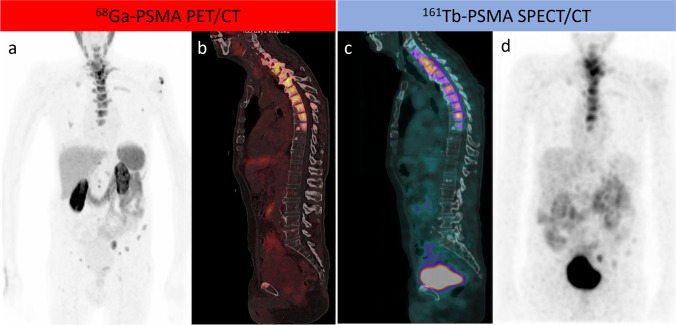
Recently, our nuclear medicine team at King Hussein Cancer Center in Amman, Jordan, published the first-in-human SPECT/CT imaging results following 161Tb-PSMA radioligand therapy [11]. The patient received an empiric, well-tolerated dose of 161Tb-PSMA-617 (5,550 MBq); protocols for whole-body planar and SPECT/CT scanning have been developed based on the decay scheme that revealed two gamma energies with high frequencies (48.9 keV and 74.5 keV). The radionuclide’s spatiotemporal distribution in target to non-target potentially dose-limiting organs was determined by acquiring time sequential planar images at 18 h, 69 h, and 90 h post-injection (p.i.). At 69 h p.i., SPECT/CT images were acquired from the lower cervical level to the pelvis, allowing for more refined image-derived activity quantification and tissue kinetic characterization and allowing visualization of all previously identified PSMA-avid primary and metastatic bone lesions on the 68Ga-PSMA PET/CT scan (Fig. 1). Our positive experience with the first radiotheranostic application of 161Tb-PSMA in mCRPC in Jordan and Asia was disclosed with no acute or treatment-related adverse events [11]. In our department, four patients with advanced, heavily pretreated mCRPC have received 161Tb-PSMA radioligand therapy (compassionate use under the local regulatory framework and international ethical and radiation safety standards). After receiving 161Tb-PSMA, patients did not report any acute negative side effects, and there were no notable changes in vital parameters during the initial assessment. At the subsequent follow-up of the four patients, there were no clinically significant changes in relevant laboratory values (hematologic, renal, and hepatic panels), as defined by the Common Terminology Criteria for Adverse Events (CTCAE v5.0) [12]. While the influence of the treatment on the biochemical response is still being evaluated, preliminary results suggest a favorable activity, with patients reporting an improvement in their physical functioning and other quality-of-life measures. This ground-breaking radiotheranostic approach marks an important milestone in our PSMA-radioligand therapy program, which was launched in 2017, and holds great promise for improving the outcomes of mCRPC patients in Jordan and the Middle East [13, 14].
Fig. 1.
A patient with metastatic castration-resistant prostate cancer who progressed on hormonal therapy and was referred for PSMA-RLT. As shown in a maximum intensity projection (MIP) and b sagittal fused PET/CT, 68Ga-PSMA PET/CT revealed widespread PSMA-avid bone metastases that were extensive sclerotic in the thoracic spine. 161Tb-PSMA SPECT/CT 24 h after a 5.5 GBq theraputic dose of 161Tb-PSMA RLT demonstrated adequate localization of the 161Tb-PSMA in the metastatic bone deposits, which were extensively expressed in the thoracic spine as demonstrated by sagittal fused SPECT/CT and MIP (c, d)
Expanding the PSMA RLT in Prostate Cancer
PSMA-targeted radioligand therapy (RLT) using 177Lu has shown impressive results in retrospective and prospective studies [15, 16]. However, 161Tb is a radionuclide that possesses similar chemical and pharmacokinetic properties to 177Lu, both in vivo and in vitro. Additionally, it behaves similarly with regard to radioligand-specific cell uptake and internalization. Moreover, 161Tb emits a modest fraction of photons which makes it suitable for post-therapy imaging [11, 17]. While 161Tb has a similar physical half-life of 6.9 days and β-particles of a slightly higher energy (mean energy 161Tb: 154 keV vs 177Lu: 133 keV), its co-emitted Auger and conversion electrons theoretically result in disseminated higher tumor-absorbed doses. A preclinical evaluation of 161Tb-PSMA RLT showed superiority to 177Lu-PSMA in in vitro experiments and a preventative tumor model [17]. This potential advantage, when compared to 177Lu, is expected to broaden the applicability of radiotheranostics to treat small lesions and established metastatic disease, which may lead to better control of micrometastatics and possibly lower recurrence rates. Table 1 compares the characteristics of 177Lu and 161Tb radionuclides [18], demonstrating that 161Tb is a promising radionuclide because it combines the advantages of a medium-energy β−emission with those of Auger electrons.
Table 1.
The characteristics of 177Lu and 161Tb radionuclides [18]
| Radionuclide | 177Lu | 161Tb |
|---|---|---|
| Half-life (d) | 6.647 | 6.906 |
| Type of decay (%) | β− (100%) | β− (100%) |
| β-Particle mean energy (keV) | 133.3 | 154.3 |
| Conversion electrons (keV per decay) | 13.52 | 39.28 |
| Conversion electrons energy range (keV) | 6.2–206.3 | 3.3–98.3 |
| Auger and Coster–Kronig electrons (keV per decay) | 1.13 | 8.94 |
| Auger and Coster–Kronig electron energy range (keV) | 0.01–61.7 | 0.018–50.9 |
| Total electron energy per decay (keV) | 147.9 | 202.5 |
| Photons X and γ (total energy per decay in keV) | 35.1 | 36.35 |
| Energy per decay in keV (photons 1 electrons) | 183 | 238.9 |
| Production method | Neutron irradiation of enriched 176Lu or 176Yb targets in nuclear reactor | Neutron irradiation of enriched 160Gd targets at nuclear reactor |
| Cost of production of no-carrier-added | Comparable | |
Two clinical trials for 161Tb-PSMA RLT are currently underway. The first is the VIOLET trial (NCT05521412), a phase I/II study evaluating the efficacy and safety of 161Tb-PSMA-I&T in men with mCRPC. The VIOLET trial aims to evaluate this therapy’s overall response rate, duration of response, progression-free survival, and safety profile. The findings of this trial will provide important information about the potential of 161Tb-PSMA RLT for the treatment of mCRPC. The REALITY trial (ClinicalTrials.gov Identifier: NCT04833517) is a prospective registry of targeted radionuclide therapy in patients with mCRPC. The REALITY study will assess the efficacy and safety of targeted radionuclide therapies, such as 161Tb-PSMA, in patients with mCRPC. The research will provide real-world data on the use of this therapy, such as dosing, response rates, and adverse events. These trials are expected to provide valuable insights into the potential and feasibility of 161Tb-PSMA radioligand therapy for the treatment of mCRPC.
Perspectives
The success of 177Lu-PSMA in radiotheranostics for mCRPC, backed by phases 2, 3, and randomized trials leading to approvals by the European Medicines Agency (EMA) and the USA Food and Drug Administration (FDA) [5, 19, 20], raises questions about the necessity of introducing a new radioisotope (161Tb) within the same family. Nevertheless, the increasing demand, supply shortages, and rising costs of current radiotheranostics options are major concerns that limit their accessibility worldwide [21]. Given its attractive physical characteristics, 161Tb offers a potential solution to these challenges, allowing for the expansion of effective radiotheranostics for mCRPC patients, among other indications [17, 22]. However, challenges in utilizing radiotheranostics in precision oncology may vary between developed and developing countries, with the latter facing additional hurdles in healthcare infrastructure and resources [23– 24]. Despite these challenges, it is crucial to investigate the potential benefits of introducing new radioisotopes to ensure that patients worldwide have access to optimal care. Promising approaches include the development of novel radiopharmaceuticals and the introduction of new radioisotopes, such as the success of the introduction of alpha-emitting radioisotopes, and delivery systems that can improve the availability, efficacy, and safety of radiotheranostics [25, 26]. These advances offer hope for the future of precision oncology, underscoring the need for continued research and development in this field.
Conclusion
Radiotheranostics have the potential to revolutionize cancer diagnosis and treatment, despite facing challenges such as the limited availability of radioisotopes and limited range of therapeutic radiation. However, the hard work of the nuclear medicine research community has resulted in the constant expansion of the field, with many promising approaches being introduced. The recent and preliminary evidence on the use of 161Tb as a radionuclide for theranostics in patients with mCRPC has generated great interest. Our first-in-human SPECT/CT imaging results following 161Tb-PSMA radioligand therapy have added to its therapeutic potential, and ongoing clinical trials will provide valuable insights. This new approach has the potential to support the well-established 177Lu-PSMA and improve cancer patient outcomes. The promising results highlight the importance of continuing to investigate and expand radiotheranostics’ access and applicability.
Acknowledgements
Authors would like to thank the nuclear medicine staff at King Hussein Cancer Center in Amman, Jordan.
Author Contribution
Akram Al-Ibraheem contributed to the conceptualization, design, and writing of this review manuscript. Andrew M. Scott provided critical feedback and revision of the manuscript. Both authors read and approved the final manuscript.
Data Availability
For this type of study, data sharing is not applicable as no datasets were generated or analyzed.
Compliance with Ethical Standards
Conflict of Interest
Akram Al-Ibraheem and Andrew M. Scott declare no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study, formal consent is not required and informed consent is not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roy I, Krishnan S, Kabashin AV, Zavestovskaya IN, Prasad PN. Transforming nuclear medicine with nanoradiopharmaceuticals. ACS Nano. 2022;16:5036–5061. doi: 10.1021/acsnano.1c10550. [DOI] [PubMed] [Google Scholar]
- 2.Lee DS, Cheon GJ. Nuclear theranostics in Asia: in vivo companion diagnostics. Nucl Med Mol Imaging. 2019;53:1–6. doi: 10.1007/s13139-019-00573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56–73. doi: 10.1016/j.ejca.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirmas N, Jadaan R, Al-Ibraheem A. Peptide receptor radionuclide therapy and the treatment of gastroentero-pancreatic neuroendocrine tumors: current findings and future perspectives. Nucl Med Mol Imaging. 2018;52:190–199. doi: 10.1007/s13139-018-0517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullard A. FDA approves first PSMA-targeted radiopharmaceutical. Nat Rev Drug Discov. 2022;21:327. doi: 10.1038/d41573-022-00067-5. [DOI] [PubMed] [Google Scholar]
- 6.Luining WI, Cysouw MCF, Meijer D, Hendrikse NH, Boellaard R, Vis AN, et al. Targeting PSMA revolutionizes the role of nuclear medicine in diagnosis and treatment of prostate cancer. Cancers (Basel). 2022;14:1169. doi: 10.3390/cancers14051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboagye EO, Barwick TD, Haberkorn U. Radiotheranostics in oncology: making precision medicine possible. CA Cancer J Clin. 2023; 10.3322/caac.21768. [DOI] [PubMed]
- 8.Ahn BC. Contribution of radionuclide theranostics for managing intractable malignancies. Nucl Med Mol Imaging. 2018;52:168–169. doi: 10.1007/s13139-018-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum RP, Singh A, Kulkarni HR, Bernhardt P, Rydén T, Schuchardt C, et al. First-in-humans application of 161Tb: a feasibility study using 161Tb-DOTATOC. J Nucl Med. 2021;62:1391–1397. doi: 10.2967/jnumed.120.258376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosar F, Maus S, Schaefer-Schuler A, Burgard C, Khreish F, Ezziddin S. New horizons in radioligand therapy: 161Tb-PSMA-617 in advanced mCRPC. Clin Nucl Med. 2023; 10.1097/RLU.0000000000004589. [DOI] [PubMed]
- 11.Al-Ibraheem A, Doudeen RM, Juaidi D, Abufara A, Maus S. (161)Tb-PSMA radioligand therapy: first-in-human SPECT/CT imaging. J Nucl Med. 2023; 10.2967/jnumed.122.265291. [DOI] [PubMed]
- 12.National Cancer Institute . NCI common terminology criteria for adverse events (CTCAE) data files and related documents. National Institutes of Health; 2018. [Google Scholar]
- 13.Al-Ibraheem A, Mohamedkhair A. Current status of theranostics in Jordan. Nucl Med Mol Imaging. 2019;53:7–10. doi: 10.1007/s13139-018-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Ibraheem A, Abdlkadir A, Albalooshi B, Muhsen H, Haidar M, Omar Y, et al. Theranostics in the Arab World: Achievements & Challenges. Jordan Med J. 2022;56:188–205. [Google Scholar]
- 15.Seitzer KE, Seifert R, Kessel K, Roll W, Schlack K, Boegemann M, et al. Lutetium-177 Labelled PSMA targeted therapy in advanced prostate cancer: current status and future perspectives. Cancers (Basel). 2021;13:3715. doi: 10.3390/cancers13153715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller C, Umbricht CA, Gracheva N, Tschan VJ, Pellegrini G, Bernhardt P, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1919–1930. doi: 10.1007/s00259-019-04345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hindié E, Zanotti-Fregonara P, Quinto MA, Morgat C, Champion C. Dose Deposits from 90Y, 177Lu, 111In, and 161Tb in Micrometastases of various sizes: implications for radiopharmaceutical therapy. J Nucl Med. 2016;57:759–764. doi: 10.2967/jnumed.115.170423. [DOI] [PubMed] [Google Scholar]
- 19.Oh SW, Suh M, Cheon GJ. Current status of PSMA-targeted radioligand therapy in the era of radiopharmaceutical therapy acquiring marketing authorization. Nucl Med Mol Imaging. 2022;56:263–281. doi: 10.1007/s13139-022-00764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 21.Bodei L, Herrmann K, Schoder H, Scott AM, Lewis JS. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat Rev Clin Oncol. 2022;19:534–550. doi: 10.1038/s41571-022-00652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracheva N, Müller C, Talip Z, Heinitz S, Köster U, Zeevaart JR, et al. Production and characterization of no-carrier-added 161Tb as an alternative to the clinically-applied 177Lu for radionuclide therapy. EJNMMI Radiopharm Chem. 2019;4:12. doi: 10.1186/s41181-019-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hricak H, Abdel-Wahab M, Atun R, Lette MM, Paez D, Brink JA, et al. Medical imaging and nuclear medicine: a Lancet Oncology Commission. Lancet Oncol. 2021;22:e136–ee72. doi: 10.1016/S1470-2045(20)30751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Ibraheem A, Abdlkadir AS, Mohamedkhair A, Mikhail-Lette M, Al-Qudah M, Paez D, et al. Cancer diagnosis in areas of conflict. Front Oncol. 2022;12:1087476. doi: 10.3389/fonc.2022.1087476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–138. doi: 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooijman EL, Chalashkan Y, Ling SW, Kahyargil FF, Segbers M, Bruchertseifer F, et al. Development of [(225)Ac]Ac-PSMA-I&T for targeted alpha therapy according to GMP guidelines for treatment of mCRPC. Pharmaceutics. 2021;13:715. doi: 10.3390/pharmaceutics13050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For this type of study, data sharing is not applicable as no datasets were generated or analyzed.