Abstract
To explore the autoimmune response and outcome in the central nervous system (CNS) at the onset of viral infection and correlation between autoantibodies and viruses.
Methods
A retrospective observational study was conducted in 121 patients (2016–2021) with a CNS viral infection confirmed via cerebrospinal fluid (CSF) next-generation sequencing (cohort A). Their clinical information was analysed and CSF samples were screened for autoantibodies against monkey cerebellum by tissue-based assay. In situ hybridisation was used to detect Epstein-Barr virus (EBV) in brain tissue of 8 patients with glial fibrillar acidic protein (GFAP)-IgG and nasopharyngeal carcinoma tissue of 2 patients with GFAP-IgG as control (cohort B).
Results
Among cohort A (male:female=79:42; median age: 42 (14–78) years old), 61 (50.4%) participants had detectable autoantibodies in CSF. Compared with other viruses, EBV increased the odds of having GFAP-IgG (OR 18.22, 95% CI 6.54 to 50.77, p<0.001). In cohort B, EBV was found in the brain tissue from two of eight (25.0%) patients with GFAP-IgG. Autoantibody-positive patients had a higher CSF protein level (median: 1126.00 (281.00–5352.00) vs 700.00 (76.70–2899.00), p<0.001), lower CSF chloride level (mean: 119.80±6.24 vs 122.84±5.26, p=0.005), lower ratios of CSF-glucose/serum-glucose (median: 0.50[0.13-0.94] vs 0.60[0.26-1.23], p=0.003), more meningitis (26/61 (42.6%) vs 12/60 (20.0%), p=0.007) and higher follow-up modified Rankin Scale scores (1 (0–6) vs 0 (0–3), p=0.037) compared with antibody-negative patients. A Kaplan-Meier analysis revealed that autoantibody-positive patients experienced significantly worse outcomes (p=0.031).
Conclusions
Autoimmune responses are found at the onset of viral encephalitis. EBV in the CNS increases the risk for autoimmunity to GFAP.
Keywords: autoimmune encephalitis, neuroimmunology, neurovirology, clinical neurology
WHAT IS ALREADY KNOWN ON THIS TOPIC
In previous studies, autoantibodies were seldom detected during the viral encephalitis phase.
Instead, they were usually detected only during the follow-up or autoimmune encephalitis phase after viral encephalitis.
WHAT THIS STUDY ADDS
Autoantibodies were found at the onset of viral infections in 61 of 121 patients.
The presence of Epstein-Barr virus in the cerebrospinal fluid obviously increases the risk for autoimmunity to glial fibrillar acidic protein.
Patients with autoantibodies presented worse clinical manifestations and lower modified Rankin Scale scores in follow-up.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Early detection of antibodies and application of immunotherapies may be helpful for patients with viral encephalitis.
Background
Both infectious and autoimmune aetiologies are associated with considerable morbidity from encephalitis. At the population level, the incidence and prevalence of autoimmune encephalitis are similar to those of infectious encephalitis.1 Autoimmune encephalitis is a known complication of herpes simplex virus (HSV) encephalitis.2 After antiviral treatment, some patients without detectable virus but with detectable autoantibodies, such as anti-N-methyl-D-aspartate receptor (NMDAR) IgG (NMDAR-IgG), experience a relapse of encephalitis that improves with immunosuppression, suggestive of a postinfectious autoimmune process.3 4
In addition to the association between HSV encephalitis and NMDAR-IgG, non-HSV infections antedating autoimmune encephalitis have also been found. For example, varicella zoster virus (VZV),5 Epstein-Barr virus (EBV),6 cytomegalovirus (CMV),7 adenovirus,8 enterovirus,9 HIV,10 human herpes virus type 611 and SARS-CoV-2 virus have been detected in the cerebrospinal fluid (CSF) of patients with NMDAR-IgG-associated encephalitis.12 Other autoantibodies, including those against α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR), gamma-aminobutyric acid-A receptor or gamma-aminobutyric acid-B receptor (GABABR), have also been detected after viral encephalitis.11 In one study of nine patients with HSV encephalitis, antibodies against D2-dopamine receptor and/or NMDAR were found.13 Interestingly, among intensive care patients with COVID-19 who presented with neurological syndromes, NMDAR-IgG or other neuronal autoantibodies were often detected in the CSF instead of antibodies against SARS-CoV-2.12
It remains unclear whether autoantibodies act in early events in viral encephalitis. Armangue et al reported that none of their 51 patients had antibodies against neuronal surface antigens at the onset of HSV encephalitis, but 14 of those who developed encephalitis tested positive for IgG autoantibodies during a 3-week follow-up.4 Antineuronal surface antibodies and cytokines have been detected in the serum and CSF of patients with Japanese encephalitis (JE) >3 weeks postsymptom onset.14 However, autoantibodies can also be an early event in viral encephalitis.15 In 3 of 44 patients with HSV encephalitis, NMDAR-IgG was present within the first 5–9 days of the disease.16 Similarly, in a patient with VZV encephalitis, NMDAR antibodies were detected 12 days after symptom onset.5 But there are still few cases.
Thus, the effects of early autoimmune responses in central nervous system (CNS) viral infection remain unknown. Here, we aimed to screen patients for CSF autoantibody at the onset of CNS viral infection; clarify the relationship between different viruses and autoantibody classifications and explore the outcome of early autoimmune responses in CNS viral infection.
Methods
Study design and participants
An observational study was conducted in cohort A, at only one centre to rule out potential confounders. This cohort was composed of 124 patients within 2 weeks of symptom onset, retrospectively recruited between 31 May 2016 and 5 October 2021. All patients had a CNS viral infection confirmed via CSF next-generation sequencing (NGS) in Hugobiotech (Beijing, China). Their CSF samples were stored at −80°C. Three patients were excluded, whose length of time from symptom onset to first lumbar puncture was >2 weeks.
All the CSF samples with masked data were retrospectively screened for neuronal or glial cell-specific antibody by tissue-based assay (TBA) and/or cell-based assay (CBA) at the Institute of Neuroscience and the Second Affiliated Hospital of Guangzhou Medical University. Investigators performing the autoantibody testing were blinded to the clinical data, and the treating physicians were blinded to the autoantibody detection results until the follow-up period was completed.
Clinical information, including prodromal symptoms, neurological manifestations, comorbidities, ancillary study results, treatments and outcome, was obtained by the investigators. Brain MRI data were acquired using a 3.0 T MRI scanner (Ingenia, Philips Medical Systems, The Netherlands) with a 15-channel receiver array head coil at the Department of Radiology, Third Xiangya Hospital, Central South University (Changsha, Hunan, China). All the patient received an MRI including T1, T2, fluid-attenuated inversion recovery and contrast enhancement, before their treatment. Follow-up neurological function was assessed using the modified Rankin Scale (mRS). The data of patients with GFAP-IgG were registered on an autoimmune glial fibrillar acidic protein (GFAP) astrocytopathy (GFAP-A) registry in China (GFAP-AID, ChiCTR2000041291).17
In parallel, we obtained brain tissue from eight patients with GFAP-A and EBV infection confirmed via NGS, as described in our previous study.18 Two patients as control had GFAP-A with nasopharyngeal carcinoma, whose nasopharyngeal carcinoma tissue was obtained. These patients were enrolled as cohort B to detect EBV.
Determination of autoantibody in the CSF by indirect immunofluorescence
Antibody was detected using a TBA kit (BioSystems, Costa Brava, Barcelona, Spain) with a monkey cerebellum substrate. Briefly, each diluted CSF (1:1) sample was allowed to react with tissue sections on glass slides for 2 hours at room temperature. The slides were then rinsed twice with phosphate-buffered saline (PBS) before being incubated with fluorescein-conjugated goat antihuman IgG for 30 min. Finally, the slides were rinsed with PBS, and the fluorescence pattern was examined under a microscope. Fluorescent results are judged by two researchers independently (YL and PL). If there is any dispute about the results, it can be resolved through further discussion. The neuronal/glial autoantibody pattern was defined in accordance with several previous studies.19–21 All positive samples were given titre test. Briefly, no fluorescence or fluorescence in the pure vascular endothelial cells, dura mater, choroid plexus or non-specific antinuclear antibody were judged as negative (online supplemental figure 1). The fluorescence in neurons were judged as positive, as described in a previous study.19 According to the fluorescence pattern,19 the antibodies that mainly bind to neuronal plasma membrane of the molecular layer were considered as antibodies targeting cell surface antigens, antibodies that bind in the nerve cytoplasm or nucleus are considered as antibodies targeting intracellular antigens, antibodies that bind to Purkinje cells (PC) were considered as PC-IgG. Astrocytes antibody was defined in our previous study21 and antibodies binding to antibody against myelin sheath (oligodendrocyte) are judged according to our previous study.22 Neuronal intermediate filament (NIF)-IgG was observed by another study.23
jnnp-2022-330626supp001.pdf (55.2KB, pdf)
jnnp-2022-330626supp002.pdf (252.9KB, pdf)
Samples with neuronal antibodies were retested by commercial CBA kit which included antigen to NMDAR, AMPAR, GABABR, leucine-rich glioma-inactivated 1, contactin-associated protein-like 2 (Euroimmun, Germany). Samples with positive astrocyte antibodies were routinely retested for GFAP-IgG by in-housed CBA, and the CBA-positive ones were called GFAP-IgG while the CBA-negative ones were called astrocyte antibodies. Samples with positive myelin antibodies were routinely retested for myelin oligodendrocyte glycoprotein (MOG)-IgG by commercial CBA kit (Euroimmun) and the positive ones were called MOG-IgG while the negative ones were called oligodendrocyte antibodies. Samples with NIF fluorescence were retested by in-house CBA including the antigen to α-internexin, neurofilament light chain, neurofilament medium chain, neurofilament heavy chain and peripherin protein. However, we did not further screen those target antigens for other fluorescent pattern positive samples.
Pathology
Brain tissue pathology was examined in biopsies from patients with GFAP-A, as in our previous study.18 Tissue slices (10 µm thick) were cut and serially mounted onto numbered slides to enable the distribution of molecules to be compared across adjacent serial sections. The sections were stained with H&E, and in situ hybridisation was performed as a routine procedure in our hospital.
Statistical analysis
All statistical analyses were performed using the Statistical Program for Social Sciences software V.11.0 (SPSS, Chicago, Illinois, USA). Statistical analyses were performed with the χ2 test or the Fisher’s exact test for binary and categorical data. While for continuous variables, t-test was used for normally distributed data and Mann-Whitney U test was used for non-normally distributed data. A Kaplan-Meier analysis was performed to evaluate mRS scores. The Kaplan-Meier analysis was compared between groups using log rank tests. Values of p<0.05 (two-sided) were considered to indicate a significant difference.
Results
Cohort A: virus and autoantibody study
One hundred and twenty-one patients (male:female=79:42; median age=42 (14–78) years old) were included for analysis. None of them had immune diseases before and only one patient had a history of immunotherapy. A few of them had diabetes or tumour (table 1).
Table 1.
Comparison between CNS virus infections in patients with or without autoantibody in CSF
| With autoantibody | Without autoantibody | P value | |
| N | 61 | 60 | – |
| Female/Male | 19/42 | 23/37 | 0.406 |
| Median age at onset, years (range) | 42 (15–78) | 38 (14–74) | 0.316 |
| Duration in hospital, days (range) | 18 (7–71) | 15 (5–38) | 0.013 |
| Diabetes | 4 (6.6%) | 1 (1.7%) | 0.371 |
| Tumour | 2 (3.3%) | 2 (3.3%) | 1.000 |
| Main clinical features | |||
| Fever, n (%) | 50 (82%) | 39 (65%) | 0.034 |
| Headache, n (%) | 56 (91.8%) | 51 (85.0%) | 0.242 |
| Encephalopathy, n (%) | 22 (36.1%) | 20 (33.3%) | 0.752 |
| Meningeal irritation sign, n (%) | 38 (62.3%) | 26 (43.3%) | 0.037 |
| Rectal sphincter symptoms, n (%) | 6 (9.8%) | 1 (1.7%) | 0.114 |
| Serum features | |||
| Lactate dehydrogenase level (U/L) | 47 (21–617) | 27 (13–201) | <0.001 |
| Serum sodium level (mmol/L) | 135.00 (113.00–149.00) | 138.70 (126.00–147.10) | 0.002 |
| Serum potassium level (mmol/L) | 3.98±0.39 | 3.80±0.37 | 0.015 |
| Glucose level (mmol/L) | 5.7 (2.8–12.2) | 5.29 (4.12–14.80) | 0.006 |
| CSF features at onset | |||
| WBC in CSF (cells/mm3) | 140 (5–1007) | 130 (1–1200) | 0.161 |
| Neutrophils count | 0 (0–34) | 0 (0–57) | 0.470 |
| Plasma cell count | 0 (0–16) | 0 (0–6) | 0.047 |
| Protein level (mg/L) | 1126.00 (281.00–5352.00) | 700 (76.70–2899.00) | <0.001 |
| Chloride level (mmol/L) | 119.80±6.24 | 122.84±5.26 | 0.005 |
| Glucose level (mmol/L) | 3.11 (1.08–6.94) | 3.23 (1.97–6.61) | 0.097 |
| CSF-glucose/Serum-glucose | 0.50 (0.13–0.94) | 0.60 (0.26–1.23) | 0.003 |
| Brain parenchyma abnormality | 20 (32.8%) | 16 (26.7%) | 0.462 |
| Enhancement of meninges | 26 (42.6%) | 12 (20.0%) | 0.007 |
| Treatment and outcome | |||
| Steroid treatment, n (%) | 13 (21.3%) | 11 (18.3%) | 0.681 |
| Anti-epileptic treatment, n (%) | 7 (11.5%) | 11 (18.3%) | 0.289 |
| GCS at admission | 15 (6–15) | 15 (7–15) | 0.755 |
| mRS at admission | 3 (1–5) | 3 (0–5) | 0.630 |
| mRS at discharge | 2 (0–5) | 1 (0–5) | 0.335 |
| Follow-up loss, n (%) | 16 (26.2%) | 8 (13.3%) | 0.075 |
| Follow-up time (months) | 28 (4–62) | 25 (6–64) | 0.615 |
| mRS in follow-up | 1 (0–6) | 0 (0–3) | 0.037 |
| mRS ≥1, n (%) | 22 (48.9%) | 16 (30.8%) | 0.068 |
| mRS ≥3, n (%) | 7 (15.6%) | 1 (1.9%) | 0.023 |
| Decreased memory in follow-up, n (%) | 14 (31.1%) | 8 (15.4%) | 0.065 |
CNS, central nervous system; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; mRS, modified Rankin Scale; WBC, white blood cells.
Virus and autoantibody classification
The causative viruses included: HSV 1 (HSV1, n=40), HSV 2 (HSV2, n=2), EBV (n=30), VZV (n=47), CMV (n=1) and Torque teno virus (n=1). Autoantibody was detected in 61 (50.4%) samples (neuronal, n=27; astrocyte, n=29; other, n=5).
Indirect immunofluorescence patterns of autoantibodies against neurons, astrocytes or other antigens are shown in figure 1. Classification of virus and autoantibody is shown in figure 2. At the onset of HSV infection, 17/42 (10.5%) patients had antibodies against neuronal (n=12, 28.6%), astrocyte (n=3, 7.1%) or oligodendrocyte (n=2, 4.8%) antigens. The identified antigens included NMDAR (n=1, 2.4%), GFAP (n=3, 7.1%) and MOG (n=2, 4.8%). At the onset of EBV infection, 24/30 (80.0%) patients had antibodies against neuronal antigens (n=3, 10.0%), GFAP (n=20, 66.7%) or other antigens (n=1, 3.3%); among them, three had overlapping antibody types. At the onset of VZV, 19/47 (40.4%) patients had antibodies against neuronal antigens (n=12, 25.5%), GFAP (n=5, 10.6%) or other antigens (n=2, 4.3%). The one patient with CMV had GFAP-IgG in the CSF. The patients with EBV infection were more likely to have autoantibodies (24/30), compared with patients with HSV (17/42) or VZV (19/47) infection (p<0.001). More antibodies against neuronal antigens were detected in patients with HSV (12/17) or VZV (12/19) infection (EBV 3/24, p<0.001) while more antibodies against GFAP (20/24) were detected in patients with EBV infection (HSV 3/17, VZV 5/19, p<0.001).
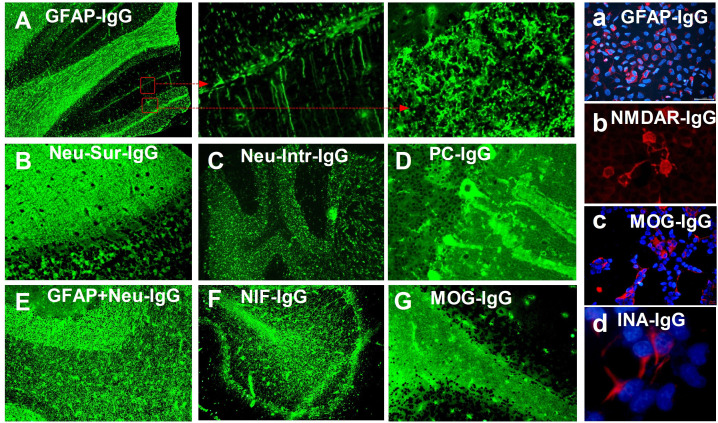
Figure 1.
Indirect immunofluorescence patterns of autoantibodies against neurons, astrocytes or other antigens. (A) Typical immunofluorescence pattern of glial fibrillar acidic protein (GFAP)-IgG in the cerebellum, showing IgG bound to the astrocytes in all layers (arrows), which was confirmed by cell-based assay (CBA, figure a). (B) Immunofluorescence pattern of neuronal cellular surface-specific antibody overlapping N-methyl-D-aspartate receptor (NMDAR)-IgG (granular layer confirmed by CBA, figure b) with unknown antigen (molecular layer) in the cerebellum. (C) Immunofluorescence pattern of neuronal nucleus-specific antibody with unknown antigen. (D) Immunofluorescence pattern of Purkinje cell (PC)-specific antibody with unknown antigen. (E) Immunofluorescence pattern of overlapping GFAP-IgG and unknown neuron-specific antibody. (F) Immunofluorescence pattern of neuronal intermediate filament (NIF), confirmed as α-internexin (INA) by CBA (figure d). (G) Typical immunofluorescence pattern of antimyelin oligodendrocyte glycoprotein (MOG) IgG in the myelin of the cerebellum, which was confirmed by CBA (figure c).
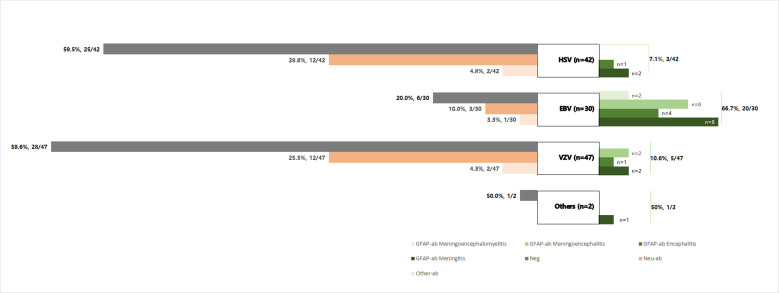
Figure 2.
Classification of virus and autoantibody. At the onset of herpes simplex virus (HSV) infection, 17/42 (10.5%) patients had antibodies against neuronal antigens (n=12, 28.6%), glial fibrillar acidic protein (GFAP) (n=3, 7.1%) or other antigens (n=2, 4.8%); at the onset of Epstein-Barr virus (EBV) infection, 24/30 (80.0%) patients had antibodies against neuronal antigens (n=3, 10.0%), GFAP (n=20, 66.7%) or other antigens (n=1, 3.3%); at the onset of varicella zoster virus (VZV), 19/47 (40.4%) patients had antibodies against neuronal antigens (n=12, 25.5%), GFAP (n=5, 10.6%) or other antigens (n=2, 4.3%). The one patient with cytomegalovirus had GFAP-IgG in the cerebrospinal fluid. The patients with EBV infection were more likely to have autoantibodies (24/30), compared with patients with HSV (17/42) or VZV (19/47) infection (p<0.001). More antibodies against neuronal antigens were detected in patients with HSV (12/17) or VZV (12/19) infection (EBV 3/24, p<0.001) while more antibodies against GFAP (20/24) were detected in patients with EBV infection (HSV 3/17, VZV 5/19, p<0.001). Ab, antibody.
Relationship between different viruses and GFAP-IgG
EBV increased the odds of having GFAP-IgG as compared with other viruses (OR 18.22, 95% CI 6.54 to 50.77, p<0.001), HSV (OR 26.00, 95% CI 6.42 to 105.26, p<0.001) and VZV (OR 16.80, 95% CI 5.07 to 55.68, p<0.001).
Comparisons between patients with autoantibody and those without autoantibody
We compared the clinical features between groups (table 1). Patients identified as having autoantibody in their CSF (CSF-positive) had a higher CSF plasma cell count (median: 0 (0–16) vs 0 (0–6), p=0.047), higher CSF protein level (median: 1126.00 (281.00–5352.00) vs 700.00 (76.70–2899.00), p<0.001), lower CSF chloride level (mean: 119.80±6.24 vs 122.84±5.26, p=0.005), lower ratios of CSF-glucose/serum-glucose (median: 0.50[0.13-0.94] vs 0.60[0.26-1.23], p=0.003), lower serum sodium level (median: 135.00 (113.00–149.00) vs 138.70 (126.00–147.10), p=0.002), higher serum potassium level (3.98±0.39 vs 3.80±0.37, p=0.015) and more enhancement of meninges (26/61 (42.6%) vs 12/60 (20.0%), p=0.007), and different mRS scores in follow-up (1 (0–6) vs 0 (0–3), p=0.037) compared with patients in whom autoantibody was not detected in their CSF (CSF-negative). Further subgroup analysis revealed that patients with GFAP-IgG had no significant differences from patients with other autoantibodies for any factors except for decreased memory in follow-up (3/21 (14.3%) vs 11/24 (45.8%), p=0.023) and CSF-glucose level (median: 2.93 (1.08–6.94) vs 3.18 (2.12–5.99), p=0.024).
Therapy and outcome
All patients received antiviral treatment and symptomatic treatment, where there was no statistical difference. Steroid treatment was administered to 6, 7 and 11 of the patients with GFAP-IgG, with other autoantibodies and without autoantibodies, respectively. Anti-epileptic treatment was administered to 3, 4 and 11 of the patients with GFAP-IgG, with other autoantibodies and without autoantibodies, respectively. No patients died while in the hospital, and only one (from the CSF-positive group) died during the follow-up period.
The CSF-positive group had longer hospital stay (18 (7–71) days vs 15 (5–38) days, p=0.013) compared with the CSF-negative group. There was no significant difference in the mRS score at admission (3 (1–5) vs 3 (0–5), p=0.630) or discharge (2 (0–5) vs 1 (0–5), p=0.335) between the CSF-positive and CSF-negative groups. A total of 97 (80.2%) patients finished the interviews through September 2021 (follow-up duration of 4–64 months). There was no statistical difference in follow-up duration between CSF-positive group and CSF-negative group (28 (4–62) vs 25 (6–64) months, p=0.615). The missed follow-up rates in the CSF-positive group and CSF-negative group were 16/61 (26.2%) and 8/60 (13.3%), respectively (p=0.075), and the median follow-up times were 28 (4–62) months and 26 (6–64) months, respectively (p=0.898). The median mRS scores in the CSF-positive and CSF-negative groups were 1 (0–6) and 0 (0–3), respectively (p=0.037). At the last follow-up, 22/45 (48.9%) and 7/45 (15.6%) of the 45 CSF-positive patients had an mRS score of ≥1 and ≥3, respectively; in contrast, 16/52 (30.8%) and 1/52 (2%) of the 52 CSF-negative patients had these respective mRS scores (22/45 (48.9%) vs 16/52 (30.8%), p=0.068 and 7/45 (15.6%) vs 1/52 (2%), p=0.023, respectively) (table 1). Only one CSF-negative (VZV infection) patient (1.9%) experienced a relapse 2 months later; this patient was diagnosed as having autoimmune encephalitis with an unclear neuronal autoantibody. No CSF-positive patients experienced a relapse of encephalitis, but one patient with EBV infection had decreased bilateral vision during follow-up. The mRS scores of the patients stratified by autoantibody presence in the CSF are shown in figure 3A-D. In each group, there were no statistical difference in the mRS scores between patients with different virus infection, except that in the CSF-negative group, patients with HSV infection were worse than those with VZV infection at admission (median: 4 (2–5) vs 3 (0–5), p<0.001).
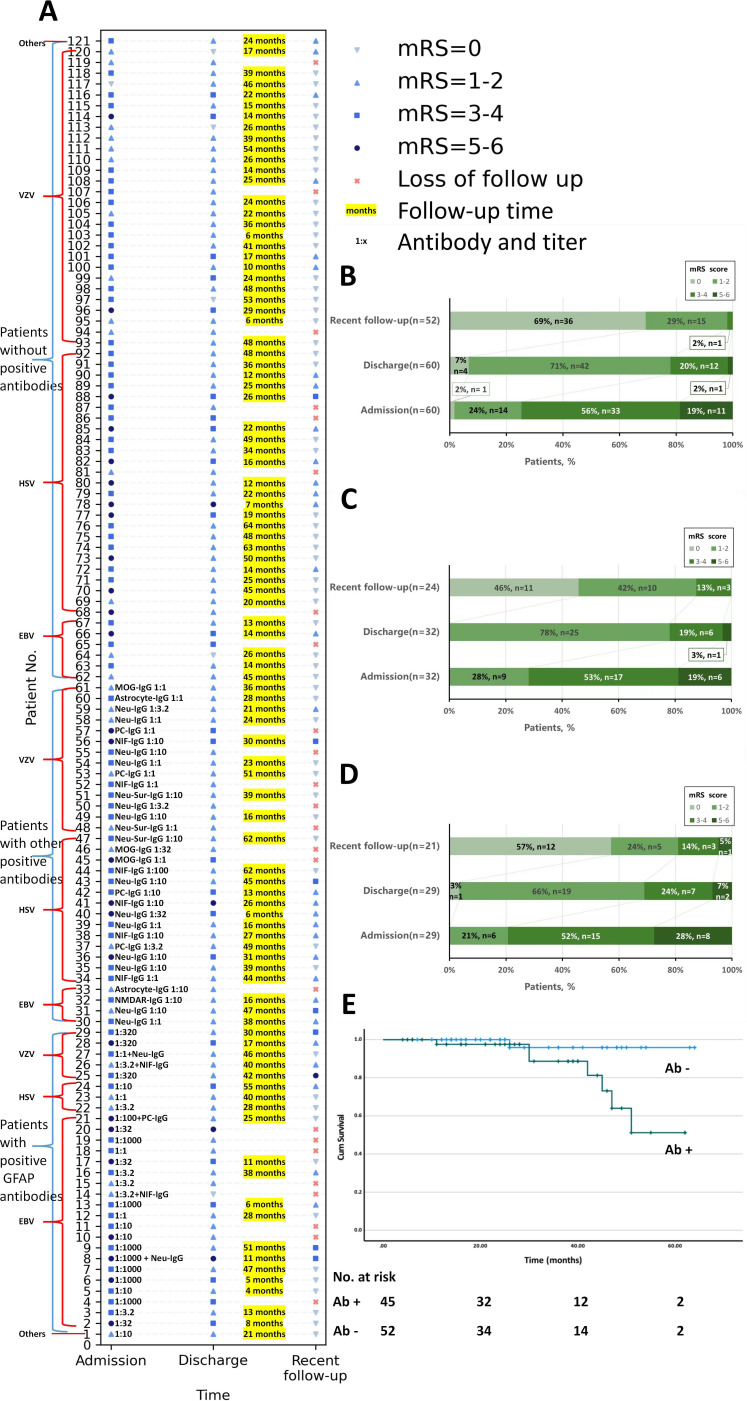
Figure 3.
Neurological status of the patients stratified by autoantibody presence in the CSF. The neurological status was measured using the mRS and was colour-coded. (A) Each patient’s mRS scores at admission, discharge and recent follow-up are shown in the chart. According to mRS scores at admission, there were no statistical differences in severity of infection between CSF-positive and CSF-negative patients. CSF-positive patients seemed to have worse neurological status at discharge, although there were no statistical differences. And CSF-positive patients had worse outcome according to the mRS scores in recent follow-up. In each group, there were no statistical difference in the mRS scores between patients with different virus infection, except that in the CSF-negative group, patients with HSV infection were worse than those with VZV infection at admission (median: 4 (2–5) vs 3 (0–5), p<0.001). (B, C, D) Bar charts of statistics on the mRS scores of patients with detectable anti-GFAP antibody (B, n=29, loss to follow-up rate (loss rate)=7/29), with other autoantibodies (C, n=32, loss rate=7/32) or without autoantibodies (D, n=60, loss rate=8/60). No significant differences were found among the loss to follow-up rates (loss rates) of the different groups (p=0.339). CSF-positive patients (n=45) with follow-up (median: 28 months; IQR: 16–42 months; range: 4–62 months). CSF-negative patients (n=52) with follow-up (median: 25 months; IQR: 15–44 months; range: 6–64 months). No significant differences were found in loss rates between CSF-positive and CSF-negative patients (p=0.075). (E) A Kaplan-Meier analysis with mRS ≥3 as the end point (log rank (Mantel-Cox), p=0.031). The CSF-positive patients were more likely to have poorer outcomes. There were no statistical differences in outcomes between patients with anti-GFAP antibodies and other antibodies. Ab, antibody; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; GFAP, glial fibrillar acidic protein; HSV, herpes simplex virus; mRS, modified Rankin Scale; VZV, varicella zoster virus.
A Kaplan-Meier analysis revealed that, compared with the CSF-negative patients, the CSF-positive patients were more likely to experience a bad outcome after the first attack (p=0.031) (figure 3E).
Cohort B
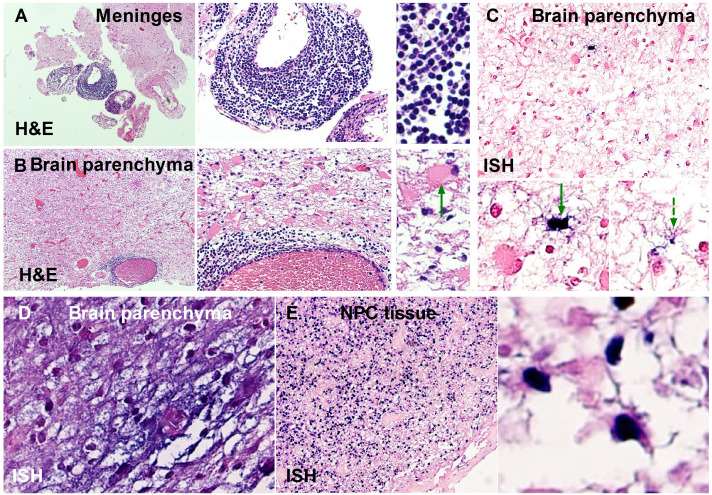
To explore the evidence of EBV infection in brain tissue, we used in situ hybridisation in previously reported brain pathological examinations from eight patients with GFAP-A and only EBV infection confirmed via NGS.18 Positive results were found in 2/8 (25.0%) patients (figure 4).
Figure 4.
Pathological evidence of EBV infection in the CNS of patients with GFAP-A. (A, B, C, D) Brain tissue was obtained from two previously reported patients18 with subacute encephalitis. (A, B, C) Tissue was obtained from patient no. 2 in the previous study.18 (D) Tissue was obtained from patient no. 6 in the previous study.18 (A, B) Inflammation was revealed in the meninges and brain parenchyma. Green arrow: astrocyte dystrophy. (C) Astrocytes with EBV infection, detected by ISH. Green solid arrow: EBV-infected astrocyte with degeneration. Green dashed arrow: EBV-infected astrocyte with normal morphology. (D) Neurons and astrocytes stained by ISH. (E) EBV-infected NPC tissue, included as a control, was obtained from the patients with GFAP-A in cohort B. CNS, central nervous system; EBV, Epstein-Barr virus; GFAP, glial fibrillar acidic protein; ISH, in situ hybridisation; NPC, nasopharyngeal carcinoma.
Discussion
Different from the spectrum of causative viruses for acute meningitis or encephalitis in China (enterovirus (48.89%), HSV (27.34%), mumps virus (12.97%) and JE virus (10.79%)),24 the three most frequent causative viruses found here were HSV (34.71%), EBV (24.79%) and VZV (38.84%). In the former study, both the serum and CSF were used for virus detection, whereas only the CSF was used in ours. Additionally, the range of patient ages and regions of residence in their study was also different from ours, in which our patients were all from South China.
At the onset of viral infections, autoimmune responses occur in the CNS, and they produce antibodies targeting neurons, astrocytes and oligodendrocytes. The presence of antibodies in the early phase of viral encephalitis was demonstrated here by typical patterns of antibodies like GFAP-IgG, mostly with high titres, and CBA confirmation of some antibodies. In contrast, in a previous study, autoantibodies were not detected during the viral encephalitis phase; instead, they were detected only during the follow-up or autoimmune encephalitis phase after viral encephalitis.4 Potential reasons that those antibodies were not recognised in previous research include: (1) methodology, for example, the Spanish team used rat brain tissue immunohistochemistry, which could be less sensitive, whereas commercial monkey brain slices with higher affinity were applied in this research and (2) focus—more attention was paid to antibodies against neuronal surface antigens, in which GFAPs are not included.4
As reported in previous studies, multiple viruses were associated with the same autoantibody, such as NMDAR-IgG,5–12 and several kinds of autoantibodies were detected after HSV encephalitis.4 11 13 In agreement with another Chinese study that found that HSV is correlated with different antibodies (NMDAR (7/7); GABA (1/7); MOG (2/7)),25 we found that HSV is correlated with NMDAR (1/17), GFAP (3/17) and MOG (2/17). Most importantly, a high correlation between EBV infection and autoantibodies, especially anti-GFAP, was found. EBV infection, compared with HSV or VZV infection, significantly increased the risk for autoimmunity to GFAP. Unlike the neurotropic viruses HSV and VZV, EBV is carried by B lymphocytes.26 27 EBV-infected B lymphocytes may pass through the damaged blood-brain barrier, thus allowing EBV to enter the CNS.26 27 Low EBV viral loads in the CNS may come from B lymphocytes, whereas high viral loads may reflect the multiplication of EBV in the cerebral parenchyma. In either case, EBV showed a higher correlation with GFAP autoimmunity in astrocytes, which has not been reported. Autoimmune GFAP-A is a clinical phenotype, characterised by the presence of GFAP-IgG, fever, headache and meningoencephalitis, whose cause and pathogenesis are still unknown.28 The present findings and our discovery of EBV in brain biopsies from patients with GFAP-A suggest that EBV infection may be a potential trigger of GFAP-A. Interestingly, astrocytes and neurons can be infected by EBV, as shown here, and affected patients can develop antibodies targeting astrocytes or neurons, but whether EBV infection makes an individual prone to developing GFAP autoimmunity is unclear.
The comparison between the CSF-positive and CSF-negative groups might be affected by the proportion of patients with anti-GFAP (29/61). Different from previous studies in which anti-NMDAR was detected in postinfectious autoimmune encephalitis,4–12 anti-GFAP were more frequent than any other autoantibodies in the present study, so more manifestations of GFAP-A were found in the CSF-positive group. For example, we found rectal sphincter symptoms in the CSF-positive group, especially among those with GFAP-IgG, although no statistically significant difference. The main clinical phenotype of GFAP-A is meningoencephalomyelitis, which means that the presence of GFAP-IgG may correlate with an affected spinal cord.28 29 Regarding serology, we observed a difference in serum sodium and potassium levels between the CSF-positive and CSF-negative groups. Hyponatraemia was also reported in a Japanese GFAP-A study in which brain MRI abnormalities in thalami were reported.30 The CSF-positive group differed from the CSF-negative group in terms of the inflammatory response as well, with higher lactate dehydrogenase levels in the serum and protein levels in the CSF, in agreement with previous reports.28–30 According to brain MRI results, 42.6% of the CSF-positive patients had affected meninges, which is much more than the 20.0% of CSF-negative patients. Meningitis is considered rare in HSV-1, VZV or EBV infection.31 Only 2 of 15 children and teenagers with a viral infection of the CNS had meninges involvement on MRI.32 Therefore, meningitis may be caused by an autoimmune response. Interestingly, lower chloride levels and ratios of CSF-glucose/serum-glucose were found in the CSF-positive group, which is similar to findings for tuberculosis of the CNS. GFAP-A with clinical features like those of tuberculosis have been reported.33 34 Another novel finding associated with tuberculosis is a self-remitting elevation of adenosine deaminase levels in the CSF of patients with GFAP-A.35 Moreover, a case series of GFAP-A masquerading as tuberculosis of the CNS was recently reported, in which lower ratios of CSF-glucose/serum-glucose were also found.36
Because this study is retrospective, we saw outcomes of antiviral therapy, most of which were natural processes of autoimmune responses. Only 24/120 (19.8%) patients were treated with short courses of low-dose steroid and there was no statistical difference between groups. Although postinfectious encephalitis was reported in 27.4% of patients with HSV,4 in the present study, patients with autoantibodies at the onset rarely experienced relapses of encephalitis (only one of the CSF-positive patients had an encephalitis relapse; however, relapsing encephalitis was also seen in only one patient from the CSF-negative group). Postulated mechanisms for postinfectious encephalitis include molecular mimicry, the release of antigenic proteins from neuronal injury that become autoimmune targets.37 But why autoimmune responses here were transient and self-limiting remains unknown. Although transient, those immune responses worsened inflammation, which was in line with the existing literature.16 First, from the clinical courses, the duration in hospital of the CSF-positive group was longer than that of the CSF-negative group. Second, in the CSF-positive group, more lesions in the meninges and the cerebral parenchyma were observed by imaging, and the CSF protein level was higher. Third, a trend towards higher mRS scores at discharge (although no statistically significant difference) and worse outcomes at follow-up by statistical and survival analysis were found in CSF-positive patients. Fourth, although at the onset, the severity of infection might be differed by species of virus, at follow-up, rather than species of virus, autoantibodies presence seemed more associated with worse outcomes. Therefore, we suggest that early identification and appropriate administration of immunotherapy may be beneficial for patient recovery. Further work is needed to evaluate the significance and feasibility of this protocol through prospective studies. It seems that the autoimmune responses at the onset of viral encephalitis rarely form chronic ones and differ from those in reported biphasic courses.4 38 We suggest that early screening of TBA results and CSF cytokines (such as CXCL10/IP-10 and IL-21, according to a recent study)39 may predict whether there will be subsequent autoimmune responses in patients with virus infections. If the TBA and the CSF cytokine results are both supportive, immunosuppressive therapies should be applied.
Limitations
Several limitations should be considered. (1) Because of the retrospective nature, uncontrolled or unknown factors might have confounded results. It is also possible that the retrospective identification of patients might have caused a selection bias. To rule out potential confounders, we included patients with virus detection in only one centre. As a further study, we have applied to conduct a multicentre prospective cohort project, which will help to more strictly observe the course of this self-limiting phenotype. It will also be helpful to observe those patients, who need immunotherapy here. (2) It is not possible to know for certain whether EBV we found in the CNS was carried in by B lymphocytes or came from virus multiplying outside of B lymphocytes, because we have not tested for EBV in the serum. Although NGS has the advantage of being able to identify a wide range of potential pathogens, it seems to be less sensitive compared with the standard amplification-based assays in the diagnosis of encephalitis, where low viral loads are common.40 To supplement evidence of EBV infection in brain tissue, we used in situ hybridisation, which partially confirmed the EBV outside of B lymphocytes. (3) Another limitation is the high loss-to-follow-up rate. In the CSF-negative group, the mRS score at discharge and the loss to follow-up rate were lower compared with those in the CSF-positive group, in which the patients lost to follow-up had mRS scores ranging from 2 to 5 at discharge. Therefore, if all patients were followed up, it would be more obvious that patients with autoantibodies had a worse prognosis. (4) For the TBA-positive samples, we could no retest them by CBA for all the known autoantibodies, which might cause omission. In the TBA-negative samples, some antibodies might also be detected in CSF if CBA which is more sensitive than TBA was applied. Therefore, there might be some undetected antibodies in both TBA-positive and TBA-negative samples. The results and conclusions in this paper were based mainly on the results of TBA. (5) Lastly, the proportion of each virus could potentially affect the comparison between CSF-positive and CSF-negative groups. A larger cohort or a case-control study based on population with specific viru infection is needed to clarify it.
Conclusions
Our work confirmed that autoimmune responses occur in the CNS at the onset of different viral infections. Those autoimmune responses are mostly transient, and rarely become chronic ones. Distinctions were demonstrated among the autoimmune responses that correlated with different viruses. EBV potentially increased the risk for autoimmunity to GFAP. Patients with autoantibodies in the CNS at the onset of viral infections presented more serious clinical manifestations and worse prognoses than patients without autoantibodies. Early detection of autoantibodies by TBA and the application of immunotherapies may be helpful.
Acknowledgments
We thank Katie Oakley, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Footnotes
Contribution: YL accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. DL and PL share the first authorship. YL and JL share the last authorship. YL and JL designed the study. DL and YL acquired, analysed and interpreted the data. DL, PL, HL and YL drafted the manuscript. DL, PL, HL and YL were involved in critical revision of the manuscript for important intellectual content. DL, PL, HL and YL contributed to statistical analysis. YL and DL obtained funding. JY, YY, XY, LJ, LX, SL, JY, CL, ZW, JY, WQ, YS, HW, HL, YW, CD, HY, XW, LZ, HF, HC, HZ, QX, GZ, TO, JW, YL, ZM, JG, CM, QZ, YL, HC, YL, HZ, SC offered administrative, technical or material support. The majority of this study was completed at the Second Affiliated Hospital of Guangzhou Medical University. All authors have read and approved the final manuscript.
Funding: This study was supported by the National Natural Science Foundation of China (81771302), GFAP-AID Programme of The Second Affiliated Hospital of Guangzhou Medical University (2020-LCYJ-XJS-03) and the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (YX202205).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol (No. 2019-hs-11, 2020-hs-54) was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University (Guangzhou China). Participants gave informed consent to participate in the study before taking part.
References
- 1. Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 2018;83:166–77. 10.1002/ana.25131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stahl JP, Mailles A. Herpes simplex virus encephalitis update. Curr Opin Infect Dis 2019;32:239–43. 10.1097/QCO.0000000000000554 [DOI] [PubMed] [Google Scholar]
- 3. Sköldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 2006;253:163–70. 10.1007/s00415-005-0941-6 [DOI] [PubMed] [Google Scholar]
- 4. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018;17:760–72. 10.1016/S1474-4422(18)30244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schäbitz W-R, Rogalewski A, Hagemeister C, et al. VZV brainstem encephalitis triggers NMDA receptor immunoreaction. Neurology 2014;83:2309–11. 10.1212/WNL.0000000000001072 [DOI] [PubMed] [Google Scholar]
- 6. Xu C-L, Liu L, Zhao W-Q, et al. Anti-N-methyl-D-aspartate receptor encephalitis with serum anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC Neurol 2011;11:149. 10.1186/1471-2377-11-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014;75:317–23. 10.1002/ana.24083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hacohen Y, Wright S, Waters P, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry 2013;84:748–55. 10.1136/jnnp-2012-303807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prüss H, Dalmau J, Harms L, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 2010;75:1735–9. 10.1212/WNL.0b013e3181fc2a06 [DOI] [PubMed] [Google Scholar]
- 10. Arboleya S, Clemente A, Deng S, et al. Anti-NMDAR antibodies in new-onset psychosis Positive results in an HIV-infected patient. Brain Behav Immun 2016;56:56–60. 10.1016/j.bbi.2016.03.011 [DOI] [PubMed] [Google Scholar]
- 11. Linnoila JJ, Binnicker MJ, Majed M, et al. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm 2016;3:e245. 10.1212/NXI.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franke C, Ferse C, Kreye J, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun 2021;93:415–9. 10.1016/j.bbi.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohammad SS, Sinclair K, Pillai S, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-D-aspartate receptor or dopamine-2 receptor. Mov Disord 2014;29:117–22. 10.1002/mds.25623 [DOI] [PubMed] [Google Scholar]
- 14. Liu B, Liu J, Sun H, et al. Autoimmune encephalitis after Japanese encephalitis in children: a prospective study. J Neurol Sci 2021;424:117394. 10.1016/j.jns.2021.117394 [DOI] [PubMed] [Google Scholar]
- 15. Prüss H. Postviral autoimmune encephalitis: manifestations in children and adults. Curr Opin Neurol 2017;30:327–33. 10.1097/WCO.0000000000000445 [DOI] [PubMed] [Google Scholar]
- 16. Prüss H, Finke C, Höltje M, et al. N-Methyl-D-Aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902–11. 10.1002/ana.23689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Jiang L, Yao H, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: to identify its diagnosis, management and prognosis (GFAP-AID) registry: study protocol for an ambispective, multicenter registry in China. Neuropsychiatr Dis Treat 2022;18:1099–105. 10.2147/NDT.S364246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan Z, Li H, Huang L, et al. CD8+ T-cell predominance in autoimmune glial fibrillary acidic protein astrocytopathy. Eur J Neurol 2021;28:2121–5. 10.1111/ene.14778 [DOI] [PubMed] [Google Scholar]
- 19. McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies: pathology and mechanisms. Acta Neuropathol 2011;122:381–400. 10.1007/s00401-011-0876-1 [DOI] [PubMed] [Google Scholar]
- 20. Peschl P, Schanda K, Zeka B, et al. Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J Neuroinflammation 2017;14:208. 10.1186/s12974-017-0984-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shan F, Long Y, Qiu W. Autoimmune glial fibrillary acidic protein astrocytopathy: a review of the literature. Front Immunol 2018;9:2802. 10.3389/fimmu.2018.02802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu T, Chen B, Yang H, et al. Screening for autoantibodies in inflammatory neurological syndrome using fluorescence pattern in a tissue-based assay: cerebrospinal fluid findings from 793 patients. Mult Scler Relat Disord 2019;28:177–83. 10.1016/j.msard.2018.12.036 [DOI] [PubMed] [Google Scholar]
- 23. Basal E, Zalewski N, Kryzer TJ, et al. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology 2018;91:e1677–89. 10.1212/WNL.0000000000006435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang L-P, Yuan Y, Liu Y-L, et al. Etiological and epidemiological features of acute meningitis or encephalitis in China: a nationwide active surveillance study. Lancet Reg Health West Pac 2022;20:100361. 10.1016/j.lanwpc.2021.100361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao Y, Wang X, Yan H, et al. Autoimmune encephalitis secondary to herpes simplex encephalitis with typical bimodal encephalitis phenotype. Chinese Journal of Nervous and Mental Diseases 2021;47:449–54. 10.3969/j.issn.1002-0152.2021.08.001 [DOI] [Google Scholar]
- 26. Soldan SS, Lieberman PM. Epstein-Barr virus infection in the development of neurological disorders. Drug Discov Today Dis Models 2020;32:35–52. 10.1016/j.ddmod.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soldan SS, Su C, Lamontagne RJ, et al. Epigenetic plasticity enables CNS-trafficking of EBV-infected B lymphocytes. PLoS Pathog 2021;17:e1009618. 10.1371/journal.ppat.1009618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol 2016;73:1297–307. 10.1001/jamaneurol.2016.2549 [DOI] [PubMed] [Google Scholar]
- 29. Iorio R, Damato V, Evoli A, et al. Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients. J Neurol Neurosurg Psychiatry 2018;89:138–46. 10.1136/jnnp-2017-316583 [DOI] [PubMed] [Google Scholar]
- 30. Kimura A, Takekoshi A, Yoshikura N, et al. Clinical characteristics of autoimmune GFAP astrocytopathy. J Neuroimmunol 2019;332:91–8. 10.1016/j.jneuroim.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 31. Bulakbasi N, Kocaoglu M. Central nervous system infections of herpesvirus family. Neuroimaging Clin N Am 2008;18:53–84. 10.1016/j.nic.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 32. Gupta K, Banerjee A, Saggar K, et al. A prospective study of magnetic resonance imaging patterns of central nervous system infections in pediatric age group and young adults and their clinico-biochemical correlation. J Pediatr Neurosci 2016;11:46–51. 10.4103/1817-1745.181244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ip B, Lam C, Ip V, et al. Autoimmune glial fibillary acidic protein astrocytopathy associated meningoencephalomyelitis and bilateral sensorineuronal deafness. Mult Scler Relat Disord 2020;40:101922. 10.1016/j.msard.2019.101922 [DOI] [PubMed] [Google Scholar]
- 34. Wang H, Chin JH, Fang B-Y, et al. Autoimmune glial fibrillary acidic protein astrocytopathy manifesting as subacute meningoencephalitis with descending myelitis: a case report. BMC Neurol 2020;20:443. 10.1186/s12883-020-02021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura S, Fujioka T, Kawashima S, et al. Self-remitting elevation of adenosine deaminase levels in the cerebrospinal fluid with autoimmune glial fibrillary acidic protein astrocytopathy: a case report and review of the literature. Intern Med 2021;60:3031–6. 10.2169/internalmedicine.6457-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quek AM, Tang D, Chin A, et al. Autoimmune glial fibrillary acidic protein astrocytopathy masquerading as tuberculosis of the central nervous system: a case series. Int J Infect Dis 2022;124:164–7. 10.1016/j.ijid.2022.09.029 [DOI] [PubMed] [Google Scholar]
- 37. Gelfand JM. Autoimmune encephalitis after herpes simplex encephalitis: insights into pathogenesis. Lancet Neurol 2018;17:733–5. 10.1016/S1474-4422(18)30279-5 [DOI] [PubMed] [Google Scholar]
- 38. Fu ZY, Ren HT, Xue LP, et al. Clinical featuers of adult patients with post-viral-encephalitis autoimmune encephalitis. Zhonghua Yi Xue Za Zhi 2020;100:1933–6. 10.3760/cma.j.cn112137-20200115-00095 [DOI] [PubMed] [Google Scholar]
- 39. Jiang JX, Fewings N, Dervish S, et al. Novel surrogate markers of CNS inflammation in CSF in the diagnosis of autoimmune encephalitis. Front Neurol 2019;10:1390. 10.3389/fneur.2019.01390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perlejewski K, Bukowska-Ośko I, Rydzanicz M, et al. Next-generation sequencing in the diagnosis of viral encephalitis: sensitivity and clinical limitations. Sci Rep 2020;10:16173. 10.1038/s41598-020-73156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330626supp001.pdf (55.2KB, pdf)
jnnp-2022-330626supp002.pdf (252.9KB, pdf)
Data Availability Statement
Data are available on reasonable request.