Abstract
Aluminum (Al) is a ubiquitous xenobiotic with known toxicity for both humans and animals. Our study was conducted to investigate the protective role of febuxostat (Feb) against aluminum chloride (AlCl3)-induced hepatorenal injury in rats. Hepatorenal injury was induced by oral administration of AlCl3 (40 mg/kg b.w.), for 2 months. Twenty-four male Sprague–Dawley rats were randomly allocated into four groups (six rats/group). The first group received the vehicle thought the experiment. The second group was considered as a control positive group. The third and fourth groups received oral treatment of Feb (10 mg/kg.b.w.) and (15 mg/kg.b.w.), respectively with AlCl3, concurrently for 2 months. Twenty-four hours, after the last treatment, serum biochemical, molecular, histopathology, and immunohistochemical studies were evaluated. Our findings showed that rats intoxicated with Alcl3 had disturbed biochemical picture. In addition, intoxication with AlCl3 increased oxidative stress and apoptosis, as demonstrated by an increase in malodialdeyde (MDA), carnitine o-acetyltransferase (Crat), and carbonic anhydrase (Car3) with a decrease in glutathione (GSH), MAP kinase-interacting serine/threonine kinase (MNK) and nuclear factor-erythroid 2-related factor 2 (Nrf2) mRNA expression. Furthermore, the levels of tumor necrosis factor-alpha (TNF-α) and the levels of caspase-3 were elevated with sever hepatic and renal pathological changes. Conversely, Feb (15 mg/kg.b.w.) could improve the serum biochemical indices and repressed MDA, Crat, and Car3 levels, whereas it increased GSH, MNK, and Nrf2 levels. Feb inhibited the apoptotic effect of AlCl3 in the liver and kidney by decreasing caspase-3 and TNF-α expression. The protective effect of Feb against AlCl3 toxicity was confirmed by histopathological findings. Moreover, molecular docking studies supported the anti-inflammatory effect of Feb due to its significant binding interactions with cyclooxygenase-1 (COX-1), NF-kappa-B-inducing kinase (NIK), and mitogen-activated protein kinases-p38 (MAPK-p38). The findings suggest that Feb system Feb can avert Alcl3-induced hepatotoxicity and nephrotoxicity by enhancing the antioxidant defense system, and inhibiting the inflammatory cascade and apoptosis.
Keywords: Aluminum, Febuxostat, Nrf2, Crat, car3, COX-1, Caspase-3, Hepatorenal injury
Introduction
Aluminum (Al) is one of the most abundant metallic elements on the planet. The availability of AL has recently drawn more attention to its biotoxicity (Al Eisa and Al Nahari 2016; Machado-Neves et al. 2023). AL is used as a food additive, in cooking pots with roughly 20% Aluminum content, in drinking water with a 0.2-mg/L concentration, as a water-purifying agent, in can bottles, in aluminum foil paper, and in antiperspirant cosmetic products. Although having a low gastrointestinal absorption capacity (less than 1%), it may accumulate over time in vital organs like the kidney, liver, and brain, where it may cause apparent neurotoxicity and cytotoxicity (Othman et al. 2020).
Additionally, AL may stimulate the pro-oxidant features of iron and copper, which results in mitochondrial dysfunction, the oxidative degradation of macromolecules, and the release of cytochrome C from the mitochondria (Al-Kahtani et al. 2020). Moreover, AL can induce the pro-oxidant features of iron and copper, resulting in mitochondrial dysfunction, oxidative degradation of macromolecules, and the release of cytochrome C from mitochondria and apoptosis. Therefore, eliminating AL toxicity through neutralization and scavenging of free radicals may be a viable option.
A selective xanthine oxidase inhibitor called febuxostat (Feb) is used to treat hyperuricemic conditions including gout and tumor lysis syndrome by lowering urate level (Pui et al. 2012). Due to their potential benefits in the treatment of several autoimmune and inflammatory illnesses, xanthine oxidase inhibitors have come under more attention. These inhibitors have anti-inflammatory, antioxidant, and immune-modulatory properties. Various experimental models had shown anti-inflammatory properties of febuxostat (Fahmi et al. 2016). In the rat model of renal ischemia–reperfusion injury, it inhibited the formation of ROS and had an antioxidative stress effect (Tsuda et al. 2012).
Determining if Feb has any potential protective benefits against Alcl3-induced hepatorenal damage in rats was the goal of the current study. Additionally, potential pathways behind in Feb-mediated effects were investigated.
Materials and methods
Experimental animals
Twenty-four adult male Sprague–Dawley rats (150–170 g) about 6 weeks old were housed in separated metal cages at the animal house, October 6 University, Giza, Egypt. Rats were housed in a well-ventilated room, under ambient laboratory conditions (22 ± 1 °C temperature, 45–55% relative humidity) with a 12-h light/12-h dark cycle. Water and food were freely available. Under approval number (RECO6U/10–2022), our study was carried out under the ethical standards documented by the Research Ethics Committee, Faculty of Dentistry, October 6 University, Giza, Egypt.
Drugs and chemicals
Aluminum Chloride (Alcl3; CAS number: 7446–70-0) and febuxostat (Feb; CAS number: 144060–53-7) were obtained from Sigma-Aldrich Co. (USA). All other chemicals and kits were of highest analytical grade. The LD50 of oral administration of Alcl3 was reported previously at 400 mg/kg b.w. (Yousef 2004). While Feb was regarded safe in rats at (15 mg/kg/day) (Takeda Pharmaceuticals America 2009).
Experimental design
Rats were divided blindly into four groups of six each, as follows: normal control group: rats were received the vehicle thought the experiment. Alcl3 group: rats were received orally Alcl3 (40 mg/kg b.w.) for 2 months (Okail et al. 2020). Alcl3 + Feb10 group: rat received oral treatment of febuxostat (10 mg/kg.b.w.) concurrently with Alcl3 for 2 months (Hwang et al. 2014; Tsuda et al. 2012). Alcl3 + Feb 15 group: rat received oral gavage treatment of febuxostat (15 mg/kg.b.w.) concurrently with Alcl3 for 2 months.
At the end of the experiment, about 2 ml blood sample were drawn from the eye’s retro-orbital plexus of the rats after being anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and centrifuged at 1500 rpm for 10 min for separation of serum (− 20 °C) for monitoring serum hepatic enzymes, total lipids, total cholesterol, triglycerides, total protein and albumin levels (El-Baz et al. 2020).
Rats were decapitated under light anesthesia, and their liver and kidney were immediately excised; and dived into two parts: part 1 being kept at − 80 °C for conducting biochemical and molecular analysis, while part 2 was fixed in 10% neutral formalin for evaluating the histopathogical and immunohistochemical alterations in liver and kidney.
Preparation of liver and kidney homogenate
The preserved liver and kidney from each rat were perfused with cold phosphate-buffered saline (PBS; pH 7.4) after being cleaned in regular saline solution and blotted over filter paper. Then, an automatic tissue homogenizer (Heidolph, Germany) was used to homogenize 1 g of tissues in 9 volumes of cold PBS (pH 7.4). The tissue homogenate was centrifuged at – 80 °C and 4000 rpm for 15 min, and the supernatant was kept at − 80 °C for evaluation of reduced glutathione (GSH) and malondialdehyde (MDA).
Evaluation of serum biochemical indices
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured colorimetrically at 546 nm (Reitman and Frankel 1957). Alkaline phosphatase (ALP) was assayed colorimetrically at 520 nm (Belfield and Goldberg 1971). Total lipids, total cholesterol and triglycerides concentrations were estimated (Zollner and Kirsch 1962), (Richmond 1973), (Fossati and Prencipe 1982). Total protein and albumin levels were evaluated (Lowry 1951), (Doumas et al. 1971).
Evaluation of hepatic and renal levels of GSH (nmol/g tissue) and MDA (nmol/g tissue)
The activity of GSH in the liver and kidney tissues was assayed colorimetrically (Beutler 1963). Levels of lipid peroxidation (LPO) in the liver and kidney tissue were determined by measuring MDA levels (Ohkawa et al. 1979).
Evaluation of oxidative stress and cell related genes
Isolation of total RNA
The standard TRIzol® Reagent extraction method was used to isolate total RNA from liver and kidney tissue samples. Tissue samples were homogenized in a mortar using liquid nitrogen and 1 ml of TRIzol® Reagent. Prior to use, RNA was dissolved in diethylpyrocarbonate (DEPC)-treated water (Salem et al. 2018).
Reverse transcription (RT) reaction
Utilizing the RevertAidTM First Strand cDNA Synthesis Kit, the complete hepatic and renal samples from Poly (A) + RNA were reversely transcribed into cDNA (Table 1).
Table 1.
Primers sequence used for qRT-PCR
| Gene | Primers sequence | NCBI reference |
|---|---|---|
| Nrf2 | F: TCT TAC GCA TTC CCC AAC CT | XM_039105942.1 |
| R: CGA CCC GCT TAA TGA CAA GG | ||
| MNK | F: CAC AGG GAC CTA AAG CCA GA | NM_001011985.2 |
| R: GCT TGT CAT AGA TGC TGG CC | ||
| Crat | F: AAG AAG CCT GAA CTT GTG CG | NM_001004085.2 |
| R: TTA GCT TCT GGG ACT TGG GG | ||
| Car3 | F: GGC GAG TTC CAG ATT CTC CT | NM_019292.4 |
| R: CTG GTC TGA GCT CAC TGT CA | ||
| GAPDH | F: AAC GAC CCC TTC ATT GAC CT | DQ403053.1 |
| R: CCC CAT TTG ATG TTA GCG GG |
(Nrf2) nuclear factor erythroid 2–related factor 2, MNK MAP kinase-interacting serine/threonine kinase, Crat carnitine O-acetyltransferase, Car3 carbonic anhydrase 3, GAPDH glyceraldehyde-3-phosphate dehydrogenase, F forward, R reverse
Real-time-polymerase chain reaction (RT-PCR)
The copy number of the rat tissues samples was ascertained using Thermo Fisher Scientific's StepOneTM Real-Time PCR System from Applied Biosystems. A melting curve analysis was carried out at 95.0 °C after of each qPCR to evaluate the effectiveness of the primers used (Table 1) (Khalil and Booles 2011).
Comet assay
Comet assay was scored in liver and kidney tissues samples from different experimental groups (Blasiak et al. 2004). The slides were stained with ethidium bromide and observed under a × 400 Zeiss epifluorescence microscope (510–560 nm, barrier filter 590 nm, 100 cell/rat). Randomly chosen non-overlapping cells were scored visually on a scale of 0–3 based on perceived comet tail length migration and relative proportion of DNA in the nucleus (class 0 = no detectable DNA damage and no tail; class 1 = tail less than the diameter of the nucleus; class 2 = tail between 1 and 2 the diameter of the nucleus; and class 3 = tail longer than 2 the diameter of the nucleus) (Collins et al. 1997; Olive et al. 1990).
Histopathological examination of hepatic and renal tissues
Liver and kidney tissues were diced and fixed in formaldehyde solution (10%) at room temperature for 48 then washed, dehydrated in alcohol, cleared in xylol, and finally embedded in paraffin wax blocks. Sections (5 μm) were cut using Leica microtome, mounted, and stained as usual with hematoxylin and eosin (Gamble et al. 2008).
Imuunohistochemical evaluation of hepatic and renal levels of caspase-3 and TNF-α
Sections were stained by stained by the IHC technique according to data sheet. TNF-α Rabbit pAb (A11534) antibody from ABclonal (catalog no. A11534; 500 W Cummings Park, Ste. 6500 Woburn, MA 01801, USA). Anti-cleaved-Caspase-3 Rabbit pAb antibody from servicebio (catalog no.GB11009: East Lake High-Tech Developing Zone, Wuhan, Hubei, China 430,079). In brief, mounted sections were deparaffinized and incubated with 3% H2O2 solution for 15 min to rinse slides with PBS and treated Incubated with primary antibody overnight at 4 °C. After rinsing in PBS peroxidase-labeled secondary antibodies were applied and incubated for 30 min. Counterstaining was done using hematoxylin (Sedik and Hassan 2022; Pedrycz and Czerny 2008).
Evaluation of pharmacodynamic, pharmacokinetics parameters and insilico toxicity of febuxostat
The pharmacokinetic parameters of Feb were evaluated by the Swiss target prediction software (http://www.swisstargetprediction.ch/). Physicochemical properties, lipophilicity, aqueous solubility, absorption, and pharmacokinetic parameters were evaluated.Glory software was also used to determine the potential metabolic route and metabolites of Feb (https://nerdd.zbh.uni-hamburg.de/glory/) (de Bruyn Kops et al. 2019). Additionally, CLC-Pred software was used to assess the toxicity of Feb against a variety of cell lines (http://www.way2drug.com/Cell-line/).
Evaluation of molecular docking results of febuxostat
Molecular docking of Feb against, cyclooxygenase-1 (COX-1, PDB ID; 6Y3C-A), NF-kappa-B-inducing kinase (NIK, PDB ID; 4DN5-A), and mitogen-activated protein kinases-p38 (MAPK-p38, PDB ID; 1A9U-A) were performed after retrieval of three-dimensional protein structures from RCSB-PDB database (https://www.rcsb.org/). Protein structures were prepared with by BIOVIA Discovery Studio (Vélizy-Villacoublay, France). Therefore, we selected the A chain of 6KDL, 4A69, and 3V2A for protein preparation by removal of water molecules and all ligands in addition to energy minimization and refinement processes. In addition, the 3D structure of Feb was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The binding free energy, binding affinity (pKi), and the ligand efficiency of Feb against prepared proteins were determined using InstaDock software (Hassan et al. 2017). Finally, BIOVIA Discovery Studio Visualizer software did the visualization of target-ligand interaction.
Statistical analysis
One-way analysis of variance (ANOVA, GraphPad Prism program 8.0, USA) and general liner models (GLM) procedure of Statistical Analysis System (1982) were used for all quantifiable comparisons in our study. Tukey’s multiple comparison and Scheffé tests were utilized to score the significance. Results are presented as mean ± SEM of six rats and the difference was documented significant when p value is ≤ 0.05.
Results
Effect of febuxostat on serum hepatic markers in rats intoxicated with Alcl3
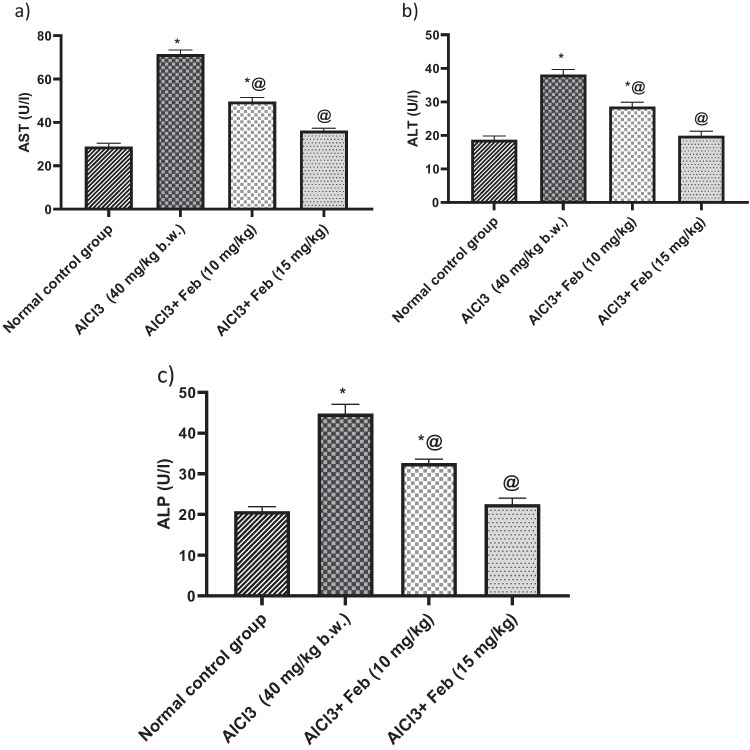
Alcl3-induced hepatorenal injury showed a highly significant elevation (p < 0.05) by 250%, 200%, and 215% in the values of AST, ALT, and ALP, respectively, as compared with normal control group. Treatment with Feb 10 mg/kg, significantly (p < 0.05) decreased AST, ALT and ALP by 69%, 75%, and 72% respectively, as compared to Alcl3 treated group. While, treatment with Feb 15 mg/kg could restore the aforementioned parameters to their normal values (Fig. 1).
Fig. 1.
Effect of febuxostat on serum hepatic markers in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, levels of AST, ALT and ALP were evaluated. *Significant difference from normal control group p < 0.05. @ Significant difference from intoxicated rats with Alcl3
Effect of febuxstat on urea, creatinine, and uric acid levels in rats intoxicated with Alcl3
Administration of Alcl3 for 2 months was associated with increased levels of urea, creatinine and uric acid (p < 0.05) by 248%, 180%, and 3.5-folds, as compared with a normal control group. Treatment with Feb 10 mg/kg, significantly (p < 0.05) decreased urea, creatinine, and uric acid by 67%, 75%, and 44% respectively, as compared to Alcl3-treated group. While, treatment with Feb 15 mg/kg could restore the a formentioned parameters to their normal values (Fig. 2).
Fig. 2.
Effect of febuxostat on urea, creatinine and uric acid levels in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, levels of urea, creatinine and uric acid were evaluated. *Significant difference from normal control group p < 0.05. @ Significant difference from intoxicated rats with Alcl3
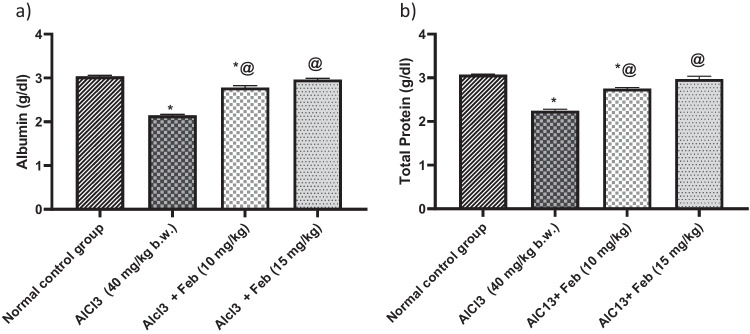
Effect of febuxstat on albumin and total protein levels in rats intoxicated with Alcl3
Administration of Alcl3 for 2 months was associated with decreased levels of albumin and T. protein (p < 0.05) by 79% and 72%, as compared with normal control group. Treatment with Feb 10 mg/kg, significantly (p < 0.05) increased albumin and T. protein by 129% and 122% respectively, as compared to Alcl3-treated group. While treatment with Feb15 mg/kg could restore the aforementioned parameters to their normal values (Fig. 3).
Fig.3.
Effect of febuxostat on albumin and total protein levels in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, levels of albumin and total protein were evaluated. *Significant difference from normal control group p < 0.05. @ Significant difference from intoxicated rats with Alcl3
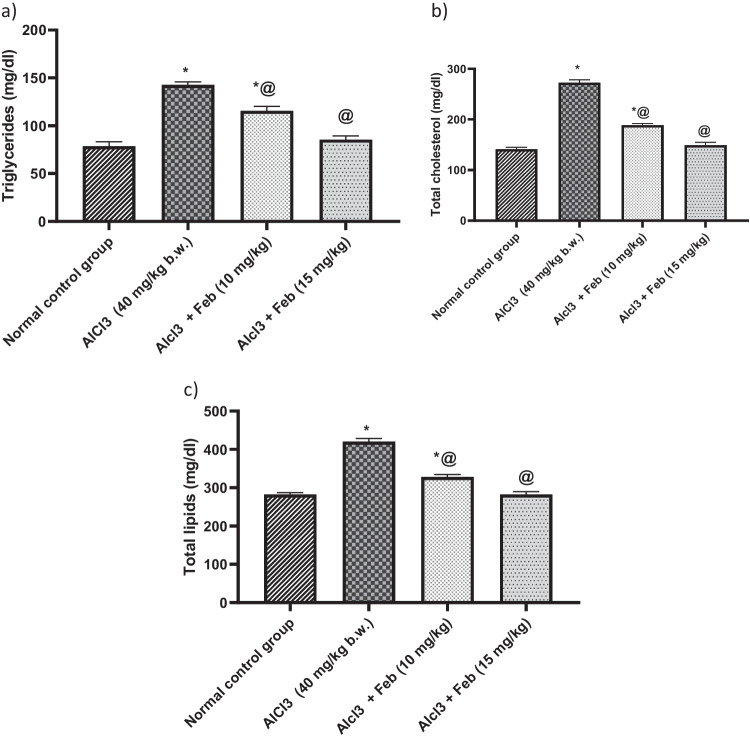
Effect of febuxstat on triglycerides, total cholesterol, and total lipids levels in rats intoxicated with Alcl3
Administration of Alcl3 for 2 months was associated with increased levels of triglycerides, totalcholesterol, and total lipids (p < 0.05) by 181%, 193%, and 148%, as compared with normal control group. Treatment with Feb 10 mg/kg, significantly (p < 0.05) decreased triglycerides, total cholesterol and total lipids by 81%, 69%, and 79% respectively, as compared to Alcl3 treated group. While treatment with Feb 15 mg/kg could restore aforementioned parameters to their normal values (Fig. 4).
Fig.4.
Effect of febuxostat on triglycerides, total cholesterol and total lipids levels in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, levels of triglycerides, total cholesterol and total lipids were evaluated. *Significant difference from normal control group p < 0.05. @ Significant difference from intoxicated rats with Alcl3
Effect of febuxstat on the hepatic and renal levels of GSH and MDA in rats intoxicated with Alcl3
Hepatic and renal levels of GSH were significantly (p < 0.05) decreased in Alcl3- exposed group by 36% and 43%, respectively, as compared to the control group. Treatment with Feb 10 mg/kg, significantly (p < 0.05) increased the hepatic and renal levels of GSH by 2.5-folds and 163% respectively, as compared to Alcl3 treated group. While treatment with Feb 15 mg/kg could normalize the hepatic and renal levels of GSH (Table 2).
Table 2.
Effect of febuxostat on the hepatic and renal values of GSH and MDA in rats intoxicated with Alcl3
| Treatment | GSH (nmol/g tissue) | MDA (nmol/g tissue) | ||
|---|---|---|---|---|
| Hepatic | Renal | Hepatic | Renal | |
| Normal control group | 39.02 ± 0.15 | 27.82 ± 0.4 | 22 ± 1.2 | 62.33 ± 1.5 |
| Alcl3 group (40 mg/kg) | 14.19 ± 0.3* | 11.99 ± 0.7* | 89 ± 3.8* | 149.7 ± 1.4* |
| Alcl3 + Feb (10 mg/kg) group | 35.26 ± 0.5*@ | 19.66 ± 0.8*@ | 45.8 ± 2.1 | 84.67 ± 1.8*@ |
| Alcl3 + Feb (15 mg/kg) group | 37.83 ± 0.4@ | 25.44 ± 0.8@ | 27.3 ± 1.8@ | 69 ± 2.1@ |
The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, hepatic and renal levels of GSH and MDA were evaluated
*Significant difference from normal control group p < 0.05
@Significant difference from intoxicated rats with Alcl3
Hepatic and renal levels of MDA were significantly (p < 0.05) increased in Alcl3-exposed group by 4-folds and 3-folds, respectively, as compared to the control group. Treatment with Feb 10 mg/kg, significantly (p < 0.05) decreased the hepatic and renal levels of MDA by 62% and 56%, respectively, as compared to Alcl3-treated group. While treatment with Feb15 mg/kg could normalize the hepatic and renal levels of MDA (Table 2).
Effect of febuxstat on the expression of Nrf2, MNK, Crat, and Car3 genes in hepatic and kidney tissues in rats intoxicated with Alcl3.
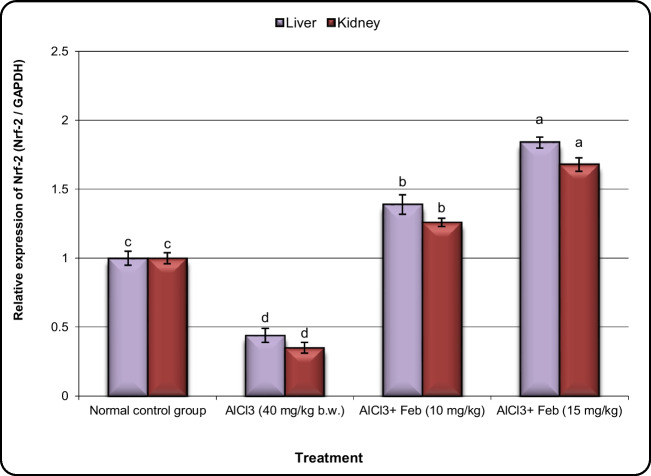
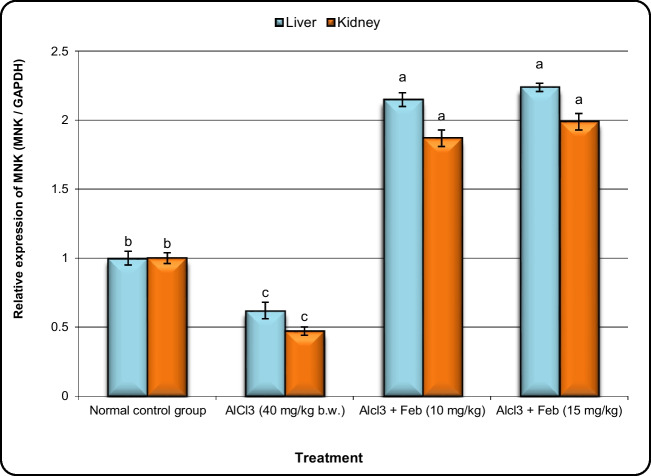
The expression levels of oxidative stress (Nrf2), cell cycle (MNK), and initial carcinogenesis (Crat and Car3) related genes in liver and kidney tissues are shown in Figs. 5, 6, 7, and 8, respectively. The Nrf2 and MNK expression levels in liver and kidney tissues were down-regulated significantly (P < 0.05) in the group of rats exposed to Alcl3 compared with control rats (Figs. 5 and 6). However, the expression levels of Nrf2 and MNK in the liver and kidney tissues of Alcl3-exposed rats treated with Feb (10 mg/kg) were increased significantly in comparison to those in rats exposed to Alcl3 only. Additionally, expression levels in liver and kidney tissues of Alcl3-exposed rats treated with Feb (15 mg/kg) were increased much more than those in Alcl3-exposed rats treated with Feb (10 mg/kg) regarding Nrf2 gene but not the case in MNK gene.
Fig.5.
Effect of febuxostat on the expression alterations of Nrf-2 gene in the liver and kidney in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of febuoxstat (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, the expression alterations of Nrf-2 gene in liver and kidney samples were evaluated. Data are presented as mean ± SD. Means with different superscripts (a,b,c) between groups in the same column are significantly different at P < 0.05
Fig.6.
Effect of febuxostat on the expression alterations of MNK gene in the liver and kidney in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, the expression alterations of MNK gene in liver and kidney samples were evaluated. Data are presented as mean ± SD. Means with different superscripts (a,b,c) between groups in the same column are significantly different at P < 0.05
Fig.7.
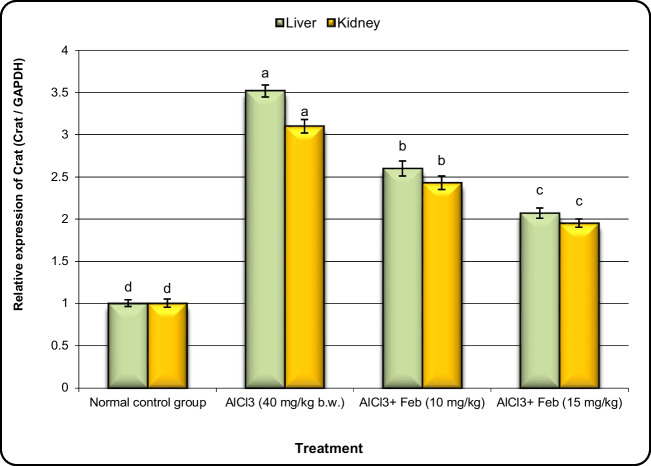
Effect of febuxostat on the expression alterations of Crat gene in the liver and kidney in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, the expression alterations of Crat gene in liver and kidney samples were evaluated. Data are presented as mean ± SD. Means with different superscripts (a,b,c,d) between groups in the same column are significantly different at P < 0.05
Fig.8.
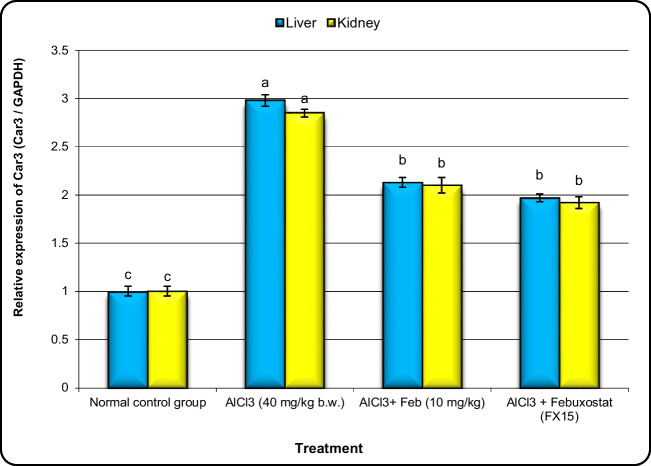
Effect of febuxostat on the expression levels of Car3 gene in the liver and kidney in rats intoxicated with Alcl3. The hepatorenal injury was induced in rats via oral administration of Alcl3 (40 mg/kg. b.w.) for 2 months. Intoxicated rats with Alcl3 were orally treated with two doses of Feb (10 mg/kg. b.w.) and (15 mg/kg. b.w.) concurrently with Alcl3 for 8 weeks. 24 h after the last treatment, the expression alterations of Car3 gene in liver and kidney samples were evaluated. Data are presented as mean ± SD. Means with different superscripts (a,b,c) between groups in the same column are significantly different at P < 0.05. Data are presented as mean ± SD. Means with different superscripts (a,b,c,d) between groups in the same column are significantly different at P < 0.05
The expression levels of Crat and Car3 genes in liver and kidney tissues were upregulated significantly (P < 0.01) in the group of rats exposed to Alcl3 compared with control rats (Figs. 7 and 8). However, the expression levels of Crat and Car3 in the liver and kidney tissues of Alcl3-exposed rats treated with Feb (10 mg/kg) were decreased significantly in comparison to those in rats exposed to Alcl3 only. Additionally, expression levels of Crat and Car3 in liver and kidney tissues of Alcl3-exposed rats treated with Feb (15 mg/kg) declined much more than those in Alcl3-exposed rats treated with Feb (10 mg/kg).
Effect of febuxstat on the rate of DNA damage in hepatic and kidney tissues in rats intoxicated with Alcl3
The rate of DNA damage in liver and kidney tissues of rats exposed to Alcl3 and treated with Feb is summarized in Tables 3 and 4, respectively.
Table 3.
Visual score of DNA damage in liver tissues of exposed to AlCl3 and treated with febuxostat
| Treatment | No. of cells | Class** | DNA damaged cells % (Mean ± SEM) |
|||||
|---|---|---|---|---|---|---|---|---|
| No of samples | Analyzed* | Comets | 0 | 1 | 2 | 3 | ||
| Normal control group | 6 | 600 | 44 | 556 | 35 | 9 | 0 | 7.33 ± 0.49c |
| Alcl3 group (40 mg/kg) | 6 | 600 | 127 | 473 | 41 | 39 | 47 | 21.17 ± 1.14a |
| Alcl3 + Feb (10 mg/kg) group | 6 | 600 | 95 | 505 | 32 | 38 | 25 | 15.83 ± 0.95b |
| Alcl3 + Feb (15 mg/kg) group | 6 | 600 | 87 | 513 | 34 | 31 | 22 | 14.50 ± 0.76b |
*Number of cells examined per a group
**Class 0 = no tail, 1 = tail length < diameter of nucleus, 2 = tail length between 1 and 2X the diameter of nucleus; and 3 = tail length > 2X the diameter of nucleus. Data are presented as mean ± SEM
a,b,c,dMean values within tissue with unlike superscript letters were significantly different (P < 0.05)
Table 4.
Visual score of DNA damage in kidney tissues of exposed to AlCl3 and treated with febuxostat
| Treatment | No. of cells | Class** | DNA damaged cells % (Mean ± SEM) |
|||||
|---|---|---|---|---|---|---|---|---|
| No of samples | Analyzed* | Comets | 0 | 1 | 2 | 3 | ||
| Normal control group | 6 | 600 | 43 | 557 | 37 | 6 | 0 | 7.18 ± 0.71c |
| Alcl3 group (40 mg/kg) | 6 | 600 | 118 | 482 | 44 | 40 | 34 | 19.67 ± 0.88a |
| Alcl3 + Feb (10 mg/kg) group | 6 | 600 | 89 | 511 | 36 | 32 | 21 | 14.84 ± 1.17b |
| Alcl3 + Feb (10 mg/kg) group | 6 | 600 | 81 | 519 | 38 | 29 | 14 | 13.52 ± 0.78b |
*Number of cells examined per a group
**Class 0 = no tail, 1 = tail length < diameter of nucleus, 2 = tail length between 1 and 2X the diameter of nucleus; and 3 = tail length > 2X the diameter of nucleus. Data are presented as mean ± SEM
a,b,c,dMean values within tissue with unlike superscript letters were significantly different (P < 0.05)
The rates of DNA damage in the group of rats exposed to Alcl3 were increased significantly (P < 0.01) compared with that in control rats (Tables 1 and 3). However, the DNA damage in the liver and kidney tissues of Alcl3-exposed rats treated with Feb (10 mg/kg) were decreased significantly in comparison to those in rats exposed to Alcl3 only. Moreover, the DNA damage in the liver and kidney tissues of Alcl3-exposed rats treated with Feb (15 mg/kg) were reduced much more than those in Alcl3-exposed rats treated with Feb (10 mg/kg) but without significant differences.
Effect of febuxstat on the histopathological picture in the liver and kidney in rats intoxicated with Alcl3
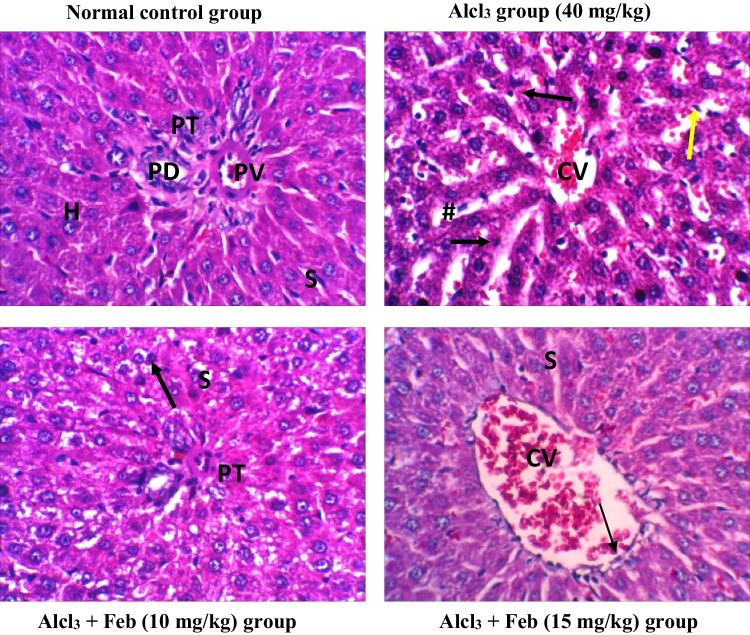
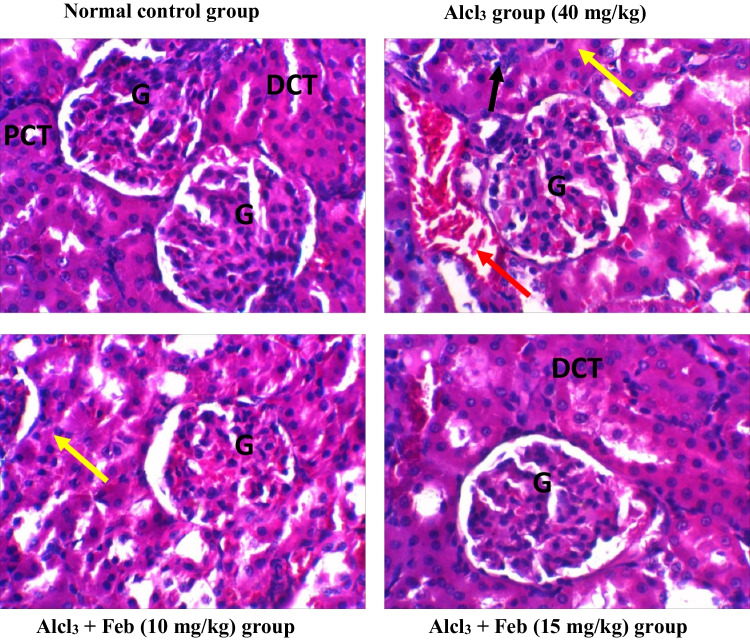
Alcl3-induced severe pathological alterations, marked loss of architecture, severe inflammatory cells were observed in the liver and kidney tissue. However, Alcl3 + Feb (15 mg/kg) group showed mild inflammation with few coagulative necrosis in some hepatic cells and nephrocytes. While Alcl3 + Feb (15 mg/kg) group showed normal histological appearance in liver and kidney tissues with an increase in Kupffer cells for regeneration and little hemorrhage and congestion in kidney tissue (Figs. 9 and 10).
Fig. 9.
photomicrographs of H&E staining rat liver sections of control and different experimental groups. Normal control group showing normal liver tissue histology with normal hepatic cell aggregation. Hepatocyte (H) appears in usual shape rows separated by hepatic sinusoids (S), normal hepatic portal tract (PT) with the normal portal vein (PV) and bile duct (PD). AlCl3 group (40 mg/kg) showing complete loss of normal hepatic cord architecture with necrotic. Degeneration and necrotic hepatocyte (black arrow) around congested central veins (CV) and inflammatory cell aggregation (Yellow arrow) and swollen sinusoids (#). Alcl3 + Feb (10 mg/kg) group showing mild shrunken portal tract (PT), mild coagulative necrosis of some hepatic cells (black arrow) slightly swelled hepatic sinusoids (S). Alcl3 + Feb (15 mg/kg) group showing most hepatic cord preserve the normal histological appearance, with congestion central vein (CV) lined with normal endothelial cells (thin arrow) and normal sinusoids (S), with an increase in Kupffer cells (Kc) for regeneration. Magnification X: 400
Fig. 10.
photomicrographs of H&E staining rat kidney sections of control and different experimental groups. Normal control group showed a normal structure of the kidney with glomerulus (G), proximal convoluted tubule (PCT) and distal convoluted (DCT). Alcl3 group (40 mg/kg) showed severe congestion and hemorrhage of glomerular tuft (G), swelling of renal tubules (yellow arrow) with renal casts in lumen (black arrow), Edema in the interstitial tissue with lymphocytic cells infiltration around glomeruli and renal tubules (red arrows). Alcl3 + Feb (10 mg/kg) group showed mild congestion of glomerular tuft (G) and some tubules suffered cloudy swelling (yellow arrow). Alcl3 + Feb (15 mg/kg) group showed improvement in the kidney histology architecture resembling the control group with still little swelling cells and mild hemorrhage. Magnification X: 400
Effect of febuxstat on the expression levels of Caspase-3 in the liver and kidney in rats intoxicated with Alcl3
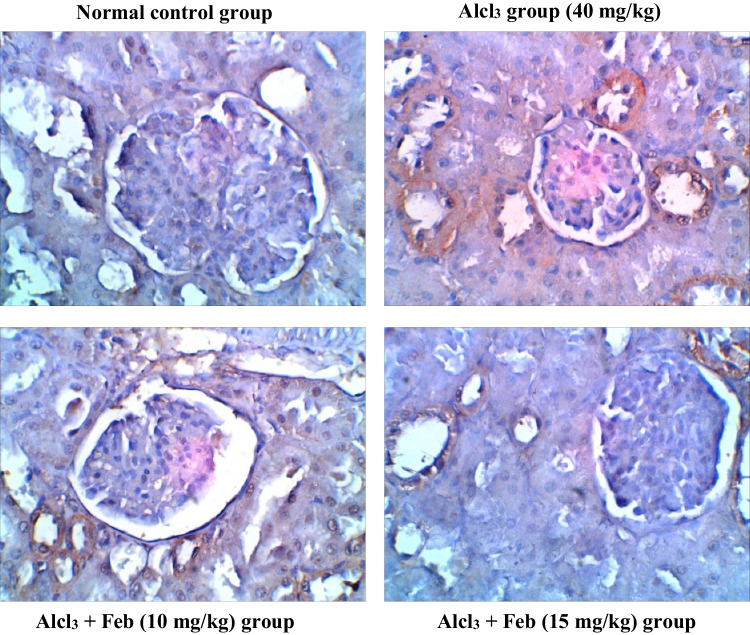
Oral administration of theAlcl3 group (40 mg/kg) for 2 months revealed marked increase in the number of hepatic and renal Caspase-3 immunolabeled cells around central veins suggesting inflammatory response and apoptosis. Alcl3 + Feb (10 mg/kg) group showing a slight decrease in hepatic and renal Caspase-3 immunolabeled cells, indicate a slight improvement in the liver and kidney section. Alcl3 + Feb (15 mg/kg) the group showed a significant reduction in Caspase-3 immunolabeled cells, nearly normal to control group (Figs. 11 and 12).
Fig. 11.
Immunohistochemical staining of Caspase-3 in liver sections from control and experimental groups. Normal control group revealing rare immunolabeled hepatic cells of Caspase -3. Alcl3 group (40 mg/kg) revealed brown staining with a slight increase in the number of Caspase-3 immunolabeled hepatocytes (that were observed around central veins suggesting inflammatory response and slight increase in apoptosis. Alcl3 + Feb (10 mg/kg) group showing slight decrease in Caspase-3 immunolabeled cells, indicating a slight improvement in the liver section. Alcl3 + Feb (15 mg/kg) group showed slight decrease in Caspase-3 immunolabeled cells, nearly normal to the control group
Fig. 12.
Immunohistochemical staining of Caspase-3 in kidney sections from control and experimental groups. Normal control group revealing negligible caspase-3 immunopositivity in the cortical regions of the kidney, glomerulus not stained brown. Alcl3 group (40 mg/kg) revealed strong increase in Caspase-3 expression in cortical areas especially in the proximal convoluted tubules as an inflammatory marker, whereas in the glomerulus was less Caspase-3 expression. Alcl3 + Feb (10 mg/kg) group showing partial inhibition in Caspase-3 expression indicating weak immune staining in distal convoluted tubules of cortical regions. Alcl3 + Feb (10 mg/kg) group showing noticed few cells were positive expression for cleaved Caspase-3 almost normal if compared to a normal control group
Effect of febuxstat on the expression levels of TNF-α in the liver and kidney in rats intoxicated with Alcl3
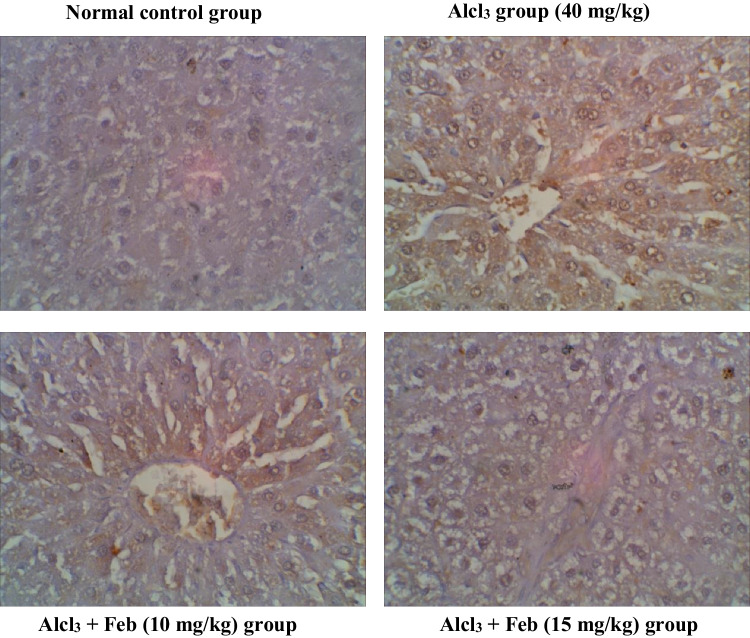
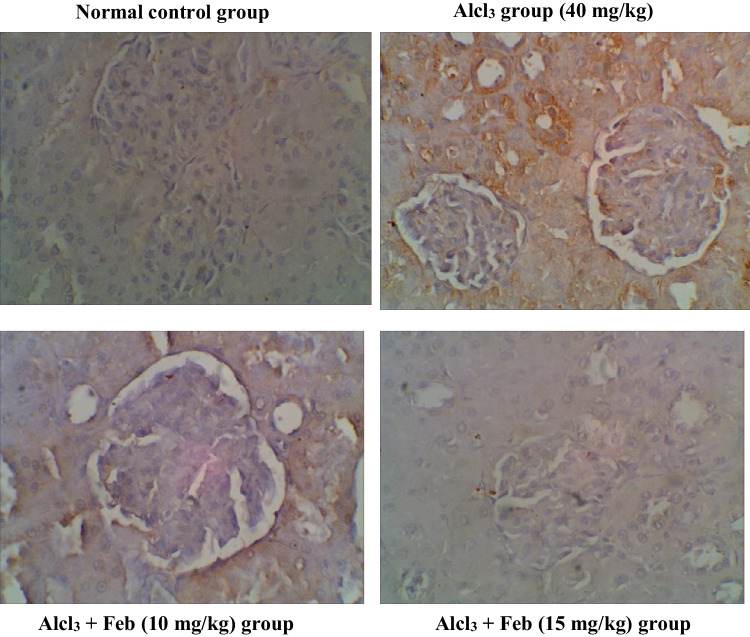
Oral administration of the Alcl3 group (40 mg/kg) for 2 months revealed marked increase in the number of hepatic and renal TNF-α, indicating mononuclear inflammatory cells to starting the necrosis process. Alcl3 + Feb (10 mg/kg) the group showed a slight decrease in hepatic and renal TNF-α, indicating a slight improvement in liver and kidney section. Alcl3 + Feb (15 mg/kg) the group showed a significant reduction in TNF-α, nearly normal to control group (Figs. 13 and 14).
Fig. 13.
Immunohistochemical staining of TNF—α in liver sections from control and experimental groups. The normal control group revealed no expression of TNF-α immunostained hepatic cells. Alcl3 group (40 mg/kg) revealed a strong increase in TNF-α expression of apoptotic hepatocytes indicating hepatic injury. Alcl3 + Feb (10 mg/kg) group showing reduced expression of TNF-α of hepatocytes. Alcl3 + Feb (10 mg/kg) group showed a marked reduction in the expression of TNF-α in the liver section showing somewhat normal cells. Brown color indicates immunopositivity for each stain, CV: central vein. H: hepatocyte. Magnification: 40X
Fig. 14.
Immunohistochemical staining of TNF—α in kidney sections from control and experimental groups. The normal control group revealed no TNF-α positive stained cells in the cortical region of kidney tissue. Alcl3 group (40 mg/kg) showed that glomerular sections have high TNF-positive immunostaining indicating mononuclear inflammatory cells as starting the necrosis process. Alcl3 + Feb (10 mg/kg) group showing moderate TNF-α immune-reactive renal tubular cells as amelioration in kidney tissue. Alcl3 + Feb (10 mg/kg) group showing very weak TNF-α immune-reactive cells nearly normal if compared to control group staining. Brown color indicates immunopositivity for each stain, G: Glomerulus. CT: proximal convoluted tubules. Magnification: 40X
Results of pharmacokinetic of febuoxstat by Swiss target prediction software
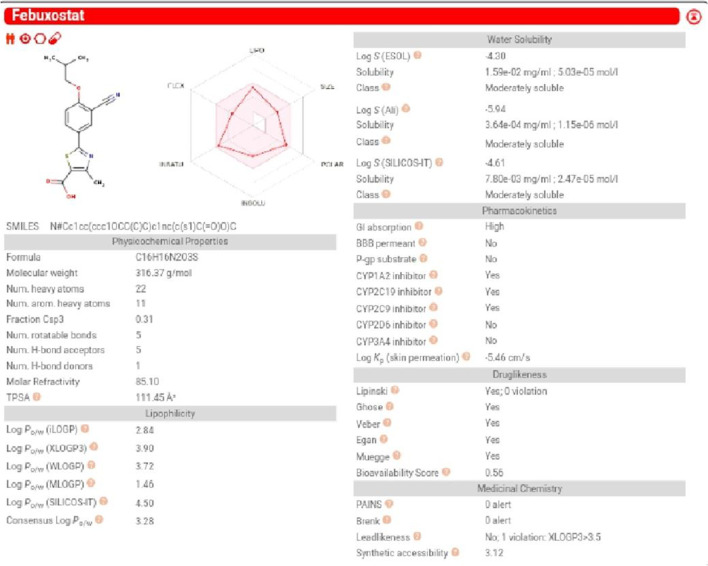
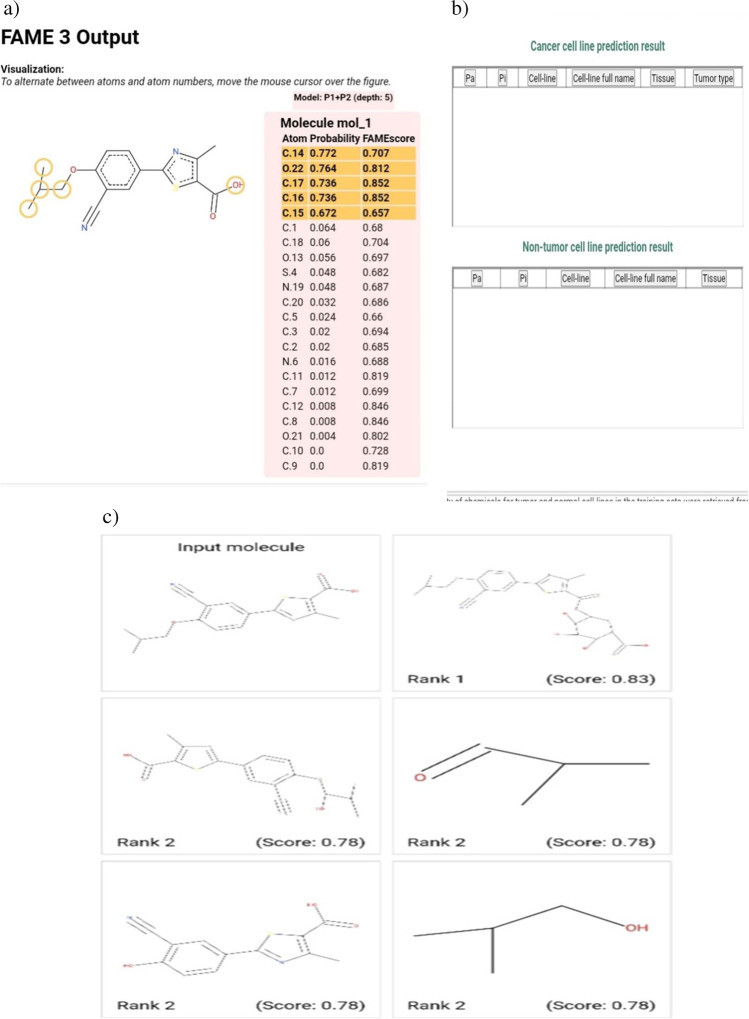
Feb exhibits high GIT absorption, moderate water solubility, and low BBB permeability. Additionally, the Feb adhered to most drug-likeness rules, including the Lipinski rule. Feb showed no toxicity against the various cell lines (Figs. 15 and 16).
Fig. 15.
Evaluation of the physic-chemical and pharmacokinetic properties of febuxostat using Swiss target prediction software. The physicochemical properties that were evaluated are absorption, distribution, bioavailability and water solubility. Febuoxostat followed Lipinski, Ghose, Veber, Egan and Mueggr rules, while bioavailability scores, 0.56
Fig. 16.
Evaluation of FAME1, possible metabolites and toxicity against cell lines. Swiss target prediction software was applied to score FAME1, products of phase 1 metabolism and toxicity against cell lines
Results of molecular docking of febuxstat
Molecular docking scores and interactions of Feb against target proteins are represented in Table 5 and Figs. 1, 2, and 3. Regarding cyclooxygenase-1 (COX-1), Feb made four hydrophobic (ARG83, PRO86 (2), and LYS473), and three hydrogen (SER85, ARG467, and GLU520) bonds during its binding with the COX-1 binding site (Fig. 1). Feb bound with the amino acid residues in the NF-kappa-B-inducing kinase (NIK) binding site by pKi value of 5.43 (Table 5 and Fig. 2). Feb bound and interacted with NIK binding site by two charge (ARG363 (2)), six hydrophobic (ARG363, ARG366, ARG368, PRO372 (2), and LEU433), and one hydrogen (ARG363).
Table 5.
Molecular docking results of febuxostat against NF-kappa-B-inducing kinase, MAPK-P38, and Cyclooxygenase-1 (COX-1)
| Febuoxostat | Protein | Binding free energy (kcal/mol) | pKi | Ligand efficiency (kcal/mol/non-H atom) | |
|---|---|---|---|---|---|
| NF-kappa-B-inducing kinase (NIK) PDB ID; 4dn5_A | Scores | − 7.4 | 5.43 | 0.3364 | |
| Interactions |
Charge: ARG363 (2) Hydrophobic: ARG363, ARG366, ARG368, PRO372 (2), and LEU433 Hydrogen: ARG363 |
||||
|
MAPK-P38 PDB ID; 1a9u_A |
Scores | − 6.2 | 4.55 | 0.2818 | |
| Interactions |
Hydrophobic: VAL30, TYR35, VAL38, and ARG173 Hydrogen: TYR35 |
||||
|
Cyclooxygenase-1 (COX-1) PDB ID; 6Y3C-A |
Scores | − 6.6 | 4.84 | 0.3 | |
| Interactions |
Hydrophobic: ARG83, PRO86 (2), and LYS473 Hydrogen: SER85, ARG467, and GLU520 |
||||
Molecular docking was utilized to illustrate the interaction between febuxostat against NIK, MAPK-P38, and COX1. Binding free energy, PKi, and ligand efficiency were scored. In addition, the number and type of chemical interactions were detected
Results in Table 5 and Fig. 3 showed the pKi values of Feb is (4.55) towardMAPK-p38. Feb interacted with MAPK-p38 binding site interacted by four hydrophobic (VAL30, TYR35, VAL38, and ARG173) and one hydrogen (TYR35).
Feb bound and interacted with the binding site of interleukin-1 beta convertase by two charge (ARG161 and ARD163) and four hydrophobic (TRP145, ILE155 (2), and LEU196) bonds with pKi value of 3.81.
Discussion
Aluminum (AL) is widely spread in the crust of the earth; t makes up about 8% of all the mineral elements. It is easy to enter our body because it is a component of cooking utensils and medications including anti-acids, deodorants, and food additives so toxicity of aluminum is considered due to its availability (Hassan and Kadry 2021b). Because of its great availability, AL can accumulate in the liver and kidney, making humans especially sensitive to its toxicity (Al Dera 2016). These findings suggest that the potential role of febuxostat (Feb) against Alcl3 toxicity-induced hepatorenal injury in rats. Febuxostat is available in two dose levels (10 and 15 mg/kg/day), with the later dose appearing to be the most efficient.
Based on our research, we can state with certainty that Feb has the greatest ability to scavenge ROS, activate antioxidant enzymes, and block the inflammatory cascade and apoptosis in order to prevent Alcl3-induced hepatotoxicity and nephrotoxicity.
Hence, Feb is essential for the creation of novel therapeutic strategies intended to combat Alcl3-induced hepatotoxicity and nephrotoxicity. Our findings showed that, when compared to the control group, the Alcl3-exposed group had significantly (p 0.05) lower levels of GSH and MDA in the liver and kidneys, When compared to the Alcl3-treated group, treatment with Feb 10 mg/kg significantly (p 0.05) enhanced the levels of GSH and MDA in the liver and kidneys. Although a treatment with Feb15 mg/kg could restore normal GSH and MDA levels to the liver and kidneys.
Feb administration to intoxicated rats could improve the antioxidant status and ameliorated the changes in biochemical indices and histopathological picture. Al is not a redox active metal, but it can produce reactive oxygen species (ROS). Aqueous solution of AlCl3 which administered orally for three months with a 10 mg/kg/day can lead to oxidative stress and cell damage in a variety of tissues, including the liver and kidney (Zatta et al. 2002).
Lipid peroxidation (MDA) is regarded as the primary indicator of oxidative damage in the toxicity of many xenobiotics (Mężyńska et al. 2019). As a result of increased free radical production, which reacts with polyunsaturated fatty acids to produce hydroperoxides and start a chain reaction that damages cell membranes by oxidation. Due to Feb’s potent antioxidant properties, administration of 15 mg/kg of Feb was successful in bringing down the elevated levels of MDA. This is in line with earlier research showing powerful antioxidant effects of Feb in a dose febuxostat (10 mg/kg; p.o.) for 21 days (Khames et al. 2017). Additionally, it has been demonstrated that Feb lowers ROS and MDA levels in the aortas of streptozocin-diabetic rats and atherosclerotic mice which administrate Feb 0.027 mg/mL with drinking water for 12 weeks (Nomura et al. 2014).
The human body possesses unique antioxidant redox hemostasis to maintain and stabilize the balance of oxidative molecules (Hamid et al. 2010). In the current study, Alcl3-induced decrease in hepatic and renal GSH content, indicating impairment in antioxidant defense system. These findings are matching with earlier study that showed that Alcl3 causes oxidative stress, which lowers the activity of GSH and inhibits the levels of antioxidant enzymes in various tissues (Kumar and Gill 2014). This study documented that the antioxidant enzymes activity was inhibited due to reduced GSH biosynthesis either from increased intracellular Al levels or excessive free radical production (Cheraghi and Roshanaei 2019). Supplementation of Alcl3-intoxicated rats with Feb could normalize the hepatic and renal activity of GSH indicating its ability to restore redox homeostasis. Previous research revealed that oral febuxostat (10 and 15 mg/kg/day, respectively) for 14 days beginning 7 days before cisplatin injection (Fahmi et al. 2016).
Our findings demonstrated that the Alcl3-treated group, Feb 10 mg/kg treatment significantly (p 0.05) lowered AST, ALT, and ALP. However, the aforementioned parameters might be returned to normal values with treatment with Feb 15 mg/kg. Exposure to Alcl3-induced disruption in hepatic and renal membranes leading to subsequent leakage of these biomarkers into the systemic circulation. Our findings concur with those of an earlier study that found that higher hepatic and kidney function indices are considered the chief symptoms of Alcl3 in a dose of (34 mg/kg which equal to 1/25 LD 50), induced hepatotoxicity and nephrotoxicity (El-Demerdash et al. 2022). Pretreatment with Feb significantly reduced Alcl3-induced hepatotoxicity and nephrotoxicity, as evidenced by reducing serum hepatic and kidney values. These results are consistent with a prior study that documented the ameliorative role of Feb during exposure to xenobiotics (Battelli et al. 2018).
Hepatotoxicity is reflected by significant alteration in lipid metabolism. Our findings revealed that administration of Alcl3 orally for 2 months caused a marked increment in the values of total lipids, triglycerides, and total cholesterol. As previously stated, Alcl3 administration leads to elevated MDA-induced disruption in membrane integrity causing significant impairment in lipid metabolism. Alterations in enzyme activities are considered the main factors in triggering hypertriglyceridemia and hypercholesterolemia and those results were in line with (Newairy et al. 2009). On the other hand, administration of Feb (15 mg/kg) could restore the forementioned parameters and our results were agreed (Heikal et al. 2019) that reported that Feb showed strong anti-inflammatory and antioxidant properties by lowering serum levels of proinflammatory cytokines and lipid peroxidation index and increasing antioxidant enzyme activity in high-fat diet rabbits.
Hepatotoxicity is thought to be indicated by abnormal protein metabolism. The administration of Alcl3 in the current study caused a marked reduction in the levels of albumin and total protein. This finding is in agreement with (Al-Eisa et al. 2017), administration of aluminium chloride in adose 30 mg/kg every other day intraperitoneally for 8 weeks revealed the decrease in total protein and albumin in Alcl3-treated animals might be related to alterations in the synthesis and/or metabolism of protein. On the other hand, administration of Feb (10 and 15 mg/kg) to Alcl3 intoxicated rats showed normalization in the levels of albumin and total protein indicating the occurrence of conformational changes in the albumin and protein levels and those findings are matched with prior study (Maresh et al. 2020).
The master regulator of several antioxidant enzymes, nuclear factor-erythroid 2-related factor 2 (Nrf2) modifies cellular redox equilibrium and detects the presence of oxidative stress. This is accomplished by increasing the activity of enzymes involved in antioxidant defense, including ferritin, glutathione reductase, glutathione peroxidase, heme oxygenase-1 (HO-1), and superoxide dismutase (SOD) (Younis et al. 2021). This redox sensitive transcription factor is also present in the cells to protect it from oxidative stress (Kensler et al. 2007). All tissues have constitutively high amounts of Nrf2, which might fluctuate depending on how much detoxification an organ needs to perform (Shelton and Jaiswal 2013). When exposed to oxidative stress, Nrf2 dissociates from Keap1 and moves to the nucleus where it binds with the antioxidant-response element (ARE) to control the transcription of antioxidant genes (Lewis et al. 2010). Nrf2 is kept in the cytosol by attaching to several proteins, including its cytosolic inhibitor, Kelch like-ECH-associated protein 1 (Keap1) (Wei et al. 2011).
According to this study, rats exposed to Alcl3 had significantly lower levels of Nrf2 and MNK expression in their liver and kidney tissues than did control rats (P 0.05). However, compared to rats merely exposed to Alcl3, rats treated with Feb (10 mg/kg) after being exposed to Alcl3 had considerably higher expression levels of Nrf2 and MNK in their liver and kidney tissues. Moreover, Alcl3-exposed rats treated with Feb (15 mg/kg) had considerably higher expression levels of the Nrf2 gene in their liver and kidney tissues than Alcl3-exposed rats treated with Feb (10 mg/kg), but not of the MNK gene. Our findings are consistent with thoseof (Chen et al. 2021) who found a connection between Nrf2 dysregulation and Alcl3 toxicity which induced by oral administration of 175 mg/kg of AlCl3 for 25 days. This suggests that increased oxidative stress created by the Alcl3 environment signals the cell to produce more Nrf2, but despite increased production, it fails to reach the nucleus to augment the transcription machinery. And thus may explain the mechanisms of action of AL in our body that produces free radicals via, increases the ability to uptake iron; thus, oxidative stress may contribute the Alcl3 toxicity (Al-Kahtani and Morsy 2019).
One of our most interesting results is that the oral administration of Feb (10 mg/kg and 15 mg/kg b.w.) concurrent with Alcl3 upregulated the hepatic and renal levels of the Nrf2 gene. This would imply that one of the Feb’s primary defense mechanisms against Alcl3-induced toxicity is activation of Nrf2. Our results are consistent with those of (Omizo et al. 2020), who hypothesized that Feb was dissolved in drinking at a concentration of 0.03 mg/L for 8 weeks might facilitate the nuclear translocation of Nrf2, that potentiate the renal antioxidant capacity. Additionally, previous studies showed that elevated Nrf2 expression was associated with reduced inflammation in hepatic ischemia–reperfusion injury; therefore, we could conclude that Nrf2 mediates antioxidant, the anti-inflammatory, and antiapoptotic effects (Zhong et al. 2013).
Recent research also revealed that the activation of numerous signaling pathways, including PI3Kphosphatidylinositol 3-kinase/Akt, protein kinase B PKC protein kinase C, and MNK mitogen-activated protein kinase, allows the release of Nrf2 from Keap1 and subsequent translocation for the induction of diverse antioxidant and detoxifying enzyme expressions (Martin et al. 2004). MAP kinase-interacting serine/threonine kinase (MNK) was discovered as a subfamily of murine serine/threonine kinase by screening a mouse embryo library with a novel ERK-interacting clone as a probe (Guo et al. 2017). The MNK pathway controls a number of cellular processes; cancer, neurodegeneration, and inflammation have all been linked to dysregulation of MAPK kinase pathways (Hassan and Kadry 2021a). JNK, MNK P38 and extracellular signal-regulated kinase (ERK) are members of the MAPK family. Its activation has recently been claimed to have a crucial role in the pathophysiology of hepatic encephalopthy (Khalil et al. 2022).
Our current study revealed that Alcl3-induced a significant downregulation in hepatic and renal MNK (ERK). This could imply that MNK (ERK) activation is one of the Feb’s key protective mechanisms against Alcl3-induced toxicity. Furthermore, the effect of Feb on MNK (ERK) expression in the liver and kidneys of treated rats were correlated with the activities of antioxidant enzymes in this study. Activating MNK (ERK) can prevent oxidative stress-induced cell apoptosis and thus consistent with these findings, in our study, co-treatment with Alcl3 and Feb (10 and 15 mg/kg) cause activation of ERK and alleviated ROS generation, which reveals that MNK, Alcl3 and oxidative stress may influence each other. Our results are consistent with (Khan et al. 2017) who demonstrated that Feb pretreatment activate the ERK1/2 pathway and suppressing the p38/JNK/NF-κBp65/T TNF-α pathway and thus reduced the heart dysfunction brought on by reperfusion injury.
The basic mechanism of carcinogenesis is oxidative stress (OS), and methylation of its associated genes may contribute to the development of cancer. A crucial part of several metabolic processes is played by the mitochondrial matrix enzyme that CRAT generates, which catalyzes the inter conversion of acetyl-CoA and acetyl carnitine. According to studies, CRAT not only regenerates free CoA but also buffers the mitochondrial acetyl-CoA pool, both of which have an impact on the actions of a number of oxidative enzymes. Insufficient CRAT activity may worsen number of metabolic disturbances and raise oxidative stress levels (Seiler et al. 2014).
When compared to control rats, the group of rats treated to Alcl3 showed a substantial upregulation in the expression levels of the Crat and Car3 genes in the liver and kidney tissues. However, compared to rats exposed to Alcl3 alone, animals treated with Feb (10 mg/kg) showed significantly lower levels of Crat and Car3 expression in the liver and kidney tissues. Additionally, rats exposed to Alcl3 who were given Feb (15 mg/kg) had significantly lower Crat and Car3 expression levels in their liver and kidneys than rats exposed to Alcl3 who were given Feb (10 mg/kg).
So, this will decrease oxidative stress levels and enhance different metabolic processes. Our results are consistent with Gao et al. 2016.
Carbonic anhydrase (car3) is a family of metalloenzymes, and its active site contains a zinc ion that has an important role in carcinogenesis. The primary job of car3 is to catalyze the reversible hydrolysis of carbon dioxide into bicarbonate. Car3 takes involvement in the transport of carbon dioxide, calcification, and photosynthesis. In mammals, car3 participates in the metabolism-related synthesis of glycogen, urea, and lipids and controls ion transport, pH value, and water homeostasis (Bolt et al. 2005). Furthermore, the ability of Feb to reduce the levels of crat and car 3 (procarcinogenic genes) in the hepatic and renal tissue of the Alcl3-feb groups supported this assumption. These findings are in parallel with Szollosi et al. 2020 who showed that car3 enhances the capacity of hepatocellular carcinoma cells to invade through the FAK signaling pathway. In this study it was observed that the Alcl3 group showed over expression of car3 when compared to the control group. While, other researchers have made use of car3 inhibitors, such as acetazomide, methazolamide, ethoxzolamide, dichlorophenamide, dorzolamide, and brinzolamide. According to certain research, both in vivo and in vitro cancer cell growth, proliferation, migration, and colony formation may be considerably decreased by car3 inhibitors (Karakuş et al. 2018). This assumption is in harmony with Prat et al. 1985. Who revealed that a link between car3 and tumor metastasis.
According to the results of molecular docking studies, Feb showed strong binding capacity with the core targets of inflammatory cascades especially, COX-1, MAPK-p38 and NF-κB.
Conclusion
Our current study used two dose levels of febuxostat (10 and 15 mg/kg/day), where, the latter dose appears the most effective. Based on our findings, we can conclusively report that Feb can avert Alcl3-induced hepatotoxicity and nephrotoxicity due to its greatest activity to scavenge ROS, triggering the activity of antioxidant enzymes, and inhibiting the inflammatory cascade and apoptosis. As a result, Feb is crucial for the development of cutting-edge therapeutic approaches that aimed to counteract Alcl3-induced hepatotoxicity and nephrotoxicity.
Acknowledgements
NA
Abbreviations
- Feb
Febuxostat
- Al
Aluminum
- Crat
Carnitine o-acetyltransferase
- Car3
Carbonic anhydrase
- MNK
MAP kinase-interacting serine/threonine kinase
- MDA
Malondialdehyde
- GSH
Glutathione
- Nrf2
Nuclear factor erythroid 2-related factor 2
- TNF-α
Tumor necrosis factor-alpha
- COX-1
Cyclooxygenase-1
- NIK
NF-kappa-B-inducing kinase
- MAPK-p38
Mitogen-activated protein kinases-p38
- Alcl3
Aluminum chloride
- ROS
Reactive oxygen species
- PBS
Phosphate-buffered saline
- NRC
National Research Centre
- ANOVA
One-way analysis of variance
- LPO
Lipid peroxidation
- OS
Oxidative stress
- ARE
Antioxidant-response element
- HO-1
Heme oxygenase-1
- SOD
Superoxide dismutase (SOD)
- DEPC
Diethylpyrocarbonate
- GLM
General liner models
- AST
Alanine amino transferase
- ALT
Aspartate amino transferase
- ALP
Alkaline phosphatase
- BBB
Blood brain barrier
Authors’ contributions
Conceptualization: Ahmed A. Sedik; methodology: Ahmed A. Sedik, Soha A. Hassan, Heba I. Shafey, Wagdy K. B. Khalil, Noha A. Mowaad; formal analysis and investigation: Ahmed A. Sedik, Soha A. Hassan, Heba I. Shafey, Wagdy K. B. Khalil, Noha A. Mowaad; writing—original draft preparation: Ahmed A. Sedik, Soha A. Hassan, Heba I. Shafey, Wagdy K. B. Khalil, Noha A. Mowaad; writing—final draft: Ahmed A. Sedik, Noha A. Mowaad. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). NA.
Data availability
All data are available upon request.
Declarations
Ethics approval
The study was carried out in accordance with the ethical standards documented by the Research Ethics Committee, Faculty of Dentistry, October 6 University, Giza, Egypt, under approval number (RECO6U/10–2022).
Consent to participate
NA.
Consent for publication
All authors have approved the final manuscript for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al Dera HS. Protective effect of resveratrol against aluminum chloride induced nephrotoxicity in rats. Saudi Med J. 2016;37:369. doi: 10.15537/smj.2016.4.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Eisa R, Al Nahari H. Protective effect of royal jelly against the liver toxicity caused by aluminum chloride (AlCl3) in adult male rats. Adv Environ Biol. 2016;10:113–127. [Google Scholar]
- Al-Eisa R, Khouja H, Al-Nahari H (2017) Turmeric (Curcuma Longa) protection against the liver toxicity caused by aluminum chloride (AlCl3) in Adult Male Rats. Int J Pharm Res Allied Sci 6(2)
- Al-Kahtani M, Morsy K. Ameliorative effect of selenium nanoparticles against aluminum chloride-induced hepatorenal toxicity in rats. Environ Sci Pollut Res. 2019;26:32189–32197. doi: 10.1007/s11356-019-06417-y. [DOI] [PubMed] [Google Scholar]
- Al-Kahtani M, Abdel-Daim MM, Sayed AA, El-Kott A, Morsy K. Curcuminphytosome modulates aluminum-induced hepatotoxicity via regulation of antioxidant, Bcl-2, and caspase-3 in rats. Environ Sci Pollut Res. 2020;27:21977–21985. doi: 10.1007/s11356-020-08636-0. [DOI] [PubMed] [Google Scholar]
- Battelli MG, Bortolotti M, Polito L, Bolognesi A (2018) The role of xanthine oxidoreductase and uric acid in metabolic syndrome. BiochimicaetBiophysicaActa (BBA)-Molecular Basis of Disease 1864:2557–2565 [DOI] [PubMed]
- Belfield A, Goldberg D. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12:561–573. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- Beutler E. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Blasiak J, Arabski M, Krupa R, Wozniak K, Zadrozny M, Kasznicki J, Zurawska M, Drzewoski J. DNA damage and repair in type 2 diabetes mellitus. Mutat Res/fundamental Mol Mechan Mutagenesis. 2004;554:297–304. doi: 10.1016/j.mrfmmm.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Bolt RJ, Wennink J, Verbeke J, Shah GN, Sly WS, Bökenkamp A. Carbonic anhydrase type II deficiency. Am J Kidney Dis. 2005;46(A50):e71–e73. [PubMed] [Google Scholar]
- Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J Biol Sci. 2021;28:4232–4239. doi: 10.1016/j.sjbs.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheraghi E, Roshanaei K (2019) The protective effect of curcumin against aluminum chloride-induced oxidative stress and hepatotoxicity in rats. Pharma Biomed Res 5(1):11–18
- Collins A, Dušinská M, Franklin M, Somorovská M, Petrovská H, Duthie S, Fillion L, Panayiotidis M, Rašlová K, Vaughan N. Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen. 1997;30:139–146. doi: 10.1002/(sici)1098-2280(1997)30:2<139::aid-em6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- deBruyn Kops C, Stork C, Šícho M, Kochev N, Svozil D, Jeliazkova N, Kirchmair J. GLORY: generator of the structures of likely cytochrome P450 metabolites based on predicted sites of metabolism. Front Chem. 2019;7:402. doi: 10.3389/fchem.2019.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clinicachimicaacta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- El-Baz FK, Salama A, Salama RAA (2020) Dunaliella salina attenuates diabetic neuropathy induced by STZ in rats: involvement of thioredoxin. BioMed research international 2020:1295492 [DOI] [PMC free article] [PubMed]
- El-Demerdash FM, Hussien DM, Ghanem NF, Al-FargaAM (2022) Bromelain modulates liver injury, hematological, molecular, and biochemical perturbations induced by aluminum via oxidative stress inhibition. BioMed Res Int 2022:5342559 [DOI] [PMC free article] [PubMed]
- Fahmi AN, Shehatou GS, Shebl AM, Salem HA. Febuxostat protects rats against lipopolysaccharide-induced lung inflammation in a dose-dependent manner. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:269–278. doi: 10.1007/s00210-015-1202-6. [DOI] [PubMed] [Google Scholar]
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Gamble M, Banks I, Bancroft JD (2008) Managing the laboratory. Theory and Practice of Histological Techniques, vol 1
- Gao T, Joyce BT, Liu L, Zheng Y, Dai Q, Zhang Z, Zhang W, Shrubsole MJ, Tao M-H, Schwartz J. DNA methylation of oxidative stress genes and cancer risk in the Normative Aging Study. Am J Cancer Res. 2016;6:553. [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Peng G, Li E, Xi S, Zhang Y, Li Y, Lin X, Li G, Wu Q, He J. MAP kinase-interacting serine/threonine kinase 2 promotes proliferation, metastasis, and predicts poor prognosis in non-small cell lung cancer. Sci Rep. 2017;7:10612. doi: 10.1038/s41598-017-10397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid A, Aiyelaagbe O, Usman L, Ameen O, Lawal A. Antioxidants: its medicinal and pharmacological applications. Afr J Pure Appl Chem. 2010;4:142–151. [Google Scholar]
- Hassan SA, Kadry MO. Neurodegenerative and hepatorenal disorders induced via aluminum chloride in murine system: impact of β-secretase, MAPK, and KIM. Biol Trace Elem Res. 2021;199:227–236. doi: 10.1007/s12011-020-02132-9. [DOI] [PubMed] [Google Scholar]
- Hassan NM, Alhossary AA, Mu Y, Kwoh C-K. Protein-ligand blind docking using QuickVina-W with inter-process spatio-temporal integration. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-15571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikal MM, Shaaban AA, Elkashef WF, Ibrahim TM. Effect of febuxostat on biochemical parameters of hyperlipidemia induced by a high-fat diet in rabbits. Can J Physiol Pharmacol. 2019;97:611–622. doi: 10.1139/cjpp-2018-0731. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Lee KH, Jang HH, Lee SR, Woo JS, Lee HJ, Jung KH, Kim W. Febuxostat contributes to improvement of endothelial dysfunction in an experimental model of streptozocin-induced diabetic rats. Int J Cardiol. 2014;171:e110–e112. doi: 10.1016/j.ijcard.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Karakuş F, Eyol E, Yılmaz K, Ünüvar S. Inhibition of cell proliferation, migration and colony formation of LS174T Cells by carbonic anhydrase inhibitor. Afr Health Sci. 2018;18:1303–1310. doi: 10.4314/ahs.v18i4.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Khalil WK, Booles HF. Protective role of selenium against over-expression of cancer-related apoptotic genes induced by o-cresol in rats. Arhivzahigijenurada i Toksikologiju. 2011;62:121. doi: 10.2478/10004-1254-62-2011-2074. [DOI] [PubMed] [Google Scholar]
- Khalil HM, Khalil IA, Al-Mokaddem AK, Hassan M, El-Shiekh RA, Eliwa HA, Tawfek AM, El-Maadawy WH (2022) Ashwagandha-loaded nanocapsules improved the behavioral alterations, and blocked MAPK and induced Nrf2 signaling pathways in a hepatic encephalopathy rat model. Drug Deliv Transl Res 13(1):252–274 [DOI] [PMC free article] [PubMed]
- Khames A, Gad AM, Abd El-Raouf OM. Ameliorative effects of sildenafil and/or febuxostat on doxorubicin-induced nephrotoxicity in rats. Eur J Pharmacol. 2017;805:118–124. doi: 10.1016/j.ejphar.2017.02.046. [DOI] [PubMed] [Google Scholar]
- Khan SI, Malhotra RK, Rani N, Sahu AK, Tomar A, Garg S, Nag TC, Ray R, Ojha S, Arya DS (2017) Febuxostat modulates MAPK/NF-κBp65/TNF-α signaling in cardiac ischemia-reperfusion injury. Oxidative medicine and cellular longevity 2017:8095825 [DOI] [PMC free article] [PubMed]
- Kumar V, Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41:154–166. doi: 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH. Protein measurement with the Folin phenol reagent. J biolChem. 1951;193:265–275. [PubMed] [Google Scholar]
- Machado-Neves M, Dias FCR, de Almeida Lima GD, Ribeiro IM (2023) The effect of aluminum on mitochondrial dysfunctions. In: Mitochondrial Intoxication. Academic Press, pp 205–234
- Maresh MM, Abdelaziz RR, Ibrahim TM. Febuxostat mitigates concanavalin A-induced acute liver injury via modulation of MCP-1, IL-1β, TNF-α, neutrophil infiltration, and apoptosis in mice. Life Sci. 2020;260:118307. doi: 10.1016/j.lfs.2020.118307. [DOI] [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CMR, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Mężyńska M, Brzóska MM, Rogalska J, Galicka A. Extract from Aroniamelanocarpa L. berries protects against cadmium-induced lipid peroxidation and oxidative damage to proteins and DNA in the liver: a study using a rat model of environmental human exposure to this xenobiotic. Nutrients. 2019;11:758. doi: 10.3390/nu11040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newairy A-SA, Salama AF, Hussien HM, Yousef MI. Propolis alleviates aluminium-induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol. 2009;47:1093–1098. doi: 10.1016/j.fct.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Nomura J, Busso N, Ives A, Matsui C, Tsujimoto S, Shirakura T, Tamura M, Kobayashi T, So A, Yamanaka Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci Rep. 2014;4:1–9. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Okail HA, Ibrahim AS, Badr AH. The protective effect of propolis against aluminum chloride-induced hepatorenal toxicity in albino rats. J Basic Appl Zool. 2020;81:1–11. [Google Scholar]
- Olive PL, Banáth JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the" comet" assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- Omizo H, Tamura Y, Morimoto C, Ueno M, Hayama Y, Kuribayashi-Okuma E, Uchida S, Shibata S. Cardio-renal protective effect of the xanthine oxidase inhibitor febuxostat in the 5/6 nephrectomy model with hyperuricemia. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-65706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman MS, Fareid MA, Abdel Hameed RS, Abdel Moneim AE (2020) The protective effects of melatonin on aluminum-induced hepatotoxicity and nephrotoxicity in rats. Oxidative Medicine and Cellular Longevity 2020:7375136 [DOI] [PMC free article] [PubMed]
- Pedrycz A, Czerny K. Immunohistochemical study of proteins linked to apoptosis in rat fetal kidney cells following prepregnancyadriamycin administration in the mother. Acta Histochem. 2008;110:519–523. doi: 10.1016/j.acthis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Prat M, Morra I, Bussolati G, Comoglio PM. CAR-3, a monoclonal antibody-defined antigen expressed on human carcinomas. Can Res. 1985;45:5799–5807. [PubMed] [Google Scholar]
- Pui C-H, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, Furman WL, Ribeiro RC, Spunt SL, Rubnitz JE. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children's Research Hospital, 1992 through 2007. J Clin Oncol. 2012;30:2005. doi: 10.1200/JCO.2011.40.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Richmond W. Cholesterol enzymatic colorimetric test chop-PAP method of estimation of total cholesterol in serum. Clin Chem. 1973;191:1350–1356. [PubMed] [Google Scholar]
- Salem NA, Wahba MA, Eisa WH, El-Shamarka M, Khalil W. Silver oxide nanoparticles alleviate indomethacin-induced gastric injury: a novel antiulcer agent. Inflammopharmacology. 2018;26:1025–1035. doi: 10.1007/s10787-017-0424-2. [DOI] [PubMed] [Google Scholar]
- Sedik AA, Hassan SA (2022) Attenuation of sodium fluoride-induced hepatic injury by Lactobacillus casei through inhibition of NF-κβ signalling pathway with decreased expression of caspase-3 in rats. Egypt J Chem. 10.21608/EJCHEM.2022.174724.7193
- Seiler SE, Martin OJ, Noland RC, Slentz DH, DeBalsi KL, Ilkayeva OR, An J, Newgard CB, Koves TR, Muoio DM. Obesity and lipid stress inhibit carnitineacetyltransferase activity. J Lipid Res. 2014;55:635–644. doi: 10.1194/jlr.M043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton P, Jaiswal AK. The transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene? FASEB J. 2013;27:414–423. doi: 10.1096/fj.12-217257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi DE, Hokeness K, Manzoor MK (2020) Mechanisms of Autoimmunity and Pharmacologic Treatments. Pharmacol Immunotherapeutic Drugs:207–249
- Takeda Pharmaceuticals America (2009) Uloric (febuxostat) tablets prescribing information. Deerfield, IL. Reference ID 2898038
- Tsuda H, Kawada N, Kaimori J-y, Kitamura H, Moriyama T, Rakugi H, Takahara S, Isaka Y. Febuxostat suppressed renal ischemia–reperfusion injury via reduced oxidative stress. Biochem Biophys Res Commun. 2012;427:266–272. doi: 10.1016/j.bbrc.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia–reperfusion injury. Free Radical Biol Med. 2011;51:216–224. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis NS, Elsewedy HS, Shehata TM, Mohamed ME. Geraniol Averts Methotrexate-Induced Acute Kidney Injury via Keap1/Nrf2/HO-1 and MAPK/NF-κB Pathways. Curr Issues Mol Biol. 2021;43:1741–1755. doi: 10.3390/cimb43030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef MI. Aluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicology. 2004;199:47–57. doi: 10.1016/j.tox.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Zatta P, Kiss T, Suwalsky M, Berthon G. Aluminium (III) as a promoter of cellular oxidation. Coord Chem Rev. 2002;228:271–284. [Google Scholar]
- Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol vis Sci. 2013;54:3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner N, Kirsch K. Serum total lipids determination colorimetrically. Z GesExp Meal. 1962;1335:54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request.