Abstract
Objective
To examine if individual-level and area-level socioeconomic status (SES) modifies the association of moderate-to-vigorous physical activity (MVPA), domain-specific physical activity and sedentary behaviour with all-cause mortality (ACM) and incident cardiovascular disease (CVD).
Methods
We used self-reported (International Physical Activity Questionnaire short form) and accelerometer-measured physical activity and sedentary behaviour data from the UK Biobank. We created an individual-level composite SES index using latent class analysis of household income, education and employment status. The Townsend Index was the measure of area-level SES. Cox proportional hazards regression models stratified across SES were used.
Results
In 328 228 participants (mean age 55.9 (SD 8.1) years, 45% men) with an average follow-up of 12.1 (1.4) years, 18 033 deaths and 98 922 incident CVD events occurred. We found an increased ACM risk of low physical activity and high sedentary behaviour and an increased incident CVD risk of low accelerometer-measured moderate-to-vigorous physical activity (ACCEL_MVPA) and high sitting time. We observed statistically significant interactions for all exposures in ACM analyses by individual-level SES (p<0.05) but only for screen time in area-level SES–ACM analysis (p<0.001). Compared with high self-reported moderate-to-vigorous physical activity (IPAQ_MVPA), adjusted ACM HRs for low IPAQ_MVPA were 1.14 (95% CI 1.05 to.25), 1.15 (95% CI 1.06 to 1.24) and 1.22 (95% CI 1.13 to 1.31) in high, medium and low individual-level SES, respectively. There were higher detrimental associations of low ACCEL_MVPA with decreasing area-level SES for both outcomes and of high screen time with ACM in low area-level SES.
Conclusion
We found modest evidence suggesting that the detrimental associations of low MVPA and high screen time with ACM and incident CVD are accentuated in low SES groups.
Keywords: Physical activity, Sedentary Behavior
What is already known on this topic?
Low socioeconomic status (SES) groups have a higher prevalence of unhealthy lifestyles and may suffer disproportionate harm.
Studies incorporating composite SES index, multiple domains of physical activity, sedentary behaviour and use of both self-report and device-measured assessments are limited.
What are the new findings?
Our results suggest that there is a stronger inverse association of self-reported moderate-to-vigorous physical activity (MVPA) with all-cause mortality (ACM) in low compared with high individual-level SES groups.
We found higher detrimental associations of low accelerometer-measured moderate-to-vigorous physical activity with ACM and incident cardiovascular disease in low area-level SES; patterns were less clear for individual-level SES.
The detrimental associations of high self-reported screen time with ACM were stronger in low area-level SES.
Effect modification by SES was less clear for physical activity domains and sitting time.
How might it impact on clinical practice in the future?
We recommend primary prevention interventions that tackle physical inactivity and excessive sedentary behaviour be tailored to the needs of low SES groups.
Considering the variability in the interaction effects across SES measures, it may be important to target both low individual-level and area-level SES groups.
Background
Socioeconomic inequalities in health are a global challenge.1 2 They signify a range of differences in socioeconomic status (SES) as determined by an individual’s economic and social position in relation to others, based on income, education, employment status or occupation and ethnicity.1 3 Generally, individuals of low SES or those living in low socioeconomic areas have a higher prevalence of detrimental health-related behaviours4 and may have less favourable health outcomes such as higher morbidity and mortality.1 5–8 Even for a similar level of exposure to risk factors, low SES groups may suffer worse overall health outcomes (a phenomenon termed as vulnerability hypothesis).9 10 Overall, low SES may increase both exposure to chronic disease risk factors and increase the vulnerability of morbidity and impaired health on exposure.11 12
The relationships between individual-level and area-level SES, physical activity and sedentary behaviour have been extensively researched. Self-reported leisure-time physical activity (LTPA) is positively associated with high individual (education,13 employment,14 income13) and area-level SES.13 15 Studies using device-measured physical activity, which captures leisure time as well as occupational and incidental physical activity, have shown both direct16 17 and inverse18 associations between physical activity and SES. Of the various SES measures used, some of the most consistent positive associations with physical activity are reported for education.19 The detrimental associations of physical inactivity and sedentary behaviour with higher risks of cardiovascular diseases (CVDs) and premature mortality are also well established.20–22
In considering how to reduce socioeconomic inequalities in health, it is important to understand the interaction between SES and health behaviours in jointly determining future health outcomes.23 24 The scant evidence on the association between SES, physical activity, sedentary behaviour and health outcomes is unclear5 6 and less consistent between studies employing self-report and objective physical activity measures.25 For example, a previous study reported more consistent and stronger associations of education and occupational social class with device-measured physical activity than with self-report.25 In a UK Biobank analysis, Foster et al 5 found a significant interaction between a composite lifestyle behaviour score and area-level SES (Townsend Index) for risk of all-cause mortality (ACM) and CVD mortality, but not CVD incidence.5 Compared with the most healthy lifestyle, the association of the least healthy lifestyle with ACM was more pronounced in lower area-level SES.5 Another recent study reported lower ACM and CVD risk among groups with healthy lifestyles, with stronger associations among low individual-level SES.6 Both studies used composite lifestyle scores comprising multiple behavioural factors (eg, alcohol, smoking and diet).5 6 The physical activity component was limited to self-reported moderate-to-vigorous physical activity (MVPA)5 or LTPA,6 and sedentary behaviour was limited to television (TV) viewing time,5 which is a poor proxy of overall sedentary time.26
Social patterning (differences across the SES spectrum) in physical activity is more prominent for physical activity domains (eg, transportation, occupational, household and leisure-time) than for total physical activity.27 28 For example, European adults from high SES participate mostly in LTPA.28 In contrast, adults from low SES mostly participated in occupational physical activity, while no variations by SES were observed for total physical activity and active commuting.28 Another study reported higher device-measured sedentary behaviour and lower TV viewing among higher SES.29 No studies, to our knowledge, have examined how SES modifies the association of multiple domains of self-reported and device-measured physical activity and sedentary behaviour with mortality and incident CVD. Differential reporting bias could be more crucial in the context of SES, with another UK cohort (Whitehall II) reporting a weaker correlation between self-reported and device-measured physical activity data in low SES than in high SES groups and for moderate-intensity activities than vigorous activities.30 In another study, Gorzelitz et al concluded that discordance between self-reported and device-measured physical activity data was inversely correlated to educational level.31 Accelerometry devices can capture very short bouts of MVPA as well as lower-intensity activities performed in any domain and overcome other important limitations of self-report measurements (eg, recall or social desirability bias).32 33 However, motion sensor devices such as accelerometers cannot capture domain-specific activities and can be logistically challenging to implement in low-resource settings due to higher time and resource requirements.34 Using both self-reported and device-measured physical activity is recommended for a more complete understanding of the associations of physical activity with prospective health outcomes.34 Further, understanding the role of SES in determining the associations of total physical activity, domains and sedentary behaviour with health outcomes is essential to narrow health disparities, a gap identified by the 2020 WHO Guideline Development Group.34
The primary aim of this study was to examine whether individual-level SES modifies the association of total and domain-specific physical activity and sedentary behaviour with ACM and incident CVD. The secondary aim was to examine the same effect modification by area-level SES. We hypothesised that the detrimental associations of low physical activity and high sedentary behaviour with outcomes would be stronger in low SES (vulnerability hypothesis).
Methods
Study design and participants
We used data from the UK Biobank, a prospective, population-based cohort study that recruited adults aged 40–69 years between 2006 and 2010.35 We excluded participants with missing covariates, socioeconomic information or exposures; poor self-rated health; prevalent CVD (self-reported or hospital admission); or an event (death or CVD event) within 2 years of recruitment (online supplemental figures S1 and S2).
bjsports-2022-105435supp001.pdf (639.1KB, pdf)
Exposures
Online supplemental text S1 provides full descriptions of the exposure variables. Here, we summarise their main attributes:
Questionnaire-based physical activity: Weekly self-reported moderate-to-vigorous physical activity (IPAQ_MVPA) was measured using an adaption of the International Physical Activity Questionnaire short form.36 It has moderate validity (r=0.52) for measuring MVPA among adults in the UK compared with accelerometer data.37 Such correlations with accelerometry are higher than most other self-reported instruments.38 We calculated total weekly IPAQ_MVPA volume (metabolic equivalent (MET)-minutes/week; number of minutes/week×standardised MET value of walking, and moderate and vigorous activities) and categorised participants into three groups: low (<600 MET-min/week), medium (600–3000 MET-min/week) and high (≥3000 MET-min/week).36
Device-measured physical activity: Accelerometer-measured moderate-to-vigorous physical activity (ACCEL_MVPA) was derived in a subsample of participants using data from the Axivity AX3 accelerometer worn on their dominant wrist for 24 hours/day for 1 week.39 We used previously established procedures40 41 to calibrate data and identify non-wear and only included participants with at least four valid monitoring days (at least one of those days being a weekend). We used a previously validated machine learning activity recognition scheme that uses raw acceleration signals to identify and quantify time spent in different intensities in 10 s windows.42 Using the total weekly time spent in ACCEL_MVPA, we classified participants into tertiles for this study. The use of tertiles provided the optimal balance between physical activity exposure resolution and exposure group size.
Domain-specific physical activity: Weekly household physical activity volume was based on frequency and duration of light and heavy do-it-yourself activities (such as home maintenance, gardening, digging, carpentry, etc) and categorised into tertiles. Weekly LTPA volume was based on the frequency and duration of walking for pleasure, other exercises and strenuous sports43 and categorised into tertiles.
Sedentary behaviour: The study includes two forms of sedentary behaviours: accelerometer-measured sitting time and self-reported screen time. We categorised participants into tertiles of total weekly sitting time using the information from the Axivity AX3 accelerometer using the same process defined earlier. We created ‘screen time’ tertiles using self-reported daily hours spent watching TV and non-occupational computer use.44
Outcomes
We examined associations with ACM and incident CVD. Incident CVD was defined as an event (fatal or non-fatal attributed to International Classification of Diseases, 10th Revision (codes I00–I99)), after baseline assessment. Participants were followed up until an event or censoring (30 September 2021 for England/Wales and 31 October 2021 for Scotland due to rolling data linkage updates).
Effect modifiers
Online supplemental Text S2 and Table S1 provide detailed descriptions of the socioeconomic indices. In brief, we examined effect modification by two composite socioeconomic indices: individual-level SES index and area-level SES (Townsend Index).45 The individual-level composite SES index was created using latent class analysis of three socioeconomic variables (household income, education and employment status)6 and categorised as high, medium and low SES (online supplemental text S2). Since the model with four latent classes failed to converge, we used the model with three latent classes. ‘High SES’ had a higher proportion of participants with college or university degree and before tax household income of £52 000 or greater (see online supplemental Table S1). The proportion of unemployed, those with less than high school education (labelled as ‘none’ in the UK Biobank) and those with household income less than £18 000 were higher in class labelled ‘low SES’. The Townsend Index is derived from the respondent’s postcode and reflects unemployment, non-car ownership, non-home ownership and household overcrowding.45 We categorised it into thirds using tertiles, where the lowest third indicated high area-level SES.
Covariates
Online supplemental table S2 provides complete descriptions of the covariates. We selected variables a priori from the relevant literature.5 6 We adjusted analyses for sex, ethnicity, sleep score (derived using morning chronotype, sleep duration, insomnia, snoring and daytime sleepiness),46 dietary pattern score (from the intake of fruits, vegetables, fish, red meat and processed meat),47 smoking and alcohol consumption.
Statistical analysis
We used multivariable-adjusted Cox proportional hazards regression stratified by socioeconomic indices, with age (scaled in years) as the underlying time scale. To address the impact of reverse causality, we have excluded the initial 2 years of follow-up and any events within it.5 39 48 49 The reference groups were the optimum category/tertile of the exposure variables (high physical activity/low sedentary behaviour). Model 1 (main effects) for all exposures was adjusted for the aforementioned covariates, Townsend Index and education. For IPAQ_MVPA and LTPA analyses, we additionally adjusted for screen time; screen time analyses were adjusted for IPAQ_MVPA; ACCEL_MVPA analyses were adjusted for sitting time and vice versa; household physical activity analyses were adjusted for LTPA and screen time. There was no evidence of multicollinearity between the variables entered in the model (variation inflation factor ≤1.16).
Multiplicative interaction terms between exposures and individual-level and area-level SES were included in models 2 and 3, respectively. We evaluated interactions between exposures (physical activity/sedentary behaviour) and socioeconomic indices using likelihood ratio tests comparing models with and without a cross-product term. The p value for interaction was obtained using continuous variables. Proportional hazard assumption was tested using Schoenfeld residuals50 and was satisfied. For CVD incidence analyses, we used the Fine and Grey subdistribution method51 to account for competing risks (non-CVD related deaths).
We conducted several sensitivity analyses. First, we additionally adjusted ACM models stratified by individual-level SES for body mass index (BMI). Second, we repeated ACM models for physical activity exposures by adjusting for self-rated health instead of excluding those with poor self-rated health. Third, we excluded the first 3 years of follow-up and events within these years to reduce potential reverse causation.6 To further check the sensitivity of the estimates, we calculated E-values that indicate the strength of association an unmeasured confounder would need to have with exposure and outcome to explain away the observed exposure–outcome association.52 All analyses were performed using Stata/MP V.17.0, with two-sided p values of <0.05 considered statistically significant. Study reporting conforms to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines53 (see online supplemental STROBE checklist).
Patient and public involvement
Patients and the public were not involved in the design or conduct of this study.
Results
Sample characteristics
We analysed data from 328 228 participants (mean age 55.9 (8.1) years, 45% men). The low, medium and high IPAQ_MVPA levels consisted of 15%, 48.6% and 36.4% participants. Over the mean follow-up period of 12.2 (1.4) years (3 922 258 person-years), 18 033 deaths and 98 922 incident CVD events occurred. Participant characteristics across IPAQ_MVPA and ACCEL_MVPA levels are presented in table 1 and online supplemental table S3). Online supplemental table S4 shows the distribution of exposure variables across individual-level SES.
Table 1.
Baseline characteristics of participants stratified by level of self-reported MVPA (n=328 228)
| Characteristics | Total population (N=328 228) | IPAQ_MVPA (n=3 10 499) | |||
| High (n=113 053) | Medium (n=150 763) | Low (n=46 683) | P value | ||
| Mean age (SD) (years) | 55.9±8.1 | 56.2±8.2 | 55.6±8.1 | 55.1±7.8 | <0.001 |
| Men | 148 522 (45.2) | 52 285 (46.2) | 68 212 (45.2) | 21 873 (46.9) | <0.001 |
| White ethnicity or race | 313 783 (95.6) | 108 619 (96.1) | 144 313 (95.7) | 44 393 (95.1) | <0.001 |
| Household income (£) | <0.001 | ||||
| Less than 18 000 | 65 250 (19.9) | 25 634 (22.7) | 25 367 (16.8) | 7469 (16.0) | |
| 18 000–30 999 | 82 782 (25.2) | 31 830 (28.2) | 35 434 (23.5) | 10 351 (22.2) | |
| 31 000–51 999 | 88 932 (27.1) | 30 340 (26.8) | 41 676 (27.6) | 13 256 (28.4) | |
| 52 000–100 000 | 71 789 (21.9) | 20 335 (18.0) | 37 255 (24.7) | 12 388 (26.5) | |
| Greater than 100 000 | 19 475 (5.9) | 4914 (4.3) | 11 031 (7.3) | 3219 (6.9) | |
| Education | <0.001 | ||||
| None | 43 483 (13.2) | 18 350 (16.2) | 14 578 (9.7) | 4640 (9.9) | |
| O/CSE or equivalent | 88 309 (26.9) | 33 348 (29.5) | 37 505 (24.9) | 12 432 (26.6) | |
| A/NVQ/professional or equivalent | 77 006 (23.5) | 27 462 (24.3) | 34 761 (23.1) | 11 006 (23.6) | |
| College/university | 119 430 (36.4) | 33 893 (30.0) | 63 919 (42.4) | 18 605 (39.9) | |
| Employment | <0.001 | ||||
| Employed | 311 760 (95.0) | 107 396 (95.0) | 143 745 (95.3) | 44 382 (95.1) | |
| Unemployed | 16 468 (5.0) | 5657 (5.0) | 7018 (4.7) | 2301 (4.9) | |
| Townsend Index tertile | <0.001 | ||||
| First | 111 076 (33.8) | 36 884 (32.6) | 52 896 (35.1) | 16 539 (35.4) | |
| Second | 110 210 (33.6) | 38 274 (33.9) | 50 534 (33.5) | 15 760 (33.8) | |
| Third | 106 942 (32.6) | 37 895 (33.5) | 47 333 (31.4) | 14 384 (30.8) | |
| Smoking status | <0.001 | ||||
| Never | 182 037 (55.5) | 61 552 (54.4) | 85 281 (56.6) | 25 936 (55.6) | |
| Previous | 113 664 (34.6) | 39 835 (35.2) | 52 203 (34.6) | 15 601 (33.4) | |
| Current | 32 527 (9.9) | 11 666 (10.3) | 13 279 (8.8) | 5146 (11.0) | |
| Alcohol status | <0.001 | ||||
| Never | 11 384 (3.5) | 3859 (3.4) | 4634 (3.1) | 1742 (3.7) | |
| Previous | 9530 (2.9) | 3520 (3.1) | 3893 (2.6) | 1357 (2.9) | |
| Current | 307 314 (93.6) | 105 674 (93.5) | 142 236 (94.3) | 43 584 (93.4) | |
| Sleep pattern | <0.001 | ||||
| Poor | 22 062 (6.7) | 7168 (6.3) | 9361 (6.2) | 3752 (8.0) | |
| Intermediate | 185 713 (56.6) | 62 458 (55.2) | 84 495 (56.0) | 28 091 (60.2) | |
| Healthy | 120 453 (36.7) | 43 427 (38.4) | 56 907 (37.7) | 14 840 (31.8) | |
| Diet pattern | <0.001 | ||||
| Poor | 20 120 (6.1) | 6314 (5.6) | 8370 (5.6) | 4150 (8.9) | |
| Reasonable | 201 082 (61.3) | 66 724 (59.0) | 92 368 (61.3) | 30 747 (65.9) | |
| Good | 107 026 (32.6) | 40 015 (35.4) | 50 025 (33.2) | 11 786 (25.2) | |
| Body mass index | <0.001 | ||||
| Normal weight | 112 801 (34.4) | 41 601 (36.8) | 53 460 (35.5) | 12 892 (27.6) | |
| Overweight | 141 884 (43.2) | 49 178 (43.5) | 65 555 (43.5) | 19 801 (42.4) | |
| Obesity | 73 543 (22.4) | 22 274 (19.7) | 31 748 (21.1) | 13 990 (30.0) | |
| Self-rated health | |||||
| Excellent | 61 350 (18.7) | 24 460 (21.6) | 29 240 (19.4) | 5966 (12.8) | <0.001 |
| Good | 201 826 (61.5) | 69 432 (61.4) | 94 153 (62.5) | 27 827 (59.6) | |
| Fair | 65 052 (19.8) | 19 161 (16.9) | 27 370 (18.2) | 12 890 (27.6) | |
Participants’ self-reported moderate-to-vigorous physical activity (IPAQ_MVPA) measured using the IPAQ was categorised as low (<600 MET-min/week), medium (600–<3000 MET-min/week), and high (≥3000 MET-min/week). Townsend Index (including measures of unemployment, non-car ownership, non-home ownership and household overcrowding), derived from respondents’ postcode was used as an indicator of area-level SES. We categorised Townsend Index into tertiles where the lowest score indicated the highest area-level SES. Employment status is categorised as employed (includes paid employment or self-employed, retired, paid or voluntary work or student) and unemployed (includes looking after home and/or family, unable to work and unemployed). O/CSE or equivalent is O level/ Certificate of Secondary Education or equivalent. A/NVQ/professional or equivalent is A level/ National Vocational Qualification, other professional qualifications such as nursing, teaching or equivalent. Sleep pattern is derived using sleep duration, chronotype, insomnia, snoring and dozing. Diet pattern is derived using intake of fruits and vegetables, fish (oily and non-oily), red meat (beef, pork and lamb) and proceeded meat intake. Body mass index is categorised as normal weight (18.5–<25 kg/m2), overweight (25.0–<30 kg/m2) and obesity (≥ 30 kg/m2).
Values in the table are frequencies and percentages unless otherwise stated. Differences between groups was tested using one-way analysis of variance for age and using χ2 test for other variables.
IPAQ, International Physical Activity Questionnaire; IPAQ_MVPA, self-reported moderate-to-vigorous physical activity; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity; SES, socioeconomic status.
All-cause mortality
Whole sample
We found detrimental associations of low IPAQ_MVPA (HR 1.15, 95% CI 1.10 to 1.20), low ACCEL_MVPA (HR 1.62, 95% CI 1.39 to 1.89) and low household physical activity (HR 1.06, 95% CI 1.01 to 1.12) with ACM (online supplemental table S5). The HRs for mortality were higher among participants in medium and lowest tertiles of LTPA, compared with those in the highest LTPA tertile. Participants in the highest screen time and sitting time tertile were at 12% (9%–17%) and 19% (2%–39%) higher hazard of mortality than those in the lowest tertile, respectively (online supplemental table S5). For individual-level SES, we observed significant likelihood ratio tests (p<0.05) for all exposures. The multiplicative interaction term was only significant for screen time (p value for screen time×area-level SES<0.001).
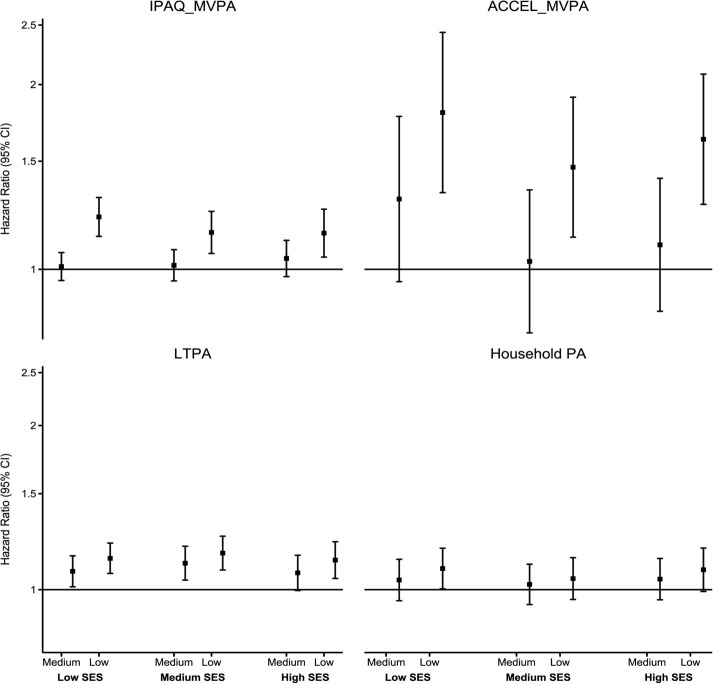
Stratified by individual-level SES
Figure 1 shows the stratified association of MVPA and domain-specific physical activity with ACM across individual-level SES. There was no statistically significant association of medium IPAQ_MVPA and ACCEL_MVPA with ACM across all levels of individual-level SES. However, there was a stronger detrimental association of low IPAQ_MVPA with ACM in low SES. For example, compared with high IPAQ_MVPA, ACM HRs for low IPAQ_MVPA were 1.14 (95% CI 1.05 to 1.25) in high SES, 1.15 (95% CI 1.06 to 1.24) in medium SES and 1.22 (95% CI 1.13 to 1.31) in low SES. We observed no clear individual-level SES gradient in the associations of ACCEL_MVPA with ACM, though there was a slightly more pronounced detrimental association of low ACCEL_MVPA in low SES. HRs for low ACCEL_MVPA were 1.80 (95% CI 1.33 to 2.43) in low SES 1.47 (95% CI 1.13 to 1.91) in medium SES and 1.67 (95% CI 1.27 to 2.08) in high SES. Low LTPA was inversely associated with mortality in all groups, with less clear SES patterning. We observed some evidence of higher mortality HRs of medium LTPA among low and medium SES groups only (HR 1.07, 95% CI 0.99 to 1.16 in high SES; HR 1.12, 95% CI 1.04 to 1.20 in medium SES; and HR 1.08, 95% CI 1.01 to 1.15 in low SES). There was no association of household physical activity with ACM across SES groups (figure 1).
Figure 1.
Association of PA with all-cause mortality across individual-level SES. Small squares denote point estimates of the HR, and the bars indicate 95% CIs. Reference: high PA. Y axis is in log scale. Individual-level SES was created using latent class analysis of three socioeconomic factors (household income, education and employment status) and categorised into low, medium and high. IPAQ_MVPA: participants’ PA measured using the IPAQ was categorised as low (<600 MET-min/week), medium (600–< 3000 MET-min/week) and high (≥3000 MET-min/week). Low SES: high IPAQ_MVPA (2882/32 501), medium IPAQ_MVPA (2751/30 856,1.01 (0.96–1.06)), low IPAQ_MVPA (997/9208, 1.22 (1.13–1.31)). Medium SES: High (2088/46 120), medium (2447/53 386, 1.01 (0.96–1.08)), low (892/17 658, 1.15 (1.06–1.24)). High SES: high (1280/34 432), medium (2426/66 521, 1.04 (0.97–1.11)), low (784/19 817, 1.14 (1.05–1.25)) ACCEL_MVPA: device-measured total PA was measured using the Axivity AX3 triaxial accelerometer worn on the participant’s dominant wrist for a 7-day period. The total number of minutes spent on MVPA (a sum of moderate and vigorous activities) was extracted and categorised into tertile-based thirds. ‘Low’ indicates the first tertile; ‘medium’ indicates the second tertile; and ‘high’ indicates the third tertile. Low SES: high ACCEL_MVPA (70/2695), medium ACCEL_MVPA (109/2884, 1.30 (0.95–1.77)), low ACCEL_MVPA (194/3407, 1.80 (1.33–2.43). Medium SES: high (103/6461), medium (129/6521, 1.03 (0.79–1.35)), low (211/6275, 1.47 (1.13–1.91)). High SES: high (121/9330), medium (142/8699, 1.10 (0.85–1.41)), low (229/7726, 1.67 (1.27–2.08)). LTPA was calculated using the frequency and duration of walking for pleasure, other exercises and strenuous sports in the last 4 weeks and was categorised into tertile-based thirds. Low SES: high LTPA (1811/21 186), medium LTPA (1816/20 970, 1.08 (1.01–1.15)), low LTPA (2041/22 726, 1.14 (1.07–1.22)). Medium SES: high (1430/33 481), medium (1606/35 277, 1.12 (1.04–1.20)), low (1671/36 695, 1.17 (1.09–1.25)). High SES: high (1365/39 621), medium (1428/39 546, 1.07 (0.99–1.16)), low (1252/33 835, 1.13 (1.05–1.22)). Household PA was assessed by asking participants the frequency and duration of light and heavy do-it-yourself activities in the last 4 weeks and categorised into tertile-based thirds. Low SES: high household PA (1419/15 351), medium household PA (1323/14 910, 1.04 (0.95–1.14)), low household PA (1578/16 931, 1.09 (1.00–1.19)). Medium SES: high (1349/26 268), medium (1266/27 675, 1.02 (0.94–1.11)), low (1185/26 505, 1.05 (0.96–1.14)). High SES: high (1153/27 564), medium (1175/31 809, 1.04 (0.96–1.14)), low (1006/28 341, 1.09 (0.99–1.19)). ACCEL_MVPA, accelerometer-measured moderate-to-vigorous physical activity; IPAQ, International Physical Activity Questionnaire; IPAQ_MVPA, self-reported moderate-to-vigorous physical activity; LTPA, leisure-time physical activity; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity; PA, physical activity; SES, socioeconomic status.
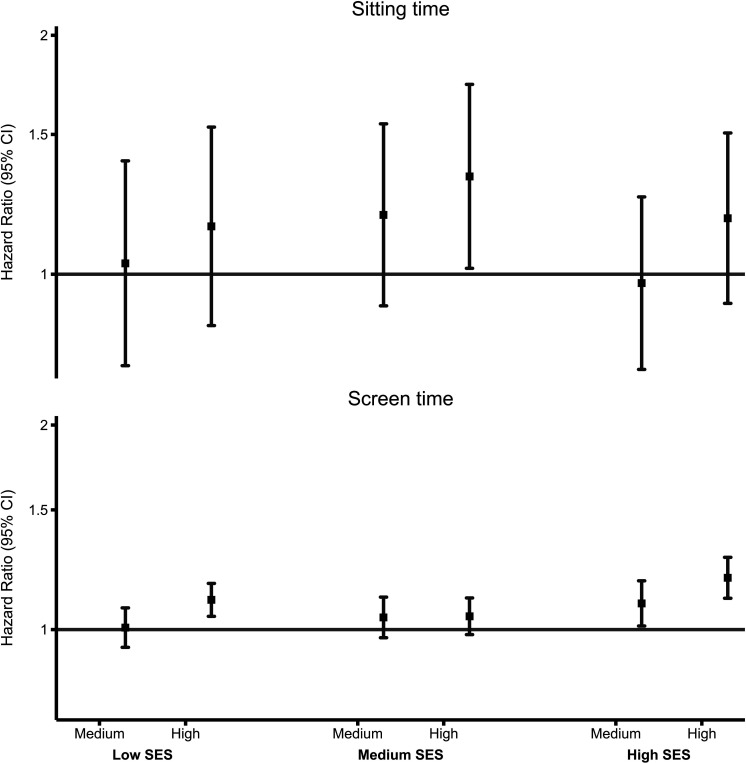
We found no evidence of association of sitting time with ACM across all individual-level SES groups (except the highest tertile in medium SES (HR 1.33, 95% CI 1.02 to 1.73) (figure 2). High screen time was detrimentally associated with ACM only among low and high SES groups, with a more pronounced association in high SES. For example, compared with low screen time, ACM HRs for high screen time were 1.10 (95% CI 1.04 to 1.17) in low SES, 1.04 (95% CI 0.98 to 1.11) in medium SES and 1.19 (95% CI 1.11 to 1.28) in high SES (figure 2).
Figure 2.
Association of sedentary behaviour with all-cause mortality across individual-level SES. Small squares denote point estimates of the HR, and the bars indicate 95% CIs. ‘Low’ indicates the first tertile; ‘medium’ indicates the second tertile; and ‘high’ indicates third tertile. Reference: lowest/first tertile; Y axis is in log-scale. Individual-level SES was created using latent class analysis of three socioeconomic factors (household income, education and employment status) and was categorised into low, medium and high. Sitting time: device-measured sitting time was measured using the Axivity AX3 triaxial accelerometer worn on participant’s dominant wrist for a 7-day period. The total number of minutes of sitting time was extracted and categorised into tertile-based thirds. Low SES: low sitting time (79/2735), medium sitting time (107/2820, 1.03 (0.77–1.39)), high sitting time (187/3431, 1.15 (0.86–1.53)). Medium SES: low (101/7026), medium (140/6340, 1.19 (0.91–1.55)), high (202/5891, 1.33 (1.02–1.73)). High SES: low (114/8394), medium (146/8918, 0.97 (0.76–1.25)), high (232/8443, 1.18 (0.92–1.51)) Screen time: Screen time was derived using daily hours spent watching television and non-occupational and categorised into tertile-based thirds. Low SES: low screen time (2488/30 194), medium screen time (1628/17 857, 1.01 (0.94–1.08)), high screen time (3493/33 018, 1.10 (1.04–1.17)). Medium SES: low (2278/55 968), medium (1401/29 164, 1.04 (0.97–1.12)), high (2096/38 388; 1.04 (0.98–1.11)). High SES: low (2251/71 359), medium (984/23 852, 1.09 (1.01–1.18)), high (1404/28 317, 1.19 (1.11–1.28)). SES, socioeconomic status.
Results were largely consistent with the main models when we further adjusted individual-level SES models of physical activity (online supplemental figure S3) and sedentary behaviour (online supplemental figure S4) for BMI. When we adjusted the main physical activity models for self-rated health (instead of excluding participants with poor self-rated health), the detrimental associations of low IPAQ_MVPA and low LTPA with ACM were attenuated in medium and high SES (online supplemental figure S5). Removing the first 3 years of follow-up did not appreciably change the results obtained in the main analysis (online supplemental figure S6).
Stratified by area-level SES
Low IPAQ_MVPA and ACCEL_MVPA were associated with higher ACM risk in all area-level SES groups (online supplemental figure S7). We observed higher ACM HRs of low ACCEL_MVPA in low and medium SES. For example, HRs for low ACCEL_MVPA were 1.78 (95% CI 1.36 to 2.29), 1.71 (95% CI 1.31 to 2.25) and 1.41 (95% CI 1.08 to 1.84) in low, medium and high area-level SES groups, respectively. The detrimental associations of medium and low tertiles of LTPA were more pronounced in medium SES. We found clear detrimental associations of low household physical activity in the low SES group only (online supplemental figure S7).
We observed a clear gradient of stronger detrimental associations of screen time with ACM with decreasing area-level SES (online supplemental figure S8). For example, compared with the lowest screen time tertile, ACM HRs for high screen time were 1.07 (95% CI 1.01 to 1.14) in high, 1.13 (95% CI 1.06 to 1.20) in medium and 1.22 (95% CI 1.15 to 1.29) in low SES groups. There was no association of sitting time with ACM across all area-level SES groups.
Incident CVD
Whole sample
Compared with high ACCEL_MVPA, participants in medium (HR 1.11, 95% CI 1.05 to 1.17) and lowest (HR 1.14, 95% CI 1.07 to 1.21) tertiles were at an increased incident CVD risk. Our results showed detrimental associations of the highest sitting time tertile (HR 1.11, 95% CI 1.05 to 1.18) with incident CVD (online supplemental table S5). We did not find statistically significant associations of self-reported physical activity and sedentary behaviour exposures with incident CVD. The multiplicative interaction term was not significant for all exposures.
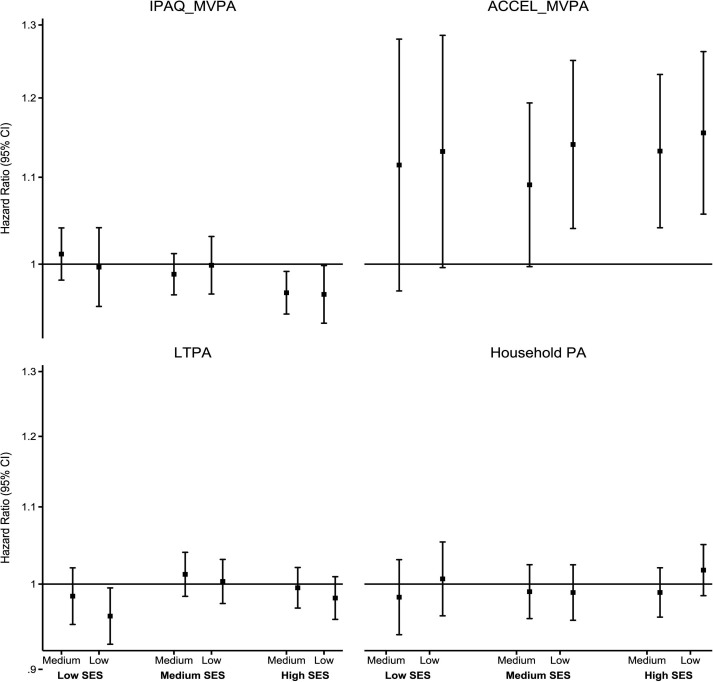
Stratified by individual-level SES
Figure 3 shows the stratified association of MVPA and domain-specific physical activity with incident CVD across individual-level SES. The individual-level SES patterns of the association of IPAQ_MVPA with incident CVD were less clear. We observed clear detrimental associations of the lowest tertile of ACCEL_MVPA in medium and high SES and that of medium tertile in high SES only. For example, HRs for low ACCEL_MVPA were 1.13 (95% CI 0.99 to 1.28) in low SES, 1.14 (95% CI 1.04 to 1.25) in medium SES and 1.15 (95% CI 1.06 to 1.26) in high SES, respectively. There was no association of LTPA and household physical activity with incident CVD across SES groups (figure 3).
Figure 3.
Association of PA with incident CVD across individual-level SES. Small squares denote point estimates of the sub-HR, and the bars indicate 95% CI. Reference: high PA; Y axis is in log scale. Individual-level SES was created using latent class analysis of three socioeconomic factors (household income, education and employment status) and was categorised into low, medium and high. IPAQ_MVPA: participants’ PA measured using the IPAQ was categorised as low (<600 MET-min/week), medium (600–<3000 MET-min/week) and high (≥3000 MET-min/week). Low SES: high IPAQ_MVPA (9612/29 796), medium IPAQ_MVPA (9155/28 142, 1.01 (0.98–1.04)), low IPAQ_MVPA (2672/8276, 1.00 (0.95–1.04)). Medium SES: high (13 948/43 887), medium (15 994/50 705, 0.99 (0.97–1.01)), low (5375/16 783, 1.00 (0.97–1.03)). High SES: high (10 658/33 107), medium (20 215/64 038, 0.97 (0.95–0.99)), low (6083/19 105, 0.97 (0.94–1.00)). ACCEL_MVPA: device-measured total PA was measured using the Axivity AX3 triaxial accelerometer worn on the participant’s dominant wrist for a 7-day period. The total number of minutes spent on MVPA (a sum of moderate and vigorous activities) was extracted and categorised into tertile-based thirds. ‘Low’ indicates the first tertile; ‘medium’ indicates the second tertile; and ‘high’ indicates third tertile. Low SES: high ACCEL_MVPA (617/2534), medium ACCEL_MVPA (794/2686, 1.11 (0.97–1.28)), low ACCEL_MVPA (1045/3099, 1.13 (0.99–1.28)). Medium SES: high (1281/6234), medium (1023/6238, 1.09 (0.99–1.19)), low (1693/5942, 1.14 (1.04–1.25)). High SES: high (5942/9080), medium (1633/8402, 1.13 (1.04–1.23)), low (1723/7366, 1.15 (1.06–1.26)). LTPA was calculated using the frequency and duration of walking for pleasure, other exercises and strenuous sports in the last 4 weeks and categorised into tertile-based thirds. Low SES: high LTPA (6360/19 384), medium LTPA (6198/19 180, 0.98 (0.95–1.02)), low LTPA (6581/20 802, 0.96 (0.93–0.99)). Medium SES: high (10 034/31 895), medium (10 712/33 502, 1.01 (0.98–1.04)), low (11 114/34 965, 1.00 (0.98–1.03)). High SES: high (12 152/38 123), medium (12 158/38 075, 0.99 (0.97–1.02)), low (10 339/32 599, 0.98 (0.96–1.01)). Household PA was assessed by asking participants the frequency and duration of light and heavy do-it-yourself activities in the last 4 weeks and categorised into tertile-based thirds. Low SES: high household PA (4576/14 106), medium: high household PA (4377/13 657, 0.98 (0.94–1.03)), low: high household PA (5024/15 399, 1.01 (0.96–1.05)). Medium SES: high (7880/24 847), medium (8378/26 283, 0.99 (0.96–1.02)), low (7979/25 241, 0.99 (0.96–1.02)). High SES: High (8364/26 384), medium (9666/30 612, 0.99 (0.96–1.02)), low (8780/27 288, 1.02 (0.99–1.05)).ACCEL_MVPA, accelerometer-measured moderate-to-vigorous physical activity; CVD, cardiovascular disease; IPAQ, International Physical Activity Questionnaire; IPAQ_MVPA, self-reported moderate-to-vigorous physical activity; LTPA, leisure-time physical activity; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity; PA, physical activity; SES, socioeconomic status.
Sitting time (except highest tertile in high SES) and screen time were not associated with incident CVD across all individual-level SES groups (online supplemental figure S9). Compared with participants in the lowest sitting time tertile, high SES participants in the highest tertile were at 13% higher hazard of incident CVD (HR 1.13, 95% CI 1.03 to 1.23).
Stratified by area-level SES
We observed a clear SES gradient of association of low ACCEL_MVPA with incident CVD; the detrimental associations became stronger with decreasing area-level SES (online supplemental figure S10). For example, compared with high ACCEL_MVPA, HRs of low ACCEL_MVPA were 1.20 (95% CI 1.09 to 1.32), 1.13 (95% CI 1.03 to 1.24) and 1.14 (95% CI 0.98 to 1.32) in low, medium and high area-level SES, respectively. IPAQ_MVPA, LTPA and household physical activity were not associated with incident CVD across all SES groups (online supplemental figure S10).
The deleterious association of high sitting time tertile with incident CVD was observed in medium SES only (online supplemental figure S11). Screen time was not associated with incident CVD across all area-level SES strata.
We have provided e-values for all significant associations in online supplemental table S6. More than half of all e-values for significant associations in the main analysis had an HR of >1.50. For example, an unmeasured confounder would have to have an association of 3.00 with the exposure and outcome to explain away the observed HR of 1.80 of low ACCEL_MVPA and ACM association in low individual-level SES, but weaker confounding could not do so.
Discussion
This study investigated if SES modifies the association of physical activity and sedentary behaviour with ACM and incident CVD. We found detrimental associations of low MVPA with ACM and incident CVD and of high screen time with ACM, with some evidence of stronger detrimental associations in low SES groups. Our findings suggested some variability in the interaction effects of SES on exposure–outcome associations, depending on the SES and physical activity measure we tested. SES patterns were clearer for individual-level SES while using self-reported MVPA and for area-level SES while using device-measured MVPA. These findings may inform public health policy and practice by identifying vulnerable individuals and priority target groups for physical inactivity and sedentary behaviour interventions.
SES may influence an individual’s access to health information, treatment choices, compliance to treatment regimens, quality of care and social support, resulting in differential prognosis for similar risk factors or health conditions.54 Previous studies have suggested that low socioeconomic groups may suffer disproportionate harm from unhealthy behaviours such as smoking10 55 and alcohol consumption.56 However, there is limited evidence on the interaction of SES and physical activity and sedentary behaviour for prospective health outcomes.5 Studies using a single individual-level SES measure have shown inconsistent results. For example, Moore et al 57 found a stronger beneficial association of higher LTPA with mortality among those with a college education than those with high school or less education (HR 0.62, 95% CI 0.59 to 0.65, vs HR 0.57, 95% CI 0.54 to 0.59). In contrast, Arem et al reported no interaction of education and LTPA for mortality risks.20 In our study, the detrimental associations of low physical activity and high sedentary behaviour were more pronounced in low SES, suggesting that SES may interact with physical activity and sedentary behaviour for mortality and incident CVD risks. This finding supports the vulnerability hypothesis, which suggests unhealthy lifestyles may inflict more harm in low socioeconomic groups5 10 and is consistent with studies on other unhealthy behaviours such as smoking10 55 and alcohol consumption.56
We found some gradient of stronger detrimental associations of self-reported MVPA with ACM in low individual-level SES, but the patterns were not clear for incident CVD. Though there was detrimental association of low self-reported MVPA in all SES groups, we found some evidence of more pronounced detrimental association in low SES. For example, participants of low, medium and high individual-level SES with low MVPA were at 22%, 15% and 14% higher hazard of ACM, respectively, compared with those with high MVPA (with approximately 50% overlap in the 95% CI of the low and high SES). These findings are in line with previous studies that have shown more consistent and stronger detrimental associations of unhealthy lifestyles in low SES than their affluent counterparts.5 6 A previous UK Biobank analysis showed a higher mortality risk among those with the least healthy lifestyles in the most deprived fifth compared with the least deprived one (HR 2.47, 95% CI 2.04 to 3.00, vs HR 1.65, 95% CI 1.25 to 2.19).5 Besides a higher prevalence of unhealthy lifestyle factors,5 56 other potential explanations for these higher ACM hazards in low SES include exposure to chronic stressors, poor access to information, lower levels of social support,4 5 58 and limited health literacy. Targeted primary prevention interventions aimed at increasing physical activity in low SES groups may partly address socioeconomic inequalities in health. Combining approaches such as ‘high-risk strategy’ (focusing on those who are physically inactive and/or highly sedentary) and ‘vulnerable population approach’ (focusing on lower SES groups) might be useful.59
Regarding device-measured physical activity, our results showed higher ACM and incident CVD risk of low device-measured MVPA, and these associations were accentuated with decreasing area-level SES. Effect modification by individual-level SES was less clear for device-measured physical activity, where the high SES group had more pronounced detrimental associations with incident CVD. The differential findings between self-reported and device-measured physical activity exposures may be due to differential measurement properties of the two approaches,60 the selective nature of self-reported physical activity instruments (eg, capturing bouts lasting at least 10 continuous minutes and mostly LTPA) and a weaker correlation of these two measurement approaches in low SES.30 31
The socioeconomic patterning of the physical activity domains–mortality association was unclear. Our findings are in agreement with a previous study,20 which found no statistically significant interaction (p=0.090) by education in the LTPA–mortality associations. At the same time, it contradicts another study57 that reported stronger beneficial associations of LTPA with mortality among those with higher education. These inconsistencies in the literature highlight the complex role of SES in physical activity domains–outcome associations and suggest the need for future research to better understand the interaction effects of SES and any underlying mechanisms. We observed no association of LTPA and household physical activity with incident CVD across SES groups (for both individual- and area-level SES), which could partly be due to the lack of overall association between these domains and incident CVD in our study (online supplemental table S5). For sedentary behaviour, we found detrimental associations of high screen time with ACM, and these associations became stronger with decreasing area-level SES. In contrast, the effect modification of SES on the associations of sitting time with both outcomes was less clear.
Our results indicated variability in the interaction effects based on the SES measure used. SES patterns were clearer for individual-level SES (self-reported MVPA) and for area-level SES (device-measured MVPA). A possible explanation is that area-level SES is more reflective of total movement as captured by accelerometry, while individual-level SES reflects better leisure time PA, which is what questionnaires capture mostly. Previous studies have also shown mixed results depending on the SES measure used. Foster et al,5 in their previous UK Biobank analysis, reported a higher disproportionate risk of a least healthy lifestyle on ACM in low individual-level and area-level SES.5 In contrast, Zhang et al 6 reported stronger lifestyles–mortality associations for individual-level SES than that for area-level SES and attributed this to less sensitivity of postcode-derived SES to social causes of health, individual differences, confusion with environmental health determinants and low reliability for heterogenous and mobile communities.6 However, area-level SES might also contribute to health inequalities through differential access to material resources (physical activity infrastructures, health facilities, etc), crime, overcrowding and differences in individual-level SES (eg, limited access to quality schools).23 Our findings further add nuance to the literature and highlight the complex role of SES in health behaviours–outcome associations. Taken together, interventions targeting physical inactivity and high sedentary behaviour in low SES groups (individual-level and area-level) might provide the greatest return. We recommend incorporating both individual- and area-level SES measures in future studies to better understand this relationship.
Strengths and limitations
To our knowledge, this is the first study examining the interaction effect of area-level and individual-level socioeconomic indices and domain-specific physical activity and sedentary behaviour with ACM and incident CVD using both self-reported and device-measured data. Using two SES indicators (individual level and area level) provided a comprehensive understanding of possible interaction effects. We accounted for competing risks using a subdistribution hazard model and excluded underweight participants and those with poor self-rated health with possible undiagnosed, subclinical conditions. E-values indicated that it is less likely that the associations we observed are due to unmeasured confounding.
UK Biobank has a low response rate (5.5%) and a higher prevalence of affluent participants of white ethnic background than the general UK population.61 However, recent evidence shows that physical activity estimates of long-term health outcomes (including ACM and CVD mortality) are not materially affected by poor representativeness and low response rates.62 Possible misreporting of physical activity participation31 and covariates between high and low SES might have affected our results. Greater misreporting of physical activity participation in low SES participants31 might have attenuated the associations, suggesting possibility of even stronger real associations. Despite extensive measures we took (excluding participants with poor self-rated health, prevalent CVD or an event (death or CVD event) within up to 3 years of recruitment), reverse causality is still a possibility, and this study’s observational nature limits inferences about causality.
Conclusion
Compared with higher SES groups, low SES groups showed modest evidence of more pronounced inverse associations of MVPA with ACM and incident CVD, and direct association of screen time with ACM. Our results suggested some variability in the interaction effects based on the SES and physical activity measures we tested. We observed consistent and clear interactions of individual-level SES in the association of self-reported MVPA with ACM. In comparison, area-level SES showed some evidence of interactions in the associations of device-measured MVPA with both outcomes and of screen time with ACM. Results were less clear for physical activity domains and device-measured sitting time. Public health interventions targeting physical activity and sedentary behaviour might need to focus on both low SES individuals as well as low SES areas for greater returns. Further research is needed to establish this evidence and better understand the mechanisms underlying these findings.
Acknowledgments
The data underlying this article were provided by UK Biobank under Application Number 25813. The authors thank all the participants and professionals who contributed to the UK Biobank.
Footnotes
Twitter: @SusanPaudel8, @M_Stamatakis
Correction notice: This article has been corrected since it published Online First. The size of the figures have been increased for clarity.
Contributors: SP and ES conceptualised the study. SP carried out the analysis and prepared the original manuscript. MA, PP, MH and ES contributed to the composition and editing of the full manuscript over several rounds of revisions. All authors have read and agreed on the final version of the manuscript. SP is the guarantor and accepts full responsibility for the work and the conduct of the study.
Funding: This work was supported by a National Health and Medical Research Council Leadership Level 2 Investigator Grant (ES, grant code APP1194510).
Competing interests: None declared.
Patient and public involvement: Patients and the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants, and the National Health Service National Research Ethics Service (Ref 11/NW/0382) approved the use of deidentified data from the UK Biobank. The participants consented to the use of deidentified data and health records and gave informed consent to participate in the study before taking part.
References
- 1. Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099–104. 10.1016/S0140-6736(05)71146-6 [DOI] [PubMed] [Google Scholar]
- 2. Marmot M, Allen J, Goldblatt P. n.d. A social movement, based on evidence, to reduce inequalities in health: fair society, healthy lives (the marmot review). Social Science & Medicine 1982;71:1254–8. [DOI] [PubMed] [Google Scholar]
- 3. Khanolkar AR, Chaturvedi N, Kuan V, et al. Socioeconomic inequalities in prevalence and development of multimorbidity across adulthood: a longitudinal analysis of the MRC 1946 national survey of health and development in the UK. PLoS Med 2021;18:e1003775. 10.1371/journal.pmed.1003775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol 2010;36:349–70. 10.1146/annurev.soc.012809.102529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster HME, Celis-Morales CA, Nicholl BI, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health 2018;3:e576–85.:S2468-2667(18)30200-7. 10.1016/S2468-2667(18)30200-7 [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y-B, Chen C, Pan X-F, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ 2021;373:n604. 10.1136/bmj.n604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stringhini S, Carmeli C, Jokela M, et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet 2017;389:1229–37.:S0140-6736(16)32380-7. 10.1016/S0140-6736(16)32380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lago S, Cantarero D, Rivera B, et al. Socioeconomic status, health inequalities and non-communicable diseases: a systematic review. Z Gesundh Wiss 2018;26:1–14. 10.1007/s10389-017-0850-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaneda T, Zimmer Z, Fang X, et al. Gender differences in functional health and mortality among the Chinese elderly: testing an exposure versus vulnerability hypothesis. Res Aging 2009;31:361–88. 10.1177/0164027508330725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pampel FC, Rogers RG. Socioeconomic status, smoking, and health: a test of competing theories of cumulative advantage. J Health Soc Behav 2004;45:306–21. 10.1177/002214650404500305 [DOI] [PubMed] [Google Scholar]
- 11. Diderichsen F, Evans T, Whitehead M. The social basis of disparities in health. In: Challenging inequities in health: From ethics to action 1. 2001: 12–23. [Google Scholar]
- 12. Hoven H, Siegrist J. Work characteristics, socioeconomic position and health: a systematic review of mediation and moderation effects in prospective studies. Occup Environ Med 2013;70:663–9. 10.1136/oemed-2012-101331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrell L, Hollingsworth B, Propper C, et al. The socioeconomic gradient in physical inactivity: evidence from one million adults in England. Soc Sci Med 2014;123:55–63.:S0277-9536(14)00692-3. 10.1016/j.socscimed.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 14. Sport England . Active lives adult survey may 2020/21 report. London: Sport England, 2021. [Google Scholar]
- 15. Scottish Government . Scottish health survey 2019 - headline findings on physical activity, health and well-being. Scottish Government; 2019. Available: https://www.nature.scot/doc/scottish-health-survey-2019-headline-findings-physical-activity-health-and-well-being#Adult+physical+activity [Google Scholar]
- 16. Lindgren M, Börjesson M, Ekblom Ö, et al. Physical activity pattern, cardiorespiratory fitness, and socioeconomic status in the SCAPIS pilot trial — a cross-sectional study. Preventive Medicine Reports 2016;4:44–9. 10.1016/j.pmedr.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindsay T, Westgate K, Wijndaele K, et al. Descriptive epidemiology of physical activity energy expenditure in UK adults (the fenland study). Int J Behav Nutr Phys Act 2019;16:126.:126. 10.1186/s12966-019-0882-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. da Silva IC, van Hees VT, Ramires VV, et al. Physical activity levels in three Brazilian birth cohorts as assessed with RAW triaxial wrist accelerometry. Int J Epidemiol 2014;43:1959–68. 10.1093/ije/dyu203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gidlow C, Johnston LH, Crone D, et al. A systematic review of the relationship between socio-economic position and physical activity. Health Education Journal 2006;65:338–67. 10.1177/0017896906069378 [DOI] [Google Scholar]
- 20. Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–67. 10.1001/jamainternmed.2015.0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the pure study. Lancet 2017;390:2643–54.:S0140-6736(17)31634-3. 10.1016/S0140-6736(17)31634-3 [DOI] [PubMed] [Google Scholar]
- 22. Xu C, Furuya-Kanamori L, Liu Y, et al. Sedentary behavior, physical activity, and all-cause mortality: dose-response and intensity weighted time-use meta-analysis. J Am Med Dir Assoc 2019;20:1206–1212.S1525-8610(19)30400-1. 10.1016/j.jamda.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 23. Boylan JM, Robert SA. Neighborhood Ses is particularly important to the cardiovascular health of low Ses individuals. Soc Sci Med 2017;188:60–8.:S0277-9536(17)30427-6. 10.1016/j.socscimed.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hussein M, Diez Roux AV, Mujahid MS, et al. Unequal exposure or unequal vulnerability? contributions of neighborhood conditions and cardiovascular risk factors to socioeconomic inequality in incident cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2018;187:1424–37. 10.1093/aje/kwx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapteyn A, Banks J, Hamer M, et al. What they say and what they do: comparing physical activity across the USA, England and the Netherlands. J Epidemiol Community Health 2018;72:471–6. 10.1136/jech-2017-209703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamatakis E, Ekelund U, Ding D, et al. Is the time right for quantitative public health guidelines on sitting? A narrative review of sedentary behaviour research paradigms and findings. Br J Sports Med 2019;53:377–82. 10.1136/bjsports-2018-099131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stalsberg R, Pedersen AV. Are differences in physical activity across socioeconomic groups associated with choice of physical activity variables to report? Int J Environ Res Public Health 2018;15:922. 10.3390/ijerph15050922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beenackers MA, Kamphuis CBM, Giskes K, et al. Socioeconomic inequalities in occupational, leisure-time, and transport related physical activity among European adults: a systematic review. Int J Behav Nutr Phys Act 2012;9:1–23.:116. 10.1186/1479-5868-9-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stamatakis E, Coombs N, Rowlands A, et al. Objectively-assessed and self-reported sedentary time in relation to multiple socioeconomic status indicators among adults in England: a cross-sectional study. BMJ Open 2014;4:e006034. 10.1136/bmjopen-2014-006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabia S, van Hees VT, Shipley MJ, et al. Association between questionnaire- and accelerometer-assessed physical activity: the role of sociodemographic factors. Am J Epidemiol 2014;179:781–90. 10.1093/aje/kwt330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorzelitz J, Peppard PE, Malecki K, et al. Predictors of discordance in self-report versus device-measured physical activity measurement. Ann Epidemiol 2018;28:427–31.:S1047-2797(17)31137-7. 10.1016/j.annepidem.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-Response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366:l4570. 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warren JM, Ekelund U, Besson H, et al. Assessment of physical activity-a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European association of cardiovascular prevention and rehabilitation. Eur J Cardiovasc Prev Rehabil 2010;17:127–39. 10.1097/HJR.0b013e32832ed875 [DOI] [PubMed] [Google Scholar]
- 34. DiPietro L, Al-Ansari SS, Biddle SJH, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 who physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act 2020;17:143.:143. 10.1186/s12966-020-01042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sudlow C, Gallacher J, Allen N, et al. Uk Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. The IPAQ Group . IPAQ-scoring protocol-international physical activity questionnaire [Available from]. n.d. Available: https://sites.google.com/site/theipaq/scoring-protocol
- 37. Cleland C, Ferguson S, Ellis G, et al. Validity of the International physical activity questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol 2018;18:176.:176. 10.1186/s12874-018-0642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Helmerhorst HJF, Brage S, Warren J, et al. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act 2012;9:1–55.:103. 10.1186/1479-5868-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramakrishnan R, Doherty A, Smith-Byrne K, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK Biobank cohort study. PLoS Med 2021;18:e1003487. 10.1371/journal.pmed.1003487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sipos M, Paces P, Rohac J, et al. Analyses of triaxial accelerometer calibration algorithms. IEEE Sensors J 2011;12:1157–65. 10.1109/JSEN.2011.2167319 [DOI] [Google Scholar]
- 41. Ahmadi MN, Nathan N, Sutherland R, et al. Non-wear or sleep? evaluation of five non-wear detection algorithms for RAW accelerometer data. J Sports Sci 2020;38:399–404. 10.1080/02640414.2019.1703301 [DOI] [PubMed] [Google Scholar]
- 42. Pavey TG, Gilson ND, Gomersall SR, et al. Field evaluation of a random forest activity classifier for wrist-worn accelerometer data. J Sci Med Sport 2017;20:75–80.:S1440-2440(16)30108-6. 10.1016/j.jsams.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 43. Chudasama YV, Khunti KK, Zaccardi F, et al. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med 2019;17:108. 10.1186/s12916-019-1339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Celis-Morales CA, Lyall DM, Steell L, et al. Associations of discretionary screen time with mortality, cardiovascular disease and cancer are attenuated by strength, fitness and physical activity: findings from the UK Biobank study. BMC Med 2018;16:77.:77. 10.1186/s12916-018-1063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the north routledge. 1988. [Google Scholar]
- 46. Huang B-H, Duncan MJ, Cistulli PA, et al. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med 2022;56:718–24. 10.1136/bjsports-2021-104046 [DOI] [PubMed] [Google Scholar]
- 47. Rutten-Jacobs LC, Larsson SC, Malik R, et al. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank participants. BMJ 2018;363:k4168. 10.1136/bmj.k4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walmsley R, Chan S, Smith-Byrne K, et al. Reallocation of time between device-measured movement behaviours and risk of incident cardiovascular disease. Br J Sports Med 2021;56:1008–17.:bjsports-2021-104050. 10.1136/bjsports-2021-104050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ekelund U, Tarp J, Fagerland MW, et al. Joint associations of accelero-meter measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med 2020;54:1499–506. 10.1136/bjsports-2020-103270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bradburn MJ, Clark TG, Love SB, et al. Survival analysis Part III: multivariate data analysis -- choosing a model and assessing its adequacy and fit. Br J Cancer 2003;89:605–11. 10.1038/sj.bjc.6601120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 52. Linden A, Mathur MB, VanderWeele TJ. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: the evalue package. The Stata Journal 2020;20:162–75. 10.1177/1536867X20909696 [DOI] [Google Scholar]
- 53. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9.:S1743-9191(14)00212-X. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 54. Grintsova O, Maier W, Mielck A. Inequalities in health care among patients with type 2 diabetes by individual socio-economic status (Ses) and regional deprivation: a systematic literature review. Int J Equity Health 2014;13:1–14.:43. 10.1186/1475-9276-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Birch S, Jerrett M, Eyles J. Heterogeneity in the determinants of health and illness: the example of socioeconomic status and smoking. Soc Sci Med 2000;51:307–17. [PubMed] [Google Scholar]
- 56. Jones L, Bates G, McCoy E, et al. Relationship between alcohol-attributable disease and socioeconomic status, and the role of alcohol consumption in this relationship: a systematic review and meta-analysis. BMC Public Health 2015;15:.:400. 10.1186/s12889-015-1720-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moore SC, Patel AV, Matthews CE, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 2012;9:e1001335. 10.1371/journal.pmed.1001335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stringhini S, Dugravot A, Shipley M, et al. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med 2011;8:e1000419. 10.1371/journal.pmed.1000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diderichsen F, Hallqvist J, Whitehead M. Differential vulnerability and susceptibility: how to make use of recent development in our understanding of mediation and interaction to tackle health inequalities. Int J Epidemiol 2019;48:268–74. 10.1093/ije/dyy167 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Nie J, Ferrari G, et al. Association of physical activity intensity with mortality: a national cohort study of 403 681 us adults. JAMA Intern Med 2021;181:203–11. 10.1001/jamainternmed.2020.6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–34. 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stamatakis E, Owen KB, Shepherd L, et al. Is cohort representativeness passé? poststratified associations of lifestyle risk factors with mortality in the UK Biobank. Epidemiology 2021;32:179–88. 10.1097/EDE.0000000000001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2022-105435supp001.pdf (639.1KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available.