Abstract
Previous research suggests a broad range of deficits in major depressive disorder. Our goal was to update the current assumptions and investigate the extent of cognitive impairment in depression in the acute and remitted state. A systematic review of the existing literature between 2009 and 2019 assessing the risk of bias within the included studies was performed. Of the 42 articles reviewed, an unclear risk of bias was shown overall. The risk of bias mainly concerned the sample selection, inadequate remedial measures, as well as the lack of blinding the assessors. In the acute phase, we found strong support for impairment in processing speed, learning, and memory. Follow-up studies and direct comparisons revealed less pronounced deficits in remission, however, deficits were still present in attention, learning and memory, and working memory. A positive correlation between the number of episodes and cognitive deficits as well as depression severity and cognitive deficits was reported. The results also demonstrate a resemblance between the cognitive profiles in bipolar disorder and depression. Comparisons of depression with schizophrenia led to unclear results, at times suggesting an overlap in cognitive performance. The main findings support the global deficit hypothesis and align with results from prior meta-analyses and reviews. Recommendations for future research are also presented.
Keywords: Depression, Neuropsychology, Cognition, Deficits, Impairment
Introduction
Cognitive dysfunction is considered one of the core symptoms of depression in the current diagnostic and statistical manual of mental disorders [1]. It is described as a reduced ability to think, concentrate, or make decisions. The cognitive level of performance in patients with major depressive disorder (MDD) is of high practical relevance because cognitive deficits are associated with a lowered ability to function in everyday life, reduced psychotherapeutic treatment success, and increased suicidality [2, 3].
Previous research has shown deficits in the areas of executive functions (EF), memory, psychomotor speed, and attention in patients with MDD as compared to healthy controls (HC) [4, 5]. These deficits range from mild to severe and can partially persist after remission from depression [4–6]. Hammar and Ardal [4] summarize deficits in attention, EF, and memory after remission. They point out that a reduction of depressive symptoms does not necessarily lead to cognitive improvement. This could be due to cognitive impairments being trait markers rather than state markers of depression [7]. So far, research on the connection between symptom severity and cognitive impairment has been inconsistent, but overall, a tendency for a positive correlation is reported [8, 9]. Besides these studies, systematic reviews and meta-analyses comparing the evidence of cognitive impairment in depression with other disorders are rather rare. Bora et al. [10] state that there is only a small difference in cognitive functioning between schizophrenia (SCH) and affective psychoses, despite the popular belief that these are two qualitatively distinguishable disorders. The authors revealed that, among other factors, more severe negative symptoms moderated the negative effect on cognitive functions. Similar results are reported by Stefanopoulou et al. [11] who also found quantitative rather than qualitative differences comparing MDD, bipolar disorder (BD), and SCH. The cognitive profiles in euthymic BD patients point to similar results as shown in remitted depressed (RD) patients [12].
In our systematic review, we aim to summarize the research findings of the last 10 years on neuropsychological deficits of adults suffering from MDD. Based on the latest research on cognitive deficits in MDD described above, we expect (1) homogeneous results concerning the impaired areas: EF, memory, attention, and psychomotor speed. Regarding the course of the deficits, we hypothesize (2) only a partially restored cognitive performance profile after remission. In addition, we assume (3) a positive correlation between the number of episodes and cognitive impairment as well as (4) a positive correlation between severity of depression and cognitive impairment. Additionally, we will summarize results considering the frequency of cognitive deficits in depression and differences between MDD and BD as well as MDD and SCH.
Methods
To guarantee a transparent and reproducible research process [13], we (a) disclosed all systematic review data, including risk of bias coding, on the Open Science Framework (OSF; see https://osf.io/hn3w8/), (b) adhered to the PRISMA 2020 reporting guidelines [14], (c) pre-registered our introduction and method section on the Open Science Framework before starting with data collection (see https://osf.io/5by6j), (d) hereby allow other researchers to re-analyze our data including our entire literature hits from databases in common file formats, and (e) recruited expertise.
Inclusion criteria
We included studies published in English examining the neuropsychological functions of at least 18-year-old participants who received a depressive disorder diagnosis according to international diagnostic manuals (e.g., ICD-10, DSM-IV, and DSM-5). The selected studies measured cognitive functions by reliable, valid, and objective neuropsychological tests. The test data could reflect the current status, a follow-up (e.g., 1 year after onset of illness), or compare cognitive deficits with other diseases.
Consequently, we excluded studies on animals, biological studies that aim at identifying disorder-specific genes, and studies in which the participants mainly suffer from comorbid psychological diseases (e.g., dementia, addiction). Furthermore, no family studies or research with a focus on the effects of interventions (e.g., therapeutic effects) were considered. We did not include studies examining social cognitions (e.g., perspective taking, empathy) or studies with participants not meeting depression criteria.
Information sources and search
We chose the online databases PsycINFO, Scopus, and PubMed for our literature search. The search term was created by adapting search terms of already conducted reviews. Our search term was applied to the titles of the primary studies. For all databases, we used the following search term: “(depress*) AND (cogniti* OR neuropsychological) AND (impairment* OR function* OR deficit*)”. For PsycINFO we deactivated the option “linked full text”, set the publication year to 2009–2019, set the publication type to “Peer-reviewed Journal”, and activated the box “English”. In Scopus, we set the date range to 2009–2019, set the document type to “article”, and access type to “All”. The only filter activated in PubMed was restricting the search to studies published within the last ten years. Studies published until the 1st of October 2019 were included.
Study selection and data collection
Three members of our research team were responsible for the selection process. Non-relevant studies were excluded and assigned to different categories according to why they were rejected. On the other hand, studies that appeared relevant were downloaded in a RIS-format and saved in Citavi. The main author (DK) double-checked the excluded and included studies. For included studies, we extracted data for the following variables: author names, publication year, date and place of the study, diagnosis and age of patients, applied psychological tests, and the outcome of the tests.
Outcome measures
To compare the results of different studies, we used statistical values of reliable and valid standardized neuropsychological tests. All reported differences in our review were based on statistically significant results. We did not rely on descriptive evaluations (e.g., “better”, “higher scores”).
Assessment of the risk of bias in individual trials
To assess the risk of bias in individual studies, we used and adapted the Cochrane risk of bias tool for randomized-controlled trials [15]. We omitted the items assessing random sequence generation and allocation concealment because they only apply to randomized-controlled trials. Instead, we assessed selection bias and verified if a clear and thorough diagnostic procedure was applied. A detailed description and explanation of our items as well as citations of the primary studies to support our evaluation is provided in our excel coding sheet on the OSF (see https://osf.io/hn3w8/). We summarized the assessment of risk of bias within and across trials primarily by following an example by Higgins et al. [15] (see Table 1).
Table 1.
Summary assessments of risk of bias within and across studies (adapted from Higgins et al. [15])
| Risk of bias | Interpretation | Within trial | Across trials |
|---|---|---|---|
| Low risk of bias | Bias, if present, is unlikely to alter the results seriously | Low risk of bias for all key items | The majority of trials carry a low risk of bias |
| Unclear risk of bias | A risk of bias raises some doubt about the results | Low or unclear risk of bias for all key items | The majority of trials carry a low or unclear risk of bias |
| High risk of bias | Bias may alter the results seriously | High risk of bias for one or more key items | The majority of trials carry a high risk of bias |
Results
Included studies
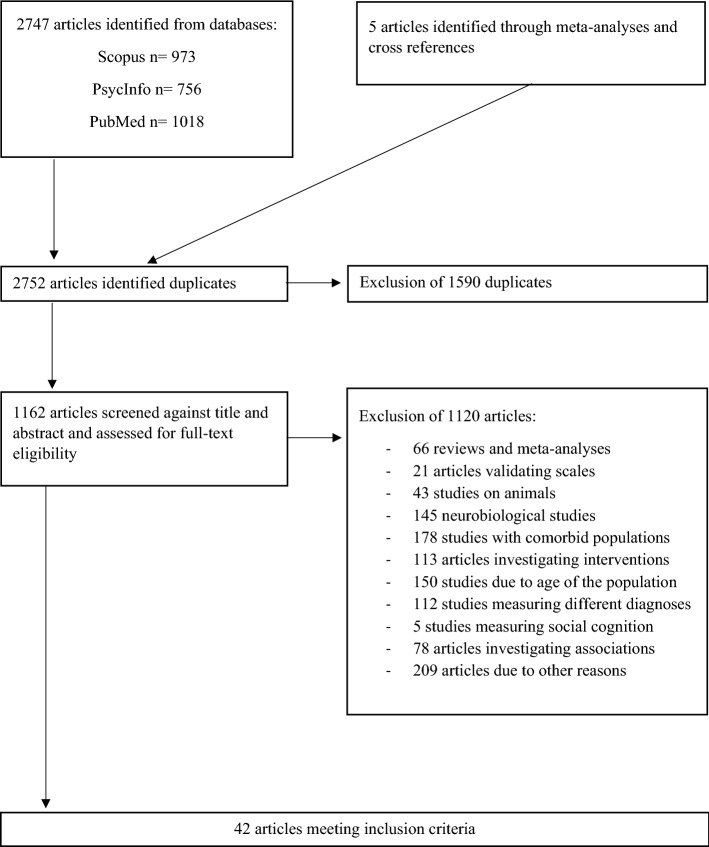
A total of 1162 articles were screened for eligibility. After exclusion of 1120, we included a total of 42 studies [16–57]. Figure 1 illustrates our search, screening, and selection process.
Fig. 1.
Flowchart of the selection process (adapted from Page et al.[14])
For example, a study by Eraydin et al. [58] was excluded because the depression diagnostics were conducted through an online tool which limited the reliability. Likewise, we excluded a study by Ambaw et al. [59] which used only one cognitive screening test (MMSE), that had not been validated in the study’s country and applied unclear inclusion criteria.
Risk of bias
Based on the modified Cochrane Risk of Bias Tool, ten studies carried a high risk of bias (see Table 2). Eight of these studies showed methodological deficits in the selection process, one study reported not blinding the assessors, and one study did not address incomplete outcome data. A closer look of the studies showing a weaker selection process revealed differences in the characteristics of the experimental and the control group. These sample differences, which were not adequately corrected, led to comparisons of heterogeneous groups. The remaining studies showed an unclear risk of bias, mainly due to the absence of any blinding measurements.
Table 2.
Summary assessment of the risk of bias within studies by applying the Cochrane Risk of Bias Tool (Higgins et al. [15])
| Author, year | Selection bias | Clear diagnostics | Blinding (patients) | Detection bias | Incomplete outcome data addressed | Free of selective reporting | Summary assessment |
|---|---|---|---|---|---|---|---|
| Albert [16] | ? | + | ? | ? | ? | + | ? |
| Ardal [17] | + | ? | ? | ? | + | + | ? |
| Baune [18] | ? | + | ? | ? | ? | + | ? |
| Bhardwaj [19] | + | + | ? | ? | + | + | ? |
| Boeker [20] | ? | ? | ? | ? | ? | + | ? |
| Castaneda [21] | ? | ? | ? | + | + | + | ? |
| Constant [22] | – | ? | ? | ? | + | + | – |
| Daniel [23] | ? | ? | ? | + | + | + | ? |
| Gooren [24] | ? | ? | + | ? | ? | + | ? |
| Gruber [25] | + | ? | ? | ? | ? | + | ? |
| Grützner [26] | + | + | ? | ? | + | ? | ? |
| Halvorsen [27] | ? | + | ? | ? | + | + | ? |
| Hammar [28] | + | ? | ? | ? | + | + | ? |
| Hasselbalch [29] | ? | ? | ? | + | + | + | ? |
| Hsu [30] | + | ? | ? | ? | + | + | ? |
| Jia [31] | ? | ? | ? | ? | + | + | ? |
| Kaygusuz [32] | – | + | ? | ? | + | ? | – |
| Keilp [33] | ? | ? | ? | ? | ? | + | ? |
| Leposavic [34] | ? | ? | ? | ? | ? | + | ? |
| Liu [35] | ? | + | ? | ? | ? | + | ? |
| Lyche [36] | – | + | ? | ? | + | + | – |
| Lyche [37] | – | + | ? | ? | + | + | – |
| Maalouf [38] | + | + | ? | ? | + | + | ? |
| Mak [39] | ? | + | ? | ? | + | ? | ? |
| McClintock [40] | – | + | + | + | ? | + | – |
| Moniz [41] | ? | ? | ? | ? | ? | + | ? |
| Neu [42] | – | + | ? | ? | + | + | – |
| Peters [43] | ? | + | ? | ? | + | + | ? |
| Preiss [44] | + | + | ? | – | + | + | – |
| Preiss [45] | + | + | ? | ? | + | + | ? |
| Rampacher [46] | + | + | ? | ? | + | + | ? |
| Reppermund [47] | + | + | ? | ? | - | ? | – |
| Roca [48] | ? | + | ? | ? | + | + | ? |
| Schaub [49] | ? | ? | ? | ? | + | + | ? |
| Schmid [50] | + | + | ? | ? | ? | + | ? |
| Schulze [51] | ? | ? | ? | ? | + | + | ? |
| Schwert [52] | ? | + | ? | ? | + | + | ? |
| Sostaric [53] | + | ? | ? | ? | + | + | ? |
| Taconnat [54] | + | ? | ? | ? | ? | + | ? |
| Talarowska [55] | – | ? | ? | ? | ? | + | – |
| Wekking [56] | – | ? | ? | ? | ? | ? | – |
| Zaremba [57] | + | ? | ? | ? | ? | + | ? |
Bold print represents the summary assessment
Notes + : represents a low risk of bias; ?: represents an unclear risk of bias; -: represents a high risk of bias
Overall, our set of included studies carries an unclear risk of bias (see Table 1). Therefore, the results and conclusions of this review must be interpreted with caution.
Currently depressed (CD) patients vs. HC group
Information processing speed
The vast majority of studies demonstrated a significant reduction of information processing speed in CD patients [16, 24, 35, 36, 39, 41, 42, 47, 51, 52, 54, 57]. Three studies did not show significant differences [27, 28, 30]. An exemplary overview of assignments of tests to cognitive functions is presented in Table 3. Table 4 shows all the included studies for the comparison between CD and HC.
Table 3.
Assignments of tests to neuropsychological functions
| Cognitive function | Cognitive sub-function | Test |
|---|---|---|
| Attention | Alertness | CalCAP, COGBAT Alertness, TEA phasic alertness task |
| Divided attention | COGBAT: Divided Attention, TAP divided attention | |
| Sustained attention | Continuous performance task (CPT) | |
| Executive function | Cognitive flexibility | CANTAB intra-extradimensional set shift (IED), TMT B, Wisconsin Card Sorting Test (WCST) |
| Inhibition |
D-KEFS Color–Word Interference Test, Stroop test |
|
| Planning | Tower test | |
| Information processing speed | CANTAB rapid visual information processing (RVIP), TMT A | |
| Learning and memory | Verbal | Rey‘s Auditory Verbal Learning Test, Wechsler Memory Scale (WMS), Word memory task (WMT), CANTAB paired associates learning (PAL) |
| Visual | Benton Visual Retention Test, Doors test, Wechsler Memory Scale (WMS), | |
| Verbal fluency | Animal naming, Controlled Oral Word Association Test (COWA), D-KEFS Verbal Fluency | |
| Visuospatial ability | RBANS visuospatial ability | |
| Working memory | Digit span, Paced Auditory Serial Addition Test (PASAT) |
D-KEFS Delis-Kaplan Executive Function System, CalCAP California Computerized Assessment Package, CANTAB Cambridge Neuropsychological Test Automated Battery, COGBAT Cognitive Basic Assessment, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, TAP Testbatterie zur Aufmerksamkeitsprüfung, TEA Test of Everyday Attention, TMT Trail Making Test
Table 4.
Characteristics and main results of studies investigating currently depressed samples
| Author & year | Currently depressed group | Healthy control group | Cognitive tests | Main results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (% female) | Age | Characteristics | Symptom severity | n (% female) | Age | Symptom severity | ||||||
| Albert [16] | 91 (66.3) | 35.9, 9.0 | Recurrent MDD, IP | 24.0, 4.4 (MADRS) | 105 (64.8) | 30.2, 9.1 | 0.7, 1.1 (MADRS) | Logical Memory 1 & 2, Benton Visual Retention Test, RVLT, COWA, TMT A + B, Animal Naming, Stroop Color, Symbol-Digit Modality, Digit Span Forward & Backward |
D < HC: processing speed D = HC: WM, visual memory, EF |
|||
| Ardal [17] | 19 (52.6) | 42.5, 10 | Acute unipolar MDD, IP and OP | 22.2, 3.6 (HDRS) | 19 (52.6) | 42, 9.7 | – | Stroop test (Hugdahl version) | D < HC: inhibition | |||
| Baune [18] | 26 (missing / wrong) | 46.0, 12.1 | MDD, OP | 18.0, 5.9 (HAMD-D 17) | 206 (61.2) | 47.5, 15.2 | – | RBANS |
D < HC: immediate memory, visuospatial/construction, language, attention, D = HC: delayed memory |
|||
| Boeker [20] | 28 (46.4) | 39.7, 11.4 | MDD, IP | 25.9, 8.2 (BDI); 28.5, 7.0 (HDRS-21) | 28 (46.4) | 35.0 (SD error) | – | CANTAB | D < HC: visual learning, memory, WM, EF, sustained attention | |||
| Constant [22] | 25 (64.0) | 45.8, 11.0 | MDD, OP | 25.6, 8.6 (BDI); 22.0, 5.8 (GDS) | 29 (62.1) | 47.5, 12.6 | 2.4, 3.2 (BDI), 4.0, 3.3 (GDS) | TEA 1.5, PASAT, Word memory test, Doors test | D < HC: alertness, WM, verbal and visual memory | |||
| Gooren [24] | 102 (72) | 52.4, 11.9 | MDD, IP | 19.7, 4.7 (MES) | 85 (68.2) | 52.6, 68.2 | – | RAVLT, TMT A, Verbal Fluency Test, WMS | D < HC: verbal learning, verbal memory, processing speed, verbal fluency, visual memory | |||
| Gruber [25] | 18 (78) | 46.5, 10.7 | CD, IP | 4.3, 1.0 (CGI); 19.9, 10.7 (BDI); 20.1, 10.7 (MADRS) | 18 (78) | 44.6, 11.6 | – | Computer-based behavioral experiment measuring process- and circuit-specific WM tasks |
D < HC: articulatory rehearsal component of verbal WM D = HC: non-articulatory maintenance of phonological information, visuospatial WM |
|||
| Halvorsen [27] | 37 (73) | 37.5, 12.0 | CD, OP | 25.3, 9.2 (BDI-II) | 50 (78) | 38, 12.7 | 3.1, 2.9 (BDI-II) | D-KEFS: Color-Word and Verbal Fluency, WCST-64, TMT A + B, calCAP, Halstead-Reitan Seashore Rhythm Test, WAIS-III: digit span forward, digit span backward, digit symbol coding, |
D < HC: WM, processing speed D = HC: EF, attention, verbal fluency |
|||
| Hammar [28] | 24 (75) | 38.1, 11.4 | Recurrent MDD, acute, IP | 22.4, 4.5 (HDRS); 27.1, 5.2 (MADRS) | 24 (75) | 37.1, 11.5 | – | D-KEFS: TMT, Colour-Word Interference Test, Verbal Fluency Test, Tower Test |
D < HC: inhibition, inhibition/switching, category fluency, color naming D = HC: processing speed, cognitive flexibility, planning, word reading, letter fluency, category switching |
|||
| Hsu [30] | 26 (76.9) | 23.1, 6.2 | CD, OP | 23.2, 10.2 (BDI-II) | 29 (62) | 24.1, 6.8 | 5.9, 7.9 (BDI-II) | D-KEFS: Color-Word interference test, TMT A + B, Emotional Stroop Task |
D < HC: selective attention, inhibition D = HC: psychomotor speed, cognitive flexibility/set shifting |
|||
| Jia [31] | 62 (52.6) | 35.6, 12.7 | FE drug naïve depressed OP | – | 90 (66.7) | 35, 10.7 | – | RBANS |
FED < HC: language, delayed memory FED = HC: Immediate memory, attention, visuoconstruction |
|||
| Jia [31] | 111 (65.8) | 41.6, 12.1 | Medicated FE unipolar depressed IP | – | 90 (66.7) | 35, 10.7 | – | RBANS |
MD < HC: immediate memory, delayed memory, language, MD = HC: attention, visuoconstruction |
|||
| Liu [35] | 30 (66.7) | 27.8, 7.2 | MDD, IP and OP | 24.1, 4.5 (HDRS-24) | 30 (53.3) | 24.5, 3.0 | – | WAIS-RC: digit symbol-coding, digit span; WMS-RC; modified WCST, TMT-B, VFT; modified Stroop Color Word Test: |
D < HC: psychomotor speed, WM, visual memory, attention switching, verbal fluency D = HC: attention, cognitive flexibility, response inhibition |
|||
| Lyche [36] | 37 (62.2) | 44.2, 12.3 | MDD without anxiety | 21.4, 11.1 (BDI) | 91 (69.3) | 35.8, 12.0 | 2.1, 2.7 (BDI) | WAIS-III: picture completion, similarities; CANTAB: intra-extra dimensional, spatial WM, stop signal task |
D < HC: psychomotor speed D = HC: set shifting, WM, inhibition |
|||
| Lyche [37] | 37 (62.2) | 44.2, 12.3 | MDD | 21.3, 11.1 (BDI) | 92 (68.5) | 35.7, 12.0 | 2.1, 2.7 (BDI) | D-KEFS: Color Word Interference Test, Attentional Network Test |
D < HC: alertness D = HC: switching/inhibition, inhibition, EF |
|||
| Mak 2018 | 35 (57.1) | 24.9, 4.4 | MDD | 23.2, 5.4 (MADRS) | 35 (65.7) | 22.9, 3.2 | 0.2, 0.4 (MADRS) | TMT, Digit Span, WCST, Category fluency test, Chinese AVLT, WMS |
D < HC: processing speed, cognitive flexibility D = HC: attention switching, WM, verbal fluency, verbal and visual memory |
|||
| Maloof [38] | 20 (80) | 34.2, 9.4 | acute recurrent unipolar depressed, OP | 24.8, 5.8 (HAM-D) | 28 (67.9) | 31.9, 9.4 | – |
CANTAB: Rapid Visual Processing (RVP), Stockings of Cambridge (SOC), Delayed matching to Sample task (DMS) |
D < HC: EF D = HC: sustained attention, memory |
|||
| Moniz [41] | 20 (65) | 44.3, 14.8 | MDD non suicide attempters | 17.2, 7.3 (HAM-D); 2.3, 1.0 (BSI-D) | 20 (65) | 43.3, 14.9 | – | Go/No-Go Task, ToL, Victoria Stroop Test, WCST, Finger Tapping Task, TMT, Verbal Fluency Test, AVLT | D < HC: processing speed, cognitive flexibility, motor speed, planning, inhibition, EF | |||
| Neu [42] | 67 (67.2) | 51.7, 12.0 | MDD, IP | 19.1, 4.7 (MES) | 63 (69.8) | 52.4, 11.3 | – | RAVLT, TMT A, verbal fluency, WMS-R (Subscale Visual Memory) | D < HC: verbal learning and memory, processing speed, verbal fluency, visual memory | |||
| Reppermund [47] | 53 (52.3) | 43.5, 8.0 | MDD, IP | 25.1, 5.1 (HAMD) | 13 (53.8) | 46.4, 9.5 | – |
TAP: Alertness + Divided Attention, ZVT, Aufmerksamkeits-Belastungstest d2, WMS, verbal fluency tasks, SPM, CANTAB |
D < HC: verbal learning and memory, WM, attention, processing speed, EF | |||
| Schmid [50] | 30 (47) | 26.2, 5.9 | FE MDD, OP | 24.6, 3.7 (MADRS) | 30 (47) | 26.2, 5.7 | – | D-KEFS: Colour-Word Interference, verbal fluency CWIT, TMT, Tower Test |
D < HC: inhibition, semantic fluency D = HC: mental flexibility, phonemic fluency, planning, problem solving |
|||
| Schulze [51] | 34 (59) | 26.2, 5.9 | Moderate depressive disorder OP | – | 76 (59) | 24.9, 5.7 | – | MWT-A, LPS-3, ToH, WCST, TAP: WM, attention, CPT |
D < HC: working speed D = HC: set shifting, planning, inhibition, WM, sustained attention |
|||
| Schwert [52] | 103 (69) | 42.8, 13.02 | Acute recurrent MDD, OP | 18.1, 5.5 (HAMD-17); 26.6, 9.7 (BDI-II) | 103 (69) | 42.7, 12.5 | – | COGBAT: TMT A + B, Alertness, Divided Attention, N-back verbal, Figuraler Gedächtnis Test, Go-NoGo, ToL |
D < HC: processing speed, divided attention, verbal WM, figural memory, inhibition D = HC: alertness, cognitive flexibility, planning |
|||
| Taconnat [54] | 21 (71) | 29.7, 5.5 | MDD, IP | 11.8, 3.4 (HADS, Depression) | 24 (67) | 28.5, 4.6 | 7.1, 3.3 (HADS depression) | WCST, letter-comparison test, COWA: categorical fluency | D < HC: EF, cognitive speed, categorical fluency | |||
| Zaremba [57] | 106 (60) | 37.7, 13.3 | MDD IP and OP | 14.64, 4.25 (HDRS-17) | 120 (56) | 37.4, 13.5 | 1.40, 1.68 (HDRS-17) | WAIS-R: digit symbol substitution test, TMT A, RAVLT, WMS: Spatial Span, Letter-Number Sequences |
D < HC: processing speed, verbal learning and memory, visuospatial learning and memory D = HC: WM |
|||
MDD Major Depressive Disorder, CD Currently depressed, IP inpatients, OP outpatients, FE first episode, D < HC significant differences in favor of HC, D = HC no significant differences, p < .05, EF executive Functions, WM working memory
AVLT Auditory Verbal Learning Test, BDI Beck Depression Inventory, CalCAP California Computerized Assessment Package, CANTAB Cambridge Neuropsychological Test Automated Battery, COGBAT Cognitive Basic Assessment, COWA Controlled Oral Word Association, CPT Continuous Performance Task, D-KEFS Delis-Kaplan Executive Function System, HADS Hospital Anxiety and Depression Scale, HAMD/HDRS Hamilton Depression Rating Scale, LPS Leistungsprüfsystem, MADRS Montgomery-Asberg Depression Rating Scale, MMSE Mini-Mental Status Examination, MWT Mehrfachwahl-Wortschatz-Intelligenz-Test, PASAT Paced Auditory Serial Addition Test, RAVLT Rey Auditory Verbal Learning Test, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, SPM Raven’s Standard Progressive Matrices, TAP Testbatterie zur Aufmerksamkeitsprüfung, TEA Test of Everyday Attention, TMT Trail Making Test, ToL Tower of London, ToH Tower of Hanoi, WAIS Wechsler Adult Intelligence Scale, WCST Wisconsin Card Sorting Test, WMS Wechsler Memory Scale, ZVT Zahlenverbindungstest
Attention
A slight majority of studies investigating attention found a significantly reduced performance for depressed patients. Deficits were predominantly apparent for alertness [22, 27, 37]. Schwert [52] also found a reduced alertness on a non-significant level (p = 0.067). Additionally, divided attention [52], sustained attention [20], selective attention [30], and attention switching [35] was significantly impaired. Reppermund et al. [47] stated that there are significant deficits in attention and showed a reduced performance in the depressed sample, but withheld results compared to the HC group. Two other studies’ results regarding the RBANS domain attention were inconsistent [18, 31]. Unimpaired performance was shown in attention switching [39] sustained attention [38, 51], and in attention span (WAIS digit span forward) [27, 35].
Verbal and visual learning and memory
Significant differences between HC and CD patients are mainly found in verbal learning and memory [22, 24, 42, 47, 57] and in visual learning and memory [20, 22, 24, 35, 42, 52, 57]. Jia [31] showed that first episode drug-naïve depressive patients had deficits in delayed, but not in immediate memory, whereas medicated depressive patients presented deficits in immediate and delayed memory. By contrast, Baune [18] found differences just in the immediate memory (RBANS). Three other authors failed to show differences in visual memory [16], verbal and visual memory [39], and immediate and delayed visual memory [38].
Visuospatial
In the domain visuoconstruction, two studies [18, 31] showed ambiguous results using the RBANS to examine the drawing of a geometrical figure and the organizing of lines according to their angles.
Working memory (WM)
There is a similar amount of evidence for deficits in WM [20, 22, 27, 35, 47, 52] and for an unimpaired performance [16, 36, 39, 51, 57]. Gruber [25] dedicated an entire study to investigating different facets of WM and showed significantly reduced scores for CD patients in verbal WM tasks requiring the articulatory rehearsal mechanism controls. Additionally, CD patients scored worse, but no significant results were found in other WM tasks (non-articulatory maintenance of phonological information; visuospatial rehearsal/pattern maintenance).
Verbal fluency
Most studies investigating language used categorical fluency tests to examine verbal semantic fluency. Significant differences in favor of the HC were shown in multiple studies [24, 35, 42, 54]. Schmid [50] also found differences in semantic fluency, but no differences in phonemic fluency or switching category. The same results were found in Hammar’s study [28] using the D-KEFS to examine multiple verbal fluency categories. Depressed patients had significantly worse results in semantic fluency, but not phonemic fluency or in a switching category condition. A similar trend is shown in Halvorsen et al.’s study [27] also through the D-KEFS: they found no differences in phonemic (p = 0.56) and switching category (p = 0.53), but a tendency towards a difference in semantic fluency (p = 0.1). Mak [39] could not find differences in verbal semantic fluency.
Executive function
There is a similar amount of studies showing deficits in inhibition in CD patients [17, 28, 30, 41, 50, 52] and studies showing no deficits [35–37, 51]. While most studies report no significant differences in cognitive flexibility [27, 28, 30, 35, 36, 47, 50–52], two studies found differences [39, 41]. Planning did not differ between groups [28, 50–52] except in Moniz’s study [41]. For visual problem solving, there is evidence of deficits [47] and evidence of similar performance [50]. Albert [16] found no differences in EF measured by multiple tests, which included an assessment of cognitive flexibility. Also, Lyche [37] using the Attentional Network Test, found no significant deficits for the domain EF. Deficits for the depressed sample for EF was shown using the tasks “Stockings of Cambridge” (SOC) [38] and “intra-extradimensional set shift” [20]. The Wisconsin Card Sorting Test (WCST) also revealed deficits [41, 54].
Remitted depressed (RD) patients vs. HC group
Information processing speed
The majority of studies did not find a decrease of the information processing speed in RD patients [19, 26, 27, 30, 43, 57] (see Table 5). However, Halvorsen [27] showed a slower reaction in the RD sample. Preiss [45] showed deficits in the hospitalized sample but not in the non-hospitalized one. Differences between the groups were found in four studies [29, 34, 44, 56].
Table 5.
Characteristics and main results of studies investigating remitted depressed samples
| Remitted depressed group | Healthy control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author and year | n (% female) | Age | sample | Symptom severity | n (female) | Age | Symptom severity | Cognitive tests | Main results |
| Baune [18] | 44 (missing /wrong) | 44.2, 15.9 | previous MDD | 6.8, 4.3 (HAMD-D 17) | 206 (61.2) | 47.5, 15.2 | N/A | RBANS |
RD < HC: immediate memory, attention, RD = HC: visuospatial/construction Language Delayed memory |
| Bhardway [19] | 20 (10.0) | 34.3, 8.2 | Recovered | 3.5, 2.0 (HDRS) | 20 (15.0) | 33.0, 7.5 | 2.5, 2.0 (HDRS) | WCST, WAIS, MMSE, Vocabulary Test |
RD < HC: planning, problem solving RD = HC: WM, visuomotor speed, shifting attention |
| Daniel [23] | 25 (64.0) | 50.6, 8.3 | MDD remitted | 3.2, 1.4 (HAM-D) | 29 (62.1) | 47.5, 12.6 | N/A | MMSE, Babcock Story Recall Test, WCST, TMT-B, Stroop Color & Word, WAIS-R: Symbol-Number Association, Digit Span | RD < HC: EF, WM RD = HC: verbal memory |
| Grützner [26] | 65 (80) | 38.9, 14.3 | Fully and partial remitted | 8.75, 5.1 (HAMD), 15.56, 9.93 (BDI-II) | 65 (80) | 38.9, 14.2 | 1.85, 1.99 (HAMD); 2.62, 3.17 (BDI-II) | COGBAT: TMT A + B, Alertness, Divided Attention, Selective attention; Nback verbal; Figural Memory Test, CVLT; Go-NoGo-INHIB, ToL, WAIS: Symbol Coding Task; |
RD < HC: attention, learning, memory, WM RD = HC: information processing speed, EF |
| Halvorsen [27] | 81 (87.7) | 37.4, 9.6 | recovered MDD | 7.7, 6.7 (BDI-II) | 50 (78) | 38, 12.7 | 3.1, 2.9 (BDI-II) | D-KEFS: Color-Word and Verbal Fluency, WCST, TMT A + B, calCAP, Halstead-Reitan Seashore Rhythm Test, WAIS-III: digit span forward, digit span backward, digit symbol coding |
RD < HC: processing speed RD = HC: WM, EF, information processing, attention, verbal fluency |
| Hasselbalch [29] | 88 (68) | 59.8, 9.2 | Remitted MDD | 2.8, 2.4 (HDRS-17) | 50 (70) | 59.7, 8 | 1.7, 1.7 (HDRS-17) | TMT A + B, Symbol Digit Modalities Test, RAVLT, CCR, RCFT, Familiar Faces, Boston Naming Test, phonological fluency test, semantic fluency test, Stroop test incongruent/ interference, WCST, Letter-Number-Sequencing |
RD < HC: attention, visuomotor speed RD = HC: memory, verbal function, EF |
| Hsu [30] | 30 (66.7) | 23.9, 6.3 | Formerly depressed | 7.6, 7.2 (BDI-II) | 29 (62) | 24.1, 6.8 | 5.9, 7.9 (BDI-II) | D-KEFS: Color-Word interference test, TMT A + B, Emotional Stroop Task | RD = HC: selective attention, inhibition, psychomotor speed, cognitive flexibility |
| Leposavic [34] | ? | 48.3, 7.8 | Endogenous depression, remit-ed, IP | 7.3, 1.5 (BDI); 6.0, 2.2 (HRSD) | ? | 47.7, 6.6 | N/A | MMSE, TMT A + B, RCFT RAVLT, WCST |
RD < HC: processing speed, attention switching, visual and verbal memory, WM, prolonged attention |
| Peters [43] | 62 (72.3) | 20.9, 1.6 | Remitted MDD youth OP | 2.7, 3.4 (HAM-D) | 43 (57.5) | 20.7 (1.7) | 0.4, 1.0 (HAMD) | Stroop Color and Word Test, COWAT, WAIS-IV: Digit Symbol, TMT A + B, Parametric Go/NoGo Task |
RD < HC: cognitive control RD = HC: verbal fluency, processing speed, Conceptual reasoning & set shifting, processing speed with interference resolution |
| Preiss [44] | 97 (52.6) | 46.3, 12.0 | RD | 11.8, 7.0 (BDI-II), 4.4, 3.0 (MADRS) | 97 (52.6) | 46.1, 12.8 | 6.6, 6.0 (BDI-II) | AVLT, TMT A + B | RD < HC: verbal learning and memory, processing speed, cognitive flexibility |
| Preiss [45] | 46 (45.7) | 47.3, 10.4 | RD, previously hospitalized | 11.8, 6.9 (BDI-II), 4.3, 3.0 (MADRS) | 92 (54.3) | 46.2, 12.0 | 6.3 (5.7) (BDI-II) | AVLT, TMT A + B |
RD < HC: delayed recall, processing speed RD = HC: learning, EF |
| Preiss [45] | 46 (63) | 43.5, 13.0 | RD, non- hospitalized | 11.3, 7.2 (BDI-II), 4.5, 3.2 (MADRS) | 92 (54.3) | 46.2, 12.0 | 6.3 (5.7) (BDI-II) | AVLT, TMT A + B |
RD < HC: delayed recall, RD = HC: Learning, EF, processing speed |
| Wekking [56] | 137 (75) | 44.9, 9.4 | Remitted MDD | 3.7, 2.9 (HDRS) | Normative data | Stroop Color-Word Test; Memory Comparison Task; Digit Span; Rivermead Behavioral Memory Tests: Story Recall, Dutch CVLT |
RD < HC: processing speed, WM, verbal memory RD = HC: EF |
||
| Zaremba [57] | 119 (67) | 38.5, 13.9 | Remitted MDD OP | 2.60, 2.23 (HDRS-17) | 120 (56) | 37.4, 13.5 | 1.40, 1.68 (HDRS-17) | WAIS-R: Digit symbol substitution test, TMT A, RAVLT, WMS, WAIS-3: Letter-Number Sequences |
RD = HC: processing speed, verbal learning and memory, visuospatial learning and memory, WM |
MDD Major Depressive Disorder, RD Remitted depressed, IP inpatients, OP outpatients, FE first episode, D < HC significant differences in favor of HC, D = HC no significant differences, p < 0.05, EF executive Functions, WM working memory. AVLT Auditory Verbal Learning Test, BDI Beck Depression Inventory, CalCAP California Computerized Assessment Package, CCR Category Cued Recall, COGBAT Cognitive Basic Assessment, COWA Controlled Oral Word Association, CVLT California Verbal Learning Test, D-KEFS Delis-Kaplan Executive Function System, HADS Hospital Anxiety and Depression Scale, HAMD/HDRS Hamilton Depression Rating Scale, MADRS Montgomery-Asberg Depression Rating Scale, MMSE Mini-Mental Status Examination, RAVLT Rey Auditory Verbal Learning Test, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, RCFT Rey-Osterrieth Complex Figure Test, TMT Trail Making Test, ToL Tower of London, WAIS Wechsler Adult Intelligence Scale, WCST Wisconsin Card Sorting Test, WMS Wechsler Memory Scale
Attention
Deficits in attention were observed in various tests [18, 26, 29, 34], while several other studies showed comparable performances in shifting attention [19] and selective attention [30] or in general attention [27].
Verbal and visual learning and memory
Significant deficits in RD patients were found in visual and verbal memory [26, 34, 44], in verbal immediate and delayed recall [56], and in just the immediate memory [18]. Slightly different results were found in Daniel’s study [23] in verbal memory, p = 0.05; the difference did not reach significance. Preiss [45] revealed deficits in the delayed recall but not in learning. Other studies showed no differences in delayed memory [18] and verbal and nonverbal memory [29]. Similarly, Zaremba [57] showed unimpaired performance in verbal and visual learning and memory.
Visuospatial
Comparable results are shown for both groups by one study [18].
Working memory
For WM the results are relatively inconsistent. Some studies demonstrated significant differences [23, 26, 34, 56], while others did not [19, 27, 57].
Verbal fluency
When testing for language, particularly for verbal fluency, no differences between the two groups were reported [27, 29, 43].
Executive functions
No differences were found for inhibition [26, 30]. Most studies showed no deficits in cognitive flexibility assessed by the TMT-B [26, 30, 45] or a verbal fluency task with a switching condition [27]. One study reported impairment in the RD group [44]. For planning, two studies reported no differences [26, 29], one reported differences [19]. Bhardway [19] pointed out reduced problem solving. Peters [43] demonstrated differences in cognitive control (Go/NoGo Task), but revealed similar performance between the groups in set shifting and conceptual reasoning. Daniel [23] showed deficits in a general EF factor, contrary to multiple other studies [27, 29, 56].
For a summarized overview for currently and remitted depressed vs. healthy control groups please see Table 6.
Table 6.
Summary of main results for currently and remitted depressed vs. healthy control groups
| Currently depressed vs. HC | Remitted depressed vs. HC | |
|---|---|---|
| D < < < HC | Information processing speed, verbal learning and memory, visual learning and memory | – |
| D < < HC | Attention, inhibition, verbal fluency, WM | Attention, verbal learning and memory, visual learning and memory, WM |
| D < HC | – | Information processing speed, planning |
| D = HC | Cognitive flexibility, planning | Cognitive flexibility, verbal fluency |
Notes A cognitive domain was included if it was investigated by at least 3 studies. HC: healthy control group
< < < : strong tendency (more than 75% of studies report deficits for depressed group)
< < : moderate tendency (more than 50% of studies report deficits for depressed group)
< : weak tendency (more than 25% of studies report deficits for depressed group)
= : similar (25% or less of studies report deficits for depressed group)
CD vs. RD patients
Baune [18] showed deficits in the domains attention and construction in favor of the RD group (RBANS). No differences were found for immediate and delayed memory and for language.
Halvorsen [27] found deficits exclusively in the acute phase for WM. Other domains did not show any further discrepancies.
Follow up studies
In a follow up study, Ardal and Hammar [17] investigated whether depressive symptoms persist after recovery of a recurrent depressive episode. Using the Stroop test for cognitive inhibition, they tested a sample of 38 participants in the acute phase, 6 months later, and after recovery 10 years later. They found a significant difference between the depressed group and the HC in all three stages, indicating that neuropsychological impairment in the acute phase persists over the lifetime.
In another follow-up study, Boeker [20] showed that despite clinical recovery through assessment of depression severity no improvements were found in WM, EF, and sustained attention. However, better results were shown by RD patients in learning and memory.
In a sample of 79 depressed patients, Roca [48] found that after six months, RD patients were significantly better than non-remitted in processing speed, WM, selective attention/response inhibition, planning, verbal fluency, and especially in set-shifting. No improvement was shown for cognitive inhibition.
First episode depressed vs. recurrent depressed
In-patients with a first episode (n = 50) or a recurrent depressive disorder (n = 160, average of 4.4 episodes) were tested in Talarowska’s study [55]. The depressive ratings were similar. Significantly better results in favor of the first episode group were found for information processing speed, learning, visual and verbal memory, WM, EF, and verbal fluency. This tendency was already visible when comparing patients with one episode with others with two episodes.
Kaygusuz [32] examined differences between first episode and recurrent depression. The recurrent depression group was more educated than the first episode group and no differences between the two groups were apparent.
Severity of depression
Multiple studies reported a negative correlation of neuropsychological functioning with depression severity. Talarowska [55] revealed a negative correlation between symptom severity and performance regarding information processing speed, learning, verbal memory, EF, and verbal fluency. Boeker [20] found an association of depression severity and learning and memory. Likewise, Kaygusuz [32] showed a negative correlation of attention, encoding, learning, naming, and mental speed with depression severity after dividing the sample in mild and severe depression. Schwert [52] showed that the severity of MDD predicted significantly worse results in planning and divided attention. Finally, Liu [35] found that depression severity correlated with decreased WM performance.
Contrary results were published by Reppermund [47] who tested 25 facets of cognitive performance and only found one correlation (WM) with depression severity. In further studies, no association between depression severity and global cognitive functioning or verbal or figural memory [40] and cognitive performance [50] was reported.
Additional comparisons
Five studies summarized the frequency of cognitive impairment. All of the following studies except the last one define scores lower than the 16th percentile–one standard deviation below the mean of the control group – as impaired.
The McClintock [40] study focused on the frequency of cognitive dysfunction and on correlations with symptom severity of a severely unipolar depressed group of patients referred for ECT. The authors found out that 41% of the MDD group were impaired in the MMSE, 29% were impaired in verbal memory, and 52% were impaired in visual memory.
Schwert [52] found impairment rates for acute unipolar depression of 77% in attention, 57% in EF, 39% in figural memory, 31% in WM, and 23% in processing speed. While 52% of the HC group showed no cognitive impairment at all, cognitive impairment was observed at 26% in the MDD group. 2% of the CD group were impaired in all five tested domains (0% in the HC).
In Reppermund’s study [47], the highest rates of impairment for patients in acute depression were found in tasks involving EF (60%) and alertness (57%). At discharge, when 43 of 53 patients were considered remitted, significant improvement was found in 10 of the 25 test scores. For example, impairment was still found in EF (57%), alertness (40%), and divided attention (47%).
In a sample of RD patients, Preiss [45] found that 34% of the former hospitalized and 20% of the never hospitalized patients presented cognitive deficits.
Defining scores lower than the 5th percentile (1.63 SD below the mean) as cognitive impairment, Wekking [56] investigated remitted, former depressed patients. Of this sample the highest impairment was found for different assessments of memory (9–34%) and for speed of memory processing (13–28%). The impairment found for WM was 13–15%, for speed of information processing 11–16%, and for EF 2%.
Comparing groups of CD patients with depressed BD-I patients and euthymic remitted BD-I patients, Maalouf [38] revealed that just the MDD and BD depressed group were impaired on EF, but not the BD euthymic patients. In Daniel’s study [23] RD and remitted BD-I patients were compared. The performances of neither the EF nor the WM nor the verbal memory differentiated. Similar to these findings, Liu [35] did not find any differences using an extensive test battery on patients in an acute depressive state with either the diagnosis depression or BD-II. Testing another sample with treatment naïve MDD and BD-II patients in an acute depressive state, Mak [39] found significantly slower psychomotor speed for the MDD group. Like the previous studies, no differences were shown in learning and memory, frontal EF, and verbal fluency.
When compared to patients with SCH, Schaub [49] revealed significantly better results for MDD in the domains verbal and visual short-term memory, verbal fluency, visual-motor coordination, information processing, and selective attention. Practical reasoning, general verbal abstraction, spatial-figural functioning, and speed of cognitive processing did not differ. Conducting a larger-scaled study with 102 patients with a diagnosis of depression without psychotic features and 72 patients with SCH, Gooren [24] demonstrated an overlap between the two disorders: patients with unipolar depression revealed better results in verbal fluency and visual memory, verbal learning and processing speed were comparable. The better result in delayed memory in SCH could be due to better learning. Sostaric [53] also pointed out a certain overlap regarding cognitive performance in MDD and SCH, with the sample of hospitalized patients in both groups showing significant lower scores in information processing speed, shifting of attention, and in visual and verbal learning and memory compared to normative data. The SCH group achieved significantly better results in WM and in the visual delayed recall.
Castaneda [21] investigated two groups of either pure MDD patients or comorbid MDD patients, with mostly anxiety disorders and substance abuse/dependence. No statistically significant differences were found in verbal and visual short-term memory, verbal long-term memory and learning, attention, processing speed, and EF.
The comparison of neuropsychological deficits in suicide attempters and non-attempters with a history of unipolar or bipolar depression revealed that past suicide attempters performed significantly worse in attention, memory, and WM [33]. Suicide attempters also achieved worse, but non-significant differences in learning, language fluency, and impulse control. Moniz [41] showed poorer cognitive inhibition in suicide attempters compared to non-attempters. However, the suicide attempters presented better results in planning.
Constant [22] compared the cognitive performance between participants with chronic fatigue syndrome and MDD with results showing similar reaction times as well as performance on memory tasks and alertness. For WM, depressed participants showed a worse performance than participants with chronic fatigue syndrome.
Leposavić [34] investigated hospitalized depressed vs. demented patients. Depressed patients showed significantly better performances in processing speed, attention switching, visual and verbal memory, WM, and prolonged attention.
To compare patients with MDD and OCD, Rampacher [46] matched the groups according to depression severity. They found significant differences in visual organization and problem solving in favor of MDD. No significant differences could be shown for verbal and visual memory, delayed visual response, visuo-motor speed/set shifting, and verbal fluency.
Discussion
As previous research suggests a broad range of deficits in Major Depressive Disorder, we aimed to update the available evidence and systematically review studies published between 2009 and 2019, investigating cognitive impairment in adult depressive patients in the acute and remitted state. Additionally, we assessed a possible risk of bias of the included primary studies and compared the neuropsychological profiles of depressive patients to those suffering from BD or SCH.
The majority of included studies focused on an experimental design with a CD and a HC group. Large differences in favor of the control group were found in information processing speed with 12 out of 15 studies reporting significant differences using mainly the TMT-A. Besides that, strong tendencies were found for deficits in verbal and visual learning and memory. For verbal fluency, moderate tendencies for deficits were shown for semantic tests, but not for phonemic or for category switching tasks. Nine out of 15 studies revealed deficits in attention, mainly a reduced alertness. Sustained attention and attention switching remained unclear. Additionally, inhibition and WM deficits were reported in more of 50% of studies testing these areas. For WM, tasks that require the articulatory rehearsal mechanism controls seem to be especially affected. Studies testing for EF in broader domain mainly showed deficits. For cognitive flexibility, two out of 11 and for planning one out of five studies showed significant differences. With respect to visuospatial skills and visual problem solving, one study supports deficits in these domains while another study contradicts these findings. For a summarized overview, please see Table 6. The strong tendency for deficits in information processing speed, learning and memory, and verbal fluency is in compliance with former summaries [4, 5, 48] and our hypothesis (1). EF which is usually described as one of the most impaired domains [4, 5] showed a weaker, but still moderate tendency for deficits in our systematic review of the current literature. This was mainly due to results on inhibition and WM. Other features of the broad field of EF, e.g., planning and cognitive flexibility assessed mainly through the TMT-B, do not seem to be impaired in CD patients.
Comparing samples of RD patients with HC, no clear tendency for deficits in the RD group was reported as it was shown for CD patients. The highest ratio of impaired function was found for visual learning and memory in three out of four studies. Other moderate tendencies were seen for verbal learning and memory, attention, and WM. Information processing speed was impaired in 40% of the studies. Notably, Preiss [45] showed deficits in a former hospitalized sample, but not in the non-hospitalized one. Planning was found to be deficient in one out of three studies. No differences were revealed for cognitive flexibility and verbal fluency. In addition, EF was not found to be impaired. Two studies focusing on the direct comparison between CD and RD found deficits for attention, construction, and WM in the acute sample. Follow up studies likewise suggest a persistence of cognitive impairment after remission. Ardal and Hammar [17] found ongoing deficits in cognitive inhibition and propose that cognitive inhibition could be an irreversible vulnerability marker. Also, Boeker [20] showed persisting deficits in EF, WM, and sustained attention suggesting these to be trait markers. However, impairment in visual learning and memory showed a significant increase after recovery and could be state marker according to the authors. In Roca’s study [48] improvements for the RD sample were revealed in most domains. Cognitive inhibition was found to be impaired like in Ardal’s study [17]. The heterogenous results between Boeker [20] and Roca [48] could be due to defining the “recovered” depressed sample. Boeker’s recovered group shows a mean of 10.5 (SD: 8) in the HDRS 21 while Roca’s group had to score lower than 7 in the HDRS 17. Consequently, in Boeker’s “recovered” sample there is an inclusion of patients responding well to the treatment, but not remitted according to the ACNP Task Force [60]. These findings line up with previous studies and our hypothesis (2) that just a partial cognitive improvement is achieved in remission.
The included studies revealed a greater cognitive impairment in patients suffering from recurrent episodes than in first episode depressed patients. Severity of depression was found to have a positive correlation with cognitive impairment in five out of eight studies. For the most part, more dominant deficits were found for learning, memory, attention, processing speed, WM, and EF. This confirms our hypotheses (3, 4) and lines up with prior research [9, 10]. It is possible that the prescription of medication might have narrowed down the span of reported depressive symptoms consequently leading to false identification of severely depressed patients as just moderately depressed. Mixing up the groups would end up obscuring possible greater differences. We cannot rule out that additional treatment constitutes a factor in the more impaired group. However, in the review process, insufficient data could be collected on, for example, medication.
Most studies investigating differences of the cognitive pattern for depression and BD showed similar results if the groups were in an equal state. Few differences were found in samples of euthymic or depressed patients with either diagnosis. These results align with current research demonstrating no worse performance by BD samples [61, 62] as well as contradicts other current research [63, 64].
Three of the included studies which investigated differences between MDD and SCH suggested a partial overlap in deficits. Besides a partial overlap, a heterogeneity in results was observed, likely due to the inclusion of different subtypes of SCH, for example, overrepresentation of the better performing paranoid subtype [65], the mostly uncontrolled influence of medication, and other factors.
Comorbidity with mostly anxiety disorders did not seem to affect cognitive performance. Former suicide attempters in general showed more cognitive deficits than non-attempters, with inhibition being one of the most evaluated factors. Comparisons of MDD with chronic fatigue syndrome showed overall similar results. One study found significantly better results for depressed patients compared with a demented sample. Patients with an obsessive–compulsive disorder performed significantly worse than depressive patients in visual organization and problem-solving tasks. Not enough studies reported on these comparisons to draw reasonable conclusions. Nevertheless, the studies emphasized that other diagnoses seem to impact the cognitive performance in a different, often more impairing way.
Our review revealed that just some studies reported frequencies of cognitive impairment and those that did reported a broad range of frequencies. Based on the assumption that impairment is defined by one SD under the mean of the control group, one study found that 74% of the CD showed deficits compared to up to 34% of RD. In the acute phase, frequencies of 57–77% in attention, 57–60% in EF, and 29–52% in memory were seen. At discharge, Reppermund [47] discovered an impairment of 57% in EF and of 40% in alertness. Memory functions were shown as being one of the most impaired cognitions. Surprisingly, when investigating the frequency of cognitive deficits in patients in the acute state of depression, only around 30–50% were affected by memory deficits. A possible explanation could be a high variability in the extent of memory deficits, leading nonetheless to differences in mean comparisons.
Overall, the presented results for the acute phase do not consistently support the general cognitive effort hypothesis, which states that automatic processes are normal but that tasks requiring effortful processing are impaired [66]. Likewise, the included studies did not show specific impairment in memory or EF in the acute phase of depression [67]. The evidence instead speaks in favor of the global-diffuse hypothesis, which expects an extensive reduced cognitive performance in multiple areas [69] with underlying impairment in attentional processes. Impairment in attention was present in up to 77% of the current sample of studies, even more severe than EF. In line with this, multiple review articles [70, 71] on neuroimaging studies suggest that attentional deficits in major depression are accompanied by reduced connectivity within frontoparietal control systems, as well as imbalanced connectivity between control systems and networks involved in internal or external attention [70]. This leads to a favoring of internal thoughts at the cost of engaging with the external world in depression, and may partially explain the well-documented bias towards rumination, as well as the global cognitive deficits, as summarized in the current review.
Moreover, our results support the common pathway disorder hypothesis. Supporters of this hypothesis see the global deficit based on impaired functional networks with attentional and executive elements common in different diagnoses [72, 73]. Persistent impairment after remission points to sustained neurocognitive deficits rather than a state character of these impairments. However, the development of neurocognitive impairments over time should be examined in more detail: studies examining neurocognitive functions prior to the first MDD episode and with long-term follow-up provide tentative evidence for a progressive decline in neurocognitive functioning [74].
Study limitations and recommendations for future research
Evaluating the risk of bias across studies leads to an unclear risk in our study set. Therefore, the current results should be interpreted with caution. The vast majority of studies with a high risk of bias showed limitations in the selection process. Therefore, the current results are limited due primarily to methodological deficits in the selection of clinical groups and HC groups (i.e. no correction for differences between groups concerning age, gender, or educational level). Furthermore, most studies did not quantify the duration and number of episodes of depression in clinical groups, did not consider putative interaction effects between medication and cognitive performance in clinical groups, and paid little attention to comorbid psychiatric or neurological illnesses that could moderate the correlation between depression severity and cognitive deficits. Furthermore, the matching of clinical and healthy groups was limited, particularly concerning the estimation of premorbid cognitive performance levels of clinical patients with those of the HC group. Despite common reporting of education levels, an explicit assessment of premorbid intelligence was only conducted by 48% of the studies. Concerning the blinding procedure, only four out of 42 studies adequately encountered a possible detection bias by blinding assessors. Conducting a meta-analysis could be an option for future research. We, however, favored a systematic review approach because of a broader, and therefore, more heterogeneous range of hypotheses.
Given that one quarter of the included studies bear a high risk of bias according to our risk assessment, adherence to a standardized methodology is essential for future studies, especially concerning the selection. In Table 7 we provide five practical recommendations for future researchers.
Table 7.
Five practical recommendations to lower the risk of bias and to improve the quality of neuropsychological research
| 1. Match clinical and control groups based on premorbid intelligence measurements of the patients (e.g. National adult reading test, NART) as well as age, gender, and educational level |
|
2. Control for moderating effects of medication on the correlation between depression symptom severity and cognitive deficits 3. Improve the characterization of clinical groups concerning the duration and number of depressed episodes, as well as putative comorbid psychiatric or neurological illnesses that may impact on cognitive performance |
| 4. Neatly execute blinding procedures of assessors to avoid an overestimation of differences between clinical and healthy groups |
| 5. Apply a generally used cut-off when reporting clinically significant cognitive impairments (e.g. one standard deviation below the mean) |
Conclusions
Current studies about CD patients reveal strong support for deficits in processing speed, learning and memory, and impairment in attention, inhibition, verbal fluency, and WM. Despite remission of the depressed syndrome, evidence for persistent deficits in attention, learning and memory, and WM is reported. Nevertheless, RD patients show smaller deficits than in acute state, as shown in direct comparisons and in follow-up studies. Evidence for a positive correlation between number of episodes and cognitive deficits and as well as between depression severity and cognitive deficits is reported. Most studies did not find differences in the cognitive profiles of patients with MDD and BD I or II. For a comparison with SCH heterogeneous results were reported, partially suggesting an overlap of the cognitive profiles. Specific studies are needed for a further understanding of differences in the cognitive profiles between depression and other disorders. Attentional deficits were found in up to 77% of acute MDD patients. The results support the assumption of global deficits and the final common pathway disorder hypothesis for cognitive dysfunction in patients suffering from MDD. Due to an unclear risk of bias across our study set, these results should be interpreted cautiously. Based on our risk of bias assessment, we derive recommendations for future research to lower the risk of bias and to improve the quality of neuropsychological research.
Acknowledgements
The authors thank all involved staff of the Section for Clinical Psychology and Psychophysiology for their assistance with conducting the systematic review.
Author contributions
All authors contributed to the conception of the systematic review. SK and DK had the idea for the article. DK and CW performed the literature search, risk of bias assessment, and summary of the data. DK, CW, and NT wrote the first draft of the manuscript. All authors critically revised the work and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Availability of data and materials
Supplemental data are available for open access at https://osf.io/hn3w8/.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical standards
Due to our systematic review approach, the manuscript does only contain clinical studies or aggregated patient data of our primary studies.
References
- 1.American Psychiatric Association Diagnostic and statistical manual of mental disorders (5th ed.)
- 2.Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 2018;269:157–171. doi: 10.1016/j.psychres.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Weightman MJ, Knight MJ, Baune BT. A systematic review of the impact of social cognitive deficits on psychosocial functioning in major depressive disorder and opportunities for therapeutic intervention. Psychiatry Res. 2019;274:195–212. doi: 10.1016/j.psychres.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Hammar Å, Årdal G. Cognitive functioning in major depression-a summary. Front Hum Neurosci. 2009 doi: 10.3389/neuro.09.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roca M, Vives M, López-Navarro E, García-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. 2015;43(5):187–193. [PubMed] [Google Scholar]
- 6.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. 2011;134(1–3):20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- 8.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24(1):9. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- 9.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1–3):1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195(6):475–482. doi: 10.1192/bjp.bp.108.055731. [DOI] [PubMed] [Google Scholar]
- 11.Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. Int Rev Psychiatry. 2009;21(4):336–356. doi: 10.1080/09540260902962149. [DOI] [PubMed] [Google Scholar]
- 12.Cullen B, Ward J, Graham NA, Deary IJ, Pell JP, Smith DJ, Evans JJ. Prevalence and correlates of cognitive impairment in euthymic adults with bipolar disorder: a systematic review. J Affect Disord. 2016;205:165–181. doi: 10.1016/j.jad.2016.06.063. [DOI] [PubMed] [Google Scholar]
- 13.Lakens D, Hilgard J, Staaks JPC. On the reproducibility of meta-analyses: six practical recommendations. BMC Psychology. 2016;4(1):1–10. doi: 10.1186/s40359-016-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert KM, Potter GG, Boyd BD, Kang H, Taylor WD. Brain network functional connectivity and cognitive performance in major depressive disorder. J Psychiatr Res. 2019;110:51–56. doi: 10.1016/j.jpsychires.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Årdal G, Hammar A. Is impairment in cognitive inhibition in the acute phase of major depression irreversible? Results from a 10-year follow-up study. Psychol Psychother. 2011;84(2):141–150. doi: 10.1348/147608310X502328. [DOI] [PubMed] [Google Scholar]
- 18.Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176(2–3):183–189. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj A, Wilkinson P, Srivastava C, Sharma M. Cognitive deficits in euthymic patients with recurrent depression. J Nerv Ment Dis. 2010;198(7):513–515. doi: 10.1097/NMD.0b013e3181e4c5ba. [DOI] [PubMed] [Google Scholar]
- 20.Boeker H, Schulze J, Richter A, Nikisch G, Schuepbach D, Grimm S. Sustained cognitive impairments after clinical recovery of severe depression. J Nerv Ment Dis. 2012;200(9):773–776. doi: 10.1097/NMD.0b013e318266ba14. [DOI] [PubMed] [Google Scholar]
- 21.Castaneda AE, Marttunen M, Suvisaari J, Perl J, Saarni SI, Aalto-Setl T, Aro H, Lnnqvist J, Tuulio-Henriksson A. The effect of psychiatric co-morbidity on cognitive functioning in a population-based sample of depressed young adults. Psychol Med. 2010;40(1):29–39. doi: 10.1017/S0033291709005959. [DOI] [PubMed] [Google Scholar]
- 22.Constant EL, Adam S, Gillain B, Lambert M, Masquelier E, Seron X. Cognitive deficits in patients with chronic fatigue syndrome compared to those with major depressive disorder and healthy controls. Clin Neurol Neurosurg. 2011;113(4):295–302. doi: 10.1016/j.clineuro.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Daniel BD, Montali A, Gerra ML, Innamorati M, Girardi P, Pompili M, Amore M. Cognitive impairment and its associations with the path of illness in affective disorders: a comparison between patients with bipolar and unipolar depression in remission. J Psychiatr Pract. 2013;19(4):275–287. doi: 10.1097/01.pra.0000432597.79019.e2. [DOI] [PubMed] [Google Scholar]
- 24.Gooren T, Schlattmann P, Neu P. A comparison of cognitive functioning in acute schizophrenia and depression. Acta Neuropsychiatr. 2013;25(6):334–341. doi: 10.1017/neu.2013.21. [DOI] [PubMed] [Google Scholar]
- 25.Gruber O, Zilles D, Kennel J, Gruber E, Falkai P. A systematic experimental neuropsychological investigation of the functional integrity of working memory circuits in major depression. Eur Arch Psychiatry Clin Neurosci. 2011;261(3):179–184. doi: 10.1007/s00406-010-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grützner TM, Sharma A, Listunova L, Bartolovic M, Weisbrod M, Roesch-Ely D. Neurocognitive performance in patients with depression compared to healthy controls: association of clinical variables and remission state. Psychiatry Res. 2019;271:343–350. doi: 10.1016/j.psychres.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 27.Halvorsen M, Hifdt RS, Myrbakk IN, Wang C, Sundet K, Eisemann M, Waterloo K. Cognitive function in unipolar major depression: a comparison of currently depressed, previously depressed, and never depressed individuals. J Clin Exp Neuropsychol. 2012;34(7):782–790. doi: 10.1080/13803395.2012.683853. [DOI] [PubMed] [Google Scholar]
- 28.Hammar Å, Strand M, Årdal G, Schmid M, Lund A, Elliott R. Testing the cognitive effort hypothesis of cognitive impairment in major depression. Nord J Psychiatry. 2011;65(1):74–80. doi: 10.3109/08039488.2010.494311. [DOI] [PubMed] [Google Scholar]
- 29.Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology. 2012;26(5):642–651. doi: 10.1037/a0029301. [DOI] [PubMed] [Google Scholar]
- 30.Hsu KJ, Davison GC. Compounded deficits: the association between neuropsychological impairment and attention biases in currently depressed, formerly depressed, and never depressed individuals. Clin Psychol Sci. 2017;5(2):286–298. doi: 10.1177/2167702617692998. [DOI] [Google Scholar]
- 31.Jia QF, Chen P, Zhu HL, Chen SS, Gu XC, Yin XY, Wu YH, Yin GZ, Hui L. Cognitive impairments in first-episode drug-naïve versus medicated depressive patients: RBANS in a Chinese population. Psychiatr Q. 2019;90(3):471–480. doi: 10.1007/s11126-019-09641-4. [DOI] [PubMed] [Google Scholar]
- 32.Kaygusuz CC, Arisoy O, Boztas MH, Sercan M. Comparison of first episode and recurrent major depression patients in terms of cognitive function. Dusunen Adam. 2013;26(4):320–332. doi: 10.5350/DAJPN2013260401. [DOI] [Google Scholar]
- 33.Keilp JG, Beers SR, Burke AK, Melhem NM, Oquendo MA, Brent DA, Mann JJ. Neuropsychological deficits in past suicide attempters with varying levels of depression severity. Psychol Med. 2014;44(14):2965–2974. doi: 10.1017/S0033291714000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leposavić I, Leposavić L, Gavrilović P. Depression vs. dementia: a comparative analysis of neuropsychological functions. Psihologija. 2010;43(2):137–153. doi: 10.2298/PSI1002137L. [DOI] [Google Scholar]
- 35.Liu T, Zhong S, Wang B, Liao X, Lai S, Jia Y. Similar profiles of cognitive domain deficits between medication-naïve patients with bipolar II depression and those with major depressive disorder. J Affect Disord. 2019;243:55–61. doi: 10.1016/j.jad.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 36.Lyche P, Jonassen R, Stiles TC, Ulleberg P, Landrø NI. Cognitive control functions in unipolar major depression with and without co-morbid anxiety disorder. Front Psychiatry 1 (DEC) 2010 doi: 10.3389/fpsyt.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyche P, Jonassen R, Stiles TC, Ulleberg P, Landrø NI. Attentional functions in major depressive disorders with and without comorbid anxiety. Arch Clin Neuropsychol. 2011;26(1):38–47. doi: 10.1093/arclin/acq095. [DOI] [PubMed] [Google Scholar]
- 38.Maalouf FT, Klein C, Clark L, Sahakian BJ, LaBarbara EJ, Versace A, et al. Impaired sustained attention and executive dysfunction: bipolar disorder versus depression-specific markers of affective disorders. Neuropsychologia. 2010;48(6):1862–1868. doi: 10.1016/j.neuropsychologia.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak A, Lau D, Chan A, So S, Leung O, Wong S, Lam L, Leung CM, Lee S. Cognitive impairment in treatment-naïve bipolar II and unipolar depression. Sci Rep. 2018 doi: 10.1038/s41598-018-20295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClintock SM, Cullum CM, Husain MM, Rush AJ, Knapp RG, Mueller M, Petrides G, Sampson S, Kellner CH. Evaluation of the effects of severe depression on global cognitive function and memory. CNS Spectr. 2010;15(5):304–313. doi: 10.1017/S109285290002753X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moniz M, de Jesus SN, Pacheco A, Gonçalves E, Viseu J, Brás M, Silva D, Batista S. The influence of planning and response inhibition on cognitive functioning of non-psychotic unipolar depressed suicide attempters. Eur J Psychol. 2017;13(4):717–732. doi: 10.5964/ejop.v13i4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neu P, Gooren T, Niebuhr U, Schlattmann P. Cognitive impairment in schizophrenia and depression: a comparison of stability and course. Appl Neuropsychol Adult. 2019;26(3):215–228. doi: 10.1080/23279095.2017.1392962. [DOI] [PubMed] [Google Scholar]
- 43.Peters AT, Jacobs RH, Crane NA, Ryan KA, Weisenbach SL, Ajilore O, Lamar M, Kassel MT, Gabriel LB, West AE, Zubieta J-K, Langenecker SA. Domain-specific impairment in cognitive control among remitted youth with a history of major depression. Early Interv Psychiatry. 2017;11(5):383–392. doi: 10.1111/eip.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preiss M, Kucerova H, Lukavsky J, Stepankova H, Sos P, Kawaciukova R. Cognitive deficits in the euthymic phase of unipolar depression. Psychiatry Res. 2009;169(3):235–239. doi: 10.1016/j.psychres.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 45.Preiss M, Kucerova H, Lukavsky J, Sos P, Stepankova H, Cermakova R. Cognitive deficits in hospitalized and never hospitalized remitted unipolar depressive patients. Eur J Psychiatry. 2010;24(3):129–135. doi: 10.4321/S0213-61632010000300001. [DOI] [Google Scholar]
- 46.Rampacher F, Lennertz L, Vogeley A, Schulze-Rauschenbach S, Kathmann N, Falkai P, Wagner M. Evidence for specific cognitive deficits in visual information processing in patients with OCD compared to patients with unipolar depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34(6):984–991. doi: 10.1016/j.pnpbp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Reppermund S, Ising M, Lucae S, Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med. 2009;39(4):603–614. doi: 10.1017/S003329170800411X. [DOI] [PubMed] [Google Scholar]
- 48.Roca M, López-Navarro E, Monzón S, Vives M, García-Toro M, García-Campayo J, Harrison J, Gili M. Cognitive impairment in remitted and non-remitted depressive patients: a follow-up comparison between first and recurrent episodes. Eur Neuropsychopharmacol. 2015;25(11):1991–1998. doi: 10.1016/j.euroneuro.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Schaub A, Neubauer N, Mueser KT, Engel R, Möller H-J. Neuropsychological functioning in inpatients with major depression or schizophrenia. BMC Psychiatry. 2013 doi: 10.1186/1471-244X-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid M, Hammar A. Cognitive function in first episode major depressive disorder: poor inhibition and semantic fluency performance. Cogn Neuropsychiatry. 2013;18(6):515–530. doi: 10.1080/13546805.2012.754748. [DOI] [PubMed] [Google Scholar]
- 51.Schulze C, Zimmermann R, Gschwandtner U, Pflueger MO, Rapp C, Studerus E, Riecher-Rössler A. Can cognitive deficits facilitate differential diagnosis between at-risk mental state for psychosis and depressive disorders? Early Interv Psychiatry. 2013;7(4):381–390. doi: 10.1111/eip.12004. [DOI] [PubMed] [Google Scholar]
- 52.Schwert C, Stohrer M, Aschenbrenner S, Weisbrod M, Schröder A. Neurocognitive profile of outpatients with unipolar depressive disorders. J Clin Exp Neuropsychol. 2019;41(9):913–924. doi: 10.1080/13803395.2019.1634180. [DOI] [PubMed] [Google Scholar]
- 53.Šoštarič M, Zalar B. The overlap of cognitive impairment in depression and schizophrenia: a comparative study. Psychiatr Danub. 2011;23(3):251–256. [PubMed] [Google Scholar]
- 54.Taconnat L, Baudouin A, Fay S, Raz N, Bouazzaoui B, El-Hage W, Isingrini M, Ergis A-M. Episodic memory and organizational strategy in free recall in unipolar depression: the role of cognitive support and executive functions. J Clin Exp Neuropsychol. 2010;32(7):719–727. doi: 10.1080/13803390903512645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talarowska M, Zajàczkowska M, Gałecki P. Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatr Danub. 2015;27(1):38–43. [PubMed] [Google Scholar]
- 56.Wekking EM, Bockting C, Koeter M, Schene AH. Cognitive functioning in euthymic recurrently depressed patients: relationship with future relapses and prior course of disease. J Affect Disord. 2012;141(2–3):300–307. doi: 10.1016/j.jad.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 57.Zaremba D, Schulze Kalthoff I, Förster K, Redlich R, et al. The effects of processing speed on memory impairment in patients with major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;92:494–500. doi: 10.1016/j.pnpbp.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Eraydin IE, Mueller C, Corbett A, Ballard C, Brooker H, Wesnes K, Aarsland D, Huntley J. Investigating the relationship between age of onset of depressive disorder and cognitive function. Int J Geriatr Psychiatry. 2019;34(1):38–46. doi: 10.1002/gps.4979. [DOI] [PubMed] [Google Scholar]
- 59.Ambaw A, Desalegn GT. Magnitude and correlates of cognitive impairment among major depressive disorder patients in Addis Ababa: institution based cross-sectional study. BMC Res Notes. 2019;12(1):135. doi: 10.1186/s13104-019-4184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 61.Xu G, Lin K, Rao D, Dang Y, Ouyang H, Guo Y, Ma J, Chen J. Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. J Affect Disord. 2012;136(3):328–339. doi: 10.1016/j.jad.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62(8):917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 63.Stoddart SDR, Craddock NJ, Jones LA. Differentiation of executive and attention impairments in affective illness. Psychol Med. 2007;37(11):1613–1623. doi: 10.1017/S0033291707000712. [DOI] [PubMed] [Google Scholar]
- 64.Cotrena C, Branco LD, Shansis FM, Fonseca RP. Executive function impairments in depression and bipolar disorder: association with functional impairment and quality of life. J Affect Disord. 2016;190:744–753. doi: 10.1016/j.jad.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Dillon C, Taragano F, Sarasola D, Iturry M, Serrano C, Raczkowski A, Allegri R. Estudio de los rendimientos cognitivos en pacientes esquizofrénicos (forma paranoide vs residual) (Cognitive performance in schizophrenia (paranoid vs residual subtype)) Vertex (Buenos Aires, Argentina) 2007;18(73):170–175. [PubMed] [Google Scholar]
- 66.Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol Gen. 1979;108(3):356–388. doi: 10.1037/0096-3445.108.3.356. [DOI] [Google Scholar]
- 67.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 68.Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol. 1997;19(4):587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- 69.Landrø NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(4):233–240. [PubMed] [Google Scholar]
- 70.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Treadway MT, Pizzagalli DA. Imaging the pathophysiology of major depressive disorder - from localist models to circuit-based analysis. Biol Mood Anxiety Disord. 2014;4(1):1–13. doi: 10.1186/2045-5380-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mialet JP, Pope HG, Yurgelun-Todd D. Impaired attention in depressive states: a non-specific deficit? Psychol Med. 1996;26(5):1009–1020. doi: 10.1017/s0033291700035339. [DOI] [PubMed] [Google Scholar]
- 73.Zihl J, Grön G, Brunnauer A. Cognitive deficits in schizophrenia and affective disorders: evidence for a final common pathway disorder. Acta Psychiatr Scand. 1998;97(5):351–357. doi: 10.1111/j.1600-0447.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 74.Allott K, Fisher CA, Amminger GP, Goodall J, Hetrick S. Characterizing neurocognitive impairment in young people with major depression: state, trait, or scar? Brain Behav. 2016;6(10):e00527. doi: 10.1002/brb3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplemental data are available for open access at https://osf.io/hn3w8/.