Abstract
Small RNAs (sRNAs) of the fungal pathogen Botrytis cinerea can enter plant cells and hijack host Argonaute protein 1 (AGO1) to silence host immunity genes. However, the mechanism by which these fungal sRNAs are secreted and enter host cells remains unclear. Here, we demonstrate that B. cinerea utilizes extracellular vesicles (EVs) to secrete Bc-sRNAs, which are then internalized by plant cells through clathrin-mediated endocytosis (CME). The B. cinerea tetraspanin protein, Punchless 1 (BcPLS1), serves as an EV biomarker and plays an essential role in fungal pathogenicity. We observe numerous Arabidopsis clathrin-coated vesicles (CCVs) around B. cinerea infection sites and the colocalization of B. cinerea EV marker BcPLS1 and Arabidopsis CLATHRIN LIGHT CHAIN 1, one of the core components of CCV. Meanwhile, BcPLS1 and the B. cinerea-secreted sRNAs are detected in purified CCVs after infection. Arabidopsis knockout mutants and inducible dominant-negative mutants of key components of the CME pathway exhibit increased resistance to B. cinerea infection. Furthermore, Bc-sRNA loading into Arabidopsis AGO1 and host target gene suppression are attenuated in those CME mutants. Together, our results demonstrate that fungi secrete sRNAs via EVs, which then enter host plant cells mainly through CME.
Subject terms: Virulence, Plant molecular biology, Fungal host response
Fungal pathogen Botrytis cinerea can send sRNAs to plant cells to suppress plant immunity. Here the authors demonstrate that B. cinerea utilizes extracellular vesicles (EVs) to secrete Bc-sRNAs, which are then internalized by plant cells through clathrin-mediated endocytosis (CME).
Introduction
Communication between hosts and microbes/parasites involves the exchange of bioactive molecules, such as proteins and metabolites. Recent studies show that RNA molecules, particularly regulatory small RNAs (sRNAs), also travel between hosts and interacting organisms to induce gene silencing in trans, a mechanism termed “cross-kingdom RNA interference” (RNAi)1–14. Since the initial discovery of cross-kingdom RNA trafficking3, this regulatory mechanism has subsequently been observed between plants and many fungal pathogens11,12,15–17, oomycete pathogens13, and parasitic plants14. Interestingly, cross-kingdom sRNA trafficking has been found to be important even for symbiosis, specifically in mycorrhizal interactions10 and between prokaryotic Rhizobia and host soybean roots8. This mechanism is not limited to plant-microbe/parasite interactions and has also been observed in animal-pathogen/parasite systems4–6. In many of these interactions, the transferred microbial sRNAs can utilize host AGO proteins to silence host immune response genes3,8,11–13. Given the importance of these sRNA effectors in pathogen virulence, anti-fungal strategies that target and silence genes in the RNAi machinery, such as the Dicer-like (DCL) proteins that generate sRNAs, have been shown to be effective for plant protection16,18–22. Further protection could likely be achieved by inhibiting the transport processes that enable cross-kingdom trafficking of these sRNAs. However, how these transferred sRNAs are delivered and internalized by plant cells is still a mystery.
Extracellular vesicles (EVs) are used across the kingdoms of life to package and deliver functional molecules into recipient cells or organisms2,6,23–26. These EVs can provide protection to their cargos from degradation by extracellular enzymes, which is especially important for RNA trafficking. Indeed, EVs have been utilized by plants to deliver plant small RNAs to fungal pathogens during cross-kingdom RNAi2. Furthermore, many fungal species have been shown to secrete EVs, including yeast and filamentous fungi27–30. Although, only recently have EVs from plant pathogenic fungi such as the maize smut pathogen Ustilago maydis31, the wheat pathogen Zymoseptoria tritici32, and the cotton pathogen Fusarium oxysporum f. sp. vasinfectum33 been characterized. Still, it remains unclear whether plant fungal pathogens secrete EVs to deliver sRNAs into interacting plant cells.
Endocytosis is the primary route for eukaryotic cells to internalize plasma membrane proteins, lipids, and extracellular materials into the cytoplasm34,35. In plants, endocytosis is involved in many critical biological processes, including growth, development, signal transduction, nutrient uptake, and plant-microbe interactions36–39. In the past decades, substantial progress has been made in characterizing the function of endocytosis in plant immune responses. For instance, several plasma-membrane localized receptor proteins involved in the plant defense response are internalized through the endocytosis pathway36,39–41. Analogous to animal and yeast cells, clathrin-mediated endocytosis (CME) is the main route to internalize extracellular and plasma membrane materials in plants42. Clathrin is a triskelion-shaped complex composed of three clathrin heavy chains (CHCs) and three clathrin light chains (CLCs)34. By interacting with the plasma membrane, cargo proteins, adaptor protein 2 complex (composed of α, β2, μ2, and σ2 subunits) and other accessory protein factors, clathrin-coated pits (CCPs) can be formed34,43. The CCPs will then bud off from the plasma membrane to form clathrin-coated vesicles (CCVs) to deliver cargos into cells34. CME also contributes to the internalization of protein effectors from the fungal pathogen Magnaporthe oryzae and the oomycete pathogen Phytophthora infestans into plant cells44,45. In addition to CME, other sterol-sensitive clathrin-independent endocytosis (CIE) pathways exist in plants. The most well-studied proteins in the CIE pathway are membrane microdomain-associated flotillin1 and remorins46,47. Whether CME and/or CIE pathways play a role in the internalization of fungal sRNA effectors and EVs is unknown.
Here, we find that B. cinerea secretes EVs to deliver fungal sRNAs, and the B. cinerea tetraspanin protein BcPLS1 is essential for B. cinerea pathogenicity and EV-mediated Bc-sRNA secretion. Furthermore, utilizing genetics, biochemistry, and cell biology approaches, we demonstrate that CME is the major pathway for the uptake of fungal EVs and their sRNA cargo.
Results
B. cinerea can secrete BcPLS1-positive EVs during infection
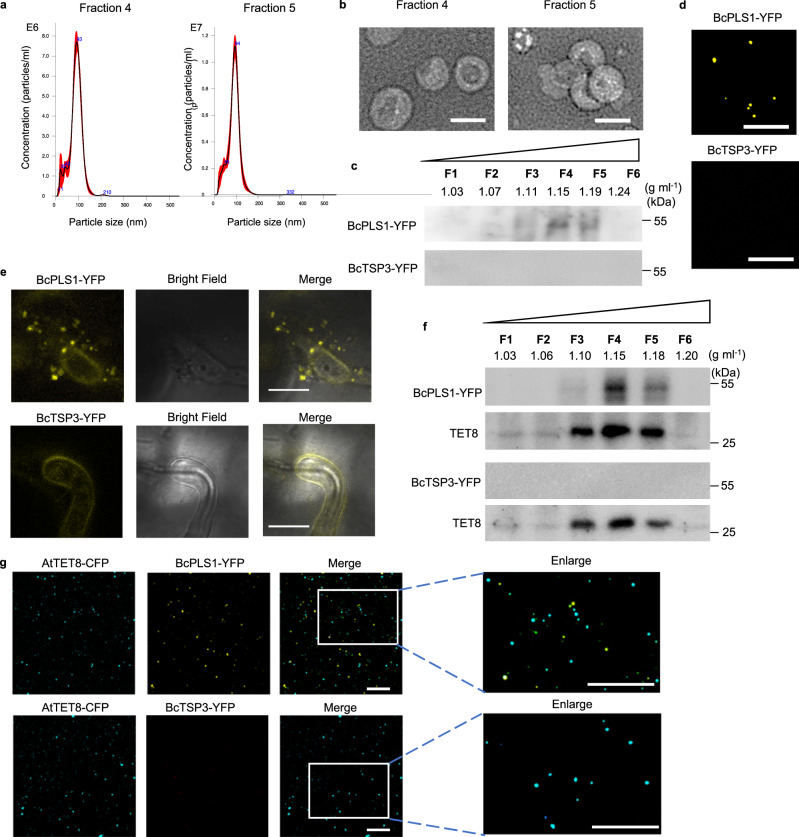
B. cinerea EVs were first isolated from the supernatants of wild-type strain B05.10 liquid culture using the traditional differential ultracentrifugation (DUC) method27. Nanoparticle tracking analysis (NTA) was used to determine the size distribution of isolated EVs, and a peak at 113 nm was observed for B. cinerea EVs (Supplementary Fig. 1a). The isolated EVs were further examined by transmission electron microscopy (TEM), which confirmed the typical cup-shaped form of EVs (Supplementary Fig. 1b). To further purify EVs obtained from B. cinerea liquid culture, the isolated EVs were subjected to sucrose gradient centrifugation as per the minimal information guidelines for studies of extracellular vesicles 2018 (MISEV2018)48. Analysis using NTA indicated that EVs were predominantly concentrated in sucrose fractions four and five with peak diameters of 93 nm and 94 nm, respectively (Fig. 1a). EVs were observed in both fractions and exhibited typical cup-shaped structures as confirmed by negative staining and TEM analysis (Fig. 1b). The morphology and size distribution of the isolated B. cinerea EVs were comparable to EVs from other species, including plants and plant pathogenic fungi31–33,49, suggesting that B. cinerea has the ability to secrete EVs into the external environment.
Fig. 1. B. cinerea secretes BcPLS1-positive EVs during infection.
a The size distributions of the B. cinerea EVs isolated by sucrose gradients in fractions 4 and 5 were measured using nanoparticle tracking analysis. The data of 4 measurements in one replicate are presented here. Similar data were obtained in three biological replicates. The red area represents the standard error. b Transmission electron microscopy images of B. cinerea EVs from fractions 4 and 5. Scale bar, 100 nm. c Western blot analysis of BcPLS1 and BcTSP3 in sucrose gradient fractions purified B. cinerea EVs from liquid culture. d B. cinerea EVs isolated from BcPLS1-YFP and BcTSP3-YFP liquid culture were examined by confocal microscopy. Scale bar, 10 μm. e BcPLS1-YFP labeled B. cinerea EVs were observed outside the fungal cells at the site of infection on Arabidopsis leaves. Samples were pretreated with 0.75 M sorbitol for 30 min to induce plasmolysis before imaging. Scale bars 10μm. f BcPLS1-YFP and BcTSP3-YFP were examined in sucrose gradient fractionated EVs that were isolated from BcPLS1-YFP or BcTSP3-YFP B. cinerea strains infected wild-type Arabidopsis using western blot. Arabidopsis TET8 was used as plant exosome control. g EVs isolated from BcPLS1-YFP or BcTSP3-YFP B. cinerea strains infected Arabidopsis TET8-CFP plants were purified using sucrose gradients, and the EVs from fraction four were examined using confocal microscopy. Scale bars, 10μm. Source data are provided as a Source Data file.
Some tetraspanin proteins, such as CD63 in mammalian cells and tetraspanin 8 (TET8) in plant cells, are known to be markers for EVs2,50. In B. cinerea, two tetraspanin coding genes, Punchless 1 (BcPLS1) and Tetraspanin 3 (BcTSP3), have been discovered51. Although these two proteins display limited amino acid sequence similarity, they share conserved structural hallmarks, including four transmembrane domains (TM1to TM4), a small extracellular loop (ECL1), an intracellular loop (ICL), and a large extracellular loop (ECL2) (Supplementary Fig. 2a, b). To determine whether these tetraspanin proteins are associated with B. cinerea EVs, both BcPLS1 and BcTSP3 proteins were tagged with a fluorescent protein YFP and inserted into the B. cinerea genome by homologous recombination. The EVs were isolated and separated using sucrose gradients from the liquid culture of both transgenic strains. As shown in Fig. 1c, BcPLS1 was found in the EV fractions three to five at the density of 1.11–1.19 g ml–1 and mainly concentrated at fraction four. However, BcTSP3 was hardly detected in isolated EV fractions, although it can be expressed in B. cinerea cells (Fig. 1c and Supplementary Fig. 2c). Meanwhile, BcPLS1-YFP-positive EV signals can be observed under confocal microscopy (Fig. 1d).
Both transformed strains were then used to challenge wild-type Arabidopsis plants and samples were examined by confocal microscopy ten hours post-inoculation. As shown in Fig. 1e, BcPLS1-YFP-labeled vesicles were clearly observed outside of the fungal cells around infection sites. However, it was difficult to detect secreted vesicle signals from BcTSP3-YFP-infected plants. EVs were then isolated from BcPLS1-YFP or BcTSP3-YFP infected plants and separated using sucrose gradients. Both BcPLS1-YFP and Arabidopsis exosome marker TET8 were concentrated in the same fractions (Fig. 1f). To further confirm that B. cinerea secretes EVs during infection, BcPLS1-YFP or BcTSP3-YFP strains were used to inoculate Arabidopsis TET8-CFP transgenic plants. EVs were isolated from the apoplastic fluid of infected plants using sucrose gradients, and fraction four was examined under confocal microscopy. The EVs from BcPLS1-YFP-infected TET8-CFP plants exhibited two distinct fluorescent protein-labeled signals (Fig. 1g). These results demonstrate that during infection, B. cinerea can secrete BcPLS1-positive EVs.
B. cinerea secretes small RNA effectors through EVs
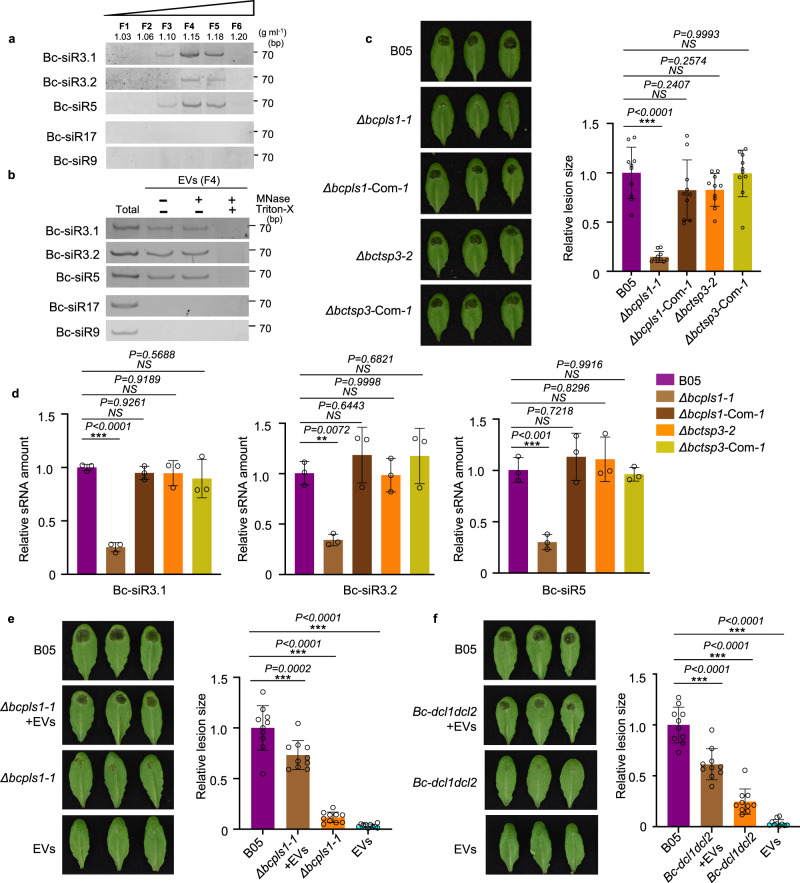
Our previous work demonstrated that B. cinerea can transfer sRNA effectors into host plant cells to suppress host immunity and promote infection3. To study whether B. cinerea uses EVs to transport these sRNA effectors, we examined fungal sRNAs using sRNA stem-loop reverse transcription-polymerase chain reaction (RT-PCR) analysis in EVs isolated from B. cinerea liquid culture52. Three previously discovered sRNA effectors, Bc-siR3.1, Bc-siR3.2, and Bc-siR5, were detected in B. cinerea EVs isolated by sucrose gradient fractionation. These sRNAs were concentrated primarily in fractions four and five, which also contained high levels of BcPLS1 (Fig. 2a). Additionally, EVs from fraction four were used for a Micrococcal Nuclease (MNase) protection assay. As shown in Fig. 2b, the sRNA effectors can still be detected after MNase treatment unless vesicles were ruptured with Triton X-100 (Fig. 2b and Supplementary Fig. 3). This result indicates that B. cinerea sRNAs are localized within and protected by EVs.
Fig. 2. B. cinerea secretes small RNA effectors through EVs.
a B. cinerea sRNAs were examined in B. cinerea EVs isolated by sucrose gradient fractionation. b B. cinerea sRNAs can be protected from micrococcal nuclease treatment by EVs. F4, B. cinerea EV samples were collected from fraction 4 after sucrose gradient centrifugation. Bc-siR17 and Bc-siR9 were used as negative controls. c The Δbcpls1 strain showed strongly reduced virulence, whereas the Δbctsp3 strain showed similar virulence with the wild-type B05 strain. Both Δbcpls1 and Δbctsp3 complementary strains showed similar virulence with the wild-type B05 strain. d Bc-sRNAs were examined by Real-time PCR in EVs isolated from B05, Δbcpls1, Δbctsp3, Δbcpls1 complementary, and Δbctsp3 complementary strains. The data are presented as mean ± s.d. Similar results were obtained in three biologically independent experiments. e Wild-type B. cinerea EVs partially complement the virulence of B. cinerea Δbcpls1 mutant. Premixing of wild-type B. cinerea EVs from sucrose gradient fraction 4 with Δbcpls1 mutant spores partially complement the weak virulence of Δbcpls1 on Arabidopsis leaves. f Wild-type B. cinerea EVs partially complement the virulence of B. cinerea dcl1dcl2 mutant. Premixing of wild-type B. cinerea EVs from sucrose gradient fraction 4 with dcl1dcl2 mutant spores partially complements the weak virulence of dcl1dcl2 on Arabidopsis leaves. Relative lesion size was determined 2 days after infection. The statistical data in c, e and f are presented as mean ± s.d., n = 10 biological replicates. The statistical analysis was performed using ANOVA Dunnett’s multiple comparisons test. The small open circles represent individual values. The error bars indicate s.d. **P < 0.01, ***P < 0.001. Source data are provided as a Source Data file.
To assess the role of tetraspanin proteins in B. cinerea small RNA delivery, deletion mutants of BcPLS1 and BcTSP3 were generated by targeted gene replacement in wild-type B. cinerea strain B05 (Supplementary Fig. 4a). Two independent mutant strains of each gene Δbcpls1 and Δbctsp3 were complemented by expressing BcPLS1 and BcTSP3, respectively (Supplementary Fig. 4a). On minimal medium (MM), but not potato dextrose agar, the growth rate of the Δbcpls1 strain was reduced compared to the wild-type and Δbctsp3 strains. (Supplementary Fig. 5). The spores of both mutants were collected and used to inoculate wild-type Arabidopsis plants to assess their virulence. The Δbcpls1 mutant strain showed a significant reduction in virulence with the lesion size being reduced by 84% compared with the wild-type strain (Fig. 2c). However, infection with Δbctsp3 resulted in lesions similar to the wild-type strain (Fig. 2c). To further understand the functions of BcPLS1 and BcTSP3 in EV-mediated sRNA secretion from B. cinerea, EVs were isolated from the culture supernatants of both the Δbcpls1 and Δbctsp3 strains and Bc-sRNA amounts were examined by real-time PCR and sRNA stem-loop RT-PCR. Compared with the wild-type strain, significantly lower levels of Bc-sRNAs were detected in Δbcpls1 EVs, while similar amounts of Bc-sRNAs were detected in Δbctsp3 EVs (Fig. 2d and Supplementary Fig. 6a). Further, we found the EV amount released by Δbcpls1 was significantly reduced compared with wild-type and Δbctsp3 strains (Supplementary Fig. 6b). This result indicates that BcPLS1 is important for EV-mediated sRNA secretion in B. cinerea.
To further confirm the function of BcPLS1 in B. cinerea virulence and EV secretion, we premixed Δbcpls1 spores with EVs isolated from wild-type B. cinerea and used the mixture to infect Arabidopsis plants. As shown in Fig. 2e, EVs from wild-type B. cinerea can partially restore the virulence of Δbcpls1. The lesion size increased from 12% of the wild-type strain to 73% of the wild-type strain. This result demonstrates that the cargoes in B.cinerea EVs are important for fungal virulence.
To determine whether sRNAs in the EVs contribute to the fungal virulence, we premixed spores from a B. cinerea mutant dcl1dcl2 strain with EVs isolated from wild-type B. cinerea culture supernatants and then inoculated Arabidopsis plants. The dcl1dcl2 mutant cannot generate sRNA effectors, such as Bc-siR3.1, Bc-siR3.2, and Bc-siR5, and exhibits a very weak virulence phenotype3. However, when premixed with EVs from wild-type B. cinerea, which contain sRNA effectors, B. cinerea dcl1dcl2 mutant pathogenicity was dramatically recovered. The lesion size increased from 24% of the wild-type strain to 61% of the wild-type strain (Fig. 2f). We then further used EVs isolated from the dcl1dcl2 mutant to complement the phenotype of Δbcpls1. Results showed that B. cinerea dcl1dcl2 mutant EVs could only restore the virulence of Δbcpls1 from 18% of the wild-type strain to 32% of the wild-type strain (Supplementary Fig. 7). This modest increase may be attributed to the other cargoes in the dcl1dcl2 EVs, because fungal EVs have been found to serve as a mode of transport for various virulence-related proteins and toxic metabolites53. These results indicate that fungal DCL1/DCL2-generated sRNA effectors encapsulated in B. cinerea EVs play an important role in fungal virulence.
Clathrin-mediated endocytosis is involved in plant immunity against B. cinerea
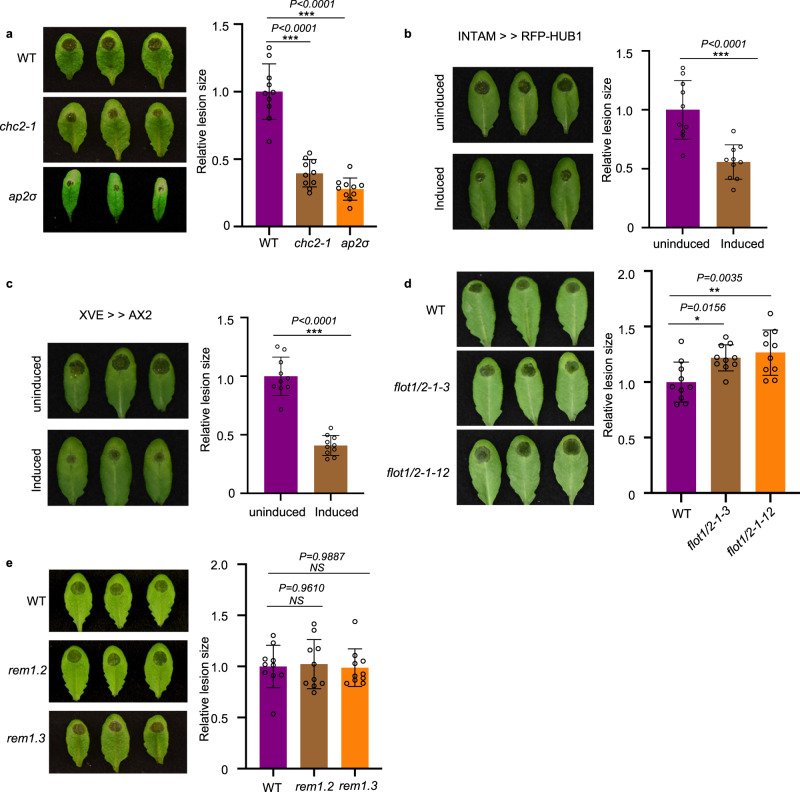
In the mammalian system, multiple endocytic pathways are used to internalize EVs and their cargoes from various origins54. To determine whether endocytosis pathways are also involved in the uptake of B. cinerea EVs and their sRNA cargoes, we first treated wild-type Arabidopsis leaves with plant CME-specific inhibitor ES9-17 or CIE-specific inhibitor MβCD55,56. Vesicle internalization was strongly suppressed by ES9-17 but not by MβCD (Supplementary Fig. 8a, c), suggesting that CME is likely the major pathway for EV uptake. However, we found that ES9-17 can also suppress the growth of B. cinerea (Supplementary Fig. 8b). So, the chemical treatment may cause a broad impact on both plants and fungi. Therefore, we chose genetic methods to ascertain the involvement of the CME pathway in the plant immune response to B. cinerea infection. We performed B. cinerea infection on two Arabidopsis CME pathway mutants, chc2 and ap2σ, which carry mutations in CLATHRIN HEAVY CHAIN 2 and ADAPTOR 2 SIGMA SUBUNIT, respectively. Compared with wild-type plants, both chc2-1 and ap2σ mutants displayed reduced disease susceptibility to B. cinerea (Fig. 3a). The relative lesion size on chc2-1 and ap2σ mutants was significantly reduced by 60% and 73% compared with wild-type plants, respectively (Fig. 3a). Furthermore, we took advantage of inducible lines that expressed the C-terminal part of clathrin heavy chain (INTAM≫RFP-HUB1) or the clathrin coat disassembly factor Auxilin2 (AX2) (XVE≫AX2). Overexpression of both lines blocked CME in Arabidopsis41,57. After induction, both INTAM≫RFP-HUB1 and XVE≫AX2 lines showed significantly reduced susceptibility to B. cinerea infection (Fig. 3b, c). The relative lesion size on INTAM≫RFP-HUB1 and XVE≫AX2 was reduced by 45% and 59% compared with control plants. These results confirm that CME contributes to plant susceptibility to B. cinerea infection.
Fig. 3. Clathrin-mediated endocytosis (CME) is involved in plant susceptibility against B. cinerea.
a Arabidopsis chc2-1 and ap2σ mutants exhibited enhanced disease resistance to B. cinerea infection. b, c INTAM≫RFP-HUB1(b) and XVE≫AX2 (c) transgenic plants showed enhanced resistance phenotype to B. cinerea infection after induction by 2 μM 4-Hydroxytamoxifen or 5 μM estradiol, respectively. d Arabidopsis flot1/2 double mutants showed increased susceptibility to B. cinerea infection. e Arabidopsis rem1.2 and rem1.3 mutants exhibited similar susceptibility to B. cinerea compared with the wild-type. Relative lesion size was determined by comparing lesion size to the size of the entire leaf 2 days after infection. The data are presented as mean ± s.d., n = 10 biological replicates. The statistical analysis in b and c was performed using unpaired two-tailed Student’s t-test. The statistical analysis in a, d and e were performed using ANOVA Dunnett’s multiple comparisons test. The small open circles represent individual values. The error bars indicate s.d. *P < 0.05, **P < 0.01, ***P < 0.001. Source data are provided as a Source Data file.
Next, we tested the possible function of the CIE pathway in plant immunity against B. cinerea infection. We examined the response of CIE-pathway mutants flot1/2, remorin 1.2, and remorin 1.3 after infection by wild-type B. cinerea. Because there is a close homolog of FLOT1 in Arabidopsis named FLOT2, we generated flot1/2 double mutants by knocking out FLOT2 in the flot1 mutant background using the Crispr-Cas9 system (Supplementary Fig. 9). Two independent lines were inoculated by B. cinerea, both of them showed a slightly more susceptible phenotype compared with wild-type (Fig. 3d). The homozygous knockout mutants of rem1.2 and rem1.3 T-DNA insertion lines were available and used for pathogen assays. No noticeable difference was observed between rem1.2 or rem1.3 and wild-type plants when challenged by B. cinerea spores (Fig. 3e). These results indicate that CME plays a more important role than CIE in plant susceptibility and the internalization of B. cinerea sRNAs into plant cells.
Bc-sRNA loading into host AGO1 and subsequent host target gene suppression is attenuated in CME mutants
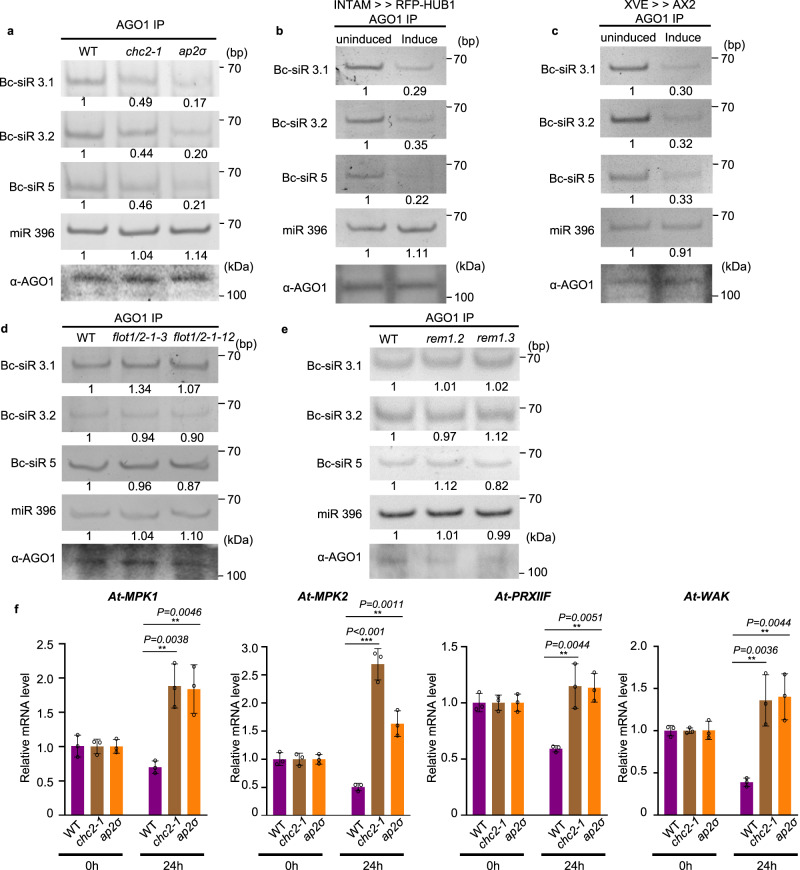
B. cinerea sRNAs can hijack the host plant RNAi machinery and silence plant immunity-related genes by binding to Arabidopsis Argonaute protein AGO13. If CME is a critical factor in the internalization of EVs and Bc-sRNAs into plant cells, the deficiency of CME is likely to impact the level of sRNAs incorporated into the AGO1 protein. To determine whether CME is crucial for the uptake of B. cinerea sRNAs, we immunoprecipitated AGO1 from B. cinerea-infected wild-type Arabidopsis and CME mutants, including chc2-1 and ap2σ. As shown in Fig. 4a, the fungal sRNA effectors, Bc-siR3.1, Bc-siR3.2, and Bc-siR5, were all detected in the AGO1-associated fraction precipitated from infected wild-type plants. However, the amount of B. cinerea sRNAs loaded into AGO1 was significantly reduced by around 60% and 80% in chc2-1 and ap2σ, respectively, compared to wild-type plants. This observation was further supported by similar results obtained from INTAM≫RFP-HUB1 and XVE≫AX2 lines (Fig. 4b, c).
Fig. 4. Bc-sRNA loading into plant AGO1 and target gene suppression was reduced in CME mutants.
a The loading of Bc-siR3.1, Bc-siR3.2, and Bc-siR5 into AGO1 of wild-type, chc2-1, and ap2σ mutants was examined with AGO1-IP followed by RT-PCR. b, c, The loading of Bc-siR3.1, Bc-siR3.2, and Bc-siR5 into AGO1 of induced INTAM≫RFP-HUB1 (b) and XVE≫AX2 (c) transgenic plants was examined with AGO1-IP followed by RT-PCR. d, e The loading of Bc-siR3.1, Bc-siR3.2, and Bc-siR5 into AGO1 of wild-type, flot1/2 (d), rem1.2, and rem1.3 mutants (e) was examined with AGO1-IP followed by RT-PCR. In a–e, miR396 served as a positive control. AGO1 was detected by western blot. f, Suppression of host target genes by Bc-sRNAs was attenuated in CME mutants. The expression of Bc-siR3.1 target At-PRXIIF, Bc-siR3.2 target At-MPK1 and At-MPK2, and Bc-siR5 target At-WAK in chc2-1 and ap2σ mutants compared with those in wild-type infected plants after B. cinerea infection was examined by real-time RT-PCR. The data are presented as mean ± s.d. Similar results were obtained in three biologically independent experiments. The statistical analysis was performed using ANOVA Dunnett’s multiple comparisons test. The small open circles represent individual values. The error bars indicate s.d. *P < 0.05, **P < 0.01, ***P < 0.001. Source data are provided as a Source Data file.
We also assessed whether CIE contributes to Bc-sRNA uptake by challenging flot1/2, rem1.2, and rem1.3 mutants with wild-type B.cinerea. Unlike CME mutants, the amount of AGO1-associated Bc-sRNAs in flot1/2, rem1.2, and rem1.3 mutants was similar to wild-type plants (Fig. 4d, e). As the total amount of AGO1 protein was not decreased in any of the CME and CIE mutants (Supplementary Fig. 10), the decrease of Bc-sRNAs associated with AGO1 in CME mutants can be attributed to a reduction in the Bc-sRNA internalization of plant cells, rather than a decrease in the amount of AGO1 protein. Thus, CME is a key pathway for Bc-sRNA trafficking into host plant cells.
B. cinerea sRNA effectors have a suppressive effect on predicted host target genes during infection3,58. If CME is truly essential for cross-kingdom trafficking of Bc-sRNAs, we expect to observe attenuated suppression of host target genes in CME mutants. Thus, we quantified the transcript levels of Bc-siR3.1 target gene At-PRXIIF, Bc-siR3.2 target genes At-MPK1 and At-MPK2, and Bc-siR5 target gene At-WAK, at 0 and 24 hours post-infection. As expected, the expression of these genes was suppressed in wild-type plants after 24 hours of infection. However, this suppressive effect was attenuated in the chc2-1, ap2σ, induced INTAM≫RFP-HUB1 and XVE≫AX2 mutants shown by no reduction in target gene transcript levels 24 hours after infection (Fig. 4f and Supplementary Fig. 11a, b). We performed a similar experiment to explore the role of the CIE pathway in target gene suppression using the flot1/2, rem1.2, and rem1.3 mutants. Unlike CME mutants, all target genes were still significantly suppressed in the CIE mutants (Supplementary Fig. 12a, b). These results again demonstrate that CME is the major pathway for Bc-sRNA uptake.
B. cinerea induces accumulation of clathrin-coated vesicles around infection sites
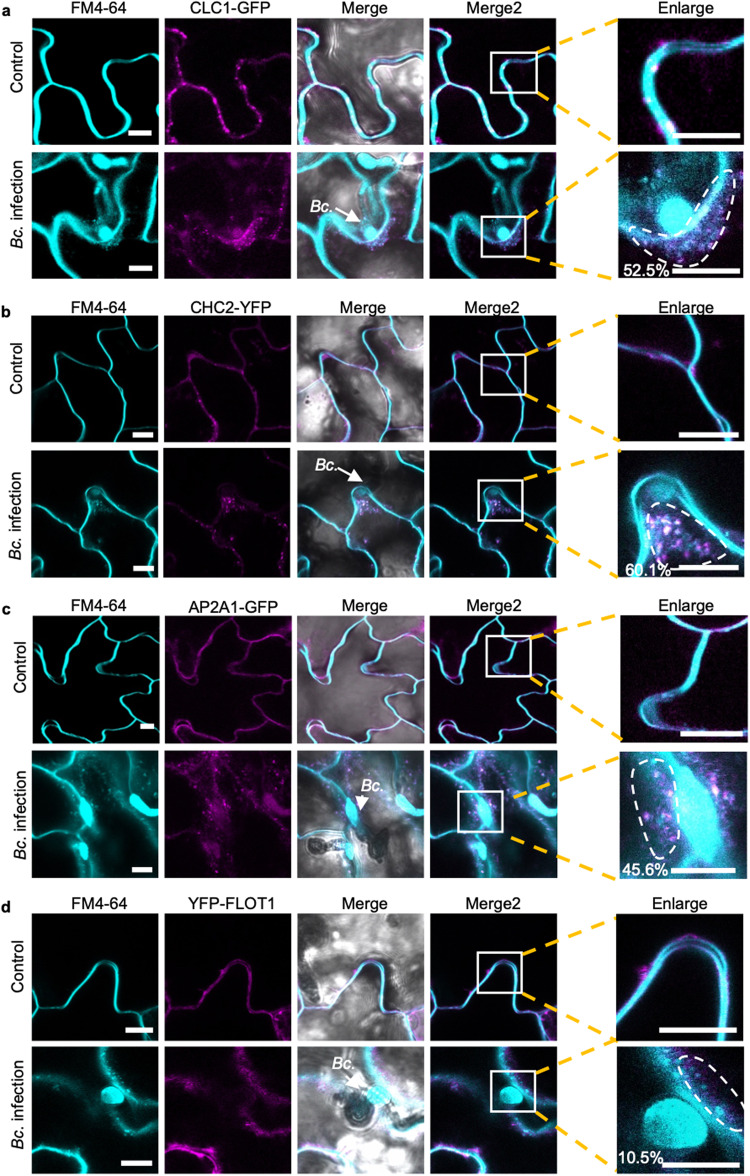
To further validate the role of CME in the internalization of B. cinerea sRNA effectors into host plant cells, Arabidopsis CLATHRIN LIGHT CHAIN 1 (CLC1)-GFP and CLATHRIN HEAVY CHAIN 2 (CHC2)-YFP transgenic plants were challenged by B. cinerea. Eight hours post inoculation, the infected plants were stained with the endocytic tracer FM4-64 and subjected to confocal microscopy to examine the role of endocytosis in plant response38. Numerous distinct fluorescently labeled endocytic vesicles were observed around B. cinerea infection sites, indicating the activation of endocytosis during B. cinerea infection (Fig. 5a, b). Meanwhile, we detected co-localization between CLC1-GFP or CHC2-YFP with the FM4-64 signal on vesicles at the infection sites, with co-localization rates of 52% and 60%, respectively (Fig. 5a, b). These results suggest that B. cinerea infection induces the accumulation of CCVs around infection sites. In addition to clathrin, AP2 adaptor complexes are important components for CME in plant cells59. AP2A1-GFP plants were also treated with wild-type B. cinerea spores. Similar to CHC2-YFP and CLC1-GFP plants, clear colocalization (46%) was observed on the endocytic vesicles at the infection sites between AP2A1-GFP with FM4-64 (Fig. 5c). We also generated the YFP-tagged FLOT1 transgenic line and monitored the endocytosis through FM4-64 staining following inoculation with B. cinerea. As shown in Fig. 5d, endocytic FM4-64 signals can also be observed, but the percentage of colocalization (10%) between YFP-FLOT and FM4-64 was limited. These results further confirm that CME is more involved than CIE in B. cinerea infection of Arabidopsis.
Fig. 5. Clathrin-coated vesicles accumulate around B. cinerea infection sites.
a–c Confocal microscopy showed that the CLC1-GFP (a), CHC2-YFP (b) and AP2A1-GFP (c) signal overlapped with endocytic FM4-64 vesicle signals when treated with B. cinerea. Untreated plants served as control. d Confocal microscopy of B. cinerea infection on Arabidopsis YFP-FLOT1 plants. The scale bars in a–d, are 10 μm. The arrows indicate the location of B. cinerea (Bc.). The degree of colocalization between CLC1-GFP, CHC2-YFP, AP2A1-GFP, and YFP-FLOT1 and FM4-64 signals was quantified using three independent images. Regions of interest (ROIs) that are used for measuring colocalization percentage were marked in the enlarged panel. Source data are provided as a Source Data file.
B. cinerea EVs and sRNAs are associated with clathrin-coated vesicles
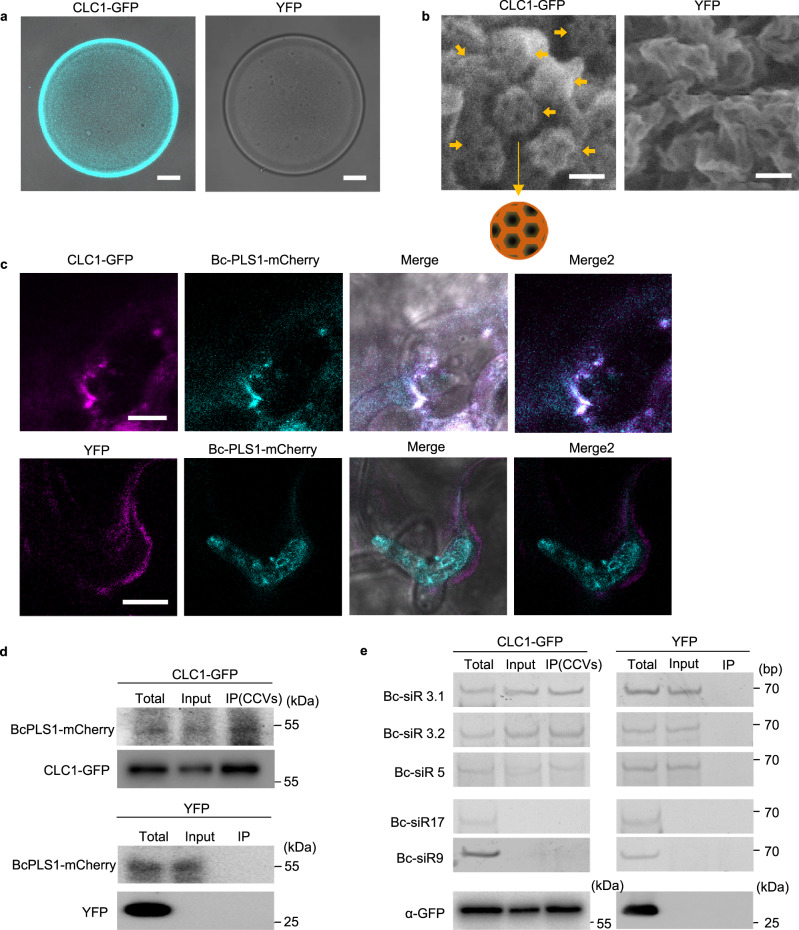
Unlike in animal cells, plant CCVs are not disassembled immediately after scission from the plasma membrane, which allows the isolation of CCVs by immunocapture60. We isolated plant CCVs from CLC1-GFP transgenic plants after infection with B. cinerea using a two-step procedure comprising differential centrifugation, followed by immunoaffinity purification with antibodies against GFP. GFP signal was clearly detected on the surface of GFP-TRAP agarose beads after incubation with isolated CCVs (Fig. 6a). To examine whether the immuno-captured CCVs were intact, the GFP-TRAP beads were subjected to scanning electron microscopy (SEM) analysis after pull down. Figure 6b shows that most of the immuno-captured CCVs were intact with typical cage structures on the surface of GFP-TRAP agarose beads. We further inoculated CLC1-GFP plants with the BcPLS1-mCherry B. cinerea strain. After ten hours of inoculation, colocalization of CLC1-GFP and BcPLS1-mCherry signals could be observed around the infection sites (Fig. 6c). Further, BcPLS1-mCherry can be detected in the CCV fractions isolated from infected plants (Fig. 6d). The Bc-sRNA effectors that we examined previously were also present in the immunopurified CCVs (Fig. 6e). These results provide conclusive evidence that B. cinerea EVs are responsible for transporting Bc-sRNAs and enabling their internalization through plant CME.
Fig. 6. CME mediates the uptake of B. cinerea secreted sRNAs.
a CLC1-GFP labeled CCVs can be pulled down by GFP-Trap Agarose beads. Arabidopsis YFP plant was used as a negative control. Scale bar, 10 μm. b Intact CCVs can be observed on the surface of GFP-Trap Agarose beads after CCV pull down from CLC1-GFP plants using the scanning electron microscope (SEM). A schematic figure of CCV is presented. The solid arrows indicate different intact CCVs. Scale bar, 100 nm. c BcPLS1-mCherry colocalizes with Arabidopsis CLC1-GFP during infection. d BcPLS1-mCherry signals can be detected in GFP-Trap Agarose beads-isolated CCVs from BcPLS1-mCherry infected CLC1-GFP plants using western blot. YFP plant was used as a negative control. e B. cinerea-secreted sRNAs can be detected in purified plant CCVs by RT-PCR. In d and e, the crude CCVs fraction obtained from B. cinerea-infected CLC-GFP or YFP plants through sucrose gradients was used for immunoprecipitation. Bc-siR17 and Bc-siR9 were negative controls. CLC1-GFP and YFP protein was detected by western blot using an anti-GFP antibody. Source data are provided as a Source Data file.
Discussion
Cross-kingdom RNAi is emerging as a critical regulatory mechanism across interaction systems. While recent studies show that plant and animal hosts utilize extracellular vesicles to protect and transport sRNAs to their fungal pathogens2,5, how fungi transported sRNAs to their hosts was still unknown. Here, we demonstrate that, just like their plant hosts, B. cinerea utilizes EVs to protect and transport sRNAs to plants, providing the first example of fungi utilizing EVs for cross-kingdom RNA transport. Recently, the algal species Emiliania huxleyi was found to secrete vesicles containing sRNAs that influence viral infection and the population dynamics of interacting marine microorganisms61,62. Furthermore, bacterial EVs isolated from seawater were found to transport DNA transposons that facilitate horizontal gene transfer of nutrient-acquisition and bacteriophage-resistance genes among subpopulations of marine picocyanobacterium Prochlorococcus63. These studies demonstrate that EVs can survive in harsh environment and provide perfect protection for vulnerable RNA and DNA during transport, highlighting the under-appreciated role of EVs in cross-kingdom/organismal communication, host-microbial interaction and microbial evolution23.
We show that plant cells internalize fungal EVs through clathrin-mediated endocytosis. CME is one of the major pathways for plant uptake of environmental molecules, with extensive studies demonstrating its vital role in different biological processes38,64, including plant defense responses39,41,65–67. While the plant CME pathway has recently been implicated in the uptake of protein effectors from fungal and oomycete pathogens44,45, we demonstrate here that it is also critical for the plant uptake of fungal sRNA effectors and fungal EVs. Although in mammals, intraorganismal EV internalization into recipient cells can occur through CME68, the mechanisms of internalization of foreign EVs from one organism to another in a different kingdom had never been reported until this study. We found that numerous CCVs accumulated around infection sites, indicating the activation of CME during B. cinerea infection. Additionally, the B. cinerea EV marker protein BcPLS1 is colocalized with plant CME component protein CLC1 during fungal infection and can be detected in the immunopurified plant CCVs. These findings strongly support that fungal EVs are internalized by the plant CME pathway. Furthermore, the EV-delivered sRNAs were also detected in the immunopurified CCVs, providing additional evidence for the role of plant CME in the uptake of fungal EV-delivered sRNAs.
These new insights into the mechanistic basis for cross-kingdom RNA communication between fungi and plants can help in the development of translational applications. For example, in B. cinerea and many other pathogens, such as Fusarium graminearum, Penicillium italicum, Colletotrichum gloeosporioides, and Valsa mali, sRNA effectors are generated by DCL proteins and used to promote virulence, and mutation in the DCL genes attenuates the virulence of these pathogens3,69–72. Consequently, targeting and silencing DCL and other components of the RNAi machinery, even transiently, has been proven to provide powerful protection against these pathogens by hampering their ability to synthesize sRNA effectors16,18–22. Specifically, using spray-induced gene silencing (SIGS) to suppress the expression of both fungal DCL1 and DCL2 genes can effectively protect plants from infection by various pathogens, including fungal pathogens B. cinerea16,18–20, F. graminearum21, and oomycete pathogen Plasmopara viticola22. Recent application of modern nanotechnology in SIGS20,73–75, especially the utilization of artificial nanovesicles that mimic EVs for RNA protection19,73, largely improves the efficacy of SIGS for crop protection. Here, by elucidating how sRNA effectors are transported from fungal pathogens into plants, we provide additional mechanistic targets for disease control. Specifically, we demonstrate that the EV-associated B. cinerea tetraspanin protein, BcPLS1, is an important protein for fungal EV secretion and virulence. Previous studies on PLS1 in both the B. cinerea T4 strain and Magnaporthe grisea showed that Δpls1 mutants exhibit weak virulence76,77, and the M. grisea PLS1 protein was found to be mainly expressed during infection and localized to appressorial membranes77. Consistently, we found the Δbcpls1mutant showed weak virulence on plants (Fig. 2c) and the BcPLS1 protein can be observed on the appressorial membranes (Fig. 1e). Furthermore, we detected BcPLS1 in the isolated B. cinerea EVs, and found that the delivery of B. cinerea sRNA effectors is compromised in the Δbcpls1 mutant, suggesting that BcPLS1-positive EVs are critical for sRNA effector transport. Thus, we have expanded the repetoire of effective genetic targets, such as PLS1, for RNAi-based anti-fungal strategies such as SIGS. Combining existing effective gene targets with PLS1 and other EV proteins targeting in SIGS will likely provide even more effective crop protection by targeting not only sRNA biogenesis, but also the trafficking mechanism of sRNA effectors from pathogens to plants.
Overall, our study helps to elucidate the underlying molecular mechanisms of EV-mediated sRNA trafficking during “cross-kingdom RNAi” and will further inform efforts in other organisms that also deliver sRNAs into interacting species. Given how widespread the phenomenon of cross-kingdom RNAi is in different branches of life, this will have strong rammifications for anti-pathogen measures in agriculture and human health.
Methods
Fungal strains and culture conditions
Botrytis cinerea strain B05.10 was used as a host strain for gene replacement and fluorescent labeling experiments, and as a wild-type (WT) control in all experiments. WT, mutants, and transgenic strains were all grown on complete media Potato Dextrose Broth with 1.5% Agar (PDA) at room temperature. The minimal medium (MM) utilized for measuring growth rate was prepared according to a previous study78. The MM was prepared as follows (per liter of distilled H2O): sucrose, 30 g; KH2PO4, 1 g; MgSO4•7H2O, 0.5 g; KCl, 0.5 g; FeSO4•7H2O, 10 mg; NaNO3, 2 g; agar, 20 g; trace element solution, 0.2 ml. The trace element solution contains (per 95 ml of distilled H2O): citric acid, 5 g; ZnSO4•7H2O, 5 g; Fe(NH4)2(SO4)2•6H2O, 1 g; CuSO4•5H2O, 0.25 g; MnSO4•H2O, 50 mg; H3BO4, 50 mg; NaMoO4•2H2O, 50 mg.
EV isolation
EV isolation from fungal culture supernatant was performed as previously described27. In order to eliminate the possibility of contamination from the culture medium, the medium was initially ultracentrifuged at 100,000 g for 1 hour to eliminate any particles. Subsequently, the medium was filtered using a 0.22 μm filter prior to its use for B. cinerea culture. 106 B. cinerea spores were cultured for 48 hours at room temperature with shaking on the rocker in 100 ml of purified YEPD (0.3% yeast extract, 1% peptone, 2% glucose, pH 6.5) medium. The culture supernatant was collected by centrifugation at 3000 g for 15 mins at 4 °C. The supernatant was then filtered through a 70 μm cell strainer to further remove cells. The cell-free supernatant was centrifuged at 10,000 g for 30 mins to remove smaller debris and a second round of centrifugation at 10,000 g for 30 mins was performed to ensure the removal of aggregates. The resulting supernatant was filtered through a 0.45 μm filter and ultracentrifuged at 100,000 g for 1 h to precipitate vesicles. Vesicles were washed once in phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4), and the final pellets were resuspended in PBS.
EV isolation from infected plants was performed as previously described2,49. Apoplastic fluid was extracted from B. cinerea-infected Arabidopsis leaves by vacuum infiltration with infiltration buffer (20 mM MES, 2 mM CaCl2, 0.1 M NaCl, pH 6.0) and then centrifuged at 900 g for 10 mins. Cellular debris was further removed from the supernatant by centrifugation at 2000 g for 30 minutes, filtration through a 0.45 μm filter and finally centrifugation at 10,000 g for 30 mins. EVs were then collected by ultracentrifugation at 100,000 g for 1 hr and washed with infiltration buffer at 100,000 g for 1 hour.
Sucrose gradient separation of EVs
EVs from B. cinerea liquid culture and B. cinerea-infected Arabidopsis were purified by discontinuous sucrose density gradient centrifugation25. 10–90% sucrose stocks (w/v), including 10, 16, 22, 28, 34, 40,46, 52, 58, 64, 70 and 90%, were prepared using infiltration buffer (20 mM MES, 2 mM CaCl2, 0.1 M NaCl, pH 6.0). The discontinuous gradient was prepared by layering 1 ml of each solution in a 15-ml ultracentrifuge tube. 100 μl EVs were premixed with 1 ml of 10% sucrose stock and loaded on top of the sucrose gradient. Then, samples were centrifuged in a swinging-bucket rotor for 16 h at 100,000 g, 4 °C and six fractions (2 ml each) were collected from top to bottom. Collected fractions were transferred to new ultracentrifuge tubes and each sample was diluted to 12 ml using infiltration buffer, followed by a final centrifugation for 1 h at 100,000 g, 4 °C to obtain pellets. The pellet was then resuspended in 50 μl infiltration buffer for further analysis.
Nanoparticle tracking analysis
A NanoSight NS300 fitted with a blue laser (405 nm) with NanoSight NTA software version 3.1 (Malvern Panalytical) was used to determine the size distribution and concentration of the B. cinerea EVs. EV samples were diluted 50 times using 0.22 μm filtered PBS and injected into the flow cell at a flow rate of 50 units. Four 60 second videos were taken for each sample and used to determine the size distribution and concentration of the EVs.
Transmission electron microscopy analysis
B. cinerea EVs were imaged using negative staining. Briefly, 10 μl of EVs were deposited onto 3.0 mm copper Formvar-carbon-coated grids (EMS) for 1 min and excess sample was removed from the grids using filter paper. The grids were then stained with 1% uranyl acetate for 30 s and blotted before a second round of staining with 1% uranyl acetate 2 min. The samples were air-dried before visualization with a Talos L120 transmission electron microscope, operated at 120 kV.
sRNA expression analyses
RNA was extracted using the Trizol extraction method for both total RNA and EV samples. sRNA RT-PCR was performed as previously described2. The PCR products were detected on a 12% PAGE gel. Quantitative PCR was performed with the CFX96 real-time PCR detection system (Bio-Rad) using SYBR Green mix (Bio-Rad). All primer sequences are provided in Supplementary Data 1.
To determine if sRNAs were localized within vesicles, EVs from sucrose gradient-purified fraction four were treated with 10 U of micrococcal nuclease (MNase) (Thermo Fisher) with or without Triton-X-100. For Triton-X-100 treatment, vesicles were incubated with 1% Triton-X-100 on ice for 30 minutes before nuclease treatments. Nuclease treatment was carried out at 37 °C for 15 minutes followed by RNA isolation.
Transformation
The homologous recombination-based method was used to knock out B. cinerea genes as described previously3. The 5′- and 3′-regions of BcPLS1 and BcTSP3 were amplified and inserted into a modified pBluescript KS(+) plasmid (PBS-HPH) in which a hygromycin resistance cassette containing the hph gene of E. coli under the control of the trpC promoter of Aspergillus nidulans had been inserted between HindIII and SalI digestion sites. The full-length fragments containing the 5′- and 3′-regions of each specific gene and the hph cassette were transformed into the protoplasts of wild-type B. cinerea. The transformants were purified by the isolation of a single spore on PDA containing 70 μg ml−1 hygromycin.
The complementary lines were generated by expressing the CDS of BcPLS1 or BcTSP3 in the Δbcpls1 or Δbctsp3 mutants, respectively. The CDS of both BcPLS1 and BcTSP3 were cloned separately into the pENTR vector (Life Technologies), then into the destination vector pBCC, which carries nourseothricin (NTC) N-acetyl transferase (NAT) conferring nourseothricin resistance. The specific primers 4-5-S and 4-3-A (Supplementary Data 1) were used to amplify dsDNA fragments containing promotor PtrpC, inserted genes, and NAT for B. cinerea transformation The positive transformants were selected on PDA plates with 50 μg ml-1 Nourseothricin Sulfate (Goldbio) and 70 μg ml−1 hygromycin.
The BcPLS1-YFP and BcTSP3-YFP strains were generated by amplifying and inserting the stop codon removed CDS region and 3’ UTR region into the modified PBS-HPH, in which the YFP gene sequence has been inserted between BamHI and HindIII. The BcPLS1-mCherry was generated by inserting the stop codon removed CDS region and 3’ UTR region into the modified PBS-HPH with the mCherry sequence inserted at the C terminal. The transformation and selection conditions were the same as the homologous recombination-based gene knock-out. All primers used are provided in Supplementary Data 1.
Plant materials and growth conditions
Arabidopsis thaliana wild-type and mutant lines used in this study were all Columbia 0 (Col-0) backgrounds. The chc2-1(SALK_028826)79, ap2σ (SALK_141555)59, rem1.2 (SALK_117639)80, rem1.3 (SALK_023886C)80 T-DNA insertion lines were ordered from the Arabidopsis Biological Resource Center (Ohio State University, USA). The CLC1-GFP81 and AP2A1-GFP81 lines were provided by Dr. Eugenia Russinova’s lab. The INTAM > > RFP-HUB157 and XVE > > AX241 lines were provided by Dr. Jiří Friml. The CHC2-YFP, TET8-CFP, and YFP-only transgenic lines were constructed using the Agrobacterium-mediated flower dipping method as described previously82. The flot1/2 double mutant was generated using Crispr-Ca9 system in the flot1 (SALK_203966C) mutant background. The designed guide RNA sequences are listed in Supplementary Data 1. Both wild-type and mutant plants were grown at 22 °C under 12 h light per day.
Plasmid construction
The vector for CHC2-YFP was constructed as follows: Arabidopsis genomic DNA was used as a template with primers CHC2-Pentr-F and CHC2-Pentr-R to amplify the whole sequence of CHC2. The final PCR product was cloned into pENTR/SD/D-TOPO (Life Technologies), and then into the destination vector pEarleyGate 101 by LR reactions (Life Technologies). For the YFP-FLOT1 construct, the full-length CDS of FLOT1 was cloned into the pENTR/SD/D-TOPO vector (Life Technologies), then into the destination vector pEARLYGATE 104 for YFP tagging (Life Technologies). For the TET8-CFP construct, the full-length CDS of TET8 was cloned into the pENTR/SD/D-TOPO vector (Life Technologies), then into the destination vector pEARLYGATE 102 for CFP tagging (Life Technologies). For the construct to express YFP, the YFP sequence was amplified from pEARLYGATE 101 and then cloned into pENTR/SD/D-TOPO vector and finally into pEARLYGATE 100 (Life Technologies). Primer sequences are listed in Supplementary Data 1.
Chemical solutions and treatments
ES9-17 (Sigma Aldrich) was dissolved in DMSO to create a 30 mM stock solution. mβCD (Sigma Aldrich) was dissolved in deionized water to create a 200 mM stock solution. 4-Hydroxytamoxifen (Sigma Aldrich) was dissolved in DMSO to make a 2 mM stock solution. Estradiol (Sigma Aldrich) was dissolved in DMSO to make a 5 mM stock solution. For the chemical inhibitor treatments, Arabidopsis plant leaves were incubated in liquid 1/2 Murashige and Skoog (MS) medium with 30 μM or 10 mM mβCD for 24 hours. DMSO and deionized water were used as controls. To induce the expression of RFP-HUB1 or AX2, 2 μM 4-Hydroxytamoxifen or 5 μM estradiol was used to pretreat the plant leaves in liquid 1/2 MS medium for 24 hours.
CCV purification
The clathrin-coated vesicle isolation procedure was adapted from previously established protocols83,84. 50 grams of Arabidopsis leaves were homogenized for 1 min in 2 volumes of resuspension buffer (100 mM MES, pH 6.4, 3 mM EDTA, 0.5 mM MgCl2, 1 mM EGTA, protease inhibitors (Roche)) using a Waring Blender. The homogenate was squeezed through eight layers of cheesecloth and three layers of Miracloth (Sigma). Then the homogenate was centrifuged in four increments to obtain a microsomal pellet: 500 g, 5 min; 5000 g, 10 min; 20,000 g, 30 min; 100,000 g, 60 min. The pellet was then resuspended in 6 ml resuspension buffer. Then the resuspended pellet was loaded on top of the discontinuous sucrose gradients, which were made from three sucrose stocks (10%, 35%, and 50%) in the resuspension buffer. The gradient was composed of 1 ml of 50% sucrose, 5 ml of 35% sucrose, and 3 ml of 10% sucrose in a Beckman 14 × 95 mm Ultra-Clear centrifuge tube. The gradients were centrifuged at 116,000 g 50 min. Samples were collected from 10% and 10/35% interface and resuspended in the resuspension buffer to make the sucrose concentration lower than 5%. The samples were then centrifuged at 200,000 g for 50 min, and the pellets were collected as crude CCVs.
Further immunoisolation of CCVs was performed as follows: GFP-Trap® Agarose (ChromoTek) was equilibrated in the resuspension buffer. Plant crude CCVs from CLC1-GFP and YFP were incubated with the GFP-Trap® Agarose for 2 hr at 4 °C. After, beads were washed three times with resuspension buffer. The RNA from purified CCVs was extracted using TRIzol Reagent (Invitrogen) following immunoisolation. Subsequently, the immunoisolated CCVs on GFP-Trap Agarose beads were further analyzed by a scanning electron microscope (SEM).
Confocal microscopy
Arabidopsis seedlings were imaged on a Leica TCS SP5 confocal microscope (Leica Microsystems). Briefly, leaves were infiltrated with 10 μM FM4-64 dye for 30 mins before examination. Samples were imaged using a 40x water immersion dip-in lens mounted on a Leica TCS SP5 confocal microscope (Leica Microsystems). The excitation wavelength was 488 nm for GFP, 514 nm for YFP, and 559 nm for FM4-64. Emission was detected at 500–550 nm for GFP and YFP, 570–670 nm for FM4-64. The colocalization percentage was processed by means of the ImageJ (Fiji) software package. More specifically, the regions of interest (ROI) were selected to include vesicles accumulated around B. cinerea infection area. The total area of FM4-64 (red) and overlapped area (yellow) were measured and used for colocalization calculation. The average of three independent values was presented in the figures. The method for intracellular/PM signal intensity ratio quantification was described previously55. Non-saturated images were converted in ImageJ (Fiji) to 8-bit and regions of interest (ROIs) were selected based on the PM or cytosol localization. Intensity values per ROI of PM and intracellular signals were measured and used for calculation.
Pathogen assay
B. cinerea was cultured on PDA plates for 10 days at room temperature before spores were collected and diluted into inoculation medium (0.1% sabouraud maltose broth buffer, Difco) for a final concentration of 105 spores per mL. Four-week-old plants were then inoculated by applying a single 10 μl droplet of B. cinerea per leaf. Photos were taken 2 days after inoculation and analyzed by ImageJ software to measure the lesion size.
For pathogen assay using a mixture of WT or Bc-dcl1/dcl2 B. cinerea EVs with Δbcpls1 and Bc-dcl1/dcl2, 106 B. cinerea spores were cultured for 48 hours at room temperature with shaking on the rocker in 100 ml of YEPD (0.3% yeast extract, 1% peptone, 2% glucose, pH 6.5) medium. B. cinerea EVs were isolated and purified using sucrose gradients as described above, then EVs from fraction four were resuspended in 100 μl PBS. The spores were collected and diluted into 2 x inoculation medium (0.2% sabouraud maltose broth buffer, Difco) for a final concentration of 2 × 105 spores per mL. Equal volumes of EVs and spores from mutant strains were mixed. Four-week-old plants were then inoculated by applying a single 10 μl droplet of EVs and spores mixture per leaf. Equal volumes of EVs and 2 x inoculation medium were mixed to inoculate plants as the negative control. Photos were taken 2 days after inoculation and analyzed by ImageJ software to measure the lesion size.
Real-time RT-PCR
Total RNA was extracted from plant tissues using TRIzol and the concentration was measured at 260 nm using a Nanodrop 2000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Equal amounts of total RNA were treated with DNase I enzyme (Roche, Basel, Switzerland) and converted into first-strand cDNA using SuperScript III (Invitrogen, Carlsbad, CA). The cDNA was then used to check target gene expression by real-time RT-PCR with gene-specific primers. Primer sequences are listed in Supplementary Data 1.
Western blotting
Protein samples were resolved in a 12% SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to nitrocellulose membranes (GE Healthcare), blocked with 5% skimmed milk in TBST (Tris-buffered saline, 0.1% Tween 20) and probed with anti-GFP (Sigma-Aldrich), anti-mCherry (abcam) or anti-TET8. Then the signals were detected using the enhanced chemiluminescence method (GE Healthcare).
AGO1 immunoprecipitation
Arabidopsis AGO1 IP was performed with 5 g fresh leaves collected 24 hr after spray inoculation with B. cinerea. Tissues were collected and ground in liquid nitrogen before resuspension in 5 ml of extraction buffer (20 mM Tris–HCl at pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.5% NP40, proteinase inhibitor cocktail; Sigma). The extract was then precleared by incubation with 50 ul of Protein A-agarose beads (Roche) at 4 °C for 30 min. The precleared extract was incubated with 10 ul of AGO1-specific antibody for 6 hours. 50 ul of Protein A-agarose beads (Roche) was then added to the extract before overnight incubation. Immunoprecipitates were washed three times (5 min each) in wash buffer (20 mM Tris–HCl at pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.5% Triton x-100, proteinase inhibitor cocktail; Sigma). RNA was extracted from the bead-bound AGO1 with TRIzol reagent (Invitrogen). Proteins were detected by boiling the bead-bound AGO1 in SDS-loading buffer before running in a SDS-PAGE gel.
Scanning electron microscope (SEM)
The structure of CCVs isolated using GFP-Trap Agarose was examined using field emission scanning electron microscopy Nova NanoSEM 450 (FEI) at 15 kV. The samples were washed three times with PBS (pH 7.2) and fixed with 2.5% glutaraldehyde overnight at 4 °C. Samples were dehydrated with gradient alcohol solutions (10%, 30%, 50%, 70%, 80%, and 90%) for 10 min, 100% ethanol for 20 min, and 100% acetone for 30 min. Subsequently, samples were dried by CO2 under critical point. Samples were sputter coated with gold and fixed on the stab using carbon tape before observation and imaging.
Statistical analysis
The statistical analyses were performed using analysis of variance (ANOVA), Dunnett’s multiple comparisons test, and unpaired two-tailed Student’s t-test using GraphPad Prism (9.0.2). The statistical tests and n numbers, including sample sizes or biological replications, are described in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Dr. Eugenia Russinova for providing CLC1-GFP and AP2A1-GFP seeds, Dr. Jiří Friml for providing the INTAM > > RFP-HUB1 and XVE > > AX2 lines, Shumei Wang for generating the Arabidopsis YFP-only transgenic line and Rachael Hamby and Lida Halilovic for editing the paper. This work was supported by National Institute of Health (R35GM136379), National Science Foundation (IOS 2020731), United State Department of Agriculture (2021-67013-34258), United States Department of Agriculture National Institute of Food and Agriculture (2019-70016-29067) and the CIFAR ‘Fungal Kingdom’ fellowship to H. J., by National Natural Science Foundation of China (32272029), Hubei Provincial Natural Science Foundation of China (2022CFA079) to Q. C.
Source data
Author contributions
H.J. conceived the idea and supervised the project. B.H. and H.J. designed the experiments. B.H. performed most of the experiments and analyzed data. H.W. developed key protocols and performed part of the data analysis. Q.C. validated mutant lines and performed pathogen assay. A. Chen performed the nanoparticle tracking and TEM analysis. A. Calvo generated constructs for B. cinerea transformation. G.L. generated the flot1/2 double mutant lines. B.H., A.Chen, and H.J. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available within the paper, Supplementary Information, and Source Data. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-40093-4.
References
- 1.Cai Q, et al. Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annu Rev. Plant Biol. 2021;72:497–524. doi: 10.1146/annurev-arplant-081720-010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Q, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiberg A, et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui C, et al. A fungal pathogen deploys a small silencing RNA that attenuates mosquito immunity and facilitates infection. Nat. Commun. 2019;10:4298. doi: 10.1038/s41467-019-12323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halder LD, et al. Candida albicans Induces Cross-Kingdom miRNA Trafficking in Human Monocytes To Promote Fungal Growth. mBio. 2022;13:e0356321. doi: 10.1128/mbio.03563-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck AH, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gualtieri C, Leonetti P, Macovei A. Plant miRNA Cross-Kingdom Transfer Targeting Parasitic and Mutualistic Organisms as a Tool to Advance Modern Agriculture. Front Plant Sci. 2020;11:930. doi: 10.3389/fpls.2020.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren B, Wang X, Duan J, Ma J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science. 2019;365:919–922. doi: 10.1126/science.aav8907. [DOI] [PubMed] [Google Scholar]
- 9.Kusch S, Singh M, Thieron H, Spanu PD, Panstruga R. Site-specific analysis reveals candidate cross-kingdom small RNAs, tRNA and rRNA fragments, and signs of fungal RNA phasing in the barley-powdery mildew interaction. Mol. Plant Pathol. 2023;24:570–587. doi: 10.1111/mpp.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong-Bajracharya J, et al. The ectomycorrhizal fungus Pisolithus microcarpus encodes a microRNA involved in cross-kingdom gene silencing during symbiosis. Proc. Natl Acad. Sci. USA. 2022;119:e2103527119. doi: 10.1073/pnas.2103527119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji HM, et al. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. N. Phytol. 2021;232:705–718. doi: 10.1111/nph.17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang BS, Li YC, Guo HS, Zhao JH. Verticillium dahliae Secretes Small RNA to Target Host MIR157d and Retard Plant Floral Transition During Infection. Front Plant Sci. 2022;13:847086. doi: 10.3389/fpls.2022.847086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunker F, et al. Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. Elife. 2020;9:e56096. doi: 10.7554/eLife.56096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahid S, et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature. 2018;553:82–85. doi: 10.1038/nature25027. [DOI] [PubMed] [Google Scholar]
- 15.Zhang, T. et al. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants2, 16153 (2016). [DOI] [PubMed]
- 16.Wang M, et al. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants. 2016;2:16151. doi: 10.1038/nplants.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, et al. Puccinia striiformis f. sp. tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. N. Phytol. 2017;215:338–350. doi: 10.1111/nph.14577. [DOI] [PubMed] [Google Scholar]
- 18.Duanis-Assaf D, et al. Double-stranded RNA targeting fungal ergosterol biosynthesis pathway controls Botrytis cinerea and postharvest grey mould. Plant Biotechnol. J. 2022;20:226–237. doi: 10.1111/pbi.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam MT, et al. Minicell-based fungal RNAi delivery for sustainable crop protection. Micro. Biotechnol. 2021;14:1847–1856. doi: 10.1111/1751-7915.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niño-Sánchez J, et al. BioClay™ prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022;64:2187–2198. doi: 10.1111/jipb.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werner BT, Gaffar FY, Schuemann J, Biedenkopf D, Koch AM. RNA-Spray-Mediated Silencing of Fusarium graminearum AGO and DCL Genes Improve Barley Disease Resistance. Front Plant Sci. 2020;11:476. doi: 10.3389/fpls.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haile ZM, et al. Double-Stranded RNA Targeting Dicer-Like Genes Compromises the Pathogenicity of Plasmopara viticola on Grapevine. Front Plant Sci. 2021;12:667539. doi: 10.3389/fpls.2021.667539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buzas, E. I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol.23, 236–250 (2023). [DOI] [PMC free article] [PubMed]
- 24.Rizzo J, Rodrigues ML, Janbon G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front Cell Infect. Microbiol. 2020;10:346. doi: 10.3389/fcimb.2020.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He B, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants. 2021;7:342–352. doi: 10.1038/s41477-021-00863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues ML, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peres da Silva R, et al. Extracellular vesicle-mediated export of fungal RNA. Sci. Rep. 2015;5:7763. doi: 10.1038/srep07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bleackley MR, Dawson CS, Anderson MA. Fungal Extracellular Vesicles with a Focus on Proteomic Analysis. Proteomics. 2019;19:e1800232. doi: 10.1002/pmic.201800232. [DOI] [PubMed] [Google Scholar]
- 30.Vallejo MC, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 2012;7:e39463. doi: 10.1371/journal.pone.0039463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon S, et al. mRNA Inventory of Extracellular Vesicles from Ustilago maydis. J. Fungi (Basel) 2021;7:562. doi: 10.3390/jof7070562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill EH, Solomon PS. Extracellular vesicles from the apoplastic fungal wheat pathogen Zymoseptoria tritici. Fungal Biol. Biotechnol. 2020;7:13. doi: 10.1186/s40694-020-00103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleackley MR, et al. Extracellular Vesicles From the Cotton Pathogen Fusarium oxysporum f. sp. vasinfectum Induce a Phytotoxic Response in Plants. Front Plant Sci. 2019;10:1610. doi: 10.3389/fpls.2019.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 35.Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA. Endocytotic cycling of PM proteins. Annu Rev. Plant Biol. 2005;56:221–251. doi: 10.1146/annurev.arplant.56.032604.144150. [DOI] [PubMed] [Google Scholar]
- 36.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barberon M, et al. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl Acad. Sci. USA. 2011;108:E450–E458. doi: 10.1073/pnas.1100659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23:1920–1931. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mbengue M, et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc. Natl Acad. Sci. USA. 2016;113:11034–11039. doi: 10.1073/pnas.1606004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spallek T, et al. ESCRT-I mediates FLS2 endosomal sorting and plant immunity. PLoS Genet. 2013;9:e1004035. doi: 10.1371/journal.pgen.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortiz-Morea FA, et al. Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc. Natl Acad. Sci. USA. 2016;113:11028–11033. doi: 10.1073/pnas.1605588113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paez Valencia J, Goodman K, Otegui MS. Endocytosis and Endosomal Trafficking in Plants. Annu Rev. Plant Biol. 2016;67:309–335. doi: 10.1146/annurev-arplant-043015-112242. [DOI] [PubMed] [Google Scholar]
- 43.Fan L, Li R, Pan J, Ding Z, Lin J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015;20:388–397. doi: 10.1016/j.tplants.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, et al. Uptake of oomycete RXLR effectors into host cells by clathrin-mediated endocytosis. Plant Cell. 2023;35:2504–2526. doi: 10.1093/plcell/koad069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira-Garcia E, et al. Clathrin-mediated endocytosis facilitates the internalization of Magnaporthe oryzae effectors into rice cells. Plant Cell. 2023;35:2527–2551. doi: 10.1093/plcell/koad094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell. 2012;24:2105–2122. doi: 10.1105/tpc.112.095695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffaele S, et al. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell. 2009;21:1541–1555. doi: 10.1105/tpc.108.064279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Théry C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Wang S, Cai Q, Jin H. Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 2021;63:2020–2030. doi: 10.1111/jipb.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J. Proteom. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Lambou K, et al. Fungi have three tetraspanin families with distinct functions. BMC Genom. 2008;9:63. doi: 10.1186/1471-2164-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007;3:12. doi: 10.1186/1746-4811-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutter BD, Innes RW. Extracellular vesicles in phytopathogenic fungi. Extracell. Vesicles Circul. Nucleic Acids. 2023;4:90–106. doi: 10.20517/evcna.2023.04. [DOI] [Google Scholar]
- 54.Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles3, 24641–4655 (2014). [DOI] [PMC free article] [PubMed]
- 55.Dejonghe W, et al. Disruption of endocytosis through chemical inhibition of clathrin heavy chain function. Nat. Chem. Biol. 2019;15:641–649. doi: 10.1038/s41589-019-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, et al. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell. 2011;23:3780–3797. doi: 10.1105/tpc.111.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robert S, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143:111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M, Weiberg A, Dellota E, Jr., Yamane D, Jin H. Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017;14:421–428. doi: 10.1080/15476286.2017.1291112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan L, et al. Dynamic analysis of Arabidopsis AP2 sigma subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development. 2013;140:3826–3837. doi: 10.1242/dev.095711. [DOI] [PubMed] [Google Scholar]
- 60.Narasimhan M, et al. Evolutionarily unique mechanistic framework of clathrin-mediated endocytosis in plants. Elife. 2020;9:e52067. doi: 10.7554/eLife.52067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schatz, D. et al. Ecological significance of extracellular vesicles in modulating host-virus interactions during algal blooms. ISEM J15, 3714–3721 (2021) [DOI] [PMC free article] [PubMed]
- 62.Schatz, D. et al. Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga. Nat Microbiol2, 1485–1492 (2017). [DOI] [PubMed]
- 63.Hackl, T. et al. Novel integrative elements and genomic plasticity in ocean ecosystems. Cell186, 47–62.e16 (2023). [DOI] [PubMed]
- 64.Di Rubbo, S. et al. The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell25, 2986–2997 (2013). [DOI] [PMC free article] [PubMed]
- 65.Postma J, et al. Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity. N. Phytol. 2016;210:627–642. doi: 10.1111/nph.13802. [DOI] [PubMed] [Google Scholar]
- 66.Bar M, Avni A. EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J. 2009;59:600–611. doi: 10.1111/j.1365-313X.2009.03897.x. [DOI] [PubMed] [Google Scholar]
- 67.Sharfman M, et al. Endosomal signaling of the tomato leucine-rich repeat receptor-like protein LeEix2. Plant J. 2011;68:413–423. doi: 10.1111/j.1365-313X.2011.04696.x. [DOI] [PubMed] [Google Scholar]
- 68.Tian T, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q, et al. Dicer-like Proteins Regulate the Growth, Conidiation, and Pathogenicity of Colletotrichum gloeosporioides from Hevea brasiliensis. Front Microbiol. 2017;8:2621. doi: 10.3389/fmicb.2017.02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin C, Zhu H, Jiang Y, Shan Y, Gong L. Silencing Dicer-Like Genes Reduces Virulence and sRNA Generation in Penicillium italicum, the Cause of Citrus Blue Mold. Cells. 2020;9:363. doi: 10.3390/cells9020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng H, et al. Dicer-Like Genes Are Required for H(2)O(2) and KCl Stress Responses, Pathogenicity and Small RNA Generation in Valsa mali. Front Microbiol. 2017;8:1166. doi: 10.3389/fmicb.2017.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Werner BT, et al. Fusarium graminearum DICER-like-dependent sRNAs are required for the suppression of host immune genes and full virulence. PLoS One. 2021;16:e0252365. doi: 10.1371/journal.pone.0252365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiao L, et al. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol. J. 2023;21:854–865. doi: 10.1111/pbi.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitter N, et al. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants. 2017;3:16207. doi: 10.1038/nplants.2016.207. [DOI] [PubMed] [Google Scholar]
- 75.Luksiene Z, Rasiukeviciute N, Zudyte B, Uselis N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem Photobio. B. 2020;203:111656. doi: 10.1016/j.jphotobiol.2019.111656. [DOI] [PubMed] [Google Scholar]
- 76.Gourgues M, Brunet-Simon A, Lebrun MH, Levis C. The tetraspanin BcPls1 is required for appressorium-mediated penetration of Botrytis cinerea into host plant leaves. Mol. Microbiol. 2004;51:619–629. doi: 10.1046/j.1365-2958.2003.03866.x. [DOI] [PubMed] [Google Scholar]
- 77.Clergeot PH, et al. PLS1, a gene encoding a tetraspanin-like protein, is required for penetration of rice leaf by the fungal pathogen Magnaporthe grisea. Proc. Natl Acad. Sci. USA. 2001;98:6963–6968. doi: 10.1073/pnas.111132998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Correll JC, Klittich CJR, Leslie JF. Nitrate Nonutilizing Mutants of Fusarium oxysporum and Their Use in Vegetative Compatibility Tests. Genetics. 1987;77:1640–1646. [Google Scholar]
- 79.Larson ER, Van Zelm E, Roux C, Marion-Poll A, Blatt MR. Clathrin Heavy Chain Subunits Coordinate Endo- and Exocytic Traffic and Affect Stomatal Movement. Plant Physiol. 2017;175:708–720. doi: 10.1104/pp.17.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang D, et al. Salicylic acid-mediated plasmodesmal closure via Remorin-dependent lipid organization. Proc. Natl Acad. Sci. USA. 2019;116:21274–21284. doi: 10.1073/pnas.1911892116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dejonghe W, et al. Mitochondrial uncouplers inhibit clathrin-mediated endocytosis largely through cytoplasmic acidification. Nat. Commun. 2016;7:11710. doi: 10.1038/ncomms11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006;1:641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- 83.Reynolds GD, August B, Bednarek SY. Preparation of enriched plant clathrin-coated vesicles by differential and density gradient centrifugation. Methods Mol. Biol. 2014;1209:163–177. doi: 10.1007/978-1-4939-1420-3_13. [DOI] [PubMed] [Google Scholar]
- 84.Harley SM, Beevers L. Isolation and partial characterization of clathrin-coated vesicles from pea (Pisum sativum L.) cotyledons. Protoplasma. 1989;150:103–109. doi: 10.1007/BF01403665. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available within the paper, Supplementary Information, and Source Data. Source data are provided with this paper.