Abstract
Rationale
Participation in high-intensity exercise in early life might act as stressor to the airway barrier.
Objectives
To investigate the effect of intense exercise and associated exposure to air pollution on the airway barrier in adolescent elite athletes compared with healthy controls and to study exercise-induced bronchoconstriction (EIB) in this population.
Methods
Early-career elite athletes attending ‘Flemish-Elite-Sports-Schools’ (12–18 years) of 4 different sport disciplines (n=90) and control subjects (n=25) were recruited. Presence of EIB was tested by the eucapnic voluntary hyperventilation (EVH) test. Markers at mRNA and protein level; RNA-sequencing; carbon load in airway macrophages were studied on induced sputum samples.
Results
444 genes were differentially expressed in sputum from athletes compared with controls, which were related to inflammation and epithelial cell damage and sputum samples of athletes contained significantly more carbon loaded airway macrophages compared with controls (24%, 95% CI 20% to 36%, p<0.0004). Athletes had significantly higher substance P (13.3 pg/mL, 95% CI 2.0 to 19.2) and calprotectin (1237 ng/mL, 95% CI 531 to 2490) levels as well as IL-6, IL-8 and TNF-α mRNA levels compared with controls (p<0.05). The incidence of EIB in athletes was 9%. The maximal fall in forced expiratory volume in 1 s (%) after EVH test in athletes was significantly associated with prior PM10 and PM2.5 exposure.
Conclusion
Early-career elite athletes showed increased markers of air pollution exposure, epithelial damage and airway inflammation compared with controls. Acute exposure to increased air pollution PM10 levels was linked to increased airway hyper-reactivity.
Trial registration number
Keywords: airway epithelium, exercise
WHAT IS ALREADY KNOWN ON THIS TOPIC
Exercise-induced bronchoconstriction (EIB) is a prevalent condition in elite athletes, associated to the type of sport and the environment of exercise.
WHAT THIS STUDY ADDS
Sputum analysis of adolescent elite athletes showed increased carbon load in airway macrophages, levels of epithelial damage and airway inflammation compared with healthy controls. Furthermore, the airway response to eucapnic voluntary hyperventilation (EVH) testing was associated with prior particulate matter (PM) exposure in these athletes. Finally, underlying mechanisms of EIB are suggested.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of our study highlight the association of exposure to air pollution and airway hyper-reactivity in adolescent elite athletes compared with healthy control subjects. Our study is further evidence that action should be taken to reduce PM and improve air quality during training sessions and sports competitions.
Introduction
Although regular physical activity is of utmost importance to prevent worsening of asthma and other chronic diseases,1 excessive physical activity may induce a stress reaction in the airways resulting in epithelial damage and inflammation.2 It is known that exercise itself can induce bronchoconstriction in otherwise healthy subjects. This phenomenon is called exercise-induced bronchoconstriction (EIB) and is defined as a transient, reversible airway narrowing occurring during or after exercise.3 Elite athletes have a high risk to develop EIB, ranging from 30% to 70% of the athletes.4 Endurance sports and sports in combination to environmental factors such as chlorine or cold air are linked to the appearance of EIB.5 6 Indeed, the International Olympic Committee (IOC) systematic review and meta-analysis found the highest prevalence of lower airway dysfunction in endurance (25.1%), aquatic (39.9%) and winter (29.5%) athletes.7 Several mechanisms contributing to EIB have been described: airway cooling-rewarming, airway dehydration, epithelial cell damage resulting in the release of different inflammatory mediators and neurogenic inflammation.8 Worldwide, different school programmes exist, with the aim of selecting and training future elite athletes already at a young age. Furthermore, it has been demonstrated that EIB can already be present early on in the sports career or during adolescence.9 10
The relationship between air pollution, EIB and elite sports has not been extensively studied. Nowadays, in high traffic areas, air pollution is a major issue and the negative effects of pollutants on the airways have been shown repeatedly.11 12 It appears to be related to increased oxidative stress and inflammation in airways as well as systemic inflammation, even compromising sports performance.13 14 During exercise, ventilation increases up to 150 L/min in healthy adults and even beyond 200 L/min in elite athletes, resulting in an elevated inhalation and exposure of potential harmful environmental triggers.4 15 16 The term air pollution includes particulate matter (PM) and gaseous compounds like ozone (O3).17 PM is categorised based on particle seize: PM10 (<10 µm), PM2.5 (<2.5 µm) and ultrafine particles (<0.1 µm). Daigle et al already demonstrated increased ultrafine carbon deposition during exercise compared with rest,18 and McDonnell et al demonstrated a decrease in forced expiratory volume in 1 s (FEV1) after exercise in exposure of O3 compared with the same exercise performed in a filtered air environment.19
Sputum induction is an important non-invasive tool of airway sampling.20 Furthermore, transcriptomic analysis on these sputum samples by next-generation sequencing (RNA-Seq) allows high-throughput and detailed characterisation of gene expression profiles.21 In this way, we wanted to study the inflammatory response to exercise and associated environmental exposures (in particular air pollution) in early-career athletes, since the potential of repetitive bouts of high intensity exercise may lead to chronic inflammation, compared with healthy controls. We hypothesise that the increased ventilation rate of adolescent elite athletes, in a strongly polluted area as Belgium, is associated with airway hyper-reactivity and is reflected in their transcriptomic pattern.
Methods
Subjects
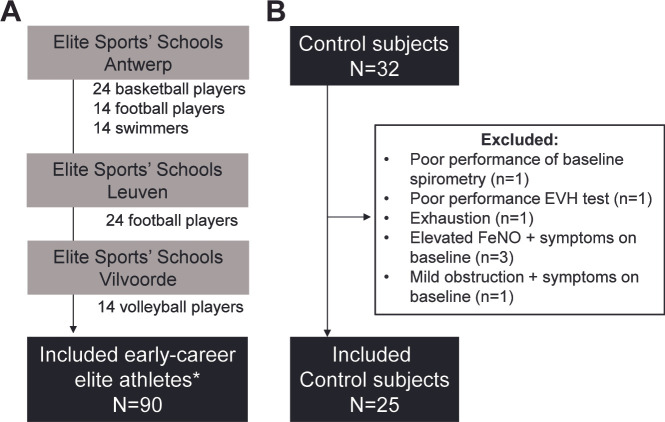
Early-career elite athletes of the four most prevalent sport disciplines attending ‘Flemish Elite Sports’ Schools’ (12–18 years) were recruited. An overview of the number of athletes per sport discipline in each Elite Sports’ Schools is presented in figure 1A. Athletes were included in this study from January 2019 to December 2019. Control subjects, performing less than 6 hours of sport/week, were recruited to and included from January 2020 to March 2021 (figure 1B). Athletes received one visit at their Elite Sports’ Schools. The study visit of control subjects was performed in UZ Leuven.
Figure 1.
Recruited subjects. (A) Included athletes (n=90) in elite Sports’ Schools in Antwerp, Leuven and Vilvoorde. *None were excluded. (B) Included controls (n=25). Exclusion criteria are shown.
Study design
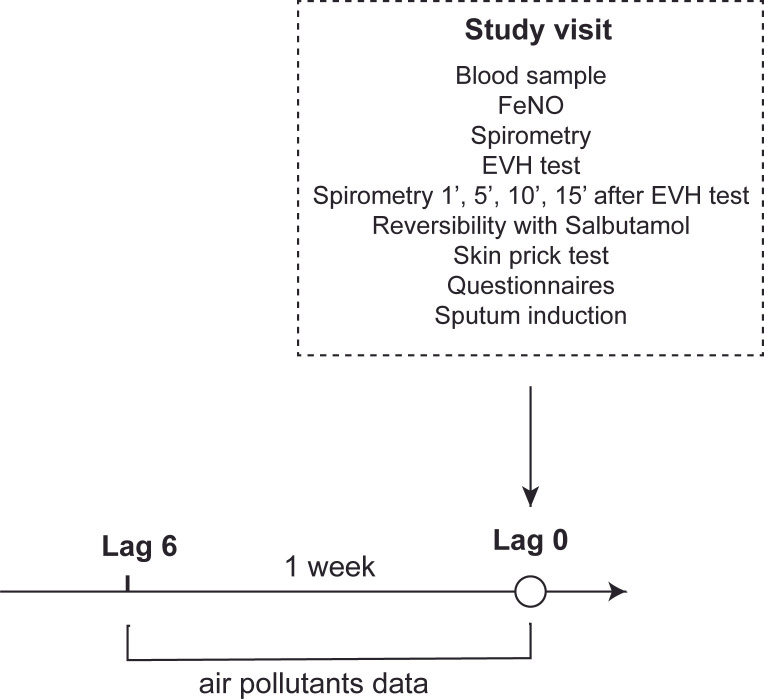
The study design is presented in figure 2.
Figure 2.
Study design. Demonstrating order of different interventions at the study visit. EVH, eucapnic voluntary hyperventilation.
FeNO
Fractional exhaled nitric oxide (FeNO) levels were measured with portable device ‘Niox Vero’ (Accuramed, Belgium), recorded in parts per billion (ppb).
Spirometry and eucapnic voluntary hyperventilation test
Spirometry was performed using a portable spirometer (Spirolab III Spirometer, MIR). The eucapnic voluntary hyperventilation (EVH) test (EucapSys, SMTEC, Switzerland) was performed according to American Thoracic Society (ATS) guidelines and adapted for this young age group.10 22 Briefly, subjects inhaled a dry air mixture containing 5% CO2 at room temperature for 6 min. The target ventilation to be maintained was 21×FEV1, equivalent to 70% of MVV. The EVH test was considered to be positive if the fall in FEV ≥10% at one of the time points (1’, 5’, 10’, 15’) after the EVH test (the measurement after 1’ not taken into account). After the EVH test, reversibility testing was done using 400 µg of salbutamol.
Skin prick tests
A skin prick test for nine common aero-allergens was performed on all subjects: grass pollen, mixed tree pollen (hazel, birch and alder), birch pollen, weed pollen, house dust mite (Dermatophagoides pteronyssinus), cat, dog, Alternaria Alternata, Aspergillus fumigatus (HAL Allergy, Leiden, The Netherlands). A subject was considered to be atopic if at least one allergen was positive (≥3 mm and at least the size of the histamine control).23
Questionnaires
The Allergy Questionnaire for Athletes (AQUA),24 25 as well as the asthma control test,26 was filled in by all subjects. Additionally, a self-made exposure questionnaire, based on previous cohort studies,10 27 was filled in to assess presence of airway symptoms (eg, wheezing, dyspnoea, coughing, rhinorrhoea, …), medication use, family history of allergies and exposure at home (pets, smoking) and hours of sports a week.
Sputum induction
Subjects inhaled salt solutions of respectively 3%, 4% and 5% during 7 min each. After inhalation, sputum was collected by spitting in a collection tube and processed by the selected plug method, to minimise contamination with saliva, like previously described.27–31 Sputum induction was not performed in swimmers, due to lack of consent in that particular sport branch. Cytospins (Shandon cytocentrifuge) were prepared from 12 500 and 25 000 sputum cells for differential cell counts and were stained with Diff-Quik. The remaining cells were lysed for mRNA analysis at −80°C. The sputum supernatants were stored at −80°C for further analysis.
Sample analysis
Carbon load in airway macrophages
Black carbon (BC) load in airway macrophages was determined as previously described by Bai et al.32 Briefly, digital images of 25 randomly selected airway macrophages from each cytospin slide were obtained at ×1000 magnification. Cells were manually delineated and the ImageJ software (NIH, Maryland, USA) automatically counted the number of particles and percentage area occupied by BC in the indicated area. The percentage of loaded macrophages was determined manually.
RNA isolation, cDNA synthesis, qPCR
Sputum levels of chemokines (CCL3, IL-8), cytokines (IL-1α, IL-1β, IL-6, IL-17A, IL-17F, IFN-γ, TNF-α), tight junctions (CLDN1, CLDN15, OCLN, ZO-1) and enzymes (CHIT1) were measured using real time qPCR.27–31 More information can be found in online supplemental material and methods.
thorax-2022-219651supp001.pdf (1.9MB, pdf)
RNA-Seq
Sequencing of mRNA was performed on, respectively, the Illumina 4000 (Illumnia, San Diego, USA). A detailed description on library preparation, bioinformatics processing and differential gene expression analysis is available in online supplemental data.
Serum and sputum supernatant biomarker analysis
Serum clara cell protein 16 (CC16) and uric acid levels; and sputum supernatant uric acid, surfactant protein D (SpD), human high mobility group protein B1 (HMGB1) and substance P were measured by ELISA. More information can be found in online supplemental material and methods.
Environmental exposure data
The average air pollution data, more specifically PM2.5, PM10, BC and O3 were obtained from ‘Belgian Interregional Environment Agency’ (IRCEL). Average regional levels of these marker for each athlete’s Elite Sports’ School/boarding school address were estimated using a spatial temporal interpolation method. For control subjects, the residence address was used. This model provides interpolated daily pollutant values in 4×4 km2 grids from the Belgian telemetric air quality networks. Individual daily mean concentrations (µg/m3) were calculated during a 7-day period prior EVH testing. The average outdoor temperature and humidity were obtained from ‘Royal Meteorological Institute of Belgium’. Environmental data were used to evaluate the association with response to EVH test. Exposure levels of each pollutant were divided into three subgroups based on data display IRCEL and WHO guidelines (2019): PM2.5 low (<10 µg/m3), immediate (10–25 µg/m3) and high (>25 µg/m3); PM10 low (<20 µg/m3), immediate (20–50 µg/m3) and high (>50 µg/m3); O3 low (<50 µg/m3), immediate (50–100 µg/m3) and high (>100 µg/m3).
Statistical analysis
Statistical analysis was performed with Graphpad Prism V.9 (Graphpad Software, San Diego, USA). Normality was tested with Shapiro-Wilk test. To compare the means of two normally distributed groups, parametric t-tests were used, if not normally distributed, the Mann-Whitney test was used. Welch’s correction was applied to correct for unequal sample size. Bonferroni was applied to correct for multiple testing. For normally distributed data one-way analysis of variance with Tukey’s multiple comparisons test was used to compare a parameter between more than two groups, the Kruskal-Wallis test with Dunn’s multiple comparisons test for non-normally distributed data. Contingency tables were tested with Fisher’s exact test or χ2 test. Correlation was studied by the Pearson or Spearman correlation test, depending on normality. IBM SPSS V.28 Statistics (SPSS, Chicago, USA) was used for linear regression analysis. The absolute value of the maximal fall in FEV1 was log-transformed to obtain homoscedasticity. To investigate the association between the maximal fall in FEV1 and air pollution exposures, we adjusted the models for age-squared, gender, body mass index (BMI), atopic state, humidity and temperature. Since the dependent variable (maximal fall in FEV1) was log transformed, the resulting regression coefficients and their 95% CIs were transformed to [10ˆ(B – 1) × 100]. This transformation allows interpreting the coefficient as the percentage of change in maximal fall in FEV1. A difference was considered significant when p<0.05.
Results
Subject characteristics
Ninety adolescent elite athletes from four different sport disciplines were recruited: basketball (n=24), football (n=38), volleyball (n=14) and swimming (n=14) and 25 healthy controls between the ages of 12 and 18 were included. An overview of the subject characteristics is shown in table 1. All athletes performed sport activities on a high level, with a median training load of 18–21 hours/week. Baseline FEV1% predicted of all athletes exceeded 80%, except for 1 football player previously diagnosed with asthma by physician having an FEV1% of 69%. Significantly higher baseline FEV1% and forced vital capacity (FVC%) predicted values were observed in swimmers (mean±SD: 121%±11%) compared with all other sport disciplines and controls. In addition, 33 of 90 early-career athletes (37%) were atopic, mostly to grass pollen (n=25/33), followed by house dust mite (n=22/33), of which 25 were polysensitised.
Table 1.
Subject characteristics
| Control subjects | Athletes | P value | Sport disciplines | ||||
| Basketball players | Football players | Volleyball players | Swimmers | ||||
| No (n=) | 25 | 90 | 24 | 38 | 14 | 14 | |
| Age (years) | 15.59±1.64 | 15.53±1.41 | 0.9225 | 15.88±1.30 | 15.39±1.22 | 16.64±1.08 | 14.21±1.31 |
| Gender (M/F) | 13/12 | 51/39 | 0.8204 | 20/4 | 18/20 | 4/10 | 9/5 |
| BMI | 19.84±3.00 | 20.79±3.06 | 0.1677 | 22.35±2.53** | 19.53±3.56 | 21.35±1.65 | 21.00±2.10 |
| Atopy (n=) | 9 (36%) | 42 (47%) | 0.3423 | 10 (42%) | 14 (37%) | 7 (50%) | 2 (14%) |
| FEV1 (L) | 3.83±0.77 | 4.11±0.88 | 0.1391 | 4.78±0.86** | 3.57±0.66 | 4.29±0.50 | 4.25±0.89 |
| FEV1% predicted | 104.4±9.5 | 108.0±12.4 | 0.1890 | 104.6±10.3 | 105.0±11.8 | 107.6±11.7 | 121.1±10.7*** |
| FVC (L) | 4.35±0.89 | 4.81±1.09 | 0.0569 | 5.58±1.08** | 4.12±0.81 | 5.00±0.50 | 5.17±1.24 |
| FVC% predicted | 103.5±10.0 | 109.6±14.1 | 0.0447 | 105.1±12.5 | 106.1±11.9 | 109.7±11.1 | 126.6±13.0**** |
| FeNO (ppb) | 10.0 (7.5–14.5) | 14.0 (10.0–23.0) | 0.0114 | 18.5 (13.3–39.0)* | 12.5 (9.0–22.3) | 13.0 (10.0–18.0) | 13.5 (9.8–22.5) |
| Achieved target ventilation (%) | 97.2±15.6 | 94.7±16.3 | 0.4924 | 95.9±18.8 | 94.3±13.8 | 94.0±17.8 | 92.4±12.6 |
| Training years | / | 10 (9–11) | / | 10 (9–11) | 10 (9–12) | 10 (7–11) | 10 (9–11) |
| Hours of sports a week | 4 (3–5) | 20 (16–22) | <0.0001 | 20 (18–22)**** | 18 (15–20)**** | 20 (20–22)**** | 21 (16–22)**** |
| Smoking state (yes/no) | 3/22 | 1/89 | 0.0318 | 0/24 | 1/37 | 0/14 | 0/14 |
| Sputum total cell count (×106) | 0.4 (0.3–0.9) | 1.0 (0.5–1.8) | 0.0341 | 1.2 (0.5–4.7) | 0.9 (0.4–1.5) | 0.9 (0.6–2.0) | NA |
| Sputum macrophages (%) | 98.0 (92.3–98.8) | 96.4 (89.2–99.1) | 0.4288 | 94.0 (59.0–100.0) | 97.7 (91.4–99.25) | 94.1 (93.6–98.3) | NA |
| Sputum neutrophils (%) | 1.0 (0–5.6) | 1.2 (0–9.8) | 0.9382 | 1.0 (0–40.0) | 1.2 (0–−6.6) | 2.8 (0–6.2) | NA |
| Sputum eosinophils (%) | 0 (0–0) | 0 (0–0) | 0.8758 | 0 (0–0) | 0 (0–0) | 0 (0–0) | NA |
| Sputum lymphocytes (%) | 0.8 (0.3–1.2) | 0.6 (0–2.0) | 0.9068 | 0 (0–1.7) | 1.3 (0.4–2.5) | 0.6 (0–1.4) | NA |
P value represents the p value obtained with statistical analysis among controls and whole athletes’ group. Normally distributed data are represented as mean±SD and analysed viat-test. Non-parametric data are represented as median with IQR and analysed with Mann-Whitney U test.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared with control subjects (one-way ANOVA for parametric data and Kruskall-Wallis test for non-parametric data).
ANOVA, analysis of variance; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NA, not available.
Cellular composition of the sputum
In total 73 sputum samples of athletes (81%) (swimmers were not included due to lack of consent) and 18 sputum samples of controls (72%) were collected (n=91). Of all samples, 65% yielded a sufficient quality to perform differential cell count (n=59) to phenotype airway inflammation (table 1, online supplemental figure E1). The proportion of cell types (and overall total yields) did not differ between athletes and controls. The median percentage of squamous epithelial cells on the total cell counts was 16.7% (P25–P75: 6.6%–30.6%). The differential cell count of the sputum samples from these subjects was dominated by macrophages (median: 97%, P25–P75: 91%–99%), followed by neutrophils (median: 1%, P25–P75: 0%–8%).
BC particles in airway macrophages
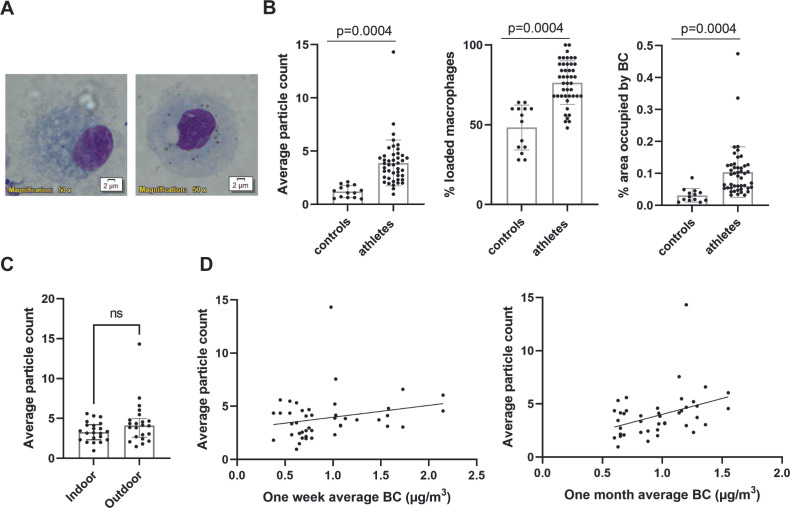
To assess personal exposure to combustion derived particles, carbon loading in airway macrophages was determined. Of the in total 91 collected sputum samples, 58 samples (64%) yielded a sufficient quality and number of airway macrophages (figure 3A and online supplemental figure E1). There was no association between the carbon content of airway macrophages and age, weight, height or BMI of the subjects. Sputum samples of athletes contained significantly more loaded airway macrophages compared with controls (p<0.0004) (figure 3B). Similarly, the number of particles in macrophages and percentage area occupied by BC were significantly increased in athletes compared with controls (p<0.0004) (figure 3B). However, no significant difference was observed between indoor and outdoor athletes (figure 3C). One week and the past one month average BC exposure did both not differ between controls and athletes (online supplemental figure E2). The carbon load in macrophages was positively associated with the estimated past one-month average BC (r=0.3996, p=0.0072) rather than with the shorter time period of one-week average BC in athletes (r=0.1570, p=0.3087) (figure 3D).
Figure 3.
Increased carbon load in airway macrophages in athletes compared with controls. (A) Illustration of images captured for analysis showing airway macrophages stained by diff-Quick with increasing BC load. (B) The average particle count per macrophage, percentage of loaded macrophages and the percentage area occupied by BC for each participant was calculated by a blinded researcher. For each participant, 25 macrophages were counted. (Normality confirmed, unpaired t-test with Welch’s correction), controls (controls: n=14, athletes n=44) (C) Comparison of indoor (n=22) and outdoor (n=22) average BC load (normality confirmed, unpaired t-test). (D) Correlation between one week and one month average BC (µg/m3) and the average particle count observed in athletes (n=44). (Spearman correlation). BC, black carbon; ns, not significant.
Airway inflammation in controls and athletes
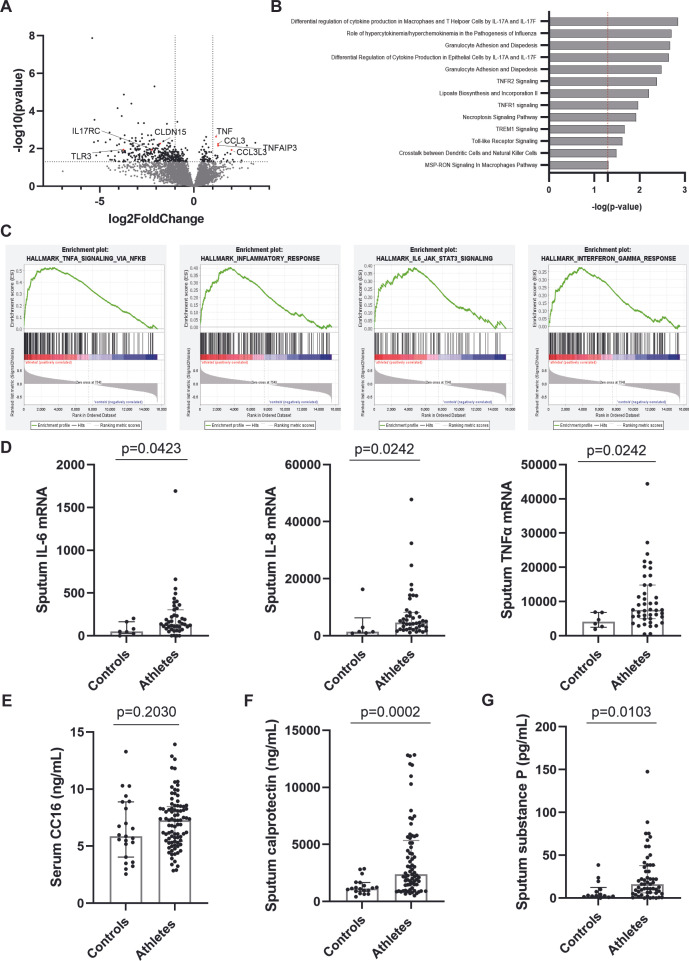
To assess the transcriptomic difference between airway cells of athletes and controls in depth, RNA-Seq was performed on isolated RNA from 48 sputum samples (selected based on quality requirements, online supplemental figure E1). A total of 444 genes were differentially expressed between controls (n=11) and athletes (n=37) (p<0.05). Seventy-seven genes were upregulated and 367 genes were downregulated in athletes compared with controls. DEGs were mapped onto a volcano plot (figure 4A, online supplemental table E2). Specifically, DEGs related to inflammation and epithelial cell damage including TNF, CCL3, CLDN15, TNAIP3, IL17RC and TLR3, respectively, were observed. To identify related mechanisms, DEGs were subjected to Ingenuity Pathways Analysis (IPA) analysis. Significant canonical pathway analysis revealed that those DEGs play key roles in gene networks involved in cell death and survival and immune cell trafficking (figure 4B). Similarly, gene set enrichment analysis demonstrated that gene set of airway inflammation, including TNF-α, INF-γ and IL-6 were enriched for athletes (figure 4C).
Figure 4.
Profiling of serum and induced sputum samples in controls and athletes. (A) Volcano plot with the magnitude expressed as log2 fold change (x-axis) and significance expressed as −log10 of the adjusted p value (y-axis) of differential expression analysis. Genes of interest are labelled. (B) Selected significantly enriched or downregulated pathways based on Ingenuity Pathways Analysis (IPA) analysis listed according their p value. (C) Significant enrichment plots at False Discovery Rate (FDR) <25%. (D) Following cytokine mRNA levels (controls: n=6, athletes: n=43) were measured via qPCR: IL-6, IL-8 and TNF-α. (E) Serum CC16 levels in controls vs athletes were measured via ELISA (controls: n=23, athletes: n=83). Sputum supernatants (controls: n=18, athletes: n=73) calprotectin (F) and substance P levels (G) were measured via ELISA. (Mann-Whitney U test).
After enrichment analysis, several key genes were identified and validated using qPCR (controls: n=6, athletes: n=43, online supplemental figure E3). We observed significantly elevated transcription levels of TNF-α and CCL3 in sputum of athletes compared with controls even correlating with the number of sports/weeks. CLDN15 was not significantly decreased in athletes compared with controls, but was significantly different among the different sport disciplines (p=0.0135), with the highest levels observed in volleyball players. Besides TNF-α, athletes had significantly higher levels of sputum IL-6 and IL-8 mRNA levels compared with controls (figure 4D, p<0.05). Furthermore, IL-6 levels were significantly higher in outdoor athletes compared with indoor athletes (p=0.0049). Other measured cytokines (IL-1α, IL-1β, IL-17A, IL-17F, IFN-γ) did not show a significant difference between controls and athletes (online supplemental figure E4).
Damage-associated molecular pattern, including HMGB-1 and SpD, was not detectable in sputum from controls, while present in sputum of athletes (n=24, respectively) (data not shown). Serum CC16 levels, described as marker of epithelial damage in serum from athletes in previous cohorts, were surprisingly not significantly elevated in athletes compared with controls (figure 4E) and also did not correlate with the maximal fall in FEV1 after EVH test. A significant higher calprotectin level in sputum supernatant, a marker for neutrophilic inflammation, was however observed in athletes when compared with controls (figure 4F, p=0.0002). Sputum neutrophil levels furthermore correlated positively with calprotectin levels in sputum supernatant (r=0.3216, p=0.0312). In addition, athletes demonstrated increased levels of substance P in sputum supernatant compared with controls (figure 4G, p=0.0103).
EVH response in young elite athletes
Eight elite athletes (9%) tested positive for EIB (≥10% fall in FEV1) (online supplemental table E7). Because of clinical symptoms during the test (dyspnoea), one additional athlete did not achieve three post-EVH spirometry measurements and salbutamol (Ventolin) was given early after symptom occurrence and hence she was excluded from analysis. Of the EIB+ athletes (n=8) three were atopic (online supplemental figure E5). Of these atopic EIB+ athletes, two athletes were basketball players (2/24) and one was a volleyball player (1/14). In total three of the non-atopic EIB positive athletes were swimmers (3/14) and two were football players (2/38). Also, three control subjects (12%) tested positive for EIB of which two were atopic. The EIB response was mild in seven athletes and three controls, that is, 10%–25% fall in FEV1 and was considered to be moderate (≥25% to <50%) in one athlete. Nine subjects had their maximal fall in FEV1 (%) under threshold of 10% within 10 min after the EVH test and two controls had a decline in FEV1 later on, 15 min after the EVH test. Preliminary comparison between atopic EIB+ (n=2) and EIB− (n=4) athletes by RNA-Seq can be found in online supplemental file (please refer to figure E5 and table E4).
Air pollution, epithelial barrier integrity and severity of bronchoconstriction during EVH test
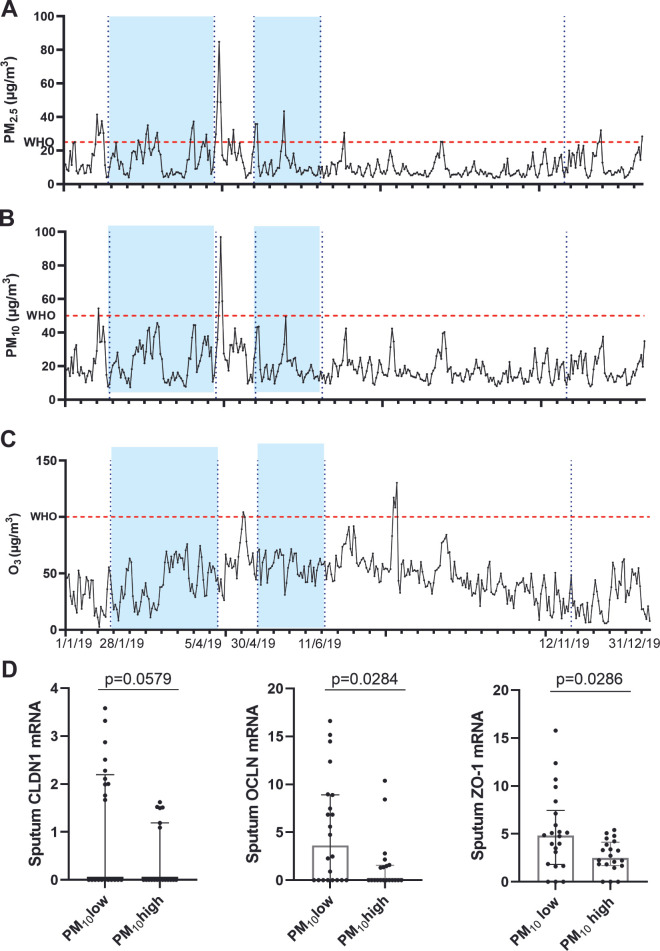
The association of environmental exposures such as prior air pollution exposure, outdoor temperature and outdoor humidity with expression of tight junctional proteins was evaluated in all athletes. Figure 5 shows the temporal analysis of pollutant levels during the period of inclusions. Significantly lower mRNA levels of OCLN and ZO-1 in athletes exposed to higher levels of PM10 (>20 µg/m3, n=22) compared with athletes exposed to lower levels (<20 µg/m3, n=23) are observed (p=0.0284, p=0.0286) (figure 5D). The same trend is observed for CLDN1 mRNA levels (p=0.0579) (figure 5D).
Figure 5.
Air pollution exposure prior EVH test. (A–C) Daily mean levels of each pollutant considered during the study period averaged over the three included Elite Sport’s Schools. The flashing red line refers to the maximum concentration of each pollutant established by WHO.57 The dotted blue lines refer to the time frame the athletes participated in the study. (G) Effect of the most robust air pollutant PM10 on tight junction mRNA expression of CLDN1, OCLN and ZO-1. (Mann-Whitney) (PM10low: n=22, PM10high: n=20, outliers were removed based on Grubbs). EVH, eucapnic voluntary hyperventilation. O3, ozone; PM2.5, particulate matter < 2.5 µm; PM10, particulate matter < 10 µm.
Table 2 shows the associations between daily air pollution levels and changes in maximal fall in FEV1 after EVH test, adjusted for humidity, temperature, age-squared, gender, BMI and atopic state in a single pollutant model. The spearman correlation between meteorological factors and other pollutants are presented in online supplemental table E5. The maximal fall in FEV1 was significantly associated with both PM2.5 and PM10. For example, the maximal fall in FEV1 was 10.45% lower for each 1 µg/m3 increment in average PM2.5 and 10.42% lower for each 1 µg/m3 increase in PM10, indicating a reduction in maximal fall in FEV1 with increasing air pollution exposure. Concretely, for an athlete with a maximal fall in FEV1 of −5%, the maximal fall will decrease to −5.5% when exposed to one unit more of PM. In the multipollutant model, considering both PM2.5 and PM10, the association with PM10 appeared to be the most robust (table 3).
Table 2.
Single pollutant models for PM2.5, PM10 and O3
| Adj* B-coefficient | Adj* 95% CI | P value | |
| PM2.5 | −10.45 | −10.21 to −10.72 | <0.001 |
| PM10 | −10.42 | −10.21 to −10.67 | <0.001 |
| O3 | −10.07 | −9.93 to −10.21 | 0.289 |
Adjusted relative changes (%) with their 95% CI in maximal fall in FEV1.
*Adjusted for humidity, temperature, age-squared, gender, BMI and atopic state.
BMI, body mass index; FEV1, forced expiratory volume in 1 s; O3, ozone; PM10, particulate matter < 10 µm; PM2.5, particulate matter < 2.5 µm.
Table 3.
Two-pollutant model with PM2.5 and PM10
| Adj* B-coefficient | Adj* 95% CI | P value | |
| PM2.5 | −9.38 | −8.05 to −10.38 | 0.163 |
| PM10 | −11.27 | −10.07 to −12.59 | 0.036 |
Adjusted relative changes (%) with their 95% CI in maximal fall in FEV1
*Adjusted for humidity, temperature, age-squared, gender, BMI-squared and atopic state.
BMI, body mass index; FEV1, forced expiratory volume in 1 s; PM10, particulate matter < 10 µm; PM2.5, particulate matter < 2.5 µm.
Discussion
Our results clearly indicate that in the airways of early-career elite athletes, who are exposed to intense physical exercise and air pollution, the epithelial barrier is affected and airway inflammation occurs. Increased carbon load in sputum macrophages of these athletes is observed, whether or not performing outdoor sport activities. Probably, it is the combination of environmental triggers (both intense exercise and air pollution) which impacts the airways. Remarkably, the airway response to EVH testing in athletes was associated with prior PM exposure, suggesting that acute air pollution could induce increased bronchial reactivity of the airways, which is particularly relevant in athletes with high ventilatory demands. In athletes’ sputum samples, genes related to epithelial cell damage, airway inflammation (IL-6, IFN-γ, TNF-α) and immune trafficking are clearly upregulated compared with control subjects. Moreover, based on preliminary RNA-Seq analysis between EIB+ and EIB− athletes, the impact of tight/gap epithelial damage, oxidative stress and (neuro)inflammation can be envisioned in research on mechanisms of EIB.
It is known that acute and/or chronic exposure to intense physical exercise can induce airway inflammatory reactions including cytokine release.33 34 Indeed, many studies have consistently shown that local and/or systemic levels of IL-1α, IL-1β, IL-6, IL-8 and IL-10 are increased in adult athletes after exercise.27 35 However, few studies focus on local inflammatory markers in early-career athletes without asthma.36 We here confirm increased sputum mRNA levels of IL-6, IL-8 and TNF-α in early-career elite athletes. TNF-α can be released from activated macrophages and is able to induce cytokine release from the epithelium. The epithelial barrier might furthermore be directly impacted by intense physical exercise. Epithelial damage was suggested in earlier studies,10 27 37 38 and we here also observed elevated damage-associated molecular pattern (HMGB-1) in the sputum of the athletes compared with controls, which may feature as early inducers of local inflammation. Furthermore, the impact of the epithelial barrier is reflected by the downregulation of CLDN15, a component of the tight junctions, in athletes compared with controls. We observed increased sputum neutrophilia in athletes related to the hours of physical training, although no pathological neutrophilic inflammation (defined as sputum neutrophil count >63%) was observed. However, also sputum calprotectin levels were significantly increased in athletes compared with controls, pointing to the potential start of neutrophilic airway inflammation in young elite athletes. In line with this, Decaesteker et al 39 demonstrated a significant increase in sputum supernatant calprotectin levels after exposure to exercise, as well as to other environmental conditions (hypoxia and cold).
Furthermore, histological analysis revealed clear uptake of BC particles by the macrophages, confirming their activity, which was increased in athletes compared with controls, likely the result of their high ventilatory demands during exercise. Chronic PM exposure has been linked to higher airway macrophage BC load.32 40–42 Although the exact lifespan of human macrophages is unknown, PM2.5 6-month exposures are most strongly associated with airway macrophage BC content, rather than with shorter time periods considered.40 43 We found a significant correlation between airway macrophages BC load and athletes’ average BC exposure during 1 month in average, but not for a shorter period of 1 week. Besides, PM2.5 and PM10 might both impact the airway epithelial barrier. We found significant lower levels of OCLN and ZO-1 levels in athletes exposed to higher levels of PM10 compared with athletes exposed to lower levels.
Strikingly, the maximal fall in FEV1 post-EVH test in athletes was significantly associated with prior PM exposure (at lag3). Previous studies also demonstrated a significant negative correlation between spirometric indices (FEV1 and FVC) and the exposure to air pollution 3–6 days prior.44 45 For the EVH test, such correlation was not described before. However, variability in the results of the EVH test has repeatedly been reported.46–48 The algorithm for the evaluation of asthma and EIB in athletes by Boulet and O’Byrne in New England Journal of Medicine already suggested to repeat the bronchoprovocation in case of a negative result in a period of more intense training or during exposure to relevant allergens or environmental conditions.49 This environmental condition might very well be PM exposure. In addition, the recent position paper of European Academy of Allergy and Clinical Immunology highlights the importance of repeat assessment and requirement of in season testing.50
Emphasis thus far was on the risk of airway barrier damage and inflammation by intense physical exercise. However, the potential beneficial advantages were less studied at the gene expression level. To our knowledge, this is the first study that focuses on a more in-depth transcriptomic profiles from human sputum samples of early-career athletes compared with healthy controls by RNA-Seq. This analysis confirmed the role of epithelial damage, immune trafficking and airway inflammation. However, several other genes were found to be differentially expressed between controls and athletes. Of these CCL3 (MIP-1α) was clearly upregulated. CCL3 is known to be involved in neutrophilic inflammation and might be produced by macrophages, lymphocytes, neutrophils, eosinophils, fibroblasts and mast cells.51 52 IL1-β can induce its expression in airway epithelial cells by activating nuclear factor (NF)-kB. Its expression can be related to our observation of sputum supernatant calprotectin level. Furthermore, the expression profile in our athletes’ cohort was significantly associated with the gene set that is upregulated in response to IFN-γ. The rise in INF-γ should be considered as beneficial for immune state, since it is an anti-inflammatory cytokine.53 In line with previous studies, we found a downregulation of TLR3 in athletes compared with controls.54 55 This may contribute to the higher reported susceptibility for infections in athletes.54 However, on a long term, a decrease in TLR might also be beneficial due to reduced inflammatory capacity of leukocytes, limiting chronic inflammation. In addition, we found significantly lower IL17RC, which is a coreceptor to respond to IL-17A and IL-17F.56 This downregulation might also act as a protective response of athletes against immune inflammation. Taken together our results point towards a type 1 and 17 inflammation on intense exercise in early-career elite athletes.
We are aware of a major limitation of the study that inflammation in swimmers could not be documented due to the absence of sputum samples of swimmers who, as a sport discipline, did not consent to have a sputum induction done. As a result, sufficient qualitive RNA to use for RNA-Seq analysis, was only available from atopic EIB+ athletes, and hence interesting information is missing about the underlying mechanism in non-atopic EIB+ athletes. Another limitation of our study is that the exposure to air pollution was estimated based on environmental measurements in the area where the control subject or athlete resides (either at home or as an intern in the elite sport school) but were not measured by personal samplers, accordingly not taking into account other personal factors such as time spent indoors versus outdoors. In addition, we have no control over the amount of single exposure in each subject. However, this is best resembling the real-life situation, in which athletes are exposed to different triggers at the same moment in time. Lastly, we are aware that our cross-sectional and observational design do not permit causal relations to be drawn. Therefore, mice models are needed.
In conclusion, high intensity exercise and exposure to air pollution in early-career athletes are associated with increased levels of epithelial damage and airway inflammation compared with controls. Acute exposure to increased air pollution PM10 levels may be associated with increased airway hyper-reactivity.
Acknowledgments
W. Bossuyt, V. Brys, A. Cortés Calabuig and the UZ Leuven Genomics Core for RNA-Seq and bioinformatics expertise. We are very grateful to C. Wang of the Centre for Environmental Sciences, Hasselt University, for sharing expertise and critical insights. In addition, we thank our colleagues from the Allergy and Clinical Immunology Research Group, Ahmad Kasran, Backaert Wout, Staels Frederik and Ieven Toon to supervise as doctor the visits at the 'Elite sports’ schools'. Furthermore, we thank the participating athletes for their cooperation and the 'Elite sports’ schools' of Antwerp, Leuven and Vilvoorde in Flanders.
Footnotes
Contributors: DMB, JL, MR and LD conceptualised and designed the study. JG, A-CJ, ED, CG, VV, JS and SA contributed to the recruitment of athletes and/or performing of the experiments. JG, A-CJ, SFS, ED, TD, KP, VV, JS, SA, BDW, JL, SV, MR, LD and DMB contributed to the analysis and interpretation. JG drafted the article, which was critically revised and edited by DMB, who is guarantor. All authors revised the manuscript and approved the final version.
Funding: This work was supported by Applied Biomedical Research with a Primary Social finality project (TBM) grant of the Flemish government, Belgium (T001417N). DMB and LD are recipients of a senior researcher fellowship from the Fund for Scientific Research Flanders (FWO).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. We will provide the data in open access to allow analysis over different study cohorts in for example a systematic review.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethical Committee of KU/UZ Leuven (S59778). Participants gave informed consent to participate in the study before taking part.
References
- 1. GINA . GINA main report - global initiative for asthma. Available: https://ginasthma.org/gina-reports/ [Accessed 1 Mar 2022].
- 2. Frey A, Lunding LP, Ehlers JC, et al. More than just a barrier: the immune functions of the airway epithelium in asthma pathogenesis. Front Immunol 2020;11:761. 10.3389/fimmu.2020.00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official american thoracic society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013;187:1016–27. 10.1164/rccm.201303-0437ST [DOI] [PubMed] [Google Scholar]
- 4. Atchley TJ, Smith DM. Exercise-induced bronchoconstriction in elite or endurance athletes: pathogenesis and diagnostic considerations. Ann Allergy Asthma Immunol 2020;125:47–54. 10.1016/j.anai.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 5. Bougault V, Turmel J, Boulet L-P. Bronchial challenges and respiratory symptoms in elite swimmers and winter sport athletes: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 2010;138:31S–37S. 10.1378/chest.09-1689 [DOI] [PubMed] [Google Scholar]
- 6. Parsons JP, Mastronarde JG. Exercise-Induced bronchoconstriction in athletes. Chest 2005;128:3966–74. 10.1378/chest.128.6.3966 [DOI] [PubMed] [Google Scholar]
- 7. Price OJ, Sewry N, Schwellnus M, et al. Prevalence of lower airway dysfunction in athletes: a systematic review and meta-analysis by a subgroup of the IOC consensus group on “ acute respiratory illness in the athlete. ” Br J Sports Med 2022;56:213–22. 10.1136/bjsports-2021-104601 [DOI] [PubMed] [Google Scholar]
- 8. Couto M, Kurowski M, Moreira A, et al. Mechanisms of exercise-induced bronchoconstriction in athletes: current perspectives and future challenges. Allergy 2018;73:8–16. 10.1111/all.13224 [DOI] [PubMed] [Google Scholar]
- 9. de Aguiar KB, Anzolin M, Zhang L. Global prevalence of exercise-induced bronchoconstriction in childhood: a meta-analysis. Pediatr Pulmonol 2018;53:412–25. 10.1002/ppul.23951 [DOI] [PubMed] [Google Scholar]
- 10. Jonckheere A-C, Seys S, Dilissen E, et al. Early-onset airway damage in early-career elite athletes: a risk factor for exercise-induced bronchoconstriction. J Allergy Clin Immunol 2019;144:1423–5. 10.1016/j.jaci.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 11. Goossens J, Jonckheere A-C, Dupont LJ, et al. Air pollution and the airways: lessons from a century of human urbanization. Atmosphere 2021;12:898. 10.3390/atmos12070898 [DOI] [Google Scholar]
- 12. Thurston GD, Kipen H, Annesi-Maesano I, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J 2017;49:1600419. 10.1183/13993003.00419-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rundell KW. Effect of air pollution on athlete health and performance. Br J Sports Med 2012;46:407–12. 10.1136/bjsports-2011-090823 [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Zhu T, Kipen H, et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing olympics. Res Rep Health Eff Inst 2013:5–174. [PMC free article] [PubMed] [Google Scholar]
- 15. Bruce XRM. The control of ventilation during exercise: a lesson in critical thinking. Adv Physiol Educ 2017;41:539–47. 10.1152/advan.00086.2017 [DOI] [PubMed] [Google Scholar]
- 16. Kippelen P, Fitch KD, Anderson SD, et al. Respiratory health of elite athletes-preventing airway injury: a critical review. Br J Sports Med 2012;46:471–6. 10.1136/bjsports-2012-091056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet 2014;383:1581–92. 10.1016/S0140-6736(14)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daigle CC, Chalupa DC, Gibb FR, et al. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol 2003;15:539–52. 10.1080/08958370304468 [DOI] [PubMed] [Google Scholar]
- 19. McDonnell WF, Horstman DH, Hazucha MJ, et al. Pulmonary effects of ozone exposure during exercise: dose-response characteristics. J Appl Physiol Respir Environ Exerc Physiol 1983;54:1345–52. 10.1152/jappl.1983.54.5.1345 [DOI] [PubMed] [Google Scholar]
- 20. Weiszhar Z, Horvath I. Induced sputum analysis: step by step. Breathe 2013;9:300–6. 10.1183/20734735.042912 [DOI] [Google Scholar]
- 21. Metzker ML. Sequencing technologies-the next generation. Nat Rev Genet 2010;11:31–46. 10.1038/nrg2626 [DOI] [PubMed] [Google Scholar]
- 22. Van der Eycken S, Schelpe A, Marijsse G, et al. Feasibility to apply eucapnic voluntary hyperventilation in young elite athletes. Respir Med 2016;111:91–3. 10.1016/j.rmed.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 23. Heinzerling L, Mari A, Bergmann K-C, et al. The skin prick test-European standards. Clin Transl Allergy 2013;3:3. 10.1186/2045-7022-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonini M, Braido F, Baiardini I, et al. AQUA: allergy questionnaire for athletes. development and validation. Med Sci Sports Exerc 2009;41:1034–41. 10.1249/MSS.0b013e318193c663 [DOI] [PubMed] [Google Scholar]
- 25. Jonckheere A-C, Seys SF, Dilissen E, et al. AQUA© questionnaire as prediction tool for atopy in young elite athletes. Pediatr Allergy Immunol 2018;29:648–50. 10.1111/pai.12949 [DOI] [PubMed] [Google Scholar]
- 26. Schatz M, Sorkness CA, Li JT, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117:549–56. 10.1016/j.jaci.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 27. Seys SF, Hox V, Van Gerven L, et al. Damage-Associated molecular pattern and innate cytokine release in the airways of competitive swimmers. Allergy 2015;70:187–94. 10.1111/all.12540 [DOI] [PubMed] [Google Scholar]
- 28. Seys SF, Daenen M, Dilissen E, et al. Effects of high altitude and cold air exposure on airway inflammation in patients with asthma. Thorax 2013;68:906–13. 10.1136/thoraxjnl-2013-203280 [DOI] [PubMed] [Google Scholar]
- 29. Seys SF, Grabowski M, Adriaensen W, et al. Sputum cytokine mapping reveals an “ IL-5, IL-17A, IL-25-high ” pattern associated with poorly controlled asthma. Clin Exp Allergy 2013;43:1009–17. 10.1111/cea.12125 [DOI] [PubMed] [Google Scholar]
- 30. Truyen E, Coteur L, Dilissen E, et al. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax 2006;61:202–8. 10.1136/thx.2005.052399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bullens DMA, Truyen E, Coteur L, et al. Il-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006;7:135. 10.1186/1465-9921-7-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bai Y, Bové H, Nawrot TS, et al. Carbon load in airway macrophages as a biomarker of exposure to particulate air pollution; a longitudinal study of an international panel. Part Fibre Toxicol 2018;15:1–10. 10.1186/s12989-018-0250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci 2019;8:201–17. 10.1016/j.jshs.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki K. Cytokine response to exercise and its modulation. Antioxidants 2018;7:17. 10.3390/antiox7010017 [DOI] [Google Scholar]
- 35. Santos J de MBD, Bachi ALL, Luna Junior LA, et al. The relationship of IL-8 and IL-10 myokines and performance in male marathon runners presenting exercise-induced bronchoconstriction. Int J Environ Res Public Health 2020;17:2622. 10.3390/ijerph17082622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goossens J, Decaesteker T, Jonckheere A-C, et al. How to detect young athletes at risk of exercise-induced bronchoconstriction? Paediatr Respir Rev 2022;44:40–6. 10.1016/j.prrv.2021.09.007 [DOI] [PubMed] [Google Scholar]
- 37. Bougault V, Turmel J, St-Laurent J, et al. Asthma, airway inflammation and epithelial damage in swimmers and cold-air athletes. Eur Respir J 2009;33:740–6. 10.1183/09031936.00117708 [DOI] [PubMed] [Google Scholar]
- 38. Chimenti L, Morici G, Paternò A, et al. Bronchial epithelial damage after a half-marathon in nonasthmatic amateur runners. Am J Physiol Lung Cell Mol Physiol 2010;298:L857–62. 10.1152/ajplung.00053.2010 [DOI] [PubMed] [Google Scholar]
- 39. Decaesteker T, Seys S, Hox V, et al. Serum and sputum calprotectin, a reflection of neutrophilic airway inflammation in asthmatics after high-altitude exposure. Clin Exp Allergy 2017;47:1675–7. 10.1111/cea.13043 [DOI] [PubMed] [Google Scholar]
- 40. Bai Y, Brugha RE, Jacobs L, et al. Carbon loading in airway macrophages as a biomarker for individual exposure to particulate matter air pollution-a critical review. Environ Int 2015;74:32–41. 10.1016/j.envint.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 41. Belli AJ, Bose S, Aggarwal N, et al. Indoor particulate matter exposure is associated with increased black carbon content in airway macrophages of former smokers with COPD. Environ Res 2016;150:398–402. 10.1016/j.envres.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fullerton DG, Jere K, Jambo K, et al. Domestic smoke exposure is associated with alveolar macrophage particulate load. Trop Med Int Health 2009;14:349–54. 10.1111/j.1365-3156.2009.02230.x [DOI] [PubMed] [Google Scholar]
- 43. Jacobs L, Emmerechts J, Mathieu C, et al. Air pollution related prothrombotic changes in persons with diabetes. Environ Health Perspect 2010;118:191–6. 10.1289/ehp.0900942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steinvil A, Fireman E, Kordova-Biezuner L, et al. Environmental air pollution has decremental effects on pulmonary function test parameters up to one week after exposure. Am J Med Sci 2009;338:273–9. 10.1097/MAJ.0b013e3181adb3ed [DOI] [PubMed] [Google Scholar]
- 45. Chang Y-K, Wu C-C, Lee L-T, et al. The short-term effects of air pollution on adolescent lung function in taiwan. Chemosphere 2012;87:26–30. 10.1016/j.chemosphere.2011.11.048 [DOI] [PubMed] [Google Scholar]
- 46. Hull JH, Ansley L, Price OJ, et al. Eucapnic voluntary hyperpnea: gold standard for diagnosing exercise-induced bronchoconstriction in athletes? Sports Med 2016;46:1083–93. 10.1007/s40279-016-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rizzo JÂ, Rodrigues Filho E de A, Gonçalves AV, et al. Reproducibility of eucapnic voluntary hyperpnoea for exercise-induced bronchoconstriction diagnosis in asthmatic children and adolescents. Pediatr Allergy Immunol 2021;32:1700–8. 10.1111/pai.13610 [DOI] [PubMed] [Google Scholar]
- 48. Price OJ, Ansley L, Hull JH. Diagnosing exercise-induced bronchoconstriction with eucapnic voluntary hyperpnea: is one test enough? J Allergy Clin Immunol Pract 2015;3:243–9. 10.1016/j.jaip.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 49. Boulet L-P, O’Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med 2015;372:641–8. 10.1056/NEJMra1407552 [DOI] [PubMed] [Google Scholar]
- 50. Price OJ, Walsted ES, Bonini M, et al. Diagnosis and management of allergy and respiratory disorders in sport: an EAACI Task force position paper. Allergy 2022;77:2909–23. 10.1111/all.15431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonsignore MR, Morici G, Vignola AM, et al. Increased airway inflammatory cells in endurance athletes: what do they mean? Clin Exp Allergy 2003;33:14–21. 10.1046/j.1365-2222.2003.01557.x [DOI] [PubMed] [Google Scholar]
- 52. Belda J, Ricart S, Casan P, et al. Airway inflammation in the elite athlete and type of sport. Br J Sports Med 2008;42:244–8; 10.1136/bjsm.2007.036335 [DOI] [PubMed] [Google Scholar]
- 53. Vijayaraghava A, K R. Alteration of interferon gamma (IFN-γ) in human plasma with graded physical activity. J Clin Diagn Res 2014;8:BC05–7. 10.7860/JCDR/2014/9502.4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev 2006;12:34–53. [PubMed] [Google Scholar]
- 55. Collao N, Rada I, Francaux M, et al. Anti-Inflammatory effect of exercise mediated by Toll-like receptor regulation in innate immune cells-a review. Int Rev Immunol 2020;39:39–52. 10.1080/08830185.2019.1682569 [DOI] [PubMed] [Google Scholar]
- 56. Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol 2010;32:33–42. 10.1007/s00281-009-0185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. WHO/Europe . Air quality guidelines global update 2005. 2006. Available: www.euro.who.int [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thorax-2022-219651supp001.pdf (1.9MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. We will provide the data in open access to allow analysis over different study cohorts in for example a systematic review.