Abstract
Background
To evaluate the association of age with long-term outcome after thrombectomy.
Methods
In a retrospective cohort study based on routine healthcare data from Germany between 2010 and 2018, we included 18 506 patients with acute ischaemic stroke treated with mechanical thrombectomy. Association between age and mortality, disability, and level of care at 1 year was assessed.
Results
The median age was 76 years, 36.3% were aged ≥80 years and 55.8% were women. Patients aged ≥80 compared with those <80 years had a higher mortality (55.4% vs 28.5%; adjusted HR 1.13; 95% CI 1.05 to 1.31), more often had moderate/severe disability (35.5% vs 33.2%, adjusted HR 1.14; 95% CI 1.06 to 1.23) and less frequently had no/slight disability (17.4% vs 41.0%) at 1 year. Older age was associated with a higher likelihood of living in a nursing home (13.4% vs 9.2%, adjusted HR 1.09; 95% CI 0.97 to 1.22) and a lower likelihood of living at home (33.8% vs 62.8%) at 1 year. These associations were also robust when analysed in patients with no disability prior to stroke. Factors most strongly associated with worse 1-year outcomes in elderly patients were chronic limb-threatening ischaemia (67.9% vs 56.4%; HR 1.59, 95% CI 1.38 to 1.82), dementia at baseline (65.2% vs 47.3%; HR 1.29, 95% CI 1.17 to 1.44) and ventilation >48 hours (79.3% vs 52.2%; HR 2.91, 95% CI 2.66 to 3.18).
Conclusions
In this large ‘real-world’ cohort, outcomes after mechanical thrombectomy were strongly associated with age. Of patients aged ≥80 years more than half were dead and less than one-fifth were functionally independent at 1 year. Certain comorbidities and ventilation >48 hours were associated with even worse outcomes.
Keywords: cerebrovascular disease, stroke
What is already known on this topic
Although an aging population leads to a higher frequency of age-related diseases such as ischemic stroke, patients aged 80 years or older are severely underrepresented in randomised controlled trials of mechanical thrombectomy. Therefore, current knowledge of outcomes after thrombectomy in elderly patients is based on an overall small number of patients.
What this study adds
This German nationwide real-world analysis of patients treated for acute ischemic stroke with mechanical thrombectomy found that outcomes were strongly related to age. Of patients aged 80 years and older, more than half were dead and less than one-fifth were functionally independent after one year.
How this study might affect research, practice or policy
Our study provides evidence on the outcome of elderly stroke patients treated with mechanical thrombectomy and identifies clinically relevant comorbidities that may help guide treatment decisions in elderly stroke patients.
Introduction
Clinical trials have shown that mechanical thrombectomy is a highly effective therapy in acute ischaemic stroke due to proximal large artery occlusion. Overall, 11 randomised controlled trials, which recruited a total of about 1050 patients treated with mechanical thrombectomy, demonstrated that early treatment with mechanical thrombectomy devices is safe and effective for reducing disability in patients who had a stroke.1–11 As a result, mechanical thrombectomy has been accepted as the standard of care for reperfusion therapy in acute ischaemic stroke.7 However, within these randomised controlled trials on mechanical thrombectomy, only about 20% of all included patients are aged 80 years or older.7 12 Therefore, findings on outcomes after thrombectomy in the elderly are based on an overall small number of patients. Conclusions on outcomes are further limited by rather short observations in large clinical trials of only 3 months.7 12
To address the current knowledge gap, our study was designed to investigate the association of age on long-term outcome after mechanical thrombectomy, in particular in patients aged 80 years and older, in a real-world cohort of patients who had a stroke with a longer follow-up.
Methods
Data source and study population
In brief, the German remuneration system is based on the ‘German Diagnosis Related Groups’ (G-DRG) system. Please refer to online supplemental methods for further information.
jnnp-2022-330506supp001.pdf (757.8KB, pdf)
The Allgemeine Ortskrankenkasse (AOK) is a consortium of 16 regional health insurances, which build together the largest public German health insurance; it is currently responsible for more than 26 million people representing about 30% of the entire German population. All patients’ data are stored in a central computerised database at the AOK Research Institute (WIdO, Berlin), from which we obtained aggregated and anonymised data of all patients who fulfilled the following criteria. All patients ≥18 years hospitalised with an encoded main diagnosis of acute ischaemic infarction, who received mechanical thrombectomy (International Statistical Classification of Diseases and Related Health ProblemsICD-10 GM I63 and OPS code 8–836.80 or 8–836.81) in the years 2010 to 2018 were included in the analysis. Patients with incomplete basic information or incomplete insurance status within 2 years before index were excluded from the analysis (online supplemental figure 1). This hospitalisation was defined as the index hospitalisation. Patients were grouped according to age at the index hospitalisation into distinct subgroups as follows: < 60 years, 60–69 years, 70–79 years, 80–84 years, 85–89 years, 90–94 years and >94 years. Baseline characteristics included also other encoded diagnoses for, for example, hypertension, diabetes mellitus, dyslipidaemia, prior stroke, atrial fibrillation, dementia at or within 2 years before index hospitalisation. Definitions of all variables are presented in online supplemental table 1.
We further obtained and analysed data from the Research Data Centers of the Federal Statistical Office and the Statistical Offices of the Laender (Statistisches Bundesamt, DESTATIS; https://www.destatis.de) for the years 2010–2018 with respect to mortality and care situation in patients aged. The database comprises all people living in Germany.
The data present here were assessed in the GenderVasc research project.
Outcomes
With a median follow-up time of 42.7 months, patients were followed up for a minimum of 1 year and a maximum of 9 years after index hospitalisation. The main clinical outcomes were as follows: (1) overall survival (OS); (2) degree of disability and (3) care situation. Patients were observed from date of admission to end of follow-up, given by death from any cause, exit from the data base or end of study (31 December 2019). The degree of disability was derived from the so-called care level or care-grade assigned by the German healthcare system and was divided into no/slight disability and moderate to severe disability. From 1995 to 2017, three levels of care based on the severity of the health condition were distinguished: care level 1 (considerable need for care): a person requires help at least once a day, and an average of at least 90 min of help every day of the week; care level 2 (severe need for care): a person needs help at least three times a day and requires an average of at least 180 min of help every day of the week; care level 3 (extreme need for care): a person needs round-the-clock help every day and requires an average of at least 300 min of help every day of the week.13 14 Since 2017, the previous definition of the above-mentioned three care levels (‘Pflegestufen’) was replaced by five care grades (‘Pflegegrade’) based on physical, mental and psychological disabilities. The scale goes from ‘grade 1: Little impairment of independence’ up to ‘Grade 5: Hardship cases’ and is determined by impairments of independence or incapacitation in six fields (modules), which are weighted as follows: mobility (10%), cognitive and communicative abilities (15%), behaviour patterns and psychological problems (15%), level of self-sufficiency (40%), health restrictions, demands and stress due to therapies (20%), and structure of everyday life and social contacts (20%).13 14 The different care grades are divided as follows: care grade 1: slight loss of independence, care grade 2: considerable loss of independence, care grade 3: severe loss of independence, care grade 4: very severe loss of independence and care grade 5: very severe loss of independence. We distinguished no/slight disability (care level=0 or care grade ≤1) from moderate to severe disability (care level >0 or care grade >1).
Data accessibility
A detailed description of data accessibility is provided in online supplemental methods.
Statistical analysis
Patient data were grouped for their age for the descriptive analysis. Qualitative data were tested via two-sided χ2 test and quantitative data were tested using a two-sided Wilcoxon test. The 1-year OS rate was estimated with a Kaplan-Meier estimator, all p values of the test procedures described above are purely descriptive and unadjusted. Univariate analysis for the degree of disability and the care situation were evaluated using multistate models. Statistical analyses were performed using SAS software V.9.4, SAS Institute and R V.4.1.0, R foundation, Vienna, Austria. The actual state probability 1 year after thrombectomy was calculated using Nelson-Aalen estimator and presented with unadjusted 95% CIs. Furthermore, all primary endpoints were analysed using multivariable Cox regression model. To analyse the outcome 1 year after thrombectomy, patients without an event within 1 year were censored after 1 year. The models included risk profiles of patients at baseline. All presented CIs and p values are standard unadjusted and purely descriptive. HRs and unadjusted 95% CI for all features are shown in the tables and figures. All analyses are intended to be fully explorative (hypotheses generating), not confirmatory and are interpreted accordingly.
Results
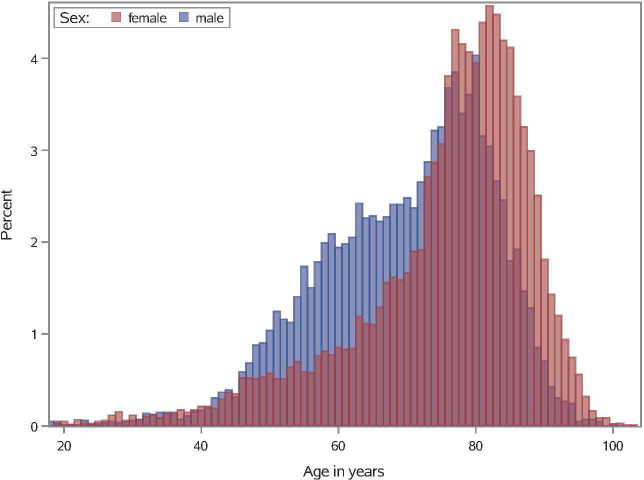
During the study period, a total of 18 506 patients with acute ischaemic stroke treated with mechanical thrombectomy were registered. Patient median age was 76 years (IQR 11 years) and 55.8% were female (table 1). Among the study population, 6729 patients were aged ≥80 years (36.4%) and 11 777 (63.6%) <80 years, who received mechanical thrombectomy. The age distribution of the study population is shown in figure 1. Patient baseline characteristics for each age group are shown in table 1. Patients aged 80 years and older were more likely to have comorbidities, such as arterial hypertension (97.0% vs 84.7%), atrial fibrillation (72.7% vs 42.3%), dementia (5.2% vs.<1%), chronic heart failure (52.8% vs 28.3%) and chronic kidney disease (26.1% vs 21.6%). Prior to admission, elderly patients were more often in need of care (outpatient care or nursing home care) compared with patients <80 years. Systemic thrombolysis was equally conducted among all age groups. Similarly, in-hospital parameters, such as ventilation >48 hours and acute renal failure, were equally distributed in all age groups. Overall, there were 4889 (26.4%) in-hospital deaths, 2426 (49.6%) of whom were aged ≥80 years and 2463 (50.4%) <80 years.
Table 1.
Baseline characteristics of study population
| All patients | <60 years | 60–69 years | 70–79 years | 80–84 years | 85–89 years | ≥90 years | P value | |
| No of patients (%) | 18 506 (100) | 3005 (16.2) | 3059 (16.5) | 5713 (30.9) | 3484 (18.8) | 2298 (12.4) | 947 (5.1) | <0.001 |
| Demographics | ||||||||
| Age, median (IQR), years | 76 (11) | 52 (9) | 65 (4) | 76 (4) | 82 (2) | 87 (2) | 92 (4) | <0.001 |
| Women, n (%) | 10 325 (55.8) | 1249 (41.6) | 1238 (40.5) | 3146 (55.1) | 2228 (64.0) | 1699 (73.9) | 765 (80.8) | <0.001 |
| Comorbidities, n (%) | ||||||||
| Hypertension | 16 495 (88.7) | 1957 (65.1) | 2648 (86.6) | 5366 (93.9) | 3361 (96.5) | 2242 (97.6) | 921 (97.3) | <0.001 |
| Diabetes mellitus | 7213 (39.0) | 588 (19.6) | 1118 (36.6) | 2559 (44.8) | 1582 (45.4) | 990 (43.1) | 376 (39.7) | <0.001 |
| Dyslipidaemia | 11 675 (63.0) | 1533 (51.0) | 1929 (63.1) | 3805 (66.6) | 2342 (67.2) | 1480 (64.4) | 568 (60.0) | <0.001 |
| Prior stroke | 4741 (25.6) | 618 (20.6) | 705 (23.1) | 1500 (26.3) | 984 (28.2) | 665 (28.9) | 269 (28.4) | <0.001 |
| Prior myocardial infarction | 1415 (7.6) | 155 (5.2) | 226 (7.4) | 492 (8.6) | 301 (8.6) | 181 (7.9) | 60 (6.3) | <0.001 |
| Atrial fibrillation | 9877 (53.4) | 449 (14.9) | 1134 (37.1) | 3400 (59.5) | 2422 (69.5) | 1741 (75.8) | 731 (77.2) | <0.001 |
| Dementia | * | <10 | 16 (0.5) | 82 (1.4) | 137 (3.9) | 137 (6.0) | 78 (8.2) | <0.001 |
| Chronic heart failure | 6879 (37.2) | 440 (14.6) | 757 (24.8) | 2131 (37.3) | 1641 (47.1) | 1327 (57.8) | 583 (61.6) | <0.001 |
| Chronic kidney disease | 5616 (30.3) | 247 (8.2) | 549 (18.0) | 1752 (30.7) | 1479 (42.5) | 1109 (48.3) | 480 (50.7) | <0.001 |
| Cancer | 4007 (21.7) | 288 (9.6) | 516 (16.9) | 1380 (24.2) | 950 (27.3) | 629 (27.4) | 244 (25.8) | <0.001 |
| Level of care before admission, n (%) | ||||||||
| Outpatient care | 3743 (20.2) | 112 (3.7) | 249 (8.1) | 793 (13.9) | 939 (27.0) | 1055 (45.9) | 595 (62.8) | <0.001 |
| Nursing home care | 915 (4.9) | 15 (0.5) | 42 (1.4) | 152 (2.7) | 200 (5.7) | 272 (11.8) | 234 (24.7) | <0.001 |
| In hospital parameters, n (%) | ||||||||
| Systemic thrombolysis | 8157 (44.1) | 1308 (43.5) | 1340 (43.8) | 2598 (45.5) | 1520 (43.6) | 973 (42.3) | 418 (44.1) | 0.152 |
| Ventilation >48 hours | 4595 (24.8) | 882 (29.4) | 872 (28.5) | 1533 (26.8) | 794 (22.8) | 424 (18.5) | 90 (9.5) | <0.001 |
| Acute renal failure | 973 (5.2) | 99 (3.3) | 130 (4.3) | 305 (5.3) | 225 (6.5) | 163 (7.1) | 51 (5.4) | <0.001 |
| In hospital death | 4889 (26.4) | 373 (12.4) | 589 (19.3) | 1501 (26.3) | 1159 (33.3) | 874 (38.0) | 393 (41.5) | <0.001 |
Differences between age groups for categorical and continuous variables were tested via χ2 and Kruskal-Wallis test, respectively.
P value ‘for comparisons of patients in all groups’.
*This number was removed because of data protection, that is, cell number was smaller than 5.
Figure 1.
Age distribution of study population. Histogram shows the age distribution of female (red) and male individuals (blue). The x-axis shows age (2-year interval); y-axis shows percentage of female patients and male patients for a given age group.
Association of age and comorbidities with mortality
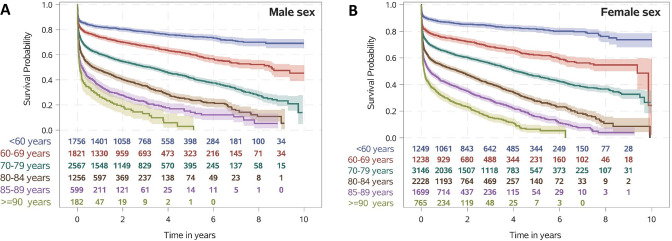
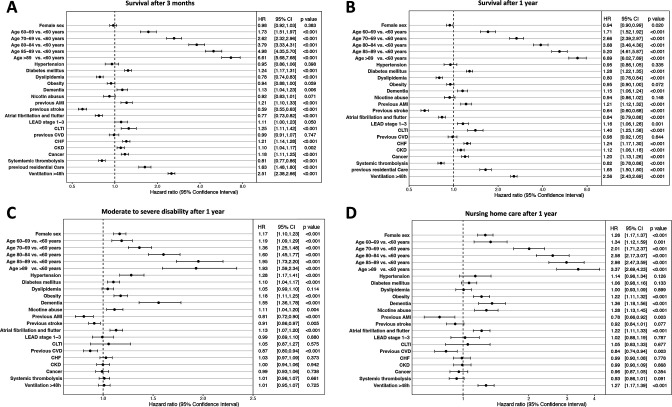
The OS in patients who had a stroke treated with mechanical thrombectomy reported over a period of up to 9 years after the index hospitalisation as related to age groups and sex is shown by Kaplan-Meier curves in figure 2. Patients aged ≥80 years had notably lower survival rates throughout the whole period of follow-up. One-year survival decreased with age (figure 2). The 1-year mortality in patients who had a stroke over 80 years of age treated with thrombectomy was 55.4% compared with a 1-year mortality of 19.3% in the general population over 80 years of age (online supplemental figure 2A). The differences in survival rates of distinct age groups maintained across time after index hospitalisation. Multivariable Cox regression analysis showed that the patient factor most strongly associated with death was older age at the time of index hospitalisation (figure 3B). Moreover, comorbidities with a statistically noticeable increased HR for death were observed for diabetes mellitus (HR 1.28; 95% CI 1.22 to 1.35), previous acute myocardial infarction (HR 1.21; 95% CI 1.12 to 1.32), chronic limb-threatening ischaemia (HR 1.40; 95% CI 1.25 to 1.58), chronic heart failure (HR 1.24; 95% CI 1.17 to 1.30), previous residential care (HR 1.65; 95% CI 1.50 to 1.80) and ventilation >48 hours (HR 2.56; 95% CI 2.43 to 2.69). In contrast, dyslipidaemia (HR 0.80; 95% CI 0.76 to 0.84), previous stroke (HR 0.64; 95% CI 0.60 to 0.68), atrial fibrillation and flutter (HR 0.84; 95% CI 0.79 to 0.88) and systemic thrombolysis (HR 0.82; 95% CI 0.78 to 0.86) were associated with improved OS. The effect of various cofactors on the OS HR was comparable at 3 months and 1 year (figure 3A, B). In the adjusted analyses, patients aged >80 years with certain comorbidities had strongly increased HR for death at 1-year follow-up: chronic kidney disease (HR 1.26; 95% CI 1.15 to 1.37), dementia (HR 1.29; 95% CI 1.17 to 1.44), previous acute myocardial infarction (HR 1.37; 95% CI 1.23 to 1.53), diabetes mellitus (HR 1.44; 95% CI 1.31 to 1.57), chronic limb-threatening ischaemia (HR 1.59; 95% CI 1.38 to 1.82) and ventilation >48 hours (HR 2.91; 95% CI 2.66 to 3.18) (table 2).
Figure 2.
Kaplan-Meier survival curves in patients who had ischaemic stroke treated with mechanic thrombectomy for all-cause mortality by age (<60 years, 60–69 years, 70–79 years, 80–84 years, 85–89 years and ≥90 years). (A) Survival probability in male sex. (B) Survival probability in female sex.
Figure 3.
Forest plots of outcome parameters. (A) Forest plot of overall survival HR and 95% CIs analysed by multivariable Cox regression model at 3 months. (B) Forest plot of overall survival HR and 95% CIs analysed by multivariable Cox regression model at 1 year. (C) Forest plot analysed by multivariable Cox regression model representing the HR and 95% CIs for moderate to severe disability. (D) Forest plot analysed by multivariable Cox regression model representing the HR and 95% CIs for nursing home care. AMI, acute myocardial infarction; CHF, chronic heart failure; CKD, chronic kidney failure; CLTI, chronic limb-threatening ischaemia; CVD, cerebrovascular disease; LEAD, lower extremity arterial disease.
Table 2.
Multivariable Cox regression for overall survival
| Comorbidities | HR | 95% CI | P value |
| Age ≥80 years+ventilation >48 hours | 2.91 | 2.66 to 3.18 | <0.001 |
| Age ≥80 years+diabetes mellitus | 1.44 | 1.31 to 1.57 | <0.001 |
| Age ≥80 years+previous myocardial infarction | 1.37 | 1.23 to 1.53 | <0.001 |
| Age ≥80 years+chronic limb-threatening ischaemia | 1.59 | 1.38 to 1.82 | <0.001 |
| Age ≥80 years+chronic kidney disease | 1.26 | 1.15 to 1.37 | <0.001 |
| Age ≥80 years+dementia | 1.29 | 1.17 to 1.44 | <0.001 |
P value ‘for comparisons of patients in all groups’.
Association of age and comorbidities with functional outcomes
Associations between patient baseline characteristics and the level of disability and care at 1 year after stroke are shown in figure 3C, D. Multivariable Cox regression analysis showed that the likelihood of moderate to severe disability noticeably increased with age (figure 3C). Along with age, hypertension (HR 1.28; 95% CI 1.17 to 1.41), obesity (HR 1.18; 95% CI 1.11 to 1.25), dementia (HR 1.55; 95% CI 1.36 to 1.78), and atrial fibrillation and flutter (HR 1.13; 95% CI 1.07 to 1.20) showed a higher likelihood of moderate to severe disability (figure 3C). In multivariable Cox regression, older age, obesity (HR 1.22; 95% CI 1.11 to 1.32), dementia (HR 1.36; 95% CI 1.18 to 1.56), nicotine abuse (HR 1.28; 95% CI 1.13 to 1.45), atrial fibrillation and flutter (HR 1.22; 95% CI 1.11 to 1.33), and ventilation >48 hours (HR 1.27; 95% CI 1.17 to 1.39) were independently associated with higher likelihood of living in a nursing home 1 year after stroke (figure 3D). Parameters noticeably associated with a decreased likelihood of living in a nursing home were previous acute myocardial infarction (HR 0.78; 95% CI 0.66 to 0.92) and previous cerebrovascular disease (HR 0.84; 95% CI 0.74 to 0.94) (figure 3D).
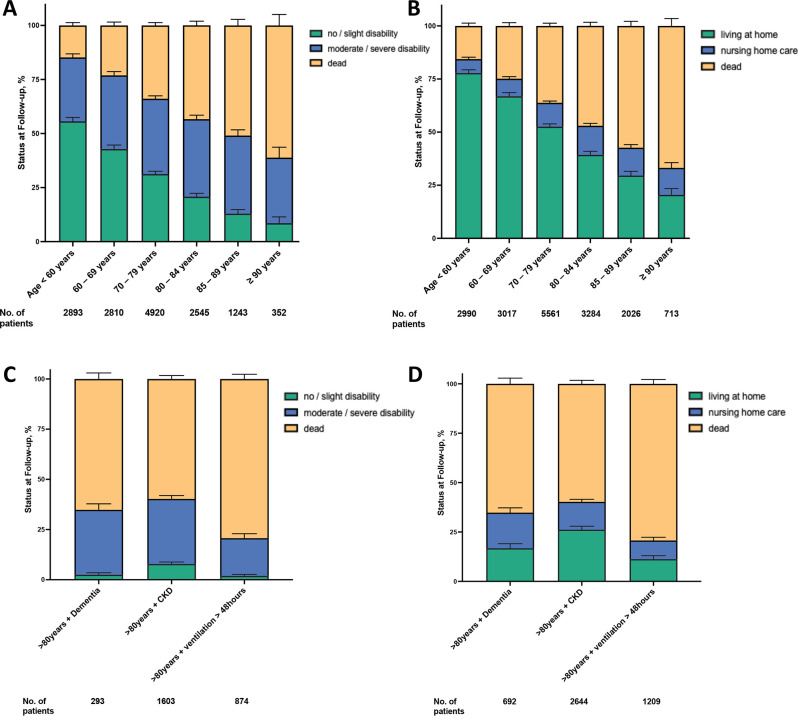
Unfavourable outcomes defined as moderate to severe disability, living in a nursing home and death were independently linked to older age (figure 4A, B). Of all patients treated with mechanical thrombectomy, 34.3% (5064/14 763) had no/slight disability, 33.8% (4996/14 763) had moderate to severe disability and 31.8% (4695/14 763) were dead at 1 year (figure 4A). In the subgroup analyses for age, in comparison to patients aged <80 years, patients aged ≥80 years had lower rates of no/slight disability (17.4% vs 41.0) and had higher rates of moderate to severe disability (35.5% vs 33.2%, adjusted HR 1.14; 95% CI 1.06 to 1.23, p<0.001) and mortality (47.2% vs 25.8%; adjusted HR 1.13; 95% CI 1.05 to 1.22, p<0.001) (figure 4A). At 1-year follow-up, 52.9% (9305/17 7591) of patients from all age groups were living at home, 10.6% (1864/17 7591) were living in a nursing home and 36.5% (6421/17 7591) were dead (figure 4B). Patients aged ≥80 years compared with those <80 years were less likely living at home (33.8% vs 62.8%) and had higher rates of nursing home care (13.4% vs 9.2%, adjusted HR 1.09; 95% CI 0.97 to 1.22, p=0.143) and mortality (52.9% vs 27.9%) (figure 4B). Of patients with stroke aged 80–84 years 13.7% lived in a nursing home after mechanical thrombectomy, whereas 5.9% of the general population aged 80–84 years live in a nursing home. Patients aged >85 years who have undergone thrombectomy less likely live in a nursing home compared with subjects from the general population of that age (13.0% vs 18.5%) (online supplemental figure 2B). Furthermore, as expected, the comparison of outcomes in patients >80 years of age with and without thrombectomy showed that patients without thrombectomy had lower mortality and were less likely to have moderate to severe disability or to be living in a nursing home (online supplemental figure 3A, B). Certainly, the outcome of patients who had a stroke with thrombectomy and also of those without thrombectomy is age-dependent. However, we focused our study on quantifying the worse outcome in patients >80 years of age. In sensitivity analyses, these associations were robust when evaluated both, only patients with no disability living at home prior to stroke and the entire study cohort. In patients aged >80 years the following parameters were most strongly associated with increased HR for moderate to severe disability: chronic kidney disease (HR 1.13; 95% CI 1.03 to 1.25), ventilation >48 hours (HR 1.15; 95% CI 1.05 to 1.27), diabetes (HR 1.25; 95% CI 1.14 to 1.37) and dementia (HR 1.77; 95% CI 1.52 to 2.06) (figure 4C, table 3). In patients aged >80 years the following comorbidities were most strongly associated with increased HR for nursing home care: chronic kidney disease (HR 1.08; 95% CI 1.03 to 1.25), diabetes (HR 1.16; 95% CI 1.00 to 1.34), ventilation >48 hours (HR 1.40; 95% CI 1.21 to 1.62) and dementia (HR 1.48; 95% CI 1.24 to 1.76) (figure 4D, table 4). A subgroup analysis showed that patients who had prior need for care had an increased mortality rate compared with patients without prior need for care (56.9% vs 22.6%) after mechanical thrombectomy.
Figure 4.
Ordinal outcomes for age subgroups and comorbidities for status at 1-year follow-up. (A) Disability status (no/slight disability, moderate/severe disability or dead) for distinct age subgroups (<60 years, 60–69 years, 70–79 years, 80–84 years, 85–89 years and ≥90 years) at 1-year follow-up. (B) Care situation (living at home, nursing home care or dead) for distinct age subgroups (<60 years, 60–69 years, 70–79 years, 80–84 years, 85–89 years and ≥90 years) at 1-year follow-up. (C) Disability status (no/slight disability, moderate/severe disability or dead) for patients aged >80 years with distinct comorbidities (dementia, chronic kidney disease or ventilation >48 hours) at 1-year follow-up. (D) Care situation (living at home, nursing home care or dead) for patients aged >80 years with distinct comorbidities (dementia, chronic kidney disease or ventilation >48 hours) at 1-year follow-up.
Table 3.
Multivariable Cox regression for moderate to severe disability
| Comorbidities | HR | 95% CI | P value |
| Age ≥80 years+ventilation >48 hours | 1.15 | 1.05 to 1.27 | <0.001 |
| Age ≥80 years+diabetes mellitus | 1.25 | 1.14 to 1.37 | <0.001 |
| Age ≥80 years+previous myocardial infarction | 0.92 | 0.80 to 1.05 | <0.001 |
| Age ≥80 years+chronic limb-threatening ischaemia | 1.19 | 0.97 to 1.46 | <0.001 |
| Age ≥80 years+chronic kidney disease | 1.13 | 1.03 to 1.25 | <0.001 |
| Age ≥80 years+dementia | 1.77 | 1.52 to 2.06 | <0.001 |
P value ‘for comparisons of patients in all groups’.
Table 4.
Multivariable Cox regression for nursing home care
| Comorbidities | HR | 95% CI | P value |
| Age ≥80 years+ventilation >48 hours | 1.40 | 1.21 to 1.62 | <0.001 |
| Age ≥80 years+diabetes mellitus | 1.16 | 1.00 to 1.34 | <0.001 |
| Age ≥80 years+previous myocardial infarction | 0.85 | 0.70 to 1.04 | <0.001 |
| Age ≥80 years+chronic limb-threatening ischaemia | 1.14 | 0.88 to 1.49 | <0.001 |
| Age ≥80 years+chronic kidney disease | 1.08 | 0.93 to 1.25 | <0.001 |
| Age ≥80 years+dementia | 1.48 | 1.24 to 1.76 | <0.001 |
P value ‘for comparisons of patients in all groups’.
Discussion
This German nationwide real-world analysis of patients treated with mechanical thrombectomy for acute ischaemic stroke represents the cohort with the largest number of patients aged ≥80 years and reports clinically valuable findings. First, outcomes after mechanical thrombectomy were strongly associated with age. Second, of patients aged 80 years and older more than half were dead and less than one-fifth were functionally independent at 1 year. Third, in patients aged ≥80 years with dementia, more than 80% were either dead or had moderate to severe disability at 1-year follow-up. Fourth, more than a quarter of patients who had a stroke aged ≥80 years with ventilation duration >48 hours were dead at 1-year follow-up.
Our findings provide thorough information regarding the association of age on long-term outcome after mechanical thrombectomy. The most recent analysis of pooled data from clinical trials of mechanical thrombectomy showed a clinically and statistically significant absolute reduction in mortality in patients aged ≥80 years.7 12 However, the power of these trials to probe for mortality effects in elderly patients was limited by the small sample size of patients aged 80 years or older. In addition, data from recent large clinical trials are limited to 3 months outcome data. Our population of 6729 patients who had an acute stroke aged ≥80 years treated with mechanical thrombectomy in the current study was substantially larger than the pooled clinical trial sample and outcome data were analysed for a follow-up period up to 9 years. Hence, with a larger dataset, the current study found statistically noticeable higher mortality rates and grades of disability associated with age. Of note, data obtained from previous studies are exclusively obtained from subgroup analyses.7 12
Older age has often been used as an exclusion criterion for mechanical thrombectomy, and in this respect, two randomised clinical trials on mechanical thrombectomy in patients with acute ischaemic stroke had an upper age limit.10 11 However, an ageing population leads to a higher frequency of age-related diseases such as dementia, cancer and stroke, in particular in patients with multimorbidity.15 Our data provide novel findings regarding the relation between certain comorbidities and patient characteristics with outcomes after acute ischaemic stroke. Our analysis of pretreatment factors validates the importance of age and comorbidities in poor outcomes among patients treated with mechanical thrombectomy. Moreover, our data identify the most important parameters associated to outcomes as diabetes, chronic limb-threatening ischaemia and dementia. We further identified ventilation >48 hours as an important posttreatment factor. Indeed, comorbidities and other clinical effect modifiers worsening outcome in elderly patients identified in our study are already known to have a significant impact on stroke outcome. For instance, in a recent cohort study, prolonged ventilation time (>24 hours) in patients undergoing mechanical thrombectomy for anterior circulation large vessel occlusion was associated with independently predicted unfavourable functional outcome at 3 months poststroke.16 Moreover, it has been shown that patients with diabetes and acute ischaemic stroke have more in-hospital complications, worse discharge outcomes, higher mortality and stroke recurrence at 90 days, compared with pre-diabetes and no diabetes.17 However, all of these studies were not primarily focusing on the association with older age. Surprisingly, certain pretreatment factors, such as dyslipidaemia, prior stroke and atrial fibrillation/flutter were associated with improved survival. The better outcome in patients with atrial fibrillation may be due, for example, to the fact that cardiogenic emboli can be removed more effectively by mechanical thrombectomy compared with vascular occlusions in other causes of stroke.18 A further potential reason for counterintuitive results might be that patients with these prediagnoses had undergone regular medical check-ups beforehand and were therefore better adjusted with regard to any risk factors for possible complications. Our data show, that frailty and disability are further cofactors regarding the neurological outcome in elderly patients. Patients over 80 years of age who have previously lived in a nursing home have twice the risk of mortality when mechanical thrombectomy is performed compared with patients without prior inpatient care. Therefore, the degree of previous disability or frailty is an important factor in the prognostic evaluation of mechanical thrombectomy in elderly patients.
According to current stroke guidelines, patients aged 80 years or older, with acute ischaemic stroke due to large vessel occlusion within 6 hours of symptom onset should be treated with mechanical thrombectomy plus best medical treatment, and those between 6 and 24 hours from time last known well should be treated with mechanical thrombectomy plus best medical treatment, if they meet the eligibility criteria of the DEFUSE-3 or DAWN trials.19 In randomised clinical trials, there was no evidence of reduced treatment effect in elderly patients with good or independent premorbid function and, moreover, a clinically and statistically significant reduction in mortality in stroke patients aged 80 years or older treated with mechanical thrombectomy was reported.12 Based on the data from our study, physicians should be aware of the association of age and comorbidities with mortality and neurological outcomes in elderly stroke patients when undergoing mechanical thrombectomy.
Strengths and limitations
The present study has several strengths, including a large sample size with 9-year follow-up data from the largest public German health insurance. For our data analysis, there are the generally recognised limitations of the retrospective study design, which harbour the risk of selection and information bias. In general, data were retrospective data not collected for research purpose. However, due to legal regulations and integrated monitoring systems, the reliability of the database is extremely high. A further limitation of our study is that some residual confounding by unmeasured factors cannot be excluded. Such factors are likely to be associated with the expectation of good outcome and thus with the decision to perform a mechanical thrombectomy. The spectrum of outcomes observed in our study must be interpreted against this background. The data reported are dependent on the accuracy and completeness of abstraction from the medical record. Therefore, the focus of our study was on ‘hard’ endpoints such as all-cause death, which is very unlikely to be incorrectly coded. Precise coding rules for main and secondary diagnoses as well as procedures were used in this field and have not been changed for more than 15 years in Germany and regarding the diagnoses analysed in this study. Since a complete coding is necessary for a correct, complete reimbursement of the treatment costs of a hospital, a possible undercoding should be considered unlikely. A further potential weakness is the lack of a control group of patients who had a stroke with large vessel occlusion without mechanical thrombectomy. However, data on the type of vessel occlusion in patients who had ischaemic stroke are not systematically recorded in the G-DRG system and are therefore not available. However, a direct comparison between patients who had a stroke treated and not treated with thrombectomy is not meaningful because these cohorts differ significantly with respect to the type of occlusion and other clinical parameters and thus the outcome.
Conclusions
In conclusion, this cohort study demonstrates that outcomes after mechanical thrombectomy were strongly associated with age. Patients aged 80 years and older undergoing mechanical thrombectomy were dead in more than half of the cases and less than one-fifth were functionally independent at 1-year follow-up compared with younger patients. Certain comorbidities and ventilation >48 hours were associated with even worse outcomes.
Footnotes
CB and JK contributed equally.
Contributors: CB, JK, HR and JM contributed to the conception and design of the study; CB, JK, JF, CLM, PD, TR and CG contributed to the acquisition and analysis of data; CB, JK, HW, HR and JM contributed to drafting the text or preparing the figures; all authors read and critically revised the manuscript. HR and JM These authors jointly supervised this work. JM acted as guarantor.
Funding: This project 'GenderVasc' on which this publication is based was funded by The Federal Joint Committee, Innovation Committee (G-BA, Innovationsfonds, number 01VSF18051. GenderVasc is a cooperation project with the AOK Research Institute of the AOK (WIdO).
Competing interests: CB has received speaking fees from Chugai Pharma. HW receives honoraria for acting as a member of Scientific Advisory Boards from Abbvie, Alexion, Argenx, Bristol Myers Squibb/Celgene, Janssen, Merck, Novartis and Sandoz. Speaker honoraria and travel support from Alexion, Biogen, Bristol Myers Squibb, Genzyme, Merck, Neurodiem, Novartis, Ology, Roche, TEVA and WebMD Global. HW is acting as a paid consultant for Abbvie, Actelion, Argenx, BD, Biogen, Bristol Myers Squibb, EMD Serono, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant. Janssen. Lundbeck, Merck, NexGen, Novartis, PSI CROl, Roche, Sanofi, Swiss Multiple Sclerosis Society, UCB and Worldwide Clinical Trials. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., Alexion. Amicus Therapeutics Inc., Argenx, Biogen, CSL Behring, F. Hoffmann - La Roche, Genzyme, Merck KgaA, Novartis, Roche Pharma and UCB Biopharma. HR received Consulting fees from PLURISTEM, NEOVASC; Payment honoraria for lectures, presentations from DAICHI SANKYIO, DIAPLAN, MEDUPDATE, STREAMEDUP, CORVIA; Participation in Advisory Boards from BMS, PFIZER, NOVONORDISK; the department received research grants from BARD, PFIZER, BMS. JM has received grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung (BMBF), Else Kröner-Fresenius-Stiftung, EVER Pharma Jena, and Ferrer International, travel grants from Boehringer Ingelheim, and speaking fees from Bayer Vital and Chugai Pharma.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The authors confirm that the data used in this study cannot be made available in the manuscript, the online supplemental files or in a public repository due to German data protection laws (‘Bundesdatenschutzgesetz’, BDSG). Data are stored on a secure drive in the AOK Research Institute (WIdO), to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the sickness fund. For assistance in obtaining access to the data, please contact wido@wido.bv.aok.de.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee of the Landesaerztekammer Westfalen-Lippe and the Medical Faculty of the University of Muenster (No 2019-21-f-S). Participants gave informed consent to participate in the study before taking part.
References
- 1. Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–47. 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 2. Mocco J, Zaidat OO, von Kummer R, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke 2016;47:2331–8. 10.1161/STROKEAHA.116.013372 [DOI] [PubMed] [Google Scholar]
- 3. Muir KW, Ford GA, Messow C-M, et al. Endovascular therapy for acute ischaemic stroke: the pragmatic ischaemic stroke thrombectomy evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry 2017;88:38–44. 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 6. Khoury NN, Darsaut TE, Ghostine J, et al. Endovascular thrombectomy and medical therapy versus medical therapy alone in acute stroke: a randomized care trial. J Neuroradiol 2017;44:198–202. 10.1016/j.neurad.2017.01.126 [DOI] [PubMed] [Google Scholar]
- 7. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 8. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 9. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 10. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 11. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 12. Campbell BCV, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy. Stroke 2016;47:798–806. 10.1161/STROKEAHA.115.012360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Health FM of . The german healthcare system. Strong. Reliable. Proven. 2020. [Google Scholar]
- 14. Long-term care guide - bundesgesundheitsministerium · PDF file with a view to the 2020s, when baby boomers will retire, we have initiated a large number of… which long-term care [PDF document]. fdocuments.in. Available: https://fdocuments.in/document/long-term-care-guide-bundesgesundheitsministerium-with-a-view-to-the-2020s-when.html [Accessed 17 Jul 2022].
- 15. Rechel B, Grundy E, Robine J-M, et al. Ageing in the European Union. Lancet 2013;381:1312–22. 10.1016/S0140-6736(12)62087-X [DOI] [PubMed] [Google Scholar]
- 16. Fandler-Höfler S, Heschl S, Kneihsl M, et al. Ventilation time and prognosis after stroke thrombectomy: the shorter, the better! Eur J Neurol 2020;27:849–55. 10.1111/ene.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akhtar N, Kamran S, Singh R, et al. The impact of diabetes on outcomes after acute ischemic stroke: a prospective observational study. J Stroke Cerebrovasc Dis 2019;28:619–26. 10.1016/j.jstrokecerebrovasdis.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 18. Lin C-J, Luo C-B, Chien C, et al. Better endovascular mechanical thrombectomy outcome in atrial fibrillation patients with acute ischemic stroke: a single-center experience. J Chin Med Assoc 2020;83:756–60. 10.1097/JCMA.0000000000000377 [DOI] [PubMed] [Google Scholar]
- 19. Turc G, Bhogal P, Fischer U, et al. European stroke organisation (ESO)-European Society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by stroke alliance for Europe (safe). Eur Stroke J 2019;4:6–12. 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330506supp001.pdf (757.8KB, pdf)
Data Availability Statement
A detailed description of data accessibility is provided in online supplemental methods.
Data are available on reasonable request. The authors confirm that the data used in this study cannot be made available in the manuscript, the online supplemental files or in a public repository due to German data protection laws (‘Bundesdatenschutzgesetz’, BDSG). Data are stored on a secure drive in the AOK Research Institute (WIdO), to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the sickness fund. For assistance in obtaining access to the data, please contact wido@wido.bv.aok.de.